Abstract
The Transient Receptor Potential (TRP) channels are a family of cationic ion channels widely distributed in mammalian tissues. In general, the global genetic disruption of individual TRP channels result in phenotypes associated with impairment of a particular tissue and/or organ function. An exception is the genetic ablation of the TRP channel TRPM7, which results in early embryonic lethality. Nevertheless, the function of TRPM7 in oocytes, eggs and pre-implantation embryos remains unknown. Here, we described an outward rectifying non-selective current mediated by a TRP ion channel in immature oocytes (germinal vesicle stage), matured oocytes (metaphase II eggs) and 2-cell stage embryos. The current is activated by specific agonists and inhibited by distinct blockers consistent with the functional expression of TRPM7 channels. We demonstrated that the TRPM7-like channels are homo-tetramers and their activation mediates calcium influx in oocytes and eggs, which is fundamental to support fertilization and egg activation. Lastly, we showed that pharmacological inhibition of the channel function delays pre-implantation embryo development and reduces progression to the blastocyst stage. Our data demonstrate functional expression of TRPM7-like channels in mouse oocytes, eggs and embryos that may play an essential role in the initiation of embryo development.
Following the fusion of a sperm with a mature oocyte, hereafter referred as egg, fertilization triggers initiation of embryo development whose first step is the egg activation. Prior to maturation, immature oocytes are arrested in prophase I of meiosis I, the germinal vesicle stage (GV). Oocytes remain arrested at this stage until puberty, when following the establishment of regular cycles and a surge of luteinizing hormone (LH), they resume meiosis (oocyte maturation), in preparation for fertilization. During maturation, oocytes undergo GV Breakdown, GVBD, complete meiosis I, and re-arrest at the metaphase II of meiosis II (MII stage), which is the stage of ovulation and fertilization1. Egg activation is widely thought to be triggered by increases in the intracellular concentrations of free Calcium ([Ca2+]i), calcium oscillations, which are induced by release of the fertilizing spermatozoon’s “sperm factor”, identified as phospholipase C zeta 1 (PLC ζ)2,3. The [Ca2+]i oscillations initiated by the sperm last several hours and their number, amplitude and frequency impact the developmental competence of the zygote4. Despite the intracellular nature of the [Ca2+]i oscillations, Ca2+ influx from the surrounding media is required to support these oscillations5,6. Indeed, studies in the mouse have shown that [Ca2+]i oscillations cease or show reduced frequency in the absence of extracellular Ca2+, which results in depletion of the internal Ca2+ stores5 and preclude egg activation and initiation of development7. Ca2+ influx is also required during maturation, as the oocyte’s internal Ca2+ stores dramatically increase in content during this process8. Nevertheless, Ca2+ influx is not required for the resumption of meiosis, although organization of the first spindle and release of the first polar body are dependent on extracellular Ca2+ and its influx9. Thus, despite a pivotal role for Ca2+ influx both during oocyte maturation and egg activation, the molecular identity and function of the Ca2+-permeant channel(s) that underlie it is unknown.
Several channels have been proposed to mediate Ca2+ influx during maturation and fertilization in mammalian oocytes. Voltage-activated Ca2+ (CaV) channels have been reported to be expressed in oocytes of different species10, including the mouse11, where they recently have been shown to contribute to the increase in intracellular Ca2+ store contents during oocyte maturation12. Despite this contribution, Cacnah−/− female mice showed only a slight reduction in litter size12 suggesting that these channels are dispensable for fertility. The Calcium Release Activated Current (CRAC) channels (also known as SOCE (Store-Operated Calcium Entry)), were described to be active in mouse GVs, but their function decreases as maturation progresses8,13. Neither CRAC nor CaVs have been shown to have essential functions during maturation or fertilization12,14,15,16.
The transient receptor potential channels, TRPs, are other possible mediators of Ca2+ influx in oocytes and eggs. TRP channels are a family of proteins that include ~30 members grouped in six subfamilies. TRP channels are in general cationic non-selective, weakly voltage sensitive, able to respond to different physiological stimuli and have a wide spectrum of physiological functions17. A member of the TRP family, TRPV3, was shown to mediate Ca2+ influx in mouse eggs. Selective activation of TRPV3 channels provokes Ca2+ entry and parthenogenetic egg activation (parthenogenesis), although its physiological function in eggs remains undetermined, as Trpv3-deficient (Trpv3−/−) mice are fertile15,18.
Most of the TRPs show tissue-specific expression and the generation of genetically modified mice has been helpful to understand their physiological function17. The ubiquitously expressed and member of the Melastatin TRP subfamily, TRPM7, is a bifunctional protein, “chanzyme”, with serine/threonine kinase and ion channel activities19,20. TRPM7 has been shown to activate downstream of protein G-coupled receptors and respond to intracellular and extracellular Mg2+, pH, and phosphate inositol diphosphate (PIP2)21. The cellular functions of TRPM7, like the majority of TRP channels, have been difficult to elucidate in part due to the lack of specific pharmacological tools. Recently, new and specific TRPM7 inhibitors and agonists have been shown to effectively target the channel under physiological conditions. Here, we took advantage of NS8593, a compound initially reported to be a K+ channel blocker22 and, of the small organic molecule, Naltriben23, to study and confirm the expression of TRPM7 channels in oocytes, eggs and embryos. It is worth noting that unlike the lack of dramatic phenotypes observed in most genetically modified mice that target TRP channels, Trpm7 knock-out mice are embryonic lethal before E7.5 of embryogenesis24,25, which further supports the need to study TRPM7 expression and function in gametes and early pre-implantation embryos. In this study, our results obtained by a combination of electrophysiological, imaging studies and in-vitro embryo culture using TRPM7 antagonists and agonists, show functional expression of the channel in oocytes, eggs and 2-cell embryos, and suggest a role in pre-implantation development, as in the presence of TRPM7 specific inhibitors, development is curtailed or slowed.
Results
Strontium oscillations are not mediated by TRPV3 channel in GV oocytes
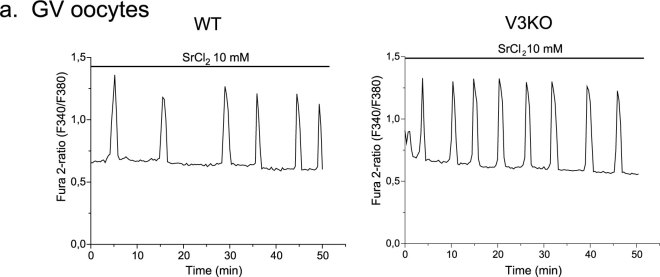
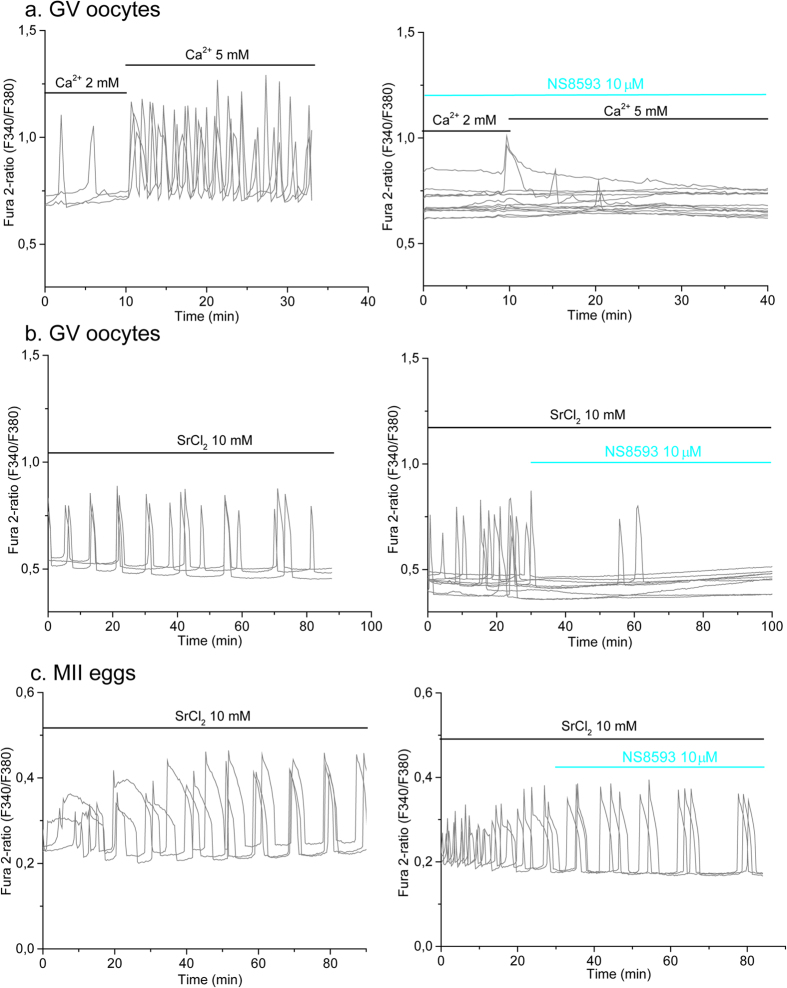
Exposure to Sr2+ promotes oscillations in both GV oocytes and MII eggs26. Nevertheless, in mice, eggs lacking TRPV3 channels are unable to mount Sr2+-induced oscillations and undergo egg activation, confirming the identity of the channel responsible for promoting Sr2+ influx in eggs15. Whether or not GV oocytes from Trpv3 KO mice are capable of displaying oscillations when exposed to Sr2+ is not known. This possibility should not be discounted given that during oocyte maturation new protein expression changes the protein profile of cytoplasmic proteins27 as well as of membrane proteins28, raising the possibility that different channels mediate Sr2+ influx in oocytes vs. eggs. To test the capacity of Trpv3−/− GV oocytes to mount Sr2+ oscillations, we exposed Wild Type (WT) and Trpv3−/− GV oocytes to Sr2+ (Fig. 1). In WT oocytes, exposure to Sr2+ induced the expected oscillations (Fig. 1a, left panel), which were comparable to those observed in Trpv3−/−oocytes (Fig. 1a, right panel). Importantly, the Sr2+ induced oscillations in WT and Trpv3−/− oocytes were unaffected by temperature, which also rules out TRPV3 as the active channel at GV, as TRPV3 is a temperature-sensitive channel29,30,31 and the measurements here were done at RT. These results support our previous findings showing that functional expression of TRPV3 channels is not detectable at the GV stage and increases during maturation15. Therefore, the channel(s) mediating Sr2+ influx in WT or Trpv3−/− oocytes is not TRPV3.
Figure 1. GV oocytes show Sr2+-induced Ca2+ oscillations that are not mediated by TRPV3 channels.
(a) Spontaneous oscillations induced by Sr2+ at RT in a WT GV oocyte (left panel, n = 8, oocytes that show 3 or more oscillations) and in a Trpv3−/− oocyte (right panel, n = 6).
Oocytes and eggs express a non-selective cationic conductance that is blocked by external Mg2+
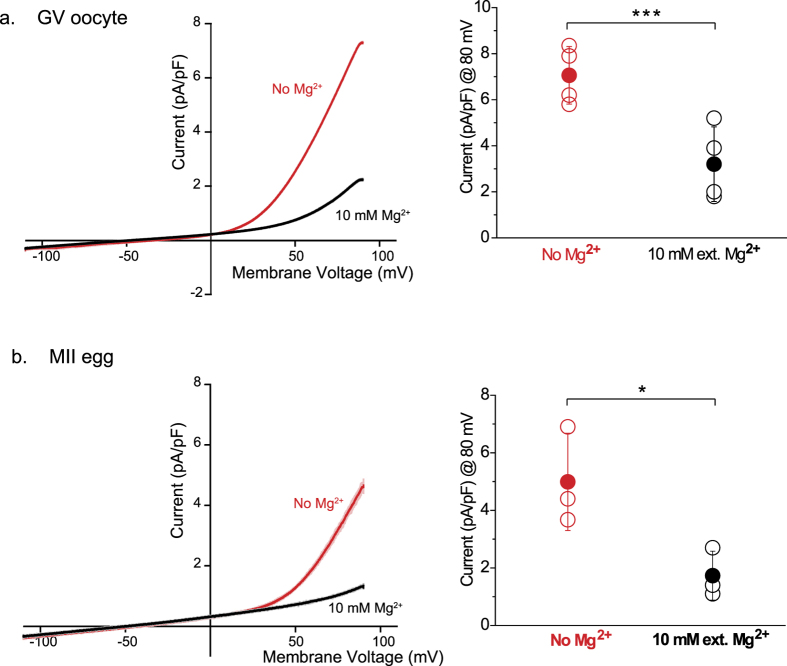
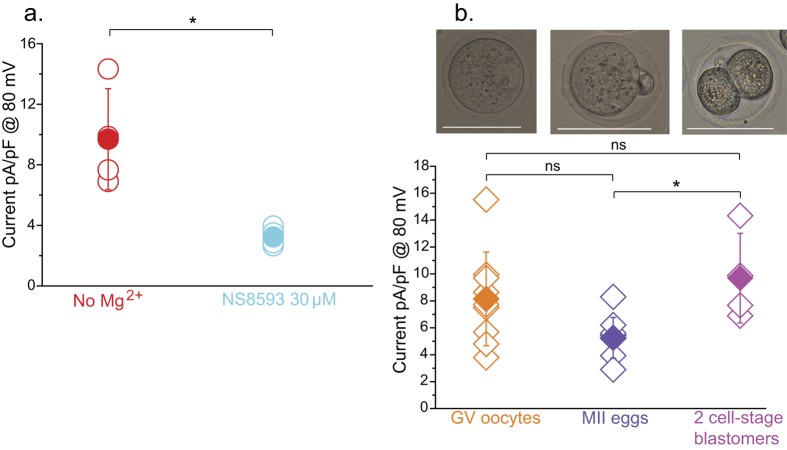
Extracellular magnesium (Mg2+) is a well-known inhibitor of some cationic non-selective channels members of the TRP family, including TRPV3 channels32, which are expressed in mouse eggs15. Moreover, extracellular and intracellular Mg2+ also blocks TRP channels of the Melastatin sub-family, TRPM6 and TRPM720,33. We therefore, tested in mouse oocytes and eggs the function of non-cationic channels blocked by this divalent cation. In order to avoid interference, if any, by TRPV3 channels in our electrophysiological recordings, we used Trpv3−/− oocytes and eggs. In those gametes and, using internal and external solutions without Mg2+, a voltage ramp evoked outwardly rectifying currents with properties characteristics of TRPM7 and/or TRPM6 (Fig. 2a,b, left panel, red trace). Addition of 10 mM extracellular Mg2+ blocked the TRPM7 or TRPM6-like current in both cells (Fig. 2a,b, left panel, black trace), reducing its magnitude (current amplitudes are summarized in Fig. 2a,b, right panels). This data shows for the first time the presence of currents that are consistent with functional expression of TRM7- and/or TRPM6-like channels on the plasma membrane (PM) of mouse oocytes and eggs.
Figure 2. A cationic non-selective channel blocked by external Mg2+ is expressed in oocytes and eggs.
(a,b) Whole-cell voltage clamp recordings from oocytes and eggs. (a) Left panel: Current evoked from a voltage ramp from −100 to +100 mV in the absence (red trace) and presence of 10 mM external Mg2+ (black trace) in GV oocytes. Right panel: Averaged current recorded at +80 mV (7, 1 ± 1, 25 pA/pF for recordings in absence of Mg2+; n = 4. 3, 2 ± 1, 62 pA/pF for recordings in presence of external Mg2+; n = 4). (b) Current evoked from a voltage ramp from −100 to +100 mV in the absence (red trace) and presence of 10 mM external Mg2+ (black trace) in MII eggs. Right panel: Averaged current recorded at +80 mV. (5 ± 1, 7 pA/pF for recordings in absence of Mg2+; n = 3. 1, 7 ± 0, 85 pA/pF for recordings in presence of external Mg2+; n = 3). ± SD. ***P < 0.001, *P < 0.05.
Pharmacological assessment of the current suggests expression of TRPM7 channels in mouse oocytes and eggs
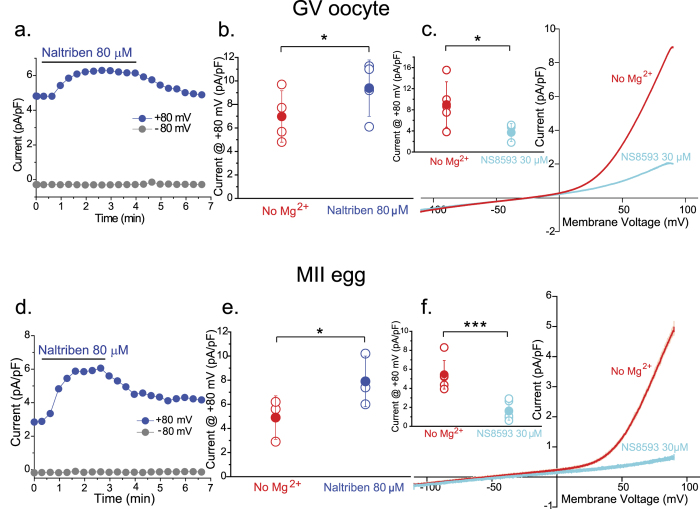
To test the functional expression of TRPM7 in oocytes and eggs, we made use of a series of compounds with specificity for this channel and determined how they affected its function. TRPM7 current can be blocked by modulators targeting small conductance Ca2+-activated K+ channels (SK)22. NS8593, a gating modulator of the KCa 2.1–2.3 channels, was recently found to block with higher selectivity over other TRP channels, currents mediated by native and transfected TRPM7 channels22. On the other hand, Naltriben, an organic compound, was recently shown to selectively activate TRPM7 channels under physiological conditions23. We therefore, took advantage of these pharmacological agents and performed electrophysiological recordings in oocytes and eggs in the absence of intracellular and extracellular Mg2+. We also performed these studies in Trpv3−/− oocytes and eggs, as some modulators of TRPM7 channels can affect the activity of TRPV3 channels34,35. In response to a voltage ramp, Naltriben elicited a TRPM7-like current in both oocytes (Fig. 3a,b) and eggs (Fig. 3d,e), and in its presence the average current amplitude was higher than the basal current in both cell types (summarized, Fig. 3b,e). Addition of NS8593 effectively blocked the TRPM7- like current (Fig. 3c,f). Collectively, these results are consistent with functional expression of TRPM7-like channels in mouse oocytes and eggs.
Figure 3. Current responses to drugs targeting TRPM7 channels in mouse oocytes and eggs.
(a,f) Whole-cell voltage clamp recordings from oocytes (a–c) and eggs (d–f). (a) Whole-cell patch clamp recording in response to 80 μM Naltriben (red bar). (b) Averaged current recorded at +80 mV (7 ± 2, 19 pA/pF for recordings in basal conditions (No external Mg2+, red symbol); n = 4. 9, 4 ± 2, 4 pA/pF for current responses to 80 μM Naltriben; n = 4). (c) Current evoked from a voltage ramp from −100 to +100 mV in response to 30 μM NS8593 (cyan). Insert: Averaged current recorded at +80 mV (9 ± 4, 3 pA/pF for basal responses; n = 5. 3, 7 ± 1, 7 pA/pF for responses to 30 μM NS8593; n = 5). (d) Representative trace of a voltage-clamp recording on an egg in response to 80 μM Naltriben (red bar). (e) Averaged current recorded at +80 mV (4, 9 ± 1, 8 pA/pF for recordings in basal conditions (No external Mg2+, red symbol); n = 3. 7, 9 ± 2, 1 pA/pF for current responses to 80 μM Naltriben; n = 3). (f) Current-Voltage (I-V) relation in response to a voltage ramp (−100 mV to +100 mV) in basal conditions (no external Mg2+, red trace) and in response to 30 μM NS8593 (cyan). Insert: Averaged current recorded at +80 mV (5, 4 ± 1, 5 pA/pF for basal responses; n = 6. 1, 6 ± 0, 9 pA/pF for responses to 30 μM NS8593; n = 6). ± SD. ***P < 0.001, *P < 0.05.
Naltriben induces Ca2+ influx in mouse oocytes and eggs
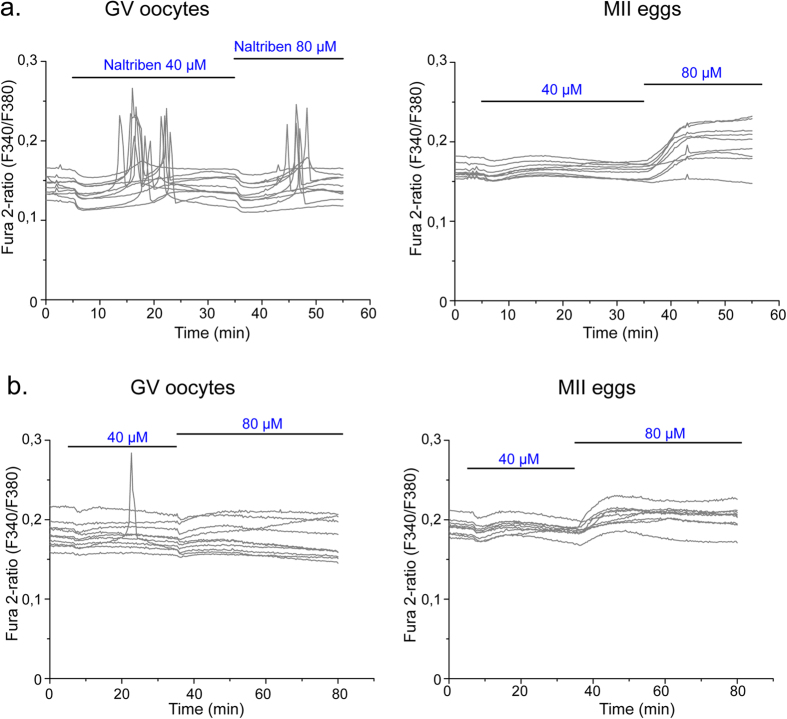
Ca2+ influx is required to fill the stores during maturation and to refill them following fertilization27, although the channels and/or transporters through which this is accomplished are unknown. To further evaluate the capacity of TRPM7 to mediate Ca2+ influx in oocytes and eggs, we tested the [Ca2+]i responses induced by the TRPM7-specific activator Naltriben. WT oocytes responded to a wide range of Naltriben concentrations with brief [Ca2+]i peaks, which were preceded by a transient decrease in baseline [Ca2+]i (Fig. 4a left panel). On the other hand, MII eggs required higher concentration of Naltriben and the response seems to be sustained (Fig. 4a, right panel). To confirm that [Ca2+]i increases induced by Naltriben were caused by Ca2+ influx and not due to intracellular off targets effects of the drug, we exposed the cells to Naltriben in media without Ca2+. Oocytes and eggs failed to show [Ca2+]i increase in response to the agonist in absence of Ca2+ in the media (Fig. 4b, left and right panels). These results suggest that Naltriben-induced [Ca2+]i increases in oocytes and eggs are caused by Ca2+ influx mediated by TRPM7 channels.
Figure 4. The TRPM7 agonist Naltriben induces [Ca2+]i responses in oocytes and eggs.
Changes in [Ca2+]i were induced by repeated application of 40 and 80 μM Naltriben (black horizontal bars) in Ca2+-containing media (upper panels) or in Ca2+ free-media (lower panels). (a) Left panel, GV oocytes. n = 9. Right panel: MII eggs. n = 9. (b) Left panel, GV oocytes. n = 10. Right panel: MII eggs. n = 10. Note that in Ca2+ free-media application of Naltriben failed to induce major [Ca2+]i changes.
NS8593 blocked Ca2+ and Sr2+ induced oscillations in oocytes but not Sr2+-induced oscillations in eggs
GV oocytes display spontaneous [Ca2+]i oscillations which are inhibited by high concentration of Nickel, an unspecific blocker of Ca2+ channels36. The channel(s) that mediates Ca2+ influx supporting these oscillations in oocytes is unknown. As we have shown in this study, TRPM7-like channels expressed in oocytes are able to mediate Ca2+ influx. Therefore, to further evaluate the role of TRPM7 channels supporting Ca2+ oscillations in oocytes, we tested the efficacy of NS8593 in blocking these [Ca2+]i responses. As expected, WT oocytes displayed spontaneous [Ca2+]i oscillations in presence of 2 mM extracellular Ca2+ (Fig. 5a, left panel). The frequency of the oscillations rapidly increased by bringing the external Ca2+ concentration to 5 mM (see Fig. 5a, left panel), suggesting enhanced Ca2+ influx. The addition of NS8593 to the culture media prevented both spontaneous and Ca2+ (5 mM) enhanced oscillations (Fig. 5a right panel). These results suggest that TRPM7-like channels may underlie [Ca2+]i oscillations in GV oocytes.
Figure 5. NS8593 blocks [Ca2+]i and Sr2+ induced oscillations in oocytes.
Spontaneous [Ca2+]i oscillations were induced and measured at RT in response to increasing concentrations of extracellular Ca2+ and 10 mM Sr2+ in WT oocytes. (a) Left panel, [Ca2+]i oscillations were evaluated in response to increased extracellular Ca2+ (black horizontal bars). Figure shows representative traces for 3 oocytes. n = 9. Right panel: Oocytes were exposed to 10 μM NS8593 (cyan bar) and Ca2+ oscillations were evaluated after increasing external Ca2+ concentration from 2 or 5 mM (black horizontal bars). n = 13. (b) Left panel: Representative traces for 3 oocytes showing Sr2+-induced oscillations. n = 5, oocytes that show 3 or more oscillations. Right panel: Sr2+-induced oscillations were blocked by exposure to 10 μM NS8593 (cyan bar). n = 9. (c) Oscillations induced by Sr2+ at 33–35 °C (left panel, n = 5. Figure shows representative traces for 3 eggs) are not blocked by 10 μM NS8593 (cyan bar; right panel, n = 7. Figure shows representative traces for 3 eggs).
As previously shown by others and us, external Sr2+ induces periodic Ca2+ oscillations in oocytes (Fig. 5b left panel)26 and eggs15,26 (Fig. 5c left panel), although the channel(s) that mediate this influx in GV oocytes remains unknown. We therefore examined if NS8593 could block Sr2+ induced oscillations in WT GV oocytes and eggs. We found that whereas addition of NS8593 blocked oscillations in GV oocytes (Fig. 5b right and left panel) it was without effect in MII eggs (Fig. 5c right and left panel). Our results suggest that a common channel, most likely TRPM7-like channels, mediate Ca2+ and Sr2+ influx in GV oocytes. In agreement with our previous data15, these results also point out that TRPM7 channel does not contribute to the Sr2+-induced oscillations observed in eggs, clearly indicating that different channels mediate Sr2+ influx in GV oocytes and MII eggs. Finally, our data showed that NS8593 does not inhibit TRPV3 channels.
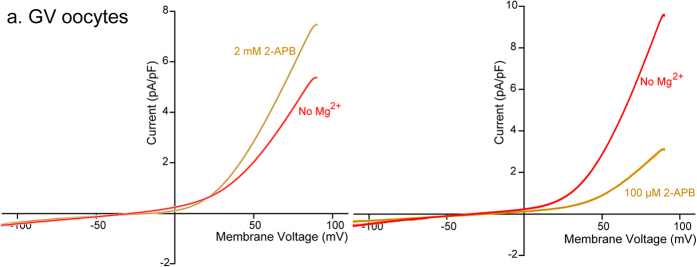
TRPM7 homomers and not heteromers with TRPM6 are expressed in oocytes
Like TRPM7, TRPM6 channels belong to the subfamily Melastatin of TRP channels and, contain an α serine/threonine kinase domain. TRPM6 homo-oligomers show slightly higher permeability to Mg2+ than Ca2+, and are modulated by Mg2+ and ATP37. TRPM6 has been shown to form hetero-tetramers with TRPM738, and it was of interest to determine the structural composition of the TRPM-like channels expressed in oocytes and eggs. To accomplish this, we used electrophysiological recordings and 2-APB, which besides being both an unspecific Ca2+ channel blocker and an agonist of TRPV3 channels34,39, it has dual pharmacological effects on TRPM6 and TRPM7 channels; this effect can be used to discriminate whether these channels are assembled in homomeric or heterotetramic configurations35. To avoid any contamination of the TRPV3 currents in our recordings15,40, we performed the electrophysiological recordings in Trpv3−/− oocytes (Fig. 6) and eggs (see Supplementary Fig. S1). We used two concentrations of 2-APB to examine the currents in response to a voltage ramp; the low concentration was 100 μM, and the high concentration was 2 mM. The low concentration of 2-APB was expected to block TRPM7 channels but increase currents through TRPM6 homo-oligomers or M6-M7 hetero-tetramers, whereas the high concentration of 2-APB was expected to activate TRPM7 currents41. Accordingly, the cationic currents evoked by a voltage ramp in oocytes showed a significant increase when exposed to 2 mM 2-APB (Fig. 6a, left panel), whereas exposure to 100 μM 2-APB resulted in a marked inhibition of the current (Fig. 6a, right panel). Together, these results suggest that TRPM7 homo-tetramers mediate the current recorded in oocytes and eggs.
Figure 6. Modulation of native TRPM7 current by 2-APB in oocytes.
Whole-cell voltage clamp recordings from oocytes. Current evoked from a voltage ramp from −100 to +100 mV. Left panel. 2-APB 2 mM significantly increased TRPM7 current in GV oocytes (n = 4). Right panel. 2-APB 100 μM blocked TRPM7 current (n = 4).
TRPM7 functional expression persists post-fertilization
Fertilization triggers the completion of the second meiosis, rendering eggs into zygotes and leading to the first mitotic cleavage and to the 2-cell stage embryo. Ca2+ influx is required to support oscillations during the zygote stage, although for how long these channels remain functional in the zygote and/or embryos is not known. Here, we examined the functional expression of TRPM7-like channels 40 h post-fertilization, which corresponds to the late 2-cell stage. To accomplish this, recovered zygotes were allowed to cleave to 2-cell stage embryos and the blastomeres were mechanically separated and treated as units. To detect the presence of the TRPM7-like current in these cells, we evaluated the current response to a voltage ramp using agonists and inhibitors of the channel. We found TRPM7-like channels are expressed in 2-cell blastomeres, as the detected cationic non-selective current was blocked by NS8593 (Fig. 7a). In support of the presence of an active TRPM7 channel in embryos, its agonist Naltriben increased the non-selective current expressed in blastomeres (Supplementary Fig. S2). To assess whether the recorded current was similar to that detected in oocytes and eggs, we compared the density of the currents in 2-cell stage blastomeres to that in oocytes and eggs. Our results show that the TRPM7-like current seemed to experience a decline from the GV to the MII stage, with fertilization inducing an increase in the current that now appears to similar to that detected in GV oocytes (Fig. 7b). Altogether, we interpret our results to mean that TRPM7 channels are functionally expressed in both GV oocytes and eggs and post-fertilization at least until the 2-cell stage embryos.
Figure 7. TRPM7 channel is expressed after fertilization.
(a) Whole-cell patch clamp recordings in blastomeres from 2-cell stage obtained after fertilization. Averaged current at +80 mV is showed. Red symbols: Basal current, 9, 7 ± 3, 3 pA/pF, n = 4. Cyan: Current in response to 30 μM NS8593, 3, 2 ± 0, 6 pA/pF, n = 4. (b) Comparison of basal current density between GV oocytes (orange symbols; 8, 1 ± 3, 5 pA/pF, n = 9), MII eggs (blue diamonds; 5, 2 ± 1, 5 pA/pF, n = 9) and 2-cell stage blastomers (violet symbols; 9, 7 ± 3, 3 pA/pF, n = 4) in absence of external Mg2+ measured at +80 mV. ± SD. *P < 0.05. Scale bar: 50 μm.
TRPM7 blockade prevented pre implantation development in mouse embryos
The finding that the TRPM7-like current is detectable in embryos led us to further evaluate the possible function of TRPM7 on early pre-implantation embryo development. To accomplish this we examined the progression of embryo development in the presence of the TRPM7 inhibitor NS8593. WT zygotes were collected 15 h post hCG injection and exposed to the drug soon after with the notion to avoid any possible effect of NS8593 on sperm entry and on initiation of [Ca2+]i oscillations. We found that whereas cleavage to the 2-cell stage was undisturbed in the presence of the inhibitor, subsequent development was greatly affected and progression of the blastocysts was largely prevented (Table 1). To determine when embryo progression was affected, we evaluated embryonic cleavages at shorter intervals. As shown in Table 2 embryos treated with NS8593 cleaved to the 4-cell stage at lower rates (p < 0.05) (Table 2). In addition, and because NS8593 also target SKCa2+ channels, we exposed WT zygotes to the KCa2+ 2.1–2.3 (SK1, SK2, and SK3 channels) inhibitor apamin42, and evaluated embryo development progression in zygotes. Apamin had no effect on development (Supplementary Table S1), excluding SKs channels as NS8593 targets in mouse embryos. Next, we evaluated the impact of on Trpv3−/− embryos, as in these embryos there could be possible compensation of channel activities after fertilization. Trpv3−/− zygotes were collected 20 h post hCG injection, as we noted that embryos of this strain are unable to proceed past the 2-cell stage if collected 15 h post hCG injection. Similarly to what we observed in WT embryos, Trpv3−/− embryos showed delayed developmental progression, which became evident between the 8-cell and morula stages (evaluation at 60 h); progression to the blastocysts stage was also diminished (see Supplementary Table S2). Another specific TRPM7 inhibitor, Waixenicin A (ref. 21), was not used for embryo culture studies, as at the concentrations needed to block oscillations in oocytes and eggs, it caused cell lysis (Supplementary Table S3). In total, our results showed that TRPM7 function is required for normal pre-implantation development, as disruption of the function of the channel impaired development, delaying progression to the morula stage, and inhibiting blastocyst formation.
Table 1. NS8593 inhibits pre-implantation embryo development to the blastocyst stage of mouse WT CD1 zygotes.
| Treatment | #2nd PB/2PNa | #2-Cells (%)b | #Blastocysts (%)b |
|---|---|---|---|
| Control | 55 | 41 (75%) | 27 (66%) |
| NS8593-10 μM | 59 | 47 (80%) | 1 (0.02%) |
aZygotes were collected 15 h post hCG. Four replicates, PB: Polar Body, PN: Pro-Nucleus.
b2-cell embryos were evaluated at 24 h and blastocysts at 96 h post collection, respectively.
Table 2. NS8593 delays developmental progression of mouse WT CD1 zygotes prior to the morula stage.
| Treatment | #2nd PB/2PNa | #2-Cells (%)b | #4-cells (%) | #8 -cells (%) |
|---|---|---|---|---|
| Control | 32 | 26 (81) | 26 (100) | 21 (81) |
| NS8593-10 μM | 35 | 31 (88) | 21 (69) | 6 (29) |
aZygotes were collected 15 h post hCG. Two replicates, PB: Polar Body, PN: Pro-Nucleus.
b2-cell embryos were evaluated at 24 h post collection, 4-cell embryos at 48 h and 8-cell embryos at 72 h.
Discussion
Here we have examined the functional expression of a member of the TRP family of channels, TRPM7, in mouse oocytes, eggs and 2-cell embryos. Using direct measurements of ion currents by whole-cell voltage-clamp along with Ca2+ imaging studies and specific agonists and inhibitors of TRPM7, we found that homo-tetramer TRM7-like channels are functionally expressed in oocytes, eggs and after fertilization. Accordingly, Ca2+ influx was enhanced by the TRPM7-specific agonist Naltriben, and spontaneous [Ca2+]i (and Sr2+) induced oscillations were inhibited by the TRPM7-specific antagonist NS8593. These results suggest a role for TRPM7 in Ca2+ influx and homeostasis in oocytes and eggs. Furthermore, our results suggest that this channel is important for pre-implantation development, as in vitro culture of embryos in the presence of a specific inhibitor prevented or delayed progression to the blastocyst stage.
GV oocytes undergo spontaneous [Ca2+]i oscillations following release from antral follicles36. These oscillations are dependent on Ca2+ release from intracellular [Ca2+]]i stores, but ultimately depend on extracellular Ca2+ and Ca2+ influx; these oscillations are not required for the GVBD to take place36. Oscillations during GV stage can also be observed by replacing external Ca2+ with Sr2+. Exposure to Sr2+ is a well-known method of activation of rodent eggs, as Sr2+ induces persistent oscillations in MII eggs that promotes parthenogenesis26,43,44. In this study, we demonstrated that the channel that permeates Sr2+ in oocytes is not the same that permeates it in eggs, as whereas Trpv3−/− eggs are unable to mount Sr2+ oscillations15, Trpv3−/− GV oocytes do so normally. TRPV3 channels, which are responsible for Sr2+ influx in mouse eggs, are temperature sensitive29,30,31, and the oscillations induced by Sr2+ in oocytes can occur at RT. Accordingly, Sr2+ (and Ca2+) induced oscillations in GV oocytes may be mediated by the same channel(s). Consistent with this notion, NS8593 inhibited [Ca2+]i and Sr2+ oscillations in oocytes, but not in eggs. These results suggest that differential expression exists among oocytes and eggs regarding PM channels that mediate cation influx and efflux. Our results showing functional expression of TRPM7-like channels in GV oocytes suggest this channel, which is permeable to divalent cations including Sr2+, is a candidate to mediate Ca2+ and Sr2+ influx in oocytes.
In this study, we used voltage-clamp and several pharmacological approaches to elucidate the molecular identity of a native cationic non-selective channel(s) present in oocytes, eggs and embryos. Their presence was further supported by the expression of the corresponding transcripts, as previously reported45. The channel(s) was blocked by extracellular Mg2+, which is consistent with expression of TRPM746. We therefore used a TRPM7- specific inhibitor NS8593, which selectively inhibited native TRPM7-like currents in several cells lines22. We also used the TRPM7 agonist Naltriben to determine if it activates similar current to those observed in oocytes and eggs. Naltriben was identified using an aquorin bioluminescence assay to screen small molecules with agonist activity on the TRPM7 channel. Naltriben was determined as the most specific towards TRPM7 among more than 20 compounds that were shown to have activity23. The electrophysiological responses and the inhibitory and stimulatory effects of NS8593 and Naltriben, respectively, on the studied currents in oocytes, eggs and embryos are consistent with functional expression of TRPM7-like currents in the germ cells here recorded.
Since TRPM7 has been reported to form heteromers with its homolog TRPM638, we examined whether or not the currents in our cells were mediated by the homo-tetramer TRPM7 channels. To accomplish this we used the non-selective Ca2+ blocker 2-APB47, which has dual and concentration- dependent actions on TRPM7 currents35. Our results showing that 100 μM 2-APB blocked the current in oocytes and eggs, whereas 2 mM worked as an agonist of the channels confirmed the presence of TRPM7 channels in these cells as well as showed that there are of homo-tetrameric composition. Lastly, embryos generated from a Trpm7 knock-out mouse model died before day 7.5 of embryogenesis (E7.5) showing that TRPM7 is essential to support embryonic development24,25. Nevertheless, whether TRPM7 function is required at earlier stages of development was not determined. Consequently, and given our findings of functional expression of TRPM7-like channels in oocytes, eggs and 2-cell stage embryos, we investigated whether or not its inhibition could compromise pre-implantation embryo development. To that end, we cultured in vivo fertilized zygotes in the presence of NS8593. We noticed that the WT zygotes treated with the inhibitor showed slower cleavage rates prior to reaching the morula stage, and were unable to reach the blastocyst stage. The delayed development was also observed in Trpv3−/− embryos, in agreement with the indispensable role of TRPM7 channels suggested by genetic studies24. Our results are consistent with a crucial function of TRPM7 channels post-fertilization.
As mentioned, deletion of the mouse Trpm7 gene, showed it to be the first TRP channel with an essential and non-redundant role in embryogenesis24. Later, it was shown that induced global Trpm7 disruption at E7-E9, was lethal but not after day E1425. Interestingly, TRPM7 was found not to be required for the survival of many differentiated cells, but it was indispensable for the survival of pluripotent stem cells and some multipotent stem cells25. Consistent with those findings, downregulation of TRPM7 by morpholino injection in Xenopus embryos caused severe gastrulation defects, including a short and curved axis and a widely, opened neural tube48. Our results showed functional expression of TRPM7 channels before and after fertilization, which suggests a role for TRPM7 in mediating Ca2+ influx in these cells and possibly prior to the blastocyst stage. The precise role of TRPM7 on early cleavage state embryos will require more detailed and specific approaches such as knock-down of its expression and function. Our attempts in this direction using siRNA probes and/or a TRPM7 antibody did not cause any developmental phenotype likely due to high levels and stability of maternally contributed TRPM7 and low antibody specificity. These experiments suggest that maternal TRPM7 channels are essential for early development and studies involving oocyte specific Trpm7-KO will become crucial to elucidate the function of Trpm7 during pre-implantation embryo development.
The broad permeability of TRPM7 towards divalent cations raised questions regarding its function in the regulation of cellular Ca2+ levels49. Initially, TRPM7 was reported as a Mg2+ regulated channel20, which was confirmed by findings showing inhibition of the TRPM7 current by high intracellular and extracellular Mg2+ concentrations; these properties, in fact, were used to differentiate it from CRAC channels33. Further, the permeability to and blockade by Mg2+ contribute to its essential role in the regulation of cellular Mg2+ concentrations, which was later demonstrated in TRPM7-deficient cells whose impaired viability and proliferation were rescued by supplementation with Mg2+ extracellular50. In addition, Mg2+ has been shown to play a fundamental role during development in humans and its deficiency has been linked to birth defects, including spina bifida, defects in fetal growth and development51. Moreover, the impaired gastrulation phenotype caused by depletion of TRPM7 in Xenopus embryos can be prevented by Mg2+ supplementation, by expression of the TRPM7 homologous channel TRPM6, or by the expression of a Mg2+ transporter48. Additionally, heterozygous animals lacking the α-kinase domain of TRPM7 showed signs of hypomagnesaemia, and stem cells lacking this domain displayed proliferation arrest that was rescued by Mg2+ supplementation52. The Mg2+ requirement for oocyte maturation and egg activation in mammals has not been assessed carefully, although, a recent study showed that lower concentrations of Mg2+ in-vitro fertilization, IVF, and embryo culture media, 0, 2 mM instead of 1, 2 mM Mg2+, improved development to the blastocyst stage53. Importantly, the estimated total intracellular Mg2+ concentration in somatic cells is between 14 to 20 mM, which is actively maintained by Mg2+ transporters and ion channels54; the expression of these proteins has not been described in mammalian oocytes and eggs. Thus, it is presently unknown whether TRPM7 contributes to Mg2+ regulation in mammalian oocytes and eggs. It is worth noting that in prawn and shrimp oocytes, external Mg2+ is the signal that stimulates resumption of meiosis at the time of spawning55,56, demonstrating that changes in the concentration of this ion are important for the initiation of development. The study of the regulation of Mg2+ cellular homeostasis and the role of TRPM7 in Mg2+ transport in oocytes needs to be addressed.
Zinc (Zn2+) is another ion that permeates TRPM7 channels35,49. Zn2+ has been shown to tightly regulate oocyte maturation as intracellular deficiency in Zn2+ provokes arrest in meiotic telophase and fertilization of these oocytes results in inviable embryos57. Zn+ is also fundamental for cell cycle resumption after fertilization and release of Zn2+ to the extracellular media, “Zn2+ sparks”, occurs nearly simultaneously with initiation of [Ca2+]i oscillations58. During oocyte maturation, Zn2+-transporters such as ZIP6 and ZIP10 have been shown to be responsible of the accumulation of Zn2+ 59, although their function after fertilization remains unclear. TRPM7 conducts Zn2+ efficiently49, and given that the cytosolic concentration the Zn2+ can regulate the binding of the α-kinase domain of TRPM7 to transcription factors containing zinc-fingers, it cannot be ruled out that TRPM7 contributes to gene expression in early embryos. Furthermore, the cleaved kinase domain of TRPM7 can bind components of the Polycomb Repressor Complex 1 (PRC1)60, which are proteins associated with transcriptional silencing of genes during oogenesis and embryonic development61. Therefore, even when TRPM7 could not be the major mediator of Zn2+ influx/efflux in oocytes and embryos, its activity regulating Zn2+ homeostasis might have an important role in epigenetic changes during embryo development.
In summary, we demonstrated that homo-tetramers of TRPM7-like channels are expressed in mouse oocytes, eggs and embryos. We showed that TRPM7 is able to mediate Ca2+ influx in oocytes, eggs and embryos, suggesting a role of this channel during oocyte maturation, egg activation and early embryo development. Furthermore, we showed that the blockade of the TRPM7 function post-fertilization leads to delayed embryonic development resulting in inhibition of development to the blastocyst stage. The generation of specific oocyte Trpm7-KO mice by targeting Trpm7 in early stages of oocyte and follicle development would be a major step forward to elucidate the function of Trpm7 on oogenesis, fertilization and pre-implantation embryo development in mammals.
Methods
Oocyte and embryo collection
Six-to-twelve-week-old females (CD1, Trpv3−/− colony18) were superovulated with intraperitoneal (i.p.) injection of 5 IU pregnant mare’s serum gonadotropin (PSMG, Sigma), followed 48 h later by i.p. injection of 5 IU of human chorionic gonadotropin (hCG, Sigma). Ovulated eggs (cumulus masses) were obtained by pulling the oviducts open with fine forceps in a HEPES-buffered culture medium (M2 medium, Millipore) 13–16 h after administration of hCG. In order to obtain 2 cell-stage embryos, females were mated with C57BL/6 males at the same time of the hCG injection. 2 cell-stage embryos were collected 40 h post hCG-injection. Cumulus cells were removed using hyaluronidase (Sigma) and gentle aspiration through a pipette. The zona pellucidae (ZP) were removed by exposure to Tyrode’s acid solution (pH 2.5) for a few seconds followed by thorough washing in M2 medium. Blastomers were separated mechanically by gentle aspiration through a pipette. GV oocytes were collected from the ovaries of 5- to 12-week-old Trpv3−/− or CD-1 female mice. Females were injected with 5 IU PMSG and cumulus cell-enclosed oocytes were recovered 42–46 h later into Chatot, Ziomek, or Bavister (CZB) medium and 100 μM isobutyl-1-methylxanthine (IBMX). Experimental protocols completed at Aarhus University were performed according to the Danish national and Institutional regulations and approved by the Animal Experiments Inspectorate under the Danish Ministry of Justice (permit numbers 2015-15-0201-00800, 2012-15-2935-00002).
Calcium imaging
[Ca2+]i imaging was carried out as previously described40. In brief, [Ca2+]i was measured using the Ca2+ sensitive dyes Fura-2-acetoxymethyl ester (Fura 2-AM, Molecular Probes; Invitrogen). Eggs were loaded with 1.25 μM Fura-2AM supplemented with 0.02% pluronic acid (Molecular Probes) for 20–30 min at room temperature. To estimate [Ca2+]i, oocytes/eggs were thoroughly washed and attached to glass-bottom chambers. For Fig. 1, responses to SrCl2 were recorded in FCS-free (serum free) HEPES-buffered Chatot, Ziomek, or Bavister (HCZB) medium, where external Ca2+ was replaced by 10 mM SrCl2, and under mineral oil. Ca2+ measurements were performed on the stage of an Olympus IX70 microscope equipped with an Olympus LUCPlanFL N × 20 objective (N.A. 0.45) and an EasyRatioPro fluorescence imaging system (Photon Technology International, NJ, USA). The oocytes were exited at 340 and 380 nm and emission was recorded at 510 nM. Intracellular Ca2+ ([Ca2+]i) was expressed from 340/380 fluorescence ratio. For Fig. 4, oocytes and eggs were recorded in FCS-free (serum free) TL-HEPES medium containing Ca2+ (2.04 mM), and Mg2+ (0.492 mM) under mineral oil. For Fig. 5, oocytes and eggs were recorded in FCS-free TL-HEPES containing the Ca2+ concentration described in the figure. For the Sr2+- induced oscillations measurements, Ca2+ was replaced by 10 mM SrCl2. Oocytes and eggs were monitored using inverted microscope (Nikon) fluorescence measurements. Fura 2-AM was excited between 340 nm and 380 nm by a filter wheel (Ludl Electronic Products Ltd.), and fluorescence was obtained every 20 s. After passing through a 510 nm, the emitted light was collected by a Cool Photometrics SenSys CCD camera (Roper Scientific, Tucson, AZ) to calculate fluorescence ratios of 340/380 nm in the whole egg.
Electrophysiology
Whole-cell currents were measured at 22–24 °C using an HEKA 10 USB amplifier. Electrophysiology recordings were performed on the same day of oocyte, egg or embryo isolation up to 8 hours post-surgery. Eggs and embryo were maintained in human tubal fluid medium (HTF, EMD Millipore) and GV oocytes in CZB medium (EMD Millipore) at 37 °C and 5% CO2. Data were analyzed using Igor Pro and Origin 7.0 (OriginLab). Pipettes of 1–3 MΩ resistance were made from glass capillaries (593600, A-M systems). The intracellular solution contained (in mM): 142 Cs-Methanesulfonate, 10 HEPES, 3 NaATP, 0.3 NaGTP, 5 EGTA, 3 mM CaCl2 (free 100 nM) pH: 7.3–7.4. Concentration of Calcium was calculated using WincMax Chelator. The external solution for giga seal formation contained (in mM): 125 NaCl, 6 KCl, 20 mM CaCl2, 1.2 MgCl2, 20 mM HEPES-NaOH, pH: 7.3–7.4. TRPM7 basal responses to agonists or blockers, were measured in an external solution containing (in mM): 140 mM NaMES, 10 mM HEPES, 10 mM glucose, 4 mM KCl, and 2 mM CaCl2. The solution of NaMES was used in order to avoid chloride currents24. The osmolarity of all solutions was 290–320 mOsm. All voltages were corrected for calculated junction potentials present between the internal and external solution before seal formation. TRPM7 currents were activated by voltage ramps from 100 mV to −100 mV (600 ms, every 2 s), in the presence of the agonist or blockers. The holding potential (HP) was 0. Statistical analyses were performed using GraphPad Prism: t-test, paired, two-tailed P value (Figs 1, 3 and 7a) and One-way ANOVA (Fig. 7b).
Chemicals
NS8593 (SIGMA), Naltriben (SIGMA).
In vitro pre-implantation embryo development
Zygotes were collected from the oviducts of 6 to 8-weeks-old super ovulated CD1 and TRPV3KO females mated to CD1 males. CD1 zygotes were recovered 15 h post-hCG whereas TRPV3KO zygotes were collected at 20 h post-hCG, as earlier collection greatly reduced in vitro pre-implantation development even in control embryos. After collection, zygotes were cultured in KSOM media (Specialty Media) with or without 10 μM NS8593 in four-well dishes (Thermo Scientific Nunc, Fisher). Assessment of embryo development was made every 12 or 24 h according to the experimental design until 96 h of culture, where blastocysts rates were assessed. At least two replicates were performed per strain and Chi square tests were performed for significance.
Additional Information
How to cite this article: Carvacho, I. et al. TRPM7-like channels are functionally expressed in oocytes and modulate post-fertilization embryo development in mouse. Sci. Rep. 6, 34236; doi: 10.1038/srep34236 (2016).
Supplementary Material
Acknowledgments
I.C. is recipient of a Carlsberg Foundation Postdoctoral Fellowship (2013_01_0438), and was further supported by a grant from the Danish National Research Foundation (DNRF) (PUMPKIN DNRF85 to KLH). These studies were supported in part by a NIH grant HD 51872 to R.A.F. We thank Dr. David E. Clapham for kindly share the Trpv3−/− mice colony. We are most grateful to Dr. Vladimir Matchkov for assistance in Calcium imaging experiments, Dr. Ulf Simonsen and Dr. Søren Peter Olesen for generous access to the electrophysiology set-up, Dr. Patricio Rojas for support with IgorPro analysis. We thank Changli He for overall general laboratory support. We thanks Dr. Matthias Piesche for helpful comments and discussion.
Footnotes
Author Contributions I.C. performed electrophysiological recordings; I.C., G.A. and H.C.L. carried out calcium imaging measurements; G.A., K.M. and K.L.-H. performed in vitro pre-implantation embryo development assays. I.C. prepared the figures; I.C., G.A., H.C.L., K.L.-H. and K.M. analyzed the data. I.C. and R.A.F designed the experiments; I.C., R.A.F. and K.L.-H. directed research activities and wrote the manuscript.
References
- Swain J. E. & Pool T. B. ART failure: oocyte contributions to unsuccessful fertilization. Hum. Reprod. Update 14, 431–446 (2008). [DOI] [PubMed] [Google Scholar]
- Saunders C. M. et al. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development 129, 3533–3544 (2002). [DOI] [PubMed] [Google Scholar]
- Yoon S. Y. et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca(2+) release and are unable to initiate the first step of embryo development. J. Clin. Invest. 118, 3671–3681 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozil J. P., Banrezes B., Toth S., Pan H. & Schultz R. M. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev. Biol. 300, 534–544 (2006). [DOI] [PubMed] [Google Scholar]
- Kline D. & Kline J. T. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J. Biol. Chem. 267, 17624–17630 (1992). [PubMed] [Google Scholar]
- Miao Y. L. & Williams C. J. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol. Reprod. Dev. 79, 742–756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston N. J., McGuinness O., Johnson M. H. & Maro B. The exit of mouse oocytes from meiotic M-phase requires an intact spindle during intracellular calcium release. J. Cell Sci. 108 (Pt 1), 143–151 (1995). [DOI] [PubMed] [Google Scholar]
- Cheon B., Lee H. C., Wakai T. & Fissore R. A. Ca2+ influx and the store-operated Ca2+ entry pathway undergo regulation during mouse oocyte maturation. Mol. Biol. Cell 24, 1396–1410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombes R. M., Simerly C., Borisy G. G. & Schatten G. Meiosis, egg activation, and nuclear envelope breakdown are differentially reliant on Ca2+, whereas germinal vesicle breakdown is Ca2+ independent in the mouse oocyte. J. Cell Biol. 117, 799–811 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. Calcium at fertilization and in early development. Physiol. Rev. 86, 25–88 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou P., Bountra C., Bland K. P. & House C. R. Calcium action potentials in unfertilized eggs of mice and hamsters. Q. J. Exp. Physiol. 69, 365–380 (1984). [DOI] [PubMed] [Google Scholar]
- Bernhardt M. L. et al. CaV3.2 T-type channels mediate Ca(2)(+) entry during oocyte maturation and following fertilization. J. Cell Sci. 128, 4442–4452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Palermo G. & Machaca K. Downregulation of store-operated Ca2+ entry during mammalian meiosis is required for the egg-to-embryo transition. J. Cell Sci. 126, 1672–1681 (2013). [DOI] [PubMed] [Google Scholar]
- Chen C. C. et al. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science 302, 1416–1418 (2003). [DOI] [PubMed] [Google Scholar]
- Carvacho I., Lee H. C., Fissore R. A. & Clapham D. E. TRPV3 channels mediate strontium-induced mouse-egg activation. Cell Rep. 5, 1375–1386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y. L., Stein P., Jefferson W. N., Padilla-Banks E. & Williams C. J. Calcium influx-mediated signaling is required for complete mouse egg activation. Proc. Natl. Acad. Sci. USA 109, 4169–4174 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. J., Sweet T. B. & Clapham D. E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 62, 381–404 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X. et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 141, 331–343 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels L. W., Yue L. & Clapham D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001). [DOI] [PubMed] [Google Scholar]
- Nadler M. J. et al. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature 411, 590–595 (2001). [DOI] [PubMed] [Google Scholar]
- Fleig A. & Chubanov V. Trpm7. Handb Exp Pharmacol 222, 521–546 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubanov V. et al. Natural and synthetic modulators of SK (K(ca2+)) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7. Br. J. Pharmacol. 166, 1357–1376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T. et al. Activation of TRPM7 channels by small molecules under physiological conditions. Pflugers. Arch. 466, 2177–2189 (2014). [DOI] [PubMed] [Google Scholar]
- Jin J. et al. Deletion of Trpm7 disrupts embryonic development and thymopoiesis without altering Mg2+ homeostasis. Science 322, 756–760 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J. et al. The channel kinase, TRPM7, is required for early embryonic development. Proc. Natl. Acad. Sci. USA 109, E225–E233 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. et al. Strontium promotes calcium oscillations in mouse meiotic oocytes and early embryos through InsP3 receptors, and requires activation of phospholipase and the synergistic action of InsP3. Hum. Reprod. 20, 3053–3061 (2005). [DOI] [PubMed] [Google Scholar]
- Wakai T., Vanderheyden V. & Fissore R. A. Ca2+ signaling during mammalian fertilization: requirements, players, and adaptations. Cold Spring Harb. Perspect. Biol. 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti E., Boni R., Gallo A. & Silvestre F. Ion currents modulating oocyte maturation in animals. Syst. Biol. Reprod. Med. 59, 61–68 (2013). [DOI] [PubMed] [Google Scholar]
- Xu H. et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418, 181–186 (2002). [DOI] [PubMed] [Google Scholar]
- Smith G. D. et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418, 186–190 (2002). [DOI] [PubMed] [Google Scholar]
- Peier A. M. et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 296, 2046–2049 (2002). [DOI] [PubMed] [Google Scholar]
- Luo J., Stewart R., Berdeaux R. & Hu H. Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. J. Invest. Dermatol. 132, 2158–2165 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M. & Lewis R. S. Separation and characterization of currents through store-operated CRAC channels and Mg2+-inhibited cation (MIC) channels. J. Gen. Physiol. 119, 487–507 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H. Z. et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 279, 35741–35748 (2004). [DOI] [PubMed] [Google Scholar]
- Li M., Jiang J. & Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 127, 525–537 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll J. & Swann K. Spontaneous cytosolic calcium oscillations driven by inositol trisphosphate occur during in vitro maturation of mouse oocytes. J. Biol. Chem. 267, 11196–11201 (1992). [PubMed] [Google Scholar]
- Chubanov V. & Gudermann T. Trpm6. Handb Exp Pharmacol 222, 503–520 (2014). [DOI] [PubMed] [Google Scholar]
- Chubanov V. et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc. Natl. Acad. Sci. USA 101, 2894–2899 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. K., Lee H., Mizuno A., Suzuki M. & Caterina M. J. 2-aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 24, 5177–5182 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. C., Yoon S. Y., Lykke-Hartmann K., Fissore R. A. & Carvacho I. TRPV3 channels mediate Ca(2+) influx induced by 2-APB in mouse eggs. Cell Calcium 59, 21–31 (2016). [DOI] [PubMed] [Google Scholar]
- Sah R. et al. Timing of myocardial trpm7 deletion during cardiogenesis variably disrupts adult ventricular function, conduction, and repolarization. Circulation 128, 101–114 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adelman J. P., Maylie J. & Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu. Rev. Physiol. 74, 245–269 (2012). [DOI] [PubMed] [Google Scholar]
- Whittingham D. G. & Siracusa G. The involvement of calcium in the activation of mammalian oocytes. Exp. Cell Res. 113, 311–317 (1978). [DOI] [PubMed] [Google Scholar]
- Kline D. & Kline J. T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 149, 80–89 (1992). [DOI] [PubMed] [Google Scholar]
- Park S. J., Shirahige K., Ohsugi M. & Nakai K. DBTMEE: a database of transcriptome in mouse early embryos. Nucleic Acids Res. 43, D771–D776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates-Withers C., Sah R. & Clapham D. E. TRPM7, the Mg(2+) inhibited channel and kinase. Adv. Exp. Med. Biol. 704, 173–183 (2011). [DOI] [PubMed] [Google Scholar]
- Clapham D. E., Runnels L. W. & Strubing C. The TRP ion channel family. Nat. Rev. Neurosci. 2, 387–396 (2001). [DOI] [PubMed] [Google Scholar]
- Liu W. et al. TRPM7 regulates gastrulation during vertebrate embryogenesis. Dev. Biol. 350, 348–357 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteilh-Zoller M. K. et al. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J. Gen. Physiol. 121, 49–60 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C. et al. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114, 191–200 (2003). [DOI] [PubMed] [Google Scholar]
- Komiya Y., Su L. T., Chen H. C., Habas R. & Runnels L. W. Magnesium and embryonic development. Magnes. Res. 27, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryazanova L. V. et al. TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat. Commun. 1, 109 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick J. R. et al. The beneficial effects of reduced magnesium during the oocyte-to-embryo transition are conserved in mice, domestic cats and humans. Reprod. Fertil. Dev. 27, 323–331 (2015). [DOI] [PubMed] [Google Scholar]
- Komiya Y. & Runnels L. W. TRPM channels and magnesium in early embryonic development. Int. J. Dev. Biol. 59, 281–288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudeau M. & Goudeau H. External Mg2+ triggers oscillations and a subsequent sustained level of intracellular free Ca2+, correlated with changes in membrane conductance in the oocyte of the prawn Palaemon serratus. Dev Biol 177, 178–189 (1996). [DOI] [PubMed] [Google Scholar]
- Lindsay L. L., Hertzler P. L. & Clark W. H. Jr. Extracellular Mg2+ induces an intracellular Ca2+ wave during oocyte activation in the marine shrimp Sicyonia ingentis. Dev. Biol. 152, 94–102 (1992). [DOI] [PubMed] [Google Scholar]
- Kim A. M., Vogt S., O’Halloran T. V. & Woodruff T. K. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat. Chem. Biol. 6, 674–681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A. M. et al. Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs. ACS Chem. Biol. 6, 716–723 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B. Y. et al. Maternally-derived zinc transporters ZIP6 and ZIP10 drive the mammalian oocyte-to-egg transition. Mol. Hum. Reprod. 20, 1077–1089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapivinsky G., Krapivinsky L., Manasian Y. & Clapham D. E. The TRPM7 chanzyme is cleaved to release a chromatin-modifying kinase. Cell 157, 1061–1072 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posfai E. et al. Polycomb function during oogenesis is required for mouse embryonic development. Genes Dev. 26, 920–932 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.