Abstract
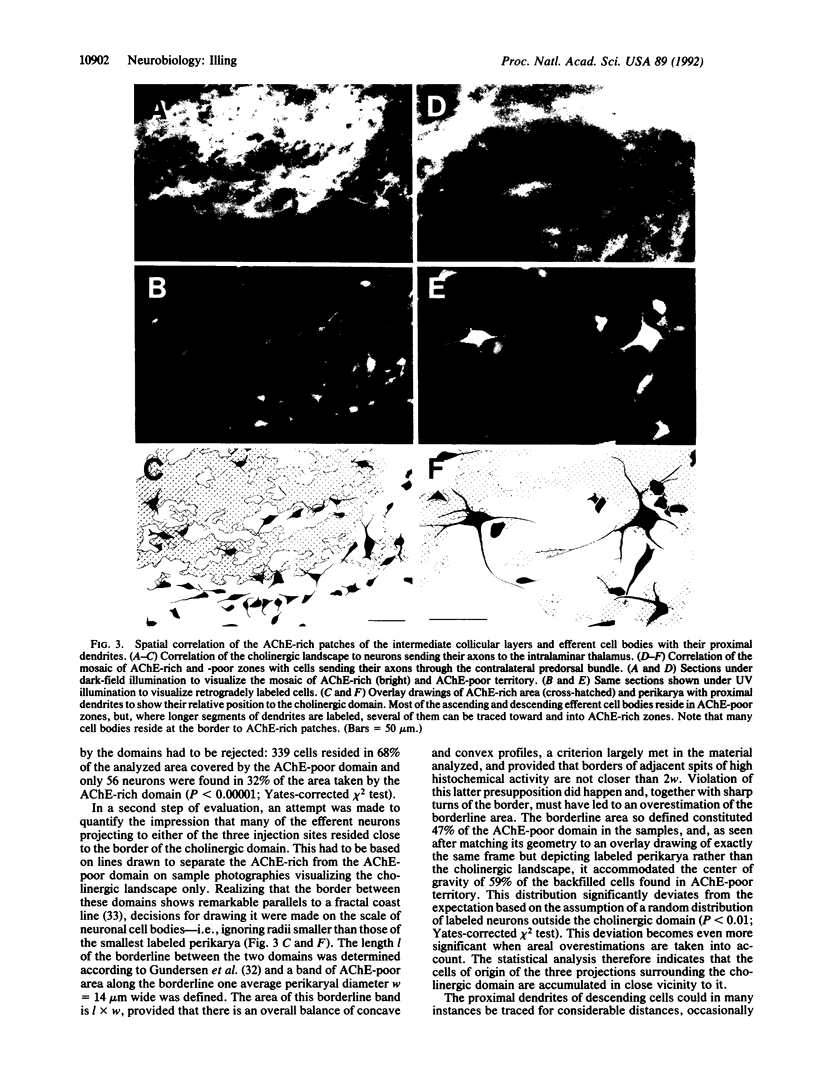
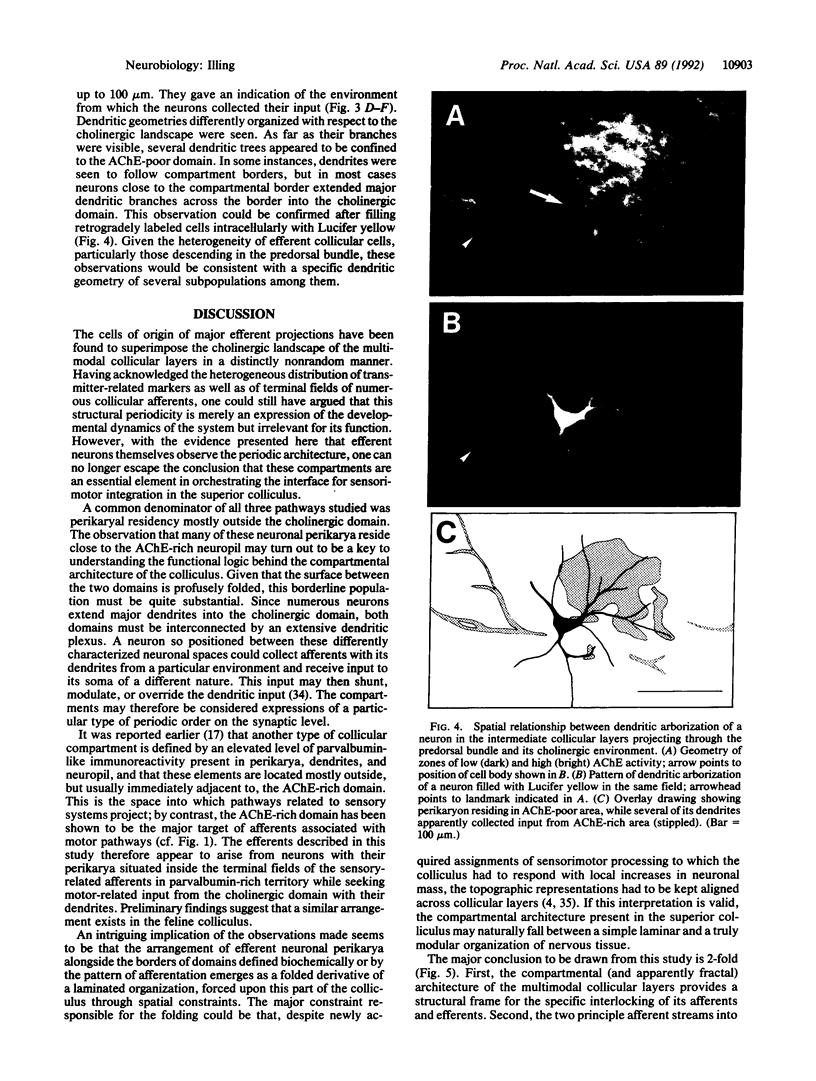
The superior colliculus is a layered structure in the mammalian midbrain serving multimodal sensorimotor integration. Its intermediate layers are characterized by a compartmental architecture. These compartments are apparent through the clustering of terminals of major collicular afferents, which in many instances match the heterogeneous distribution of tissue components such as acetylcholinesterase, choline acetyltransferase, substance P, and parvalbumin. The present study was undertaken to determine whether efferent cells observe this compartmental architecture. It was found that subpopulations of both descending and ascending collicular efferents originate from perikarya situated in characteristic positions relative to the collicular compartments defined by elevated acetylcholinesterase activity and that their dendrites appear to be specifically coordinated with the heterogeneous environment. With the specific interlocking of afferent and efferent neurons through spatially distinguished neural networks, the compartmental architecture apparently constitutes an essential element for the determination of information flow in the superior colliculus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behan M., Appell P. P., Graper M. J. Ultrastructural study of large efferent neurons in the superior colliculus of the cat after retrograde labeling with horseradish peroxidase. J Comp Neurol. 1988 Apr 8;270(2):171–184. doi: 10.1002/cne.902700203. [DOI] [PubMed] [Google Scholar]
- Beninato M., Spencer R. F. A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry. J Comp Neurol. 1986 Nov 22;253(4):525–538. doi: 10.1002/cne.902530409. [DOI] [PubMed] [Google Scholar]
- Bickford M. E., Hall W. C. Collateral projections of predorsal bundle cells of the superior colliculus in the rat. J Comp Neurol. 1989 May 1;283(1):86–106. doi: 10.1002/cne.902830108. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Azizi S. A., Mihailoff G. A., Woodward D. J. The tectopontine projection the the rat with comments on visual pathways to the basilar pons. J Comp Neurol. 1981 Oct 20;202(2):287–307. doi: 10.1002/cne.902020212. [DOI] [PubMed] [Google Scholar]
- Chalupa L. M., Rhoades R. W. Responses of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus. J Physiol. 1977 Sep;270(3):595–626. doi: 10.1113/jphysiol.1977.sp011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger U. C., Hubel D. H. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975 May;38(3):690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Ennis M., Shipley M. T., Behbehani M. M. A double-labeling method for AChE and fluorescent retrograde tracers. Brain Res Bull. 1990 Jan;24(1):113–118. doi: 10.1016/0361-9230(90)90294-a. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hall W. C., Fitzpatrick D., Klatt L. L., Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol. 1989 Sep 22;287(4):495–514. doi: 10.1002/cne.902870408. [DOI] [PubMed] [Google Scholar]
- Harting J. K., Van Lieshout D. P. Spatial relationships of axons arising from the substantia nigra, spinal trigeminal nucleus, and pedunculopontine tegmental nucleus within the intermediate gray of the cat superior colliculus. J Comp Neurol. 1991 Mar 22;305(4):543–558. doi: 10.1002/cne.903050403. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J., Midtgaard J. Dendrite processing in more ways than one . Trends Neurosci. 1989 Sep;12(9):313–315. doi: 10.1016/0166-2236(89)90036-2. [DOI] [PubMed] [Google Scholar]
- Huerta M. F., Frankfurter A. J., Harting J. K. The trigeminocollicular projection in the cat: patch-like endings within the intermediate gray. Brain Res. 1981 Apr 27;211(1):1–13. doi: 10.1016/0006-8993(81)90063-9. [DOI] [PubMed] [Google Scholar]
- Illing R. B. Choline acetyltransferase-like immunoreactivity in the superior colliculus of the cat and its relation to the pattern of acetylcholinesterase staining. J Comp Neurol. 1990 Jun 1;296(1):32–46. doi: 10.1002/cne.902960104. [DOI] [PubMed] [Google Scholar]
- Illing R. B., Graybiel A. M. Complementary and non-matching afferent compartments in the cat's superior colliculus: innervation of the acetylcholinesterase-poor domain of the intermediate gray layer. Neuroscience. 1986 Jun;18(2):373–394. doi: 10.1016/0306-4522(86)90160-0. [DOI] [PubMed] [Google Scholar]
- Illing R. B., Graybiel A. M. Convergence of afferents from frontal cortex and substantia nigra onto acetylcholinesterase-rich patches of the cat's superior colliculus. Neuroscience. 1985 Feb;14(2):455–482. doi: 10.1016/0306-4522(85)90303-3. [DOI] [PubMed] [Google Scholar]
- Illing R. B., Vogt D. M., Spatz W. B. Parvalbumin in rat superior colliculus. Neurosci Lett. 1990 Dec 11;120(2):197–200. doi: 10.1016/0304-3940(90)90037-a. [DOI] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc Natl Acad Sci U S A. 1983 May;80(9):2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leise E. M. Modular construction of nervous systems: a basic principle of design for invertebrates and vertebrates. Brain Res Brain Res Rev. 1990 Jan-Apr;15(1):1–23. doi: 10.1016/0165-0173(90)90009-d. [DOI] [PubMed] [Google Scholar]
- Mandelbrot B. How long is the coast of britain? Statistical self-similarity and fractional dimension. Science. 1967 May 5;156(3775):636–638. doi: 10.1126/science.156.3775.636. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo J. J., Senba E., Matsutani S., Takatsuji K., Fukui H., Tohyama M. Laminar and segregated distribution of immunoreactivities for some neuropeptides and adenosine deaminase in the superior colliculus of the rat. J Comp Neurol. 1989 Feb 15;280(3):410–423. doi: 10.1002/cne.902800307. [DOI] [PubMed] [Google Scholar]
- Norita M. Neurons and synaptic patterns in the deep layers of the superior colliculus of the cat. A Golgi and electron microscopic study. J Comp Neurol. 1980 Mar 1;190(1):29–48. doi: 10.1002/cne.901900104. [DOI] [PubMed] [Google Scholar]
- Redgrave P., Odekunle A., Dean P. Tectal cells of origin of predorsal bundle in rat: location and segregation from ipsilateral descending pathway. Exp Brain Res. 1986;63(2):279–293. doi: 10.1007/BF00236845. [DOI] [PubMed] [Google Scholar]
- Schnurr B., Spatz W. B., Illing R. B. Similarities and differences between cholinergic systems in the superior colliculus of guinea pig and rat. Exp Brain Res. 1992;90(2):291–296. doi: 10.1007/BF00227240. [DOI] [PubMed] [Google Scholar]
- Shepherd G. M. The neuron doctrine: a revision of functional concepts. Yale J Biol Med. 1972 Dec;45(6):584–599. [PMC free article] [PubMed] [Google Scholar]
- Sparks D. L. Neural cartography: sensory and motor maps in the superior colliculus. Brain Behav Evol. 1988;31(1):49–56. doi: 10.1159/000116575. [DOI] [PubMed] [Google Scholar]
- Sprague J. M. The role of the superior colliculus in facilitating visual attention and form perception. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1286–1290. doi: 10.1073/pnas.88.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein B. E. Development of the superior colliculus. Annu Rev Neurosci. 1984;7:95–125. doi: 10.1146/annurev.ne.07.030184.000523. [DOI] [PubMed] [Google Scholar]
- Tauchi M., Masland R. H. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984 Nov 22;223(1230):101–119. doi: 10.1098/rspb.1984.0085. [DOI] [PubMed] [Google Scholar]
- Tokunaga A., Otani K. Dendritic patterns of neurons in the rat superior colliculus. Exp Neurol. 1976 Aug;52(2):189–205. doi: 10.1016/0014-4886(76)90164-3. [DOI] [PubMed] [Google Scholar]
- Wallace M. N. Spatial relationship of NADPH-diaphorase and acetylcholinesterase lattices in the rat and mouse superior colliculus. Neuroscience. 1986 Oct;19(2):381–391. doi: 10.1016/0306-4522(86)90268-x. [DOI] [PubMed] [Google Scholar]
- Williams M. N., Faull R. L. The nigrotectal projection and tectospinal neurons in the rat. A light and electron microscopic study demonstrating a monosynaptic nigral input to identified tectospinal neurons. Neuroscience. 1988 May;25(2):533–562. doi: 10.1016/0306-4522(88)90257-6. [DOI] [PubMed] [Google Scholar]
- Yamasaki D. S., Krauthamer G. M., Rhoades R. W. Superior collicular projection to intralaminar thalamus in rat. Brain Res. 1986 Jul 23;378(2):223–233. doi: 10.1016/0006-8993(86)90925-x. [DOI] [PubMed] [Google Scholar]