Abstract
Two common missense variants in APOL1 (G1 and G2) have been definitively linked to CKD in black Americans. However, not all individuals with the renal-risk genotype develop CKD, and little is known about how APOL1 variants drive disease. Given the association of APOL1 with HDL particles, which are cleared by the kidney, differences in the level or quality of mutant APOL1‑HDL particles could be causal for disease and might serve as a useful risk stratification marker. We measured plasma levels of G0 (low risk), G1, and G2 APOL1 in 3450 individuals in the Dallas Heart Study using a liquid chromatography-MS method that enabled quantitation of the different variants. Additionally, we characterized native APOL1‑HDL from donors with no or two APOL1 risk alleles by size-exclusion chromatography and analysis of immunopurified APOL1‑HDL particles. Finally, we identified genetic loci associated with plasma APOL1 levels and tested for APOL1-dependent association with renal function. Although we replicated the previous association between APOL1 variant status and renal function in nondiabetic individuals, levels of circulating APOL1 did not associate with microalbuminuria or GFR. Furthermore, the size or known components of APOL1‑HDL did not consistently differ in subjects with the renal-risk genotype. Genetic association studies implicated variants in loci harboring haptoglobin-related protein (HPR), APOL1, and ubiquitin D (UBD) in the regulation of plasma APOL1 levels, but these variants did not associate with renal function. Collectively, these data demonstrate that the risk of renal disease associated with APOL1 is probably not related to circulating levels of the mutant protein.
Keywords: chronic kidney disease, Epidemiology and outcomes, genetic renal disease, human genetics, Pathophysiology of Renal Disease and Progression, risk factors
Individuals of African descent have long been known to be at significantly increased risk for CKD, compared with individuals from other ethnic backgrounds. This increased risk has recently been attributed to two alleles in the APOL1 gene, termed G1 and G2, that confer resistance to selected human-infective substrains of African Trypanosomes, including Trypanosoma brucei rhodesiense.1,2 Positive selection for resistance to these and potentially other pathogens is thought to explain the high prevalence of these alleles in the West African and black American populations, with 10%–15% of the black American population carrying two copies of the risk alleles and thus at an increased risk of a variety of forms of CKD. The risk conferred by the renal-risk genotype (RRG) varies for different diseases with odds ratios (OR) ranging from 2.9 for lupus nephritis to >29 for HIV associated nephropathy.1,3–6 The varying OR for different types of renal disease and incomplete penetrance suggest that though the APOL1 RRG is a potent risk factor for the development of renal disease, it is not sufficient on its own to induce renal dysfunction. The nature of the additional contributing factors is unknown, but has been hypothesized to be related to induction of APOL1 by innate immune effectors including Toll-Like Receptor ligands and interferons as might be seen in HIV infection.7
A single copy of either one of the APOL1 risk variants confers resistance to trypanosomiasis, but two copies of the risk alleles are required for increased susceptibility to CKD.1 Although this mode of inheritance would typically imply a loss-of-function model, several lines of evidence suggest that the APOL1 risk variants are actually gain-of-function toxic proteins. The evidence against a loss-of-function model (in which APOL1 is a protective factor in the kidney) include: (1) APOL1 does not appear to be required for normal kidney function because a human homozygous null individual has been identified with apparently normal kidney function after several years of follow-up,8,9 (2) the restricted species distribution of the APOL1 gene,10,11 and (3) evidence for increased cytotoxic effects of G1 and G2 APOL1 when expressed in a variety of model systems.7,12–14
The bulk of APOL1 is found in the plasma, where it circulates as part of a specialized HDL particle consisting of APOA1, APOL1 and the hemoglobin binding protein Haptoglobin-Related Protein (HPR), which also serves as the receptor for uptake by trypanosomes.15 A subset of APOL1‑HDL has also been shown to bind IgM and forms the distinct lytic factor TLF‑2.16 Because HDL is cleared in part through the kidney and because IgM may contribute to the pathophysiology of FSGS and other forms of glomerular disease,17–19 it is possible that the renal toxicity is dependent on the APOL1 in the circulation, rather than that expressed in the kidney. A mismatch between APOL1 mRNA localization and protein abundance in distinct renal cell types including the podocyte has recently been reported, and podocytes readily take up HDL, suggesting that APOL1 from the circulation may be taken up by distinct cell types in the kidney and contribute to the pathology of APOL1 nephritis.20 In either scenario, circulating variant APOL1 could contribute to kidney disease. A strong relationship between the levels of APOL1 risk variants in the circulation and CKD would provide correlative evidence that the circulating pool contributes to the pathophysiology of APOL1-related nephropathy.
Although APOL1 risk alleles confer a greatly increased susceptibility to a variety of renal diseases, the majority of individuals with the risk genotype are asymptomatic. The reasons for this incomplete penetrance are unknown and it is currently not possible to identify those carriers of the RRG at greatest risk for developing CKD. Therefore, developing predictive biomarkers that could discriminate between low- and high-risk individuals is of significant interest. If circulating APOL1 contributes to renal disease, it is plausible that levels of APOL1 might identify those at greatest risk of developing CKD. Circulating APOL1 may also predict CKD risk even if the circulating fraction is not causal, but merely highly correlated with renal expression levels. Previous work has suggested that there was no association between plasma APOL1 and renal disease in a small HIV-associated CKD cohort,21 but, to date, no study has examined the relationship between APOL1 genotype, plasma APOL1 levels, and renal disease. We have recently developed a liquid chromatography (LC)‑MS method to specifically monitor the levels of distinct APOL1 variants in plasma.22 Here, we use this methodology to ask whether there is an association between the levels of APOL1 variants in renal disease in a large cross-sectional cohort, and to determine whether levels of circulating APOL1 could plausibly be used to distinguish those at greatest risk for developing renal disease.
Results
Study Population
Characteristics of the Dallas Heart Study (DHS) participants are shown in Table 1. A total of 3450 individuals (52% black, 44% male, mean age 44±10 years) with available measurements of circulating APOL1 were included in the current study. Black participants had a higher burden of established CKD risk factors, such as diabetes, obesity, and hypertension, compared with white participants (14.2% versus 6.7%, 52.4% versus 35.8%, and 28.2% versus 11.5%, respectively, all P<0.05). However, they had a similar prevalence of obesity and diabetes as Hispanic participants (P>0.05). Despite a high rate of obesity, black participants had more favorable fasting lipoprotein profiles compared with white or Hispanic participants (P<0.05).
Table 1.
Baseline characteristics of the DHS participants (n=3450)
| Characteristic | Black (n=1788) | White (n=1004) | Hispanic (n=588) | P Value |
|---|---|---|---|---|
| Male, n (%) | 755 (42.2) | 474 (47.2)a | 245 (41.7)c | 0.02 |
| Mean age (yr) | 44.8 (±10.2) | 44.9 (±10.0) | 40.2 (±9.1)b,c | <0.001 |
| Mean BMI (kg/m2) | 31.7 (±8.2) | 29 (±6.6)a | 30.3 (±6.5)b,c | <0.001 |
| Obesity, n (%) | 786 (52.4) | 330 (35.8)a | 241 (48.2)c | <0.001 |
| Mean fasting glucose (mg/dl) | 91 (±11.7) | 91.3 (±10.3)a | 94 (±10.5)b,c | <0.001 |
| Mean HOMA-IR (U) | 4.6 (±4.8) | 3.5 (±4.1)a | 4.4 (±4.3)b,c | <0.001 |
| Diabetes, n (%) | 254 (14.2) | 67 (6.7)a | 68 (11.6)c | <0.001 |
| Mean systolic BP (mmHg) | 130.2 (±20.0) | 119.9 (±15.4)a | 118.6 (±17.0)b | <0.001 |
| Mean diastolic BP (mmHg) | 81 (±10.6) | 75.9 (±8.8)a | 74.6 (±9.2)b | <0.001 |
| Hypertension, n (%) | 503 (28.2) | 115 (11.5)a | 61 (10.4)b | <0.001 |
| Median ACR (mg/g) | 3.1 (1.8 to 6.6) | 2.6 (1.8 to 4.1)a | 3.1 (2.1 to 5.2)c | <0.001 |
| Median GFR (ml/min per 1.73 m2) | 102.4 (88.0 to 116.8) | 90.3 (80.5 to 101.9)a | 104 (93.1 to 120.0)c | <0.001 |
| CKD, n (%) | ||||
| All (n=3262) | 207 (12) | 46 (4.8)a | 36 (6.3) | 0.002 |
| Nondiabetic (n=2878) | 129 (8.7) | 31 (3.5)a | 22 (4.4) | 0.002 |
| Diabetic (n=384) | 78 (31.2) | 15 (22.4) | 14 (20.9) | 0.51 |
| Microalbuminuria, n (%) | ||||
| All (n=3256) | 181 (10.5) | 33 (3.4)a | 35 (6.1)c | <0.001 |
| Nondiabetic (n=2872) | 112 (7.6) | 20 (2.2)a | 21 (4.2)c | <0.001 |
| Diabetic (n=383) | 69 (27.7) | 13 (19.4) | 14 (20.9) | 0.70 |
| GFR<60, n (%) | ||||
| All (n=3379) | 55 (3.1) | 19 (1.9) | 3 (0.5)b | 0.02 |
| Nondiabetic (n=2990) | 38 (2.5) | 13 (1.4) | 1 (0.2)b | 0.01 |
| Diabetic (n=389) | 17 (6.7) | 6 (9.0) | 2 (2.9) | 0.32 |
| GFR<30, n (%) | 14 (0.8) | 0 (0.0) | 1 (0.2) | 0.09 |
| Mean total cholesterol (mg/dl) | 177.9 (±40.2) | 183.9 (±38.6)a | 182 (±40)b | <0.001 |
| Mean LDL cholesterol (mg/dl) | 104.8 (±36.6) | 108.4 (±34.5)a | 107.1 (±33.3)b | <0.001 |
| Mean HDL cholesterol (mg/dl) | 52.3 (±15.3) | 48.6 (±15.2)a | 45.7 (±11.2)b,c | <0.001 |
| Median triglycerides (mg/dl) | 85 (62 to 123) | 109 (75 to 166)a | 119 (81 to 176)b,c | <0.001 |
| Mean total lipoprotein HDL particles (nmol/L) | 33 (±6.4) | 33.8 (±6.4)a | 32.2 (±5.8)c | <0.001 |
| Mean lipoprotein large HDL (nmol/L) | 7.3 (±4.0) | 6.1 (±4.0)a | 5.8 (±3.2)b | <0.001 |
| Mean lipoprotein medium HDL (nmol/L) | 3.9 (±4.1) | 5.6 (±5.2)a | 4.9 (±4.9)b,c | <0.001 |
| Mean lipoprotein small HDL (nmol/L) | 21.7 (±5.8) | 22.2 (±6.0)a | 21.4 (±5.5) | 0.02 |
| Mean HDL lipoprotein particle size (nm) | 9.1 (±0.5) | 8.9 (±0.4)a | 8.9 (±0.4)b | <0.001 |
| Median CRP (mg/L) | 3.6 (1.4 to 8.4) | 2.3 (0.9 to 4.9)a | 2.8 (1.3 to 6.3)c | <0.001 |
| Median IL-6 (pg/ml) | 16.9 (0.0 to 35.8) | 18.8 (0.0 to 41.6)a | 15.1 (0.0 to 29.1)b,c | <0.001 |
| Median IL-18 (mg/L) | 498 (336.5 to 750) | 561 (407 to 832)a | 532 (364.5 to 763)b | <0.001 |
| Median IL1AP (ng/ml) | 5428 (3916 to 7561) | 6241 (4357 to 8602)a | 5637 (4132 to 7811)c | <0.001 |
| Median TNFR1A (ng/ml) | 0.6 (0.4 to 0.9) | 0.6 (0.4 to 0.9)a | 0.6 (0.4 to 0.8) | 0.05 |
| CXCL1>0 ng/ml, n (%) | 241 (15.0) | 35 (3.8)a | 43 (8.1)b,c | <0.001 |
| CXCL2>0 ng/ml, n (%) | 521 (32.3) | 276 (30.1) | 114 (21.4)b,c | <0.001 |
| Median CXCL10 (ng/ml) | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.4)a | 0.3 (0.2–0.5)b,c | <0.001 |
| Median CD40 ligand (ng/ml) | 1.4 (0.8–2.6) | 1.4 (0.8 to 2.3) | 1.4 (0.8–2.4) | 0.58 |
| Mean plasma APOA1 (μM) | 64.1 (±18.4) | 61.5 (±17.9)a | 60.5 (±16.2)b | <0.001 |
| Mean plasma total APOL1 (nM) | 862.5 (±296.8) | 627.2 (±209.5)a | 695.5 (±235.5)b,c | <0.001 |
Data are shown as mean (±SD), median (25th to 75th percentile), or n (%), as indicated. Characteristics are compared across ethnic groups using linear regression for continuous data and logistic regression for categorical data. Models are adjusted for age, gender, BMI, systolic BP, and diabetes, where appropriate. Obesity is defined as BMI>30 kg/m2. Microalbuminuria, ACR≥17 mg/g in men or ≥25 mg/g in women. CKD is defined as the presence of microalbuminuria and/or GFR<60 ml/min per 1.73 m2. HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein; CXCL, C-X-C motif ligand; CD40, cluster of differentiation 40.
European Americans differ from black participants at P<0.05.
Hispanic participants differ from black participants at P<0.05.
Hispanic participants differ from white participants at P<0.05.
Population Characteristics of Plasma APOL1
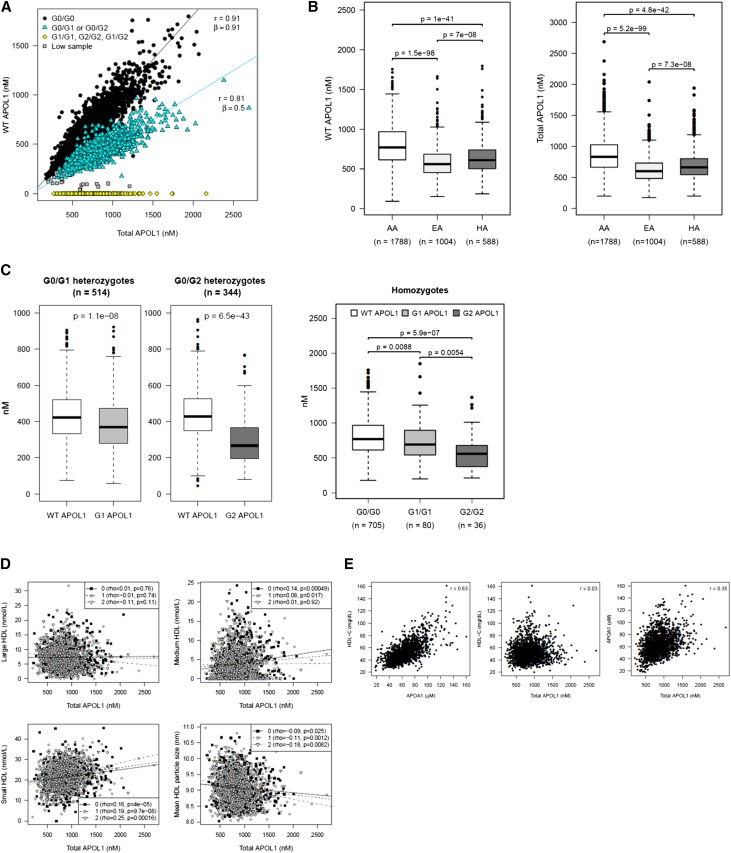
APOL1 was measured in stored plasma samples from 3450 subjects in the DHS using a LC‑MS method described previously.22 Multiple peptides from APOL1 were measured, including a carboxy‑terminal–specific peptide (LNILNNNYK) that enabled us to quantify the levels of G0, G1, or G2 variant protein in the plasma, as well an internal peptide (ALDNLAR) that served as a control for quantitation. Correlation between values derived from the carboxy‑terminal peptide and the internal peptide were high, and consistent between APOL1 from different genotypes, demonstrating the high reproducibility and quality of the assays (Figure 1A). The slope of the linear regression line for single risk allele carriers was slightly more than half of that for the nonrisk allele carriers, suggesting that G0 APOL1 may circulate at slightly higher concentration than G1 or G2 APOL1 in these individuals (Figure 1C).
Figure 1.
Population characteristics of plasma APOL1 levels in the DHS. Data on G0, G1, or G2 APOL1 (LNILNN[NY]K) or full-length APOL1 (ALDNLAR) are presented. (A) APOL1 levels as determined by the carboxy‑terminal LNI peptide or the internal ALD peptide are highly correlated. The relationship was estimated by linear regression without an intercept. (B) Plasma APOL1 levels by ethnic status in DHS. APOL1 circulates at higher levels in black participants than in other ethnic groups. WT APOL1 refers to protein as quantified by the LNILNNNYK peptide. Total APOL1 refers to protein as quantified by the internal ALD peptide. (C) G1 and G2 APOL1, as measured by the LNI peptide, circulate at levels below G0 APOL1 in either one or two risk allele carriers. (D) APOL1, as measured by the ALD peptide, is poorly correlated with overall HDL levels, but is correlated with small HDL. (E) APOL1 is more significantly correlated with plasma APOA1 compared with HDL.
Plasma APOL1 circulates across a 20‑fold concentration range, from approximately 100 to 2000 nM. Levels of total APOL1 (as defined by the internal peptide) were significantly higher (~50% elevated) in black participants compared with individuals of European (P=5.2×10–99) and Hispanic (P=4.8×10–42) origin (Figure 1B, right panel). Similar results were obtained when the analysis was restricted to WT allele homozygotes (G0/G0) (data not shown). Consistent with the correlations seen between the internal and carboxy‑terminal peptides, the levels of G1 and G2 APOL1 were lower than G0 APOL1 in heterozygotes and homozygous individuals with a more pronounced effect on the G2 variant (approximately 50% reduction compared with G0/G0; P=6.5×10–43 for G0/G2, P=5.9×10–7 for G2/G2) suggesting some change in biogenesis or elimination of this variant compared with the others (Figure 1C).
Association of APOL1 Genotypes with Baseline Characteristics and CKD Phenotypes in DHS Black Participants
As previously described, there was no association between APOL1 RRG and most baseline population characteristics, although subjects with the RRG had an elevated BP (P=0.02) and a higher prevalence of hypertension (P=0.01), consistent with an increased risk of CKD (Table 2). In addition, RRG carriers had slightly lower fasting glucose (P=0.04), and lower total cholesterol (P=0.01) and LDL cholesterol levels (P=0.03). We did not see a difference across genotypes in the total plasma HDL cholesterol levels, or HDL lipoprotein particle distribution (Table 2). APOL1 genotype did not correlate with C-reactive protein or other inflammatory markers (Table 2). Consistent with our observation of lower circulating levels of G2 APOL1 variants, RRG carriers had significantly decreased levels of total APOL1 (P<0.001, Table 2). As had been previously shown, carriers of the RRG among nondiabetic, but not diabetic individuals, had a higher median albumin-to-creatinine ratio (ACR) and a higher prevalence of microalbuminuria and GFR<60 ml/min per 1.73 m2 compared with non-RRG individuals (Table 2).23 The association was confirmed when using genotype calls based on the LC‑MS protein analysis (Supplemental Table 1).
Table 2.
Characteristics of black participants of the DHS by the number of APOL1 risk alleles (n=1736)
| Characteristic | 0 copies | 1 copy | 2 copies | P Value | P Value |
|---|---|---|---|---|---|
| (n=667) | (n=837) | (n=232) | (additive model) | (recessive model) | |
| Male, n (%) | 276 (41.4) | 351 (41.9) | 103 (44.4) | 0.49 | 0.45 |
| Mean age (yr) | 44.6 (±10.3) | 44.9 (±10.5) | 45.5 (±9.4) | 0.25 | 0.34 |
| Mean BMI (kg/m2) | 32 (±8.5) | 31.9 (±8.3) | 31.5 (±7.6) | 0.75 | 0.74 |
| Mean fasting glucose (mg/dl) | 91.5 (±11.3) | 90.8 (±11.7) | 90.1 (±12.2) | 0.04 | 0.14 |
| Mean HOMA-IR (U) | 4.7 (±4.7) | 4.6 (±4.5) | 4.4 (±6.0) | 0.06 | 0.22 |
| Diabetes, n (%) | 95 (14.2) | 122 (14.6) | 27 (11.6) | 0.17 | 0.23 |
| Mean systolic BP (mmHg) | 130.4 (±20.5) | 129.3 (±19.5) | 132.6 (±20.4) | 0.32 | 0.02 |
| Mean diastolic BP (mmHg) | 81.1 (±10.8) | 80.5 (±10.4) | 82 (±10.6) | 0.50 | 0.07 |
| Hypertension, n (%) | 192 (28.8) | 215 (25.7) | 80 (34.6) | 0.42 | <0.01 |
| Median ACR (mg/g) | |||||
| All (n=1674) | 2.9 (1.8 to 5.8) | 3.0 (1.8 to 6.1) | 4.1 (2.1 to 11.2) | 0.003 | <0.001 |
| Nondiabetic (n=1434) | 2.8 (1.7 to 5.0) | 2.9 (1.7 to 5.2) | 4.0 (2.1 to 10.8) | 0.001 | <0.001 |
| Diabetic (n=240) | 5.3 (2.4 to 24.2) | 7.0 (2.4 to 30.0) | 7.3 (2.7 to 18.6) | 0.50 | 0.80 |
| Median GFR (ml/min per 1.73 m2) | |||||
| All (n=1736) | 103.0 (88.2 to 117.4) | 101.7 (88.1 to 116.1) | 101.7 (86.1 to 115.0) | 0.18 | 0.15 |
| Nondiabetic (n=1492) | 103.8 (90.0 to 117.6) | 101.7 (88.4 to 115.5) | 101.9 (86.2 to 115.0) | 0.05 | 0.09 |
| Diabetic (n=244) | 98.1 (80.2 to 116.0) | 101.3 (82.7 to 121.7) | 101.3 (87.6 to 114.8) | 0.19 | 0.69 |
| CKD, n (%) | |||||
| All (n=1680) | 63 (9.8) | 89 (11) | 46 (20.3) | <0.001 | <0.001 |
| Nondiabetic (n=1439) | 34 (6.2) | 51 (7.4) | 39 (19.5) | <0.001 | <0.001 |
| Diabetic (n=241) | 29 (30.5) | 38 (31.9) | 7 (25.9) | 0.89 | 0.46 |
| Microalbuminuria, n (%) | |||||
| All (n=1674) | 56 (8.7) | 77 (9.5) | 39 (17.4) | 0.001 | <0.001 |
| Nondiabetic (n=1434) | 30 (5.5) | 45 (6.5) | 32 (16.2) | <0.001 | <0.001 |
| Diabetic (n=240) | 26 (27.4) | 32 (27.1) | 7 (25.9) | 0.91 | 0.71 |
| GFR<60, n (%) | |||||
| All (n=1736) | 16 (2.4) | 23 (2.7) | 15 (6.5) | 0.01 | 0.002 |
| Nondiabetic (n=1492) | 11 (1.9) | 12 (1.7) | 14 (6.8) | 0.004 | <0.001 |
| Diabetic (n=244) | 5 (5.3) | 11 (9.0) | 1 (3.7) | 0.99 | 0.42 |
| GFR<30, n (%) | 5 (0.7) | 3 (0.4) | 6 (2.6) | 0.08 | 0.003 |
| Mean total cholesterol (mg/dl) | 177.2 (±37.4) | 180.1 (±43.1) | 173 (±37.3) | 0.28 | 0.01 |
| Mean LDL cholesterol (mg/dl) | 104.8 (±34.3) | 106.2 (±38.9) | 100.7 (±35.1) | 0.27 | 0.03 |
| Mean HDL cholesterol (mg/dl) | 52.2 (±15.3) | 52.3 (±15.5) | 52.7 (±15.1) | 0.53 | 0.74 |
| Median triglycerides (mg/dl) | 85 (61 to 120) | 87 (63 to 125) | 79 (61 to 125) | 0.95 | 0.20 |
| Mean total lipoprotein HDL particles (nmol/L) | 32.8 (±6.6) | 33.3 (±6.3) | 33 (±6.4) | 0.63 | 0.46 |
| Mean lipoprotein large HDL (nmol/L) | 7.4 (±4) | 7.3 (±3.9) | 7.5 (±4.2) | 0.34 | 0.31 |
| Mean lipoprotein medium HDL (nmol/L) | 4 (±4.2) | 4 (±4) | 3.8 (±3.9) | 0.69 | 0.64 |
| Mean lipoprotein small HDL (nmol/L) | 21.4 (±5.9) | 22 (±5.6) | 21.6 (±6.3) | 0.70 | 0.30 |
| Mean HDL lipoprotein particle size (nm) | 9.1 (±0.5) | 9.1 (±0.5) | 9.1 (±0.5) | 0.30 | 0.13 |
| Median CRP (mg/L) | 3.5 (1.4 to 8.3) | 3.8 (1.4 to 8.5) | 3.5 (1.5 to 7.7) | 0.79 | 0.67 |
| Median IL-6 (pg/ml) | 16.4 (0 to 34.9) | 17.6 (0 to 37.4) | 17.8 (0 to 32.1) | 0.59 | 0.92 |
| Median IL-18 (mg/L) | 498 (332 to 748) | 505.5 (339 to 769) | 480 (338 to 752) | 0.51 | 0.75 |
| Median IL1AP (ng/ml) | 5325 (3837 to 7315) | 5475 (3896 to 7905) | 5550 (4214 to 7230) | 0.21 | 0.55 |
| Median TNFR1A (ng/ml) | 0.6 (0.4 to 0.9) | 0.6 (0.4 to 0.9) | 0.6 (0.4 to 0.8) | 0.89 | 0.51 |
| CXCL1>0 ng/ml, n (%) | 96 (15.9) | 104 (13.8) | 36 (17.5) | 0.98 | 0.31 |
| CXCL2>0 ng/ml, n (%) | 188 (31) | 248 (33) | 68 (32.9) | 0.47 | 0.81 |
| Median CXCL10 (ng/ml) | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.4) | 0.3 (0.2 to 0.5) | 0.45 | 0.36 |
| Median CD40 ligand (ng/ml) | 1.3 (0.8 to 2.4) | 1.4 (0.8 to 2.7) | 1.4 (0.8 to 2.6) | 0.81 | 0.98 |
| Mean plasma APOA1 (μM) | 63.8 (±18.4) | 64.3 (±18.3) | 64.7 (±18.6) | 0.33 | 0.65 |
| Mean plasma total APOL1 (nM) | 887.1 (±300.8) | 862.1 (±296.2) | 797.6 (±283.5) | <0.001 | <0.001 |
Data are shown as mean (±SD), median (25th to 75th percentile), or n (%), as indicated. Characteristics are compared across genotypes using linear regression for continuous data and logistic regression for categorical data. Models are adjusted for age, gender, BMI, systolic BP, and diabetes, where appropriate. Microalbuminuria, ACR≥17 mg/g in men or ≥25 mg/g in women. CKD is defined as the presence of microalbuminuria and/or GFR<60 ml/min per 1.73 m2. HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein; CXCL, C-X-C motif ligand; CD40, cluster of differentiation 40.
Association of APOL1 Levels with Baseline Characteristics and CKD Phenotypes in DHS Black Participants
We next tested whether circulating APOL1 levels correlated with renal disease risk factors, and plasma lipoprotein and cytokine levels. Because APOL1 and lipoprotein levels differed significantly across ethnic groups, we restricted this analysis to black individuals. Although APOL1 circulates as part of an HDL particle, there was no association between total plasma APOL1 and HDL cholesterol levels (Figure 1D, middle panel and Table 3). Despite the absence of an association with HDL, APOL1 levels were strongly positively correlated with plasma APOA1 levels (r=0.39, Table 3), even though HDL and APOA1 levels are strongly positively correlated with each other (Figure 1E).
Table 3.
Correlations of total APOL1 plasma levels with clinical characteristics and plasma lipoprotein and cytokine levels of DHS black participants
| Characteristic | No. subjects | Spearman rho | P Value | Partial r | P Value |
|---|---|---|---|---|---|
| Fasting glucose (mg/dl) | 1490 | 0.07 | 0.001 | 0.07 | 0.02 |
| HOMA-IR (U) | 1460 | 0.14 | <0.001 | 0.08 | 0.003 |
| Systolic BP (mmHg) | 1786 | 0.04 | 0.11 | 0.05 | 0.08 |
| Diastolic BP (mmHg) | 1786 | 0.08 | <0.001 | 0.08 | 0.003 |
| ACR | 1723 | 0.04 | 0.11 | 0.04 | <0.10 |
| GFR | 1788 | 0.00 | 0.88 | 0.01 | 0.63 |
| Total cholesterol (mg/dl) | 1788 | 0.18 | <0.001 | 0.16 | <0.001 |
| LDL cholesterol (mg/dl) | 1787 | 0.13 | <0.001 | 0.10 | <0.001 |
| HDL cholesterol (mg/dl) | 1788 | 0.03 | 0.16 | 0.04 | 0.13 |
| Triglycerides (mg/dl) | 1788 | 0.14 | <0.001 | 0.14 | <0.001 |
| Total lipoprotein HDL particles (nmol/L) | 1712 | 0.22 | <0.001 | 0.21 | <0.001 |
| Lipoprotein large HDL (nmol/L) | 1712 | −0.04 | 0.09 | −0.05 | 0.09 |
| Lipoprotein medium HDL (nmol/L) | 1712 | 0.09 | <0.001 | 0.09 | 0.001 |
| Lipoprotein small HDL (nmol/L) | 1712 | 0.17 | <0.001 | 0.18 | <0.001 |
| HDL lipoprotein particle size (nm) | 1712 | −0.11 | <0.001 | −0.12 | <0.001 |
| Plasma APOA1 (μM) | 1741 | 0.35 | <0.001 | 0.39 | <0.001 |
| CRP (mg/L) | 1782 | 0.14 | <0.001 | 0.10 | <0.001 |
| IL-6 (pg/ml) | 1664 | 0.07 | 0.004 | 0.05 | 0.04 |
| IL-18 (mg/L) | 1188 | 0.06 | 0.04 | 0.07 | 0.01 |
| IL1AP (ng/ml) | 1663 | 0.03 | 0.21 | 0.03 | 0.19 |
| TNFR1A (ng/ml) | 1660 | −0.02 | 0.49 | −0.02 | 0.37 |
| CXCL10 (ng/ml) | 1660 | 0.03 | 0.28 | 0.01 | 0.69 |
| CD40 ligand (ng/ml) | 1495 | −0.05 | 0.06 | −0.05 | 0.09 |
Correlation coefficients were calculated for all black participants with available clinical phenotypes, lipid and cytokine data, and plasma APOL1 measurements. Partial Pearson correlation coefficient (r) was determined from linear regression models, adjusted for age, gender, BMI, and the number of APOL1 risk alleles, with plasma APOL1 levels as a predictor and each characteristic as the outcome. HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein; CXCL, C-X-C motif ligand; CD40, cluster of differentiation 40.
Circulating APOL1 levels were positively associated with C-reactive protein (P<0.001, Table 3), IL‑6 (P=0.04), and IL‑18 (P=0.01), but were not associated with other inflammatory markers. There was no correlation between total plasma APOL1 levels and urine ACR or GFR (P>0.05, Table 3).
Association of Plasma APOL1 Levels with Renal Function
To determine if levels of variant-specific APOL1 were associated with CKD, we used multivariable logistic regression models adjusted for age, gender, and BP. The analyses of each protein variant (G0, G1, or G2) were restricted to individuals carrying corresponding alleles. Although total levels of the variant protein (G1+G2 APOL1) were associated with increased incidence of microalbuminuria, GFR, and total CKD in nondiabetic individuals, these associations became nonsignificant when corrected for the number of risk alleles (Table 4). Similar results were obtained when analyzing individuals with or without the RRG separately (Table 5), and in the combined black cohort (including both patients with diabetes and nonpatients with diabetes, Supplemental Tables 2 and 3). Moreover, no significant association was detected when the analysis was done using the ratio of G1/G2 to G0 APOL1 levels in single risk allele carriers (data not shown). Thus, there is no evidence for a cryptic risk of renal disease in those individuals in whom risk allele APOL1 exceeds that of G0 APOL1 in the circulation.
Table 4.
Association between circulating APOL1 levels and CKD in nondiabetic DHS black participants
| Outcome | APOL1 protein | N subjects | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| CKD | Total APOL1 | 1479 | 0.95 (0.78 to 1.15) | 0.58 | 0.99 (0.82 to 1.21) | 0.96 |
| WT APOL1 | 1239 | 1.02 (0.81 to 1.28) | 0.86 | 1.19 (0.87 to 1.62) | 0.28 | |
| G1 APOL1 | 588 | 1.22 (0.96 to 1.55) | 0.11 | 1.14 (0.85 to 1.52) | 0.38 | |
| G2 APOL1 | 402 | 1.25 (0.92 to 1.70) | 0.16 | 1.24 (0.88 to 1.75) | 0.23 | |
| G1+G2 APOL1 | 892 | 1.44 (1.19 to 1.75) | <0.001 | 1.17 (0.92 to 1.50) | 0.2 | |
| Microalbuminuria | Total APOL1 | 1474 | 0.91 (0.74 to 1.12) | 0.39 | 0.95 (0.77 to 1.18) | 0.63 |
| WT APOL1 | 1237 | 1.02 (0.79 to 1.30) | 0.89 | 1.19 (0.85 to 1.66) | 0.32 | |
| G1 APOL1 | 584 | 1.17 (0.90 to 1.52) | 0.24 | 1.07 (0.79 to 1.47) | 0.65 | |
| G2 APOL1 | 400 | 1.08 (0.76 to 1.55) | 0.66 | 1.06 (0.73 to 1.55) | 0.75 | |
| G1+G2 APOL1 | 887 | 1.32 (1.07 to 1.63) | <0.001 | 1.07 (0.82 to 1.39) | 0.64 | |
| GFR<60 ml/min/1.73 m2 | Total APOL1 | 1534 | 0.82 (0.58 to 1.17) | 0.27 | 0.88 (0.62 to 1.25) | 0.47 |
| WT APOL1 | 1287 | 0.94 (0.61 to 1.44) | 0.78 | 0.83 (0.47 to 1.48) | 0.53 | |
| G1 APOL1 | 603 | 1.10 (0.71 to 1.72) | 0.66 | 0.98 (0.58 to 1.63) | 0.92 | |
| G2 APOL1 | 415 | 1.61 (0.96 to 2.68) | 0.07 | 1.80 (0.96 to 3.38) | 0.07 | |
| G1+G2 APOL1 | 920 | 1.64 (1.20 to 2.24) | 0.002 | 1.20 (0.79 to 1.81) | 0.39 | |
The analysis includes nondiabetic DHS black participants with available data on microalbuminuria, GFR and total CKD. The analysis of allele-specific protein levels is restricted to individuals who carry at least one copy of the corresponding allele. For example, the analysis of WT APOL1 levels is confined to individuals with 0 or 1 copies of the G1 and G2 alleles. The analysis of total levels of the variant protein (G1+G2 APOL1) is restricted to individuals with at least one copy of the variant alleles (G1 or G2). Model 1 includes age, gender, and systolic BP as covariates. Model 2 is additionally adjusted for the number of copies of the risk alleles. OR are calculated per 1 SD increase in circulating APOL1 levels. N subjects, number of individuals included in each analysis. 95% CI, 95% confidence interval.
Table 5.
Association between circulating APOL1 levels and CKD in nondiabetic DHS black participants stratified by the number of APOL1 risk alleles
| Outcome | APOL1 protein | One risk allele | Two risk alleles | ||||
|---|---|---|---|---|---|---|---|
| N | OR (95% CI) | P Value | N | OR (95% CI) | P Value | ||
| CKD | Total APOL1 | 692 | 1.03 (0.77 to 1.38) | 0.83 | 199 | 0.9 (0.61 to 1.33) | 0.59 |
| WT APOL1 | 692 | 1.28 (0.84 to 1.95) | 0.25 | – | – | – | |
| G1 APOL1 | 512 | 1.19 (0.79 to 1.77) | 0.40 | 75 | 1.2 (0.78 to 1.86) | 0.41 | |
| G2 APOL1 | 370 | 1.07 (0.69 to 1.67) | 0.77 | 31 | 2.49 (0.81 to 7.66) | 0.11 | |
| G1+G2 APOL1 | 692 | 1.15 (0.75 to 1.79) | 0.52 | 199 | 1.19 (0.88 to 1.59) | 0.26 | |
| Microalbuminuria | Total APOL1 | 690 | 1.00 (0.73 to 1.37) | 0.99 | 196 | 0.8 (0.51 to 1.24) | 0.31 |
| WT APOL1 | 690 | 1.19 (0.75 to 1.90) | 0.46 | – | – | – | |
| G1 APOL1 | 509 | 1.01 (0.65 to 1.57) | 0.98 | 74 | 1.27 (0.80 to 2.01) | 0.31 | |
| G2 APOL1 | 369 | 0.84 (0.52 to 1.37) | 0.49 | 30 | 2.78 (0.64 to 12.14) | 0.17 | |
| G1+G2 APOL1 | 690 | 1.06 (0.66 to 1.69) | 0.82 | 196 | 1.09 (0.79 to 1.50) | 0.60 | |
| GFR<60 ml/min per 1.73 m2 | Total APOL1 | 715 | 1.25 (0.75 to 2.08) | 0.38 | 204 | 0.81 (0.43 to 1.52) | 0.51 |
| WT APOL1 | 715 | 1.32 (0.66 to 2.64) | 0.44 | – | – | – | |
| G1 APOL1 | 526 | 1.36 (0.70 to 2.66) | 0.37 | 76 | 0.63 (0.24 to 1.62) | 0.34 | |
| G2 APOL1 | 379 | 1.93 (0.91 to 4.11) | 0.09 | 35 | 3.99 (0.05 to 339.87) | 0.54 | |
| G1+G2 APOL1 | 715 | 1.63 (0.72 to 3.68) | 0.24 | 204 | 1.08 (0.66 to 1.77) | 0.76 | |
The analysis includes nondiabetic DHS black participants with available data on microalbuminuria, GFR and total CKD. The analysis of allele-specific protein levels is restricted to individuals who carry at least one copy of the corresponding allele. For example, the analysis of WT APOL1 levels is confined to individuals with 0 or 1 copies of the G1 and G2 alleles. The analysis of total levels of the variant protein (G1+G2 APOL1) is restricted to individuals with at least one copy of the variant alleles (G1 or G2). OR are calculated per 1 SD increase in circulating APOL1 levels. N, number of individuals included in each analysis. 95% CI, 95 % confidence interval.
Characterization of the Size Distribution and Protein Complement of APOL1‑HDL
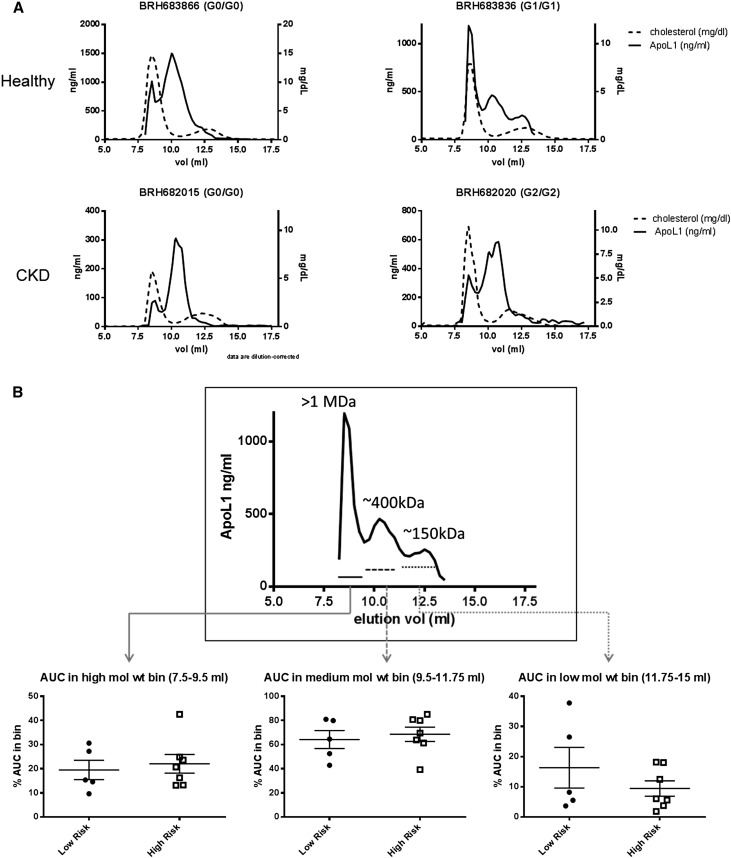
Although no association was detected between the levels of circulating APOL1 variants and renal disease, it remained possible that changes in the quality rather than quantity of the APOL1 particle contributed to renal dysfunction. Therefore, plasma and matched DNA from 59 healthy young black males and 14 subjects with ESRD was procured. Individuals were genotyped by Sanger sequencing and the size distribution of APOL1‑HDL was monitored by size-exclusion chromatography followed by quantitation of total cholesterol or APOL1 across the fractions. APOL1 migrated in three predominant molecular mass bins, at >1 MDa, 300–600 kDa, and a lower molecular mass bin of 100–200 kDa, a distribution that roughly matches what has been reported for density-gradient purified APOL1‑HDL (Figure 2A). The high mol wt APOL1 corresponds to the IgM-bound particle known as TLF‑2 and there was no evidence for any significant amount of monomeric APOL1 in plasma, consistent with previous reports.16 There was no association between any of the different size bins and APOL1 risk genotype, in either healthy subjects or those with ESRD (Figure 2B).
Figure 2.
Size distribution of APOL1 particles in EDTA plasma from subjects with or without the RRG, with or without ESRD as measured by size-exclusion chromatography on a Superdex 200 column. (A) Representative chromatograms of total cholesterol (dashed) or APOL1 (solid) in neat plasma separated by size-exclusion chromatography. The cholesterol peak eluting at 8 ml represents LDL and VLDL eluting in the void volume of the column, the heterogeneous cholesterol peak between 12 and 13 ml represents HDL. A fraction of APOL1 consistently elutes with VLDL/LDL and represents IgM-bound APOL1. The remaining APOL1 elutes largely between the HDL peak and the void volume suggesting a mol wt greater than the bulk of the HDL pool. Traces from healthy subjects and subjects with ESRD with or without the RRG are shown. (B) APOL1 levels in the normalized high, medium and low mol wt pools in five subjects without the RRG or seven subjects with the RRG are shown. Differences between low- and high-risk genotypes were tested by t test. No statistically significant changes (P<0.05) were noted.
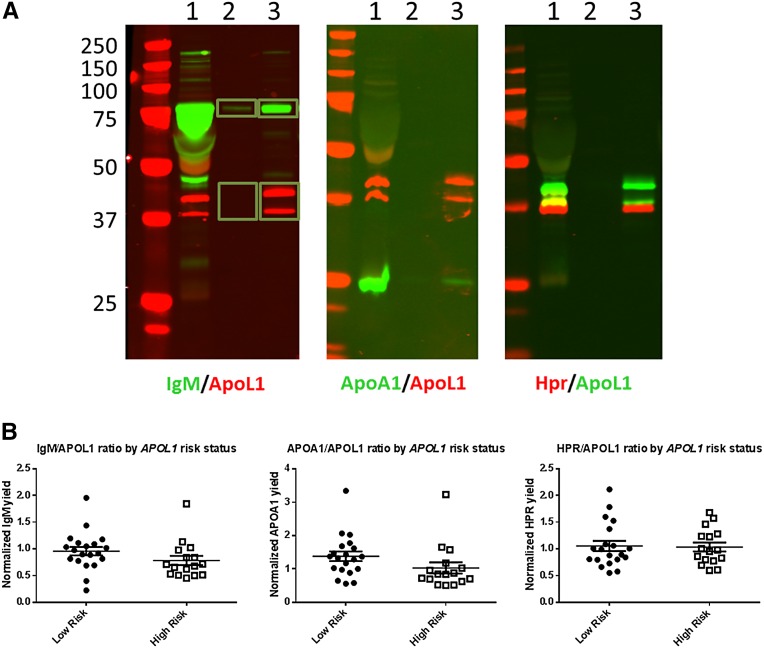
The APOL1‑HDL particle contains three known protein components, including APOA1, HPR, and IgM.16,24 To determine whether there were any changes in the protein complement of APOL1‑HDL, intact APOL1‑HDL was immunopurified from plasma, and the relative amounts of the known APOL1‑HDL proteins were then assessed using semiquantitative immunoblotting. Again, we found no association between the APOL1-normalized amounts of APOA1, HPR, or IgM and RRG in either healthy subjects or those with ESRD (Figure 3). These results are comparable with those reported recently by Weckerle et al., although in that study, an RRG associated decrease in HPR association was noted specifically in the IgM containing APOL1 complex.25
Figure 3.
Analysis of the protein complement of APOL1‑HDL in subjects with or without the RRG. (A) Representative images of semiquantitative immunoblotting to determine the relative amounts of APOL1‑HDL proteins within the particle. APOL1‑HDL was immunopurified from EDTA plasma under detergent-free conditions, and proteins were eluted with Laemmli buffer and separated via SDS‑PAGE. One percent of input (lane 1) and 10% of immunopurified material from control (lane 2) or APOL1 (lane 3) immunoprecipitations was loaded in each lane and blotted with antibodies specific for the proteins noted under each image. Shown is an overlay of signal from the 680 nm channel (rabbit antibodies) and the 800 nm channel (mouse antibodies). Yellow indicates areas of overlap in the overlay. Signal was quantified separately for regions of interest for each channel. The yield of APOA1, HPR, and IgM were determined relative to the input amount and normalized for the yield of APOL1 calculated for each image. (B) APOL1‑HDL was purified from EDTA plasma using from 20 subjects without and 16 subjects without the RRG and the relative levels of known APOL1‑HDL associated proteins were determined by semiquantitative immunoblotting, as detailed above. Differences between low- and high-risk genotypes were tested by t test. No statistically significant changes (P<0.05) in the APOL1 normalized levels of IgM, APOA1, or HPR were noted.
Exome-Wide Association Analysis
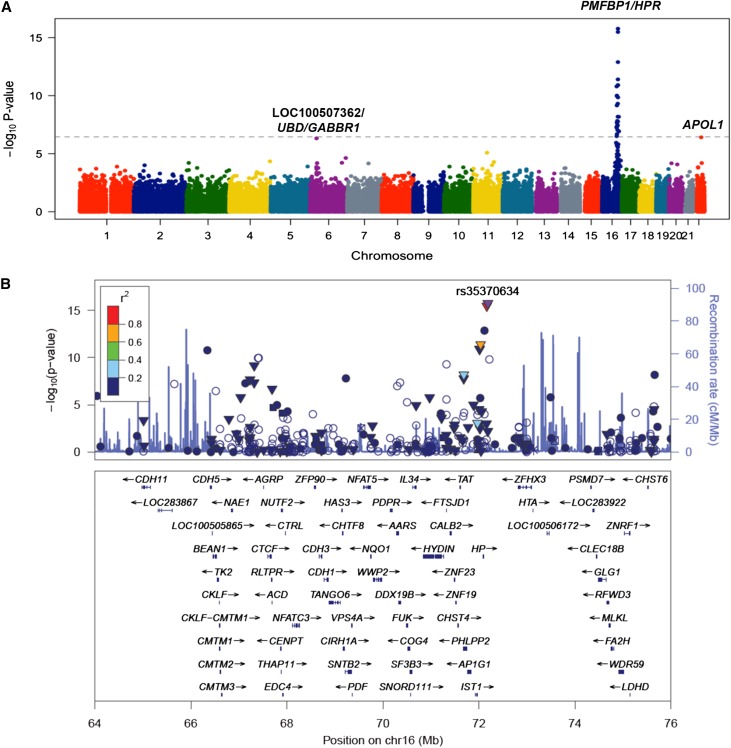
To determine if levels of circulating APOL1 were influenced by genetic variation in APOL1 or other genomic loci, we performed an association analysis using variants on the Illumina HumanExome chip. A total of 144782 variants were tested for association with APOL1 levels in 3354 DHS participants with available genotypes and APOL1 data. Several variants on chromosome 16, in a 10 Mb locus containing the HPR gene reached exome-wide significance (lead single nucleotide polymorphism [SNP]: PMFBP1 E48K rs35370634, P=2.1×10–16). In addition, two variants approached our exome-wide significance threshold: a coding variant in APOL1 (G96R, rs41297245, P=4.2×10–7) and an intergenic SNP, located approximately 22 kb downstream of the ubiquitin D (UBD) gene on chromosome 6 (rs1233421, P=4.9×10–7).
Fine-mapping analysis of the chromosome 16 locus (position between 66 and 76 Mb) revealed several independent SNPs (defined as R2<0.2 with the index SNP) associated with APOL1 levels (Figure 4, Supplemental Table 4). Specifically, an intronic variant in HPR (rs2000999) previously shown to be associated with lipid26,27 and haptoglobin levels,28 remained independently associated with circulating APOL1 levels, after conditioning on the index SNP (rs35370634) (P=3.8×10–14). In addition, three other SNPs in the HPR locus remained associated with APOL1 levels in joint analysis with P<3.5×10–7. Together, these five variants on chromosome 16 (with P<3.5×10–7 in joint analysis), APOL1 G96R rs41297245, and rs1233421 explained 8.5% of variance in APOL1 levels, after adjustments for demographic variables and APOL1 G1 and G2 genotypes (11.8% of variance in black participants).
Figure 4.
Exome-wide association analysis for circulating APOL1 levels in DHS. (A) A Manhattan plot showing highly significant genome-wide association between the HPR locus on chromosome 16 and APOL1 levels. Each dot represents a SNP plotted against its chromosomal position on the x-axis and the strength of the association (–log10 P-value) on the vertical axis. The dashed line denotes the Bonferroni-corrected exome-wide significance threshold. (B) Regional plot of association with plasma total APOL1 levels at the HPR locus. Each dot represents a SNP plotted against its chromosomal position (using hg19 coordinates). The vertical axis shows –log10 P-values from single-SNP analysis for association with APOL1 levels. The most significant SNP at the locus is shown in purple color. The remaining SNPs are color-coded according to their linkage disequilibrium with the lead SNP as indicated by the color key. The strength of linkage disequilibrium is measured by pairwise R2 values based on the DHS data. Estimated recombination rates (from HapMap) are shown by the blue line, with spikes indicating locations of frequent recombination. Genes located in the region and the direction of transcription are noted below the plots based on the data from UCSC genome browser (www.genome.ucsc.edu). (▼) Nonsynonymous variants, (▪) coding variants, and (•) SNPs with no functional annotation and (○ ) noncoding variants. The plot was generated using LocusZoom software.46
We next tested whether the variants affecting circulating APOL1 levels were associated with kidney disease. None of the variants was associated with measures of renal function, or total CKD, in the combined DHS population (Supplemental Table 5). However, the HPR rs2000999 variant was associated with GFR<60 ml/min per 1.73 m2 (OR=1.9; P<0.01 in the combined DHS population), although the association was present only in black individuals (OR=2.3; P<0.01) and not in the other ethnic groups. To test whether these associations represent the causal effect of circulating APOL1 levels on kidney disease, we further included plasma APOL1 levels as a covariate in the model. The association of the HPR rs2000999 variant with the prevalence of GFR<60 ml/min per 1.73 m2 in black participants and ACR levels in white participants remained significant in this analysis (P=0.03 and P=0.02, respectively), suggesting that the effect of genotype on kidney function is independent of its effect on the amount of APOL1 in the circulation.
Discussion
The major finding of this study is the lack of an association between the levels or characteristics of WT (G0) or risk allele (G1/G2) APOL1‑HDL in the circulation and CKD. These findings validate and extend the results of a previous study of APOL1 levels in HIV-infected CKD patients and strongly suggest that APOL1 levels are unlikely to be useful risk stratification markers in either the diseased or general at-risk populations.21
These results also add to a number of other studies that circumstantially implicate renally expressed APOL1 as the primary source of the damage in individuals with the RRG. These lines of evidence include transplant data suggesting that allograft survival tracks with donor rather than recipient genotype29–31; evidence from studies in human cells and biopsy data demonstrating that APOL1 is expressed in the podocyte and may have detrimental effects in that cell type7,12,20,32,33 and cell biologic and epidemiologic studies demonstrating that APOL1 is induced by innate immune effectors in podocytes, which may account for the variable penetrance of the RRG seen in different forms of renal disease.7,34,35 Although it is difficult to rule out the potential effects of transient changes in circulating APOL1 levels (i.e., due to infection) or a threshold effect (the damage occurs at levels above or below those monitored), the lack of an association between plasma APOL1 and prevalent renal disease in the DHS is in line with these previous observations.
Despite the lack of association between circulating APOL1 and renal disease, several interesting population characteristics were noted. APOL1 levels tend to be higher in black participants compared with those of other ethnic backgrounds, which may reflect positive selection for the innate immune effector functionality of APOL1 in the African population. Also, contrary to the findings of Bruggeman et al., levels of G1 and G2 APOL1 in the circulation were significantly lower than G0 APOL1 in both heterozygous and homozygous individuals.21
We also identified a number of genetic variants that were associated with APOL1 levels in trans. Many of these variants cluster in the HPR locus, another key component of APOL1‑HDL. These associations suggest that there may be a functional link between HPR and APOL1‑HDL particle biogenesis. Also the identification of a SNP rs1233421, which correlated with plasma APOL1 levels with near exome-wide significance, is of interest because it is located just approximately 22 kb downstream of the UBD gene (Supplemental Figure 1). UBD is a ubiquitin-like protein involved in the targeted degradation of cellular proteins, and UBD transcripts were recently shown to be upregulated in patients with APOL1-associated glomerular disease in the NEPTUNE cohort.36
The strengths of this study are the high-quality analytical methods used, which enabled the testing of a number of hypotheses regarding APOL1 levels and APOL1‑HDL–associated proteins and renal disease, including the association of specific variant proteins with CKD. This study was well powered and was done in a cohort with pre-established association between APOL1 genotype and risk of CKD. The wide range of APOL1 concentrations in the study subjects further enabled a robust test of the hypothesis that levels of APOL1 associate with CKD. Finally, this is the first study to examine in detail the size distribution and protein complement of APOL1‑HDL in risk allele carriers.
Some limitations should also be noted. First, this study was done in a population-based cohort with a relatively low level of prevalent renal dysfunction. Results might be different in a CKD disease cohort stratified by risk genotype. Second, we have limited data on the day-to-day variability in APOL1 levels in the plasma, which may influence our ability to detect associations with renal disease. Finally, we were unable to test the prognostic utility of plasma APOL1 levels due to the limited number of hard renal event in DHS. Nevertheless, the DHS likely reflects the general at-risk population more accurately than case–control cohorts and thus it remains likely that APOL1 levels have little utility in predicting renal risk in the general population. It may also be possible to revisit the prognostic utility of plasma APOL1 in the future as renal events accrue in DHS.
An association between the APOL1 RRG and cardiovascular (CV) risk in the Jackson Heart studies has recently been demonstrated.37 It was speculated that this may reflect dysfunction in the APOL1‑HDL particle. Although no obvious genotype associated changes in APOL1‑HDL size and protein complement were found in our study, and no association with CV disease was seen in this population (Supplemental Table 6), a dissociation between the effect of circulating APOL1 on renal and CV outcomes would be highly interesting and further strengthen the hypothesis that kidney expressed APOL1 is causal for the renal effects. Although the size and protein complement of APOL1‑HDL was examined, a more comprehensive evaluation of the functionality of the HDL particles was not done and is beyond the scope of this work. Further work in this area is clearly warranted and may be facilitated by investigation of the genetic drivers of plasma APOL1 identified in this report.
In sum, this study demonstrates that levels of total or mutant APOL1 in the circulation are unlikely to be useful risk stratification markers in individuals with the APOL1 RRG. This data combined with the lack of association between genetic drivers of APOL1 levels and renal disease, as well as the lack of obvious differences in the protein complement of risk allele APOL1 particles provides additional evidence in favor of models in which renally expressed APOL1 is the primary driver of kidney disease. Finally, we demonstrate that there are no obvious differences in the composition of the APOL1‑HDL particle, but do identify significant differences in the levels of circulating G2 APOL1 that may reflect underlying functional differences in the clearance or biogenesis of this variant.
Concise Methods
Study Population
The DHS is a multiethnic population-based probability sample of Dallas County residents (ages 18–65 years), with intentional oversampling of black participants. The sampling design and recruitment procedures have been previously described.38 Briefly, 3551 individuals (52% black, self-identified as [non-Hispanic] black; 29% individuals of mixed European descent, self-identified as [non-Hispanic] white; 17% Hispanics, self-identified as Hispanic; and 2% other ethnicities) completed a detailed survey, and underwent a health examination that involved measurement of BP, anthropometry, blood, and urine sample collection, and imaging studies. The ethnicity was self-assigned according to the US Census categories. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, and all subjects provided written informed consent.
Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Five BP measurements were taken using an automatic oscillometric device (series no. 52,000; Welch Allyn, Arden, NC) while subjects were in seated position. The average of the third through fifth measurements was used in the analysis. Hypertension was defined as systolic BP≥140 mmHg, diastolic BP≥90 mmHg, or a self-reported diagnosis or treatment for hypertension. Diabetes was defined as a self-reported physician diagnosis of diabetes, use of insulin or glucose-lowering medication, or a fasting glucose value of ≥126 mg/dl. Plasma levels of total cholesterol, HDL cholesterol, and triglycerides were determined using standard enzymatic assays. LDL cholesterol concentrations were estimated using the Friedewald equation.39
Measures of Kidney Function
Urine albumin and creatinine were measured from the first morning void urine samples, and ACR was calculated in milligrams per gram for each subject. Urine albumin was determined by the turbidimetric method. Serum and urine creatinine levels were measured using an alkaline picrate assay. GFR in ml/min per 1.73 m2 of body surface area was determined using the six-variable modification of diet in renal disease equations40:
 |
CKD was defined as the presence of microalbuminuria (ACR≥17 mg/g in men and ≥25 mg/g in women)41 and a GFR≥60 ml/min per 1.73 m2 body surface area (stages 1–2), or a GFR<60 ml/min per 1.73 m2 body surface area (stages 3–5), according to the National Kidney Foundation Guidelines.42,43
Genotyping
Genomic DNA was extracted from circulating leukocytes. A total of 3544 DHS participants were genotyped for the APOL1 rs73885319 (G1) and rs71785313 (G2) polymorphisms using a predesigned TaqMan AD assay (Applied Biosystems), as previously described.23 Genotype success rates were 98.3% for rs73885319 and 99.2% for rs71785313.
In addition, 3454 of the DHS participants included in this analysis were also genotyped using the Illumina Infinium HumanExome BeadChip, which captured a total of 247870 markers, including protein-altering variants (>90%), disease-associated variants from previously published genome-wide association studies, ancestry-informative markers, and other variants. Genotype calling and quality control procedures have been previously published.44
Lipoprotein Particle Analysis
Plasma lipoprotein particle profiles were measured by nuclear magnetic resonance spectroscopy using the LipoProfile-II algorithm at LipoScience, Inc. (Raleigh, NC).
Variant-specific Measurement of APOL1 in Human Plasma
APOL1 was measured in stored plasma samples from 3551 DHS subjects using an MRM‑LC–MS assay published previously using table isotope-labeled peptides as internal standards.22 This assay was also used to generate LC-MS based genotype calls. Measurements were not available for some participants due to no sample (n=96) or ID mismatch (n=3). After exclusions, APOL1 measurements were available for 3450 DHS subjects.
Procurement of Plasma for Analysis of APOL1‑HDL
Human EDTA plasma and corresponding DNA samples were obtained from Bioreclamation (Hicksville, NY). Informed consent was obtained from each of the donors for the analysis of DNA and plasma samples. DNA was prepared using the PAXgene Blood DNA system. Blood was collected into PAXgene Blood DNA tubes (Qiagen #761115), shipped at ambient temperature, and stored at 4°C until processing. DNA was prepared from whole blood using the PAXgene DNA kit (Qiagen #761133) within 2 weeks of receipt, according to the manufacturer’s directions. DNA from exon 5 was amplified using Forward and Reverse primers: APOL1‑Ex5–2F 5′AGCCAGAGCCAATCTTCAGTC3′ and APOL1‑Ex5–2R 5′GCAGATCAAGAGGTCAGGAGA3′. DNA was sequenced using the forward primer above and the reverse primer APOL1‑Ex5SeqR 5′GTTTGCATTTTGTCCTGGCCCC3′.
Size-Exclusion Chromatography of Plasma
Aliquots of frozen EDTA plasma from subjects with or without the RRG were thawed on ice. Protease and lipase inhibitors were added to 100 μl of plasma and clarified by centrifugation through an ultracentrifugation unit (Microcon, 10K MWCO). Samples (50 ul) were loaded onto a Superdex 200 10/300 column (GE Lifescience) attached to an Agilent 1100 HPLC system and fractionated at 0.4 ml/min using 1× PBS–1 mM EDTA as the mobile phase. Fractions (250 μl) were collected from 5 to 24 ml (76 fractions) and were analyzed immediately for total cholesterol (Wako Cholesterol E kit, #439–17501) or APOL1 using an in-house sandwich ELISA assay.
APOL1 ELISA
APOL1 concentration was determined through use of an electrochemiluminescence immunoassay on the Meso Scale Discovery Assay (MSD) Platform that is capable of measuring the circulating human APOL1 in the peripheral blood. The capture reagent was a rabbit monoclonal anti-APOL1 antibody, (cat# ab108315; Abcam, Inc.), rabbit anti-hAPOL1, that was conjugated to biotin and immobilized onto the surface a MSD streptavidin plate. The detection antibody was a goat anti-hAPOL1 antibody (cat# SC-18759; Santa Cruz Biotechnology), that was labeled with Ruthenium Tris‑bipyridine. The antigen–antibody complex was captured by streptavidin, which was coated on the MSD plate surface. Plates were processed into the MSD MESO SECTOR S 600 plate reader and the electrochemiluminescence signal produced was verified as directly proportional to the concentration of human APOL1 in the sample using LC‑MS–calibrated HDL as a reference. The assay was verified as fit-for-purpose for use with human plasma diluted in the size-exclusion chromatography mobile phase (PBS–1 mM EDTA) through spike-in recovery experiments.
Analysis of APOL1‑HDL Particles
Total rabbit IgG or anti-APOL1 antibody (cat# ab108315; Abcam, Inc.) was directly coupled to agarose beads using the Pierce direct immunoprecipitation kit (#26148) at 100 μg/200 μl beads slurry. One hundred microliters of EDTA plasma from subjects with or without the RRG was precleared with 100 μl of rabbit IgG beads in a microcolumn supplied in the kit for 2 hours with rotation at 4°C. Precleared plasma (45 ul) was then added to a fresh microcolumn containing 40 μl of a rabbit IgG beads or anti-APOL1 beads and rotated for another 2 hours at 4°C. Residual precleared plasma was kept as the input reference. Beads were washed five times with PBS–EDTA followed by two sequential elutions at 25 and 75 μl. Elution buffer samples were stored at –70°C prior to subsequent analysis by immunoblotting.
Semi-Quantitative Immunoblotting
Either 0.5 μl of precleared input sample or 10 μl of immunopurified APOL1 particles in a final volume of 15 μl 1× Laemmli sample buffer was run on 10% polyacrylamide gels in 1× 3‑(N-morpholono)propanesulfonic acid running buffer (Novex Bis-Tris). Positive reference samples were included on each gel for normalization. Proteins were transferred to nitrocellulose using standard wet electroblotting techniques. Membranes were blocked for 30 minutes in Odyssey blocking buffer (LI-COR) followed by incubation with anti-APOL1, anti-HPR, anti-APOA1, and anti-IgM antibodies in Odyssey blocking buffer +0.1% Tween‑20 overnight at 4°C. Membranes were washed in PBS–0.1% Tween‑20 and incubated with secondary antibody for 30 minutes. After washing, membranes were imaged using an Odyssey infrared imager and quantified using manufacturers software. Background corrected signal was used to calculate the relative yield of each protein, with subsequent normalization to APOL1 yield and positive controls on each membrane.
Statistical Analyses
Characteristics of DHS participants are summarized as mean (±SD) or median (25th to 75th percentile) for continuous data, and n (%) for categorical data. Baseline characteristics were compared across ethnic groups using linear regression for continuous data and logistic regression for categorical data. The relationship between health outcomes and APOL1 genotypes and circulating APOL1 levels was assessed using linear and logistic regression for continuous and categorical outcomes, respectively. All models were adjusted for age, gender, BMI, systolic BP, and diabetes, where appropriate. The relationship of APOL1 levels with CKD phenotypes and plasma lipoprotein and cytokine levels was assessed using the Spearman rank correlation coefficient. To account for the effect of confounders, we computed partial Pearson correlation coefficients (r) based on linear regression models adjusted for age, gender, BMI, and the number of APOL1 risk alleles, with plasma APOL1 levels as a predictor and a particular characteristic as the outcome. A logarithm or an inverse normal transformation was applied to variables with skewed distributions (e.g., BMI, BP, and plasma lipoprotein and cytokine levels) to achieve approximate normality of the residuals. P<0.05 was considered statistically significant. All statistical analyses were performed using R statistical programming language (http://www.R-project.org).
Exome-Wide Association Analysis
The association between circulating APOL1 levels and Exome Chip genotypes was tested using linear regression, assuming an additive genetic model (with genotypes coded as 0, 1, or 2 for common allele homozygotes, heterozygotes, and rare allele homozygotes, respectively) and adjusted for age, gender, and 10 leading principal components of ancestry, estimated using EIGENSTRAT45 (software version 4.2). For very rare variants (≤8 carriers), the analysis was performed using the Wilcoxon rank-sum test, without covariate adjustment. An inverse normal transformation was applied to circulating APOL1 levels to achieve approximate normality of the residuals. Exome-wide significance level was set at P<3.5×10–7, correcting for the number of variants tested (n=144 782). To determine all independently associated variants at each locus reaching exome-wide significance, we performed conditional and joint analysis using a stepwise selection procedure as follows. First, the most significant SNP with P<3.5×10–7 was included in the model, and all the remaining SNPs were tested for association with APOL1 levels conditional on the first SNP. Next, the most significant SNP with conditional P-value <3.5×10–7 was added to the model and the remaining SNPs were tested for association conditional on the selected SNPs, whereas SNPs with P>0.05 were dropped from the model. The procedure was repeated until no more variants reached a significance level of <3.5×10–7 conditional on the previously selected SNPs.
Disclosures
All authors except J.K. were employees of Merck and Company, Inc. at the time the work was done.
Supplementary Material
Acknowledgments
D.H.S. was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105.
The authors wish to thank Jonathan Cohen, Sevgi Gurkan, and Lijun Ma for helpful discussions and comments on the manuscript. The authors also wish to thank PharmaCadence for help in the measurement of plasma APOL1 in the DHS.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015101121/-/DCSupplemental.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR: Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 329: 841–845, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K: Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 128: 345–350, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, Birmingham DJ, Hebert LA, Hicks PJ, Segal MS, Edberg JC, Brown EE, Alarcón GS, Costenbader KH, Comeau ME, Criswell LA, Harley JB, James JA, Kamen DL, Lim SS, Merrill JT, Sivils KL, Niewold TB, Patel NM, Petri M, Ramsey-Goldman R, Reveille JD, Salmon JE, Tsao BP, Gibson KL, Byers JR, Vinnikova AK, Lea JP, Julian BA, Kimberly RP Lupus Nephritis–End‐Stage Renal Disease Consortium : End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 66: 390–396, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, Friedman D, Briggs W, Dart R, Korbet S, Mokrzycki MH, Kimmel PL, Limou S, Ahuja TS, Berns JS, Fryc J, Simon EE, Smith MC, Trachtman H, Michel DM, Schelling JR, Vlahov D, Pollak M, Winkler CA: APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 22: 2129–2137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Larsen CP, Beggs ML, Saeed M, Walker PD: Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 24: 722–725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB SK Investigators : Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int 83: 114–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 87: 332–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnstone DB, Shegokar V, Nihalani D, Rathore YS, Mallik L, Ashish, Zare V, Ikizler HO, Powar R, Holzman LB: APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PLoS One 7: e51546, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanhollebeke B, Truc P, Poelvoorde P, Pays A, Joshi PP, Katti R, Jannin JG, Pays E: Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N Engl J Med 355: 2752–2756, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Smith EE, Malik HS: The apolipoprotein L family of programmed cell death and immunity genes rapidly evolved in primates at discrete sites of host-pathogen interactions. Genome Res 19: 850–858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Limou S, Nelson GW, Lecordier L, An P, O’HUigin CS, David VA, Binns-Roemer EA, Guiblet WM, Oleksyk TK, Pays E, Kopp JB, Winkler CA: Sequencing rare and common APOL1 coding variants to determine kidney disease risk. Kidney Int 88: 754–763, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan X, Jhaveri A, Cheng K, Wen H, Saleem MA, Mathieson PW, Mikulak J, Aviram S, Malhotra A, Skorecki K, Singhal PC: APOL1 risk variants enhance podocyte necrosis through compromising lysosomal membrane permeability. Am J Physiol Renal Physiol 307: F326–F336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson R, Finkelstein A: Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: relevance to trypanosome lysis. Proc Natl Acad Sci U S A 112: 2894–2899, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olabisi OA, Zhang JY, VerPlank L, Zahler N, DiBartolo S 3rd, Heneghan JF, Schlondorff JS, Suh JH, Yan P, Alper SL, Friedman DJ, Pollak MR: APOL1 kidney disease risk variants cause cytotoxicity by depleting cellular potassium and inducing stress-activated protein kinases. Proc Natl Acad Sci U S A 113: 830–837, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pays E, Vanhollebeke B: Human innate immunity against African trypanosomes. Curr Opin Immunol 21: 493–498, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Raper J, Fung R, Ghiso J, Nussenzweig V, Tomlinson S: Characterization of a novel trypanosome lytic factor from human serum. Infect Immun 67: 1910–1916, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammad SM, Stefansson S, Twal WO, Drake CJ, Fleming P, Remaley A, Brewer HB Jr, Argraves WS: Cubilin, the endocytic receptor for intrinsic factor-vitamin B(12) complex, mediates high-density lipoprotein holoparticle endocytosis. Proc Natl Acad Sci U S A 96: 10158–10163, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozyraki R, Fyfe J, Kristiansen M, Gerdes C, Jacobsen C, Cui S, Christensen EI, Aminoff M, de la Chapelle A, Krahe R, Verroust PJ, Moestrup SK: The intrinsic factor-vitamin B12 receptor, cubilin, is a high-affinity apolipoprotein A-I receptor facilitating endocytosis of high-density lipoprotein. Nat Med 5: 656–661, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanović D, Holers VM, Thurman JM: IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol 24: 393–406, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI: Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol 26: 339–348, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruggeman LA, O’Toole JF, Ross MD, Madhavan SM, Smurzynski M, Wu K, Bosch RJ, Gupta S, Pollak MR, Sedor JR, Kalayjian RC: Plasma apolipoprotein L1 levels do not correlate with CKD. J Am Soc Nephrol 25: 634–644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou H, Hoek M, Yi P, Rohm RJ, Mahsut A, Brown P, Saunders J, Chmielowski RA, Ren N, Shuster D, Southwick K, Ayanoglu G, Gorman D, Laface D, Santino S, Conway J, Liu Z, Cully D, Cleary M, Roddy TP, Blom D: Rapid detection and quantification of apolipoprotein L1 genetic variants and total levels in plasma by ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 27: 2639–2647, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR: Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 22: 2098–2105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanhollebeke B, Pays E: The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol Microbiol 76: 806–814, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Weckerle A, Snipes JA, Cheng D, Gebre AK, Reisz JA, Murea M, Shelness GS, Hawkins GA, Furdui CM, Freedman BI, Parks JS, Ma L: Characterization of circulating APOL1 protein complexes in African Americans. J Lipid Res 57: 120–130, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O’Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S: Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466: 707–713, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willer CJ, Schmidt EM, Sengupta S, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Do R, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O’Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Döring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stancáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Kathiresan S, Mohlke KL, Ingelsson E, Abecasis GR Global Lipids Genetics Consortium : Discovery and refinement of loci associated with lipid levels. Nat Genet 45: 1274–1283, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Froguel P, Ndiaye NC, Bonnefond A, Bouatia-Naji N, Dechaume A, Siest G, Herbeth B, Falchi M, Bottolo L, Guéant-Rodriguez RM, Lecoeur C, Langlois MR, Labrune Y, Ruokonen A, El Shamieh S, Stathopoulou MG, Morandi A, Maffeis C, Meyre D, Delanghe JR, Jacobson P, Sjöström L, Carlsson LM, Walley A, Elliott P, Jarvelin MR, Dedoussis GV, Visvikis-Siest S: A genome-wide association study identifies rs2000999 as a strong genetic determinant of circulating haptoglobin levels. PLoS One 7: e32327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman BI, Julian BA, Pastan SO, Israni AK, Schladt D, Gautreaux MD, Hauptfeld V, Bray RA, Gebel HM, Kirk AD, Gaston RS, Rogers J, Farney AC, Orlando G, Stratta RJ, Mohan S, Ma L, Langefeld CD, Hicks PJ, Palmer ND, Adams PL, Palanisamy A, Reeves-Daniel AM, Divers J: Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 15: 1615–1622, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, Langefeld CD, Bowden DW, Hicks PJ, Stratta RJ, Lin JJ, Kiger DF, Gautreaux MD, Divers J, Freedman BI: The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant 11: 1025–1030, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, Conte S, Genovese G, Ross MD, Friedman DJ, Gaston R, Milford E, Pollak MR, Chandraker A: The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant 12: 1924–1928, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khatua, AK, Cheatham, AM, Kruzel, ED, Singhal, PC, Skorecki, K & Popik, W: Exon 4 Encoded Sequence is a Major Determinant of Cytotoxicity of Apolipoprotein L1. Am J Physiol Cell Physiol 309: C22–C37, 2015 [DOI] [PMC free article] [PubMed]
- 33.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 22: 2119–2128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horrevoets AJ, Fontijn RD, van Zonneveld AJ, de Vries CJ, ten Cate JW, Pannekoek H: Vascular endothelial genes that are responsive to tumor necrosis factor-alpha in vitro are expressed in atherosclerotic lesions, including inhibitor of apoptosis protein-1, stannin, and two novel genes. Blood 93: 3418–3431, 1999 [PubMed] [Google Scholar]
- 35.Taylor HE, Khatua AK, Popik W: The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol 88: 592–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sampson MG, Robertson CC, Martini S, Mariani LH, Lemley KV, Gillies CE, Otto EA, Kopp JB, Randolph A, Vega-Warner V, Eichinger F, Nair V, Gipson DS, Cattran DC, Johnstone DB, O’Toole JF, Bagnasco SM, Song PX, Barisoni L, Troost JP, Kretzler M, Sedor JR and the Nephrotic Syndrome Study Network : Integrative genomics identifies novel associations with APOL1 risk genotypes in black NEPTUNE subjects [published online ahead of print July 6, 2015]. J Am Soc Nephrol pii: ASN.2014111131, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, Depalma SR, Gupta N, Gabriel SB, Taylor HA Jr, Fox ER, Newton-Cheh C, Kathiresan S, Hirschhorn JN, Altshuler DM, Pollak MR, Wilson JG, Seidman JG, Seidman C: Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res 114: 845–850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH Dallas Heart Study Investigators : The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93: 1473–1480, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 40.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Mattix HJ, Hsu CY, Shaykevich S, Curhan G: Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol 13: 1034–1039, 2002 [DOI] [PubMed] [Google Scholar]
- 42.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 43.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC: Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet 46: 352–356, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D: Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38: 904–909, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ: LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26: 2336–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.