Abstract
Diabetes is manifested predominantly in males in experimental models, and compelling evidence suggests that 17β-estradiol (E2) supplementation improves hyperglycemia in humans. We previously generated a severely diabetic transgenic (Tg) mouse model by β-cell–specific overexpression of inducible cAMP early repressor (ICER) and found that male but not female ICER-Tg mice exhibit sustained hyperglycemia and develop major clinical and pathologic features of human diabetic nephropathy (DN). Thus, we hypothesized that differences in circulating hormone levels have a key role in determining susceptibility to diabetes. Here, we examined whether DN in male ICER-Tg mice is rescued by adjusting the androgen-to-E2 ratio to approximate that in normoglycemic female ICER-Tg mice. We treated hyperglycemic male ICER-Tg mice with orchiectomy (ORX), E2 pellet implantation, or both. E2 pellet implantation at an early stage of DN with or without ORX caused a rapid drop in blood glucose and a dramatic increase in β-cell number, and it markedly inhibited DN progression [namely, E2 reduced glomerulosclerosis, collagen IV deposition and albuminuria, and prevented hyperfiltration]. Furthermore, E2 pellet implantation was more effective than ORX alone and induced a remarkable improvement, even when initiated at advanced-stage DN. In contrast, induction of normoglycemia by islet transplant in ICER-Tg mice eliminated albuminuria but was less effective than E2+ORX in reducing glomerulosclerosis or collagen IV deposition and hyperfiltration. These findings indicate that E2 treatment is effective, even after establishment of DN, whereas glucose normalization alone does not improve sclerotic lesions. We propose that E2 intervention is a potential therapeutic option for DN.
Keywords: diabetes mellitus, diabetic nephropathy, diabetes, islet beta-cells, hyperglycemia
Diabetic nephropathy (DN) is a major complication of diabetes, affecting approximately 40% of patients with type 1 or 2 diabetes, and the primary cause of ESRD.1–3 Currently, there is no preventive or curative therapy, and after DN is overt, complete recovery is unlikely.
To investigate the pathophysiology and progression of CKD as well as evaluate candidate treatments, animal models are essential. For example, we have developed a severely diabetic transgenic (Tg) mouse model that displays many of the features of progressive diabetic renal pathology arising from sustained hyperglycemia.4,5 In these mice, inducible cAMP early repressor (ICER-Iγ), a member of the CREM family,6–11 is specifically overexpressed in pancreatic β-cells to suppress both insulin and cyclin A expression; this results in profound depletion of β-cells without affecting other renal constituent cells. Compared with previously reported diabetic mouse models, ICER-Tg mice characteristically develop severe diabetes early in life and maintain hyperglycemia throughout life, and this hyperglycemia slowly leads to stable development of renal manifestations.12
A marked sex difference in development of diabetes has been found in all animal models so far reported, with males being predominantly affected. Indeed, in our ICER-Tg mice, only males show sustained hyperglycemia and developed diabetic renal injury; in females, blood glucose gradually declines to near normal with age, and they do not develop diabetic renal injury.13 Similarly, in STAT3- or IRS2-knockout mice, hIAPP-Tg mice, and other various rodent models (Akita, NSY, yellow-KK, or ob/ob mouse or Wistar-fatty, Zucker-fatty, or OLETF rat), females have a lower prevalence of diabetes and exhibit normoglycemia or develop only mild hyperglycemia.14–18 This may be the main reason why male animals have been almost exclusively used in experimental studies of diabetes and diabetic renal injury, although this has rarely been mentioned explicitly.
In marked contrast, it is generally believed that the prevalence of types 1 and 2 diabetes and the development of DN as well as disease severity are similar in men and women. In a worldwide analysis by regional group, the ratio of adult men to women patients with diabetes (age >20 years old) was close to one, or there was a small excess in women in developing or developed countries.19 However, only a few studies have examined the men-to-women ratio of patients with diabetes20,21 or ESRD22 by age group; they indicate that diabetes and DN are more common in men than in women in age 40–49 years old, but the incidence in women markedly increases after age 50 years old. Thus, a plausible explanation for the apparent lack of sex difference in susceptibility in humans would be that there is a difference but that it is masked by greater incidence of diabetes or diabetic renal failure in postmenopausal women (especially black postmenopausal women) and the greater longevity of women.22–24 This idea is consistent with various clinical findings. For example, administration of estrogen or raloxifen, a selective estrogen receptor modulator, improves insulin sensitivity, blood glucose levels, plasma lipid levels, and creatinine clearance (Ccr) in postmenopausal women with type 2 diabetes, in whom the onset of diabetes coincided with menopause,25–31 but is ineffective in nonmenopausal women.25 Notably, the protective effect of endogenous 17β-estradiol (E2) against cardiovascular disease and renal disease is lost in the setting of diabetes.32,33 Thus, we speculated that alterations in levels of circulating testosterone (T) and E2 interact with multiple processes implicated in the pathogenesis and progression of diabetes and diabetic renal disease. In other words, we considered that sex difference in susceptibility to diabetes might also exist in humans but be less marked than in animal models for various reasons, including the prolonged time course (appearance of microalbuminuria in type 1 diabetes is typically 10–15 years after disease onset), the effect of hormonal changes associated with menopause, and the modifying effects of diabetes itself on the actions of estrogen.
On the basis of these considerations, we hypothesized that the levels of circulating hormones play a key role in determining susceptibility to diabetes. Indeed, we recently showed that a decreased androgen-to-E2 ratio is associated with an increase of pancreatic β-cells in severely diabetic male ICER-Tg mice.34 Also, E2 modulates disease activity in the kidney.33,35–42 Therefore, we expected that adjustment of the T-to-E2 ratio in male ICER-Tg mice to a similar level to that in normoglycemic female ICER-Tg (ICER-Tg control) mice would rescue male ICER-Tg mice from severe hyperglycemia, block progression of diabetic renal injury, and/or promote recovery from diabetic renal injury. Here, we tested this idea by adjusting the androgen-to-E2 ratio in diabetic male ICER-Tg mice by means of orchiectomy (ORX; castration) or E2 pellet implantation alone or in combination and examining the effect on various measures of diabetes severity. We also compared the results of E2 implantation in male ICER-Tg mice with early- and advanced-stage disease, and we further compared recovery from renal injury in the E2 implantation group and islet–transplanted, normoglycemic male ICER-Tg mice.
Results
Experimental Model and Blood Glucose Levels
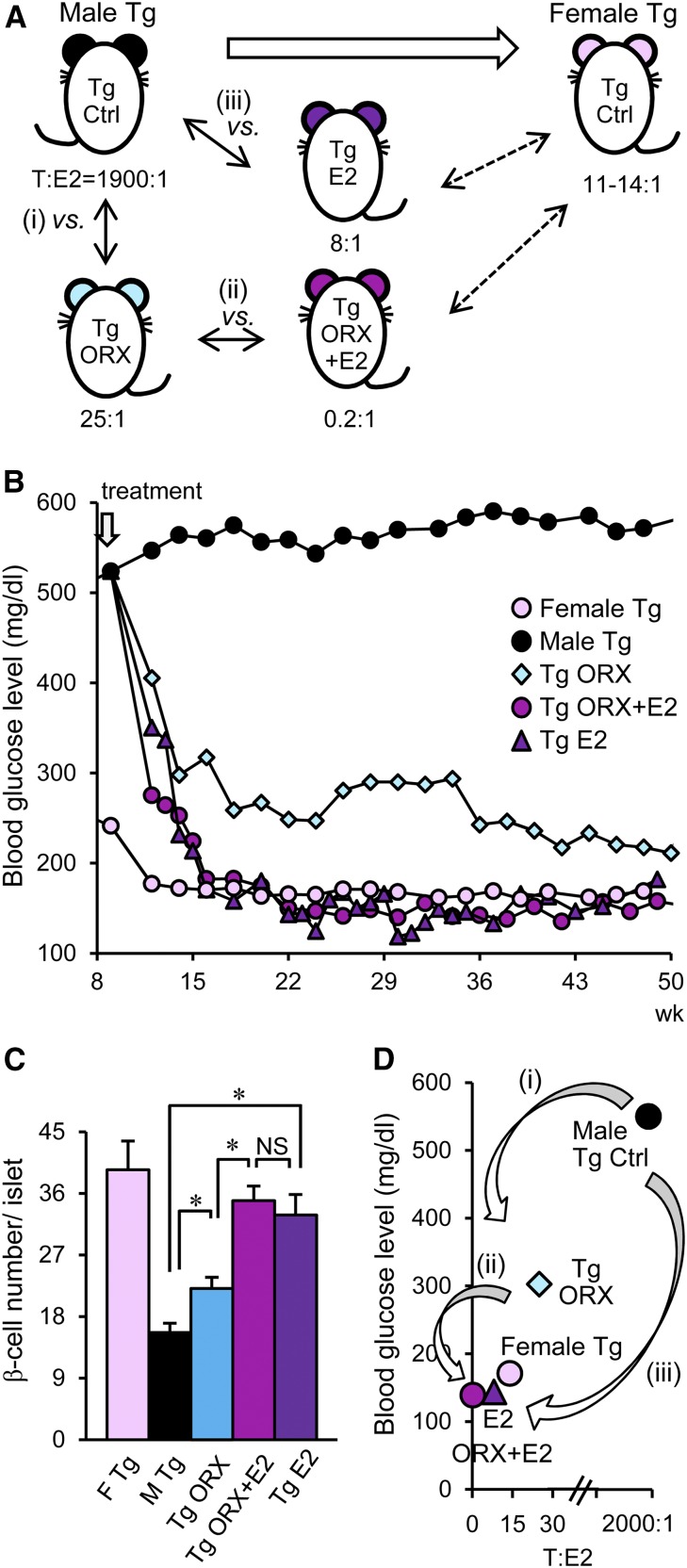
Although both male and female ICER-Tg mice have normal blood glucose levels at birth and develop hyperglycemia early in life,4 female ICER-Tg mice subsequently exhibit a gradual decline in blood glucose to nearly normal levels with age and do not develop diabetic renal injury.5,13 Therefore, we first examined whether adjustment of the circulating levels of androgen and E2 and the androgen-to-E2 ratio to those in normoglycemic female ICER-Tg control mice would block development of renal injury in diabetic male ICER-Tg mice. As shown in Figure 1A, (1) we compared ICER-Tg ORX (castrated) mice with uncastrated mice to assess the influence of androgen on diabetic renal injury (male hyperglycemic Tg control versus Tg ORX; 11 weeks old) (Figure 1A, i). (2) We also compared E2 pellet–implanted ICER-Tg ORX mice with non-E2–implanted (placebo pellets) ICER-Tg ORX mice to examine the effects of estrogen (Tg ORX versus Tg ORX + E2) (Figure 1A, ii), and (3) we compared diabetic male ICER-Tg mice (not subjected to ORX) with ICER-Tg mice implanted with E2 pellets to examine the effects of estrogen in the presence of endogenous androgen (male Tg control versus Tg E2) (Figure 1A, iii). In castrated mice, T levels were reduced (90±10 pg/ml), and a small amount of E2 remained, whereas in castrated and E2-implanted mice, T levels were reduced, and E2 was increased (572±184 pg/ml) as described previously.34 The T and E2 concentrations in male ICER-Tg control mice were 7090±210 and 3.7±1 pg/ml, respectively.
Figure 1.
The experimental model and changes in blood glucose levels. (A) Schematic illustration of differences in androgen-to-E2 ratio among normoglycemic female ICER-Tg control mice, diabetic male ICER-Tg control mice, and male ICER-Tg mice treated with ORX (castration) or E2 pellet implantation alone or in combination. (i) The influence of androgen (male Tg control versus Tg ORX). (ii) The effect of estrogen (Tg ORX versus Tg ORX + E2). (iii) The effect of E2 in the presence of endogenous androgen (male Tg control versus Tg E2). Serum sampling was done at 1–5 weeks after treatment, and the results were compared with those in age–matched 12- to 16-week-old ICER-Tg control mice. (B) Changes in blood glucose levels over time (n=12–24 per group). (C) The number of pancreatic β-cells within islets (n=6–10 pancreases per group). (D) Relationship between blood glucose level and androgen-to-E2 ratio. Ctrl, control; F, female; M, male. *P<0.05.
Blood glucose levels decreased within several weeks in ICER-Tg ORX mice but never reached the female ICER-Tg control level (Figure 1B). In contrast, in ICER-Tg ORX + E2 mice, blood glucose initially declined more rapidly followed by a gradual further decline to the female ICER-Tg control level. The number of pancreatic islet β–cells was significantly higher in ICER-Tg ORX + E2 and ICER-Tg E2 mice than in ICER-Tg ORX mice (Figure 1C). Blood glucose levels became lower with decrease of androgen-to-E2 ratio (Figure 1D). Therefore, we next analyzed improvements of histology and renal function in this model.
Experiment 1: Progression of Diabetic Renal Injury in Male ICER-Tg Control Mice and Inhibition by Treatments at an Early Stage
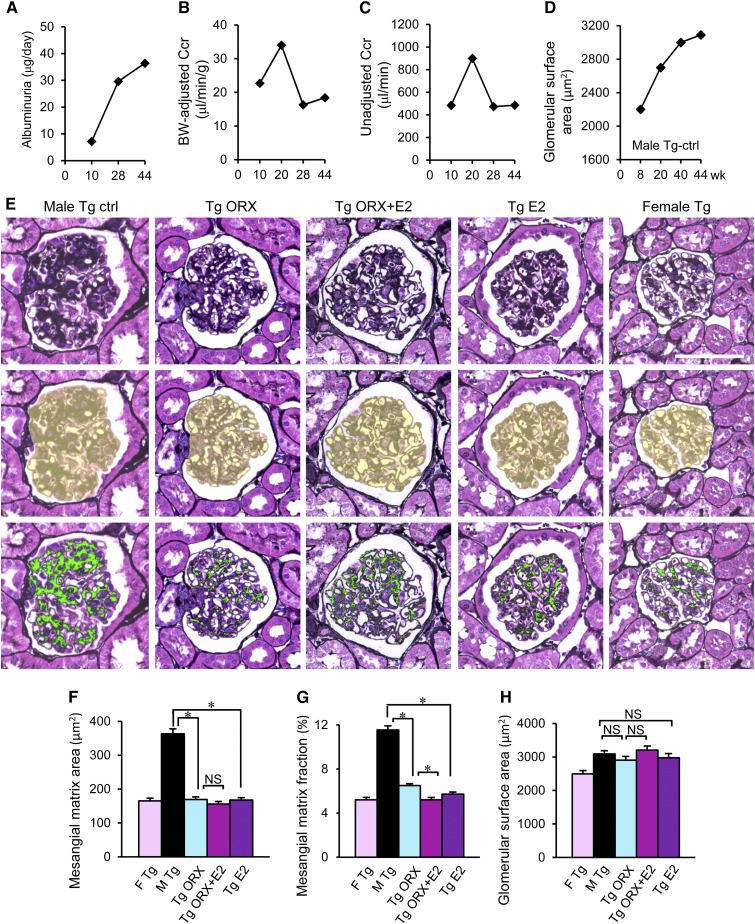
Male ICER-Tg control mice exhibit hyperglycemia because of reduced insulin secretion arising from profound depletion of pancreatic β-cells and severe diabetic renal injury caused by sustained hyperglycemia. Typical diabetic renal injury progression is seen with age in male ICER-Tg control mice: albuminuria appears at 10 weeks old and continues to increase (Figure 2A), and Ccr peaked at 20 weeks and then, declined in association with worsening glomerulosclerosis (Figure 2, B–D). Male ICER-Tg control mice are lean, and adipose tissue is rarely seen (Figure 5C). In the first experiments, we treated mice at 11 weeks old and analyzed the histologic and functional changes at 50–55 weeks old.
Figure 2.
Progression of diabetic renal injury in male ICER-Tg control mice and inhibition of glomerulosclerosis by various treatments at an early stage. (A–D) Typical progression of diabetic renal injury in male ICER-Tg control mice: increased albuminuria and glomerular surface area and hyper-Ccr. (E) A representative PASM–stained glomerulus (top panel), the assessed glomerular surface area is shown in yellow (middle panel), and the same glomerulus, in which the PASM-positive area is shown in green (bottom panel). Scale bar, 50 μm. (F and G) The mesangial matrix area (μm2) and mesangial matrix area fraction (%). Mesangial matrix area fraction was determined as the percentage of mesangial matrix area per glomerular surface area. Glomerulosclerosis was evident in male ICER-Tg control mice and improved in all of the treated mice. (H) Glomerular surface area (μm2). The bars represent the mean values determined from 50 glomeruli per kidney (n=6 mice per group) ±SEMs. BW, body weight; Ctrl, control; F, female; M, male. *P<0.05.
Figure 5.
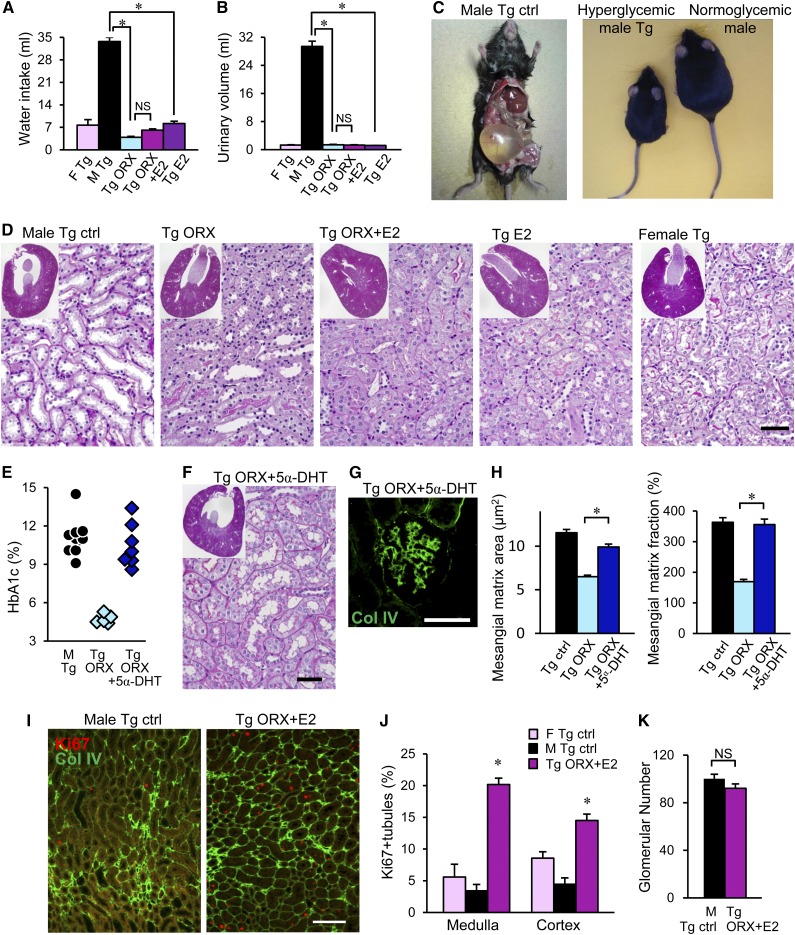
Improvements in renal injury. (A and B) Reduced daily urinary volume and water intake. (C) Male hyperglycemic ICER-Tg mice are lean, the bladder is expanded, and adipose tissue is rarely seen (52 weeks old). (D and F) Representative low–power micrographs of kidneys obtained from 50- to 55-week-old mice (insets) and a magnified view of the renal structure. The renal pelvis was greatly expanded in the male ICER-Tg control mice. Periodic acid–Schiff staining. Original magnification, ×40 in insets. (E) The blood glucose–lowering effect was blocked by 5α-DHT implantation (Tg ORX + 5α-DHT; castrated male ICER-Tg mice were implanted with 5α-DHT pellets; n=8). (G) Immunohistochemical staining with an anti-ColIV antibody. (H) Increased deposition of ECM in glomeruli, mesangial matrix area (μm2), and mesangial matrix area fraction (%) in ICER-Tg ORX + 5α-DHT mice. (I) Dual staining of Ki67 (red) as a marker of cell proliferation and ColIV (green). Ki67 was expressed in many renal tubules obtained from ORX + E2 mice at 1 week after ORX + E2 treatment but in very few renal tubules obtained from age–matched ICER-Tg control mice (24 weeks old). (J) Quantification of Ki67–positive renal tubules in renal medulla and cortex. The bars represent the mean values determined from pictures (395,850 μm2) obtained from 10 different areas in each medulla or cortex side per kidney (n=7 mice per group) ±SEMs. (K) Glomerular number at 55 weeks old. Ctrl, control; F, female; M, male. Scale bars, 50 μm in D, F, and G; 100 μm in I. *P<0.05.
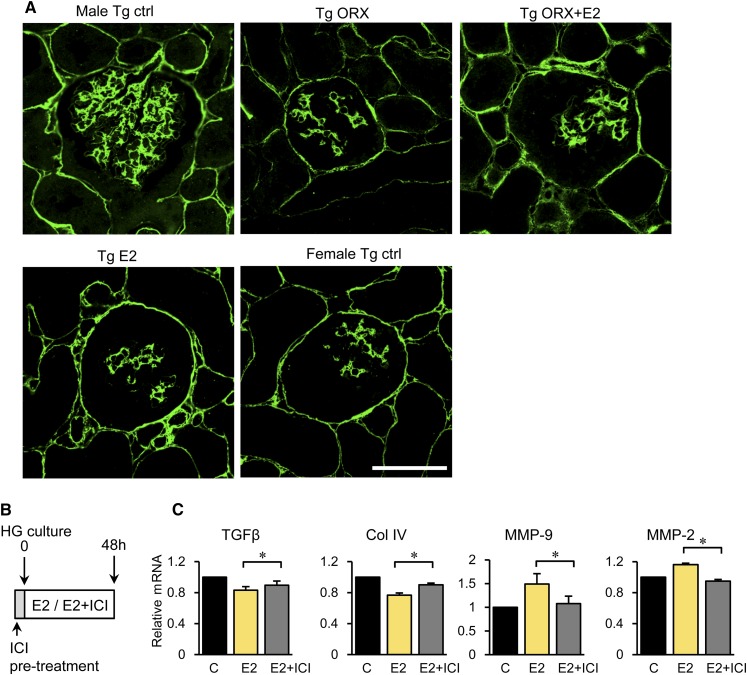
Glomerulosclerosis was evaluated by measuring periodic acid–methenamine silver (PASM) –positive areas. In hyperglycemic ICER-Tg control mice, mesangial matrix expansion was prominent (Figure 2, E–G). In ICER-Tg ORX mice, however, the PASM–positive area fraction was significantly decreased, and it became even smaller in ICER-Tg ORX + E2 mice (Figure 2G). ICER-Tg E2 mice also exhibited comparable changes in glomeruli. There was no significant difference in glomerular surface area among animals receiving different treatments (Figure 2H).
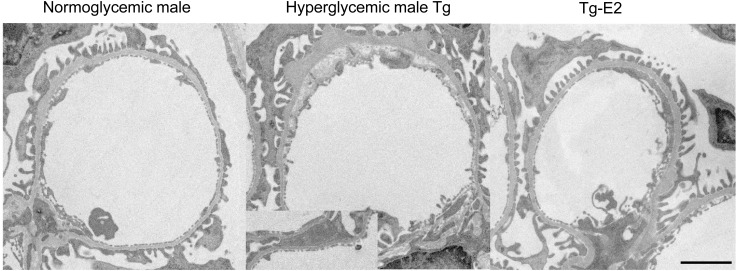
Our model rarely shows nodular lesions. However, ultrastructural study of male hyperglycemic ICER-Tg control mice revealed glomerular basement membrane thickening with partial epithelial foot process fusion and expansion of subendothelial space; E2-treated mice did not develop such abnormalities (Figure 3).
Figure 3.
Electron micrograph of the glomerulus. In male hyperglycemic ICER-Tg control mice, glomerular basement membrane thickening, expansion of subendothelial space, and partial epithelial foot process fusion (inset) were seen, whereas E2-treated mice did not exhibit these lesions. Magnification, ×3500. Scale bars, 2 μm.
Glomerular deposition of extracellular matrix (ECM) type 4 collagen (ColIV), a major component of the expanded ECM in patients and animal models with DN, was next examined. In hyperglycemic male ICER-Tg control mice, ColIV protein localization was significantly increased, mainly in mesangial areas (Figure 4A). In contrast, ColIV protein localization was markedly reduced in all treated animals.
Figure 4.
Decreased deposition of ECM in glomeruli and direct effect of E2 on glomeruli. (A) Representative glomeruli subjected to immunohistochemical staining with anti-ColIV antibody. Ctrl, control. Scale bar, 50 μm. (B) Direct effect of E2 on isolated glomeruli cultured in the presence of a high glucose (HG) concentration and stimulated with E2, PBS (control), or the combination of E2 and selective estrogen receptor antagonist ICI-182,780. (C) Expression levels of TGF-β, ColIV, MMP-9, or MMP-2 after E2 treatment. The data are means±SEMs. C, control. *P<0.05.
To determine whether these improvements are direct effects of E2, isolated glomeruli were cultured in high-glucose medium and stimulated with E2, PBS (control), or E2 plus a pure antiestrogen (ICI-182,780), which inhibits nucleocytoplasmic shuttling of the E2 receptor by blocking nuclear uptake43 (Figure 4B). Expression of TGF-β (a key inducer of ECM) and ColIV mRNA was significantly reduced, whereas expression of matrix metalloproteinase-9 (MMP-9) and MMP-2, major enzymes involved in the degradation of ECM components, was increased by E2 (Figure 4C). These effects were partially blocked by ICI-182,780. Thus, decrease of ECM in glomeruli may be at least partly caused by E2-induced increase in expression of TGF-β– and ECM-degrading enzymes.
Hyperglycemic ICER-Tg control mice show increased urine volume with increased water intake (Figure 5, A–C), which causes pelvic expansion (hydronephrosis), tubular dilation, and epithelial cell flattening (Figure 5D). These changes were not observed in ICER-Tg ORX mice, but this improvement was blocked by implantation of 5α-dihydrotestosterone (5α-DHT; the most active metabolite of T). Namely, in ICER-Tg ORX + 5α-DHT mice, hemoglobin A1c (HbA1c; biomarker of blood glucose) levels remained very high, and mesangial matrix expansion and ECM deposition were apparent with expanded renal pelvis and tubules associated with polyuria (Figure 5, E–H, Supplemental Figure 1). In contrast, in ICER-Tg ORX + E2 and ICER-Tg E2 mice, tubular morphology was normal, being similar to that in female ICER-Tg control mice (Figure 5D). Next, tubular proliferation in renal medulla and cortex 1 week after ORX + E2 treatment was evaluated using the cell proliferation marker Ki67. Expression of Ki67 in tubular cells in both medulla and cortex of ICER-Tg ORX + E2 mice was markedly increased, suggesting that tubular cell turnover was enhanced by the treatment (Figure 5, I and J). There was no change in the number of glomeruli in midtransverse kidney sections, even in the best rescued ICER-Tg ORX + E2 mice (Figure 5K).
Renal Functional Change
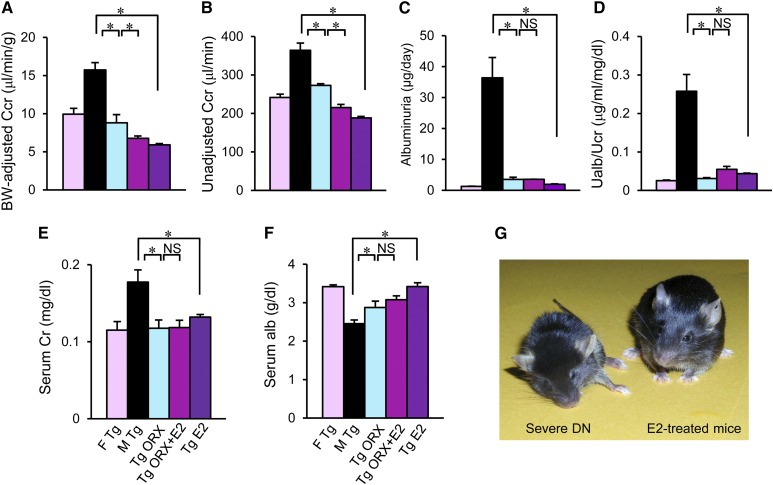
Next, change in renal function was evaluated. In hyperglycemic male ICER-Tg control mice, which showed increased urinary volume, Ccr remained higher than in female ICER-Tg control mice at 44 weeks of age. However, this glomerular hyperfiltration was significantly reduced in ICER-Tg ORX mice and further reduced in ICER-Tg ORX + E2 and ICER-Tg E2 mice (Figure 6, A and B). Albuminuria was also significantly decreased (Figure 6, C and D). Serum Cre level increased with age in male ICER-Tg control mice but was within the normal range in all treated mice (Figure 6E). This increase could be caused by the extensive muscle loss in male ICER-Tg control mice (Figure 5C). Serum albumin level was significantly higher in all treated mice than in male ICER-Tg control mice (Figure 6F). The treated mice appeared healthier than the ICER-Tg control mice with severe diabetic renal disease (Figure 6G).
Figure 6.
Renal functional changes. (A–F) Effects of various treatments on body weight–adjusted or unadjusted Ccr, albuminuria, serum Cre level, and serum albumin level at 40–44 weeks old. The data are means±SEMs (n=6–10 mice per group). Alb, albumin; BW, body weight; Cr, creatinine; F, female; M, male; Ualb, urine albumin; UCr, urine creatinine. *P<0.05. (G) Photograph of severely diabetic renal disease ICER-Tg control mice and E2-treated mice at 40 weeks old.
Experiment 2: Effects of E2 Implantation at Advanced–Stage Diabetic Renal Injury
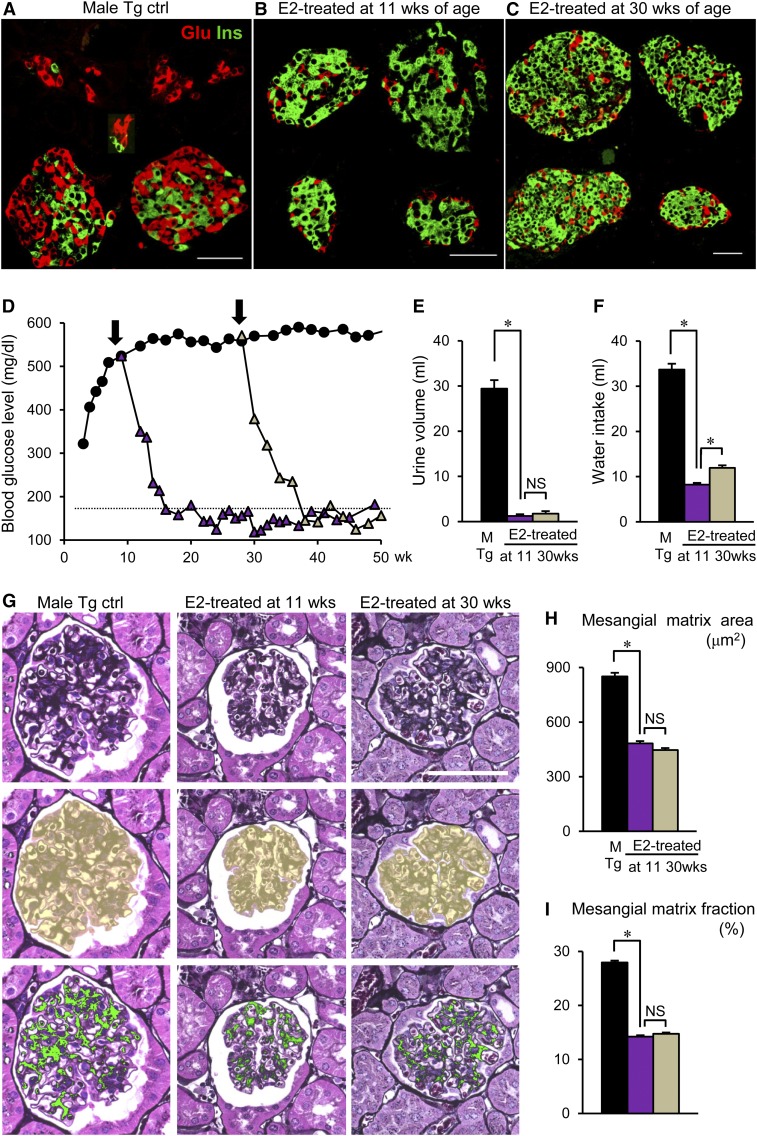
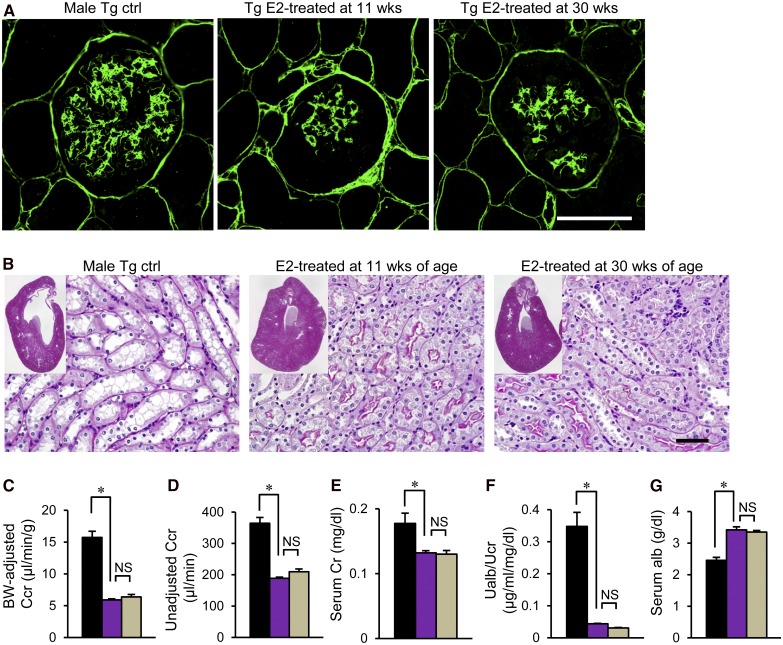
Next, to determine whether the timing of E2 implantation is important, we implanted E2 at 11 weeks of age, when albuminuria appeared (early stage), and 30 weeks of age (advanced stage): our mouse model exhibited glomerulosclerosis as early as 20 weeks.5 All mice were examined at 50–55 weeks. Compared with that in male ICER-Tg control mice (Figure 7A), the number of β-cells was dramatically increased in the groups treated at either age, resulting in marked changes of islet morphology (Figure 7, B and C) and greatly reduced blood glucose levels (Figure 7D). In both groups, glomerular sclerosis (Figure 7, G–I) and deposition of ECM (Figure 8A) were inhibited. Furthermore, expansion of the renal pelvis and tubules was improved (Figure 8B) concomitantly with reduced daily urinary volume and water intake (Figure 6, E and F). Serum Cre level and urinary albumin excretion rate were significantly decreased, Ccr was reduced, and serum albumin level was higher in both treated groups (Figure 8, C–G). These data indicate that E2 implantation is as effective against advanced–stage diabetic renal injury (after onset of glomerulosclerosis) as against early-stage injury.
Figure 7.
Effects of E2 implantation at advanced stage of diabetic renal injury. (A–C) Pancreatic islets from ICER-Tg mice treated with E2 implantation at early (11 weeks old) and advanced (30 weeks old) stages. Dual staining of insulin (Ins; green) and glucagon (Glu; red). (D) Dramatic changes in blood glucose levels were seen when E2 implantation was initiated at the two different time points (n=6–10 per group, except for the male ICER-Tg control group, for which n=24). (E and F) Daily urinary volume and water intake. (G) A representative PASM–stained glomerulus (top panel), the assessed glomerular surface area is shown in yellow (middle panel), and the same glomerulus, in which the PASM-positive area is shown in green (bottom panel). (H and I) The mesangial matrix area (μm2) and mesangial matrix area fraction (%). Mesangial matrix area fraction was determined as the percentage of mesangial matrix area per glomerular surface area. The data are means±SEMs. Ctrl, control; M, male. Scale bars, 50 μm. *P<0.05.
Figure 8.
Improvement of ECM deposition in glomeruli. (A) Representative glomeruli identified by immunohistochemical staining with an anti-ColIV antibody. (B) Representative low–power micrographs of the kidneys obtained from 50- to 55-week-old-mice (insets) and magnified views of the renal structure. Original magnification, ×40 in insets. (C–G) Body weight–adjusted or unadjusted Ccr, serum Cre, urinary albumin excretion rate, and serum albumin level. Alb, albumin; BW, body weight; Cr, creatinine; Ctrl, control; Ualb, urine albumin; UCr, urine creatinine. Scale bars, 50 μm. *P<0.05.
Experiment 3: Effects of Islet Transplantation on Renal Injury
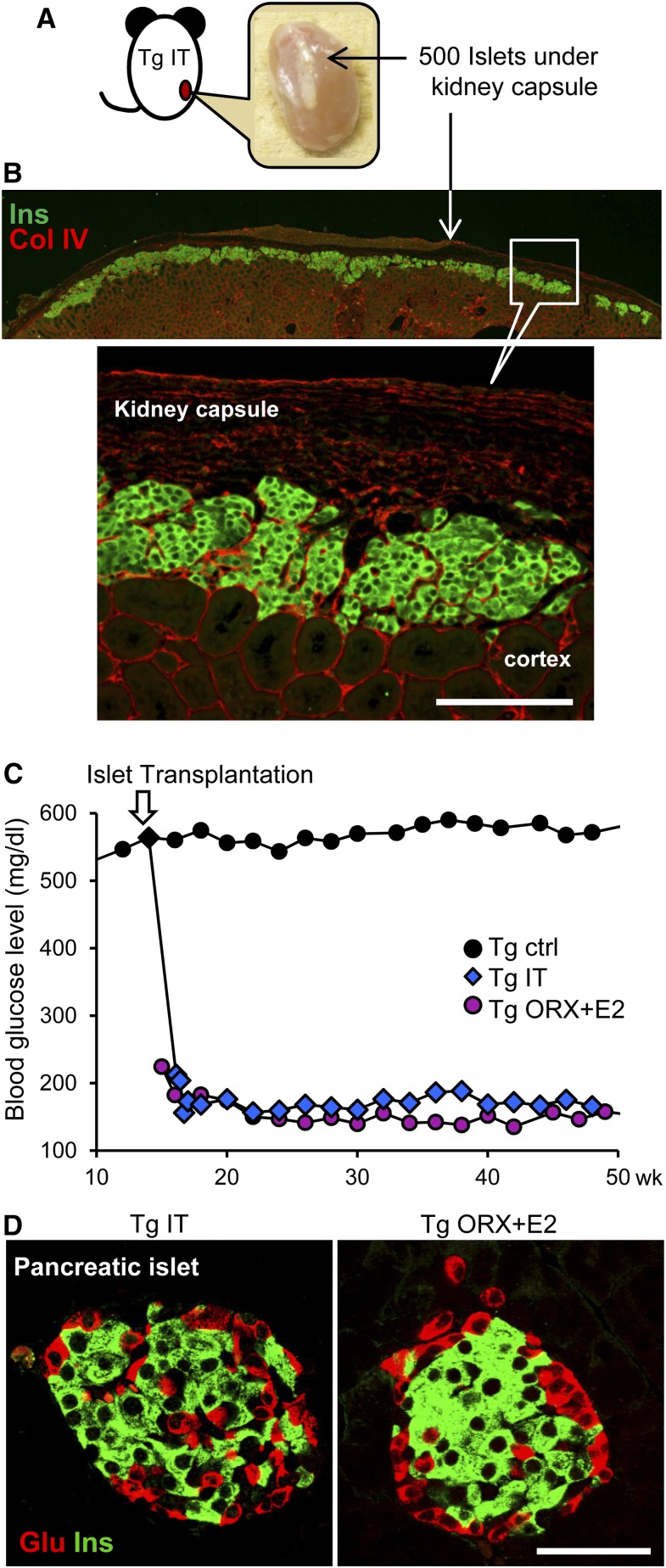
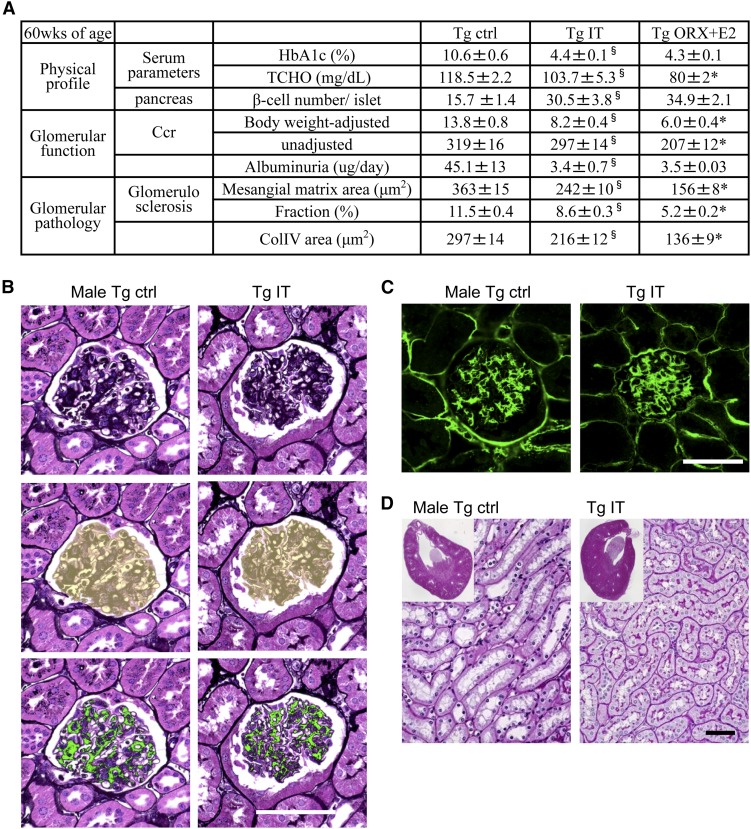
Finally, to determine whether glucose lowering alone is effective, 14-week-old male ICER-Tg mice were subjected to islet transplantation and analyzed at 60 weeks of age; 500 islets obtained from C57BL/6 wild–type mice were implanted under the kidney capsule (Figure 9A). The grafted islets were still clearly visualized by insulin staining at 46–48 weeks after transplantation (Figure 9B). Blood glucose levels in transplanted mice decreased rapidly to the level in ICER-Tg ORX + E2 mice within 1 week and then, remained at this level (Figure 9C). As in ICER-Tg ORX + E2, the number of β-cells within pancreatic islets was increased (Figure 9D). HbA1c level, serum total cholesterol level, albuminuria, and the expansion of the renal pelvis and tubules were all considerably improved by islet transplantation (Figure 10, A and D); however, the increased Ccr, glomerular sclerosis, and deposition of ECM in the glomeruli were not inhibited as effectively as in the ICER-Tg ORX + E2 mice (Figure 10, B and C). These results suggest that glucose lowering alone is effective to prevent albuminuria but cannot inhibit glomerulosclerosis.
Figure 9.
Islet transplantation and changes in blood glucose levels. (A) A kidney transplanted with 500 islets at 46–48 weeks after islet transplantation (60 weeks old). (B) Immunohistochemical staining of an islet graft under a kidney capsule with anti-insulin (green) and anti-ColIV (red) antibodies. (C) Dramatic changes were seen in the blood glucose levels after transplantation (n=12–24 per group). (D) Representative pancreatic islets with dual staining using anti-insulin (green) and antiglucagon (red) antibodies. Ctrl, control; Glu, glucagon; Ins, insulin; IT, islet transplantation. Scale bars, 100 μm in B; 50 μm in D.
Figure 10.
Effect of islet transplantation on renal injury. (A) The physical profile of male ICER-Tg mice treated with islet transplantation or ORX + E2 and age–matched diabetic ICER-Tg control mice at 60 weeks old. The data are means±SEMs (n=10–12 mice per group). *P<0.05 (Tg islet transplantation versus Tg ORX + E2; §P<0.05 (male Tg control versus Tg islet transplantation). (B) A representative PASM–stained glomerulus (top panel), the assessed glomerular surface area is shown in yellow (middle panel), and the same glomerulus, in which the PASM-positive area is shown in green (lower panel). (C) Representative glomeruli with immunohistochemical staining using anti-ColIV antibody. The mean values of mesangial matrix area (μm2), mesangial matrix area fraction (%), and ColIV-positive area (μm2) determined from 50 glomeruli per kidney (n=6 mice per group) are shown. (D) Representative low–power micrographs of kidneys obtained from 60-week-old mice (insets) and magnified views of the renal structure. Periodic acid–Schiff staining. Ctrl, control; IT, islet transplantation; TCHO, total cholesterol. Original magnification, ×40 in D, insets. Scale bars, 50 μm.
Discussion
On the basis of our hypothesis that differences in circulating hormone levels play a key role in determining susceptibility to diabetes, we expected that increasing E2 and thereby, reducing the androgen-to-E2 ratio would be an effective strategy to control initiation and progression of diabetic renal injury in male ICER-Tg mice. Indeed, ORX treatment decreased blood glucose and partially blocked progression of renal injury. Because these changes were blocked by 5α-DHT, they seemed to be caused by loss of androgen. ORX + E2 treatment proved even more effective, and E2 levels in ICER-Tg ORX + E2 mice were far higher than physiologic levels.34 Interestingly, E2 implantation alone had comparable effects to ORX + E2 treatment, which led us to the important conclusion that E2 can ameliorate diabetic renal injury, even in the presence of androgen. Therefore, we examined the direct effect of E2 on glomerular mesangial cells in the presence of a high glucose concentration. After short-term treatment, TGF-β and ColIV mRNAs were decreased, whereas MMP-2 and MMP-9 mRNAs, which affect the balance between ECM synthesis and degradation in glomeruli,35 were increased. E2 inhibitor ICI-182,78043 suppressed these changes. These results are consistent with reports that MMP-2 and/or MMP-9 activities are increased in the supernatant of isolated glomerular cells from ovariectomized (OVX) ROP-Os/− mice after in vitro E2 stimulation40 and the supernatant of isolated podocytes from E2–treated db/db diabetic mice.42
Supplementation of E2 or androgen deprivation does not induce metabolic syndrome in intact normoglycemic mouse models up to ≥32 weeks old.44 Because these mice were not originally diabetic and because their β-cells were functioning, it seems that androgen deprivation or reduction of the androgen-to-E2 ratio has quite different effects in nondiabetic and severely diabetic males, and aging is also likely to affect the response. However, we found that OVX and androgen supplementation render female ICER-Tg mice more susceptible to diabetes (A. Inada, N.L. Fujii, O. Inada, Y. Higaki, Y. Nabeshima, unpublished data). Interestingly, Elliot et al.45 reported that wild–type female mice treated with OVX plus T supplementation (OVX + T) exhibited glomerular enlargement, laminin deposition, and increased TGF-β. The androgen-to-E2 ratio of the OVX + T mice was 32:1 in the study by Elliot et al.45 This value is between that of ICER-Tg ORX mice (25:1) and male ICER-Tg mice (1900:1) in our study and consistent with our idea that the higher the ratio, the greater the exacerbation of renal injury.
Thus, the E2 level and the androgen-to-E2 ratio seem to be critical factors for blocking progression of diabetic renal injury and promoting recovery from diabetic renal injury, at least in the ICER-Tg model. A possible mechanism would be that T or 5α-DHT competitively inhibits E2 receptor complex formation.46 Kinetic analysis showed that increases in androgen concentration during preincubation led to progressive decreases in the rate of E2 and E2 receptor association in rat pituitary from a 17% decrease at 10-fold androgen excess to an 87% decrease at 1000-fold excess.46 This indicates that androgen modulates estrogenic activity, probably by altering the accessibility of E2 receptor to E2, and supports the importance of the T-to-E2 ratio.
It was long believed that, after proteinuria becomes persistent (stages 2–3), glycemic control no longer prevents deterioration of renal function (point of no return). However, strict glycemic control (e.g., by intensive insulin therapy) may protect patients from acute morbidity and mortality caused by uncontrolled diabetes and slow progression of diabetic complications,2,47–50 although this remains controversial.51,52 Importantly, we found that E2 implantation at an advanced stage, after onset of glomerulosclerosis, was as effective as at an early stage.
To determine the effect of glucose lowering alone, islet transplantation is the technique of choice, because blood glucose levels are normalized and well maintained. In male ICER-Tg mice given early–stage islet transplantation, blood glucose decreased to normal within 1–2 weeks and remained normal to ≥60 weeks. However, although albuminuria was considerably improved, glomerular sclerosis, ColIV deposition, and hyper-Ccr were not. These results provide the first evidence that normoglycemia alone is insufficient to inhibit progression of glomerular damage. Another possibility is that a very long time is needed for recovery of sclerotic lesions; this would be consistent with a clinical study by Fioretto et al.53
The effect of E2 supplementation on the renin-angiotensin system remains poorly understood. However, a sex difference in the effect of the renin-angiotensin system on the kidney has been reported.54 For example, plasma renin activity, angiotensin-converting enzyme activity and angiotensin I receptor expression are greater in kidneys in men than in kidneys in women.55–57 Furthermore, angiotensin II infusion maintained GFR in men, but GFR was decreased in women, and the decrease was greater at higher plasma E2 levels, suggesting that E2 modulates the renal response to angiotensin.58 During the luteal phase, when E2 was highest, increased renin-angiotensin system activation did not increase BP, and vasoconstriction was blunted.59 Thus, it is likely that, even if E2 increased liver angiotensin, this would not negatively affect renal function.
In conclusion, we found that diabetic renal injury in the male ICER-Tg mouse model can be rescued by supplementation of E2 and reduction of the androgen-to-E2 ratio. Pathologic and functional renal damage was reversed, even when advanced-stage disease was present. Induction of normoglycemia alone could reverse albuminuria but had no effect on glomerulosclerosis. Our findings taken together with reported in vitro, experimental, and clinical data raise the possibility that modulating the androgen-to-E2 ratio (for example, by E2 supplementation) is a potential therapeutic option for DN and a promising target for drug development.
Concise Methods
Animals
ICER-Tg mice (C57BL/6 background) were generated as described previously.4 We used two lines of ICER-Tg mice (Tg12 and Tg23), which both are severely hyperglycemic and exhibit almost the same phenotype of kidney lesion on average (Tg12 may be slightly milder). Because both lines showed similarly large and significant recoveries in blood glucose levels and kidney lesions, the data presented here are the combined results obtained with Tg12 and Tg23. Mice were housed in the University Animal Facility on a 12-hour light-dark cycle with water and standard rodent diet (CE-2; CLEA Japan) ad libitum. All animal care and experimental procedures were approved by the Animal Care and Experimentation Committee of Kyushu University.
Experimental Model
(1) To assess the influence of androgen, male ICER-Tg mice (either 11 or 30 weeks old) were subjected to ORX (castration; Tg ORX) or sham operation. The effect of castration was confirmed by implanting 12.5-mg pellets of 5α-DHT, the most active metabolite of T (Tg ORX + 5α-DHT). (2) To determine the effects of estrogens in the absence of androgens, mice were additionally implanted with 0.72-mg E2 or placebo pellets (Innovative Research of America) in a slow-release form (90 days) at the nape of the neck immediately after undergoing ORX (Tg ORX + E2). (3) To determine the effects of estrogens in the presence of endogenous androgens, mice not subjected to ORX were implanted with E2 (Tg E2) or placebo pellets at the nape of the neck. The pellets were not replaced. These treatments did not significantly affect the survival rates of mice during the study.34 Treated ICER-Tg mice and untreated male and female ICER-Tg controls were compared in the study. Body weight and morning–fed glucose levels were measured every week. Blood glucose values were measured in blood from a tail snip using a ONE-TOUCH UltraVue Device (Johnson & Johnson K.K.).
Isolation of Pancreatic Islets and Islet Transplantation
Pancreatic islets from 12- to 14-week-old C57BL/6 wild–type mice (n=10 per experiment) were isolated by collagenase digestion, separated on Ficoll gradients as described,60 and washed. Then, 500 islets were handpicked and immediately transplanted under the kidney capsule of 14-week-old male ICER-Tg control mice. Four independent isolation and transplantation experiments were performed (islet–transplanted ICER-Tg mice; n=12).
Histologic Studies
Mouse kidney halves were fixed in 10% buffered formalin and embedded in paraffin, and 2-μm-thick sections were stained with hematoxylin-eosin, periodic acid–Schiff, or PASM using standard histologic procedures. Multiple hematoxylin-eosin images (four to six pictures) were captured and merged to form a single image of the whole kidney using KEYENCE Image–Merging software.
Measurements of Glomerular Surface Area, PASM-Positive Area, and ColIV-Positive Area
The severity of mesangial matrix expansion or glomerular ColIV expression was quantified by measuring mesangial PASM–positive area or ColIV–positive area in glomeruli as follows. Briefly, ≥50 glomeruli present on each section per mouse were photographed. Noise was removed by using the eliminate-particle technique, and the area of interest was measured within the specified range using the KEYENCE Dynamic Cell Count BZ-HIC software package. The mesangial matrix area fraction was determined as the percentage of the mesangial PASM–positive area to the glomerular surface area.
Immunohistochemistry and Antibodies
Paraffin-embedded sections of pancreas and kidneys were immunostained as described previously.34 Images were captured on a KEYENCE-BZ9000 Microscope (KEYENCE Corp., Oska, Japan) or a Carl Zeiss-LSM510META Confocal Microscope (Carl Zeiss GmbH, Jena, Germany). The following antibodies were used in the immunohistochemical studies: Guinea pig anti–insulin (N1542; DAKO), rabbit antiglucagon (4030–01F; Linco Research), mouse anti-Ki67 (550609; BD Pharmingen), rat anti-ColIV (clone-H22; Shigei Medical Research Institute), Alexa Fluor 488– or 594–conjugated anti–Guinea pig (A11073; Invitrogen, Carlsbad, CA) or anti–rabbit IgG (A11037; Invitrogen), biotinylated anti-mouse (BA9200; Vector Laboratories, Burlingame, CA) or anti-rat IgG (BA9400; Vector Laboratories), Alexa Fluor 488 (S11223; Invitrogen), or 594 streptoavidin conjugate (S11227; Invitrogen).
Quantification of Ki67–Positive Renal Tubules, Glomeruli, β-Cell Number, and Whole-Pancreas Area
Sections obtained from 24-week-old mice (n=7 per group) were double immunostained for ColIV and Ki67, and renal tubules on sections of ≥10 different areas for each medulla or cortex/kidney were photographed and evaluated for expression of the cell cycle marker Ki67. Glomeruli were counted per midtransverse section of the left kidney for each mouse. For islet and β-cell quantification, sections were double immunostained for insulin and glucagon, and all islets present in one section (α- or β-cell clusters and clusters with fewer than six insulin- and glucagon-positive cells across were excluded) were photographed using a KEYENCE-BZ9000 Microscope. In total, three sections ≥150 μm apart per pancreas (in 50- to 60-week-old male mice; n=6–10 per group) were evaluated. Each section was evaluated in pairs to ensure consistency.
Measurement of Serum and Urinary Variables
Blood was obtained from the heart under anesthesia immediately before isolation of the organs. The serum total cholesterol and albumin levels were measured using a Hitachi Model 736–740 Autoanalyzer (Hitachi, Yokohama, Japan). Serum Cre and urinary Cre were measured by HPLC. Serum T levels were measured using competitive radioimmunoassay (DPC-TKTT1; Diagnostic Products Co.) with a sensitivity of 0.05 ng/ml and intra- and interassay coefficients of variation of 5%–7% and 7%–9%, respectively; recovery of various concentrations of spiked T was 99%–101%. Serum E2 levels were measured using radioimmunoassay (DPC-KE2D1; Diagnostic Products Co.) with a sensitivity of 1 pg/ml and intra- and interassay coefficients of variation of 3%–5% and 3%–6%, respectively; recovery of various concentrations of spiked E2 was 98%–101% (specificity: estrone, 4.6%; estriol, 0.24%; T and progesterone were below the limits of detection). To avoid the influence of changes in hormonal levels during pregnancy,61 all mice were nonbreeders (n=10). Because serum specimens were collected many times during the first 5-week study period and because the mean plasma concentrations of E2 or T were measured in pooled sera, age–matched ICER-Tg female samples included serum specimens collected during the 4-day estrous cycle (range =5.9–12.5 pg/ml). HbA1c was measured in blood from a tail snip using a DCA2000 Analyzer (Siemens). Urine volume and urinary parameters were measured at 40–44, 50–55, or 60 weeks of age using 24-hour urine samples from mice housed in individual metabolic cages (n=8–16 per group). During urine collection, mice were allowed free access to food and water. The measurements were repeated three times for each group. Urinary albumin was assayed with an Albumin ELISA Kit (TIA-ALBG; Serotec Co.). Body weight–adjusted Ccr was calculated by use of the following equation: Ccr = urine Cre (μg/dL) × urine volume (μL/min)/serum Cre (μg/dL)/body weight (g).
Isolation of Glomeruli and Cell Culture
Glomeruli were isolated from renal cortices of mice using the reported differential sieving method.62,63 Mesangial cells were cultured in B medium (a 3:1 mixture of minimal essential medium/F12 modified with trace elements) supplemented with 20% FCS, 1 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. The cultured cells matched the generally accepted characteristics of glomerular mesangial cells.
RNA Isolation, Reverse Transcription, and Semiquantitative PCR
For examination of the direct effect of E2, glomerular mesangial cells were maintained in the presence of 25 mM glucose (high glucose). Cells were pretreated with 1 μM ICI-182,780 (Tocris Bioscience, Bristol, United Kingdom) for 1 hour and then, stimulated with 100 μM E2 (Sigma-Aldrich, St. Louis, MO) or 100 μM E2 + 100 μM ICI-182,780 for an additional 48 hours. Each group was processed for subsequent analysis at the end of the 48-hour period. Total cellular RNA was extracted from glomerular mesangial cells using an RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s instructions and used as a template for cDNA synthesis (Superscript Reverse Transcription; Life Technologies, Carlsbad, CA). Glomerular mesangial cell cDNA was amplified by PCR using TGF-β, ColIV, MMP-2, MMP-9, and β-actin–specific oligonucleotides. The primer sequences were as follows: TGF-β (5′-ATACAGGGCTTTCGATCCAG-3′ and 5′-GTCCAGGCTCCAAATATAGG-3′), ColIV (5′-TAGGTGTCAGCAATTAGGCAG-3′ and 5′-TCACTTCAAGCATAGTGGTCCG-3′), MMP-2 (5′-ACCAGAACACCATCGAGACC-3′ and 5′-AGGGTCCACAGCTCATCATC-3′), MMP-9 (5′-GTACCGCTATGGTTACAC-3′ and 5′-CCGCGACACCAAACTGGAT-3′), and β-actin (5′-ATCCGTAAAGACCTCTATGC-3′ and 5′-AACGCAGCTCAGTAACAGTC-3′). To exclude any amplification product derived from genomic DNA that could contaminate the RNA preparation, total RNA without reverse transcription was amplified as a negative control. mRNA expression was quantified by semiquantitative PCR as described previously.11 The number of amplification cycles was selected, such that amplification of each sequence was in the exponential region of the amplification curve. For TGF-β, ColIV, MMP-2, MMP-9, and β-actin, the numbers of cycles were 30, 30, 34, 34, and 26, respectively. β-Actin was used as an internal control for differences in overall cDNA concentration and experimental variations between samples. The amount of each specific product was expressed relative to this internal control, giving a specific product-to-control gene ratio for each sample.
Electron Microscopy
For electron microscopy, specimens were obtained from kidney cortex of 48-week-old male and female ICER-Tg controls and ICER-Tg E2 mice, fixed with 2% glutaraldehyde in 0.05 mol/L phosphate buffer, dehydrated in an ethanol gradient, and embedded in epoxy resin. The sections were cut with a glass knife on a Reichert-Nissei Ultracuts Microtome (Leica Microsystems, Buffalo Grove, IL). The sections were then contrasted with uranyl acetate and lead citrate and observed with a Hitachi–H7100 Electron Microscope (Hitachi).
Data Analyses
The results are expressed as means±SEMs. Statistical significance was assessed using t tests. A value of P<0.05 was considered to be statistically significant.
Disclosures
A.I. and O.I. were the main contributors to study conception and design, acquisition and interpretation of data, obtaining funding and writing of the manuscript. Y.Y. and H.K. provided technical advice on islet isolation and transplantation. T.M. isolated glomerular mesangial cells. N.F., S.N. and H.A. provided important advice during the work and critically read the manuscript. A.F. revised the article for important intellectual content. Y.N. discussed the manuscript and contributed to the writing process. A.I. is the guarantor of this work, and as such, had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors discussed the results, commented on the manuscript and approved the final version.
Supplementary Material
Acknowledgments
We thank K. Fujishima for technical assistance, H. Matsusaka for his help in collecting the periodic acid–methenamine silver and electron microscopy data, H. Fujii for providing tissue sections, and Drs. K. Kurachi and K. Sueishi for their valuable support. We also thank Y. Shintani and other laboratory members for their support and encouragement throughout this research.
This work was supported by Ministry of Health, Labour and Welfare of Japan grants 11103412 and 11103401 (to A.I.); Japan Science and Technology Agency grants AS221Z01114G, H20-1201, and H21-1771 (to A.I.); the Ministry of Education, Culture, Sports, Science and Technology of Japan grants “Scientific Research on Innovative Areas-Stem Cell Aging and Disease” 26115002 (to Y.N.) and “City Area Program” (to A.I.); and Fukuoka Industry Science and Technology Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sex Differences and Renal Protection: Keeping in Touch with Your Feminine Side,” on pages 2921–2924.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015070741/-/DCSupplemental.
References
- 1.Rossing P: Diabetic nephropathy: Worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep 6: 479–483, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T: Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 28: 164–176, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Lim AK: Diabetic nephropathy - complications and treatment. Int J Nephrol Renovasc Dis 7: 361–381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inada A, Hamamoto Y, Tsuura Y, Miyazaki J, Toyokuni S, Ihara Y, Nagai K, Yamada Y, Bonner-Weir S, Seino Y: Overexpression of inducible cyclic AMP early repressor inhibits transactivation of genes and cell proliferation in pancreatic β cells. Mol Cell Biol 24: 2831–2841, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inada A, Nagai K, Arai H, Miyazaki J, Nomura K, Kanamori H, Toyokuni S, Yamada Y, Bonner-Weir S, Weir GC, Fukatsu A, Seino Y: Establishment of a diabetic mouse model with progressive diabetic nephropathy. Am J Pathol 167: 327–336, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foulkes NS, Borrelli E, Sassone-Corsi P: CREM gene: Use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell 64: 739–749, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Foulkes NS, Borjigin J, Snyder SH, Sassone-Corsi P: Transcriptional control of circadian hormone synthesis via the CREM feedback loop. Proc Natl Acad Sci U S A 93: 14140–14145, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molina CA, Foulkes NS, Lalli E, Sassone-Corsi P: Inducibility and negative autoregulation of CREM: An alternative promoter directs the expression of ICER, an early response repressor. Cell 75: 875–886, 1993 [DOI] [PubMed] [Google Scholar]
- 9.Stehle JH, Foulkes NS, Molina CA, Simonneaux V, Pévet P, Sassone-Corsi P: Adrenergic signals direct rhythmic expression of transcriptional repressor CREM in the pineal gland. Nature 365: 314–320, 1993 [DOI] [PubMed] [Google Scholar]
- 10.Inada A, Someya Y, Yamada Y, Ihara Y, Kubota A, Ban N, Watanabe R, Tsuda K, Seino Y: The cyclic AMP response element modulator family regulates the insulin gene transcription by interacting with transcription factor IID. J Biol Chem 274: 21095–21103, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Inada A, Weir GC, Bonner-Weir S: Induced ICER Iγ down-regulates cyclin A expression and cell proliferation in insulin-producing β cells. Biochem Biophys Res Commun 329: 925–929, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Inada A, Kanamori H, Arai H, Akashi T, Araki M, Weir GC, Fukatsu A: A model for diabetic nephropathy: Advantages of the inducible cAMP early repressor transgenic mouse over the streptozotocin-induced diabetic mouse. J Cell Physiol 215: 383–391, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Inada A, Arai H, Nagai K, Miyazaki J, Yamada Y, Seino Y, Fukatsu A: Gender difference in ICER Iγ transgenic diabetic mouse. Biosci Biotechnol Biochem 71: 1920–1926, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Kava RA, West DB, Lukasik VA, Greenwood MR: Sexual dimorphism of hyperglycemia and glucose tolerance in Wistar fatty rats. Diabetes 38: 159–163, 1989 [DOI] [PubMed] [Google Scholar]
- 15.Ueda H, Ikegami H, Yamato E, Fu J, Fukuda M, Shen G, Kawaguchi Y, Takekawa K, Fujioka Y, Fujisawa T, Nakagawa Y, Hamada Y, Shibata M, Ogihara T: The NSY mouse: A new animal model of spontaneous NIDDM with moderate obesity. Diabetologia 38: 503–508, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Haseyama T, Fujita T, Hirasawa F, Tsukada M, Wakui H, Komatsuda A, Ohtani H, Miura AB, Imai H, Koizumi A: Complications of IgA nephropathy in a non-insulin-dependent diabetes model, the Akita mouse. Tohoku J Exp Med 198: 233–244, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Gorogawa S, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, Takeda K, Akira S, Magnuson MA, Yamasaki Y, Kajimoto Y, Hori M: Insulin secretory defects and impaired islet architecture in pancreatic β-cell-specific STAT3 knockout mice. Biochem Biophys Res Commun 319: 1159–1170, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Barrado MJ, Iglesias-Osma MC, Moreno-Viedma V, Pastor Mansilla MF, Gonzalez SS, Carretero J, Moratinos J, Burks DJ: Differential sensitivity to adrenergic stimulation underlies the sexual dimorphism in the development of diabetes caused by Irs-2 deficiency. Biochem Pharmacol 81: 279–288, 2011 [DOI] [PubMed] [Google Scholar]
- 19.King H, Aubert RE, Herman WH: Global burden of diabetes, 1995-2025: Prevalence, numerical estimates, and projections. Diabetes Care 21: 1414–1431, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Wild S, Roglic G, Green A, Sicree R, King H: Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 21.The National Health and Nutrition Survey in Japan. Available at: http://www.mhlw.go.jp/file/04-Houdouhappyou-10904750-Kenkoukyoku-Gantaisakukenkouzoushinka/0000106403.pdf. Accessed April 1, 2015
- 22.Lopes AA, Port FK, James SA, Agodoa L: The excess risk of treated end-stage renal disease in blacks in the United States. J Am Soc Nephrol 3: 1961–1971, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Silbiger SR, Neugarten J: The impact of gender on the progression of chronic renal disease. Am J Kidney Dis 25: 515–533, 1995 [DOI] [PubMed] [Google Scholar]
- 24.Maric C, Sullivan S: Estrogens and the diabetic kidney. Gend Med 5[Suppl A]: S103–S113, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazer C, Meranze DR, Israel SL: Evaluation of the constitutional effects of large doses of estrogenic principle. JAMA 105: 257–263, 1935 [Google Scholar]
- 26.Gessler CJ, Halsted JA, Stetson RP: Effect of estrogenic substance on the blood sugar of female diabetics after the menopause. J Clin Invest 18: 715–722, 1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson B, Mattsson LA, Hahn L, Mårin P, Lapidus L, Holm G, Bengtsson BA, Björntorp P: Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 82: 638–643, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Brussaard HE, Gevers Leuven JA, Frölich M, Kluft C, Krans HM: Short-term oestrogen replacement therapy improves insulin resistance, lipids and fibrinolysis in postmenopausal women with NIDDM. Diabetologia 40: 843–849, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Szekacs B, Vajo Z, Varbiro S, Kakucs R, Vaslaki L, Acs N, Mucsi I, Brinton EA: Postmenopausal hormone replacement improves proteinuria and impaired creatinine clearance in type 2 diabetes mellitus and hypertension. Br J Obstet Gynaecol 107: 1017–1021, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Friday KE, Dong C, Fontenot RU: Conjugated equine estrogen improves glycemic control and blood lipoproteins in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab 86: 48–52, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Hadjadj S, Gourdy P, Zaoui P, Guerci B, Roudaut N, Gautier JF, Chabin M, Mauco G, Ragot S RADIAN Study Group : Effect of raloxifene—a selective oestrogen receptor modulator—on kidney function in post-menopausal women with Type 2 diabetes: Results from a randomized, placebo-controlled pilot trial. Diabet Med 24: 906–910, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Jain SK, Rogier K, Prouty L, Jain SK: Protective effects of 17β-estradiol and trivalent chromium on interleukin-6 secretion, oxidative stress, and adhesion of monocytes: Relevance to heart disease in postmenopausal women. Free Radic Biol Med 37: 1730–1735, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C: Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med 2: 227–237, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Inada A, Inada O, Fujii NL, Fujishima K, Inai T, Fujii H, Sueishi K, Kurachi K: β-cell induction in vivo in severely diabetic male mice by changing the circulating levels and pattern of the ratios of estradiol to androgens. Endocrinology 155: 3829–3842, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Lenz O, Elliot SJ, Stetler-Stevenson WG: Matrix metalloproteinases in renal development and disease. J Am Soc Nephrol 11: 574–581, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Potier M, Karl M, Zheng F, Elliot SJ, Striker GE, Striker LJ: Estrogen-related abnormalities in glomerulosclerosis-prone mice: Reduced mesangial cell estrogen receptor expression and prosclerotic response to estrogens. Am J Pathol 160: 1877–1885, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blush J, Lei J, Ju W, Silbiger S, Pullman J, Neugarten J: Estradiol reverses renal injury in Alb/TGF-β1 transgenic mice. Kidney Int 66: 2148–2154, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Maric C, Sandberg K, Hinojosa-Laborde C: Glomerulosclerosis and tubulointerstitial fibrosis are attenuated with 17β-estradiol in the aging Dahl salt sensitive rat. J Am Soc Nephrol 15: 1546–1556, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Mankhey RW, Wells CC, Bhatti F, Maric C: 17β-Estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. Am J Physiol Regul Integr Comp Physiol 292: R769–R777, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Karl M, Berho M, Pignac-Kobinger J, Striker GE, Elliot SJ: Differential effects of continuous and intermittent 17β-estradiol replacement and tamoxifen therapy on the prevention of glomerulosclerosis: Modulation of the mesangial cell phenotype in vivo. Am J Pathol 169: 351–361, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dixon A, Maric C: 17β-Estradiol attenuates diabetic kidney disease by regulating extracellular matrix and transforming growth factor-β protein expression and signaling. Am J Physiol Renal Physiol 293: F1678–F1690, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Catanuto P, Doublier S, Lupia E, Fornoni A, Berho M, Karl M, Striker GE, Xia X, Elliot S: 17 β-estradiol and tamoxifen upregulate estrogen receptor β expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int 75: 1194–1201, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Dauvois S, White R, Parker MG: The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 106: 1377–1388, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Inoue T, Zakikhani M, David S, Algire C, Blouin MJ, Pollak M: Effects of castration on insulin levels and glucose tolerance in the mouse differ from those in man. Prostate 70: 1628–1635, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Elliot SJ, Berho M, Korach K, Doublier S, Lupia E, Striker GE, Karl M: Gender-specific effects of endogenous testosterone: Female α-estrogen receptor-deficient C57Bl/6J mice develop glomerulosclerosis. Kidney Int 72: 464–472, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Korach KS, Muldoon TG: Inhibition of anterior pituitary estrogen-receptor complex formation by low-affinity interaction with 5 alpha-dihydrotestosterone. Endocrinology 97: 231–236, 1975 [DOI] [PubMed] [Google Scholar]
- 47.Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: A randomized prospective 6-year study. Diabetes Res Clin Pract 28: 103–117, 1995 [DOI] [PubMed] [Google Scholar]
- 48.UK Prospective Diabetes Study Group : Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 317: 713–720, 1998 [PMC free article] [PubMed] [Google Scholar]
- 49.Nyberg G, Blohmé G, Nordén G: Impact of metabolic control in progression of clinical diabetic nephropathy. Diabetologia 30: 82–86, 1987 [DOI] [PubMed] [Google Scholar]
- 50.Mulec H, Blohmé G, Grände B, Björck S: The effect of metabolic control on rate of decline in renal function in insulin-dependent diabetes mellitus with overt diabetic nephropathy. Nephrol Dial Transplant 13: 651–655, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Rossing P: Promotion, prediction and prevention of progression of nephropathy in type 1 diabetes mellitus. Diabet Med 15: 900–919, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Hovind P, Rossing P, Tarnow L, Smidt UM, Parving HH: Progression of diabetic nephropathy. Kidney Int 59: 702–709, 2001 [DOI] [PubMed] [Google Scholar]
- 53.Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Sullivan JC: Sex and the renin-angiotensin system: Inequality between the sexes in response to RAS stimulation and inhibition. Am J Physiol Regul Integr Comp Physiol 294: R1220–R1226, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Fischer M, Baessler A, Schunkert H: Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res 53: 672–677, 2002 [DOI] [PubMed] [Google Scholar]
- 56.McGuire BB, Watson RW, Pérez-Barriocanal F, Fitzpatrick JM, Docherty NG: Gender differences in the renin-angiotensin and nitric oxide systems: Relevance in the normal and diseased kidney. Kidney Blood Press Res 30: 67–80, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Sandberg K, Ji H: Sex and the renin angiotensin system: Implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther 10: 15–23, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Miller JA, Anacta LA, Cattran DC: Impact of gender on the renal response to angiotensin II. Kidney Int 55: 278–285, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Chidambaram M, Duncan JA, Lai VS, Cattran DC, Floras JS, Scholey JW, Miller JA: Variation in the renin angiotensin system throughout the normal menstrual cycle. J Am Soc Nephrol 13: 446–452, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Inada A, Inada O, Fujii H, Akashi T, Sueishi K, Fukatsu A, Nagafuchi S: Different effects of islet transplantation and Detemir treatment on the reversal of streptozotocin-induced diabetes associated with β-cell regeneration. Diabetol Int 1: 49–59, 2010 [Google Scholar]
- 61.Barkley MS, Geschwind II, Bradford GE: The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod 20: 733–738, 1979 [DOI] [PubMed] [Google Scholar]
- 62.Abe H, Matsubara T, Iehara N, Nagai K, Takahashi T, Arai H, Kita T, Doi T: Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J Biol Chem 279: 14201–14206, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Ohashi S, Abe H, Takahashi T, Yamamoto Y, Takeuchi M, Arai H, Nagata K, Kita T, Okamoto H, Yamamoto H, Doi T: Advanced glycation end products increase collagen-specific chaperone protein in mouse diabetic nephropathy. J Biol Chem 279: 19816–19823, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.