Abstract
Background
Unscheduled bleeding is the main side effect of continuous oral contraceptive pills (OCPs) and has been correlated with the up-regulation of matrix metalloprotineases (MMPs). The study objective was to determine if prophylactic administration of doxycycline (an MMP inhibitor at low subantimicrobial doses) would prevent unscheduled bleeding during the initiation of a continuous OCP.
Study Design
Subjects using cyclic hormonal contraceptives (combined OCPs, patch or ring) without unscheduled bleeding were switched to continuous OCPs (20 mcg ethinyl estradiol/100 mcg levonorgestrel). They were randomized to receive daily doxycycline [sustained-release subantimicrobial dose (40 mg daily)] or placebo for the first 84 days and then observed for an additional 28 days on the continuous OCP alone. The number of bleeding/spotting days and the time in days it took to achieve amenorrhea were compared using a t test.
Results
Sixty-five subjects were randomized. Although the use of doxycycline did not significantly decrease the number of mean bleeding/spotting days in the first 84 days of the study [doxycycline 14.75 (SE 2.30), placebo 17.78 (2.31), p=.36], women who received doxycycline had a significantly earlier onset of amenorrhea [mean last day of bleeding/spotting doxycycline 61.7 (7.7), placebo 85.2 (6.7), p=.03].
Conclusion
The coadministration of subantimicrobial-dose doxycycline during initiation of continuous OCPs results in a significant reduction in the length of time needed to achieve amenorrhea.
Keywords: Continuous oral contraceptive pills, Matrix metalloproteinases, Bleeding, Contraceptive induced amenorrhea, Doxycycline, Subantimicrobial-dose doxycycline
1. Introduction
The oral contraceptive pill (OCP) brought to market over 50 years ago was designed around a 28-day cycle that included a 7-day hormone-free interval to induce a withdrawal bleed [1,2]. Many women, however, prefer contraceptive-induced amenorrhea to monthly bleeding [3,4]. Because there is no biologic benefit to a monthly withdrawal bleed, shortening or eliminating the hormone-free interval has gained popularity as a treatment modality for menstrual-related disorders and for personal or social reasons [3,5]. Continuous administration eliminates the number of scheduled days of bleeding [5]. Unfortunately, regular bleeding is often replaced by unscheduled bleeding and spotting. Only 16% women taking continuous OCPs will experience amenorrhea in the first 3 months of use [6]. Although unscheduled bleeding decreases over time, it can lead to dissatisfaction, method discontinuation and unplanned pregnancy.
Unscheduled endometrial bleeding has been associated with up-regulation of matrix metalloproteinases (MMPs), a group of zinc-dependent proteases that degrade the extra-cellular matrix [7–9]. Progesterone is known to regulate MMP activity, with increased expression of endometrial MMP-3 and MMP-9 associated with use of the levonorgestrel intrauterine system, subdermal levonorgestrel and depot medroxyprogesterone acetate [8–11]. Levels of MMPs from endometrial samples show a positive correlation with the amount of endometrial bleeding women experience with use of levonorgestrel implants [12]. Although endometrial MMP activity with use of OCPs has not been specifically studied, a similar effect would be expected with use of this progestin-dominant method.
Independent of its antimicrobial properties, doxycycline causes chelation of the zinc atom at the active site, thereby interfering with MMP activity [13]. Doxycycline results in MMP inhibition at much lower doses (e.g., 20–40 mg/day) than is required for an antimicrobial effect (100–200 mg/day). Thus, low-dose “subantimicrobial” regimens have been developed for the treatment of inflammatory conditions like rosacea [14,15]. Since subantimicrobial-dose doxycycline can be taken on a long-term basis without antimicrobial resistance, changes to normal microbial flora or an increase in gastrointestinal side effects [15,16], we hypothesized that the approach might be useful to manage unscheduled bleeding in women initiating the use of a continuous OCP. The primary objective of this study was to evaluate whether prophylactically administered subantimicrobial-dose doxycycline would prevent bleeding and spotting in women using continuous OCPs. Secondary objectives were to describe safety, side effects and satisfaction.
2. Materials and methods
A randomized, double-blind study was conducted at Oregon Health & Science University (OHSU) and the University of Hawaii (UH) between December 2008 and May 2010. The protocol was approved by the Institutional Review Board at both institutions and was registered on ClinicalTrials.gov (identifier NCT00480532). Recruitment was done through radio, television and print advertisements. Women were invited to participate if they were between the ages of 18 and 45 years, were in good health and had no medical contraindications to OCPs. To avoid the common transition bleeding seen with combined OCP initiation, all participants were required to have been on cyclic hormonal contraceptives (combined OCPs, patch or ring) without unscheduled bleeding prior to enrollment. Potential participants were excluded if they had used an IUD or contraceptive implant in the previous 3 months, had used depot medroxyprogesterone acetate within 9 months, were smokers or had a hypersensitivity to any of the tetracyclines.
All women enrolled in the study took a continuous OCP (20 mcg ethinyl estradiol/100 mcg levonorgestrel, Aviane™, Teva Pharmaceuticals, North Wales, PA, USA) over four 28-day cycles (112 days of hormonally active pills). Subjects were randomized to either controlled-release subantimicrobial-dose doxycycline 40 mg (Oracea®, Galderma Laboratories L.P., Fort Worth, TX, USA) or placebo taken orally, once a day, for 84 days. To determine whether unscheduled bleeding was eliminated or just postponed, the study medication was discontinued after 84 days. For the remaining 28 days, bleeding patterns were observed on the continuous OCP alone.
Subjects who met the inclusion criteria using a phone screen met with study coordinators. Informed consent was obtained from all participants. In addition to collecting demographic and medical information, a physical exam was performed that included blood pressure measurement, pelvic examination, cervical cytological evaluation (not repeated if the subject had a documented result in the last 6 months) and nucleic acid amplification testing for gonorrhea and chlamydia. Subjects continued on their current cyclic hormonal contraceptive until they returned for their enrollment visit. Those subjects that had a negative screen for gonorrhea and chlamydia and cervical cytology that did not require colposcopy or an excisional procedure according to American Society for Colposcopy and Cervical Pathology guidelines [17] met criteria for enrollment. The enrollment visit was scheduled during the hormone-free interval of the current pill pack, within the first 5 days of the onset of withdrawal bleeding. After a negative urine pregnancy test, subjects were allocated equally into two groups using a computer-generated block randomization scheme. To conceal treatment groups to investigators and subjects, the OHSU Research Pharmacy packaged indistinguishable gel capsules containing either subantimicrobial-dose doxycycline or placebo into identical bottles labeled only with instructions and the subject identifier.
Subjects received OCPs, study medication and a study calendar to document daily bleeding events. Bleeding was recorded daily as bleeding, spotting or no bleeding and spotting based on standard criteria [18]. Spotting was defined as minimal blood loss that did not require new use of any sanitary protection, including liners. Bleeding was defined as blood loss that required the use of sanitary protection with a tampon, pad or liner. Bleeding episodes were defined as days of bleeding or spotting that were bounded by 2 days of no bleeding or spotting. In accordance with criteria described by Mishell et al., all bleeding in this study was defined as unscheduled [18]. One cycle was defined as 28 days.
Participants recorded the presence (yes/no) of headache, nausea, acne, mood/depression, menstrual pain, vaginal candidiasis and any other notable event on a daily basis. They were also asked to make note of whether they took the OCP and study medication. If an OCP was missed, subjects were instructed to take it as soon as they remembered and to record the date and time on their bleeding calendar. If a patient had a suspicion of pregnancy during the study period, a urine pregnancy test was performed. A follow-up phone call was done 2 weeks after randomization. Study visits took place one, three and four cycles after randomization. At three cycles (e.g., after stopping the study medication) and four cycles (e.g., study completion), subjects completed a satisfaction questionnaire that included an assessment of satisfaction with bleeding pattern and treatment using a 100-mm visual analogue scale (VAS).
The primary outcome was the number of days of bleeding and spotting for the first 84 days of the study. A two-sample t test was used to compare the mean number of days of bleeding and spotting during the first 84 days between the two groups. Because we anticipated that a large proportion of patients would have zero days of bleeding only and bleeding and spotting, our findings were confirmed with a 2-degree of freedom χ2 test. In this test, we performed two tests and combined them to arrive at a single test statistic and p value assessing whether the average number of days of bleeding differed between the two groups. This test is appropriate when a large number of subjects have a zero count for an outcome. The two parts consist of a χ2 test comparing whether the proportion of patients with no bleeding differed from those with bleeding and a t test comparing the mean number of days of bleeding for those that bled between the two groups. A t test is analogous to a χ2 test with 1 degree of freedom. Thus, by squaring the t test, we are able to sum the two χ2 values to arrive at a χ2 with 2 degrees of freedom. We analyzed whether bleeding suppression occurred earlier in the treated versus untreated groups using a χ2 test (last month of bleeding) and a t test (last day of bleeding or spotting).
To understand whether the bleeding was just postponed, we compared the mean number of days of bleeding and spotting for the last 28 days and the total study period (112 days) as well as the percentage of women who reported any bleeding or spotting in the last 28 days of the study. Trends over time in the number of days of bleeding and spotting were also compared using a repeated-measures analysis of variance (number of bleeding and spotting days for each 28-day period).
Analyses were performed based on intention to treat. In the event of incomplete data due to study discontinuation, the proportion of days with each bleeding pattern (absence of bleeding and spotting, spotting, bleeding) was calculated by dividing the number of days with each bleeding pattern by the number of women-days of participation. The proportions were then multiplied by 28 to obtain the expected number of days with each bleeding pattern during the 28-day interval in which they discontinued the study. Demographics, satisfaction, compliance with pills and medication-associated adverse effects were compared between groups using χ2 or Fisher’s Exact Tests for categorical variables and t tests for continuous factors. Statistical analyses were performed using the Statistical Program for Social Sciences (SPSS 16.0 for Windows; SPSS Inc., Chicago, IL, USA).
A sample size of 66 (33 in each group) was based on expected differences in the primary outcome of the total number of days with bleeding and spotting. Prior studies documented a mean of 19.2 (SD 12.01) days of bleeding and spotting over the first 90 days when a 100-mcg levonorgestrel/20-mcg ethinyl estradiol pill is administered continuously [19]. The sample size (beta 0.80, alpha 0.05) was calculated to detect a 50% reduction in bleeding (e.g., from 19 days to 9 days) and accounted for a 30% dropout rate.
3. Results
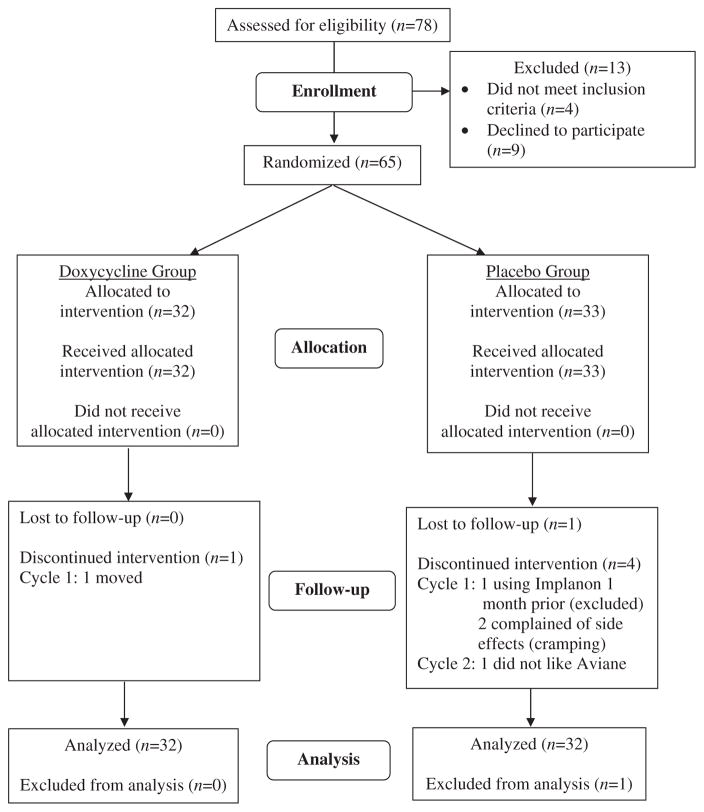
Of the 78 subjects who met the inclusion criteria using a phone screen, 65 were randomized and 59 completed all 4 cycles of the study. Thirty-six subjects were recruited at OHSU and 29 subjects were recruited at UH. Reasons for study discontinuation are illustrated in Fig. 1. Demographic characteristics were similar between the study groups (Table 1). The majority of both cohorts were switching from cyclic OCP regimens in order to avoid menstrual bleeding.
Fig. 1.
Reasons for study discontinuation.
Table 1.
Demographic and menstrual characteristics of the study population at randomization
| Doxycycline group | Placebo group | p value | ||
|---|---|---|---|---|
| n=32 | n=32 | |||
| Mean (SD) | Mean (SD) | |||
| n (%) | n (%) | |||
| Age (years)a | 28.6 (6.1) | 27.8 (5.4) | .54 | |
| Menstrual cycle length (days)a | 27.9 (1.3) | 27.6 (2.0) | .55 | |
| Body mass index (kg/m2)a | 25.7 (6.9) | 25.8 (4.8) | .97 | |
| Weight (kg)a | 68.4 (19.5) | 70.2 (15.8) | .68 | |
| Ethnicityb | Hispanic | 1 (3.1) | 5 (15.6) | .10 |
| Non-Hispanic | 31 (96.9) | 27 (84.4) | ||
| Raceb | White | 21 (65.6) | 22 (68.8) | .83 |
| Black | 1 (3.1) | 2 (6.2) | ||
| Asian | 5 (15.6) | 5 (15.6) | ||
| Native Hawaiian, Pacific Islander | 1 (3.1) | 0 (0) | ||
| Multiracial | 4 (12.5) | 3 (9.4) | ||
| Gravidityb | 0 | 20 (62.5) | 22 (68.8) | .63 |
| 1 | 4 (12.5) | 5 (15.6) | ||
| 2 or more | 8 (25.0) | 5 (15.6) | ||
| Parityb | 0 | 22 (68.8) | 26 (81.2) | .42 |
| 1 | 5 (15.6) | 4 (12.5) | ||
| 2 or more | 5 (15.6) | 2 (6.2) | ||
| Birth control method used prior to studyb | Combined OCPs | 21 (65.6) | 23 (71.9) | .86 |
| Contraceptive ring | 10 (31.2) | 8 (25.0) | ||
| Contraceptive patch | 1 (3.1) | 1 (3.1) | ||
| Painful periods prior to enrollmentb | No pain | 14 (43.8) | 10 (31.2) | .48 |
| Mild pain, does not require medication | 9 (28.1) | 8 (25.0) | ||
| Mild pain, requires medications | 9 (28.1) | 13 (40.6) | ||
| Severe pain | 0 (0) | 1 (3.1) | ||
| Reason for starting continuous COCsb | To avoid menstrual bleeding: yes | 27 (84.4) | 23 (71.9) | .18 |
| No | 5 (15.6) | 9 (28.1) | ||
| To avoid menstrual pain: yes | 6 (18.8) | 10 (31.2) | .19 | |
| No | 26 (81.2) | 22 (68.8) | ||
| To avoid premenstrual symptoms: yes | 8 (25.0) | 10 (31.2) | .39 | |
| No | 24 (75.0) | 22 (68.8) |
t Test.
χ2 Test or Fisher’s Exact Test.
The number of days of bleeding and spotting decreased for both groups over the four cycles of observation (data not shown). Although subjects receiving doxycycline demonstrated a trend toward fewer bleeding and spotting days at all comparisons, there was no significant difference between groups in the mean number of days of bleeding and spotting or bleeding only for the first 84 days and all 112 days of the study (Table 2). In the first 84 days of the study, subjects in the doxycycline group experienced a mean of 14.75 (SE 2.30) days of bleeding and spotting compared to subjects in the placebo arm [mean 17.78 (SE 2.31) days]. A 2-degree of freedom χ2 test confirmed our findings. A repeated-measures analysis of variance showed that the mean number of days of bleeding did not differ between treatment groups (p=.14) nor did bleeding patterns differ between treatment groups over time (p=.27) for the 112-day study period. However, during the last 28 days of observation, subjects in the doxycycline group recorded significantly fewer bleeding and spotting days than those in the placebo group (mean 4.10 vs. 7.91, median 0 versus 6, p=.04). Results did not differ if women switched from a cyclic OCP or a cyclic ring or patch. The mean number of days of bleeding and spotting for the entire study period in women switching from cyclic OCPs was 20.00 (SE 2.90) days versus 27.25 (SE 3.71) days in women who switched from a ring or patch (p=.15).
Table 2.
Mean and median days of bleeding only and bleeding and spotting for the first 84 days, last 28-day cycles and all 112 days of the study period
| Bleeding and spotting
|
Bleeding only
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SE) | Median (range) | Mean difference | 95% CI of difference | p value | Mean (SE) Median (range) | Mean difference | 95% CI of difference | p value | |
| First 84 days | |||||||||
| Doxycycline group, n=32 | 14.75 (2.30) | 11.0 (0, 47) | −3.03 | −9.55, 3.49 | .36 | 5.00 (1.03) 3.0 (0, 20) | −2.16 | −5.51, 1.20 | .20 |
| Placebo group, n=32 | 17.78 (2.31) | 17.0 (0, 48) | 7.15 (1.32) 4.0 (0, 28) | ||||||
| Last 28 days | |||||||||
| Doxycycline group, n=32 | 4.10 (1.23) | 0 (0, 28) | −3.81 | −7.46, 0.16 | .04 | 1.03 (0.34) 0.0 (0, 7) | −1.53 | −3.52, 0.46 | .13 |
| Placebo group, n=32 | 7.91 (1.35) | 6.0 (0, 28) | 2.56 (0.94) 0.0 (0, 28) | ||||||
| 112 days | |||||||||
| Doxycycline group, n=32 | 18.84 (3.30) | 12.0 (0, 63) | −6.84 | −16.08, 2.39 | .14 | 6.03 (1.21) 4.0 (0, 24) | −3.69 | −8.50, 1.13 | .13 |
| Placebo group, n=32 | 25.69 (3.24) | 21.0 (0, 70) | 9.72 (2.08) 5.0 (0, 56) | ||||||
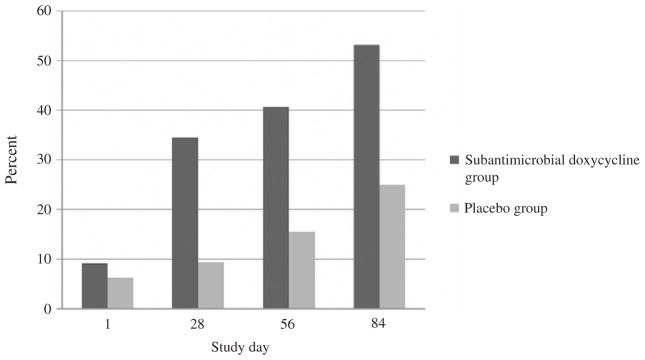
Overall, subjects randomized to receive doxycycline transitioned to amenorrhea sooner than those receiving placebo. The mean last day of bleeding or spotting was study day 61.69 (SE 7.77) for the doxycycline group compared to 85.19 (SE 6.74) for the placebo group (p=.03). Table 3 details the proportion of subjects that achieved amenorrhea during the three treatment cycles and the proportion that continued to have bleeding or spotting in the final cycle. A higher percentage of subjects receiving doxycycline experienced their last day of bleeding or spotting during cycle 1 (34.4% vs. 15.6% placebo), and this pattern continued during cycle 2 (9.4% vs. 0%) and cycle 3 (12.5 vs. 9.4%). In the last 28 days of the study, 75.0% (24/32) of subjects in the nontreatment group continued to experience bleeding compared to only 43.8% (14/32) of subjects in the treatment group (p=.05). While relatively few subjects in either group experienced no bleeding or spotting during the entire 112-day study period, significantly more subjects in the doxycycline group experienced no further bleeding or spotting after study day 28, and this advantage over placebo became wider after 56 and 84 days of study drug use (Fig. 2). There was no significant difference between groups in the duration of the longest bleeding and spotting episode during the first 84 days of the study period [doxycycline mean 7.31 days (SE 1.55), placebo mean 8.63 days (SE 1.58), p=.56] and the last 28 days of the study period [doxycycline mean 3.06 days (SE 1.08), placebo mean 4.66 days (SE 1.24), p=.34] (Table 4).
Table 3.
Subjects who experienced their last day of bleeding during a given study cycle
| Study group | Last bleeding/spotting
|
|||
|---|---|---|---|---|
| Cycle 1, n (%) | Cycle 2, n (%) | Cycle 3, n (%) | Cycle 4, n (%) | |
| Doxycycline, n=32 | 11 (34.4) | 3 (9.4) | 4 (12.5) | 14 (43.8) |
| Placebo, n=32 | 5 (15.6) | 0 (0.0) | 3 (9.4) | 24 (75.0) |
There was a significant difference between study groups (p<.05).
Fig. 2.
Cumulative percentage of subjects who had no further bleeding or spotting from the indicated study day to the end of the 112-day study period.
Table 4.
Bleeding and/or spotting episodes, length of the longest bleeding and/or spotting episode, missed study medication, missed oral contraceptive pills (OCP), and reported side effects for the study period (112 days). If subjects discontinued prematurely the proportion of bleeding and/or spotting episodes and amount of medication administered was calculated and applied to the remainder of the study period
| Doxycycline Group | Placebo Group | p value | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| group n=32 | group n=32 | |||
| Bleeding/spotting episodes | None | 3 (9.4) | 2 (6.2) | 0.89 |
| 1–3 | 17 (71.9) | 17 (65.6) | ||
| 4 or more | 12 (18.8) | 13 (28.1) | ||
| Length of longest bleeding/spotting episode (days) | None | 3 (9.4) | 2 (6.2) | 0.79 |
| 1 to 5 | 12 (37.5) | 9 (28.1) | ||
| 6 to 10 | 7 (21.9) | 9 (28.1) | ||
| 11 or more | 10 (31.2) | 12 (37.5) | ||
| Missed OCP | 0 | 20 (62.5) | 19 (59.4) | 0.08 |
| 1–2 | 9 (28.1) | 4 (12.5) | ||
| 3 or more | 3 (9.4) | 5 (28.1) | ||
| Missed study medication | 0 | 22 (68.8) | 17 (53.1) | 0.22 |
| 1–2 | 5 (15.6) | 4 (12.5) | ||
| 3 or more | 5 (15.6) | 11 (34.4) | ||
| Headache | 14 (43.8) | 23 (71.9) | 0.02 | |
| Acne | 12 (37.5) | 14 (46.9) | 0.40 | |
| Depressed mood | 7 (21.9) | 17 (53.1) | 0.01 | |
| Abdominal cramping | 9 (28.1) | 19 (59.2) | 0.02 | |
| Nausea, vomiting or diarrhea | 17 (53.1) | 20 (62.5) | 0.40 | |
| Vaginal candidiasis | 4 (12.5) | 5 (15.6) | 0.50 |
If subjects discontinued prematurely, the proportion of bleeding and/or spotting episodes and amount of medication administered were calculated and applied to the remainder of the study period.
There were significant differences in reported side effects between study groups, with subjects in the doxycycline group being less likely to report headache, depressed mood and abdominal cramping (Table 4). No major adverse outcomes were reported in either group during the treatment and posttreatment cycles. Of the three subjects who discontinued the study secondary to medication side effects, all were in the placebo group.
After 84 days of using a continuous OCP with the study medication, a minority of subjects, regardless of allocation, preferred taking the continuous OCPs with the study medication (doxycycline 16.1%, placebo 10.7%, p=.65) (Table 5). After 112 days of taking a continuous OCP, there was no difference in VAS satisfaction scores (p=.56). Half of subjects, regardless of allocation, reported that they would continue taking OCPs in a continuous fashion. A greater proportion of women who experienced no bleeding or spotting from day 28 onward preferred taking continuous to cyclic OCPs; however, this difference was not statistically significant (amenorrheic 85.7%, not amenorrheic 56.5%, p=.08).
Table 5.
Final subject satisfaction results using a 100-point VAS and preference for OC and study medication administration
| Doxycycline | Placebo | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| N (%) | N (%) | ||
| n=31 | n=28 | ||
| Assessed after 84 days | |||
| Prefer continuous OC | 22 (71.0) | 15 (53.6) | .08 |
| Cyclic OC | 7 (22.6) | 5 (17.9) | |
| No preference | 2 (6.5) | 8 (28.6) | |
| Prefer continuous OC without study medication | 14 (45.2) | 11 (39.3) | .65 |
| Continuous OC with study medication | 5 (16.1) | 3 (10.7) | |
| No preference | 12 (38.1) | 14 (50.0) | |
| Assessed after 112 days | |||
| Final satisfaction VAS | 67.0 (35.3) | 72.0 (30.9) | .56 |
| Do you plan to: | |||
| Continue taking OC continuously | 16 (51.6) | 14 (50.0) | .85 |
| Take cyclic OC | 8 (25.8) | 6 (21.4) | |
| Start a different method | 6 (19.4) | 6 (21.4) | |
| Discontinue birth control | 1 (3.2) | 2 (7.1) | |
Questions were asked after 84 days and at the end of the 112-day study period. Analysis does not include the five subjects who discontinued the study prior to this time.
4. Discussion
In this randomized, blinded, placebo-controlled study, we report the first effective medical therapy to improve bleeding patterns in women starting treatment with continuous OCPs. In our study, women using subantimicrobial doxycycline experienced fewer days of bleeding and spotting after three cycles of use and progressed to amenorrhea more rapidly than subjects that received placebo. However, the total number of days of bleeding and spotting was not significantly different between study groups.
Most women beginning a continuous OCP see amenorrhea as a goal of therapy. While unscheduled bleeding and spotting are expected during the early cycles of continuous use, persistent unscheduled bleeding represents the primary reason for dissatisfaction with the approach and discontinuation of the method. Although initiation of a 3- to 4-day pill-free break after taking at least 21 days of hormonally active pills has been recommended as a potential strategy [20,21], no effective medical treatments for unscheduled bleeding have been previously described. Our findings are robust; over 50% of the doxycycline-treated subjects achieved amenorrhea by the third cycle. This compares favorably to the results seen in a large multicenter open-label study of a continuous 20-mcg EE/90-mcg LNG pill where it took eight cycles for 50% of the subjects to achieve amenorrhea [22].
These results contrast with our earlier study that demonstrated that a higher typical antimicrobial dose of doxycycline (100 mg twice a day) initiated at the time of unscheduled bleeding and spotting and continued for 5 days did not decrease unscheduled bleeding and spotting or alter the time course to amenorrhea in women initiating continuous OCPs [23]. Taken together, these two studies suggest that continuous daily low-dose doxycycline can alter the tissue-destabilizing effects of MMPs that lead to unscheduled bleeding during use of a combined OCP. However, once bleeding becomes established, even higher-dose doxycycline cannot reverse the cascade of events that maintain endometrial stability.
The World Health Organization advises using 90-day reference intervals to analyze bleeding patterns that occur in women using noncyclic contraceptive methods [24]. We used an 84-day reference period and followed the descriptions of bleeding recommended by Mishell et al. [18] because OCPs are uniformly packaged in 28-day packs. Since bleeding patterns are affected by compliance with the pill regimen, the use of paper diaries instead of a real-time electronic data collection device to monitor pill intake and bleeding information represents a weakness of our study design [18]. However, there is no reason to suspect that compliance with the treatment regimens was affected by group assignment since the design was randomized and double blinded, with both groups taking identical OCPs and study drug capsule each day. Any inconsistencies in reporting and pill use should have been distributed evenly between the groups, leading to a dilution of the effect and rejection of the hypothesis. Furthermore, our bleeding data were comparable to those reported in other studies of continuous OCP studies using a similar pill formulation, suggesting external validity to our findings [19,23]. Finally, changes in bleeding in the treatment group are unlikely to be related to the absorption of contraceptive steroid as pharmacokinetic studies have demonstrated that concomitant use of typical doses of doxycycline does not alter contraceptive steroid levels [25,26].
The decreased number of adverse side effects in the treatment group is both interesting and reassuring. Matrix metalloprotineases have been studied in the treatment of disease states throughout the body [27–30]. Thus, it is possible that MMP inhibition has beneficial effects in other organ systems [31].
Although a Cochrane review concluded that 30-mcg EE pills have a better bleeding pattern than 20-mcg pills [32] in standard use cycles, there are no data to support the use of a 30-mcg pill for continuous use. In a randomized study of continuous use of OCPs containing 20 mcg EE in combination with either 100 mcg of LNG or 1 mg Norethindrone acetate, the addition of 10 mcg EE to either regimen did not improve bleeding patterns [19].
The dosage of doxycycline used in this study was based on an endometrial biopsy study that indicated doses of doxycycline as low as 20 mg administered twice daily inhibited endometrial MMPs in etonogestrel implant users [33]. Despite being substantially more expensive than typical-dose doxycycline ($3.80 per day versus $0.08 per day), the 40-mg controlled-release formulation allowed for once-a-day dosing that fit well with the dosing of OCPs. However, it is unclear what dose and treatment schedule of prophylactic doxycycline would optimize rates of amenorrhea, particularly in patients using a contraceptive product that contains estrogen. Future studies should incorporate sampling of the endometrium to correlate MMP activity with doxycycline dosage. Also, because we enrolled women switching from a cyclic hormonal method to continuous OCPs, our findings do not apply to women starting continuous OCPs who were not previously using a cyclic method.
Our results provide a novel and clinically useful strategy to improve bleeding outcomes in women using continuous OCPs who desire an intervention to decrease unscheduled bleeding. Although there were no differences in overall satisfaction scores between the treatment and control groups, the earlier achievement of amenorrhea may be helpful for women who are especially concerned regarding unscheduled bleeding. The benefits of the approach need to be carefully considered in view of the cost of the intervention and available alternatives including the newer extended-cycle (24–26 day) active pill regimens or the levonorgestrel intrauterine system.
Footnotes
Financial support: Funding was provided by the Fellowship in Family Planning, the Oregon Health & Science University Women’s Health Research Unit and a Research Centers in Minority Institutions award, P20 RR11091, from the National Center for Research Resources (NCRR), National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR/NIH.
References
- 1.Mishell DR., Jr Oral contraception: past, present, and future perspectives. Int J Fertil. 1991;36(Suppl 1):7–18. [PubMed] [Google Scholar]
- 2.Marks L. Sexual chemistry: a history of the contraceptive pill. New Haven: Yale University Press; 2001. [Google Scholar]
- 3.Edelman A, Lew R, Cwiak C, Nichols M, Jensen J. Acceptability of contraceptive-induced amenorrhea in a racially diverse group of US women. Contraception. 2007;75:450–3. doi: 10.1016/j.contraception.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception — an international study on acceptability. Contraception. 2003;67:1–8. doi: 10.1016/s0010-7824(02)00474-2. [DOI] [PubMed] [Google Scholar]
- 5.Miller L, Hughes JP. Continuous combination oral contraceptive pills to eliminate withdrawal bleeding: a randomized trial. Obstet Gynecol. 2003;101:653–61. doi: 10.1016/s0029-7844(03)00014-0. [DOI] [PubMed] [Google Scholar]
- 6.Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68:89–96. doi: 10.1016/s0010-7824(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 7.Galant C, Berliere M, Dubois D, et al. Focal expression and final activity of matrix metalloproteinases may explain irregular dysfunctional endometrial bleeding. Am J Pathol. 2004;165:83–94. doi: 10.1016/S0002-9440(10)63277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marbaix E, Kokorine I, Henriet P, Donnez J, Courtoy PJ, Eeckhout Y. The expression of interstitial collagenase in human endometrium is controlled by progesterone and by oestradiol and is related to menstruation. Biochem J. 1995;305(Pt 3):1027–30. doi: 10.1042/bj3051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salamonsen LA, Zhang J, Hampton A, Lathbury L. Regulation of matrix metalloproteinases in human endometrium. Hum Reprod. 2000;15(Suppl 3):112–9. doi: 10.1093/humrep/15.suppl_3.112. [DOI] [PubMed] [Google Scholar]
- 10.Vincent AJ, Malakooti N, Zhang J, Rogers PA, Affandi B, Salamonsen LA. Endometrial breakdown in women using Norplant is associated with migratory cells expressing matrix metalloproteinase-9 (gelatinase B) Hum Reprod. 1999;14:807–15. doi: 10.1093/humrep/14.3.807. [DOI] [PubMed] [Google Scholar]
- 11.Vincent AJ, Zhang J, Ostor A, et al. Matrix metalloproteinase-1 and -3 and mast cells are present in the endometrium of women using progestin-only contraceptives. Hum Reprod. 2000;15:123–30. doi: 10.1093/humrep/15.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Chegini N, Rhoton-Vlasak A, Williams RS. Expression of matrix metalloproteinase-26 and tissue inhibitor of matrix metalloproteinase-3 and -4 in endometrium throughout the normal menstrual cycle and alteration in users of levonorgestrel implants who experience irregular uterine bleeding. Fertil Steril. 2003;80:564–70. doi: 10.1016/s0015-0282(03)00797-0. [DOI] [PubMed] [Google Scholar]
- 13.Burns FR, Stack MS, Gray RD, Paterson CA. Inhibition of purified collagenase from alkali-burned rabbit corneas. Invest Ophthalmol Vis Sci. 1989;30:1569–75. [PubMed] [Google Scholar]
- 14.Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- 15.Skidmore R, Kovach R, Walker C, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. J Fam Pract. 2003;139:459–64. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- 16.Del Rosso JQ, Webster GF, Jackson M, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007;56:791–802. doi: 10.1016/j.jaad.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis. 2007;11:201–22. doi: 10.1097/LGT.0b013e3181585870. [DOI] [PubMed] [Google Scholar]
- 18.Mishell DR, Jr, Guillebaud J, Westhoff C, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception. 2007;75:11–5. doi: 10.1016/j.contraception.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Edelman AB, Koontz SL, Nichols MD, Jensen JT. Continuous oral contraceptives: are bleeding patterns dependent on the hormones given? Obstet Gynecol. 2006;107:657–65. doi: 10.1097/01.AOG.0000199950.64545.16. [DOI] [PubMed] [Google Scholar]
- 20.Sulak PJ, Carl J, Gopalakrishnan I, Coffee A, Kuehl TJ. Outcomes of extended oral contraceptive regimens with a shortened hormone-free interval to manage breakthrough bleeding. Contraception. 2004;70:281–7. doi: 10.1016/j.contraception.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Sulak PJ, Kuehl TJ, Coffee A, Willis S. Prospective analysis of occurrence and management of breakthrough bleeding during an extended oral contraceptive regimen. Am J Obstet Gynecol. 2006;195:935–41. doi: 10.1016/j.ajog.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 22.Archer DF, Jensen JT, Johnson JV, Borisute H, Grubb GS, Constantine GD. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439–45. doi: 10.1016/j.contraception.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 23.Kaneshiro B, Edelman A, Carlson N, Morgan K, Nichols M, Jensen J. Treatment of unscheduled bleeding in continuous oral contraceptive users with doxycycline: a randomized controlled trial. Obstet Gynecol. 2010;115:1141–9. doi: 10.1097/AOG.0b013e3181e0119c. [DOI] [PubMed] [Google Scholar]
- 24.Belsey EM, Machin D, d’Arcangues C. The analysis of vaginal bleeding patterns induced by fertility regulating methods. World Health Organization Special Programme of Research, Development and Research Training in Human Reproduction Contraception. 1986;34:253–60. doi: 10.1016/0010-7824(86)90006-5. [DOI] [PubMed] [Google Scholar]
- 25.Neely JL, Abate M, Swinker M, D’Angio R. The effect of doxycycline on serum levels of ethinyl estradiol, norethindrone, and endogenous progesterone. Obstet Gynecol. 1991;77:416–20. [PubMed] [Google Scholar]
- 26.Murphy AA, Zacur HA, Charache P, Burkman RT. The effect of tetracycline on levels of oral contraceptives. Am J Obstet Gynecol. 1991;164:28–33. doi: 10.1016/0002-9378(91)90617-z. [DOI] [PubMed] [Google Scholar]
- 27.Kaito K, Urayama H, Watanabe G. Doxycycline treatment in a model of early abdominal aortic aneurysm. Surg Today. 2003;33:426–33. doi: 10.1007/s10595-002-2513-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee KS, Jin SM, Kim SS, Lee YC. Doxycycline reduces airway inflammation and hyperresponsiveness in a murine model of toluene diisocyanate-induced asthma. J Allergy Clin Immunol. 2004;113:902–9. doi: 10.1016/j.jaci.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Brooks DE, Ollivier FJ. Matrix metalloproteinase inhibition in corneal ulceration. Vet Clin North Am Small Anim Pract. 2004;34:611–22. doi: 10.1016/j.cvsm.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Nikkola J, Vihinen P, Vuoristo MS, Kellokumpu-Lehtinen P, Kahari VM, Pyrhonen S. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clin Cancer Res. 2005;11:5158–66. doi: 10.1158/1078-0432.CCR-04-2478. [DOI] [PubMed] [Google Scholar]
- 31.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edelman A, Gallo MF, Nichols MD, Jensen JT, Schulz KF, Grimes DA. Continuous versus cyclic use of combined oral contraceptives for contraception: systematic Cochrane review of randomized controlled trials. Hum Reprod. 2006;21:573–8. doi: 10.1093/humrep/dei377. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Choksuchat C, Zhao Y, Ballagh SA, Kovalevsky GA, Archer DF. Effects of doxycycline on serum and endometrial levels of MMP-2, MMP-9 and TIMP-1 in women using a levonorgestrel-releasing subcutaneous implant. Contraception. 2009;79:469–78. doi: 10.1016/j.contraception.2008.12.010. [DOI] [PubMed] [Google Scholar]