Abstract
Protein engineering has been used to remodel pores for applications in biotechnology. For example, the heptameric α-hemolysin pore (αHL) has been engineered to form a nanoreactor to study covalent chemistry at the single-molecule level. Previous work has been confined largely to the chemistry of cysteine side chains or, in one instance, to an irreversible reaction of an unnatural amino acid side chain bearing a terminal alkyne. Here, we present four different αHL pores obtained by coupling either two or three fragments by native chemical ligation (NCL). The synthetic αHL monomers were folded and incorporated into heptameric pores. The functionality of the pores was validated by hemolysis assays and by single-channel current recording. By using NCL to introduce a ketone amino acid, the nanoreactor approach was extended to an investigation of reversible covalent chemistry on an unnatural side chain at the single-molecule level.
Keywords: nanoreactor, single-molecule chemistry, membrane protein, native chemical ligation, unnatural amino acid, protein semisynthesis
α-Hemolysin (αHL) is a pore-forming toxin secreted by Staphylococcus aureus. The pore contains seven subunits, and each subunit comprises 293 amino acids.1 Use of the heptameric αHL protein pore as a nanoreactor has proved profitable in studies of covalent chemistry at the single-molecule level.2−5 For example, the nanoreactor approach is advantageous because large, potentially interfering, fluorescent probes are not required. When a molecule undergoes a chemical reaction on the inner wall of the transmembrane β barrel of the αHL pore, the current carried by ions flowing through the pore is perturbed. Hence, individual reaction steps, including those that are not rate-limiting and therefore not detectable at the ensemble level, are visualized in the microsecond time domain, and the kinetics of each step can be determined.2−5 Recently, complex reaction networks4 and the motion of individual molecular walkers5 have been examined by this means. However, the chemistry carried out within engineered αHL nanoreactors has until recently been confined to the reactions of thiolates2−8 and derivatives of the side chains of cysteine residues.9,10 Lately, we expanded the range of chemistry that can be approached by introducing unnatural amino acid side chains into the αHL polypeptide by using native chemical ligation (NCL).11 By this means, an irreversible reaction of a side chain bearing a terminal alkyne was examined. In the present work, we advance the unnatural amino acid approach by introducing a ketone side chain which allows for observation of reversible chemistry. We also describe truncated pores made by the NCL approach.
Results
General Approach to Two-Fragment Ligation
Two-fragment ligations involved the reaction of an N-terminal fragment (NTF) containing a C-terminal αthioester with a C-terminal fragment (CTF) bearing an N-terminal cysteine (N-Cys). The NTF coding sequence was fused in-frame with DNA encoding a Mycobacterium xenopi DNA gyrase A (Mxe GyrA) intein–chitin binding domain (CBD), that is, NTF–intein–CBD, in the pTXB3 plasmid (New England Biolabs).12 After expression of the protein in Escherichia coli, the NTF-αthioester was cleaved from the intein–CBD with sodium 2-mercaptoethanesulfonate (MESNa), while the rest of the chimera remained bound to chitin beads.13,14 A CTF with an N-Cys can be generated by cleavage of a precursor fusion protein with a site-specific protease.15,16 However, this method may not work efficiently with proteins of poor solubility or proteins from inclusion bodies. In the latter case, the protease can be inactivated by denaturants used to solubilize a target protein, and even if cleavage is successful, an additional purification step may be required to separate the product from the protease and unwanted fragments. An alternative approach to generate N-Cys, which was taken here, is to express the polypeptide initiated by fMet-Cys. Following expression, formylmethionine (fMet) is removed by endogenous deformylation and methionine aminopeptidase activity, and the N-Cys residue undergoes condensation with pyruvic acid, an abundant metabolite, to form a thiazolidine.17 This strategy is protease-free and allows rapid overexpression of the target polypeptide (4 h) at 37 °C. The polypeptide accumulated in inclusion bodies is subsequently purified under denaturing conditions (8 M urea or 6 M guanidine hydrochloride (Gu·HCl)), and the N-Cys is unmasked with hydroxylamine or a hydroxylamine derivative.18 The use of denaturants enables the purification of polypeptides containing transmembrane regions, which are often insoluble and obtained in very low yields when processed under nondenaturing conditions (unpublished work).
Preparation of Polypeptides for Two-Fragment Ligation
We targeted three αHL polypeptides for semisynthesis by two-fragment coupling (Figure 1A): a full-length αHL monomer and two different truncated barrel mutants (TBMΔ6) (Figure S1A–C). TBMΔ6 forms an αHL heptamer in which amino acids have been removed in pairwise fashion from both of the two β strands contributed by each subunit, resulting in a β barrel shortened by 6 amino acids.19 TBMΔ6 forms conductive pores despite the short length of the barrel, presumably by inducing the formation of toroidal lipid pores that span the bilayer.19 Two TBMΔ6s bearing a different amino acid at residue 113 (Met, Phe) were chosen, as it has been shown that the mutation Met-113→Phe significantly alters the binding kinetics of cyclodextrin adaptors to the pore.20
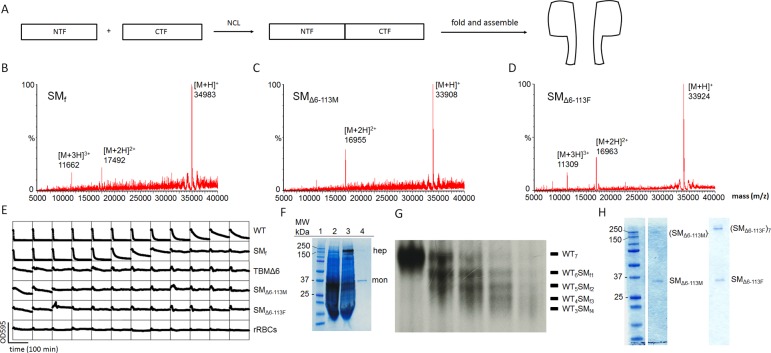
Figure 1.
Preparation of αHL pores. (A) αHL monomers were synthesized by native chemical ligation from two fragments (NTF and CTF) expressed in E. coli. Folding was performed by reducing the concentration of the denaturant (8 M urea) present during the purification of the synthetic monomers (SM). (B–D) Characterization of the synthetic αHL monomers by LC-MS. (B) SMf: [M + H]+ = 34 983 (observed mass, obs), 34 981 (calculated mass, calcd). (C) SMΔ6-113M: [M + H]+ = 33 908 (obs), 33 907 (calcd). (D) SMΔ6-113F: [M + H]+ = 33 924 (obs), 33 923 (calcd). (E) Hemolysis assays (see Supporting Information, Experimental procedures). The decrease in light scattering over time was recorded in a microplate reader at 595 nm. WT αHL monomer (row 1) lysed rRBCs, whereas TBMΔ6 (row 3) did not due to its truncated β barrel. Similarly, the full-length synthetic αHL monomer SMf (5.9 μg mL–1, in well 1) lysed rRBCs, whereas SMΔ6–113M (7.4 μg mL–1) and SMΔ6–113F (7.8 μg mL–1) did not. WT and TBMΔ6 monomers were produced by IVTT. (F) SDS-PAGE gel analysis of WT and synthetic αHL (SMf). Lane 1: molecular markers. Lane 2: radiolabeled αHL monomer (mon) produced by IVTT. Lane 3: radiolabeled WT7 pores (hep) produced in the presence of DPhPC liposomes (7 mg mL–1). Lane 4: (SMf)7 pores assembled with purified SMf in the presence of DPhPC liposomes under the same conditions comigrate with the WT7 pore. An autoradiogram is superimposed on the Coomassie Blue-stained gel. (G) Heteroheptameric pores. WT αHL (radiolabeled protein) and SM were mixed in various ratios in the presence of rRBCm to yield heteromeric WT7–nSMn (n = 0–7) pores. The heptameric pores with different numbers of SMf were separated by SDS-PAGE based on the different electrophoretic mobilities produced by D8 tails at the C-terminus of SMf. (H) Homoheptameric pores formed with SMΔ6–113M (left) and SMΔ6–113F (right). Homomeric pores were prepared in the presence of DPhPC liposomes (10 mg mL–1).
Three different NTFs (NTF126 [Ala1-Gly126], NTF113M [Ala1-Met113], and NTF113F [Ala1-Phe113]) each bearing an αthioester at the C-terminus were obtained by thiolysis of the corresponding intein–CBD fusion proteins after expression in E. coli. The pTXB3–NTF126 and pTXB3–NTF113M plasmids were prepared by cloning PCR-amplified DNA, encoding residues 1–126 and 1–113 of αHL, upstream of the intein–CBD codons (Figure S2A,B). pTXB3–NTF113F was prepared by mutagenesis of pTXB3–NTF113M by homologous recombination (Figure S2C).21 Fusion proteins were produced in E. coli (BL21(DE3), NEB) and solubilized from inclusion bodies under denaturing conditions (8 M urea). The NTF-αthioesters were obtained by on-column thiolysis of the fusion proteins bound to chitin columns with MESNa (Figure S3). The purified NTF-αthioesters were characterized by LC-MS (Figure S4, NTF126: [M + H]+ = 14 150 (obs), 14 150 Da (calcd); NTF113M: [M + H]+ = 12 838 (obs), 12 838 Da (calcd); NTF113F: [M + H]+ = 12 853 (obs), 12 854 Da (calcd)).
The pT7-SC1-CTF127 plasmid was prepared by replacing plasmid DNA encoding residues 1–127 with the codons for Met-Cys as previously reported.11 To produce CTFΔ114, pT7-SC1-CTFΔ114-DH was prepared by two successive homologous recombinations21 from pT7-TBMΔ6, which encodes TBMΔ6 (Figure S5). The codons for residues 1–113 were removed (retaining the initiator Met), and codons for a D8H6 (DH) tag were added in the first and second rounds, respectively. The two different C-terminal fragments (CTF127 [Cys127-Asn293]-D8H6, and CTFΔ114 [Cys114-Asn293, ΔPhe120-Thr125, ΔGly133-Ala138]-D8H6) were overexpressed in E. coli. Like the NTFs, the CTFs were obtained from inclusion bodies and, in this case, purified under denaturing conditions (6 M Gu·HCl) by FPLC (ÄKTA purifier, GE Healthcare Life Sciences) at room temperature by use of the His6 tag at the C-terminus (Figure S6). The N-terminal fMet was found to be absent, and the thiazolidine produced by condensation of the N-Cys with pyruvic acid was removed with 0.4 M HONH2·HCl for 4 h at room temperature. The purified CTFs were characterized by LC-MS (Figure S7, CTF127: [M + H]+ = 20 974 (obs), 20 974 Da (calcd); CTFΔ114: [M + H]+ = 21 214 (obs), 21 212 Da (calcd)).
α-Hemolysin Polypeptides by Two-Fragment Ligation
We ligated NTFs and CTFs (Figure S1) in NCL buffer [200 mM NaH2PO4 (pH 6.9), 6 M Gu·HCl, 200 mM 4-mercaptophenylacetic acid (MPAA), and 50 mM tris(2-carboxyethyl)phosphine (TCEP)] to make three different αHL constructs. In each case, an NTF and a CTF were mixed and the buffer was exchanged by dilution–concentration cycles with a centrifugal filter. NTF126 (∼0.5 mM) and CTF127 (∼0.5 mM) were coupled to produce the full-length αHL synthetic monomer (SMf). NTF113M (∼0.6 mM) and NTF113F (∼0.7 mM) were separately coupled with CTFΔ114 (∼0.8 mM) to yield two different truncated mutant monomers19 (SMΔ6–113M and SMΔ6–113F). The rate of ligation is highly dependent on the steric properties of the C-terminal amino acid residue of an NTF22,23 and the concentration of reactants. The ligation reactions were carried out for >12 h at a final concentration of ∼1 mM as previous work22 had suggested that ligations of NTFs containing C-terminal Ala, Val, Ile, Met, and Phe are completed within 9 h at a final peptide concentration of 1–3 mM. The two-fragment couplings gave SMf, SMΔ6–113M, and SMΔ6–113F (Figures S8 and S9) in 48, 46, and 50% yields, respectively.
Purification, Folding, and Functional Properties of α-Hemolysin Polypeptides
We purified the SMs (SMf, SMΔ6–113M, and SMΔ6–113F) by gel filtration in 8 M urea (Figure S8) and characterized them by LC-MS (Figure 1B–D and Figure S8, SMf: [M + H]+ = 34 983 (obs), 34 981 Da (calcd); SMΔ6–113M: [M + H]+ = 33 908 (obs), 33 907 Da (calcd); SMΔ6–113M: [M + H]+ = 33 924 (obs), 33 923 Da (calcd)). We then folded the purified SMs by diluting the 8 M urea in the purification buffer to ∼60 mM and concentrating the proteins using a centrifugal filter (MWCO 3k). The folded monomers were examined for hemolytic activity toward rabbit red blood cells (rRBCs) (Figure 1E). We observed similarities between the synthetic monomers and the WT or truncated αHL monomers produced either in E. coli or by in vitro transcription and translation (IVTT). As expected, only SMf exhibited hemolytic activity toward rRBCs.19 The specific hemolytic activity of SMf was HC50 = 92 ng mL–1, which is in the same range as that of WT αHL (HC50 = 31 ng mL–1).24 To visualize the formation of αHL heptamers, we incubated SMf at 37 °C in the presence of liposomes (10 mg mL–1, diphytanoylphosphatidylcholine, DPhPC), which produced a new band upon sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS-PAGE) corresponding to the size (∼240 kDa) of the heptamer (Figure 1F, left). We also incubated SMf in the presence of rabbit red blood cell membranes (rRBCm) in different ratios with radiolabeled WT αHL produced by IVTT. The SMf oligomerized to form heteroheptamers with different stoichiometries (WT7–nSMn, n = 0–7) (Figure 1F, right). SMΔ6–113M and SMΔ6–113F were incubated with DPhPC liposomes (10 mg mL–1) and oligomerized to form homomeric structures11 (Figure 1G).
Electrical Properties of Two-Fragment Pores and Binding of Cyclodextrins
To examine the electrical properties of the various heptameric αHL pores containing synthetic subunits [WT6SMf1, (SMΔ6–113M)7, and (SMΔ6–113F)7], we determined the mean unitary conductance values under defined conditions and measured I–V curves (Figure S10a,b). The conductance values for the αHL pores containing synthetic monomers were similar to that of pores comprising WT αHL subunits produced by IVTT.
To confirm that the transmembrane β barrels of the semisynthetic pores were intact, we evaluated the binding kinetics at the single-molecule level of cyclodextrin molecular adapters (βCD and am7βCD (heptakis(6-deoxy-6-amino)-β-cyclodextrin)) with the WT6(SMf)1, (SMΔ6–113M)7, and (SMΔ6–113F)7 pores.25,26 It was already known that the homoheptamer formed from TBMΔ6/M113F binds am7βCD very tightly.19,20 We determined the association and dissociation rate constants (kon and koff) of βCD for the three different protein pores. At least three measurements were made for each construct. βCD blocks the ionic current transiently when it is lodged within the lumen of the αHL pore. The dissociation constants (KD = koff/kon, Figure S11) of βCD for WT6SMf1 (KD = 14.5 ± 0.4 × 10–3 M, kon = 10.0 ± 0.2 × 104 M–1·s–1, and koff = 14.5 ± 0.2 × 102 s–1) and (SMΔ6–113M)7 (KD = 6.5 ± 0.2 × 10–2 M, kon = 2.4 ± 0.1 × 104 M–1·s–1, and koff = 15.7 ± 0.3 × 102 s–1) were similar to the values obtained in our previous studies for WT725 and (TBMΔ6–113M)7.19 The KD (6.1 ± 1.3 × 10–5 M) of βCD for (SMΔ6–113F)7 (Figure S12A) was smaller by 3 orders of magnitude than the KD for the (SMΔ6–113M)7 pore, which makes sense as it is known that βCD binds more tightly by 3–4 orders of magnitude to a pore formed by the full-length M113F subunit than it does to the WT pore.20 We also analyzed the binding of am7βCD to the (SMΔ6–113F)7 pore (Figure S12B) and found that it remained bound to the pore “permanently” as previously reported for the same truncated pore produced by conventional means.19 The binding kinetics of βCD and am7βCD suggest that the semisynthetic protein pores produced by two-fragment coupling, and thereby containing a Cys mutation (S114C), are very similar to the protein pores derived from WT αHL produced directly by IVTT from the corresponding genes.
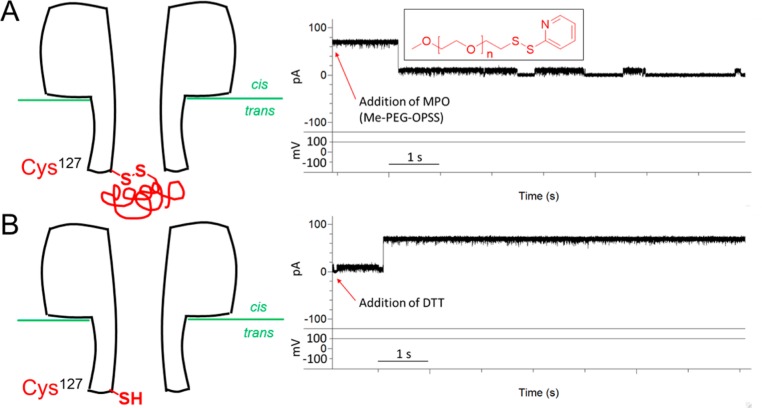
Two-Fragment Ligation Forms a Native Amide Bond
To verify the existence of a native amide bond formed between Gly126 and Cys127 in SMf, we carried out thiolate chemistry on single WT6SMf1 pores by using the side chain of Cys-127 generated by NCL. In the absence of methyl-PEG-OPSS (MPO, 5.0 kDa), the open state of WT6SMf1 had a long duration (>30 min). The addition of MPO (0.1 mM) to the trans compartment at +100 mV generated an irreversible current drop (Figure 2A), due to the formation of a disulfide bond between MPO and the side chain of Cys-127. The pore remained blocked over a range of potentials (−100 to +100 mV), indicating that the current drop is not due to simple clogging of the pore. In the presence DTT (5 mM, both compartments), the open current level was restored (Figure 2B) because the PEG chain was cleaved from the pore.
Figure 2.
Reactivity of the Cys-127 residue in WT6SMf1 αHL. (A) WT6SMf1 was eluted from a gel (Figure 1G), and a single WT6SMf1 pore was established in a planar bilayer. Me-PEG-OPSS (5 kDa, 0.1 mM, inset, n = 3) was added to the trans compartment. The current drop indicates a blockade caused by reaction of the PEG derivative with the pore through the formation of a disulfide bond with the side chain of Cys-127. (B) Addition of 5 mM DTT to both compartments cleaved the PEG chain from the WT6SMf1 pore. The buffer was 1 M KCl, 20 mM Tris·HCl (pH 8.5). The currents in (A) and (B) were filtered and sampled at 2 and 10 kHz, respectively.
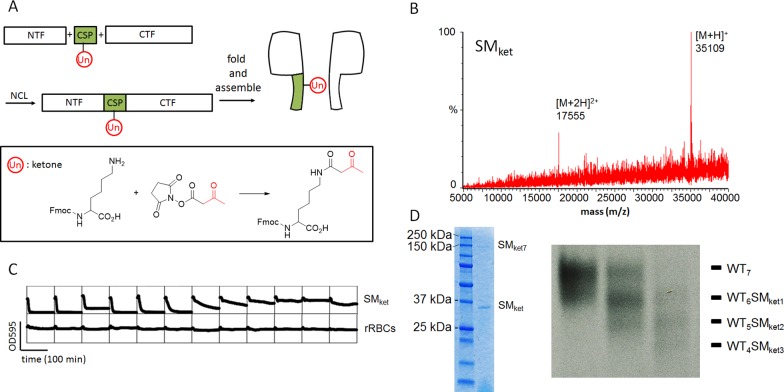
Three-Fragment Ligation To Form a Ketone-Containing αHL Polypeptide
With NTF113M and CTF127, and a synthetic central peptide, we next carried out three-fragment coupling to construct a full-length αHL monomer containing a ketone group (Figure 3A). The ketone is a versatile functional group in organic chemistry and participates in a large number of reactions.27−30 However, reactions of a ketone have not been observed yet at the single-molecule level. The synthetic methods used to obtain an unnatural amino acid containing a ketone group28,29 are not very efficient, and the techniques27,30 used to incorporate a ketone amino acid into the middle of a polypeptide chain are often arduous. We made an unnatural amino acid bearing a ketone (Fmoc-Ket-OH; Fmoc-N6-(3-oxobutanoyl)lysine), Figure 3A, inset) from Fmoc-Lys-OH and N-hydroxysuccinimidyl acetoacetate (NHA). Fmoc-Ket-OH was used for SPPS of a central segment of the polypeptide chain (CSP: Thz114ThrLeuKetTyrGlyPheAsnGlyAsnValThrGly126-Nbz), such that the Ket side chain would project into the transmembrane β barrel of an αHL pore. CSP was prepared with a C-terminal acylurea31 (Figures S13 and S14), which yields a peptide arylthioester with 4-mercaptophenylacetic acid (MPAA), accelerating the NCL reaction. We then proceeded to assemble a full-length αHL bearing the ketone group with two sequential NCL reactions (Figure S15). The final product (SMket) was purified (Figure S16) and characterized by LC-MS (Figure 3B and Figure S9D; [M + H]+ = 35 109 (obs), 35 107 Da (calcd)). A hemolysis assay showed that folded SMket (HC50 = 47 ng mL–1) had similar activity to the WT αHL monomer (Figure 3C). SMket also formed homo- and heteroheptameric pores in the presence of liposomes and rRBCm, respectively (Figure 3D).
Figure 3.
Preparation of αHL pores containing an unnatural amino acid. (A) An αHL monomer was synthesized from three fragments with a central segment (CSP) bearing an unnatural amino acid with a side chain bearing a ketone group (inset: Un, Fmoc-N6-(3-oxobutanoyl)lysine, Fmoc-Ket-OH). (B) Characterization of the synthetic αHL monomer (SMket) by LC-MS. [M + H]+ = 35 109 (obs), 35 107 (calcd). (C) Hemolysis assay for SMket. The full-length synthetic αHL monomer [SMket (0.31 mg mL–1)] lysed rRBCs. rRBCs alone (row 6 in Figure 2E) are displayed for comparison. (D) Homo- (left) and hetero- (right) heptameric pores formed with SMket. The pores were prepared by the same methods described in Figure 1F,G.
We determined the mean unitary conductance values for individual WT6SM1ket pores in 1 M KCl and 50 mM Na acetate buffer over a range of applied potentials (−100 to +100 mV) (Figure S18A). The buffer was adjusted to pH 3.4 in anticipation of an acid-catalyzed addition reaction (imine formation) on the ketone side chain (see below). The conductance of WT6SMket1 (0.93 ± 0.10 nS, n = 9) at +100 mV was similar to that of the WT7 pore (1.07 ± 0.02 nS, n = 9) under the same conditions. We also determined the association and dissociation rate constants at pH 3.4 (kon and koff) for βCD binding from the values of the mean dwell times (τon and τoff) [WT7: KD = 6.1 ± 0.2 × 10–2 M (n = 3), kon = 71.7 ± 0.1 × 102 M–1·s–1, and koff = 4.4 ± 0.2 × 102 s–1; WT6SMket1: KD = 8.6 ± 0.7 × 10–2 M (n = 3), kon = 83.7 ± 6.8 × 102 M–1·s–1, and koff = 7.2 ± 0.1 × 102 s–1] (Figure S17). The ketone residue presented by the SM subunit affects neither the electrical properties of the αHL pore nor its ability to bind the βCD adaptor.
Single-Molecule Covalent Chemistry with a Ketone-Containing αHL Pore
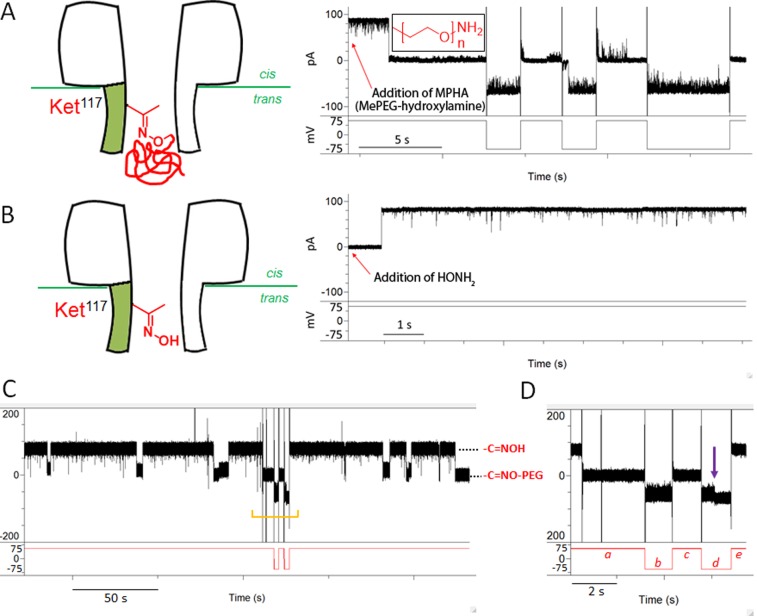
We then carried out imine chemistry with single WT6SM1ket pores. We first examined the interaction of the WT7 pore with 1.1 kDa MePEG-hydroxylamine (MPHA). The addition of 2 mM MPHA to the trans compartment at +75 mV in the presence of 1 M KCl, 50 mM Na acetate buffer (pH 3.4), produced short blockades (∼100 μs), which arise from the entry of MPHA into the lumen of the pore without covalent attachment.32 No prolonged current decrease was observed with the WT7 pore during 2 h of monitoring. We then added 2 mM MPHA to the trans side of the WT6SMket1 pore, under the same conditions, which led to a permanent current blockade at +75 mV within 10 min, presumably due to the covalent attachment of MPHA to the ketone group within the pore through imine formation. Interestingly, the modified WT6SMket1 pore opened at negative applied potentials (−100 to 0 mV) with IRES = 91% (residual current), compared with the original open state. By contrast, at positive potentials (0 to +100 mV), the pore was almost closed (IRES = 3.2%, Figure S18B) with very short openings (<50 ms). It follows that the current–voltage (I–V) characteristics of WT6SMket1-imine-PEG show virtually complete current rectification (Figure S18B). Previously, we developed a diode-like αHL pore (7R-αHL) with positively charged Arg side chains projecting into the lumen of the transmembrane β barrel,33 allowing ions to flow only at positive potentials. We used 7R-αHL to construct a bridge rectifier circuit from droplet interface bilayers. Therefore, the WT6SMket1-oxime-PEG pore, which shows the opposite electrical properties to 7R-αHL (Figure 4A), might be used in related applications. To confirm that the attachment was through imine formation, we added 20 mM NH2OH to both compartments to cleave the linkage. In ∼15 min, the current returned to its initial level (Figure 4B), suggesting that the PEG chain had been cleaved from the pore.
Figure 4.
Single-molecule reactions of the WT6SMket1 pore. (A) A WT6SMket1 pore was reacted with MePEG-hydroxylamine (MPHA, 1.1 kDa, 2 mM, inset) added to the trans compartment. Reaction occurred at a positive potential (+75 mV) and led to a permanent current blockade of the WT6SMket1 pore. The modified pore only opened at negative applied potentials. (B) The pore was restored to an open state when 20 mM HONH2·HCl was added to both compartments to release the PEG chain. The currents in (A) and (B) were filtered at 5 kHz and sampled at 25 kHz. For display, further digital filtering was carried out at 2 kHz with an 8-pole low-pass Bessel filter. The buffer was 1 M KCl, 50 mM Na acetate (pH 3.4). (C) Reversible oxime formation in a single synthetic pore containing a ketone (WT6SMket1). Oxime formation with MPHA leads to a current drop, while reversal with HONH2 returns the current to its initial level. The section defined by the orange bracket is magnified in panel D. (D) Negative potential (−75 mV, b) was applied during the PEG-oxime state (a), which opened the pore (residual current, IRES = 91%). Subsequently, a positive potential (+75 mV, c) was applied, and the pore closed. The pore became fully open (violet arrow) with IRES = 100% at negative potential (−75 mV, d), presumably when the formation of an oxime with HONH2 led to release of the pore-bound polymer. The pore was restored to an open state at a positive potential (+75 mV, e).
The observation of the ability of the WT6SMket1 pore to return its initial conductance level led us to investigate an oxime−oxime exchange reaction on the ketone side chain at the single-molecule level. In the presence of 2 mM MPA, in the trans compartment, and 10 mM of HONH2, in both the cis and trans compartment, a reversible reaction was observed (Figure 4C). During the formation of the O-alkyloxime by MPHA, the current was greatly reduced to levels in the range of 1.5 to 17 pA (Figure 4C). Subsequent transitions between this “closed” level and the open level were apparent. The open level represents the formation of an oxime with HONH2, and release of the PEG chain from the pore. The formation of an O-alkyloxime within the pore with MPHA only allowed substantial ion flow at a negative potential (−75 mV) (orange bracket in Figure 4C and a−d in Figure 4D). After transoximination with HONH2,34 the current increased from −56 to −67 pA at −75 mV (violet arrow in Figure 4D) and an open-level current of +78 pA was observed during the subsequent application of a positive potential (+75 mV).
The mean dwell time of the open pore (τon) is the mean lifetime of the oxime formed by HONH2 (o), which is the mean reaction time for O-alkyloxime (ao) formation with MPHA. Similarly, the mean dwell time of the closed pore (τoff) is the mean lifetime of the O-alkyloxime, which is the mean reaction time for oxime formation with HONH2. The measured mean lifetimes the O-alkyloxime and the oxime were 52 ± 2 s (n = 79) and 51 ± 2 s (n = 78) (Figure S19), which yield rate constants for transoximination of kf,ao = 10 M−1·s−1 and kf,o = 2 M−1·s−1, respectively, in 1 M KCl, Na acetate buffer (pH 3.4), at +75 mV, where kf,ao = kon = 1/τon·[MPHA] and kf,o = koff = 1/τoff·[NH2OH].
Conclusions
The ability to introduce unnatural amino acids into the αHL pore has the potential to provide a large variety of reactive side chains for the investigation of single-molecule covalent chemistry. We have previously produced αHL polypeptides with unnatural alkyl and aryl amino acids by using in vitro chemically acylated tRNAs.35 However, this approach is demanding and often gives poor results when more than one amino acid is introduced. The coupling of polypeptide segments by NCL has been used extensively to produce proteins36−38 and is a favorable alternative means to incorporate unnatural amino acids. In the present work, we demonstrate a variety of synthetic protein pores using αHL polypeptides and use the synthetic pore containing a ketone as single-molecule nanoreactor.
Oxime chemistry was examined in an aqueous environment at the single-molecule level with a ketone-containing αHL pore (WT6SMket1), and the work described here is the first observation of reversible covalent chemistry using an unnatural amino acid side-chain in a nanoreactor. Oxime formation from a ketone proceeds via nucleophilic addition to form a tetrahedral intermediate,39,40 followed by the elimination of water. Transoximination also proceeds reversibly through a tetrahedral intermediate that subsequently breaks down to form a new oxime and a hydroxylamine.34 In our work, no intermediates were observed in both the O-alkyloxime formation by MPHA and the transoximination reaction. Presumably, the lifetimes of the tetrahedral intermediates are too short or the current changes too small to observe under our recording conditions. We obtained rate constants for oxime and O-alkyloxime formation within the pore (kf,ao = 10 M−1·s−1 and kf,o = 2 M−1·s−1). Earlier determinations in bulk solution41,42 are in the range ∼1 to 104·M−1·s−1 and depend strongly on substituents, solvent, pH, and temperature. In our case, the partitioning of the polymer reactant, MPHA, into the pore must be considered.11,43
We have observed oxime formation by using a semisynthetic pore containing an unnatural amino acid as a nanoreactor. The ketone-containing pore expands the range of covalent chemistry that can be studied by the nanoreactor approach to reversible reactions for which statistically significant data can be acquired rapidly without tedious repeats. Taken together with our recent demonstration of alkyne chemistry,11 the versatility of the nanoreactor approach is apparent, and we look forward to developing even more ambitious possibilities, such as single-molecule catalysis, which may require the placement of several different unnatural amino acids within a single polypeptide chain.
Methods
Native Chemical Ligation
Two-Fragment Coupling
Fifty microliters of CTF (>0.5 mM), from which pyruvate had been removed, was mixed with 50 μL of NTF (>0.5 mM) in 0.4 mL of NCL buffer [200 mM NaH2PO4 (pH 6.9) containing 6 M Gu·HCl, 50 mM tris(2-carboxyethyl)phosphine (TCEP), and 200 mM 4-mercaptophenylacetic acid (MPAA)] and concentrated to 100 μL using a centrifugal filter (Amicon, MWCO 3k) at 14 000g for 20 min. The buffer containing NTF and CTF was replaced with NCL buffer by repeated (5 times) dilution and concentration with the same filter. The reaction was allowed to proceed overnight at room temperature.
Three-Fragment Coupling
CTF (0.5 mM), from which pyruvate had been removed, was mixed with CSP-Nbz (5 mM) in 0.5 mL of NCL buffer [200 mM NaH2PO4 (pH 6.9) containing 6 M Gu·HCl, 50 mM tris(2-carboxyethyl)phosphine (TCEP), and 50 mM MPAA]. After overnight reaction at room temperature, the unreacted peptide was removed by passing the mixture through a size-exclusion column (Superdex 200 10/300 GL). The product was analyzed by LC-MS.
The N-terminal Thz group was subsequently cleaved by treatment with 0.4 M HONH2·HCl in 200 mM NaH2PO4 buffer (pH adjusted to 4.0) containing 6 M Gu·HCl and 50 mM TCEP for 4 h at room temperature. For the next round of ligation, the buffer was replaced with NCL buffer containing 200 mM MPAA by repeated dilution and concentration with a centrifugal filter (Amicon, MWCO 3k). NTF-αthioester (0.6 mM) was mixed with the first ligation product (0.3 mM), and the reaction was allowed to proceed overnight. The final ligation product was purified by gel filtration followed by ion-exchange chromatography. The reaction yield was determined by quantifying the intensity of the polypeptide bands after SDS-PAGE by using ImageJ (NIH).
Acknowledgments
The authors thank Professor Tom Muir (Princeton University) for helpful discussions. This work was supported by an ERC Advanced Grant. J. Lee was supported in part by a Korean government scholarship. O.D. was supported by a Junior Research Fellowship at Christ Church, Oxford. This paper is dedicated to the memory of Dr. Stephen Cheley.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsnano.6b04663.
Experimental procedures and supplementary figures for analytical and spectral characterization data (PDF)
Author Contributions
J. Lee, A.J.B., M.A.B., S.C., O.D., and H.B. designed the research. A.J.B., M.A.B., S.C., and O.D. carried out early synthetic work. J. Lee performed recent synthetic work and characterization of the synthetic pores. J. Li and H.T. assisted with solid-phase peptide synthesis. J. Lee and H.B. wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Song L.; Hobaugh M. R.; Shustak C.; Cheley S.; Bayley H.; Gouaux J. E. Structure of Staphylococcal Alpha-Hemolysin, a Heptameric Transmembrane Pore. Science 1996, 274, 1859–66. 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- Bayley H.; Luchian T.; Shin S.-H.; Steffensen M.. Single-Molecule Covalent Chemistry in a Protein Nanoreactor. In Single Molecules and Nanotechnology; Rigler R., Vogel H., Eds.; Springer: Berlin, 2008; Vol. 12, pp 251–277. [Google Scholar]
- Lu S.; Li W. W.; Rotem D.; Mikhailova E.; Bayley H. A Primary Hydrogen-Deuterium Isotope Effect Observed at the Single-Molecule Level. Nat. Chem. 2010, 2, 921–8. 10.1038/nchem.821. [DOI] [PubMed] [Google Scholar]
- Steffensen M. B.; Rotem D.; Bayley H. Single-Molecule Analysis of Chirality in a Multicomponent Reaction Network. Nat. Chem. 2014, 6, 603–7. 10.1038/nchem.1949. [DOI] [PubMed] [Google Scholar]
- Pulcu G. S.; Mikhailova E.; Choi L.-S.; Bayley H. Continuous Observation of the Stochastic Motion of an Individual Small-Molecule Walker. Nat. Nanotechnol. 2015, 10, 76–83. 10.1038/nnano.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. H.; Luchian T.; Cheley S.; Braha O.; Bayley H. Kinetics of a Reversible Covalent-Bond-Forming Reaction Observed at the Single-Molecule Level. Angew. Chem., Int. Ed. 2002, 41, 3707–9. . [DOI] [PubMed] [Google Scholar]
- Shin S. H.; Bayley H. Stepwise Growth of a Single Polymer Chain. J. Am. Chem. Soc. 2005, 127, 10462–10463. 10.1021/ja052194u. [DOI] [PubMed] [Google Scholar]
- Choi L. S.; Bayley H. S-Nitrosothiol Chemistry at the Single-Molecule Level. Angew. Chem., Int. Ed. 2012, 51, 7972–7976. 10.1002/anie.201202365. [DOI] [PubMed] [Google Scholar]
- Hammerstein A. F.; Shin S. H.; Bayley H. Single-Molecule Kinetics of Two-Step Divalent Cation Chelation. Angew. Chem., Int. Ed. 2010, 49, 5085–90. 10.1002/anie.200906601. [DOI] [PubMed] [Google Scholar]
- Boersma A. J.; Bayley H. Continuous Stochastic Detection of Amino Acid Enantiomers with a Protein Nanopore. Angew. Chem., Int. Ed. 2012, 51, 9606–9. 10.1002/anie.201205687. [DOI] [PubMed] [Google Scholar]
- Lee J.; Bayley H. Semisynthetic Protein Nanoreactor for Single-Molecule Chemistry. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 13768–73. 10.1073/pnas.1510565112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong S. R.; Mersha F. B.; Comb D. G.; Scott M. E.; Landry D.; Vence L. M.; Perler F. B.; Benner J.; Kucera R. B.; Hirvonen C. A.; Pelletier J. J.; Paulus H.; Xu M. Q. Single-Column Purification of Free Recombinant Proteins Using a Self-Cleavable Affinity Tag Derived from a Protein Splicing Element. Gene 1997, 192, 271–281. 10.1016/S0378-1119(97)00105-4. [DOI] [PubMed] [Google Scholar]
- Evans T. C.; Benner J.; Xu M. Q. Semisynthesis of Cytotoxic Proteins Using a Modified Protein Splicing Element. Protein Sci. 1998, 7, 2256–2264. 10.1002/pro.5560071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.; Wood D. W.; Belfort G.; Derbyshire V.; Belfort M. Intein-Mediated Purification of Cytotoxic Endonuclease I-Tevi by Insertional Inactivation and Ph-Controllable Splicing. Nucleic Acids Res. 2002, 30, 4864–71. 10.1093/nar/gkf621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert T. J.; Wong C. H. New Methods for Proteomic Research: Preparation of Proteins with N-Terminal Cysteines for Labeling and Conjugation. Angew. Chem., Int. Ed. 2002, 41, 2171–4. . [DOI] [PubMed] [Google Scholar]
- Pentelute B. L.; Barker A. P.; Janowiak B. E.; Kent S. B. H.; Collier R. J. A Semisynthesis Platform for Investigating Structure Function Relationships in the N-Terminal Domain of the Anthrax Lethal Factor. ACS Chem. Biol. 2010, 5, 359–364. 10.1021/cb100003r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I. E.; De Souza D. P.; Baca M. Direct Production of Proteins with N-Terminal Cysteine for Site-Specific Conjugation. Bioconjugate Chem. 2004, 15, 658–63. 10.1021/bc049965o. [DOI] [PubMed] [Google Scholar]
- Bang D.; Kent S. B. A One-Pot Total Synthesis of Crambin. Angew. Chem., Int. Ed. 2004, 43, 2534–8. 10.1002/anie.200353540. [DOI] [PubMed] [Google Scholar]
- Stoddart D.; Ayub M.; Hofler L.; Raychaudhuri P.; Klingelhoefer J. W.; Maglia G.; Heron A.; Bayley H. Functional Truncated Membrane Pores. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 2425–30. 10.1073/pnas.1312976111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu L. Q.; Cheley S.; Bayley H. Prolonged Residence Time of a Noncovalent Molecular Adapter, Beta-Cyclodextrin, within the Lumen of Mutant Alpha-Hemolysin Pores. J. Gen. Physiol. 2001, 118, 481–493. 10.1085/jgp.118.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howorka S.; Bayley H. Improved Protocol for High-Throughput Cysteine Scanning Mutagenesis. Biotechniques 1998, 25, 764–6. [DOI] [PubMed] [Google Scholar]
- Hackeng T. M.; Griffin J. H.; Dawson P. E. Protein Synthesis by Native Chemical Ligation: Expanded Scope by Using Straightforward Methodology. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 10068–10073. 10.1073/pnas.96.18.10068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Kwon Y.; Pentelute B. L.; Bang D. Use of Model Peptide Reactions for the Characterization of Kinetically Controlled Ligation. Bioconjugate Chem. 2011, 22, 1645–1649. 10.1021/bc2002242. [DOI] [PubMed] [Google Scholar]
- Walker B.; Krishnasastry M.; Zorn L.; Kasianowicz J.; Bayley H. Functional Expression of the Alpha-Hemolysin of Staphylococcus Aureus in Intact Escherichia Coli and in Cell Lysates. Deletion of Five C-Terminal Amino Acids Selectively Impairs Hemolytic Activity. J. Biol. Chem. 1992, 267, 10902–10909. [PubMed] [Google Scholar]
- Gu L. Q.; Bayley H. Interaction of the Non-Covalent Molecular Adapter, Beta-Cyclodextrin, with the Staphylococcal Alpha-Hemolysin Pore. Biophys. J. 2000, 79, 1967–1975. 10.1016/S0006-3495(00)76445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braha O.; Webb J.; Gu L. Q.; Kim K.; Bayley H. Carriers versus Adapters in Stochastic Sensing. ChemPhysChem 2005, 6, 889–892. 10.1002/cphc.200400595. [DOI] [PubMed] [Google Scholar]
- Cornish V. W.; Hahn K. M.; Schultz P. G. Site-Specific Protein Modification Using a Ketone Handle. J. Am. Chem. Soc. 1996, 118, 8150–8151. 10.1021/ja961216x. [DOI] [Google Scholar]
- Mahal L. K.; Yarema K. J.; Bertozzi C. R. Engineering Chemical Reactivity on Cell Surfaces through Oligosaccharide Biosynthesis. Science 1997, 276, 1125–1128. 10.1126/science.276.5315.1125. [DOI] [PubMed] [Google Scholar]
- Ayers B.; Blaschke U. K.; Camarero J. A.; Cotton G. J.; Holford M.; Muir T. W. Introduction of Unnatural Amino Acids into Proteins Using Expressed Protein Ligation. Biopolymers 1999, 51, 343–354. . [DOI] [PubMed] [Google Scholar]
- Wang L.; Zhang Z.; Brock A.; Schultz P. G. Addition of the Keto Functional Group to the Genetic Code of Escherichia Coli. Proc. Natl. Acad. Sci. U. S. A. 2003, 100, 56–61. 10.1073/pnas.0234824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Canosa J. B.; Dawson P. E. An Efficient Fmoc-Spps Approach for the Generation of Thioester Peptide Precursors for Use in Native Chemical Ligation. Angew. Chem., Int. Ed. 2008, 47, 6851–5. 10.1002/anie.200705471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movileanu L.; Cheley S.; Bayley H. Partitioning of Individual Flexible Polymers into a Nanoscopic Protein Pore. Biophys. J. 2003, 85, 897–910. 10.1016/S0006-3495(03)74529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglia G.; Heron A. J.; Hwang W. L.; Holden M. A.; Mikhailova E.; Li Q. H.; Cheley S.; Bayley H. Droplet Networks with Incorporated Protein Diodes Show Collective Properties. Nat. Nanotechnol. 2009, 4, 437–440. 10.1038/nnano.2009.121. [DOI] [PubMed] [Google Scholar]
- Ciaccia M.; Di Stefano S. Mechanisms of Imine Exchange Reactions in Organic Solvents. Org. Biomol. Chem. 2015, 13, 646–654. 10.1039/C4OB02110J. [DOI] [PubMed] [Google Scholar]
- Banerjee A.; Mikhailova E.; Cheley S.; Gu L. Q.; Montoya M.; Nagaoka Y.; Gouaux E.; Bayley H. Molecular Bases of Cyclodextrin Adapter Interactions with Engineered Protein Nanopores. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 8165–8170. 10.1073/pnas.0914229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschke U. K.; Cotton G. J.; Muir T. W. Synthesis of Multi-Domain Proteins Using Expressed Protein Ligation: Strategies for Segmental Isotopic Labeling of Internal Regions. Tetrahedron 2000, 56, 9461–9470. 10.1016/S0040-4020(00)00830-9. [DOI] [Google Scholar]
- Arnold U.; Hinderaker M. P.; Nilsson B. L.; Huck B. R.; Gellman S. H.; Raines R. T. Protein Prosthesis: A Semisynthetic Enzyme with a Beta-Peptide Reverse Turn. J. Am. Chem. Soc. 2002, 124, 8522–8523. 10.1021/ja026114n. [DOI] [PubMed] [Google Scholar]
- Kienhofer A.; Kast P.; Hilvert D. Selective Stabilization of the Chorismate Mutase Transition State by a Positively Charged Hydrogen Bond Donor. J. Am. Chem. Soc. 2003, 125, 3206–3207. 10.1021/ja0341992. [DOI] [PubMed] [Google Scholar]
- Sayer J. M.; Peskin M.; Jencks W. P. Imine-Forming Elimination-Reactions 0.1. General Base and Acid Catalysis and Influence of Nitrogen Substituent on Rates and Equilibria for Carbinolamine Dehydration. J. Am. Chem. Soc. 1973, 95, 4277–4287. 10.1021/ja00794a600. [DOI] [Google Scholar]
- Sayer J. M.; Pinsky B.; Schonbrunn A.; Washtien W. Mechanism of Carbinolamine Formation. J. Am. Chem. Soc. 1974, 96, 7998–8009. 10.1021/ja00833a027. [DOI] [Google Scholar]
- Suratt E. C.; Proffitt J. R.; Lester C. T. Rate of Oxime Formation of Some Aryl Alkyl Ketones. J. Am. Chem. Soc. 1950, 72, 1561–1561. 10.1021/ja01160a036. [DOI] [Google Scholar]
- Selvaraj K.; Nanjappan P.; Ramalingam K.; Ramarajan K. Reactivities of Variously Substituted 4-Heteracyclohexanones in the Formation of Oximes. J. Chem. Soc., Perkin Trans. 2 1983, 49–52. 10.1039/p29830000049. [DOI] [Google Scholar]
- Movileanu L.; Bayley H. Partitioning of a Polymer into a Nanoscopic Protein Pore Obeys a Simple Scaling Law. Proc. Natl. Acad. Sci. U. S. A. 2001, 98, 10137–10141. 10.1073/pnas.181089798. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.