Abstract
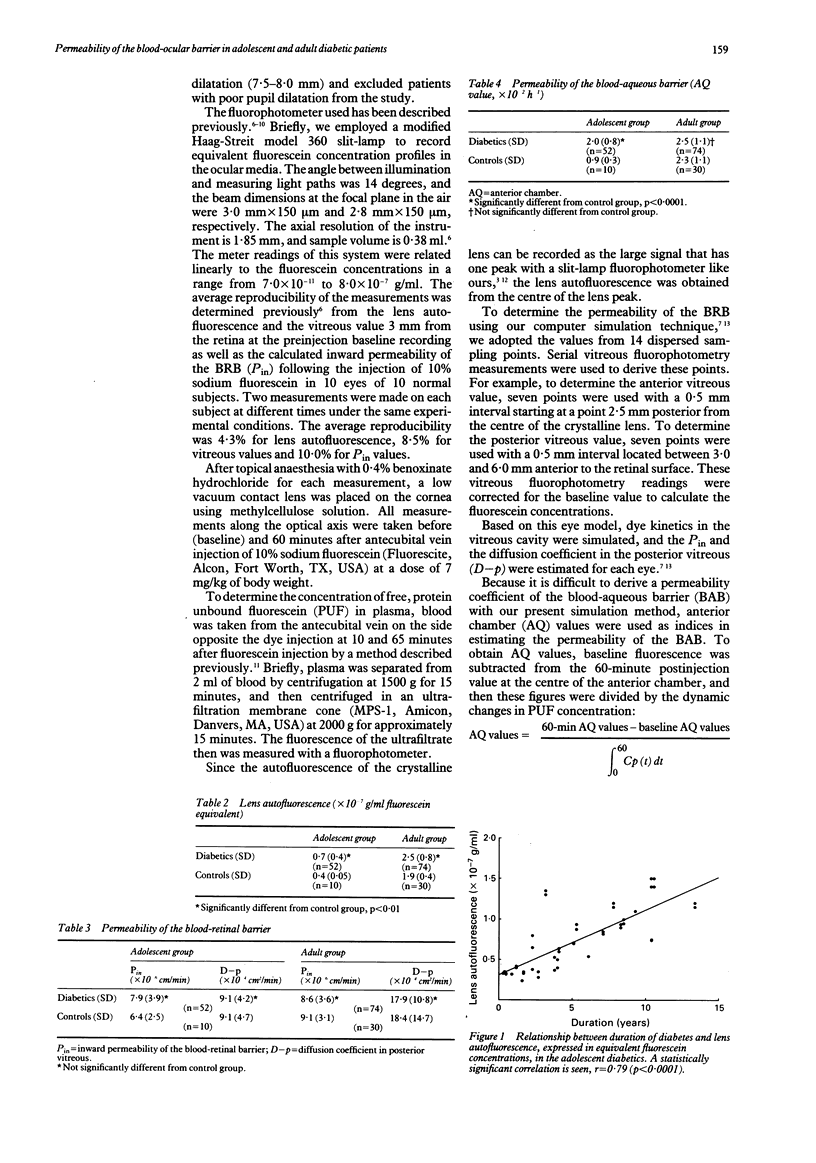
The permeability of the blood-ocular barrier was examined by fluorophotometry in adolescent and adult diabetic patients before the onset of retinopathy. The adolescent group consisted of 52 eyes of 52 insulin dependent diabetic patients aged 11 to 19 years and a control group of 10 eyes of 10 normal adolescents. The adult group consisted of 74 eyes of 74 non-insulin dependent diabetics and a control group of 30 eyes of 30 normal adults. The increase in lens autofluorescence in the adolescent diabetic patients compared with the controls was striking and showed a significant positive correlation (r = 0.79, p < 0.0001) with the duration of diabetes. Anterior chamber (AQ) values, an index of the permeability of the blood-aqueous barrier (BAB), increased in the adolescent diabetic patients compared with the controls and showed a significant positive correlation with glycosylated haemoglobin levels. No significant differences from the controls were observed regarding the permeability of the blood-retinal barrier (BRB). In the adult group there was no significant difference in either the permeability of the BRB or the AQ values between the diabetic and the control groups. Our results suggest that adolescent diabetic patients differ from adults in that BAB permeability is increased before the onset of retinopathy, suggesting that this is the cause of the striking increase in lens autofluorescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleeker J. C., van Best J. A., Vrij L., van der Velde E. A., Oosterhuis J. A. Autofluorescence of the lens in diabetic and healthy subjects by fluorophotometry. Invest Ophthalmol Vis Sci. 1986 May;27(5):791–794. [PubMed] [Google Scholar]
- Bursell S. E., Delori F. C., Yoshida A. Instrument characterization for vitreous fluorophotometry. Curr Eye Res. 1981;1(12):711–716. doi: 10.3109/02713688108998369. [DOI] [PubMed] [Google Scholar]
- Bursell S. E., Delori F. C., Yoshida A., Parker J. S., Collas G. D., McMeel J. W. Vitreous fluorophotometric evaluation of diabetics. Invest Ophthalmol Vis Sci. 1984 Jun;25(6):703–710. [PubMed] [Google Scholar]
- Cunha-Vaz J., Faria de Abreu J. R., Campos A. J. Early breakdown of the blood-retinal barrier in diabetes. Br J Ophthalmol. 1975 Nov;59(11):649–656. doi: 10.1136/bjo.59.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979 Mar-Apr;23(5):279–296. doi: 10.1016/0039-6257(79)90158-9. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Tanaka K., Taniguchi Y. Disruption of iridial blood-aqueous barrier in experimental diabetic rats. Graefes Arch Clin Exp Ophthalmol. 1982;219(4):159–164. doi: 10.1007/BF02156840. [DOI] [PubMed] [Google Scholar]
- Jedziniak J. A., Kinoshita J. H., Yates E. M., Benedek G. B. The concentration and localization of heavy molecular weight aggregates in aging normal and cataractous human lenses. Exp Eye Res. 1975 Apr;20(4):367–369. doi: 10.1016/0014-4835(75)90118-9. [DOI] [PubMed] [Google Scholar]
- Klein S., Marré E., Zenker H. J., Koza K. D. Zur Korrelation von diabetischer Irido- und Retinopathie. Fortschr Ophthalmol. 1983;79(5):428–430. [PubMed] [Google Scholar]
- Kurzel R. B., Wolbarsht M. L., Yamanashi B. S. Spectral studies on normal and cataractous intact human lenses. Exp Eye Res. 1973 Oct 10;17(1):65–71. doi: 10.1016/0014-4835(73)90168-1. [DOI] [PubMed] [Google Scholar]
- Monnier V. M., Cerami A. Nonenzymatic browning in vivo: possible process for aging of long-lived proteins. Science. 1981 Jan 30;211(4481):491–493. doi: 10.1126/science.6779377. [DOI] [PubMed] [Google Scholar]
- Sparrow J. M., Bron A. J., Brown N. A., Neil H. A. Autofluorescence of the crystalline lens in early and late onset diabetes. Br J Ophthalmol. 1992 Jan;76(1):25–31. doi: 10.1136/bjo.76.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A., Li S., Sigelman J. Age-dependent changes in the molecular size of human lens proteins and their relationship to light scatter. Invest Ophthalmol. 1974 Oct;13(10):795–798. [PubMed] [Google Scholar]
- Yoshida A., Furukawa H., Delori F. C., Bursell S. E., Trempe C. L., McMeel J. W. Effect of vitreous detachment on vitreous fluorophotometry. Arch Ophthalmol. 1984 Jun;102(6):857–860. doi: 10.1001/archopht.1984.01040030677017. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Hosaka A. [A study on blood-retinal barrier in myopia--analysis employing vitreous fluorophotometry and computer simulation]. Nippon Ganka Gakkai Zasshi. 1986 Mar;90(3):527–533. [PubMed] [Google Scholar]
- Yoshida A., Ishiko S., Kojima M., Lipsky S. N. Blood-ocular barrier permeability in monkeys. Br J Ophthalmol. 1992 Feb;76(2):84–87. doi: 10.1136/bjo.76.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Ishiko S., Kojima M. Outward permeability of the blood-retinal barrier. Graefes Arch Clin Exp Ophthalmol. 1992;230(1):78–83. doi: 10.1007/BF00166767. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Kojima M., Ogasawara H., Ishiko S. Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetic patients without retinopathy. Ophthalmology. 1991 Aug;98(8):1266–1271. doi: 10.1016/s0161-6420(91)32144-4. [DOI] [PubMed] [Google Scholar]


