Abstract
Previous work suggested that the presence of the anticodon CAU alone was enough to confer methionine acceptance to a tRNA. Conversions of Escherichia coli nonmethionine tRNAs to a methionine-accepting species were obtained by substitutions reconstructing the whole methionine anticodon loop together with preservation (or introduction) of the acceptor stem base A73. We show here that the CAU triplet alone is unable to confer methionine acceptance when transplanted into a yeast aspartic tRNA. Both non-anticodon bases of the anticodon loop of yeast tRNA(Met) and A73 are required in addition to CAU for methionine acceptance. The importance of these non-anticodon bases in other CAU-containing tRNA frameworks was also established. These specific non-anticodon base interactions make a substantial thermodynamic contribution to the methionine acceptance of a transfer RNA.
Full text
PDF
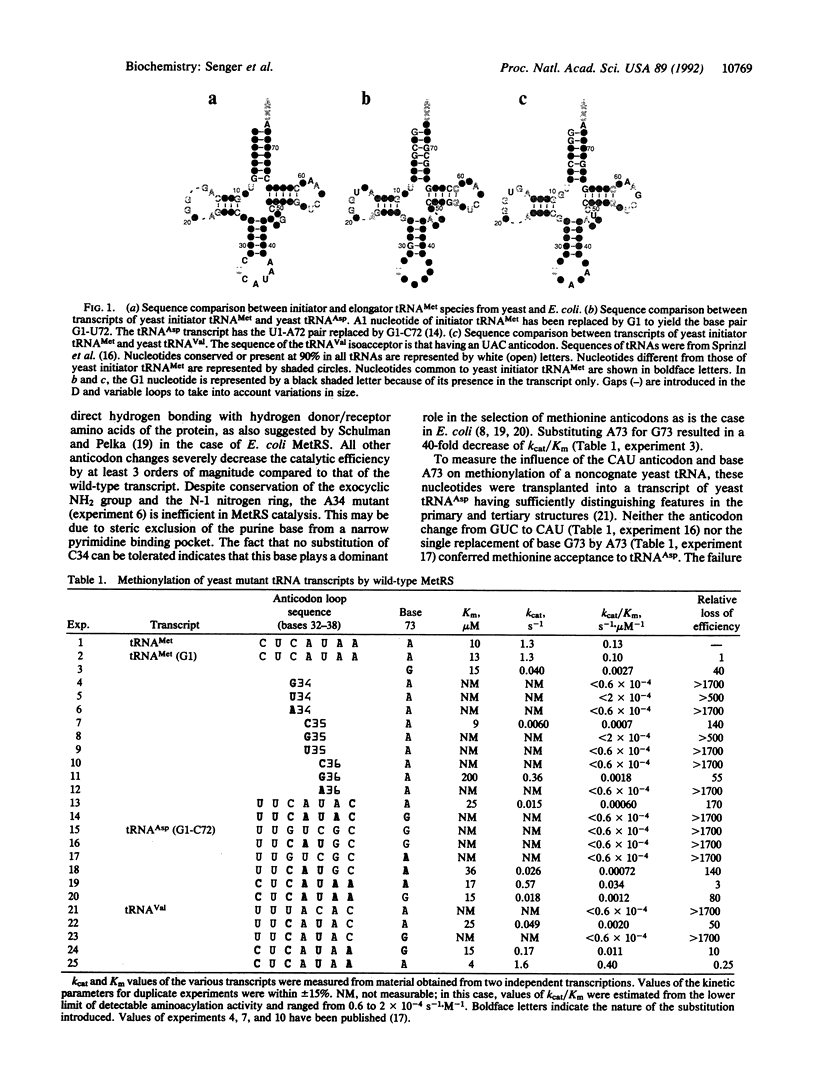


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cigan A. M., Feng L., Donahue T. F. tRNAi(met) functions in directing the scanning ribosome to the start site of translation. Science. 1988 Oct 7;242(4875):93–97. doi: 10.1126/science.3051379. [DOI] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Despons L., Senger B., Fasiolo F., Walter P. Binding of the yeast tRNA(Met) anticodon by the cognate methionyl-tRNA synthetase involves at least two independent peptide regions. J Mol Biol. 1992 Jun 5;225(3):897–907. doi: 10.1016/0022-2836(92)90409-d. [DOI] [PubMed] [Google Scholar]
- Ebel J. P., Giegé R., Bonnet J., Kern D., Befort N., Bollack C., Fasiolo F., Gangloff J., Dirheimer G. Factors determining the specificity of the tRNA aminoacylation reaction. Non-absolute specificity of tRNA-aminoacyl-tRNA synthetase recognition and particular importance of the maximal velocity. Biochimie. 1973 May;55(5):547–557. doi: 10.1016/s0300-9084(73)80415-8. [DOI] [PubMed] [Google Scholar]
- Ghosh G., Kim H. Y., Demaret J. P., Brunie S., Schulman L. H. Arginine-395 is required for efficient in vivo and in vitro aminoacylation of tRNAs by Escherichia coli methionyl-tRNA synthetase. Biochemistry. 1991 Dec 24;30(51):11767–11774. doi: 10.1021/bi00115a005. [DOI] [PubMed] [Google Scholar]
- Lee C. P., Seong B. L., RajBhandary U. L. Structural and sequence elements important for recognition of Escherichia coli formylmethionine tRNA by methionyl-tRNA transformylase are clustered in the acceptor stem. J Biol Chem. 1991 Sep 25;266(27):18012–18017. [PubMed] [Google Scholar]
- Martinis S. A., Schimmel P. Enzymatic aminoacylation of sequence-specific RNA minihelices and hybrid duplexes with methionine. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):65–69. doi: 10.1073/pnas.89.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinnel T., Mechulam Y., Blanquet S., Fayat G. Binding of the anticodon domain of tRNA(fMet) to Escherichia coli methionyl-tRNA synthetase. J Mol Biol. 1991 Jul 20;220(2):205–208. doi: 10.1016/0022-2836(91)90003-o. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Nishikawa K., Nemoto F., Kuchino Y., Nishimura S., Miyazawa T., Yokoyama S. Codon and amino-acid specificities of a transfer RNA are both converted by a single post-transcriptional modification. Nature. 1988 Nov 10;336(6195):179–181. doi: 10.1038/336179a0. [DOI] [PubMed] [Google Scholar]
- Normanly J., Abelson J. tRNA identity. Annu Rev Biochem. 1989;58:1029–1049. doi: 10.1146/annurev.bi.58.070189.005121. [DOI] [PubMed] [Google Scholar]
- Perona J. J., Rould M. A., Steitz T. A., Risler J. L., Zelwer C., Brunie S. Structural similarities in glutaminyl- and methionyl-tRNA synthetases suggest a common overall orientation of tRNA binding. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2903–2907. doi: 10.1073/pnas.88.7.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret V., Garcia A., Grosjean H., Ebel J. P., Florentz C., Giegé R. Relaxation of a transfer RNA specificity by removal of modified nucleotides. Nature. 1990 Apr 19;344(6268):787–789. doi: 10.1038/344787a0. [DOI] [PubMed] [Google Scholar]
- Rould M. A., Perona J. J., Steitz T. A. Structural basis of anticodon loop recognition by glutaminyl-tRNA synthetase. Nature. 1991 Jul 18;352(6332):213–218. doi: 10.1038/352213a0. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. Aminoacyl tRNA synthetases: general scheme of structure-function relationships in the polypeptides and recognition of transfer RNAs. Annu Rev Biochem. 1987;56:125–158. doi: 10.1146/annurev.bi.56.070187.001013. [DOI] [PubMed] [Google Scholar]
- Schimmel P. Parameters for the molecular recognition of transfer RNAs. Biochemistry. 1989 Apr 4;28(7):2747–2759. doi: 10.1021/bi00433a001. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Abelson J. Recent excitement in understanding transfer RNA identity. Science. 1988 Jun 17;240(4859):1591–1592. doi: 10.1126/science.2454505. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon loop size and sequence requirements for recognition of formylmethionine tRNA by methionyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6755–6759. doi: 10.1073/pnas.80.22.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H., Susani M. Base substitutions in the wobble position of the anticodon inhibit aminoacylation of E. coli tRNAfMet by E. coli Met-tRNA synthetase. Nucleic Acids Res. 1983 Mar 11;11(5):1439–1455. doi: 10.1093/nar/11.5.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H. Recognition of tRNAs by aminoacyl-tRNA synthetases. Prog Nucleic Acid Res Mol Biol. 1991;41:23–87. [PubMed] [Google Scholar]
- Uemura H., Imai M., Ohtsuka E., Ikehara M., Söll D. E. coli initiator tRNA analogs with different nucleotides in the discriminator base position. Nucleic Acids Res. 1982 Oct 25;10(20):6531–6539. doi: 10.1093/nar/10.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhof E., Dumas P., Moras D. Crystallographic refinement of yeast aspartic acid transfer RNA. J Mol Biol. 1985 Jul 5;184(1):119–145. doi: 10.1016/0022-2836(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Yarus M. tRNA identity: a hair of the dogma that bit us. Cell. 1988 Dec 2;55(5):739–741. doi: 10.1016/0092-8674(88)90127-4. [DOI] [PubMed] [Google Scholar]