TO THE EDITOR
Malignancies and infection remain frequent causes of morbidity and mortality in immunosuppressed human kidney transplant recipients (KTRs) (Legendre et al., 2014; Marcen, 2009). Although the goal of immunosuppression is to prolong organ graft survival, each drug class currently utilized in patients has drawbacks. The calcineurin inhibitor (CNI) tacrolimus is the current best drug to minimize acute organ rejection (Goring et al., 2014), but it is associated with the highest cancer risk (squamous cell carcinoma [SCC] included) after organ transplantation (Arichi et al., 2008; Cowlrick et al., 2008; Navarro et al., 2008).
CNIs dampen DNA repair, cell cycle checkpoint signaling, and apoptosis after ultraviolet light exposure in cultured human keratinocytes (Ming et al., 2015; Yarosh et al., 2005). CNIs also dampen transcription of the tumor suppressor p53 and reduce its ability to promote keratinocyte senescence, resulting in enhanced cellular tumorigenic potential (Dotto, 2011; Wu et al., 2010). Inhibiting calcineurin using either CNI drugs or through genetic ablation in mice reduces keratinocyte differentiation as assessed by examining keratins 1 and 10, loricrin, filaggrin, involucrin, p21waf1/ciP1, and p27kip1 (Pena et al., 2010; Santini et al., 2001). These studies were conducted by culturing keratinocytes in the presence of immunosuppressive drugs or by using mouse or mouse-human xenograft models. The consequences of systemic CNI exposure on the epidermis of human KTRs and on keratinocytes derived from these patients have not been fully characterized.
The desmosomal adhesion protein desmoglein 1 (Dsg1) is emerging as a regulator of epidermal differentiation, an important contributor to the epidermal barrier, and a key player in keratinocyte/immune cell communication (Getsios et al., 2009; Hammers and Stanley, 2013; Harmon et al., 2013; Samuelov et al., 2013). Decreased Dsg1 expression may disrupt the keratinocyte differentiation/proliferation balance, allowing SCC initiation. We recently showed that compared with unexposed controls, ultraviolet light-exposed primary human keratinocytes exhibit reduced Dsg1, concomitant with decreased differentiation and increased pro-proliferative epidermal growth factor receptor pathway signaling. Increasing Dsg1 expression, either through genetic overexpression or through treatment with a histone deacetylase inhibitor restores differentiation and decreases pro-proliferative signaling (Johnson et al., 2014a). Because differentiation is reduced in keratinocytes cultured with CNIs (Pena et al., 2010; Santini et al., 2001), we hypothesized that Dsg1 as an upstream driver of epidermal differentiation would be reduced in the epidermis of KTRs systemically treated with CNIs compared with immunocompetent patients (ICPs). Indeed, compared with normal human skin, KTRs on systemic CNIs exhibited reduced epidermal Dsg1 expression, which remained reduced in cultured primary keratinocytes derived from KTRs compared with ICPs.
A total of 16 ICPs and 15 KTRs were included in this study, which was approved through Northwestern University’s Biomedical Institutional Review Board (project numbers STU00069552 and STU00009443). KTRs gave written informed consent for participation in the study. The skin utilized for the ICP group was predominantly discarded tissue removed during abdominoplasty with 2 exceptions (buttock and breast skin, see Supplementary Table S1 online). No consent was required for use of the majority of these tissues except patient ICP-14 who was consented since tissue was sent to pathology for processing as standard of care. Characteristics of the 31 individuals who donated skin biopsies, including coded patient identifier, age, sex, ethnicity, body site from which the biopsy was taken, and immunosuppressive drug regimen of the KTRs at the time of biopsy are given in Supplementary Table S1. The majority of the KTRs (14 of 15) were on a combination of tacrolimus and mycophenolate mofetil at the time of biopsy, whereas one patient (KTR-11) was on cyclosporine (another CNI) and mycophenolate mofetil. The average immunosuppressive therapy duration at the time of biopsy was 14 months (range, 8–24 months).
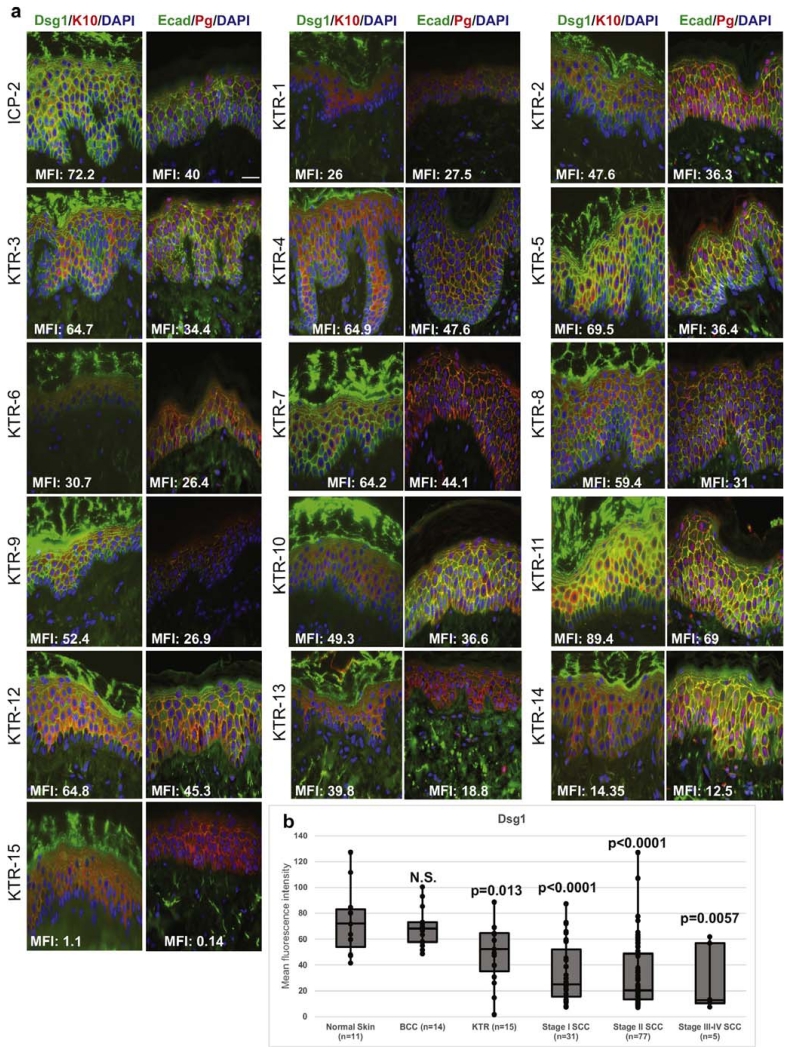
The epidermal morphologies of 1 ICP biopsy (ICP-2) and all 15 KTR biopsies appeared normal (Supplementary Figure S1 online). Histological sections from these biopsies were stained with antibodies against Dsg1, keratin 10, E-cadherin, and plakoglobin and examined using indirect immunofluorescence microscopy (Figure 1a). Histological samples were not available from many of the ICPs because the samples were purchased as vials of cultured primary keratinocytes from companies or were considered discarded tissue not archived within Northwestern University’s Skin Disease Research Center (Supplementary Table S2 online). Therefore, to compare epidermal Dsg1 expression levels between the KTR biopsies, normal human skin, and SCC tissues, human tissue microarrays were utilized. All samples were analyzed for Dsg1 mean fluorescence intensity using indirect immunofluorescence automated microscopy and analysis software (Figure 1b). Data are shown as box and whisker plots where whiskers indicate maximum and minimum Dsg1 mean fluorescence intensity, box boundaries indicate 25% and 75% values, and the midline indicates the median. Dsg1 expression was significantly reduced in skin biopsies from KTRs compared with normal human skin on the arrays (P = 0.013) and further reduced in the staged SCC tissues (P < 0.006 in each stage-control comparison). Dsg1 levels were not significantly reduced in cases of basal cell carcinoma included on the tissue microarrays compared with normal skin.
Figure 1. Epidermal Dsg1 expression was reduced in KTRs and staged SCC tissues compared with normal skin.
(a) Indirect immunofluorescence microscopy of Dsg1 (green, left panels in each column with mean fluorescence intensity [MFI]), K10 (red, left panels in each column), Ecad (green, right panels in each column with MFI), and Pg (red, right panels in each column) in abdominal skin biopsies of 1 immunocompetent patient (ICP-2) and 15 immunosuppressed kidney transplant recipients (KTR-1–15). 4′,6-Diamidino-2-phenylindole (DAPI, blue = nuclear counterstain). Bar = 50 μm. (b) Box and whisker MFI plots representing epidermal Dsg1 expression in normal human skin cores, BCCs, and staged SCCs (tissue microarrays) and in the samples shown in (a). TissueGnostics automated microscopy and tissue quest analysis software were used to determine Dsg1 MFI. Identical settings were applied for all image acquisition and analysis. NS, not significant, P< 0.05 considered significant. BCC, basal cell carcinoma; Dsg1, desmoglein 1; Ecad, E-cadherin; K10, keratin 10; Pg, plakoglobin; SCC, squamous cell carcinoma.
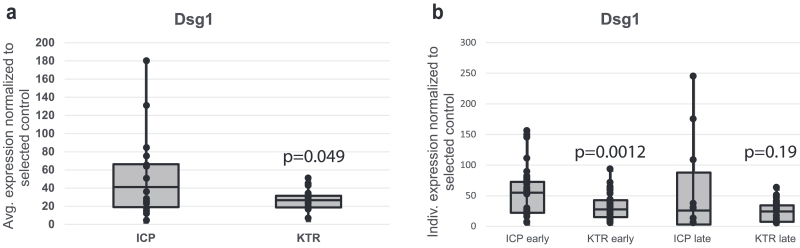
Keratinocytes cultured from all 16 ICPs and 15 KTRs (Supplementary Table S2) were induced to undergo differentiation in a high calcium medium. Expression levels of Dsg1, keratin 10, E-cadherin, and plakoglobin proteins 48 hours after inducing differentiation were examined by immunoblot and quantified using densitometry followed by normalization to a protein loading control and a reference sample loaded on each gel (Figure 2, Supplementary Figures S2 and S3 online, and Supplementary Tables S2 and S3 online). The greater variability in expression levels of all four analyzed proteins in cells derived from ICPs compared with those from KTRs may reflect the different culture initiation conditions, because ICP-derived cells were purchased from three sources compared with one source for the KTR-derived cells. When experimental replicates of cells cultured from each individual human skin biopsy were averaged (Figure 2a), Dsg1 levels were lower in keratinocytes derived from KTRs than those from ICPs (P = 0.049), whereas the expression levels of keratin 10, E-cadherin, and plakoglobin were not significantly different between groups (Supplementary Figure S2). When data points from each cell culture were considered individually rather than being averaged (Figure 2b), Dsg1 levels were significantly lower in keratinocytes derived from KTRs compared with ICPs when cultured <5 passages or <35 days (P = 0.0012). This significance was lost in keratinocytes derived from the two groups and cultured >6 passages or >36 days (P = 0.19). Analysis in this way considers each individual cell culture to be independent because cultured cells undergo changes and the cells used in this study were grown for different numbers of days and passages (Supplementary Table S2).
Figure 2. Dsg1 expression remained reduced in cultured keratinocytes derived from KTRs compared with those derived from ICPs.
(a) Protein expression levels of Dsg1 were compared using immunoblots of lysates harvested from cultured keratinocytes derived from ICPs or KTRs. Keratinocytes were grown to confluence in a low calcium-containing (0.07 mM) medium, switched to a high calcium-containing medium (1.2 mM) to induce differentiation, and harvested after 48 hours. The Dsg1 densitometry values averaged from up to five repeated experiments were lower in keratinocytes derived from KTRs than those from ICPs (P = 0.049). (b) When each densitometry data point was taken individually rather than averaging, Dsg1 levels were significantly lower in keratinocytes derived from KTRs compared with ICPs when cultured <5 passages or <35 days (early, P = 0.0012). This significance was lost in keratinocytes derived from the two groups and cultured >6 passages or >36 days (late, P = 0.19). Dsg1, desmoglein 1; ICPs, immunocompetent patients; KTRs, kidney transplant recipients.
As a readout of whether reduced Dsg1 levels correlated with reduced overall differentiation, loricrin levels were examined by immunoblot in samples harvested 72 hours after induction of differentiation in a high calcium medium. Indeed, fewer KTR-derived keratinocyte samples had detectable loricrin at that time point compared with those from ICPs (19% vs. 54% of samples, respectively). Immunoblots are shown in Supplementary Figure S3. Detailed experimental methods are provided as Supplementary Materials and Methods online.
In this study, we showed that compared with ICPs, KTRs systemically treated with CNIs and mycophenolate mofetil exhibited significantly reduced epidermal expression of Dsg1, a regulator of keratinocyte differentiation and barrier function. Although we did not functionally connect Dsg1 loss with SCC development in this study, previous reports have shown that reduction in Dsg1 expression increases epidermal growth factor receptor signaling, which may allow pro-tumorigenic keratinocyte proliferation, especially in the context of ultraviolet light exposure (Getsios et al., 2009; Hammers and Stanley, 2013; Harmon et al., 2013; Johnson et al., 2014a). Reduction in Dsg1 expression in KTRs compared with normal skin was intermediate to Dsg1 reduction found in staged human SCC tissues on tissue microarrays, consistent with the idea that Dsg1 loss is one of multiple cellular changes that occur during progression to SCC in the KTRs. Because CNIs can dampen transcriptional activation of p53 by the activating transcription factor 3 (Wu et al., 2010), it is possible that transcriptional regulation of Dsg1, such as by AP-1, p63, or grainyhead-like 1 (reviewed in Johnson et al., 2014b), may also be dampened by systemic CNI exposure, a topic for future study. Further, we previously showed that Dsg1 expression can be increased through treatment of keratinocytes with a histone deacetylase inhibitor, indicating possible epigenetic regulation of Dsg1 expression Johnson et al., 2014a). Certain histone deacetylase inhibitors have been approved for clinical use (Jawed et al., 2014), suggesting a possible method to modulate Dsg1 expression with the goal of protecting against epidermal SCC and infections in KTRs.
Supplementary Material
ACKNOWLEDGMENTS
This work was predominantly supported by a Pilot and Feasibility Grant to JLJ and JKR through Northwestern University’s Skin Disease Research Center (SDRC) sponsored by a National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant (2P30AR057216). JLJ’s salary was supported in part by a Dermatology Foundation Research Career Development Award. KJG and JLJ were supported by NIH R01 AR041836 and R01 CA122151. We acknowledge the SDRC skin tissue engineering core and morphology and phenotyping core for assistance with this project. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Northwestern University SDRC or the NIH/NIAMS. We acknowledge the support of the Northwestern University Cancer Biostatistics Core and the Center for Advanced Microscopy, both funded by a Cancer Center Support Grant (NCI CA060553) awarded to the Robert H. Lurie Comprehensive Cancer Center.
Abbreviations
- CNI
calcineurin inhibitor
- Dsg
desmoglein
- ICP
immunocompetent patient
- KTR
kidney transplant recipient
- SSC
squamous cell carcinoma
Footnotes
ORCIDs
Jodi L. Johnson: http://orcid.org/0000-0002-8328-5587
Kathleen J. Green: http://orcid.org/0000-0001-7332-5867
John J. Friedewald: http://orcid.org/0000-0002-9344-9928
June K. Robinson: http://orcid.org/0000-0003-2331-3352
CONFLICT OF INTEREST
The authors state no conflicts of interest.
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at http://dx.d0i.0rg/l0.1016/j.jid.2016.06.001.
REFERENCES
- Arichi N, Kishikawa H, Nishimura K, Mitsui Y, Namba Y, Tokugawa S, et al. Malignancy following kidney transplantation. Transplant Proc. 2008;40:2400–2. doi: 10.1016/j.transproceed.2008.07.103. [DOI] [PubMed] [Google Scholar]
- Cowlrick I, Delventhal H, Kaipainen K, Krcmar C, Petan J, Schleibner S. Three-year follow-up of malignancies in tacrolimus-treated renal recipients–an analysis of European multicentre studies. Clin Transplant. 2008;22:372–7. doi: 10.1111/j.1399-0012.2008.00796.x. [DOI] [PubMed] [Google Scholar]
- Dotto CP. Calcineurin signaling as a negative determinant of keratinocyte cancer stem cell potential and carcinogenesis. Cancer Res. 2011;71:2029–33. doi: 10.1158/0008-5472.CAN-10-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getsios S, Simpson CL, Kojima S, Harmon R, Sheu LJ, Dusek RL, et al. Desmoglein 1-dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. J Cell Biol. 2009;185:1243–58. doi: 10.1083/jcb.200809044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring SM, Levy AR, Ghement I, Kalsekar A, Eyawo O, L’ltalien GJ, et al. A network meta-analysis of the efficacy of belatacept, cyclosporine and tacrolimus for immunosuppression therapy in adult renal transplant recipients. Curr Med Res Opin. 2014;30:1473–87. doi: 10.1185/03007995.2014.898140. [DOI] [PubMed] [Google Scholar]
- Hammers CM, Stanley JR. Desmoglein-1, differentiation, and disease. J Clin Invest. 2013;123:1419–22. doi: 10.1172/JCI69071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RM, Simpson CL, Johnson JL, Koetsier JL, Dubash AD, Najor NA, et al. Desmoglein-1/Erbin interaction suppresses ERK activation to support epidermal differentiation. J Clin Invest. 2013;123:1556–70. doi: 10.1172/JCI65220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sezary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1–223.e17. doi: 10.1016/j.jaad.2013.08.033. quiz 40-2. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Koetsier JL, Sirico A, Agidi AT, Antonini D, Missero C, et al. The desmosomal protein desmoglein 1 aids recovery of epidermal differentiation after acute UV light exposure. J Invest Dermatol. 2014a;134:2154–62. doi: 10.1038/jid.2014.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Najor NA, Green KJ. Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harb Perspect Med. 2014b;4:a015297. doi: 10.1101/cshperspect.a015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014;27:19–27. doi: 10.1111/tri.12217. [DOI] [PubMed] [Google Scholar]
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–43. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ming M, Zhao B, Qiang L, He YY. Effect of immunosuppressants tacrolimus and mycophenolate mofetil on the keratinocyte UVB response. Photochem Photobiol. 2015;91:242–7. doi: 10.1111/php.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro MD, Lopez-Andreu M, Rodriguez-Benot A, Aguera ML, Del Castillo D, Aljama P. Cancer incidence and survival in kidney transplant patients. Transplant Proc. 2008;40:2936–40. doi: 10.1016/j.transproceed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Pena JA, Losi-Sasaki JL, Gooch JL. Loss of calcineurin Aalpha alters keratinocyte survival and differentiation. J Invest Dermatol. 2010;130:135–40. doi: 10.1038/jid.2009.222. [DOI] [PubMed] [Google Scholar]
- Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet. 2013;45:1244–8. doi: 10.1038/ng.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini MP, Talora C, Seki T, Bolgan L, Dotto GP. Cross talk among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expression in keratinocyte differentiation. Proc Natl Acad Sci USA. 2001;98:9575–80. doi: 10.1073/pnas.161299698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Nguyen BC, Dziunycz P, Chang S, Brooks Y, Lefort K, et al. Opposing roles for calcineurin and ATF3 in squamous skin cancer. Nature. 2010;465:368–72. doi: 10.1038/nature08996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125:1020–5. doi: 10.1111/j.0022-202X.2005.23858.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.