Abstract
Objective
To systematically evaluate the relationship between flavonoids intake and colorectal cancer risk by conducting a meta-analysis.
Results
Our meta-analysis included 18 studies involving 16,917 colorectal cancer cases in 559,486 participants in relations to flavonoids intake during six to twenty-six years of follow-up. Our results indicated that specific flavonoid subclasses, such as procyanidins (OR = 0.75; 95% CI, 0.66–0.86) and isoflavones (OR = 0.87; 95% CI, 0.78–0.98), showed protective effects against colorectal cancer risk. There was no enough evidence indicating that increased consumption of total flavonoids were significantly associated with reduced risk of colorectal cancer (OR = 0.94, 95% CI, 0.81–1.09). There was no publication bias across studies.
Methods
We performed a systematic search of PubMed, Web of Science and the Cochrane Library databases for relevant articles before December 2015. A random-effects model was used to estimate summary odds ratios and 95% confidence intervals (CIs) for associations between flavonoids and colorectal cancer risk. We assessed heterogeneity among studies by the Cochran Q and I2 statistics.
Conclusions
Our meta-analysis provides comprehensive evidence and partly supported the hypothesis that higher habitual intake of foods rich in procyanidins and isoflavones may potentially decrease colorectal cancer incidence. More prospective studies are warranted to verify this protective association.
Keywords: flavonoids, isoflavones, procyanidins, colorectal cancer
INTRODUCTION
Colorectal cancer is the third most prevalent cancer and the third leading cause of cancer-related death in men and women in America [1]. In 2014, there were estimated to be 136,830 new colorectal cancer patients and 50,310 cancer-related deaths in the United States [2]. Over the past decades, there has been substantial progress in reducing colorectal cancer morbidity and mortality due to screening programs and advanced therapies [3]. Because of huge economic burden of this disease, there is still an urgent need to tailor colorectal cancer prevention strategies.
Current epidemiologic studies to date have suggested that dietary factors play a crucial role in the development of colon cancer [4, 5], and high fruit and vegetable was generally implicated in the prevention of colorectal cancer [6–8]. A possible protective role of flavonoids against colorectal cancer has been of enormous interest recently [9–13]. Flavonoids are a diverse group of polyphenolic compounds widely available in plant-based foods, such as fruits, vegetables, herbs, tea, and juices [14]. According to their chemical structure, flavonoids can be classified as flavones, flavonols, flavanones, flavanols (flavan-3-ols), anthocyanins, isoflavoness [15]. Besides, proanthocyanidins are another important subclass of polyphenols [16].
In recent decades, accumulating studies have been conducted to investigate the relationship between diet flavonoids and colorectal cancer incidence. However, existing data is still conflicting. For example, several studies indicated that flavonoids were inversely associated with colon cancer risk [12, 13, 17–21], however, other prospective cohort studies generally failed to detect such relationship [9, 10, 22, 23]. In addition, a randomized controlled trial showed that higher intake of flavonols was associated with a 76% reduced recurrence of advanced adenoma [24]. Because of differences in study design and type of flavonoids, various studies yielded inconsistent results.
Nevertheless, Experimental studies provide evidence for potential mechanisms that relate flavonoids to cancer risk. For example, flavonoids could inhibit growth of colon cancer cell lines and colorectal carcinogenesis in animal models [25, 26]. Different subclasses of flavonoids may have varying capacities to suppress neoplasm. There are several anti-carcinogenic mechanisms of flavonoids, including antioxidative and anti-inflammatory activities [27], induction of apoptosis and suppression of angiogenesis [28, 29].
To better understand this association, we performed a meta-analysis of available studies to comprehensively evaluate dietary flavonoids intake as well as flavonoid specific subclasses in relation to colorectal cancer risk.
RESULTS
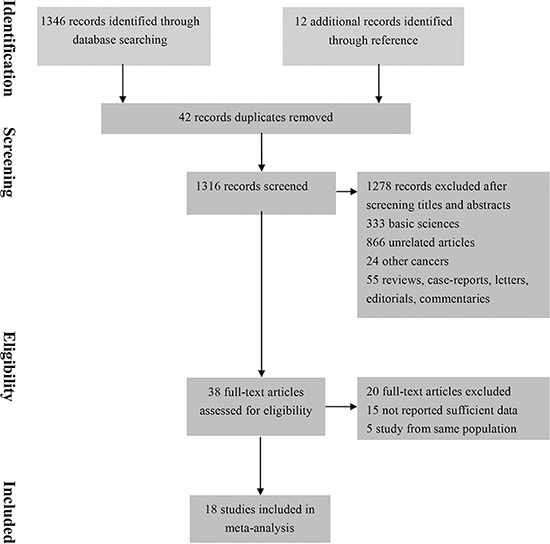
Total 1,358 studies were identified through the literature searches. After review of the titles and abstracts, 1,278 studies were excluded and remaining 38 studies were reviewed with the full texts. Thus 18 studies were finally included in final meta-analysis. (Figure 1)
Figure 1. Flow diagram summarizing study identification and selection.
Characteristics and quality of included studies
The characteristics of selected studies were outlined in Table 1. We identified five studies on total flavonoids intake and CRC risk and 16 studies that assessed subclasses of flavonoid consumption in relation to CRC incidence. These studies involved 559,486 participants with 16,917 CRC cases. Nine of them were prospective cohort studies and remaining were case-control studies. These studies were conducted in Europe (n = 9), Asia (n = 6), and America (n = 3). Food frequency questionnaires (FFQs) were used to assess exposure to certain dietary flavonoids in all but three studies [10, 18, 30], which adopted interview, food records, and diet diaries. The diagnosis of colorectal cancer was based on histologic findings or data from cancer registry.
Table 1A. Characteristics of included case-control studies on dietary flavonoids and risk of colorectal cancer.
| Study | Design | Location/ Setting | Exposure Ascertainment | Outcome assessment | Total subjects | Colon cancer cases | Confounding variables adjusted |
|---|---|---|---|---|---|---|---|
| Shin et al. 2015 | Case-control hospital-based | Korea | Validated FFQ | Medical record | 3570 | 901 | 1,4,5,7 |
| Zamora-Ros et al. 2013 | Case-control hospital-based | Spanish | Validated FFQ | Histological confirmed | 825 | 424 | 1,2,4,5,6,7,8,10,12,15 |
| Budhathoki et al. 2011 | Case-control population-based | Japan | Computer-assisted interview | Histological confirmed | 1631 | 816 | 1,2,3,4,5,13,14,15,16 |
| Rossi et al. 2010 | Case-control hospital-based | Italy | Validated FFQ | Histological confirmed | 6107 | 1953 | 1,2,3,4,5,10,11,13,15 |
| Ward et al. 2010 | Prospective case-control | Norfolk | Diet diaries | Cancer Registry | 1103 | 220 | 1,3,5,6,7,8,10,12,15,16 |
| Kyle et al. 2009 | Case-control population-based | Britain | Validated FFQ | Histological confirmed | 672 | 264 | 1,10,12,15,16 |
| Theodoratou et al. 2007 | Case-control population-based | Britain | Validated FFQ | Histological confirmed | 2912 | 1456 | 3,4,5,6,7,9,10,12,15 |
| Cotterchio et al. 2006 | Case-control population-based | America | FFQ | Histological confirmed | 2985 | 1095 | 1,2,10 |
| Rossi et al. 2006 | Case-control hospital-based | Italy | Validated FFQ | Histological confirmed | 6107 | 1953 | 1,2,3,5,6,10,13,15 |
Abbreviation: FFQ, food frequency questionnaire
1 = age, 2 = sex, 3 = body mass index, 4 = alcohol, 5 = physical activity, 6 = smoke, 7 = fibre, 8 = meat intake, 9 = fruit/vegetable intake, 10 = total energy intake (kcal/day), 11 = education 12 = NSAID, 13 = study location, 14 = occupation, 15 = family history of colorectal cancer, 16 = dietary supplements (calcium, n-3 polyunsaturated fatty acids, manganum, riboflavin, vitamin C, vitamin E, folate).
Table 1B. Characteristics of included cohort studies on dietary flavonoids and risk of colorectal cancer.
| Study | Design | Location | Time period; (years) | Exposure Ascertainment | Outcome assessment | Total subjects | Colon cancer cases | Confounding variables adjusted |
|---|---|---|---|---|---|---|---|---|
| Nimptsch et al. 2015 | Cohort | America | 26 | Validated FFQ | Histological confirmed | 118842 | 2519 | 1,3,4,5,6,8,12,15, 16,17 |
| Simons et al. 2009 | Cohort | Netherlands | 13.3 | Validated FFQ | Cancer Registry | 120852 | 2485 | 1,3,4,5,6,8,15 |
| Yang et al. 2009 | Cohort | China | 6.4 | Validated FFQ | Medical record | 68412 | 321 | 1,3,5,8,9,11,15,16, 19,20,21 |
| Wang et al. 2009 | Cohort | America | 11.5 | Validated FFQ | Medical record | 38408 | 305 | 3,4,5,6,7,9,15,16, 20,21 |
| Butler et al. 2008 | Cohort | Singapore | 10 | Validated FFQ | Cancer registry | 61321 | 961 | 1,2,18,6,4,3,11, 5,15,10 |
| Akhter et al. 2008 | Cohort | Japan | 7.6 | Validated FFQ | Medical record | 83063 | 886 | 1,3, 4,5, 6,8,9,13, 16,18, 21 |
| Mursu et al. 2008 | Cohort | Finnish | 16.2 | Food records | Cancer registry | 2590 | 55 | 1,3,6,5,4,3,10,16,7 |
| Oba et al. 2007 | Cohort | Japan | 8 | FFQ | Histological confirmed | 30221 | 213 | 1,3,4,5,6,16,21 |
| Knekt et al. 2002 | Cohort | Finnish | 6 | FFQ | Cancer Registry | 9865 | 90 | 1,2,3,6,13,14 |
Abbreviation: FFQ, food frequency questionnaire
1 = age, 2 = sex, 3 = body mass index, 4 = alcohol, 5 = physical activity, 6 = smoke, 7 = fibre, 8 = meat intake, 9 = fruit/vegetable intake, 10 = total energy intake (kcal/day), 11 = education 12 = NSAID, 13 = study location, 14 = occupation, 15 = family history of colorectal cancer, 16 = dietary supplements (calcium, n-3 polyunsaturated fatty acids, manganum, riboflavin, vitamin C, vitamin E, folate), 17 = history of endoscopy, 18 = history of diabetes mellitus, 19 = household income, 20 = menopausal status, 21 = current use of female hormones.
The overall methodological quality of studies was summarized in Table 2. Using the Newcastle–Ottawa scale (NOS) quality tool, the score of all the studies ranged from 6 to 9, indicating moderate to high quality.
Table 2A. Newcastle-Ottawa scale for assessment of quality of in included Cohort studies.
| Author | Quality assessment criteria | Overall QualityScore(max = 9) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | |||||||
| Representativeness of exposed cohort? | Selection of the non-exposed cohort? | Ascertainment of exposure? | Outcome of interest was not present at start of study? | Study control forage/gender and additional factor? | Assessment of outcome? | Was follow-up long enough for outcome to occur? | Adequacy of follow-up of cohorts? | ||
| Nimptsch et al. 2015 | * | * | * | * | ** | * | * | * | 9 |
| Simons et al. 2009 | - | * | * | * | ** | * | * | * | 8 |
| Yang et al. 2009 | - | * | * | * | ** | * | * | - | 7 |
| Wang et al. 2009 | - | * | * | * | ** | * | * | - | 7 |
| Akhter et al. 2008 | * | * | * | * | * | * | * | * | 8 |
| Mursu et al. 2008 | - | * | * | * | * | * | * | * | 7 |
| Butler et al. 2008 | * | * | * | * | ** | * | * | * | 9 |
| Oba et al. 2007 | * | * | - | * | * | * | * | * | 7 |
| Knekt et al. 2002 | * | * | - | * | * | * | * | - | 6 |
Each asterisk represents if individual criterion within the subsection were fulfilled.
Table 2B. Newcastle-ottawa scale for assessment of quality of in included case-control studies.
| Author | Quality assessment criteria | Overall Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Selection | Comparability | Outcome | |||||||
| Is the case definition adequate? | Representativeness of cases? | Selection of control? | Definition of control? | Study control for age/gender and additional factor? | Ascertainment of exposure? | Same method of cases/controls? | Non-response rate | ||
| Shin et al. 2015 | * | * | - | * | * | * | * | - | 6 |
| Zamora-Ros 2013 | * | * | - | * | ** | * | * | - | 7 |
| Budhathoki et al. 2011 | * | * | * | * | ** | * | * | - | 8 |
| Rossi et al. 2010 | * | * | * | ** | * | * | * | 8 | |
| Ward 2010 | * | - | - | * | ** | * | * | - | 7 |
| Kyle et al. 2009 | * | * | * | * | ** | * | * | * | 9 |
| Theodoratou et al. 2007 | - | * | * | * | ** | * | * | * | 8 |
| Cotterchio et al. 2006 | * | * | - | * | * | * | * | * | 7 |
| Rossi et al. 2006 | * | * | * | - | * | * | * | - | 6 |
Each asterisk represents if individual criterion within the subsection were fulfilled.
Total diet flavonoids intake and colorectal cancer risk
Five studies investigated the association of total flavonoids with incidence of colorectal cancer. The combined results indicated that no statically significant difference in colorectal cancer risk between the highest flavonoid intake and the lowest (OR = 0.94, 95% CI 0.81–1.09) (Supplementary Figure S1). There was no evidence of significant heterogeneity (P = 0.44, I2 = 0.0%). Furthermore, results for both case-control (OR = 0.81; 95% CI 0.50–1.29) and cohort studies (OR = 1.00; 95% CI 0.74–1.35) were similar. The Egger's test (P = 0.59) and Begg's test (P = 0.62) showed no evidence of publication bias in this meta-analysis.
Subclasses of diet flavonoids consumption and colorectal cancer incidence
Flavones
The correlation between high vs low intake of flavones and CRC risk were presented in five studies. The summary analysis yielded a combined risk estimate of 0.91 (95% CI, 0.78–1.05) with some evidence of heterogeneity (I2 = 56.9%, P = 0.04) (Supplementary Figure S2). There was no publication bias in analysis. We further conducted subgroup analyses by study design, sex and tumour location (Table 3). A significant association was found only for flavones intake and rectal cancer risk. However, no reduced risk of colorectal cancer was observed in the subgroup analyses by sex and design.
Table 3. Stratified analyses of flavonoid subclasses and colorectal cancer risk.
| Subgroup analysis | Pooled OR | 95% CI | Heterogeneity I2 (%) | P Value | |
|---|---|---|---|---|---|
| Flavones | |||||
| Design | Case-control | 0.83 | (0.69,1.14) | 75.6% | 0.017 |
| Cohort | 0.98 | (0.88,1.08) | 0 | 0598 | |
| Gender | Male | 0.95 | (0.87,1.05) | 6.9% | 0.342 |
| Female | 0.95 | (0.87,1.04) | 0 | 0.564 | |
| Site of tumour | Colon | 0.88 | (0.68,1.13) | 73.1% | 0.011 |
| Rectum | 0.82 | (0.70,0.97) | 0 | 0.608 | |
| Flavonols | |||||
| Design | Case-control | 0.70 | (0.62,080) | 0 | 0537 |
| Cohort | 1.01 | (0.91,1.23) | 4.9% | 0.349 | |
| Gender | Male | 0.88 | (0.77,1.01) | 39.7% | 0.191 |
| Female | 0.87 | (0.71,1.05) | 80.4% | 0.006 | |
| Site of tumour | Colon | 0.784 | (0.62,1.00) | 67.1% | 0.016 |
| Rectum | 0.82 | (0.63,1.08) | 50.5% | 0.089 | |
| Flavanones | |||||
| Design | Case-control | 1.14 | (0.93,1.38) | 42.5% | 0.156 |
| Cohort | 0.96 | (0.84,1.10) | 0 | 0.888 | |
| Gender | Male | 1.00 | (0.90,1.11) | 0 | 0.581 |
| Female | 0.98 | (0.89,1.08) | 0 | 0.716 | |
| Site of tumour | Colon | 1.03 | (0.92,1.15) | 0 | 0.653 |
| Rectum | 0.94 | (0.80,1.11) | 0 | 0.825 | |
| Flavanols | |||||
| Design | Case-control | 0.80 | (0.64,0.99) | 51.8% | 0.101 |
| Cohort | 1.01 | (0.86,1.18) | 41.3% | 0.182 | |
| Gender | Male | 1.06 | (0.94,1.19) | 36.3% | 0.208 |
| Female | 0.96 | (0.86,1.07) | 39.2% | 0.193 | |
| Siteof tumour | Colon | 0.88 | (0.69,1.12) | 68.3% | 0.013 |
| Rectum | 0.87 | (0.74,1.02) | 0 | 0.542 | |
| Anthocyanins | |||||
| Design | Case-control | 0.68 | (0.56,0.83) | 0 | 0.667 |
| Cohort | 0.92 | (0.67,1.28) | 17% | 0.272 | |
| Gender | Female | 0.87 | (0.66,1.13) | 78.6% | 0.009 |
| Male | 0.89 | (0.82,0.96) | 0 | 0.862 | |
| Site of tumour | Colon | 0.79 | (0.61,1.02) | 55.3% | 0.107 |
| Rectum | 0.88 | (0.67,1.00) | 72.3% | 0.027 | |
| Isoflavones | |||||
| Design | Case-control | 0.85 | (0.72,1.01) | 71.3% | 0.002 |
| Cohort | 0.93 | (0.83,1.04) | 0 | 0.518 | |
| Gender | Male | 0.920 | (0.78,1.08) | 50.5% | 0.049 |
| Female | 0.940 | (0.84,1.06) | 0 | 0.469 | |
| Site of tumour | Colon | 0.86 | (0.73,1.00) | 35.4% | 0.158 |
| Rectum | 0.93 | (0.78,1.10) | 25.1% | 0.237 | |
| Procyanidins | |||||
| Design | Case-control | 0.75 | (0.66,0.86) | 0 | 0.633 |
| Cohort | -* | - | - | - | |
| Gender | Male | 0.88 | (0.80,0.98) | 0 | 0.655 |
| Female | 0.84 | (0.74,0.96) | 0 | 0.695 | |
| Site of tumour | Colon | 0.81 | (0.69,0.96) | 0 | 0.555 |
| Rectum | 0.66 | (0.54,0.80) | 0 | 0.522 | |
no cohort studies were included in analysis for procyanidins.
Flavonols
The correlation between high vs low intake of flavonols and CRC risk were presented in six studies. The summary risk estimate was 0.86 (95% CI, 0.71–1.03), with considerable heterogeneity (I2 = 73.9%, P = 0.001) (Supplementary Figure S3). There was no publication bias in analysis. In subgroup analyses, the reduced risk of colorectal cancer was observed in pooled estimates of case-control studies, but not for cohort studies. There was no other significant association detected.
Flavanones
The correlation between high vs low intake of flavanones and CRC risk were presented in six studies. The summary risk estimate was 1.05 (95% CI, 0.92–1.19), with no evidence of heterogeneity (I2 = 27%, P = 0.23) (Supplementary Figure S4). No publication bias was detected in the analysis. There were no significant associations found in subgroup analyses.
Flavanols
The correlation between high vs low intake of flavanols and CRC risk were presented in seven studies. The summary risk estimate was 0.90 (95% CI, 0.78–1.04), with some heterogeneity (I2 = 56.8%, P = 0.03) (Supplementary Figure S5). No publication bias was detected in the analysis. In subgroup analyses, the association was significant in case-control studies, but not in cohort studies.
Anthocyanins
The correlation between high vs low intake of anthocyanins and CRC risk were presented in four studies. The summary risk estimate was 0.78 (95% CI, 0.61–1.01), with considerable heterogeneity (I2 = 60.2%, P = 0.057) (Supplementary Figure S6). There was no publication bias in the analysis. The subgroup analysis by design produced a significant summary risk estimate for case-control studies, but not for cohort. Furthermore, reduced risk of CRC was observed in male, but not for female.
Isoflavones
The correlation between high vs low intake of isoflavones and CRC risk were presented in eleven studies. The summary risk estimate was 0.87 (95% CI, 0.78–0.98), with considerable evidence of heterogeneity (I2 = 59.5%, P = 0.006) (Supplementary Figure S7). The result should be interpreted with caution, since significant heterogeneity existed among included studies. No publication bias was detected in the analysis. We conducted stratified analyses of eleven studies between isoflavones and colorectal cancer risk to determine the impact of differences in study design, gender, and site of tumour. No significant association was detected in either analyses.
Procyanidins
The correlation between high vs low intake of procyanidins and CRC risk were presented in four studies. The summary risk estimate was 0.75 (95% CI, 0.66–0.86), with no evidence of heterogeneity (I2 = 0, P = 0.63) (Supplementary Figure S8). There was no evidence of publication bias in analysis. The reduced risk of CRC was not only observed in male and female, but also in colon and rectum. Since studies included in this meta-analysis were all case-control, this protective association should be interpreted with caution.
DISCUSSION
In the present study, five epidemiologic studies that assessed the association between total flavonoids consumption and colorectal cancer risk in humans. Other studies evaluated the relationship between several subclasses of flavonoid and CRC risk. To our knowledge, this is the most comprehensive meta-analysis and evidence from our study indicated that total flavonoids intake were not significantly associated with reduced CRC risk. The lack of association is likely explained by the fact that limited numbers of included studies, which leaded limited power to detect an association. Furthermore, we assess potential relationships between flavonoid subclasses and CRC risk, respectively. Isoflavones and procyanidins, but not other subclasses, were inversely associated with the reduced CRC incidence. Thus, these findings partially supported flavonoid subclasses might be considered as promising candidates for potential chemopreventive agents, such as aspirin, metformin, vitamin D [31–35].
Flavonoids, as a diverse group of polyphenol, are considered as a potential anti-carcinogenic agent. Although our analyses provided some evidence of an inverse association between specific subclasses and CRC incidence, several experimental studies, both in vitro and in vivo, supported its protective role against CRC. Flavonoids have varying capacities to inhibit the development of colorectal cancer, for example, acting as antioxidants [27, 36, 37], anti-inflammatory agents [27, 38], anti-proliferative agents [39]. In vitro, flavonoids inhibiting growth of cancer cells through suppression of p21-RAS and DNMT expression [40, 41]. In addition, flavones induced effectively apoptosis through down-regulation of cyclooxygenase-2 (COX-2), nuclear transcription factor kappaB [42, 43]. However, effects of flavonoids among humans cannot be easily extrapolated from basic research. Since concentrations of flavonoids used in experimental studies were hardly reached through dietary intake, the evidence is less conclusive [39]. Therefore, whether flavonoids intake protect against colorectal cancer still needs further confirmation from epidemiologic studies and randomized clinical trials.
Our meta-analyses showed that higher consumption of isoflavones and procyanidins might be associated with lower risk of colorectal cancer. Previous meta-analysis [44] presented that soy food intake was associated with a 21% reduction in colorectal cancer risk among high intake groups in women. Isoflavones, a bioactive component rich in soy food, might have potential capacity in inhibition of cancer [45]. Our combined analysis also partially supported this hypothesise. Isoflavones, also known as phytoestrogens, might exerted anti-carcinogenic effects through hormonal and non-hormonal pathways [46, 47]. Several epidemiological studies had reported a reduced risk for CRC among high isoflavones intake [11, 12, 18, 21, 48]. The protective association was more prominent among post-menopausal women than pre-menopausal women. However, our stratified analyses were unable to detect this significant association and this may relate to limited number of included studies. Procyanidins, also known as condensed tannins, occur ubiquitously in plants. They can exert a wide variety of beneficial biological effects, such as antioxidant anti-inflammatory and anti-cancer [49]. Furthermore, this protective association was still consistent among subgroup analyses.
It is important to note several limitations of our analysis. Firstly, most results included in our analyses were case-control studies. Although the methodological quality of these observational studies was medium to high, case-control studies were prone to introduce recall bias. More prospective cohort studies need to test this association. Secondly, it was a challenge to evaluate the quantity of flavonoids intake accurately. Since the FFQ included limited flavonoid-rich food items and intake ranges, the specifically designed FFQ for flavonoids intake should be developed. In addition, flavonoid contents in food may vary depending on other factors, such as species, season and ripeness. These factors may introduce additional measurement error and therefore misestimate the relationship between flavonoids intake and cancer risk. Thirdly, bioactive compounds in food are complex and highly correlated. It is hard to completely tease apart their interaction and rule out the possibility that potential unknown components in food may co-associate with flavonoids. Further intervention studies may be required to elucidate whether the main protective effects are actually due to these flavonoids.
In conclusion, our analyses supported that several subclasses of flavonoid, procyanidins and isoflavones, may potentially protect against colorectal cancer. our results are still promising despite of the lack of sufficient evidence to show that total flavonoids were associated with reduced risk of colorectal cancer in this meta-analysis. Well-designed cohort studies are needed to further investigate the effects of exposure to dietary flavonoids and subclasses.
MATERIALS AND METHODS
Search strategy
We (HXK and SLM.) conducted a systemic search of PubMed, Web of Science and the Cochrane Library databases for all relevant studies before December 2015 independently. The following text and/or medical subject heading terms were used in the literature search: (1) flavonoid*, flavone*, flavonol*, flavanone*, flavanol*, anthocyanin*, isoflavone*, procyanidin*, (2) neoplasm, cancer, tumour, (3) colorectal, colon, rectal, large bowel. In addition, we scanned and examined the reference lists in relevant articles manually.
Eligibility criteria
Studies were eligible for this meta-analysis if they met the following criteria: (1) original article; (2) case-control or cohort studies; (3) evaluating the association between flavonoids or subclasses intake and CRC risk, and (4) reporting adjusted risk estimates with 95% CIs. In addition, case reports, editorials, reviews, animal studies or in-vitro researches were excluded. Besides, studies lacking relevant data also were excluded. When data from several publications were overlapping, we selected the publication with the most comprehensive data for inclusion in the meta-analysis.
Data extraction and quality assessment
Two authors (HXK and SLM.) independently reviewed titles and abstracts of potentially eligible articles identified by the search strategy. Two researchers (HXK and SLM.) independently extracted the following information from included studies: the first author's name, year, location, duration of follow-up, total subjects, colorectal cancer cases and adjustments for confounders. From these studies, we extracted the risk estimate of the highest relative to the lowest intake of dietary flavonoids and subclasses. Two authors independently evaluated the quality of included studies using the Newcastle–Ottawa Scale [50]. Any disagreements were resolved by discussions.
Statistical analysis
We calculated summary odds ratios with 95% CI using a random-effects model [51], since considering between-study variation. Adjusted risk estimates reported in studies were used for meta-analysis in order to account for confounding factors. We assessed heterogeneity between by Cochran's Q test and I2 statistics [52]. Significant heterogeneity was indicated if P value was < 0.1 or I2 value greater than 50% [53]. Prespecified subgroup analyses were performed to assess the potential modifying effects of the following variables on outcomes: study design, gender and tumour location. Besides we performed sensitivity analyses to test the robustness of our combined effects. We used the Egger's and Begg's test to assess publication bias [53]. A P value < 0.05 (except for Cochran's Q test) was considered statistically significant and all P values were two tailed. All statistical analyses were conducted using Stata software (version 13.0; Stata Corp, College Station, TX, United States).
SUPPLEMENTARY MATERIALS FIGURES
Footnotes
CONFLICTS OF INTEREST
No potential competing interests.
GRANT SUPPORT
The work was funded by the Zhejiang provincial medical platform 2015 specialists class B (2015 RCB016); Zhejiang province key science and technology innovation team (2013TD13); National Natural Science Foundation of China (81372623, 81302070)
Authors' contributions
SLM and HXK contributed to conception and design of the study. HXK and SLM contributed to the data acquisition, analysis and interpretation of the data. HXK and SLM contributed to writing and editing the manuscript. All authors commented on drafts of the paper and have approved the final draft of the manuscript.
REFERENCES
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA, Eheman CR, Ward EM. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290–1314. doi: 10.1002/cncr.28509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Printz C. Vegetarian diet associated with lower risk of colorectal cancer. Cancer. 2015;121:2667. doi: 10.1002/cncr.29582. [DOI] [PubMed] [Google Scholar]
- 5.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang W, Binns CW. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. The American journal of clinical nutrition. 2009;90:1112. doi: 10.3945/ajcn.2009.28320. author reply 1112–1114. [DOI] [PubMed] [Google Scholar]
- 7.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc. 2008;67:253–256. doi: 10.1017/S002966510800712X. [DOI] [PubMed] [Google Scholar]
- 8.Bhopal RS. Diet and Colorectal Cancer Incidence. JAMA Intern Med. 2015;175:1726–1727. doi: 10.1001/jamainternmed.2015.4016. [DOI] [PubMed] [Google Scholar]
- 9.Nimptsch K, Zhang X, Cassidy A, Song M, O'Reilly EJ, Lin JH, Pischon T, Rimm EB, Willett WC, Fuchs CS, Ogino S, Chan AT, Giovannucci EL, et al. Habitual intake of flavonoid subclasses and risk of colorectal cancer in 2 large prospective cohorts. American Journal of Clinical Nutrition. 2016;103:184–91. doi: 10.3945/ajcn.115.117507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mursu J, Nurmi T, Tuomainen TP, Salonen JT, Pukkala E, Voutilainen S. Intake of flavonoids and risk of cancer in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Int J Cancer. 2008;123:660–663. doi: 10.1002/ijc.23421. [DOI] [PubMed] [Google Scholar]
- 11.Rossi M, Negri E, Talamini R, Bosetti C, Parpinel M, Gnagnarella P, Franceschi S, Dal Maso L, Montella M, Giacosa A, La Vecchia C. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol Biomarkers Prev. 2006;15:1555–1558. doi: 10.1158/1055-9965.EPI-06-0017. [DOI] [PubMed] [Google Scholar]
- 12.Shin A, Lee J, Lee J, Park MS, Park JW, Park SC, Oh JH, Kim J. Isoflavone and Soyfood Intake and Colorectal Cancer Risk: A Case-Control Study in Korea. PLoS One. 2015;10:e0143228. doi: 10.1371/journal.pone.0143228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamora-Ros R, Not C, Guino E, Lujan-Barroso L, Garcia RM, Biondo S, Salazar R, Moreno V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case-control study (the Bellvitge Colorectal Cancer Study) Cancer Causes Control. 2013;24:549–557. doi: 10.1007/s10552-012-9992-z. [DOI] [PubMed] [Google Scholar]
- 14.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. The American journal of clinical nutrition. 2001;74:418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 15.Aherne SA, O'Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18:75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 16.Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ. Proanthocyanidins in health care: current and new trends. Curr Med Chem. 2004;11:1345–1359. doi: 10.2174/0929867043365288. [DOI] [PubMed] [Google Scholar]
- 17.Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary Flavonoids and the Risk of Colorectal Cancer. Cancer Epidemiology Biomarkers and Prevention. 2007;16:684–693. doi: 10.1158/1055-9965.EPI-06-0785. [DOI] [PubMed] [Google Scholar]
- 18.Budhathoki S, Joshi AM, Ohnaka K, Yin G, Toyomura K, Kono S, Mibu R, Tanaka M, Kakeji Y, Maehara Y, Okamura T, Ikejiri K, Futami K, et al. Soy food and isoflavone intake and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Scand J Gastroenterol. 2011;46:165–172. doi: 10.3109/00365521.2010.522720. [DOI] [PubMed] [Google Scholar]
- 19.Rossi M, Negri E, Parpinel M, Lagiou P, Bosetti C, Talamini R, Montella M, Giacosa A, Franceschi S, La Vecchia C. Proanthocyanidins and the risk of colorectal cancer in Italy. Cancer Causes and Control. 2009;21:243–250. doi: 10.1007/s10552-009-9455-3. [DOI] [PubMed] [Google Scholar]
- 20.Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Dietary Soy and Isoflavone Intake and Risk of Colorectal Cancer in the Japan Public Health Center-Based Prospective Study. Cancer Epidemiology Biomarkers and Prevention. 2008;17:2128–2135. doi: 10.1158/1055-9965.EPI-08-0182. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Shu XO, Li H, Chow WH, Cai H, Zhang X, Gao YT, Zheng W. Prospective cohort study of soy food intake and colorectal cancer risk in women. The American journal of clinical nutrition. 2009;89:577–583. doi: 10.3945/ajcn.2008.26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons CC, Hughes LA, Arts IC, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary flavonol, flavone and catechin intake and risk of colorectal cancer in the Netherlands Cohort Study. Int J Cancer. 2009;125:2945–2952. doi: 10.1002/ijc.24645. [DOI] [PubMed] [Google Scholar]
- 23.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland) Cancer Causes Control. 2001;12:789–796. doi: 10.1023/a:1012232008016. [DOI] [PubMed] [Google Scholar]
- 24.Bobe G, Sansbury LB, Albert PS, Cross AJ, Kahle L, Ashby J, Slattery ML, Caan B, Paskett E, Iber F, Kikendall JW, Lance P, Daston C, et al. Dietary flavonoids and colorectal adenoma recurrence in the Polyp Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2008;17:1344–1353. doi: 10.1158/1055-9965.EPI-07-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandaswami C, Lee LT, Lee PP, Hwang JJ, Ke FC, Huang YT, Lee MT. The antitumor activities of flavonoids. In Vivo. 2005;19:895–909. [PubMed] [Google Scholar]
- 26.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 27.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. The Journal of nutrition. 2005;135:2993s–3001s. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 28.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Han J, Duan J, Cui Y, Wang T, Zhang W, Liu W, Hong J, Yao M, Xiong S, Yan X. Novel antitumor activities of Kushen flavonoids in vitro and in vivo. Phytother Res. 2007;21:269–277. doi: 10.1002/ptr.2066. [DOI] [PubMed] [Google Scholar]
- 30.Ward HA, Kuhnle GG, Mulligan AA, Lentjes MA, Luben RN, Khaw KT. Breast, colorectal, and prostate cancer risk in the European Prospective Investigation into Cancer and Nutrition-Norfolk in relation to phytoestrogen intake derived from an improved database. American Journal of Clinical Nutrition. 2009;91:440–448. doi: 10.3945/ajcn.2009.28282. [DOI] [PubMed] [Google Scholar]
- 31.Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer. 2016;16:173–186. doi: 10.1038/nrc.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li P, Wu H, Zhang H, Shi Y, Xu J, Ye Y, Xia D, Yang J, Cai J, Wu Y. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: a meta-analysis. Gut. 2015;64:1419–1425. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 33.He XK, Su TT, Si JM, Sun LM. Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis. Medicine (Baltimore) 2016;95:e2749. doi: 10.1097/MD.0000000000002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Y, Zhang P, Wang F, Yang J, Liu Z, Qin H. Association between vitamin D and risk of colorectal cancer: a systematic review of prospective studies. J Clin Oncol. 2011;29:3775–3782. doi: 10.1200/JCO.2011.35.7566. [DOI] [PubMed] [Google Scholar]
- 35.Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev. 2014;8:122–146. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Zhang H, Yang X, Zhu Y, Zhang M. Evaluation of antioxidative and antitumor activities of extracted flavonoids from Pink Lady apples in human colon and breast cancer cell lines. Food Funct. 2015;6:3789–3798. doi: 10.1039/c5fo00570a. [DOI] [PubMed] [Google Scholar]
- 37.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. The Journal of nutrition. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez de Medina F, Vera B, Galvez J, Zarzuelo A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097–3108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- 39.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. The American journal of clinical nutrition. 2005;81:284S–291S. doi: 10.1093/ajcn/81.1.284S. [DOI] [PubMed] [Google Scholar]
- 40.Fini L, Selgrad M, Fogliano V, Graziani G, Romano M, Hotchkiss E, Daoud YA, De Vol EB, Boland CR, Ricciardiello L. Annurca apple polyphenols have potent demethylating activity and can reactivate silenced tumor suppressor genes in colorectal cancer cells. The Journal of nutrition. 2007;137:2622–2628. doi: 10.1093/jn/137.12.2622. [DOI] [PubMed] [Google Scholar]
- 41.Ranelletti FO, Maggiano N, Serra FG, Ricci R, Larocca LM, Lanza P, Scambia G, Fattorossi A, Capelli A, Piantelli M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int J Cancer. 2000;85:438–445. [PubMed] [Google Scholar]
- 42.Wenzel U, Kuntz S, Brendel MD, Daniel H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells. Cancer Res. 2000;60:3823–3831. [PubMed] [Google Scholar]
- 43.Peng G, Dixon DA, Muga SJ, Smith TJ, Wargovich MJ. Green tea polyphenol (-)-epigallocatechin-3-gallate inhibits cyclooxygenase-2 expression in colon carcinogenesis. Mol Carcinog. 2006;45:309–319. doi: 10.1002/mc.20166. [DOI] [PubMed] [Google Scholar]
- 44.Yan L, Spitznagel EL, Bosland MC. Soy consumption and colorectal cancer risk in humans: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:148–158. doi: 10.1158/1055-9965.EPI-09-0856. [DOI] [PubMed] [Google Scholar]
- 45.Raju J, Bielecki A, Caldwell D, Lok E, Taylor M, Kapal K, Curran I, Cooke GM, Bird RP, Mehta R. Soy isoflavones modulate azoxymethane-induced rat colon carcinogenesis exposed pre- and postnatally and inhibit growth of DLD-1 human colon adenocarcinoma cells by increasing the expression of estrogen receptor-beta. The Journal of nutrition. 2009;139:474–481. doi: 10.3945/jn.108.099200. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21:744–757. doi: 10.1081/cnv-120023773. [DOI] [PubMed] [Google Scholar]
- 47.Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, Principi MB, Bucci A, Di Leo A. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr. 2008;3:7–13. doi: 10.1007/s12263-008-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. The Journal of nutrition. 2006;136:3046–3053. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–387. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 51.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 52.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 53.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


