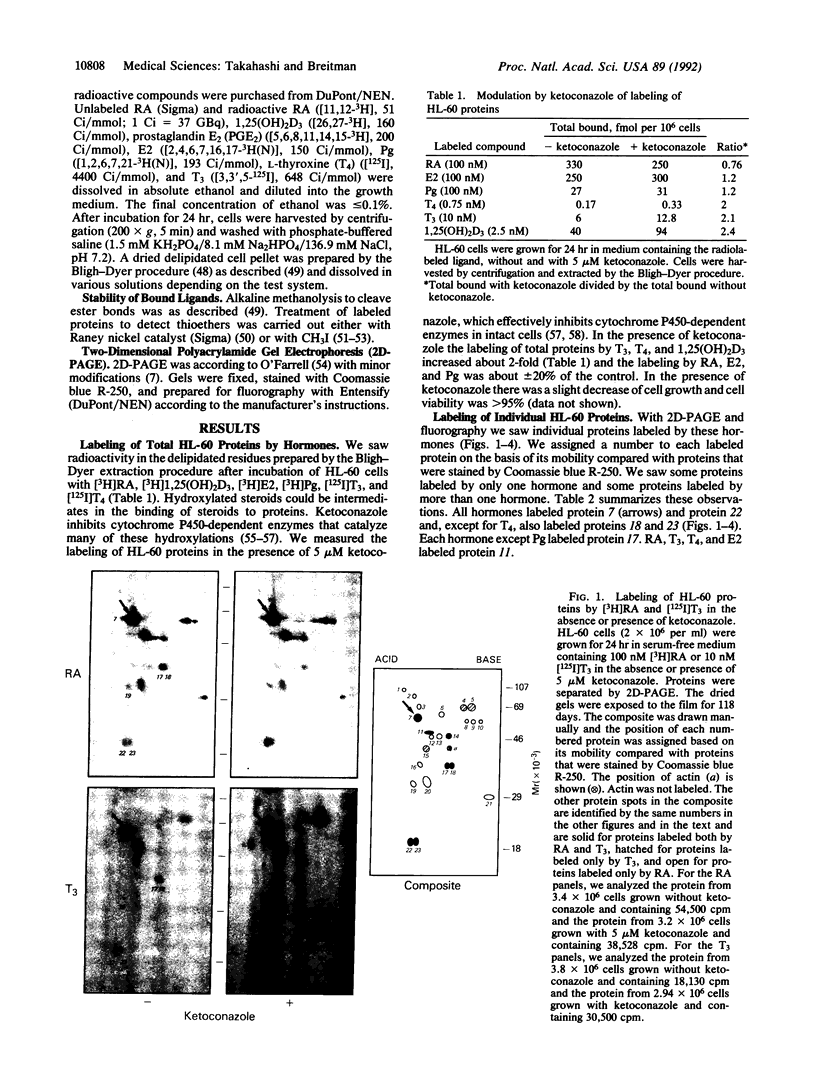
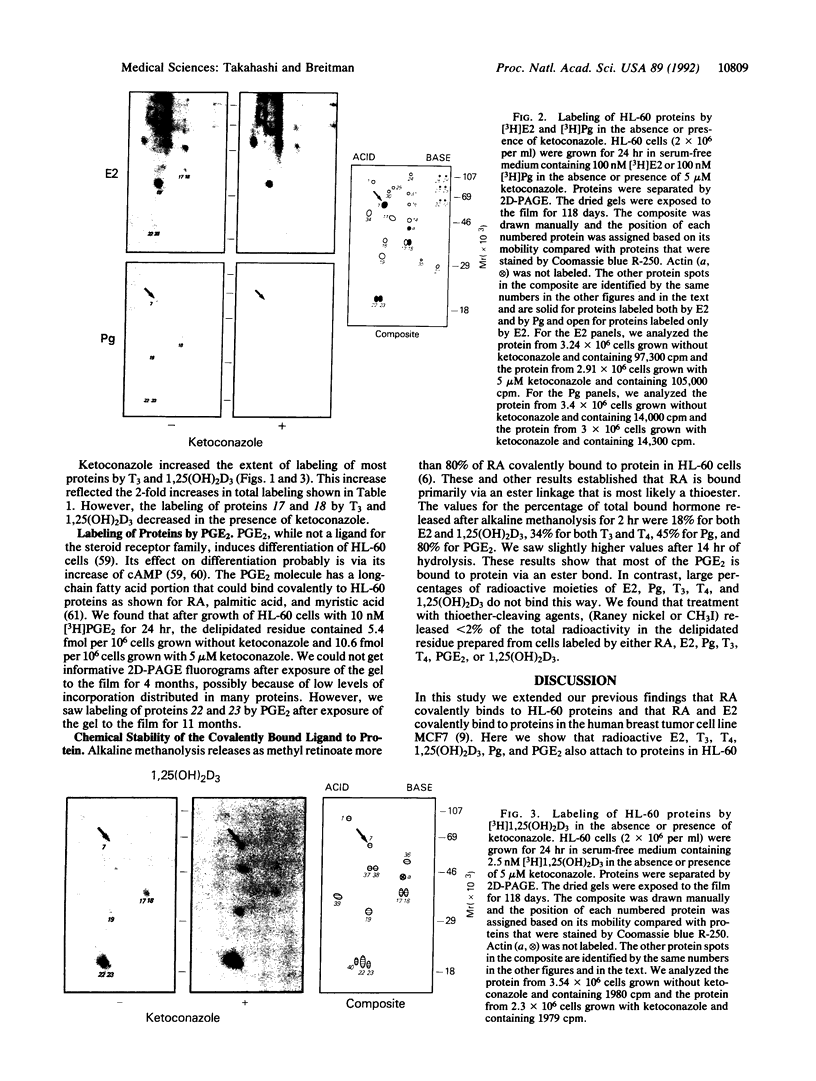
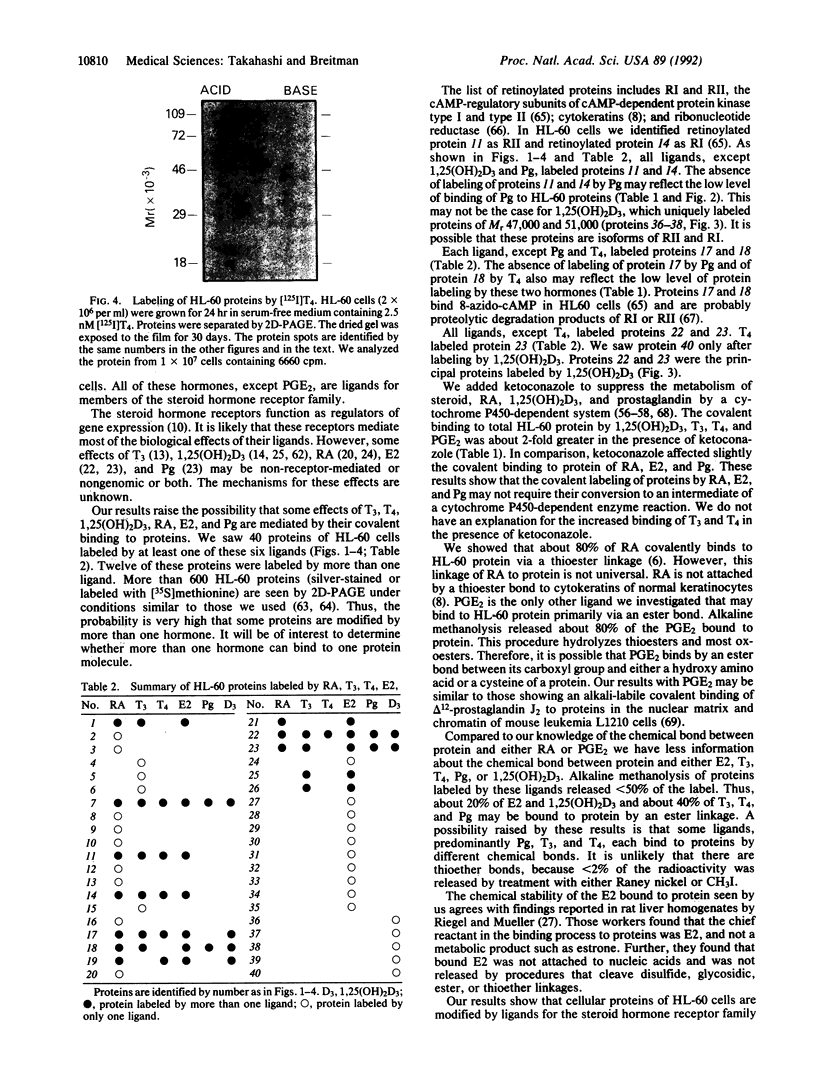
Abstract
Retinoylation, acylation with retinoic acid (RA), is a covalent modification of proteins occurring in a variety of eukaryotic cell lines. In this study, we found that proteins in HL-60 cells were labeled by 17 beta-[3H]estradiol (E2), [3H]progesterone (Pg), 1 alpha,25-dihydroxy[3H]vitamin D3 [1,25(OH)2D3], [125I]triiodothyronine (T3), [125I]thyroxine (T4), and [3H]prostaglandin E2 (PGE2). All of these hormones, except PGE2, are ligands of the steroid hormone receptor family. Addition to the growth medium of 5 microM ketoconazole, an inhibitor of cytochrome P450-dependent enzymes, increased about 2-fold the labeling of proteins by T3, T4, 1,25(OH)2D3, and PGE2. In contrast, ketoconazole did not change markedly the extent of labeling by RA, E2, or Pg. Alkaline methanolysis, which cleaves ester bonds, released variable percentages of the radioactive ligands bound to protein. These values were about 80% for RA and PGE2; 50% for T3, T4, and Pg; and 20% for E2 and 1,25(OH)2D3. Treatment with thioether-cleavage reagents, iodomethane or Raney nickel catalyst, released < 2% of the covalently bound ligands. Two-dimensional polyacrylamide gel electrophoresis patterns of labeled proteins were unique for each ligand. Proteins of M(r) 47,000 and 51,000 were labeled by RA, E2, T3, and T4. These proteins had the same mobilities as RI and RII, the cAMP-binding regulatory subunits of type I and type II cAMP-dependent protein kinases. 1,25(OH)2D3 also bound to proteins of M(r) 47,000 and 51,000. However, these proteins had pI values different from those of RI or RII. These results suggest that some activities of ligands of the steroid hormone receptor family and of PGE2 may be mediated by their covalent modification of proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderegg R. J., Betz R., Carr S. A., Crabb J. W., Duntze W. Structure of Saccharomyces cerevisiae mating hormone a-factor. Identification of S-farnesyl cysteine as a structural component. J Biol Chem. 1988 Dec 5;263(34):18236–18240. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballerini P., Lenoble M., Balitrand N., Schaison G., Najean Y., Chomienne C. Stimulatory effect of thyroid hormone on RA-induced granulocytic differentiation in leukemic cells. Leukemia. 1991 May;5(5):383–385. [PubMed] [Google Scholar]
- Baran D. T., Sorensen A. M., Shalhoub V., Owen T., Oberdorf A., Stein G., Lian J. 1 Alpha,25-dihydroxyvitamin D3 rapidly increases cytosolic calcium in clonal rat osteosarcoma cells lacking the vitamin D receptor. J Bone Miner Res. 1991 Dec;6(12):1269–1275. doi: 10.1002/jbmr.5650061202. [DOI] [PubMed] [Google Scholar]
- Bolmer S. D., Wolf G. Retinoids and phorbol esters alter release of fibronectin from enucleated cells. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6541–6545. doi: 10.1073/pnas.79.21.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt S. J., Barnes K. C., Glass D. B., Kinkade J. M., Jr Glucocorticoid-stimulated increase in chemotactic peptide receptors on differentiating human myeloid leukemia (HL-60) cells. Cancer Res. 1981 Dec;41(12 Pt 1):4947–4951. [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Replacement of serum by insulin and transferrin supports growth and differentiation of the human promyelocytic cell line, HL-60. Exp Cell Res. 1980 Apr;126(2):494–498. doi: 10.1016/0014-4827(80)90296-7. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Collins S. J., Keene B. R. Terminal differentiation of human promyelocytic leukemic cells in primary culture in response to retinoic acid. Blood. 1981 Jun;57(6):1000–1004. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Fishman J., Cerami A. Formation of covalent adducts between cortisol and 16 alpha-hydroxyestrone and protein: possible role in the pathogenesis of cortisol toxicity and systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1982 May;79(10):3320–3324. doi: 10.1073/pnas.79.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaigne S., Chomienne C., Daniel M. T., Ballerini P., Berger R., Fenaux P., Degos L. All-trans retinoic acid as a differentiation therapy for acute promyelocytic leukemia. I. Clinical results. Blood. 1990 Nov 1;76(9):1704–1709. [PubMed] [Google Scholar]
- Chen Z. X., Xue Y. Q., Zhang R., Tao R. F., Xia X. M., Li C., Wang W., Zu W. Y., Yao X. Z., Ling B. J. A clinical and experimental study on all-trans retinoic acid-treated acute promyelocytic leukemia patients. Blood. 1991 Sep 15;78(6):1413–1419. [PubMed] [Google Scholar]
- Chetrite G., Pasqualini J. R. Biological responses of ICI 164,384 and other anti-estrogens in vaginal and uterine cells of fetal guinea pig in culture. Acta Endocrinol (Copenh) 1991 Oct;125(4):401–408. doi: 10.1530/acta.0.1250401. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Munck A., Smith K. A. Glucocorticoids inhibit expression of Fc receptors on the human granulocytic cell line HL-60. Nature. 1979 May 24;279(5711):338–339. doi: 10.1038/279338a0. [DOI] [PubMed] [Google Scholar]
- Crettaz M., Baron A., Siegenthaler G., Hunziker W. Ligand specificities of recombinant retinoic acid receptors RAR alpha and RAR beta. Biochem J. 1990 Dec 1;272(2):391–397. doi: 10.1042/bj2720391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danel L., Cordier G., Revillard J. P., Saez S. Presence of estrogen binding sites and growth-stimulating effect of estradiol in the human myelogenous cell line HL60. Cancer Res. 1982 Nov;42(11):4701–4705. [PubMed] [Google Scholar]
- Danel L., Menouni M., Cohen J. H., Magaud J. P., Lenoir G., Revillard J. P., Saez S. Distribution of androgen and estrogen receptors among lymphoid and haemopoietic cell lines. Leuk Res. 1985;9(11):1373–1378. doi: 10.1016/0145-2126(85)90125-0. [DOI] [PubMed] [Google Scholar]
- De Boland A. R., Flawia M., Coso O., Boland R. A guanine nucleotide-binding protein mediates 1,25-dihydroxy-vitamin D-3-dependent rapid stimulation of Ca2+ uptake in skeletal muscle. Biochim Biophys Acta. 1991 Sep 3;1094(2):238–242. doi: 10.1016/0167-4889(91)90015-p. [DOI] [PubMed] [Google Scholar]
- Desai S. S., Appel M. C., Baran D. T. Differential effects of 1,25-dihydroxyvitamin D3 on cytosolic calcium in two human cell lines (HL-60 and U-937). J Bone Miner Res. 1986 Dec;1(6):497–501. doi: 10.1002/jbmr.5650010603. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson M. C., Sergeev I., Norman A. W. Nongenomic actions of 1,25-dihydroxyvitamin D3 in rat osteosarcoma cells: structure-function studies using ligand analogs. Endocrinology. 1991 Oct;129(4):1876–1884. doi: 10.1210/endo-129-4-1876. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Gaub M. P., Lutz Y., Ruberte E., Petkovich M., Brand N., Chambon P. Antibodies specific to the retinoic acid human nuclear receptors alpha and beta. Proc Natl Acad Sci U S A. 1989 May;86(9):3089–3093. doi: 10.1073/pnas.86.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., DiRenzo J., Kurokawa R., Han Z. H. Regulation of gene expression by retinoic acid receptors. DNA Cell Biol. 1991 Nov;10(9):623–638. doi: 10.1089/dna.1991.10.623. [DOI] [PubMed] [Google Scholar]
- Hayes M. E., Bayley D., Mawer E. B. Metabolism of 25-hydroxyvitamin D3 to 24,25-dihydroxyvitamin D3 and its sensitivity to ketoconazole in 1 alpha,25-dihydroxyvitamin D3-differentiated HL60 cells. J Mol Endocrinol. 1989 Nov;3(3):199–205. doi: 10.1677/jme.0.0030199. [DOI] [PubMed] [Google Scholar]
- Huang M. E., Ye Y. C., Chen S. R., Chai J. R., Lu J. X., Zhoa L., Gu L. J., Wang Z. Y. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988 Aug;72(2):567–572. [PubMed] [Google Scholar]
- Jungblut P. R., Seifert R. Analysis by high-resolution two-dimensional electrophoresis of differentiation-dependent alterations in cytosolic protein pattern of HL-60 leukemic cells. J Biochem Biophys Methods. 1990 Jun;21(1):47–58. doi: 10.1016/0165-022x(90)90044-d. [DOI] [PubMed] [Google Scholar]
- Koeffler H. P., Golde D. W., Lippman M. E. Glucocorticoid sensitivity and receptors in cells of human myelogenous leukemia lines. Cancer Res. 1980 Mar;40(3):563–566. [PubMed] [Google Scholar]
- Krust A., Kastner P., Petkovich M., Zelent A., Chambon P. A third human retinoic acid receptor, hRAR-gamma. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5310–5314. doi: 10.1073/pnas.86.14.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer D., Bulger W. H. Inactivation of the uterine estrogen receptor binding of estradiol during P-450 catalyzed metabolism of chlorotrianisene (TACE). Speculation that TACE antiestrogenic activity involves covalent binding to the estrogen receptor. FEBS Lett. 1990 Feb 12;261(1):59–62. doi: 10.1016/0014-5793(90)80636-w. [DOI] [PubMed] [Google Scholar]
- Lakatos P., Stern P. H. Evidence for direct non-genomic effects of triiodothyronine on bone rudiments in rats: stimulation of the inositol phosphate second messenger system. Acta Endocrinol (Copenh) 1991 Nov;125(5):603–608. doi: 10.1530/acta.0.1250603. [DOI] [PubMed] [Google Scholar]
- Liehr J. G. Vitamin C reduces the incidence and severity of renal tumors induced by estradiol or diethylstilbestrol. Am J Clin Nutr. 1991 Dec;54(6 Suppl):1256S–1260S. doi: 10.1093/ajcn/54.6.1256s. [DOI] [PubMed] [Google Scholar]
- Maltese W. A., Erdman R. A. Characterization of isoprenoid involved in the post-translational modification of mammalian cell proteins. J Biol Chem. 1989 Oct 25;264(30):18168–18172. [PubMed] [Google Scholar]
- Mangelsdorf D. J., Koeffler H. P., Donaldson C. A., Pike J. W., Haussler M. R. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984 Feb;98(2):391–398. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani C., Kupfer D. Cytochrome P-450-mediated activation and irreversible binding of the antiestrogen tamoxifen to proteins in rat and human liver: possible involvement of flavin-containing monooxygenases in tamoxifen activation. Cancer Res. 1991 Nov 15;51(22):6052–6058. [PubMed] [Google Scholar]
- Meyers C. Y., Kolb V. M., Gass G. H., Rao B. R., Roos C. F., Dandliker W. B. Doisynolic-type acids--uterotropically potent estrogens which compete poorly with estradiol for cytosolic estradiol receptors. J Steroid Biochem. 1988 Oct;31(4A):393–404. doi: 10.1016/0022-4731(88)90307-x. [DOI] [PubMed] [Google Scholar]
- Miyaura C., Abe E., Kuribayashi T., Tanaka H., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxyvitamin D3 induces differentiation of human myeloid leukemia cells. Biochem Biophys Res Commun. 1981 Oct 15;102(3):937–943. doi: 10.1016/0006-291x(81)91628-4. [DOI] [PubMed] [Google Scholar]
- Murao S., Gemmell M. A., Callaham M. F., Anderson N. L., Huberman E. Control of macrophage cell differentiation in human promyelocytic HL-60 leukemia cells by 1,25-dihydroxyvitamin D3 and phorbol-12-myristate-13-acetate. Cancer Res. 1983 Oct;43(10):4989–4996. [PubMed] [Google Scholar]
- Narumiya S., Ohno K., Fukushima M., Fujiwara M. Site and mechanism of growth inhibition by prostaglandins. III. Distribution and binding of prostaglandin A2 and delta 12-prostaglandin J2 in nuclei. J Pharmacol Exp Ther. 1987 Jul;242(1):306–311. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Olsson I. L., Breitman T. R., Gallo R. C. Priming of human myeloid leukemic cell lines HL-60 and U-937 with retinoic acid for differentiation effects of cyclic adenosine 3':5'-monophosphate-inducing agents and a T-lymphocyte-derived differentiation factor. Cancer Res. 1982 Oct;42(10):3928–3933. [PubMed] [Google Scholar]
- Pan L. Y., Guyre P. M. Individual and combined tumoricidal effects of dexamethasone and interferons on human leukocyte cell lines. Cancer Res. 1988 Feb 1;48(3):567–571. [PubMed] [Google Scholar]
- RIEGEL I. L., MUELLER G. C. Formation of a protein-bound metabolite of estradiol-16-C14 by rat liver homogenates. J Biol Chem. 1954 Sep;210(1):249–257. [PubMed] [Google Scholar]
- Reinhardt T. A., Horst R. L. Ketoconazole inhibits self-induced metabolism of 1,25-dihydroxyvitamin D3 and amplifies 1,25-dihydroxyvitamin D3 receptor up-regulation in rat osteosarcoma cells. Arch Biochem Biophys. 1989 Aug 1;272(2):459–465. doi: 10.1016/0003-9861(89)90240-3. [DOI] [PubMed] [Google Scholar]
- SZEGO C. M. The influence of the liver upon estrogen-protein binding in vitro. Endocrinology. 1953 Jun;52(6):669–678. doi: 10.1210/endo-52-6-669. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bueno A., Sancho M. J., Cobbold P. H. Progesterone and oestradiol increase cytosolic Ca2+ in single rat hepatocytes. Biochem J. 1991 Nov 15;280(Pt 1):273–276. doi: 10.1042/bj2800273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallreuter K. U., Grebe T., Pittelkow M. R., Wood J. M. [3H]-13-cis-retinoic acid covalently binds to thioredoxin reductase in human keratinocytes. Skin Pharmacol. 1991;4(1):14–20. doi: 10.1159/000210919. [DOI] [PubMed] [Google Scholar]
- Selles J., Boland R. Evidence on the participation of the 3',5'-cyclic AMP pathway in the non-genomic action of 1,25-dihydroxy-vitamin D3 in cardiac muscle. Mol Cell Endocrinol. 1991 Dec;82(2-3):229–235. doi: 10.1016/0303-7207(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Skubitz K. M., Zhen Y. S., August J. T. Dexamethasone synergistically induces chemotactic peptide receptor expression in HL-60 cells. Blood. 1982 Mar;59(3):586–593. [PubMed] [Google Scholar]
- Smith T. J., Davis F. B., Davis P. J. Retinoic acid is a modulator of thyroid hormone activation of Ca2+-ATPase in the human erythrocyte membrane. J Biol Chem. 1989 Jan 15;264(2):687–689. [PubMed] [Google Scholar]
- Sonnenschein C., Olea N., Pasanen M. E., Soto A. M. Negative controls of cell proliferation: human prostate cancer cells and androgens. Cancer Res. 1989 Jul 1;49(13):3474–3481. [PubMed] [Google Scholar]
- Swaneck G. E., Fishman J. Covalent binding of the endogenous estrogen 16 alpha-hydroxyestrone to estradiol receptor in human breast cancer cells: characterization and intranuclear localization. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7831–7835. doi: 10.1073/pnas.85.21.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinney D. C. Progesterone metabolism in hepatic microsomes. Effect of the cytochrome P-450 inhibitor, ketoconazole, and the NADPH 5 alpha-reductase inhibitor, 4-MA, upon the metabolic profile in human, monkey, dog, and rat. Drug Metab Dispos. 1990 Nov-Dec;18(6):859–865. [PubMed] [Google Scholar]
- Taetle R., Guittard J. P. Modulation of leukaemia blast colony growth by steroid hormones. Br J Haematol. 1982 Feb;50(2):247–255. doi: 10.1111/j.1365-2141.1982.tb01915.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Covalent binding of 17 beta-estradiol and retinoic acid to proteins in the human breast cancer cell line MCF-7. In Vitro Cell Dev Biol. 1989 Dec;25(12):1199–1200. doi: 10.1007/BF02621275. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoic acid acylation (retinoylation) of a nuclear protein in the human acute myeloid leukemia cell line HL60. J Biol Chem. 1989 Mar 25;264(9):5159–5163. [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoic acid acylation: retinoylation. Methods Enzymol. 1990;189:233–238. doi: 10.1016/0076-6879(90)89294-r. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoylation of HL-60 proteins. Comparison to labeling by palmitic and myristic acids. J Biol Chem. 1990 Nov 5;265(31):19158–19162. [PubMed] [Google Scholar]
- Takahashi N., Breitman T. R. Retinoylation of proteins in leukemia, embryonal carcinoma, and normal kidney cell lines: differences associated with differential responses to retinoic acid. Arch Biochem Biophys. 1991 Feb 15;285(1):105–110. doi: 10.1016/0003-9861(91)90334-f. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Jetten A. M., Breitman T. R. Retinoylation of cytokeratins in normal human epidermal keratinocytes. Biochem Biophys Res Commun. 1991 Oct 15;180(1):393–400. doi: 10.1016/s0006-291x(05)81306-3. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Liapi C., Anderson W. B., Breitman T. R. Retinoylation of the cAMP-binding regulatory subunits of type I and type II cAMP-dependent protein kinases in HL60 cells. Arch Biochem Biophys. 1991 Nov 1;290(2):293–302. doi: 10.1016/0003-9861(91)90544-s. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Abe E., Miyaura C., Kuribayashi T., Konno K., Nishii Y., Suda T. 1 alpha,25-Dihydroxycholecalciferol and a human myeloid leukaemia cell line (HL-60). Biochem J. 1982 Jun 15;204(3):713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wauwe J. P., Janssen P. A. Is there a case for P-450 inhibitors in cancer treatment? J Med Chem. 1989 Oct;32(10):2231–2239. doi: 10.1021/jm00130a001. [DOI] [PubMed] [Google Scholar]
- Walter U., Greengard P. Photoaffinity labeling of the regulatory subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:154–162. doi: 10.1016/0076-6879(83)99048-1. [DOI] [PubMed] [Google Scholar]
- Wehling M., Christ M., Theisen K. High affinity aldosterone binding to plasma membrane rich fractions from mononuclear leukocytes: is there a membrane receptor for mineralocorticoids? Biochem Biophys Res Commun. 1991 Dec 31;181(3):1306–1312. doi: 10.1016/0006-291x(91)92081-t. [DOI] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]






