Abstract
As an endoplasmic reticulum heat shock protein (HSP) 90 paralogue, glycoprotein (gp) 96 possesses immunological properties by chaperoning antigenic peptides for activation of T cells. Genetic studies in the last decade have unveiled that gp96 is also an essential master chaperone for multiple receptors and secreting proteins including Toll-like receptors (TLRs), integrins, the Wnt co-receptor, Low Density Lipoprotein Receptor-Related Protein 6 (LRP6), the latent TGFβ docking receptor, Glycoprotein A Repetitions Predominant (GARP), Glycoprotein (GP) Ib and insulin-like growth factors (IGF). Clinically, elevated expression of gp96 in a variety of cancers correlates with the advanced stage and poor survival of cancer patients. Recent preclinical studies have also uncovered that gp96 expression is closely linked to cancer progression in multiple myeloma, hepatocellular carcinoma, breast cancer and inflammation-associated colon cancer. Thus, gp96 is an attractive therapeutic target for cancer treatment. The chaperone function of gp96 depends on its ATPase domain, which is structurally distinct from other HSP90 members, and thus favors the design of highly selective gp96-targeted inhibitors against cancer. We herein discuss the strategically important oncogenic clients of gp96 and their underlying biology. The roles of cell-intrinsic gp96 in T cell biology are also discussed, in part because it offers another opportunity of cancer therapy by manipulating levels of gp96 in T cells to enhance host immune defense.
1. gp96 AND CANCER: INTRODUCTION
Heat shock proteins are a highly conserved group of chaperone molecules involved in several aspects of cellular homeostasis. Glycoprotein 96 (gp96, GRP94, Erp99, endoplasmin; thereafter after referred to as gp96) is an endoplasmic reticulum (ER) resident protein, which belongs to the HSP90 family. Constitutively expressed in virtually all cell types, gp96 expression is upregulated by interferons [1] and a multitude of stress conditions that perturb ER functions including, glucose starvation, oxidative stress, ER calcium-store depletion and the accumulation of misfolded proteins [2, 3]. Moreover, loss of gp96 is embryonically lethal [4], but this is not surprising, as gp96 is responsible for chaperoning multiple essential proteins such as TLRs (with the exception of TLR3) [5], Wnt co-receptor LRP6 [6], GARP [7], GPIb [8] and Insulin-like growth factor [4] as well as majority of the α and β integrin subunits [9, 10]. These client proteins of gp96 (Fig. 1) have been described to function at various stages of cancer development, indicating that gp96 plays a crucial role in oncogenesis, as would be discussed in depth later in this review.
Fig. 1. Model of gp96 cancer-associated clientele.
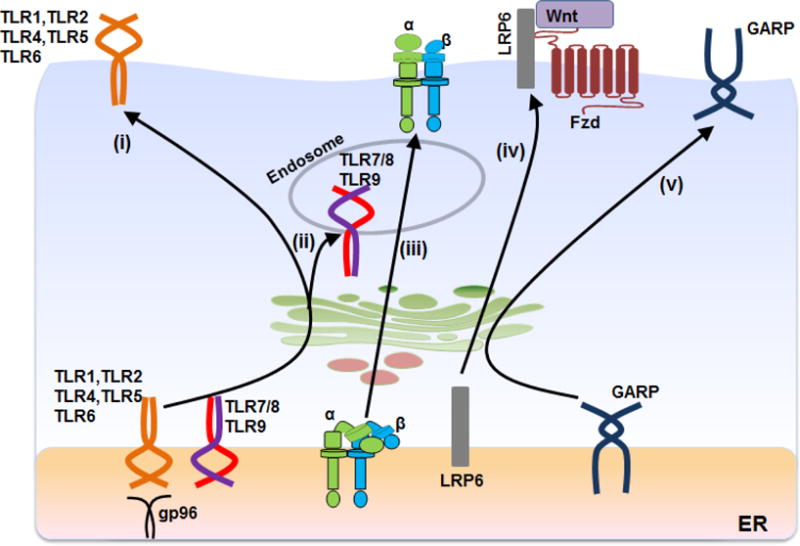
Gp96, a resident ER protein chaperones TLR1, TLR2, TLR4, TLR5 and TLR6 through the Golgi apparatus to the cell surface (i) and TLR7, TLR8 and TLR9 to endosomes (ii). Gp96 also chaperones multiple integrins (αβ subunits) (iii) and participates in canonical Wnt signaling by folding the fizzled co-receptor, LRP6 (iv). Recently, gp96 was also shown to be the key molecular chaperone for GARP (v). For clarity only relevant molecules are depicted.
Gp96 was discovered by multiple groups initially as a protein induced strongly in cells upon glucose starvation [11] and as a major calcium-binding protein in the ER [12], as well as the most abundant ER-resident protein [13]. Subsequent work identified gp96 as an active tumor rejection antigen that can induce resistance to tumor transplants in specifically immunized syngeneic recipients. Purified gp96 from two antigenically distinct chemically-induced sarcomas elicited tumor-specific immunity [14]. Previous work by our group and others have provided evidence for the immunological roles of extracellular gp96 [15–18], thus, a brief overview ensues followed by more in-depth discussions on the cell-intrinsic roles of gp96 in cancer. Moreover, loss of cellular integrity is often associated with efflux of HSPs into the extracellular environment. While multiple mechanisms have been proposed, the most rational explanation for extracellular HSPs is necrosis; a commonality among all cancers [19]. The finding that HSPs isolated from cancer or virus infected tissues, but not healthy tissues, are capable of eliciting an immune response indicates potential cross-talk between extracellular HSPs and the immune system [20]. Gp96, and to a larger extent the HSP90 family, chaperones a broad array of peptides including both normal and altered proteins [21]. Interestingly, vaccination with only purified HSPs did not elicit an immune response [22]. However, isolated gp96 cDNA from normal and tumor samples showed no noticeable differences in immunogenicity [23], and when HSPs were complexed with peptides, even poorly immunogenic peptides gained immunogenicity [22]. Together, these studies conclusively demonstrate a system in which both aspects of the HSP-antigen complex are required to mount an effective immune response.
Mechanistically, it was still unclear how HSP-antigen complexes conferred immunity. Two pieces of evidence hinted at the existence of an HSP-specific receptor: (i) immunization with extremely low concentrations of antigens chaperoned by HSPs, but not other proteins could elicit a T cell response; and (ii) inhibition of antigen presenting cells (APCs) abrogated HSP-peptide immune responses [24–26]. Support for this hypothesis arrived from multiple experiments. First, it was shown that macrophage uptake of gp96-peptide complexes results in MHC class I presentation of the peptide [20]. Second, competitive binding assays were utilized to show HSP affinity to APCs. Third, CD91 was identified as a de facto global receptor for the HSP family [27]. These findings gave credence to a model whereby the HSP-peptide complex is phagocytosed by the APC, processed intracellularly and presented to cognate CD8+ T cells via MHC class I. Interestingly, depletion of CD4+ T cell populations during the effector phase but not during the priming phase of immunization, ablated the effectiveness of tumor-derived gp96 vaccination [25]. Soon, cross-presentation of gp96-chaperoned peptides via MHC class II molecules were demonstrated experimentally [28, 29]. Combined, these findings indicated that the presence of CD4+ T cells are essential to support CD8+ T cell-mediated effector functions, but not required for the initial HSP-mediated priming of antigen-specific responses.
The extracellular roles of gp96 in immune responses have received well-deserved attentions for some time now [26]. Moreover, current work utilizing genetic knockout (KO) strategies have revealed exciting cell-intrinsic roles of gp96 in innate immunity, inflammation, cytoprotection, development, stemness and oncogenesis [5, 18, 30–32]. This review will mainly focus on the cell-intrinsic roles of gp96 in cancer development, as the basic aspects of gp96 ER function such as calcium homeostasis, mechanisms of protein folding and quality control have already been reviewed extensively elsewhere [33, 34].
2. GP96 CANCER-ASSOCIATED CLIENTELE
2.1. TLRs
Toll-like receptors are a type of pattern recognition receptors (PRRs) that recognize specific pathogen-associated molecular patterns (PAMPs) commonly present in microbes to initiate downstream immune responses. Thirteen members of the TLR family have so far been described in mammalian cells (TLR1-TLR10 in humans, TLR1-TLR9 and TLR11-TLR13 in mice). Whereas TLR3, TLR7, TLR8, TLR9 and TLR13 are localized primarily within endolysosomes and recognizes nucleic acids [35], the rest of the TLR family proteins are present on the cell surface and they largely recognize microbial membrane components. TLRs are also expressed on various immune cells, including T and B cells [36, 37], dendritic cells (DCs) [38], macrophages [39] as well as some non-immune cells such as fibroblasts and epithelial cells [40]. Upon encounter with specific microbial molecules TLR receptor are activated and initiate downstream signaling via MyD88-dependent or -independent pathways [41]. The identification of TLRs advanced the field of immunoadjuvants greatly, as most microbial immunoadjuvants work through TLR ligands [30], [42, 43]. Thus by controlling most TLRs as well as professional APCs, gp96 modulates both the innate and adaptive components of the immune system. Indeed, multiple studies have demonstrated gp96 to chaperone both cell-surface and intracellular TLRs [5, 9, 44, 45] as well as regulate the proteolytic processing and conformational stability of TLR9 [37, 46]. Moreover, gp96 plays a multi-faceted role in TLR9 biology, as its inhibition leads to a reduction in TLR9 signaling, although gp96 remains associated with TLR9 during trafficking to lysosomal compartments. Furthermore, inhibition of gp96 function also leads to increased sensitivity of TLR9 towards proteolytic degradation [46]. An important mode for the regulation of TLRs is localization and trafficking, which can be regulated in part by chaperones such as gp96. For example, the well characterized toll-like receptor, TLR4, cycles between the Golgi and plasma membrane until engaged by LPS [47], and the interaction between TLR2 and TLR4 ligands with the N-terminal domain of gp96 amplifies both innate and adaptive immune responses [48]; however, gp96 requires a co-chaperone for optimal TLR signaling under certain circumstances. For example, PRAT4A (protein associated with toll-like receptor 4) acts as a substrate-specific co-chaperone for gp96, and associates with TLR4/MD-2, for TLR4 cell surface expression [43, 49]. Further, a 2002 article by Vabulas et al., commented on how HSPs such as gp96 could act as ligands of TLRs, thus suggesting that both the innate and adaptive immune systems can be stimulated with the same ligand simultaneously [50].
TLRs are the most studied PRRs that recognize PAMPS in the context of cancer immunotherapy. This is partly due to their intrinsic roles on tumor cells, as well as their extrinsic roles on the various immune cells found within the tumor microenvironment. For example, activation of TLR9 heightens breast tumor cell proliferation and invasive phenotype [51]. TLR9 has an inverse effect in neuroblastoma cells in vitro and in vivo, as evidenced by reduced proliferation and increased apoptosis [52]. Intriguingly, TLRs that are expressed on immune cells are also expressed by glioma cells, and the same TLRs (TLR2, TLR4 and TLR9) have a tumor-promoting role in the biology of these tumors [41]. As gp96 expression is increased in multiple malignant cancers [53], it suggests gp96-regulated TLR signaling to be involved in cancer oncogenesis. As such, more research to support earlier work into gp96-based cancer vaccines [54] would further advance the field and aid our efforts to develop clinically relevant gp96 therapies.
Cancer vaccines are designed to introduce antigen in combination with adjuvants to activate DCs either ex vivo or in situ [55]. DCs are crucial for the initiation of adaptive immune responses, and are present in both mouse and human tumors [56]. The priming of cytotoxic T lymphocytes (CTLs), which are essential for antitumor immunity require DCs. As professional APCs, DCs recognize PAMPs [57, 58] and impaired cytokine production by DCs has been linked to cancer progression; thus, increasing cytokine secretion may inhibit cancer progression [59]. Indeed, Kuhn et al reported that only polyI:C and monosodium urate (MSU) plus Mycobacterium smegmatis rapidly induced monocyte-derived DCs in draining lymph nodes (LNs), which in turn increased the antitumor activity of CTLs [59]. TLR agonists are effective adjuvants since they activate the innate immune response. This activation can be harnessed to successfully treat a variety of skin cancers such as basal cell cancer, squamous cell carcinoma and malignant melanoma [60]. TLR9 agonists enhance anti-tumor immunity and inhibit tumor-associated immunosuppressive cell in a mouse cervical cancer model following recombinant lipoprotein therapy [61]. A review by Goutagny et al highlighted the plethora of ways in which PRRs such as TLRs can be targeted in cancer immunotherapy [62]. Indeed, immunotherapy is one of the more promising therapeutic strategies for the treatment of human gliomas. In 2010, a CpG phase II clinical trial was completed for patients with recurrent glioblastoma multiforme (GBM), where the therapy was well tolerated and a modest increase in survival rate was observed [41]. Since gp96 is an essential chaperone for nearly all of the TLRs, it logically follows that gp96 has a key role in potentiating TLR functions in adjuvant therapies. Furthermore, the greater specificity of gp96 in binding to substrates suggests manipulation of its client-binding domain (CBD), as an alternative strategy of targeted therapy for variety of diseases such as cancer [45].
2.2. Integrins
Integrins are cell-adhesion receptor molecules that mediate cell-cell, cell-extracellular matrix, and cell-pathogen interactions. They are non-covalently associated heterodimeric cell surface glycoproteins, consisting of α and β subunits. In humans and mice, there are 24 known αβ integrin pairs formed by 18 α subunits and 8 β subunits [63]. This diversity in subunit composition contributes to variations in ligand recognition, binding to cytoskeletal components and coupling to downstream signaling pathways. Elevated expression levels of integrins have been observed in tumors, which positively regulate their survival, proliferation, angiogenesis, migration and invasion [64–77]. These findings have made integrins the focus of intense investigation, both as cancer prognostic biomarkers and as potential therapeutic targets in malignancies. Integrin inhibitors, including antibodies, peptides and non-peptidic compounds have demonstrated promising results in preclinical, phase I and phase II clinical studies [78–87].
To date however, the clinical benefit of integrin inhibitors in phase III clinical trials has been disappointing. In part, this is thought to be due to the difficulty in targeting multiple integrins simultaneously. Recent studies show that the assembly and maturation of the majority of integrins is dependent on gp96 [5, 37, 45, 88]. The αI domain (AID), a ligand binding domain shared by seven of the eighteen α integrin subunits, has been shown to be a critical region for integrin binding to gp96, suggesting that the AID may play an important role in gp96-dependent maturation and cell surface expression of integrins [10]. Furthermore, the α7 helix region is crucial for AID binding to gp96. A cell-permeable α7 helix peptide competitively inhibits the interaction between gp96 and multiple integrins, reduces cell surface expression of multiple integrins and blocks cell invasion [10]. This is the first evidence to suggest that targeting multiple integrins may be an important strategy in developing useful cancer therapies. However, further studies are warranted to ascertain the therapeutic potential of this compound, including enhancing its intracellular delivery, its binding affinity to gp96 and its in vivo bioavailability and anti-cancer activity. Chaperone-based and client-specific inhibitors could potentially hold promise as new classes of therapeutics against cancer in the future.
2.3. LRP6
In an effort to expand the client network of gp96, Liu, et al., took an unbiased approach to immunoprecipitate gp96-clientele complex from a preleukemia B cell line, 14.GFP [6]. A tandem mass spectrometry (MS/MS) was performed to identify gp96-associated proteins. Unexpectedly, they discovered a strong interaction between gp96 and MesD [6]. MesD, so named after its critical role in mesoderm formation during early embryogenesis, has been shown to be a critical chaperone for the surface expression of LRP6 [89]. Furthermore, they found that gp96 interacts with LRP6, a co-receptor for the cell surface Wnt receptor, Frizzled, which is required for canonical Wnt signaling [90]. After maturation and surface expression, LRP6 undergoes γ-secretase–dependent regulated intramembrane proteolysis (RIP) to liberate its extracellular and intracellular domains [91]. In WT mice, LRP6 undergoes RIP to release its extracellular domain. Two forms of full-length LRP6 were identified in WT cells: Endoglycosidase H (Endo H)-resistant surface LRP6 and Endo H-sensitive LRP6 in the ER. In contrast, LRP6 does not undergo RIP or translocate to the cell surface in gp96 KO cells, as evidenced by the complete sensitivity of LRP6 in gp96KO cells. In further support of this conclusion, surface biotinylation followed by avidin pull-down and immunoblot failed to detect LRP6 on the surface of gp96 KO cells, although the same method detected similar amounts of transferrin receptor and Wnt receptor frizzled-4 (Fzd4) from WT and KO cells. Consistent with the loss of LRP6 and Wnt signaling, gp96 KO cells failed to upregulate Axin2 mRNA in response to Wnt-3a. In contrast, Wnt-3a treatment led to a dose-dependent upregulation of Axin2 mRNA in WT cells [6].
2.4. Insulin-Like Growth Factors
Other documented clients of gp96 include, the insulin-like growth factors, which are small molecular polypeptides that play important roles in cellular differentiation and metabolism [4]. Both insulin-like growth factor-I and – II were demonstrated to interact with gp96 and are not secreted in the absence of gp96 [4, 33, 92]. Indeed, conditional deletion of gp96 in striated muscle led to a systemic reduction of IGF-1, which resulted in smaller muscle growth and body size [93]. However, the roles of insulin-like growth factors in gp96-driven cancer have not been established.
2.5. Platelet Glycoprotein Ib Complex
In defining the roles of gp96 in hematopoiesis genetically, our group unexpectedly discovered that loss of gp96 causes macrothromobocytopenia in mice, a condition that resembles the human Benard-Soulier syndrome [8]. Further studies established that gp96 is a critical chaperone for the folding of platelet glycoprotein Ib complex, which is composed of GPIbα, GPIbβ, GPV and GPIX molecules [8]. Known functionally and structurally as a Von Willebrand factor receptor (VWFR), GPIb-V-IX complex is critical for platelet activation and hemostasis [94]. Interestingly, GPIbα was also reported to be ectopically expressed by various cancer cells of non-megakaryocytic cell lineage. Moreover, the cancer-associated GPIbα gene appears to be a transcriptional target for the c-Myc oncoprotein and can substitute for c-Myc in promoting growth, transformation and genomic instability [95]. It is unclear however, whether the pro-oncogenic roles of GPIbα gene product, which apparently does not depend on the functional receptor for VWFR, requires the chaperone function of gp96.
2.6. GARP
In addition to the aforementioned clients, we have shown recently that the novel latent transforming growth factor-beta (LTGFβ) docking receptor, GARP also known as LRRC32 (Leucine Rich Repeats Containing 32) is chaperoned by gp96 [7]. GARP is known to bind to latent TGFβ on the surface of Foxp3+ regulatory T cells (Tregs) and activated platelets [96]. GARP is located on chromosome 11q 13.5–14.1 in humans and 7F in mice; this is a region of synteny conserved between the two species. The GARP gene encodes for an 80 kDa transmembrane protein (663 amino acids) with an extracellular region made almost exclusively of 20 leucine rich repeats, a transmembrane domain and a short intracellular carboxyl-terminal tail of only 15 residues [97]. Structural biology studies showed that the association between latency-associated peptide (LAP) and GARP occurs through Cys192 and Cys331 present on the 7th and 12th LRR sites of GARP respectively [98]. As a latent TGFβ binding protein, the role of GARP has been linked directly to the bioavailability and activation of TGFβ. Regarding its immune modulatory properties, Foxp3 and GARP have been described as “the master and its minion” respectively [99]. Our ongoing work suggests that retroviral overexpression of GARP in naïve CD4+ T cells leads to stable reprogramming of these cells towards the induced regulatory T cell subset (unpublished observation). Additionally, surface expression of GARP is essential for the maintenance and function of Treg cells [96]. Using Treg-specific gp96 KO mice, we demonstrated recently that gp96 is essential for the cell surface expression of GARP on activated Tregs and that loss of gp96 results in instability of the Treg lineage [7]. GARP is also partially responsible for the suppressive capacity of regulatory T cells via regulation of TGFβ bioavailability. Indeed upon T cell receptor stimulation, GARP forms a complex with LTGFβ that subsequently undergoes shedding from the cell surface of regulatory T cells to yield a soluble GARP-LTGFβ complex [10]0. Soluble GARP then prevents T cell-mediated destructive inflammation by enhancing Tregs and inhibiting effector T cell activity in a preclinical humanized mouse model of xenogeneic graft-versus-host disease (GVHD) [101]. However, it is unlikely that loss of the tolerogenic function of gp96 KO Tregs is mediated solely by the absence of GARP (discussed later).
3. ROLE OF GP96 IN CANCER
3.1. Cancer-Associated Cell Surface Expression of gp96
Cell surface expression of gp96 in cancer was first described thirty years ago as a tumor rejection antigen. [14] Despite its role in the endoplasmic reticulum, it was confirmed that gp96 was anchored to the cell surface with the KDEL ER localization sequence intact [18]. Moreover, it was determined that gp96 can be expressed on the surface of cancer cells in response to stress similar to gp96 in the ER lumen [18]. In the years following, surface gp96 was observed to correlate with increased tumor immunogenicity [17] and subsequently shown to present peptides for an anti-tumor immune response [102], suggesting a key role for surface gp96 in immune responses against tumors. Continuing along the topic of immunity, we reported that surface gp96 was involved in DC maturation leading to inflammation and an increase of antigen presenting molecules and costimulators [103]. In the same study, overexpression of surface gp96 in tumor cells resulted in tumor regression through T lymphocytes (Fig. 2) [103]. We further demonstrated in a subsequent study that surface expression of gp96 promotes antigen presentation, which leads to increased memory T cell development [15]. Delving further into the relationship between surface gp96 and T lymphocytes, Banerjee et al detected surface expression of gp96 on antigen presenting cells and discovered its role in skewing activated T cells to the Th2 subset [104]. Accordingly, T cell proliferation and cytokine secretion was abrogated when surface gp96 was blocked [104]. Together, these data suggest a role for tumor-specific surface gp96 in the activation of dendritic cells and differentiation of Th2 cells, and presents a promising target for cancer immunotherapies.
Fig. 2. Summary of the roles of membrane-bound and soluble gp96 in adaptive immunity.
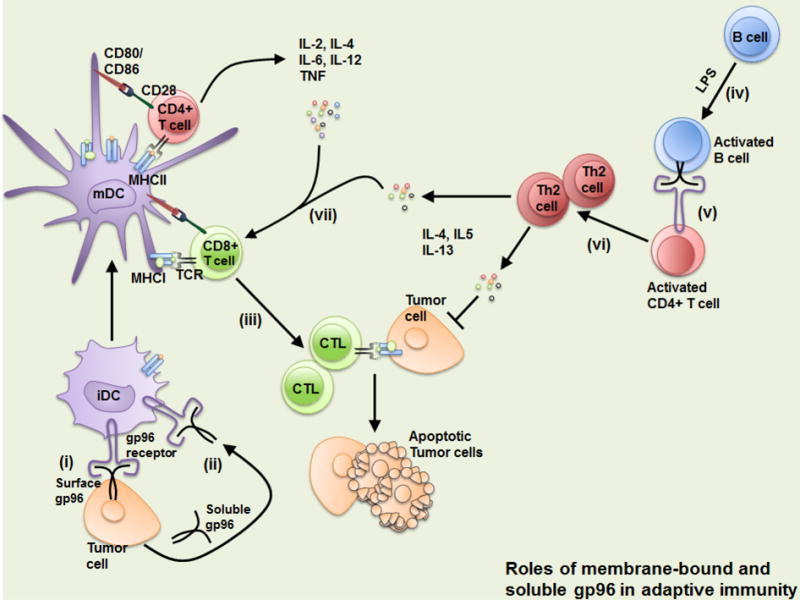
(i) Tumor-bearing membrane-bound gp96, or soluble gp96 released by tumor cells (ii), can induce maturation of immature dendritic cells (iDCs) into fully matured DCs (mDCs) via interaction with gp96 receptors on the surface of DCs. Matured DCs can induce activation of both CD4+ and CD8+ T cells, leading to apoptosis of tumor cells (iii). In some cases, LPS-activated B cells express surface gp96 (iv), which interacts with gp96 receptors such as CD91 on activated CD4+ T cells (v). Here, surface gp96 acts as a co-stimulatory molecule for T cell differentiation into the Th2 cell lineage (vi). These cells release Th2-specific cytokines which provide support for CD8 T cells in mediating tumor cell killing (vii).
Thus far, investigators have pursued several avenues of gp96-based antitumor therapies. Efforts began with cancer vaccination using tumor-derived gp96 [105]. An initial study showed that immunotherapy with gp96 was highly effective in inducing an antitumor immune response in mice bearing methylcholanthrene-induced fibrosarcomas (Meth A tumors), although this was only efficacious when given early in the tumor model course, or in conjunction with surgery [54]. Another study showed that photodynamic therapy increased surface expression of HSPs including gp96, which then contributes to therapeutic outcome via induction of inflammation and immune responses [106]. In addition, activated T cells and natural killer (NK) cells were shown to mediate antitumor immune responses against multiple myeloma [107] and lung cancer [108] when stimulated with dendritic cells pulsed with tumor-derived gp96. In the aforementioned cancer models, the primed cytotoxic T cells remained efficacious in attacking tumor cells, even after challenging a second time. The recent surge in development of gp96 inhibitors offers great therapeutic promise.
3.2. Monoclonal Antibodies Against gp96
The therapeutic targeting of surface gp96 in tumors has gathered momentum with the recent development of specific monoclonal antibodies shown to have potent gp96 inhibitory effects and consequent anti-tumor activities. Although pan-specific HSP90 inhibitors and even paralog-selective gp96 inhibitors are available, the problem with toxicities and off-target effects means developing monoclonal antibodies could provide alternative strategies to attenuating gp96 functions in cancers. Sabbatino et al. recently developed a novel monoclonal antibody (W9 mAb), which selectively targets the extracellular epitope of gp96 that is not detectable on normal cells but highly expressed on malignant cells. Further work demonstrated that W9 mAb can increase and restore sensitivity of human B-RAFV600E melanoma cells to B-RAF inhibitors [109]. A different study also demonstrated use of another monoclonal antibody, gp96 monoclonal antibody (gp96 mAb), to interfere with gp96-dependent HER2 dimerization and phosphorylation in breast cancer, and to induce anti-proliferative effects in HER2-driven cell growth both in vitro and in vivo [110]. The overall knowledge accumulated over the last thirty years across multiple cancer types provides strong emphasis for pushing forward development of useful extracellular gp96 modulators for clinical therapies.
3.3. Selective gp96 Small Molecule Inhibitors
Heat-shock protein-targeted inhibitors where initially anticipated to lack specificity and cause damage to normal as well as tumor cells, due to the abundance and ubiquitous expression of HSPs in most, if not all normal human cells. However, identification of geldanamycin (GM), a small-molecule inhibitor of HSP90 showed that tumor cells were more sensitive to HSP90-targeted drugs than untransformed cells. The selectivity of GM was demonstrated when low concentrations of the drug was described to induce differentiation, reduce cell proliferation and induce apoptosis by specifically binding to the N-terminal ATPase pocket of HSP90 [111]. Multiple groups later described unique gp96 expression patterns in various cancers, and this correlated clinically with advanced stage and poor prognosis in a variety of cancers including head and neck cancer [112], gallbladder cancer [113] and breast cancer [110]. Further work closely linked gp96 with promoting the growth and metastasis of cancers such as hepatocellular carcinoma [114], multiple myeloma [115], ovarian cancer and inflammatory colon carcinomas [116]. Since the identification of GM, multiple pan-HSP90 inhibitors have been developed and shown to disrupt various biological processes dependent on their functions [117]. However, the lack of selectivity ignited great interests in identifying paralogue-selective HSP90 inhibitors. Such inhibitors are predicted to interfere less with HSP functions in normal cells and therefore cause less toxicity, which should allow for the administration of higher drug doses and perhaps greater anticancer activity.
Among these, 5′-N-ethylcarboxamidoadenosine (NECA) was described to open up a cavity that can be accessed by the 5′-N-ethylcarboxamido moiety of NECA in gp96, but not in HSP90 [118]. Other compounds such as Compound 2 and Radamide can also access the unique 5′-hydrophobic pocket within the ATP-binding site of gp96 in a similar manner to NECA [119, 120]. Recently, Patel et al. also described novel purine-based ligands that were more than 100-fold selective for gp96 over HSP90α/β. Moreover, biochemical and crystallographic studies have showed that gp96 and other HSP90s do adopt distinct conformations and hydrolyze ATP differently when bound to nucleotides [121], despite the high degree of sequence conservation within their ATP-binding pockets. This conformational flexibility of gp96 allows the identified ligands to ‘freeze’ the protein in a state that unveils a unique pocket in gp96 due to a five amino acid (QEDGQ) insertion. Based on a strategy that combined library screening of purine-scaffold compounds and structural studies, new gp96 inhibitory ligands PU-H54, PU-WSI3 and PU-H39 were identified and described to have distinct structural features in the ATP-binding pocket; notably, Phe199 is swung away from the binding-pocket to reveal a deep hydrophobic cleft. A backward bend conformation is adopted by the X2-Ar functional group on PU-H54 that allows it to insert into the hydrophobic cleft and consequently stabilized upon contact with gp96 residue. Importantly, these distinct structural properties were not observed in the crystal structure of HSP90α bound paralog-specific inhibitors. The group then assessed the activities of their HSP90-paralogue inhibitors in whole cells, having confirmed their biochemical selectivity. Using the SKBr3 cell line, which expresses high levels of HER2 protein, gp96-selective inhibitors induced significant apoptosis with a notable reduction in cell viability [121]. Interestingly, no change in HER2 expression was observed in MCF-7 cells in presence of these inhibitors. Perhaps, due to cell specific requirements for HSP90 paralogues in the chaperoning of HER2, which is controlled by changes in the proteome. Moreover, gp96 was demonstrated to bind to HER2 specifically at the plasma membrane and stabilizes the protein in high HER2-expressing cells such as SKBr3. By contrast, gp96 inhibitors had little effect on MCF-7 cells due to the low expression of HER2 in these cells. However, HSP90 inhibitors have profound inhibitory effects in these cells as these inhibitors target cytosolic HER2. Thus, only membrane but not cytosolic HER2 molecules are substantially reduced by gp96 inhibition in SKBr3 cells in a time-dependent manner [122].
The selective inhibition of gp96 by PU-H39 was further demonstrated in vitro to interfere with the αI domain (a ligand-binding domain shared by seven integrin α-subunits) and consequently block the invasion of pre-B leukemic cells and RAW264.7 cells [10]. Additionally, the effect of PU-WS13 on gp96 was examined in a xenograft model of multiple [115] and shown to induce significant apoptosis and to block the growth of multiple myeloma cells, but not pre-B leukemic cells, thus demonstrating the dependence of myeloma growth on gp96. These studies experimentally demonstrate the possible clinical impact of developing gp96-selective inhibitors. They also show the context-specific advantages of gp96-selective inhibition over pan-HSP90 inhibition, and further emphasize the importance of further work to identify additional gp96-selective inhibitors and monoclonal antibodies.
3.4. Macrophage-Associated Colon Cancer
As both malignant cells and tumor-infiltrating immune cells express gp96, it is thus not surprising that gp96 plays a dual role in oncogenesis [6]. This chaperone promotes oncogenesis in a cancer cell-intrinsic fashion via Wnt and integrin signaling, as well as by enhancing tumor-related inflammation in a cancer cell-extrinsic manner by endowing the oncogenic functions of macrophages [123]. Macrophages express multiple pattern recognition receptors such as Toll-like receptors and can respond to TLR ligands to produce proinflammatory cytokines during infections [124]. Many functions of macrophages are regulated by TLRs, including cytokine production and antigen presentation [125]. Not surprisingly, since gp96 is a master chaperone for TLRs and integrins [9, 46], loss of gp96 from macrophages leads to a major perturbation of their function [5, 43, 44]. This has been demonstrated clearly in macrophage-associated colitis and colon cancer models. There is a strong clinical association between inflammatory bowel disease (IBD) and an increased risk of colon cancer [126, 127]. The loss of barrier functions (as is often seen in IBDs) leads to bacterial translocation across the intestinal wall, which activates macrophages through TLR ligands such as LPS. It has been postulated that chronic inflammation coupled with carcinogens triggers the upregulation of gp96 and the functional conversion of macrophages into tumor-associated macrophages (TAMs), which fuel oncogenesis by producing cytokines, reactive oxygen species, reactive nitrogen species, etc [123]. It is also known that TAMs promote cancer invasion and metastasis, while gp96 is strongly induced simultaneously in TAMs [128–132].
By using macrophage-specific gp96 KO (LysMcregp96flox/flox) mice, our group demonstrated that macrophages promote colitis and colitis-associated colon tumorigenesis in a gp96-dependent manner [116]. These macrophage-specific KO mice were more resistant to dextran sodium sulfate (DSS)-induced colitis than wild-type (WT) littermates. In response to the colitis-inducing agent and carcinogen, AOM/DSS, the colonic epithelium of WT mice harbored a higher level of β-catenin mutations within the exon 3 region of β-catenin, which was not seen in mice with gp96 deletion in macrophages [116]. Importantly, deletion of gp96 from macrophages also attenuated the production of IL-6, IL17, IL-23 and tumor necrosis factor (TNF)-α, as well as induction of genes involved in DNA mismatch repair pathways [5, 116]. These findings provide evidence that gp96 in TAMs play an important role in inflammation-driven genetic instability of gut epithelial cells [123]. On the contrary, gp96 is also involved in maintaining gut microbiota homeostasis and gut immune tolerance. Due to the tight clinical correlation between IBDs and cancer [126, 127], it was pertinent to investigate the role of gp96 and macrophages in Crohn’s disease (CD). Moreover, a lack of gp96 expression in intestinal macrophages from CD patients correlates with loss of tolerance against the host gut flora leading to chronic inflammation [133]. Thus, gp96 may serve as a key molecule in mediating gut mucosal immune tolerance. This theory is supported by the fact that gp96 is induced during differentiation of normal intestinal macrophages, but not detected in intestinal macrophages in the gut mucosa of CD patients [134]. As gp96 may have a role in tolerance induction, this observation suggests that downregulation of gp96 is responsible for the loss of tolerance against luminal bacteria found in CD patients [134, 135].
3.5. Hepatocellular Carcinoma
As with macrophage-associated cancer mentioned earlier, several recent studies have also established that gp96 plays important roles in hepatic homeostasis, steatosis and cancer development [136]. To investigate the role of gp96 in liver, Rachidi et al generated a hepatocyte-specific gp96 KO mouse model by crossing gp96flox/flox mice with Alb-cre mice. This strategy was very efficient and achieved greater than 95% deletion of gp96 in the liver of 6-weeks old KO mice. However, cre-mediated recombination was not 100% faithful, leaving out a small but definitive percentage of residual hepatocytes which retained gp96 expression [114, 137]. Nevertheless, this model allowed the investigators to discern the hepatocyte-intrinsic roles of gp96 in the baseline hepatic function, as well as during hepatic carcinogenesis. Rachidi et al found that gp96 deletion in hepatocytes resulted in a growth disadvantage, as the percentage of gp96-null hepatocytes in KO mice decreased from more than 95% in young mice (<6 month old) to less than 5% in aged mice (>12 month old). During this period of adaptation, a variety of novel metabolic disorders were identified in KO mice. First, the regenerating gp96-positive hepatocytes underwent profound steatotic changes, which raised an intriguing possibility that postulates increased metabolic demand to the liver as the underlying uniform mechanism for non-alcoholic steatohepatitis.
Second, the loss of gp96 resulted in accumulation of ceramide and sphingosine-1-phosphate, which play important roles in regulating inflammation, tumor growth and stress responses [138, 139]. These results suggest that gp96 plays important roles in maintaining the homeostasis of sphingolipid metabolism, which warrants further investigation. Importantly, this study has also unequivocally demonstrated that gp96 is an oncogenic chaperone in the liver. In response to diethyl-nitrosamine (DENA), only gp96-positive hepatocytes developed cancer. The pro-survival roles of gp96 were also confirmed by both genetic and pharmacological strategies using human hepatocarcinoma cell lines. Consistently, a recent report by Chen et al., also demonstrated that liver-specific gp96 KO mice develop abnormal tumor nodules in the advanced age, but only in gp96-positive hepatocytes [140].
The importance of gp96 in liver cancer has also been demonstrated in several other systems. For example, gp96 overexpression was shown unexpectedly to decrease the stability of the crucial tumor suppressor, p53, providing a mechanism by which ER stress can promote instability of the genome [141]. Moreover, molecular epidemiology studies also showed that there is a consistent elevation of gp96 protein and mRNA levels with progression from normal to precancerous to cancerous lesions in hepatocellular carcinoma [142]. Furthermore, supplementation of curcumin, a component of turmeric and curry which are widely used in food preparations was reported to reduce gp96 expression in liver cancer, potentially conferring future research into dietary treatments of HCC [143]. Altogether, gp96 is an important chaperone for hepatic homeostasis and an oncogenic chaperone in hepatocyte carcinogenesis. As gp96 functions in multiple important oncogenic clienteles such as TLR, IGF1, Wnt co-receptor and integrin (see below), gp96-targeted therapy may prove to be a promising therapeutic modality for liver cancer. Such gp96-targeted therapy for solid tumors will also be useful in treating hematologic tumors such as plasma cell malignancies, as the role of gp96 in these cancers is well documented [144].
3.6. Plasma Cell Malignancies
Plasma cell malignancies include multiple myeloma, Waldenstrom macroglobulinemia, monoclonal gammapathy of undetermined significance (MGUS) and others. Pathogenic malignant plasma cells characteristically produce large amounts of proteins in the form of immunoglobulins that require ER chaperones such as gp96 for quality control to prevent ER stress-induced cell death. Not surprisingly, it was found that gp96 is required for the pathogenesis of myeloma in a mouse model of disease that was driven by overexpression of the spliced variant of X-box binding protein 1 (XBP1s) [115]. Mechanistically, it was shown that gp96 chaperones Wnt signaling co-receptor LRP6, which ensures the long-term survival of myeloma cells by activating downstream target genes including survivin [145]. Thus, the dependence on gp96 to survive might be the ‘Achilles heel’ of myeloma, laying a strong foundation for development of gp96-based inhibitors against this currently incurable disease. Further work which might identify new targets of gp96, or uncover hitherto unknown functions of its cancer-associated clients, may open new avenues for developing novel gp96-targeted therapies.
4. T CELL INTRINSIC GP96: IMPLICATIONS FOR CELLULAR THERAPY
4.1. Regulatory T Cells
Regulatory T cells are a specific lineage of T cells that are generated either in the thymus for natural Tregs (nTregs) or in peripheral lymphoid tissues for inducible Treg cells (iTregs) that differentiate from naïve T cell precursors [146]. Upon egress from the thymus into peripheral and secondary lymphoid compartments, T cells are poised to activate the immune system in response to invading pathogens. Although these responses play essential roles in pathogen clearance, mechanisms exist to ensure they are directed toward harmful pathogens while remaining tolerant to self. Suppression by Treg cells that express the transcription factor, Foxp3, and are critical for maintaining immune homeostasis and tolerance, is one such mechanism by which activated T cells are prevented from directing immune responses toward self. Hence, Treg cell-mediated immune suppression becomes an obvious mechanism that tumors, which are considered “self” tissues, can co-opt in order to limit inflammation and evade immunosurveillance. Gp96 was initially described to boost antitumor immune responses which fits its role in the binding and presentation of tumor antigen peptides [14]. However, this immunogenic effect was dose-dependent, and high-dose gp96 immunization did not have cancer therapeutic effects [14]. Nevertheless, later work by Chandawarkar et al uncovered that gp96 can also act as an immune regulator when high doses of gp96 immunization (up to 100 μg) were administered [147]. They further demonstrated that immunization with high doses of gp96 can prevent myelin basic protein- or proteolipid protein-induced autoimmune encephalomyelitis in SJL mice and the onset of diabetes in non-obese diabetic (NOD) mice [148]. Similar protective effects were implicated in T cell-mediated immune disorders including IBD [135] and liver injury [149]. These studies indicated that gp96 plays different immune regulatory roles in a dose-dependent manner.
Interestingly, immune regulatory responses upon high-dose gp96 administration can be adoptively transferred by isolated CD4+ T lymphocytes from immunized mice [147, 148]. Not limited to Foxp3+ Tregs, CD4+CD25− T cells isolated from high dose gp96-immunized mice also retain their immune protective functions on type I diabetes [148]. A recent study demonstrated that high-dose gp96 immunization of mice significantly enhances Treg cell frequency and suppressive capacity, which eventually abrogates gp96-induced T cell activation [150]. Thus, emphasizing the involvement of regulatory T cells in this process. This immunization strategy provided rapid and long-lasting protection of mice against concanavalin A and anti-CD137-induced liver injury [149]. Although, the immune regulatory function of high-dose gp96 immunization was recognized decades ago, the precise mechanism at both cellular and molecular levels remains elusive. Our group has developed a unique surface gp96 expression mouse model (gp96tm) to explore this mechanism. We described recently that regulatory T cell function was enhanced in gp96tm mice and TLR4 plays a critical role in amplifying Treg cell suppressive function [36]. Our finding suggests that activation of TLR4 signaling may also contribute to Treg-mediated immune suppression upon high doses of gp96 immunization.
In contrast to the unclear mechanisms that contribute to the immune regulatory function of high dose gp96 immunization, the chaperone mechanism of gp96 in maintaining regulatory T cell function has been clearly addressed. By crossing Treg-specific Foxp3-cre transgenic mice with gp96flox/flox mice, we discovered that Treg cells cannot maintain stable Foxp3 in the absence of gp96. These KO mice have impaired suppressive function evidenced by the development of fatal autoimmune diseases [7]. Moreover, Zhang et al identified that the folding and biochemical maturation of GARP, a membrane latent-TGF-β (mLTGF-β) docking receptor, is entirely dependent on gp96 [7]. Further, in gp96-null cells, GARP cannot exit from the ER and is degraded rapidly due to a very short half-life. In addition, Tregs with high GARP expression show potent suppressive capacity [96, 151]. As discussed above, multiple integrins including integrin alpha V and β6 are also client proteins of gp96. Given the roles of these integrins in the cleavage and activation of latent TGF-β, gp96 most likely confers regulatory T cell function by controlling TGF-β activity via regulation of both GARP and integrins. These findings suggest that manipulation of gp96 levels in a Treg-specific fashion might have promising therapeutic implications in controlling autoimmune diseases and TGF-β-related pathologies such as cancers where TGFβ mediates cell invasion and metastasis. Indeed, our ongoing work suggests that inhibition of gp96 in regulatory T cells can greatly benefit adoptive T cell transfer therapy (unpublished data).
4.2. Effector T Cells
As the era of T cell immunotherapies for cancer treatment has dawned, our laboratory has aimed to investigate the role of gp96 in CD4+ and CD8+ effector T cell lineages in the context of adoptive T cell therapies. Along with calreticulin, gp96 was targeted as a cellular modulator of calcium located within the ER. Gp96 binds calcium within the ER with moderate binding affinity and may be critical for regulation of calcium-mediated signals such as mitochondrial activation and subsequent nuclear translocation of NFAT (Nuclear factor of activated T-cells), and interleukin (IL)-2 transcription within the T cells [27, 152, 153]. Unpublished data from our laboratory implicates gp96 as a key regulator of T cell calcium flux upon TCR engagement (Thaxton and Li, manuscript in preparation). Ultimately, loss of T cell-specific gp96 leads to impaired T cell activation concomitant with a loss in potential to undergo glycolysis. A downstream effect of gp96 T cell-specific deletion is the cellular-intrinsic loss of IL-2 production.
Manipulation of T cell metabolic programs is at the heart of the most promising T cell therapies for adoptive transfer to solid tumors [154]. Specifically, inhibition of AKT has been shown to cause regression of effector T cell programs toward stem cell-like characteristics coordinated with increased engraftment and cellular longevity in the tumor microenvironment [155]. Furthermore, transfer of metabolically shifted cytotoxic T cells through drug therapy has proven an effective method to alter cytotoxic T cell programs from effector cell-associated glycolysis to memory cell-associated fatty acid oxidation [156, 157]. Illumination of the role of gp96 in the upstream signaling of mitochondrial activation may prove critical in future identification of ER-mitochondrial targets for therapeutic intervention aimed to shift T cell metabolic programs.
5. PERSPECTIVE
As a genetic disorder, cancer is a complicated entity with intrinsic molecular mutations that render them to escape from normal growth regulation. Additionally, cancer cells often need to overcome extrinsic factors imposed by the host immune system, known as immunosurveillance, to progress and metastasize. Paradoxically, during the late stage of oncogenesis, tumor cells can also recruit inflammatory cells and soluble mediators to enhance tumor growth. As discussed above, gp96 can promote cancer through a cancer cell intrinsic manner via folding integrins, Wnt co-receptors, IGFs and other critical pathways. Gp96 can also promote activation of immune suppressive cells via chaperoning TLRs and activation of TGFβ (through integrin and GARP) (Fig. 3). As a critical molecule linking protein quality-control to stress and inflammation, gp96 is an attractive target for cancer therapy. Gp96-selective inhibitors should be particularly useful for cancers with a high demand for protein folding such as multiple myeloma [115, 145]. They should also be applicable for cancers with dominant component of inflammation such as inflammatory bowel disease-associated colon cancer and cancers with infectious etiology including, hepatocellular carcinoma, bladder cancer and mucosa-associated malignancies. Understanding molecularly how gp96 folds its clients shall also be essential for designing and developing better gp96 inhibitors with molecular specificity and cellular selectivity for clinical translation.
Fig. 3. Roles of molecular chaperone gp96 in oncogenesis from the vantage point of immunology.
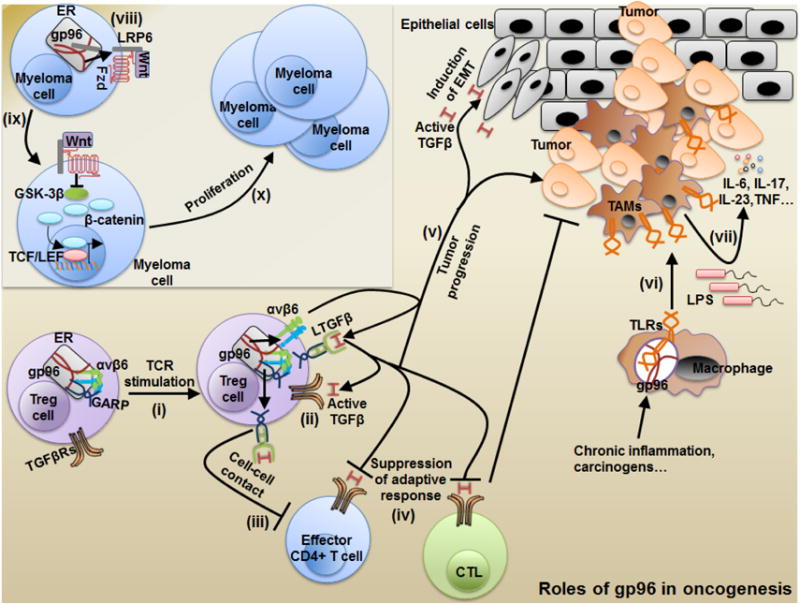
Upon TCR engagement in regulatory T cells (Tregs), gp96 mediates the surface expression of the latent TGF-β docking receptor, GARP (i). Expression of surface GARP provides a site for further binding and activation of latent TGF-β via multiple mechanisms including, cleavage by integrins such as αvβ6 integrins, which are clients of gp96 (ii). Tregs then mediate suppression of effector CD4+ and CD8+ T cells via cell-cell contact using membrane-bound active TGF-β (iii), or soluble active TGF-β released upon integrin cleavage of latent TGF-β from GARP (iv). Treg suppression of the adaptive immune response promotes tumor progression and the release of active TGF-β mediates epithelial-mesenchymal transition of normal epithelial cells (v). Furthermore in macrophages, chronic inflammation coupled with carcinogens triggers gp96 upregulation, which in-turn chaperones TLRs and augments the induction of tumor-associated macrophages (TAMs) (vi). TAMs support tumor progression by releasing cytokines including IL-6, IL-17, IL-23 and TNFα (vii). In addition, gp96 plays a major role in the oncogenesis of multiple myeloma (viii). Gp96 chaperones the Wnt signaling co-factor, LRP6, to the cell surface and this augments Wnt signaling to inhibit GSK-3β and mediate nuclear translocation of β-catenin (ix). This mechanism promotes excessive proliferation of multiple myeloma cells (x).
Acknowledgments
This work is supported by the US National Institutes of Health (P01CA186866 to Z.L., B.L., D.T.G. and G.C. and by R01CA188419, R01AI070603 to Z.L.). Z.L. is the Abney Chair Remembering Sally Abney Rose in Stem Cell Biology & Therapy and was supported by the SmartState Endowed Chair Program of South Carolina.
Biography

Zihai Li1
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
References
- 1.Chen YG, Ashok BT, Liu X, Garikapaty VP, Mittelman A, Tiwari RK. Induction of heat shock protein gp96 by immune cytokines. Cell Stress Chaperones. 2003;8:242–248. doi: 10.1379/1466-1268(2003)008<0242:iohspg>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 3.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332:462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 4.Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen SM, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Staron M, Hong F, Wu BX, Sun S, Morales C, Crosson CE, Tomlinson S, Kim I, Wu D, Li Z. Essential roles of grp94 in gut homeostasis via chaperoning canonical Wnt pathway. Proc Natl Acad Sci U S A. 2013;110:6877–6882. doi: 10.1073/pnas.1302933110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Wu BX, Metelli A, Thaxton JE, Hong F, Rachidi S, Ansa-Addo E, Sun S, Vasu C, Yang Y, Liu B, Li Z. GP96 is a GARP chaperone and controls regulatory T cell functions. J Clin Invest. 2015;125:859–869. doi: 10.1172/JCI79014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117:7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staron M, Yang Y, Liu B, Li J, Shen Y, Zuniga-Pflucker JC, Aguila HL, Goldschneider I, Li Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115:2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong F, Liu B, Chiosis G, Gewirth DT, Li Z. alpha7 helix region of alphaI domain is crucial for integrin binding to endoplasmic reticulum chaperone gp96: a potential therapeutic target for cancer metastasis. J Biol Chem. 2013;288:18243–18248. doi: 10.1074/jbc.M113.468850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AS, Bell J, Ting J. Biochemical characterization of the 94- and 78-kilodalton glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1984;259:4616–4621. [PubMed] [Google Scholar]
- 12.Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- 13.Lewis MJ, Mazzarella RA, Green M. Structure and assembly of the endoplasmic reticulum. The synthesis of three major endoplasmic reticulum proteins during lipopolysaccharide-induced differentiation of murine lymphocytes. J Biol Chem. 1985;260:3050–3057. [PubMed] [Google Scholar]
- 14.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci U S A. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai J, Liu B, Caudill MM, Zheng H, Qiao Y, Podack ER, Li Z. Cell surface expression of heat shock protein gp96 enhances cross-presentation of cellular antigens and the generation of tumor-specific T cell memory. Cancer Immun. 2003;3:1. [PubMed] [Google Scholar]
- 16.Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z. Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Natl Acad Sci U S A. 2003;100:15824–15829. doi: 10.1073/pnas.2635458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robert J, Menoret A, Cohen N. Cell surface expression of the endoplasmic reticular heat shock protein gp96 is phylogenetically conserved. J Immunol. 1999;163:4133–4139. [PubMed] [Google Scholar]
- 18.Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Binder RJ. Functions of Heat Shock Proteins in Pathways of the Innate and Adaptive Immune System. J Immunol. 2014;193:5765–5771. doi: 10.4049/jimmunol.1401417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 21.Li Z, Menoret A, Srivastava P. Roles of heat-shock proteins in antigen presentation and cross-presentation. Curr Opin Immunol. 2002;14:45–51. doi: 10.1016/s0952-7915(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava PK, Menoret A, Basu S, Binder RJ, McQuade KL. Heat shock proteins come of age: primitive functions acquire new roles in an adaptive world. Immunity. 1998;8:657–665. doi: 10.1016/s1074-7613(00)80570-1. [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: implications for protein folding and antigen presentation. EMBO J. 1993;12:3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Udono H, Levey DL, Srivastava PK. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc Natl Acad Sci U S A. 1994;91:3077–3081. doi: 10.1073/pnas.91.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- 27.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 28.Doody AD, Kovalchin JT, Mihalyo MA, Hagymasi AT, Drake CG, Adler AJ. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J Immunol. 2004;172:6087–6092. doi: 10.4049/jimmunol.172.10.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SenGupta D, Norris PJ, Suscovich TJ, Hassan-Zahraee M, Moffett HF, Trocha A, Draenert R, Goulder PJ, Binder RJ, Levey DL, Walker BD, Srivastava PK, Brander C. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J Immunol. 2004;173:1987–1993. doi: 10.4049/jimmunol.173.3.1987. [DOI] [PubMed] [Google Scholar]
- 30.Akazawa T, Ohashi T, Nakajima H, Nishizawa Y, Kodama K, Sugiura K, Inaba T, Inoue N. Development of a dendritic cell-targeting lipopeptide as an immunoadjuvant that inhibits tumor growth without inducing local inflammation. Int J Cancer. 2014;135:2847–2856. doi: 10.1002/ijc.28939. [DOI] [PubMed] [Google Scholar]
- 31.Binder RJ, Anderson KM, Basu S, Srivastava PK. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165:6029–6035. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 32.Hou J, Li X, Li C, Sun L, Zhao Y, Zhao J, Meng S. Plasma membrane gp96 enhances invasion and metastatic potential of liver cancer via regulation of uPAR. Mol Oncol. 2015;9:1312–1323. doi: 10.1016/j.molonc.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzec M, Eletto D, Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Li Z. Roles of heat shock protein gp96 in the ER quality control: redundant or unique function? Mol Cells. 2005;20:173–182. [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 36.Dai J, Liu B, Ngoi SM, Sun S, Vella AT, Li Z. TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J Immunol. 2007;178:3219–3225. doi: 10.4049/jimmunol.178.5.3219. [DOI] [PubMed] [Google Scholar]
- 37.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112:1223–1230. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagerbrand K, Westlund J, Yrlid U, Agace W, Johansson-Lindbom B. MyD88 Signaling Regulates Steady-State Migration of Intestinal CD103+ Dendritic Cells Independently of TNF-alpha and the Gut Microbiota. J Immunol. 2015;195:2888–2899. doi: 10.4049/jimmunol.1500210. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Xiao Y, Hu H, Zou Q, Li Y, Gao Y, Ge W, Cheng X, Sun SC. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat Commun. 2015;6:5930. doi: 10.1038/ncomms6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao C, Oh JH, Lee DH, Bae JS, Jin CL, Park CH, Chung JH. Toll-like receptor family members in skin fibroblasts are functional and have a higher expression compared to skin keratinocytes. Int J Mol Med. 2015;35:1443–1450. doi: 10.3892/ijmm.2015.2146. [DOI] [PubMed] [Google Scholar]
- 41.Deng S, Zhu S, Qiao Y, Liu YJ, Chen W, Zhao G, Chen J. Recent advances in the role of toll-like receptors and TLR agonists in immunotherapy for human glioma. Protein Cell. 2014;5:899–911. doi: 10.1007/s13238-014-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira SA. Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Li Y, Hao B, Bona R, Han D, Li Z. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 45.Wu S, Hong F, Gewirth D, Guo B, Liu B, Li Z. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J Biol Chem. 2012;287:6735–6742. doi: 10.1074/jbc.M111.309526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brooks JC, Sun W, Chiosis G, Leifer CA. Heat shock protein gp96 regulates Toll-like receptor 9 proteolytic processing and conformational stability. Biochem Biophys Res Commun. 2012;421:780–784. doi: 10.1016/j.bbrc.2012.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGettrick AF, O’Neill LA. Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol. 2010;22:20–27. doi: 10.1016/j.coi.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Warger T, Hilf N, Rechtsteiner G, Haselmayer P, Carrick DM, Jonuleit H, von Landenberg P, Rammensee HG, Nicchitta CV, Radsak MP, Schild H. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J Biol Chem. 2006;281:22545–22553. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Shibata T, Akashi-Takamura S, Kiyokawa T, Wakabayashi Y, Tanimura N, Kobayashi T, Matsumoto F, Fukui R, Kouro T, Nagai Y, Takatsu K, Saitoh S, Miyake K. A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J Exp Med. 2007;204:2963–2976. doi: 10.1084/jem.20071132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 51.Merrell MA, Ilvesaro JM, Lehtonen N, Sorsa T, Gehrs B, Rosenthal E, Chen D, Shackley B, Harris KW, Selander KS. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4:437–447. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 52.Brignole C, Marimpietri D, Di Paolo D, Perri P, Morandi F, Pastorino F, Zorzoli A, Pagnan G, Loi M, Caffa I, Erminio G, Haupt R, Gambini C, Pistoia V, Ponzoni M. Therapeutic targeting of TLR9 inhibits cell growth and induces apoptosis in neuroblastoma. Cancer Res. 2010;70:9816–9826. doi: 10.1158/0008-5472.CAN-10-1251. [DOI] [PubMed] [Google Scholar]
- 53.Heike M, Frenzel C, Meier D, Galle PR. Expression of stress protein gp96, a tumor rejection antigen, in human colorectal cancer. Int J Cancer. 2000;86:489–493. doi: 10.1002/(sici)1097-0215(20000515)86:4<489::aid-ijc7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.Kovalchin JT, Murthy AS, Horattas MC, Guyton DP, Chandawarkar RY. Determinants of efficacy of immunotherapy with tumor-derived heat shock protein gp96. Cancer Immun. 2001;1:7. [PubMed] [Google Scholar]
- 55.Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007;13:552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 56.Stoitzner P, Green LK, Jung JY, Price KM, Atarea H, Kivell B, Ronchese F. Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells. Cancer Immunol Immunother. 2008;57:1665–1673. doi: 10.1007/s00262-008-0487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali OA, Verbeke C, Johnson C, Sands RW, Lewin SA, White D, Doherty E, Dranoff G, Mooney DJ. Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants. Cancer Res. 2014;74:1670–1681. doi: 10.1158/0008-5472.CAN-13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K. The effect of combined IL10 siRNA and CpG ODN as pathogen-mimicking microparticles on Th1/Th2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials. 2014;35:5491–5504. doi: 10.1016/j.biomaterials.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuhn S, Hyde EJ, Yang J, Rich FJ, Harper JL, Kirman JR, Ronchese F. Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents. J Immunol. 2013;191:1984–1992. doi: 10.4049/jimmunol.1301135. [DOI] [PubMed] [Google Scholar]
- 60.Huen AO, Rook AH. Toll receptor agonist therapy of skin cancer and cutaneous T-cell lymphoma. Curr Opin Oncol. 2014;26:237–244. doi: 10.1097/CCO.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 61.Chang LS, Leng CH, Yeh YC, Wu CC, Chen HW, Huang HM, Liu SJ. Toll-like receptor 9 agonist enhances anti-tumor immunity and inhibits tumor-associated immunosuppressive cells numbers in a mouse cervical cancer model following recombinant lipoprotein therapy. Mol Cancer. 2014;13:60. doi: 10.1186/1476-4598-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goutagny N, Estornes Y, Hasan U, Lebecque S, Caux C. Targeting pattern recognition receptors in cancer immunotherapy. Target Oncol. 2012;7:29–54. doi: 10.1007/s11523-012-0213-1. [DOI] [PubMed] [Google Scholar]
- 63.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–647. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miyata S, Koshikawa N, Yasumitsu H, Miyazaki K. Trypsin stimulates integrin alpha(5)beta(1)-dependent adhesion to fibronectin and proliferation of human gastric carcinoma cells through activation of proteinase-activated receptor-2. J Biol Chem. 2000;275:4592–4598. doi: 10.1074/jbc.275.7.4592. [DOI] [PubMed] [Google Scholar]
- 65.Hollenbeck ST, Itoh H, Louie O, Faries PL, Liu B, Kent KC. Type I collagen synergistically enhances PDGF-induced smooth muscle cell proliferation through pp60src-dependent crosstalk between the alpha2beta1 integrin and PDGFbeta receptor. Biochem Biophys Res Commun. 2004;325:328–337. doi: 10.1016/j.bbrc.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 66.Hedin U, Roy J, Tran PK. Control of smooth muscle cell proliferation in vascular disease. Curr Opin Lipidol. 2004;15:559–565. doi: 10.1097/00041433-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Zhou X, Murphy FR, Gehdu N, Zhang J, Iredale JP, Benyon RC. Engagement of alphavbeta3 integrin regulates proliferation and apoptosis of hepatic stellate cells. J Biol Chem. 2004;279:23996–24006. doi: 10.1074/jbc.M311668200. [DOI] [PubMed] [Google Scholar]
- 68.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 69.Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276:17550–17558. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 70.Paulhe F, Racaud-Sultan C, Ragab A, Albiges-Rizo C, Chap H, Iberg N, Morand O, Perret B. Differential regulation of phosphoinositide metabolism by alphaVbeta3 and alphaVbeta5 integrins upon smooth muscle cell migration. J Biol Chem. 2001;276:41832–41840. doi: 10.1074/jbc.M105459200. [DOI] [PubMed] [Google Scholar]
- 71.Tsuji T. Physiological and pathological roles of alpha3beta1 integrin. J Membr Biol. 2004;200:115–132. doi: 10.1007/s00232-004-0696-5. [DOI] [PubMed] [Google Scholar]
- 72.Takanami I. Increased expression of integrin-linked kinase is associated with shorter survival in non-small cell lung cancer. BMC Cancer. 2005;5:1. doi: 10.1186/1471-2407-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo HB, Lee I, Bryan BT, Pierce M. Deletion of mouse embryo fibroblast N-acetylglucosaminyltransferase V stimulates alpha5beta1 integrin expression mediated by the protein kinase C signaling pathway. J Biol Chem. 2005;280:8332–8342. doi: 10.1074/jbc.M413532200. [DOI] [PubMed] [Google Scholar]
- 74.Felding-Habermann B, Fransvea E, O’Toole TE, Manzuk L, Faha B, Hensler M. Involvement of tumor cell integrin alpha v beta 3 in hematogenous metastasis of human melanoma cells. Clin Exp Metastasis. 2002;19:427–436. doi: 10.1023/a:1016377114119. [DOI] [PubMed] [Google Scholar]
- 75.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via alpha(v)beta3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 76.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 77.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 78.Tsuruta D, Kobayashi H, Imanishi H, Sugawara K, Ishii M, Jones JC. Laminin-332-integrin interaction: a target for cancer therapy? Curr Med Chem. 2008;15:1968–1975. doi: 10.2174/092986708785132834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 80.Bazzoni G. Signalling pathways and adhesion molecules as targets for antiangiogenesis therapy in tumors. Adv Exp Med Biol. 2008;610:74–87. doi: 10.1007/978-0-387-73898-7_6. [DOI] [PubMed] [Google Scholar]
- 81.Robertson JH, Iga AM, Sales KM, Winslet MC, Seifalian AM. Integrins: a method of early intervention in the treatment of colorectal liver metastases. Curr Pharm Des. 2008;14:296–305. doi: 10.2174/138161208783413284. [DOI] [PubMed] [Google Scholar]
- 82.Gong W, Liu Y, Huang B, Lei Z, Wu FH, Li D, Feng ZH, Zhang GM. Recombinant CBD-HepII polypeptide of fibronectin inhibits alphavbeta3 signaling and hematogenous metastasis of tumor. Biochem Biophys Res Commun. 2008;367:144–149. doi: 10.1016/j.bbrc.2007.12.110. [DOI] [PubMed] [Google Scholar]
- 83.Chen Q, Manning CD, Millar H, McCabe FL, Ferrante C, Sharp C, Shahied-Arruda L, Doshi P, Nakada MT, Anderson GM. CNTO 95, a fully human anti alphav integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- 84.Kokubo T, Uchida H, Choi ET. Integrin alpha(v)beta(3) as a target in the prevention of neointimal hyperplasia. J Vasc Surg. 2007;45(Suppl A):A33–38. doi: 10.1016/j.jvs.2007.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakuma T, Sari I, Goodman CN, Lindner JR, Klibanov AL, Kaul S. Simultaneous integrin alphavbeta3 and glycoprotein IIb/IIIa inhibition causes reduction in infarct size in a model of acute coronary thrombosis and primary angioplasty. Cardiovasc Res. 2005;66:552–561. doi: 10.1016/j.cardiores.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 86.Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF, Ellis LM. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- 87.Maubant S, Saint-Dizier D, Boutillon M, Perron-Sierra F, Casara PJ, Hickman JA, Tucker GC, Van Obberghen-Schilling E. Blockade of alpha v beta3 and alpha v beta5 integrins by RGD mimetics induces anoikis and not integrin-mediated death in human endothelial cells. Blood. 2006;108:3035–3044. doi: 10.1182/blood-2006-05-023580. [DOI] [PubMed] [Google Scholar]
- 88.Morales C, Wu S, Yang Y, Hao B, Li Z. Drosophila glycoprotein 93 Is an ortholog of mammalian heat shock protein gp96 (grp94, HSP90b1, HSPC4) and retains disulfide bond-independent chaperone function for TLRs and integrins. J Immunol. 2009;183:5121–5128. doi: 10.4049/jimmunol.0900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hsieh JC, Lee L, Zhang L, Wefer S, Brown K, DeRossi C, Wines ME, Rosenquist T, Holdener BC. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112:355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 90.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. The American journal of pathology. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. Journal of neurochemistry. 2007;101:517–529. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- 92.Ostrovsky O, Ahmed NT, Argon Y. The chaperone activity of GRP94 toward insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell. 2009;20:1855–1864. doi: 10.1091/mbc.E08-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barton ER, Park S, James JK, Makarewich CA, Philippou A, Eletto D, Lei H, Brisson B, Ostrovsky O, Li Z, Argon Y. Deletion of muscle GRP94 impairs both muscle and body growth by inhibiting local IGF production. FASEB J. 2012;26:3691–3702. doi: 10.1096/fj.11-203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lopez JA, Andrews RK, Afshar-Kharghan V, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- 95.Li Y, Lu J, Prochownik EV. Modularity of the oncoprotein-like properties of platelet glycoprotein Ibalpha. J Biol Chem. 2009;284:1410–1418. doi: 10.1074/jbc.M806222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roubin R, Pizette S, Ollendorff V, Planche J, Birnbaum D, Delapeyriere O. Structure and developmental expression of mouse Garp, a gene encoding a new leucine-rich repeat-containing protein. Int J Dev Biol. 1996;40:545–555. [PubMed] [Google Scholar]
- 98.Wang R, Zhu J, Dong X, Shi M, Lu C, Springer TA. GARP regulates the bioavailability and activation of TGFbeta. Mol Biol Cell. 2012;23:1129–1139. doi: 10.1091/mbc.E11-12-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Probst-Kepper M, Buer J. FOXP3 and GARP (LRRC32): the master and its minion. Biol Direct. 2010;5:8. doi: 10.1186/1745-6150-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gauthy E, Cuende J, Stockis J, Huygens C, Lethe B, Collet JF, Bommer G, Coulie PG, Lucas S. GARP is regulated by miRNAs and controls latent TGF-beta1 production by human regulatory T cells. PLoS One. 2013;8:e76186. doi: 10.1371/journal.pone.0076186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hahn SA, Stahl HF, Becker C, Correll A, Schneider FJ, Tuettenberg A, Jonuleit H. Soluble GARP has potent antiinflammatory and immunomodulatory impact on human CD4(+) T cells. Blood. 2013;122:1182–1191. doi: 10.1182/blood-2012-12-474478. [DOI] [PubMed] [Google Scholar]
- 102.Linderoth NA, Popowicz A, Sastry S. Identification of the peptide-binding site in the heat shock chaperone/tumor rejection antigen gp96 (Grp94) J Biol Chem. 2000;275:5472–5477. doi: 10.1074/jbc.275.8.5472. [DOI] [PubMed] [Google Scholar]
- 103.Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731–6735. doi: 10.4049/jimmunol.167.12.6731. [DOI] [PubMed] [Google Scholar]
- 104.Banerjee PP, Vinay DS, Mathew A, Raje M, Parekh V, Prasad DV, Kumar A, Mitra D, Mishra GC. Evidence that glycoprotein 96 (B2), a stress protein, functions as a Th2-specific costimulatory molecule. J Immunol. 2002;169:3507–3518. doi: 10.4049/jimmunol.169.7.3507. [DOI] [PubMed] [Google Scholar]
- 105.Ahn CY, Kim EJ, Lee I, Kwon JW, Kim WB, Kim SG, Lee MG. Effects of glucose on the pharmacokinetics of intravenous chlorzoxazone in rats with acute renal failure induced by uranyl nitrate. J Pharm Sci. 2003;92:1604–1613. doi: 10.1002/jps.10426. [DOI] [PubMed] [Google Scholar]
- 106.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 107.Qian J, Wang S, Yang J, Xie J, Lin P, Freeman ME, 3rd, Yi Q. Targeting heat shock proteins for immunotherapy in multiple myeloma: generation of myeloma-specific CTLs using dendritic cells pulsed with tumor-derived gp96. Clin Cancer Res. 2005;11:8808–8815. doi: 10.1158/1078-0432.CCR-05-1553. [DOI] [PubMed] [Google Scholar]
- 108.Shinagawa N, Yamazaki K, Tamura Y, Imai A, Kikuchi E, Yokouchi H, Hommura F, Oizumi S, Nishimura M. Immunotherapy with dendritic cells pulsed with tumor-derived gp96 against murine lung cancer is effective through immune response of CD8+ cytotoxic T lymphocytes and natural killer cells. Cancer Immunol Immunother. 2008;57:165–174. doi: 10.1007/s00262-007-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sabbatino F, Favoino E, Wang Y, Wang X, Villani V, Cai L, Yang L, Ferrone S, Ferrone CR. Grp94-specific monoclonal antibody to counteract BRAF inhibitor resistance in BRAF(V600E) melanoma. J Transl Med. 2015;13:2050. [Google Scholar]
- 110.Committee, A. S. o. P. Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81–89. doi: 10.1016/j.gie.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 111.Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 112.Chiu CC, Lin CY, Lee LY, Chen YJ, Lu YC, Wang HM, Liao CT, Chang JT, Cheng AJ. Molecular chaperones as a common set of proteins that regulate the invasion phenotype of head and neck cancer. Clin Cancer Res. 2011;17:4629–4641. doi: 10.1158/1078-0432.CCR-10-2107. [DOI] [PubMed] [Google Scholar]
- 113.Chen Y, Chen C, Ma C, Sun S, Zhang J, Sun Y. Expression of heat-shock protein gp96 in gallbladder cancer and its prognostic clinical significance. Int J Clin Exp Pathol. 2015;8:1946–1953. [PMC free article] [PubMed] [Google Scholar]
- 114.Rachidi S, Sun S, Wu BX, Jones E, Drake RR, Ogretmen B, Cowart LA, Clarke CJ, Hannun YA, Chiosis G, Liu B, Li Z. Endoplasmic reticulum heat shock protein gp96 maintains liver homeostasis and promotes hepatocellular carcinogenesis. J Hepatol. 2015;62:879–888. doi: 10.1016/j.jhep.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hua Y, White-Gilbertson S, Kellner J, Rachidi S, Usmani SZ, Chiosis G, Depinho R, Li Z, Liu B. Molecular chaperone gp96 is a novel therapeutic target of multiple myeloma. Clin Cancer Res. 2013;19:6242–6251. doi: 10.1158/1078-0432.CCR-13-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Morales C, Rachidi S, Hong F, Sun S, Ouyang X, Wallace C, Zhang Y, Garret-Mayer E, Wu J, Liu B, Li Z. Immune chaperone gp96 drives the contributions of macrophages to inflammatory colon tumorigenesis. Cancer Res. 2014;74:446–459. doi: 10.1158/0008-5472.CAN-13-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Taldone T, Ochiana SO, Patel PD, Chiosis G. Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol Sci. 2014;35:592–603. doi: 10.1016/j.tips.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soldano KL, Jivan A, Nicchitta CV, Gewirth DT. Structure of the N-terminal domain of GRP94. Basis for ligand specificity and regulation. J Biol Chem. 2003;278:48330–48338. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- 119.Immormino RM, Metzger LEt, Reardon PN, Dollins DE, Blagg BS, Gewirth DT. Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: implications for paralog-specific drug design. J Mol Biol. 2009;388:1033–1042. doi: 10.1016/j.jmb.2009.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duerfeldt AS, Peterson LB, Maynard JC, Ng CL, Eletto D, Ostrovsky O, Shinogle HE, Moore DS, Argon Y, Nicchitta CV, Blagg BS. Development of a Grp94 inhibitor. J Am Chem Soc. 2012;134:9796–9804. doi: 10.1021/ja303477g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patel PD, Yan P, Seidler PM, Patel HJ, Sun W, Yang C, Que NS, Taldone T, Finotti P, Stephani RA, Gewirth DT, Chiosis G. Paralog-selective Hsp90 inhibitors define tumor-specific regulation of HER2. Nat Chem Biol. 2013;9:677–684. doi: 10.1038/nchembio.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chavany C, Mimnaugh E, Miller P, Bitton R, Nguyen P, Trepel J, Whitesell L, Schnur R, Moyer J, Neckers L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2. J Biol Chem. 1996;271:4974–4977. doi: 10.1074/jbc.271.9.4974. [DOI] [PubMed] [Google Scholar]
- 123.Hong F, Wu BX, Li Z. Molecular regulation of macrophages in unleashing cancer-related inflammation. Oncoimmunology. 2014;3:e27659. doi: 10.4161/onci.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, Gordon S. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 125.Unanue ER. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- 126.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 127.Weedon DD, Shorter RG, Ilstrup DM, Huizenga KA, Taylor WF. Crohn’s disease and cancer. N Engl J Med. 1973;289:1099–1103. doi: 10.1056/NEJM197311222892101. [DOI] [PubMed] [Google Scholar]
- 128.Reddy RK, Dubeau L, Kleiner H, Parr T, Nichols P, Ko B, Dong D, Ko H, Mao C, DiGiovanni J, Lee AS. Cancer-inducible transgene expression by the Grp94 promoter: spontaneous activation in tumors of various origins and cancer-associated macrophages. Cancer Res. 2002;62:7207–7212. [PubMed] [Google Scholar]
- 129.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wolfram L, Fischbeck A, Frey-Wagner I, Wojtal KA, Lang S, Fried M, Vavricka SR, Hausmann M, Rogler G. Regulation of the expression of chaperone gp96 in macrophages and dendritic cells. PLoS One. 2013;8:e76350. doi: 10.1371/journal.pone.0076350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schreiter K, Hausmann M, Spoettl T, Strauch UG, Bataille F, Schoelmerich J, Herfarth H, Falk W, Rogler G. Glycoprotein (gp) 96 expression: induced during differentiation of intestinal macrophages but impaired in Crohn’s disease. Gut. 2005;54:935–943. doi: 10.1136/gut.2004.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fischbeck A, Schreiter K, Leucht K, Frey-Wagner I, Lang S, Hausmann M, Fried M, Falk W, Rogler G. Exogenous heat shock protein gp96 ameliorates CD4+CD62L+ T-cell-mediated transfer colitis. Inflamm Bowel Dis. 2014;20:1933–1941. doi: 10.1097/MIB.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 136.Chen WT, Ha D, Kanel G, Lee AS. Targeted deletion of ER chaperone GRP94 in the liver results in injury, repopulation of GRP94-positive hepatocytes, and spontaneous hepatocellular carcinoma development in aged mice. Neoplasia. 2014;16:617–626. doi: 10.1016/j.neo.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rachidi S, Sun S, Li Z. Endoplasmic reticulum heat shock protein gp96/grp94 is a pro-oncogenic chaperone, not a tumor suppressor. Hepatology. 2015;61:1766–1767. doi: 10.1002/hep.27400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 139.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen WT, Tseng CC, Pfaffenbach K, Kanel G, Luo B, Stiles BL, Lee AS. Liver-specific knockout of GRP94 in mice disrupts cell adhesion, activates liver progenitor cells, and accelerates liver tumorigenesis. Hepatology. 2014;59:947–957. doi: 10.1002/hep.26711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu B, Chu X, Feng C, Hou J, Fan H, Liu N, Li C, Kong X, Ye X, Meng S. Heat shock protein gp96 decreases p53 stability by regulating Mdm2 E3 ligase activity in liver cancer. Cancer Lett. 2015;359:325–334. doi: 10.1016/j.canlet.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 142.Wu XH, Yao DF, Su XQ, Tai BJ, Huang H, Qiu LW, Wu W, Shao YX. Dynamic expression of rat heat shock protein gp96 and its gene during development of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:616–621. [PubMed] [Google Scholar]
- 143.Ahmed HH, Shousha WG, Shalby AB, El-Mezayen HA, Ismaiel NN, Mahmoud NS. Curcumin: a unique antioxidant offers a multimechanistic approach for management of hepatocellular carcinoma in rat model. Tumour Biol. 2015;36:1667–1678. doi: 10.1007/s13277-014-2767-2. [DOI] [PubMed] [Google Scholar]
- 144.Ishii T, Udono H, Yamano T, Ohta H, Uenaka A, Ono T, Hizuta A, Tanaka N, Srivastava PK, Nakayama E. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–1309. [PubMed] [Google Scholar]
- 145.White-Gilbertson S, Hua Y, Liu B. The role of endoplasmic reticulum stress in maintaining and targeting multiple myeloma: a double-edged sword of adaptation and apoptosis. Front Genet. 2013;4:109. doi: 10.3389/fgene.2013.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chandawarkar RY, Wagh MS, Srivastava PK. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J Exp Med. 1999;189:1437–1442. doi: 10.1084/jem.189.9.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chandawarkar RY, Wagh MS, Kovalchin JT, Srivastava P. Immune modulation with high-dose heat-shock protein gp96: therapy of murine autoimmune diabetes and encephalomyelitis. Int Immunol. 2004;16:615–624. doi: 10.1093/intimm/dxh063. [DOI] [PubMed] [Google Scholar]
- 149.Zhao B, Wang Y, Zhang Y, Li Y, Zhang X, Xu Y, Chen L, Li C, Ju Y, Meng S. TAT-mediated gp96 transduction to APCs enhances gp96-induced antiviral and antitumor T cell responses. Vaccine. 2013;31:545–552. doi: 10.1016/j.vaccine.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 150.Liu Z, Li X, Qiu L, Zhang X, Chen L, Cao S, Wang F, Meng S. Treg suppress CTL responses upon immunization with HSP gp96. Eur J Immunol. 2009;39:3110–3120. doi: 10.1002/eji.200939593. [DOI] [PubMed] [Google Scholar]
- 151.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Basu S, Srivastava PK. Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity. J Exp Med. 1999;189:797–802. doi: 10.1084/jem.189.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Peters LR, Raghavan M. Endoplasmic reticulum calcium depletion impacts chaperone secretion, innate immunity, and phagocytic uptake of cells. J Immunol. 2011;187:919–931. doi: 10.4049/jimmunol.1100690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Sullivan D, Pearce EL. Targeting T cell metabolism for therapy. Trends Immunol. 2015;36:71–80. doi: 10.1016/j.it.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, Tran E, Hanada K, Yu Z, Palmer DC, Kerkar SP, Michalek RD, Upham T, Leonardi A, Acquavella N, Wang E, Marincola FM, Gattinoni L, Muranski P, Sundrud MS, Klebanoff CA, Rosenberg SA, Fearon DT, Restifo NP. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75:296–305. doi: 10.1158/0008-5472.CAN-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Crompton JG, Sukumar M, Restifo NP. Uncoupling T-cell expansion from effector differentiation in cell-based immunotherapy. Immunol Rev. 2014;257:264–276. doi: 10.1111/imr.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, Roychoudhuri R, Palmer DC, Muranski P, Karoly ED, Mohney RP, Klebanoff CA, Lal A, Finkel T, Restifo NP, Gattinoni L. Inhibiting glycolytic metabolism enhances CD8+T cell memory and antitumor function. J Clin Invest. 2013;123:4479–4488. doi: 10.1172/JCI69589. [DOI] [PMC free article] [PubMed] [Google Scholar]


