Abstract
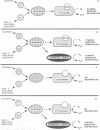
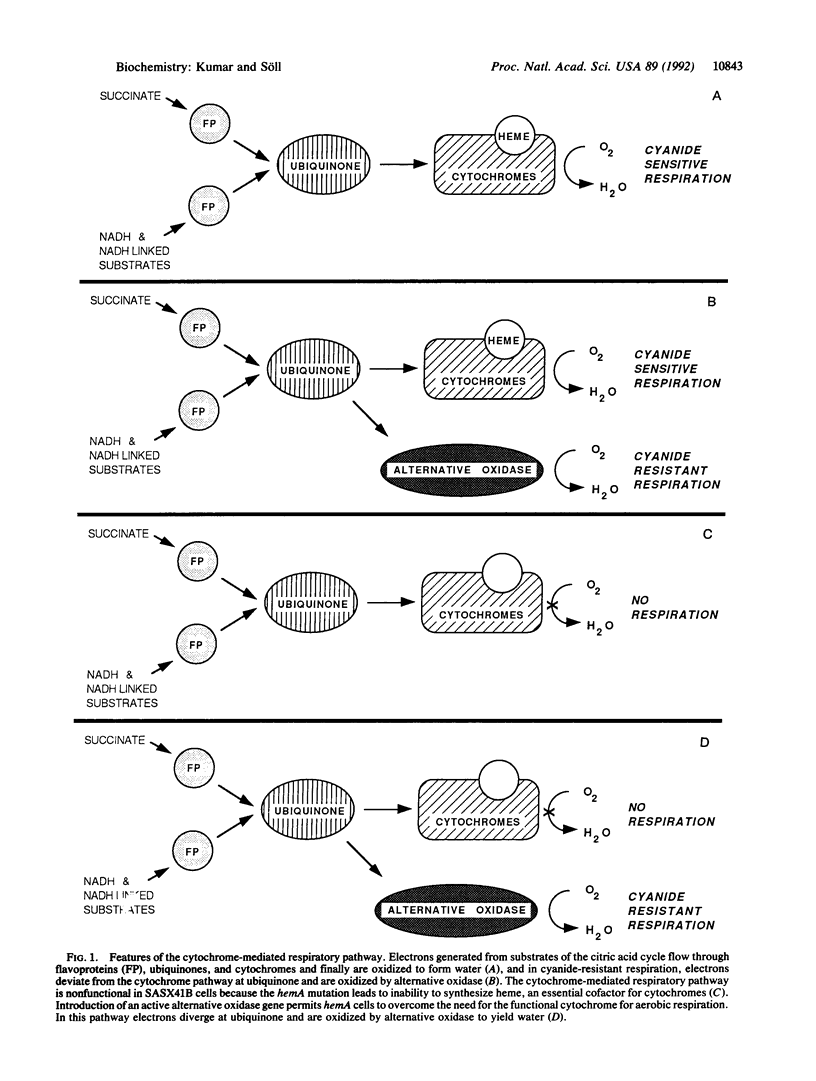
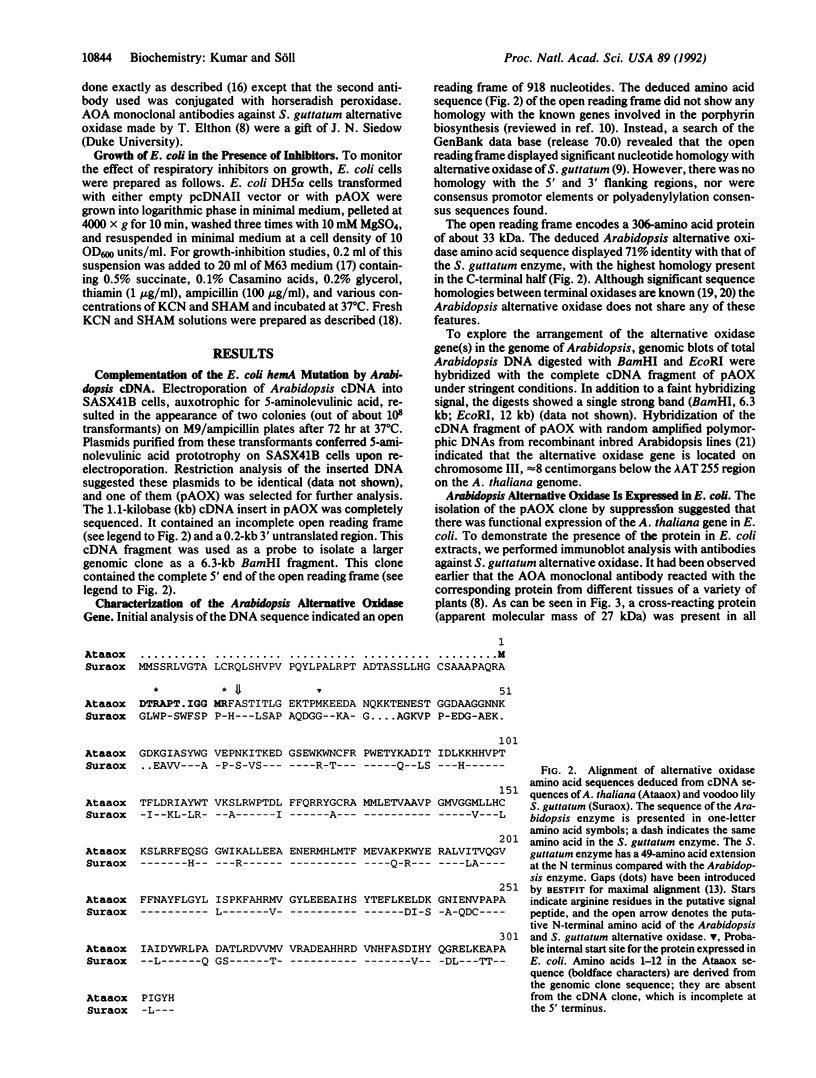
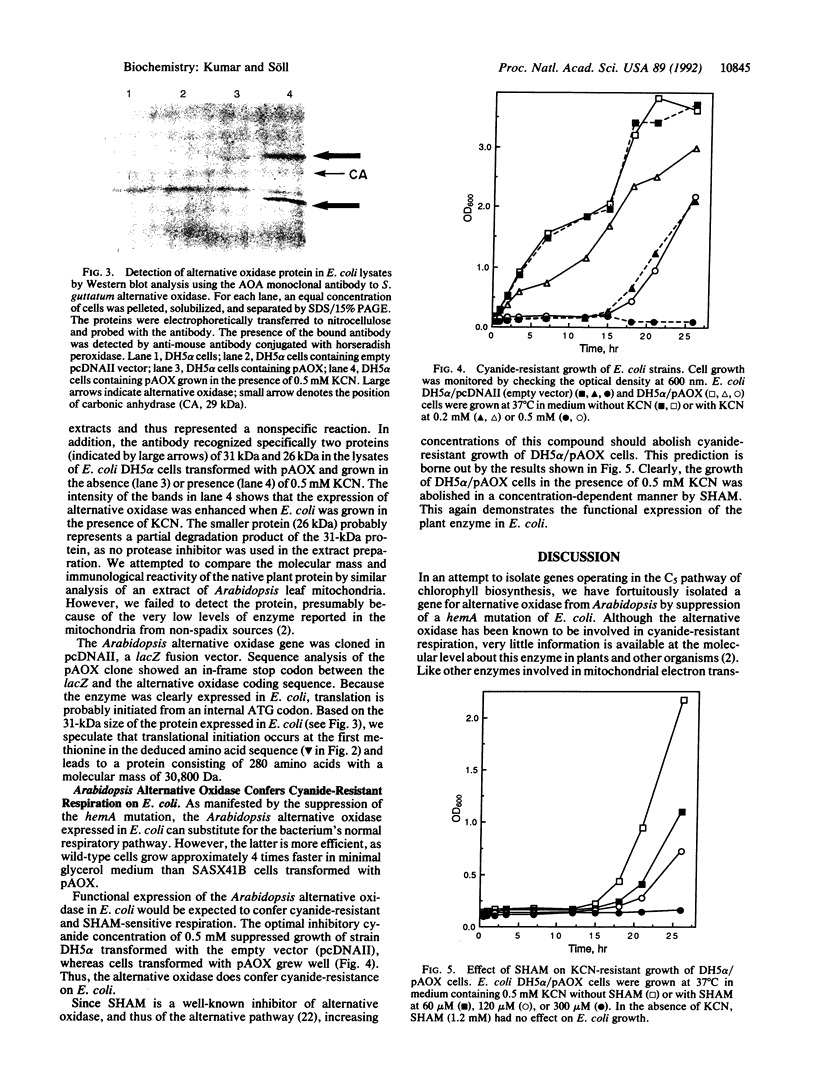
Glutamyl-tRNA reductase, encoded by the hemA gene, is the first enzyme in porphyrin biosynthesis in many organisms. Hemes, important porphyrin derivatives, are essential components of redox enzymes, such as cytochromes. Thus a hemA Escherichia coli strain (SASX41B) is deficient in cytochrome-mediated aerobic respiration. Upon complementation of this strain with an Arabidopsis thaliana cDNA library, we isolated a clone which permitted the SASX41B strain to grow aerobically. The clone encodes the gene for Arabidopsis alternative oxidase, whose deduced amino acid sequence was found to have 71% identity with that of the enzyme from the voodoo lily, Sauromatum guttatum. The Arabidopsis protein is expressed as a 31-kDa protein in E. coli and confers on this organism cyanide-resistant growth, which in turn is sensitive to salicylhydroxamate. This implies that a single polypeptide is sufficient for alternative oxidase activity. Based on these observations we propose that a cyanide-insensitive respiratory pathway operates in the transformed E. coli hemA strain. Introduction of this pathway now opens the way to genetic/molecular biological investigations of alternative oxidase and its cofactor.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Marks C. B., Lazarus R., Miller J., Stafford K., Seymour J., Light D., Rastetter W., Estell D. Production of 2-Keto-L-Gulonate, an Intermediate in L-Ascorbate Synthesis, by a Genetically Modified Erwinia herbicola. Science. 1985 Oct 11;230(4722):144–149. doi: 10.1126/science.230.4722.144. [DOI] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Cloning and expression of a structural gene from Chlorobium vibrioforme that complements the hemA mutation in Escherichia coli. J Bacteriol. 1990 Mar;172(3):1656–1659. doi: 10.1128/jb.172.3.1656-1659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandrin S. V., Rabinovich P. M., Stepanov A. I. Tri gruppy stsepleniia genov biosinteza riboflavina Escherichia coli. Genetika. 1983 Sep;19(9):1419–1425. [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Cole S. T. Nucleotide sequence coding for the flavoprotein subunit of the fumarate reductase of Escherichia coli. Eur J Biochem. 1982 Mar 1;122(3):479–484. doi: 10.1111/j.1432-1033.1982.tb06462.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D., Ratzkin B. J., Osslund T. D., Simon M. J., Wackett L. P., Gibson D. T. Expression of naphthalene oxidation genes in Escherichia coli results in the biosynthesis of indigo. Science. 1983 Oct 14;222(4620):167–169. doi: 10.1126/science.6353574. [DOI] [PubMed] [Google Scholar]
- Gennis R. B. Some recent advances relating to prokaryotic cytochrome c reductases and cytochrome c oxidases. Biochim Biophys Acta. 1991 May 23;1058(1):21–24. doi: 10.1016/s0005-2728(05)80260-9. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Henry M. F., Nyns E. D. Cyanide-insensitive respiration. An alternative mitochondrial pathway. Subcell Biochem. 1975 Mar;4(1):1–65. [PubMed] [Google Scholar]
- Hiser C., McIntosh L. Alternative Oxidase of Potato Is an Integral Membrane Protein Synthesized de Novo during Aging of Tuber Slices. Plant Physiol. 1990 May;93(1):312–318. doi: 10.1104/pp.93.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq S., Palmer J. M. Isolation of a cyanide-resistant duroquinol oxidase from Arum maculatum mitochondria. FEBS Lett. 1978 Nov 15;95(2):217–220. doi: 10.1016/0014-5793(78)80997-1. [DOI] [PubMed] [Google Scholar]
- Jahn D., Verkamp E., Söll D. Glutamyl-transfer RNA: a precursor of heme and chlorophyll biosynthesis. Trends Biochem Sci. 1992 Jun;17(6):215–218. doi: 10.1016/0968-0004(92)90380-r. [DOI] [PubMed] [Google Scholar]
- Lambowitz A. M., Slayman C. W. Cyanide-resistant respiration in Neurospora crassa. J Bacteriol. 1971 Dec;108(3):1087–1096. doi: 10.1128/jb.108.3.1087-1096.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt A., Ladenstein R., Huber R., Bolognesi M., Avigliano L., Petruzzelli R., Rossi A., Finazzi-Agró A. Refined crystal structure of ascorbate oxidase at 1.9 A resolution. J Mol Biol. 1992 Mar 5;224(1):179–205. doi: 10.1016/0022-2836(92)90583-6. [DOI] [PubMed] [Google Scholar]
- Moore A. L., Siedow J. N. The regulation and nature of the cyanide-resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta. 1991 Aug 23;1059(2):121–140. doi: 10.1016/s0005-2728(05)80197-5. [DOI] [PubMed] [Google Scholar]
- Reiter R. S., Williams J. G., Feldmann K. A., Rafalski J. A., Tingey S. V., Scolnik P. A. Global and local genome mapping in Arabidopsis thaliana by using recombinant inbred lines and random amplified polymorphic DNAs. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1477–1481. doi: 10.1073/pnas.89.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads D. M., McIntosh L. Isolation and characterization of a cDNA clone encoding an alternative oxidase protein of Sauromatum guttatum (Schott). Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2122–2126. doi: 10.1073/pnas.88.6.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Holm L., Lemieux L., Lübben M., van der Oost J. The happy family of cytochrome oxidases. Biochem Soc Trans. 1991 Aug;19(3):608–612. doi: 10.1042/bst0190608. [DOI] [PubMed] [Google Scholar]
- Schell M. A. Cloning and expression in Escherichia coli of the naphthalene degradation genes from plasmid NAH7. J Bacteriol. 1983 Feb;153(2):822–829. doi: 10.1128/jb.153.2.822-829.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonbaum G. R., Bonner W. D., Jr, Storey B. T., Bahr J. T. Specific inhibition of the cyanide-insensitive respiratory pathway in plant mitochondria by hydroxamic acids. Plant Physiol. 1971 Jan;47(1):124–128. doi: 10.1104/pp.47.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkamp E., Jahn M., Jahn D., Kumar A. M., Söll D. Glutamyl-tRNA reductase from Escherichia coli and Synechocystis 6803. Gene structure and expression. J Biol Chem. 1992 Apr 25;267(12):8275–8280. [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]