Abstract
Objective To examine the effect of transcatheter aortic valve implantation (TAVI) versus surgical replacement of an aortic valve (SAVR) in patients with severe aortic stenosis at low and intermediate risk of perioperative death.
Design Systematic review and meta-analysis
Data sources Medline, Embase, and Cochrane CENTRAL.
Study selection Randomized trials of TAVI compared with SAVR in patients with a mean perioperative risk of death <8%.
Review methods Two reviewers independently extracted data and assessed risk of bias for outcomes important to patients that were selected a priori by a parallel guideline committee, including patient advisors. We used the GRADE system was used to quantify absolute effects and quality of evidence.
Results 4 trials with 3179 patients and a median follow-up of two years were included. Compared with SAVR, transfemoral TAVI was associated with reduced mortality (risk difference per 1000 patients: −30, 95% confidence interval −49 to −8, moderate certainty), stroke (−20, −37 to 1, moderate certainty), life threatening bleeding (−252, −293 to −190, high certainty), atrial fibrillation (−178, −150 to −203, moderate certainty), and acute kidney injury (−53, −39 to −62, high certainty) but increased short term aortic valve reintervention (7, 1 to 21, moderate certainty), permanent pacemaker insertion (134, 16 to 382, moderate certainty), and moderate or severe symptoms of heart failure (18, 5 to 34, moderate certainty). Compared with SAVR, transapical TAVI was associated higher mortality (57, −16 to 153, moderate certainty, P=0.015 for interaction between transfemoral versus transapical TAVI) and stroke (45, −2 to 125, moderate certainty, interaction P=0.012). No study reported long term follow-up, which is particularly important for structural valve deterioration.
Conclusions Many patients, particularly those who have a shorter life expectancy or place a lower value on the risk of long term valve degeneration, are likely to perceive net benefit with transfemoral TAVI versus SAVR. SAVR, however, performs better than transapical TAVI, which is of interest to patients who are not candidates for transfemoral TAVI.
Systematic review registration PROSPERO CRD42016042879
Introduction
Severe symptomatic aortic stenosis is common and, without aortic valve replacement, results in a life expectancy of less than three years.1 Each year in the United States, about 75 000 patients undergo surgical aortic valve replacements (SAVR).2 Because aortic stenosis increases with age, this number will increase with the evolving population demographic.3
Transcatheter aortic valve implantation (TAVI) is an increasingly popular alternative to SAVR, at least in part because it does not require thoracotomy.4 Current practice guidelines recommend either TAVI or SAVR in patients at high surgical risk, defined as a Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score of 8% or less, but recommend SAVR over TAVI for lower risk patients.5 6 Despite this recommendation, half of the TAVI centers in Europe perform TAVI in intermediate risk patients (STS-PROM 4-8%) and 10% of centers do so in low risk patients (risk score <4%).7
The PARTNER 2A trial compared TAVI with SAVR in intermediate risk patients (risk score 4-8% or <4% with coexisting conditions that are not represented in the STS-PROM model).8 The authors claimed non-inferiority for TAVI versus SAVR for the primary composite endpoint of death from any cause or disabling stroke at two years. Two recent meta-analyses that included patients from PARTNER 2A suggested that compared with SAVR, TAVI was associated with reduced odds of major bleeding, acute kidney injury, and new onset atrial fibrillation and with increased risks of pacemaker implantation, vascular complications, and aortic regurgitation.9 10 The reviews did not, however, address the durability of valves and need for aortic reintervention after TAVI.9 10 Moreover, the reviews failed to formally rate either the quality of the evidence or the credibility of subgroup analyses (leaving the credibility of findings uncertain) or provide absolute risks, crucial for trading off the desirable and undesirable aspects of TAVI versus SAVR.
The limitations of the prior review prompted us to perform an updated systematic review and meta-analysis of randomized controlled trials of TAVI compared with SAVR for patients at low and intermediate surgical risk. We conducted this systematic review to inform recommendations11 for the first in a new series in The BMJ of trustworthy recommendations published in response to potentially practice changing evidence,12 so called Rapid Recommendations. Our review complements a co-published meta-analysis of observational data on baseline risk to inform absolute effects13 and a systematic review on patients’ values and preferences to inform the relative importance of outcomes (box 1).14
Box 1: Linked articles in this BMJ Rapid Recommendations cluster
• Foroutan F, Guyatt GH, O’Brien K, et al. Prognosis after surgical replacement with a bioprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 2016;354:i5065. doi:10.1136/bmj.i5065
• Lytvyn L, Guyatt GH, Manja V, et al. Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open 2016;6:e014327. doi:10.1136/bmjopen-2016-014327
-
• Vandvik PO, Otto CM, Siemieniuk RA, et al. Transcatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 2016;354:i5085. doi:10.1136/bmj.i5085
summary of the results from the Rapid Recommendation process
-
• Magic App (www.magicapp.org)
expanded version of the results with multilayered recommendations, evidence summaries, and decision aids for use on all devices
Methods
Protocol
The registered study protocol is available on PROSPERO (CRD42016042879).15
Information sources
A search from a previous systematic review that we judged as comprehensive included articles up to 15 July 2012.16 We complemented that review with a search of Medline, Medline in-process, Embase, and Cochrane CENTRAL from 1 January 2012 to 12 May 2016 using a combination of keywords and MeSH terms for “aortic stenosis” AND “valve replacement”, using the sensitive search filters for therapeutic interventions developed by the Health Information Research Unit at McMaster University (appendix 1).17 18 There were no restrictions on language or publication type. We also searched all references from included studies and studies citing the included studies on Google Scholar.
Study selection
We included randomized controlled trials comparing TAVI and SAVR in patients with severe aortic stenosis and a mean risk score of 8% or less. All titles and abstracts were screened in duplicate with the Covidence online service (Alfred Health, Melbourne, Australia). If either reviewer judged that the study could meet the inclusion criteria, we assessed eligibility in duplicate using the full text.
Data collection process
Two reviewers independently abstracted data and resolved conflicts by discussion. When possible, we analyzed patients in groups to which they were randomized and from the as treated population when intention to treat data were not available.
Summary measures and patient involvement
The outcomes chosen in this research paper were influenced by two people with experience of living with aortic stenosis. It was part of a wider project and is published in the Rapid Recommendation series exploring TAVI versus SAVR for people with severe aortic stenosis.11 The two patients worked with the panel to list the outcomes that were important to them; they identified several outcomes that other panel members had identified and also uniquely highlighted pain and recovery time as critical to decision making. We were not able to find direct evidence for those outcomes in the randomized controlled trials. All outcomes are consistent with the Valve Academic Research Consortium (VARC)-2 standardized endpoint definitions.19
Risk of bias and quality of evidence
We assessed risk of bias in duplicate with a modified Cochrane tool20; reviewers resolved conflicts through consensus. With respect to missing data, we judged individual trials at high risk of bias if data from more than 10% of patients were unavailable.
We rated the certainty in the evidence informing absolute effects using the GRADE approach.21 22 All authors, in consultation with the parallel Rapid Recommendations guidelines panel,11 participated in and came to consensus regarding certainty of estimates ratings. The GRADE risk of bias assessment included plausible worst case sensitivity analyses addressing missing follow-up data.23
Synthesis of results
For dichotomous outcomes we conducted a random effects meta-analysis using both Hartung-Knapp-Sidik-Jonkman (HKSJ) 95% confidence intervals and DerSimonian and Laird confidence intervals of relative risks and chose between the two for the primary report based on plausibility of results.24 We intended to pool continuous outcomes with mean differences with a similar statistical approach. We present the DerSimonian and Laird confidence intervals for all dichotomous and continuous outcomes because the other confidence intervals were implausibly wide in 15 of the 70 (21.4%) analyses (95% confidence interval of the relative effect >50 or <0.02) and implausibly narrow in two.
For symptoms of heart failure, we used ordinal regression to estimate an odds ratio across the New York Heart Association (NYHA) scores for each study at the longest follow-up. We then pooled the odds ratios with random effects, weighted by inverse variance. We assumed and tested the proportional odds across NYHA classes for each individual study, using likelihood ratio tests. In this analysis, odds ratios can be interpreted as the odds of having a 1 point increase in NYHA class.
When available, we digitized Kaplan-Meier curves and extracted patient level data on time to event25; we took this approach for mortality. We checked the proportional hazards assumption and then fitted a Cox regression model with the study as a random effects (shared frailty) variable and report hazard ratios with confidence intervals. Sensitivity analyses were performed with random effects pooling of the hazard ratios reported in individual studies and by pooling dichotomous data at the prespecified timepoints of one month and one year.
We explored effect modification for four variables: transfemoral versus transapical approach, balloon expandable versus self expanding valve, higher perioperative risk (mean risk score ≥6%) versus lower (<6%), and high versus low risk of bias for each risk of bias criterion. We expected that trials would have outcomes more favorable to TAVI than SAVR if they used a transfemoral approach, balloon expandable valves, and enrolled patients with higher perioperative risk. Subgroup analyses were performed only if there were at least two randomized controlled trials in each subgroup or a trial’s report permitted a comparison within the trial (for example, the trial reported results separately for patients with lower versus higher risk). We compared the summary estimates from each subgroup with binary, continuous, or ordinal data with a fixed effect comparison between subgroups, except when a within study subgroup was reported. In those situations, we performed two level mixed effects regression with random effects at the study level. For subgroup analyses of time to event data with extracted patient level and within trial subgroup data available, we used a shared frailty Cox model with random effects at the study level. All primary analyses were performed with STATA v13 (StataCorp, College Station, TX).
To calculate absolute effect estimates, we applied the relative effects from this review to the best estimates of baseline risk. Baseline risk estimates were derived from a systematic review of observational studies of SAVR conducted in parallel with this review for mortality and length of hospital stay.13 We used the baseline risk from the SAVR arms of the randomized controlled trials for the other outcomes.
Results
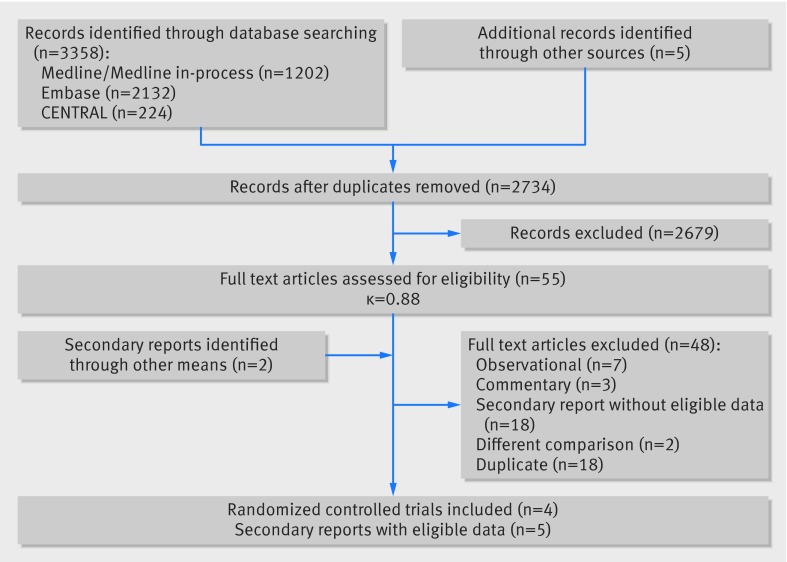
We screened 2734 unique citations, of which 55 were judged potentially eligible during screening of titles and abstracts and four were deemed eligible on full text review (fig 1). The four randomized controlled trials, all published after 2012, included 3179 patients: two trials took place in North America8 26 and two in Europe.27 28 We included additional data published in five secondary reports.29 30 31 32 33 Most patients were men (54%) and most were aged over 80 (table 1; appendix 2 provides additional study characteristics). One study included patients with a mean risk score of 7.4% but required patients with scores <8% to have additional comorbidites not included in the STS-PROM calculator.26
Fig 1 PRISMA flow diagram of studies included in review of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk
Table 1.
Characteristics of studies included in review of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis
| Trial | No randomized | Longest follow-up (months) | TAVI valve | No of women (%) | Mean (SD) age (years) | Mean (SD) risk score* | No (%) of patients | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low risk (score <4%) | NYHA class III or IV | Diabetes | Atrial fibrillation | Kidney disease | COPD | |||||||
| STACCATO | 72 | 3 | Edwards SAPIEN balloon expanding | 49 (70.0) | 81.0 (4.0) | 3.3 (1.4) | NR | 34 (48.6) | 4 (5.7) | NR | NR | 2 (2.9) |
| US Pivotal | 795 | 36 | Medtronic CoreValve self expanding | 372 (46.8) | 83.4 (6.7) | 7.4 (3.1) | 75 (9.4) | 686 (86.3) | 308 (38.7) | 351 (44.3) | 100 (12.7) | 88 (11.1) |
| NOTION | 280 | 24 | Medtronic CoreValve self expanding | 131 (46.8) | 79.1 (4.8) | 3.0 (1.6) | ~230 (81.8) | 131 (46.8) | 54 (19.3) | 74 (26.7) | 3 (1.1) | 33 (11.8) |
| PARTNER 2A | 2032 | 24 | Edwards SAPIEN XT balloon expanding | 924 (45.5) | 81.6 (6.7) | 5.8 (2.0) | ~136 (6.7) | 1558 (76.7) | 730 (35.9) | 671 (33.0) | 104 (5.1) | 627 (30.9) |
NR=not reported; NYHA=New York Heart Association; COPD=chronic obstructive pulmonary disease.
*STS-PROM (Society of Thoracic Surgeons predicted risk of mortality) risk score.
Two studies used a percutaneous retrograde approach (transfemoral),26 28 one study used a transapical approach,27 and one study used both but stratified randomization based on the heart team’s preferred approach (direct aortic approach was grouped with the transapical approach).8 Across all studies, 94.4% (n=1222) of the patients who underwent percutaneous retrograde TAVI had transfemoral access and 5.6% (n=72) had trans-subclavian access; 77.1% (n=209) of the patients who underwent non-percutaneous TAVI had transapical access and 29% (n=62) had the direct aortic approach.
Assessment of risk of bias
All four trials were at low risk of bias for allocation concealment; none blinded patients, healthcare practitioners, or data collectors, and only one attempted to blind outcome assessors8 (appendix 3). One study blinded data analysts; this study, however, had a greater degree of missing data than other studies.26 The TAVI valve industry funded three studies.8 26 28 All outcomes favoring TAVI, or transfemoral TAVI, over SAVR were robust to worst plausible sensitivity analyses.
Table 2 summarizes findings for all outcomes. Age stratified interactive summary of findings tables are available online at https://www.magicapp.org/public/guideline/aEeKpL. Appendix 4 reports abstracted outcome data by study arm.
Table 2.
GRADE summary of findings for outcomes in review of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis
| Outcome (timeframe*) | Study results (95% CI) and measurements | Absolute effect estimates (per 1000)† | Difference (95% CI) | Certainty in effect estimates (quality of evidence) | Summary | ||
|---|---|---|---|---|---|---|---|
| SAVR | TAVI | ||||||
| Transfemoral TAVI | |||||||
| Mortality‡ (2 years) | HR 0.79 (0.66 to 0.94). Based on data from 2576 patients in 3 studies; follow up 2 years | 152 | 122 | −30 (−49 to −8) | Moderate (serious imprecision) | Probably reduces risk | |
| Stroke (2 years) | RR 0.80 (0.63 to 1.01). Based on data from 2576 patients in 3 studies; follow up 2 years | 99 | 79 | −20 (−37 to 1) | Moderate (serious imprecision) | Probably reduces risk | |
| Acute kidney injury (2 years) | RR 0.38 (0.27 to 0.54). Based on data from 2576 patients in 3 studies; follow-up 2 years | 85 | 32 | −53 (−62 to −39) | High | Reduces risk | |
| Life threatening or disabling bleeding (2 years) | RR 0.39 (0.29 to 0.54). Based on data from 2576 patients in 3 studies; follow-up 2 years | 413 | 161 | −252 (−293 to −190) | High | Reduces risk | |
| Transapical TAVI | |||||||
| Mortality‡ (2 years) | HR 1.34 (0.91 to 1.97). Based on data from 552 patients in 2 studies; follow up 2 years | 196 | 253 | 57 (−16 to 153 more) | Moderate (borderline inconsistency and serious imprecision: I2=45%, wide CI) | Might increase risk | |
| Stroke (2 years) | RR 1.67 (0.97 to 2.87). Based on data from 552 patients in 2 studies; follow up 2 years | 67 | 112 | 45 (−2 to 125) | Moderate (serious imprecision: wide CI) | Probably increases risk | |
| Acute kidney injury (2 years) | RR 1.54 (0.77 to 3.07). Based on data from 552 patients in 2 studies; follow up 2 years | 43 | 66 | 23 (−10 to 89) | Low (serious imprecision and inconsistency) | Might increase risk | |
| Life threatening or disabling bleeding (2 years) | RR 0.53 (0.42 to 0.67). Based on data from 552 patients in 2 studies; follow up 2 years | 413 | 219 | −194 (−240 to −136) | High | Reduces risk | |
| TAVI v SAVR (outcomes consistent for both TAVI approaches) | |||||||
| Atrial fibrillation (2 years) | RR 0.43 (0.35 to 0.52). Based on data from 3058 patients in 3 studies; follow-up 2 years | 312 | 134 | −178 (−203 to −150) | High | Reduces risk of new onset | |
| Heart failure symptoms (NYHA ≥II) (2 years) | OR 1.29 (1.08 to 1.55). Based on data from 2146 patients in 4 studies; follow-up 2 years | 330 | 389 | 59 (17 to 103) | High | Increases risk | |
| Moderate/severe heart failure symptoms (NYHA ≥III) (2 years) | OR 1.29 (1.08 to 1.55). Based on data from 2146 patients in 4 studies; follow-up 2 years | 69 | 87 | 18 (5 to 34) | Moderate (serious imprecision) | Increases risk | |
| Aortic valve reintervention (2 years) | RR 3.25 (1.29 to 8.14). Based on data from 3058 patients in 3 studies; follow-up 2 years | 3 | 10 | 7 (1 to 21) | Moderate (serious imprecision: wide CI. Rated down for indirectness because follow-up period not long enough) | Probably increases risk | |
| Permanent pacemaker insertion (2 years) | RR 2.46 (1.17 to 5.15). Based on data from 3128 patients in 4 studies; follow-up 2 years | 92 | 226 | 134 (16 to 382) | High (I2=88% but not rated down because all studies suggested benefit) | Increases risk | |
| Myocardial infarction (2 years) | RR 0.87 (0.59 to 1.29). Based on data from 3128 patients in 4 studies; follow-up 2 years | 36 | 31 | −5 (−15 to 10) | Moderate (serious risk of bias: inadequate blinding of outcome assessors) | Might have little or no impact | |
| Health related quality of life (2 years) | Measured by: difference from baseline in KCCQ score. Minimal important difference 5 points. Scale: 0-100 (high better). Based on data from 797 patients in 1 study (US Pivotal); follow-up 2 years | Mean 18.7 points | Mean 22.2 points | 3.5 (−1.9 to 8.9) | Low (serious risk of bias and serious imprecision) | Might have little or no impact | |
| Length of index admission§ | Measured by scale (lower better). Based on data from 2032 patients in 1 study | Median 12.0 days | Median 8.0 days | −4.0 (−5 to −3) | High | Reduces length of stay | |
HR=hazard ratio, RR=relative risk, OR=odds ratio, NYHA=New York Heart Association, KCCQ=Kansas City Cardiomyopathy Questionnaire.
*Median follow-up
†Unless otherwise specified.
‡Age adjusted baseline risk of death for ages 75-85, calculated from baseline risk of death with SAVR in a linked meta-analysis of observational studies.13
§Calculated from baseline risk of death with SAVR in linked meta-analysis of observational studies.13
Outcomes favoring transfemoral but not transapical TAVI over SAVR
Mortality
At the longest follow-up (median two years), 319 of the 1578 (20.2%) patients undergoing TAVI and 340 of 1550 (21.9%) patients randomized to SAVR died (hazard ratio 0.86, 95% confidence interval 0.74 to 1.01; I2=37.6%). The one month mortality was 3.9% for TAVI and 4.0% for SAVR, despite an average predicted risk score of 5.9%.
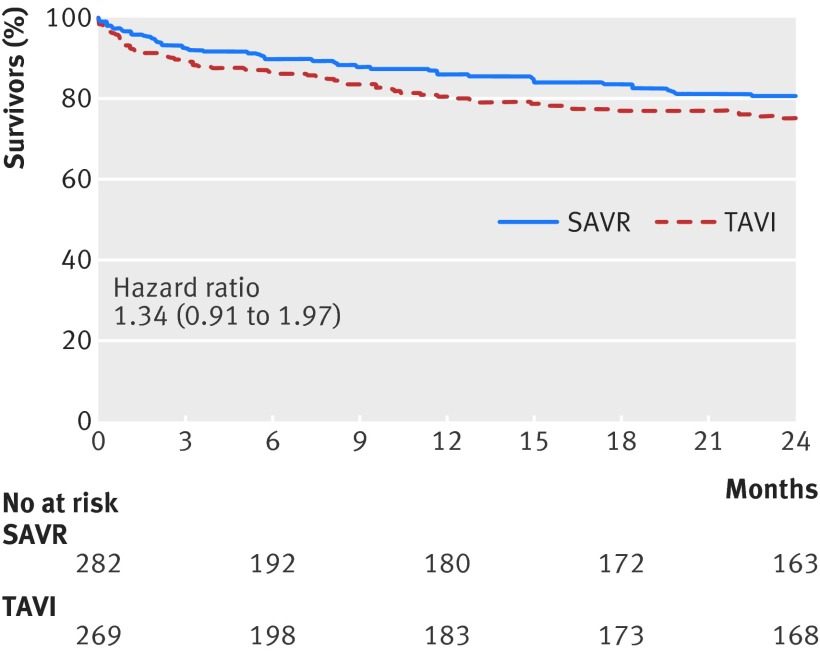
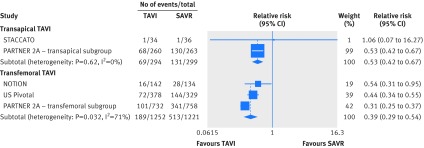
There was a significant interaction between transfemoral TAVI and transapical TAVI (P=0.015; appendix 5). Mortality was lower with transfemoral TAVI than with SAVR (hazard ratio 0.79, 95% confidence interval 0.66 to 0.94; I2=0%, 30 fewer per 1000 patients, moderate certainty; fig 2 and table 2). For transapical TAVI, the point estimate suggested harm relative to SAVR, but the confidence interval overlapped no effect (1.34, 0.91 to 1.97, I2=0%, 57 more per 1000 patients, moderate certainty; fig 3 and table 2).
Fig 2 Kaplan-Meier survival curve for transfemoral transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) for severe aortic stenosis. NOTION and PARTNER 2A provided data to 24 months, and US Pivotal provided data to 36 months
Fig 3 Kaplan-Meier survival curve for transapical transcatheter aortic valve implantation (TAVI) versus surgical aortic valve replacement (SAVR) for severe aortic stenosis. STACCATO provided data to 3 months, and PARTNER 2A provided data to 24 months
Stroke
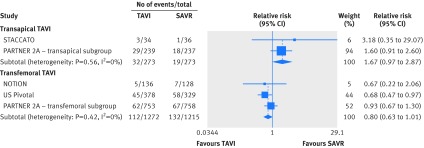
The hazard for stroke was lower with TAVI but the confidence interval overlapped no effect (hazard ratio 0.81, 95% confidence interval 0.63 to 1.01). There was an interaction by approach favoring percutaneous retrograde TAVI over transapical TAVI (P=0.012; fig 4, appendix 5). The relative risk of stroke compared with SAVR was 0.80 (0.63 to 1.01; I2=0%, 20 fewer per 1000 patients, moderate certainty; table 2) for transfemoral TAVI and 1.67 (0.97 to 2.87; I2=0%, 45 more per 1000 patients, moderate certainty; table 2) for transapical TAVI.
Fig 4 Forest plot for relative risk of stroke at longest follow-up for transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) for severe aortic stenosis, by valve approach. P=0.012 for interaction
Acute kidney injury
Acute kidney injury was less common with TAVI (relative risk 0.48, 95% confidence interval 0.27 to 0.84; I2=50%). Heterogeneity was explained by the TAVI approach (interaction P<0.001; fig 5 and appendix 5). The risk of acute kidney injury for transfemoral TAVI compared with SAVR was 0.38 (0.27 to 0.53; I2=0%, 53 fewer per 1000 patients, high certainty; table 2) and for transapical TAVI was 1.54 (0.77 to 3.07; I2=0%, 23 more per 1000 patients, low certainty; table 2).
Fig 5 Forest plot for relative risk of acute kidney injury at longest follow-up for transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) for severe aortic stenosis, by valve approach. P<0.001 for interaction
Outcomes favoring TAVI
Bleeding
The risk of life threatening or disabling bleeding was reduced with TAVI (relative risk 0.39, 95% confidence interval 0.35 to 0.45; I2=31%). Bleeding was reduced with both transfemoral TAVI (0.39, 0.29 to 0.54; I2=71%, 252 fewer per 1000 patients, high certainty; table 2) and transapical TAVI (0.53, 0.42 to 0.67; I2=0%, 194 fewer per 1000 patients, high certainty; table 2), but significantly more so with transfemoral TAVI (P=0.037 for interaction) (fig 6 and appendix 5).
Fig 6 Forest plot for relative risk of life threatening or disabling bleeding at longest follow-up for transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) for severe aortic stenosis, by valve approach. P=0.037 for interaction
Atrial fibrillation
New onset atrial fibrillation (which includes transient perioperative atrial fibrillation) was less common in patients randomized to TAVI (three studies, relative risk 0.43, 95% confidence interval 0.35 to 0.52; I2=38%, 178 fewer per 1000 patients, high certainty (table 2 and fig B in appendix 6).
Recovery time
Three trials reported length of index admission to hospital: patients in the TAVI group in the two larger studies (n=2308) were in hospital for about three and four fewer days than the SAVR group (both about 33% shorter and P≤0.001).8 28 We could not pool data because one randomized controlled trial did not report standard deviations.8 There was no significant difference in the smallest STACCATO trial, but numbers were not reported.27
Pain
No studies reported on pain after the intervention.
Outcomes favoring SAVR
Symptoms of heart failure
The TAVI group had higher odds of having 1 point worse symptoms of heart failure on the NYHA scale than the SAVR group (odds ratio 1.29, 95% confidence interval 1.08 to 1.55 (ordinal regression); I2=0%; fig C in appendix 6). For every 1000 patients, 59 (17 to 103) more patients experienced any symptoms of heart failure, of which 18 (5 to 34) were NYHA class III or IV (moderate certainty; table 2). The proportional odds assumption was not violated for any study.
Aortic valve reintervention
Aortic valve reinterventions occurred more often in the TAVI group at a median of two years (relative risk 3.25, 95% confidence interval 1.29 to 8.14; I2=0%, 7 more per 1000 patients, moderate certainty; table 2 and fig D in appendix 6).
Insertion of permanent pacemaker
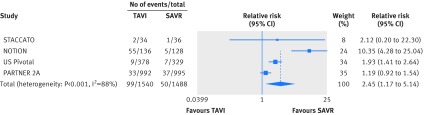
Permanent pacemaker insertion was more common with TAVI than SAVR (relative risk 2.45, 95% confidence interval 1.17 to 5.14; I2=88%, 134 more per 1000 patients, moderate certainty; table 2 and fig 7).
Fig 7 Forest plot for permanent pacemaker insertion at longest follow-up for transcatheter aortic valve implantation (TAVI) compared with surgical aortic valve replacement (SAVR) for severe aortic stenosis
Moderate or severe aortic valve regurgitation
Aortic valve regurgitation of at least moderate severity was more common in the TAVI group than in the SAVR group (three randomized controlled trials, relative risk 12.22, 95% confidence interval 5.17 to 28.88; I2=0%, 80 more per 1000 patients, high certainty; fig E in appendix 6).
Outcomes similar between groups
Myocardial infarction
There was no detectable difference in myocardial infarction between TAVI and SAVR (relative risk 0.87, 95% confidence interval 0.58 to 1.29; I2=0%, 5 fewer per 1000 patients, moderate certainty; table 2 and fig F in appendix 6).
Health related quality of life (HRQoL)
Only the US Pivotal26 and STACCATO27 trials reported HRQoL. The PARTNER 2A study protocol included HRQoL, but the primary publication did not include these data.8 The US Pivotal trial found an improvement between groups that was important to patients in overall HRQoL at one month in the TAVI group, but there was no difference with SAVR at six months and up to two years.29 30 The STACCATO trial found no differences between groups in HRQoL at three months.27
Sensitivity and other subgroup analyses
The STACCATO trial was stopped early and was the only study to exclusively use a transapical approach.27 Sensitivity analyses without STACCATO did not change statistical or clinical interpretation for any outcome.
As the included studies otherwise had similar risks of bias, subgroup analyses by risk of bias were not possible. Results at one year were similar to those at longest follow-up (appendix 7). There were no credible subgroup differences between balloon expandable and self expanding valves or between trials enrolling patients at higher or lower perioperative risk (appendix 5).
Discussion
This review shows that in patients with severe aortic stenosis, for several outcomes, transfemoral TAVI results in better outcomes relative to SAVR than the transapical approach relative to SAVR; this was true for mortality, stroke, acute kidney injury, and bleeding. These subgroup effects are highly credible. They are among a small number of a priori hypotheses with a prespecified direction, including a comparison within studies,8 chance is an unlikely explanation, and the effect is consistent across these related outcomes.34
Mortality was reduced with transfemoral TAVI compared with SAVR by about 3%, stroke by 2%, acute kidney injury by 5%, bleeding by 24%, new onset atrial fibrillation by 18%, and duration of index admission by three days. These benefits, however, come with associated harms. TAVI was associated with an increased risk of experiencing symptoms of heart failure by about 6% (2% of which were moderate or severe), permanent pacemaker insertion by about 15%, and aortic valve reintervention over the short term by about 1%.
The picture is quite different with transapical TAVI, which, though it probably shares benefits of less bleeding, less atrial fibrillation, and shorter hospital stay, increased the risk of stroke compared with SAVR by about 5% and could also increase mortality.
Strength and limitations
Strengths of this review include a comprehensive search for evidence; duplicate assessment of eligibility, risk of bias, and data abstraction; and assessments of risk of bias that included addressing loss to follow-up across studies (and found results robust to loss to follow-up).23 The review included rigorous assessment of the quality of evidence (and found the quality for many critical outcomes high and others moderate) and of the credibility of subgroup analyses (with crucial differences between transfemoral and transapical TAVR approaches). We have presented absolute and relative risks, which are crucial for making decisions between TAVI and SAVR.
Limitations include a modest total number of patients (3179) and questionable generalization of results to low risk patients (most patients were at intermediate rather than low surgical risk). The randomized controlled trials used bioprosthetic valves, typically used in older patients, in all SAVRs.5 6 Our results therefore apply only to patients who have already chosen to use a bioprosthetic valve instead of a mechanical valve. No trial reported recovery time—beyond length of hospital stay—or pain after the intervention, two outcomes that our patient representatives identified as important. The incidence of chronic pain after sternotomy is about 28% and 13% for any and moderate pain at one year, respectively, suggesting that chronic pain might be less common in TAVI.35 An unadjusted observational study that included both TAVI and SAVR patients, however, showed no difference in pain scores at three months.36 We are not able to ascertain how much of the increased risk of atrial fibrillation with SAVR represents transient postoperative atrial fibrillation—less important for patients than persistent atrial fibrillation. Further, we did not find a subgroup effect by type of TAVI valve on pacemaker insertion and thus present a single estimate of effect. We note, however, that there is evidence external to this review that self expanding valves impart a higher risk of need for pacemaker insertion than balloon expanding valves.37 Technology for TAVI38 39 and SAVR40 is continually changing, potentially further increasing the attractiveness of the TAVI option.
The most important limitation is that the relatively short duration of follow-up leaves uncertainty about one critical outcome: the need for reintervention over the longer term, a major concern with TAVI valves. We did find that TAVI is associated with a higher risk of aortic valve reintervention, although we were not able to determine whether this was because of paravalvular regurgitation or structural valve degeneration, and the absolute risk was low. The younger the patient, the greater the extent to which the uncertainty regarding the long term durability of TAVI valves is likely to influence the decision between TAVI and SAVR.
Our findings are consistent with those from recently published meta-analyses for many outcomes,9 10 but we have also provided absolute as well as relative risks and a formal rating of the quality of the evidence and documented the credibility of the crucial outcome differences between transfemoral and transapical TAVI approaches. Further, we quantified several new findings, including an increased risk of aortic valve reintervention, an increased risk of symptoms of heart failure with TAVI, and an increased risk of life threatening or disabling bleeding (rather than major bleeding, which is less important to patients) with SAVR.
In conclusion, we have clarified the trade-offs between TAVI and SAVR and identified issues of residual uncertainty. For patients with lower life expectancy (such as those aged over 85), in whom longer term valve deterioration is likely to be less of an issue, the benefits of transfemoral TAVI versus SAVR on mortality, stroke, life threatening or disabling bleeding, and a less invasive procedure are compelling. Younger patients (such as those aged 65-85), who are less concerned about the limited evidence regarding valve deterioration and the necessity for a second procedure, might (or might not) also find these mortality and morbidity benefits compelling. Even younger patients (such as those aged under 65), for whom valve longevity could be extremely important, are more likely to choose SAVR over TAVI or even to choose a mechanical over a bioprosthetic valve. Finally, patients in whom a transfemoral TAVI approach is not feasible are unlikely to view the transapical approach, which is associated with a higher rate of stroke and a possibly higher mortality rate than SAVR, as an attractive option.
What is already known on this topic
Surgical aortic valve replacement (SAVR) is more invasive than transcatheter aortic valve replacement (TAVI)
TAVI is preferred over SAVR for patients at high or extreme surgical risk
What this study adds
Success with TAVI relative to SAVR depends on the approach: transfemoral TAVI probably reduces risk of death and stroke while transapical TAVI can increase these risks
TAVI results in a 6% increased risk of symptoms of heart failure and 1% increase in valve reinterventions at two years
Long term outcomes remain uncertain
Web Extra.
Extra material supplied by the author
Appendix 1: Search strategy
Appendix 2: Additional study characteristics
Appendix 3: Risk of bias of included studies
Appendix 4: Abstracted event numbers and patients evaluated at longest follow-up
Appendix 5: Subgroup and sensitivity analyses
Appendix 6: Supplementary forest plots A-E
Appendix 7: Sensitivity analyses of outcomes at prespecified timepoints
We thank members of the Rapid Recommendations panel for critical feedback on outcome selection, GRADE judgments, and manuscript feedback, including Per Vandvik, Frederick Spencer, Rodrigo Bagur, Lyubov Lytvyn, Richard Whitlock, Trond Vartdal, David Brieger, Bert Aertgeerts, Susanna Price, Farid Foroutan, Michael Shapiro, and Ray Mertz.
Contributors: Frederick Spencer, Per Vandvik, RAS, TA, and GHG conceived the study idea. RAS performed the literature search and data analysis. RAS, TA, VM, and GHG interpreted the data analysis. RAS and VM wrote the first draft of the manuscript. TA, VM, TD, YC, and MMB acquired the data and judged risk of bias in the studies. TD extracted patient level survival data from Kaplan-Meier curves. LT provided statistical advice. TA, VM, TD, YC, MMB, and GHG critically revised the manuscript. RAS had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. RAS is guarantor.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. RAS is supported by a Vanier Canada Graduate Scholarship.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: Abstracted trial level and patient level survival data, as well as STATA code will be made publicly available on publication.
Transparency declaration: The lead author (RAS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1.Turina J, Hess O, Sepulcri F, Krayenbuehl HP. Spontaneous course of aortic valve disease. Eur Heart J 1987;8:471-83.pmid:3609042. [DOI] [PubMed] [Google Scholar]
- 2.HCUPnet. Healthcare Cost and Utilization Project Rockville, MD Agency for Healthcare Research and Quality. [Available from: http://hcupnet.ahrq.gov/. [PubMed]
- 3.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. 10.1016/S0140-6736(06)69208-8 pmid:16980116. [DOI] [PubMed] [Google Scholar]
- 4.Holmes DR Jr, , Nishimura RA, Grover FL, et al. STS/ACC TVT Registry. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. J Am Coll Cardiol 2015;66:2813-23. 10.1016/j.jacc.2015.10.021 pmid:26652232. [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Alfieri O, Andreotti F, et al. Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-96. 10.1093/eurheartj/ehs109 pmid:22922415. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura RA, Otto CM, Bonow RO, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. 10.1016/j.jacc.2014.02.537 pmid:24603192. [DOI] [PubMed] [Google Scholar]
- 7.Petronio AS, Capranzano P, Barbato E, et al. Current status of transcatheter valve therapy in Europe: results from an EAPCI survey. EuroIntervention 2016;12:890-5. 10.4244/EIJY16M06_01 pmid:27283408. [DOI] [PubMed] [Google Scholar]
- 8.Leon MB, Smith CR, Mack MJ, et al. PARTNER 2 Investigators. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. 10.1056/NEJMoa1514616 pmid:27040324. [DOI] [PubMed] [Google Scholar]
- 9.Gargiulo G, Sannino A, Capodanno D, et al. Transcatheter Aortic Valve Implantation Versus Surgical Aortic Valve Replacement: A Systematic Review and Meta-analysis. Ann Intern Med 2016;165:334-44. 10.7326/M16-0060 pmid:27272666. [DOI] [PubMed] [Google Scholar]
- 10.Siontis GC, Praz F, Pilgrim T, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of severe aortic stenosis: a meta-analysis of randomized trials. Eur Heart J 2016;ehw225 10.1093/eurheartj/ehw225 pmid:27389906. [DOI] [PubMed] [Google Scholar]
- 11.Vandvik PO, Otto CM, Siemieniuk RA, et alTranscatheter or surgical aortic valve replacement for patients with severe, symptomatic, aortic stenosis at low to intermediate surgical risk: a clinical practice guideline. BMJ 2016;354:i5085. [DOI] [PubMed] [Google Scholar]
- 12.Siemieniuk RA, Agoritsas T, Macdonald H, Guyatt GH, Brandt L, Vandvik PO. Introduction to BMJ Rapid Recommendations. BMJ 2016;354:i5191. [DOI] [PubMed] [Google Scholar]
- 13.Foroutan F, Guyatt GH, O’Brien K, et alPrognosis after surgical replacement with a biprosthetic aortic valve in patients with severe symptomatic aortic stenosis: systematic review of observational studies. BMJ 2016;354:i5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lytvyn L, Guyett GH, Manja V, et al. Patient values and preferences on transcatheter or surgical aortic valve replacement therapy for aortic stenosis: a systematic review. BMJ Open 2016;6:e0147327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemieniuk RA, Agoritsas T, Manja V, et al. PROTOCOL: Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: a systematic review and meta-analysis. PROSPERO, 2016 http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016042879. [DOI] [PMC free article] [PubMed]
- 16.Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013;2:10-23.pmid:23977554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health Information Research Unit. Search Filters for MEDLINE in Ovid Syntax and the PubMed translation 2016 http://hiru.mcmaster.ca/hiru/HIRU_Hedges_MEDLINE_Strategies.aspx.
- 18.Health Information Research Unit. Search Strategies for EMBASE in Ovid Syntax 2016 http://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx.
- 19.Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. Eur Heart J 2012;33:2403-18. 10.1093/eurheartj/ehs255 pmid:23026477. [DOI] [PubMed] [Google Scholar]
- 20.Busse JWG. G. Modification of Cochrane tool to assess risk of bias in randomized trials 2013 https://distillercer.com/wp-content/uploads/2014/02/Tool-to-Assess-Risk-of-Bias-in-Randomized-Controlled-Trials.docx.
- 21.Guyatt GH, Oxman AD, Vist GE, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. 10.1136/bmj.39489.470347.AD pmid:18436948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ. GRADE Working Group. What is “quality of evidence” and why is it important to clinicians?BMJ 2008;336:995-8. 10.1136/bmj.39490.551019.BE pmid:18456631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akl EA, Johnston BC, Alonso-Coello P, et al. Addressing dichotomous data for participants excluded from trial analysis: a guide for systematic reviewers. PLoS One 2013;8:e57132 10.1371/journal.pone.0057132 pmid:23451162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siemieniuk R, Vandvik P, Alonso-Coello P, et al. Hartung-Knapp-Sidik-Jonkman confidence intervals can be bizarrely narrow when heterogeneity is very low. Cochrane Colloquium. Seoul, Korea, 2016. [Google Scholar]
- 25.Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012;12:9 10.1186/1471-2288-12-9 pmid:22297116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams DH, Popma JJ, Reardon MJ, et al. U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-8. 10.1056/NEJMoa1400590 pmid:24678937. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen HH, Klaaborg KE, Nissen H, et alA prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention 2012;8:383-9. 10.4244/EIJV8I3A58 [DOI] [PubMed] [Google Scholar]
- 28.Thyregod HG, Steinbrüchel DA, Ihlemann N, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Valve Stenosis: 1-Year Results From the All-Comers NOTION Randomized Clinical Trial. J Am Coll Cardiol 2015;65:2184-94. 10.1016/j.jacc.2015.03.014 pmid:25787196. [DOI] [PubMed] [Google Scholar]
- 29.Arnold SV, Reynolds MR, Wang K, et al. CoreValve US Pivotal Trial Investigators. Health Status After Transcatheter or Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Increased Surgical Risk: Results From the CoreValve US Pivotal Trial. JACC Cardiovasc Interv 2015;8:1207-17. 10.1016/j.jcin.2015.04.018 pmid:26292584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S, Kumbhani DJ, Sardar P, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: an updated review of literature. Curr Cardiol Rep 2014;16:473 10.1007/s11886-014-0473-8 pmid:24585114. [DOI] [PubMed] [Google Scholar]
- 31.Deeb GM, Reardon MJ, Chetcuti S, et al. CoreValve US Clinical Investigators. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2016;67:2565-74. 10.1016/j.jacc.2016.03.506 pmid:27050187. [DOI] [PubMed] [Google Scholar]
- 32.Reardon MJ, Adams DH, Kleiman NS, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol 2015;66:113-21. 10.1016/j.jacc.2015.05.017 pmid:26055947. [DOI] [PubMed] [Google Scholar]
- 33.Søndergaard L, Steinbrüchel DA, Ihlemann N, et al. Two-Year Outcomes in Patients With Severe Aortic Valve Stenosis Randomized to Transcatheter Versus Surgical Aortic Valve Replacement: The All-Comers Nordic Aortic Valve Intervention Randomized Clinical Trial. Circ Cardiovasc Interv 2016;9:e003665 10.1161/CIRCINTERVENTIONS.115.003665 pmid:27296202. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ 2010;340:c117 10.1136/bmj.c117 pmid:20354011. [DOI] [PubMed] [Google Scholar]
- 35.Meyerson J, Thelin S, Gordh T, Karlsten R. The incidence of chronic post-sternotomy pain after cardiac surgery--a prospective study. Acta Anaesthesiol Scand 2001;45:940-4. 10.1034/j.1399-6576.2001.450804.x pmid:11576043. [DOI] [PubMed] [Google Scholar]
- 36.Kocaaslan C, Ketenci B, Yılmaz M, et al. Comparison of Transcatheter Aortic Valve Implantation versus Surgical Aortic Valve Replacement to Improve Quality of Life in Patients >70 Years of Age with Severe Aortic Stenosis. Braz J Cardiovasc Surg 2016;31:1-6.pmid:27074268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Wahab M, Neumann FJ, Mehilli J, et al. CHOICE Investigators. 1-Year Outcomes After Transcatheter Aortic Valve Replacement With Balloon-Expandable Versus Self-Expandable Valves: Results From the CHOICE Randomized Clinical Trial. J Am Coll Cardiol 2015;66:791-800. 10.1016/j.jacc.2015.06.026 pmid:26271061. [DOI] [PubMed] [Google Scholar]
- 38.Kodali S, Thourani VH, White J, et al. Early clinical and echocardiographic outcomes after SAPIEN 3 transcatheter aortic valve replacement in inoperable, high-risk and intermediate-risk patients with aortic stenosis. Eur Heart J 2016;37:2252-62. 10.1093/eurheartj/ehw112 pmid:27190101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manoharan G, Walton AS, Brecker SJ, et al. Treatment of Symptomatic Severe Aortic Stenosis With a Novel Resheathable Supra-Annular Self-Expanding Transcatheter Aortic Valve System. JACC Cardiovasc Interv 2015;8:1359-67. 10.1016/j.jcin.2015.05.015 pmid:26315740. [DOI] [PubMed] [Google Scholar]
- 40.Borger MA, Moustafine V, Conradi L, et al. A randomized multicenter trial of minimally invasive rapid deployment versus conventional full sternotomy aortic valve replacement. Ann Thorac Surg 2015;99:17-25. 10.1016/j.athoracsur.2014.09.022 pmid:25441065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Search strategy
Appendix 2: Additional study characteristics
Appendix 3: Risk of bias of included studies
Appendix 4: Abstracted event numbers and patients evaluated at longest follow-up
Appendix 5: Subgroup and sensitivity analyses
Appendix 6: Supplementary forest plots A-E
Appendix 7: Sensitivity analyses of outcomes at prespecified timepoints