Abstract
Primary aging is the progressive and inevitable process of bodily deterioration during adulthood. In skeletal muscle, primary aging causes defective mitochondrial energetics, and reduced muscle mass. Secondary aging refers to additional deleterious structural and functional age-related changes caused by diseases and lifestyle factors. Secondary aging can exacerbate deficits in mitochondrial function and muscle mass, concomitant with the development of skeletal muscle insulin resistance. Exercise opposes deleterious effects of secondary aging by preventing the decline in mitochondrial respiration, mitigating aging-related loss of muscle mass and enhancing insulin sensitivity. This review focuses on mechanisms by which exercise promotes “healthy aging” by inducing modifications in skeletal muscle.
Primary versus Secondary Aging: Setting the Stage
Most individuals wishing to be “forever young”, dream of a magic elixir to retard age-related changes in humans. While many have searched, the proverbial “fountain of youth” has not been found, and primary aging is unavoidable. Primary aging is the inevitable deterioration of cellular structure and biological function, independent of disease or harmful lifestyle or environmental factors (Holloszy, 2000). Efforts to slow or reverse primary aging have met little success (Booth et al., 2011). This may partly be related to the pre-clinical and clinical observations that various stages exist along the continuum of primary aging. For example, the adaptability or “plasticity” of skeletal muscle of individuals in the age range between 65–75 years is very different than that of individuals over 75 years. In this regard, we refer to the malleability of skeletal muscle in terms of insulin sensitivity, ‘metabolic flexibility’ and substrate preference in relation to nutrient or exercise challenges, mitochondrial function, and growth/hypertrophy response changes with aging. Overall, the plasticity of aging skeletal muscle is relatively well-preserved up to old age, but wanes significantly in very advanced age, due at least in part to a diminished up-regulation of diverse signaling and gene regulatory pathways necessary for metabolic and functional adaptations.
Efforts to increase life span and more importantly, quality of life, have been achieved by limiting secondary aging, or more specifically, the deleterious structural and functional changes that are caused by diseases and environmental factors (Holloszy, 2000). Thus, lifestyle modifications that promote health, well-being, and functional capacity minimize disease development, and consequently secondary aging. For example, even in the most physically active people, relative maximal oxygen consumption declines in the third decade of life due to primary aging, but aerobically active or sedentary lifestyles can slow or accelerate this respectively, by influencing secondary aging (Booth et al., 2011).
Regular exercise training affords protection against the aging-related changes responsible for development of insulin resistance (Rogers et al., 1990b). Strikingly physical inactivity and sedentary behavior have a deleterious effect on human health that is comparable to smoking (Bouchard et al., 2015). Because physical inactivity is an important cause of many chronic diseases, it accelerates the secondary aging process and can lead to premature death (Booth et al., 2012). Thus, physical activity and prescribed exercise are potent countermeasures against secondary aging and together, play major role in the prevention of the most deadly chronic diseases modern humans face, including cardiovascular diseases, metabolic diseases, cancer, pulmonary diseases, immune dysfunction, musculoskeletal disorders, and neurological disorders (Booth et al., 2002).
Not all Activity is the Same: Distinguishing between Physical Activity and Exercise
To set the stage for the purpose of this perspective, defining and distinguishing the differences between physical activity and exercise is important. Physical activity refers to any level of activity above seated rest that results from skeletal muscle activation and leads to movement and an increase in energy expenditure, whereas exercise refers to planned structured repetitive activity aimed to improve fitness (Caspersen et al., 1985). Physical activity is daily free living activity separate from weekly exercise, whereas exercise training is defined by a prescribed and adherent dose (i.e. mode, intensity, volume/duration, frequency) of effort or work.
The extremes of the exercise training continuum can be distinguished as endurance or resistance exercise. Endurance exercise of several minutes up to several hours at various intensities incorporating repetitive, low-resistance load increases aerobic fitness, partly reflected by a change in skeletal muscle oxidative capacity and improved function of the cardiovascular system. Conversely, resistance exercise to increase muscular mass, strength, and power consists of short-duration activity at high intensities/resistance or exercises of a single or relatively few repetitions. In order to achieve measurable functional changes in work performance, exercise training sessions should be performed three to five times weekly for several weeks. The intensity, duration, and mode of physical activity, and nutritional status, markedly affect the metabolic and molecular response to a given exercise challenge (Egan and Zierath, 2013) and in turn determine the nature of changes in skeletal muscle oxidative capacity, mass, and strength. When prescribed on a relative scale (e.g., 70% of maximum), adults of all ages are capable of exercising at the same intensity after a period of familiarization and ramping. However, in some older adults the adaptations to a particular mode of exercise training are blunted compared to young. This may reflect differences in physiologic reserve between young and old (e.g., similar resting heart rate, but far greater heart rate reserve in young).
Fighting Metabolic Disease and Muscle Atrophy: Warding off Secondary Aging
During the last half century, there has been an explosion in the diagnosis of a cluster of metabolic disorders including impaired glucose tolerance (IGT), obesity and type 2 diabetes. Strikingly, a high percentage of individuals with IGT develop type 2 diabetes within a decade. The majority of people who have IGT or type 2 diabetes are overweight or obese. Both physical inactivity and aging elevate the risk of type 2 diabetes in ‘normal’ weight and obese individuals. Moreover, increased sedentary time is associated with a greater risk for type 2 diabetes and the metabolic syndrome (van der Berg et al., 2016). Conversely, any type of physical activity is associated with reduced risk of type 2 diabetes in adults aged 70 years and over, while in adults in the 50 to 69 age range, the addition of moderate to vigorous intensity exercise appears necessary to reduce the risk of developing type 2 diabetes (Demakakos et al., 2010).
Loss of skeletal muscle mass is one of the most wide-spread, deleterious and insidious processes in aging humans. While the magnitude varies substantially across individuals, some degree of muscle atrophy impacts all individuals with aging. In 1988 at a meeting in Albuquerque, NM, I.H. Rosenberg first coined the term ‘sarcopenia’ to describe this age-related loss (penia) of flesh (sarx) (Rosenberg, 1997). Since then, classifications of sarcopenia have been proposed based on population variance in estimates of whole body lean mass (Janssen et al., 2004a; Janssen et al., 2002; Janssen et al., 2004b) or limb muscle mass (Baumgartner et al., 1998). Some of these indices have led to estimates of the attributable health care burden (Beaudart et al., 2014b; Janssen et al., 2004b). Despite the original, strict definition of sarcopenia, more recent classifications have incorporated various indices of muscle performance (e.g., strength) or mobility function (e.g., gait speed), apparently in an effort to build clinical relevance and diagnostic criteria. However, the introduction of functional correlates has only confused the diagnosis (Beaudart et al., 2015; Beaudart et al., 2014a); thus we have elected to describe the phenomenon as simply ‘aging muscle atrophy’.
Aging-related defects in mitochondrial energetics have been proposed to be causally involved in aging muscle atrophy (Gouspillou et al., 2014a). These changes are attributed to both inactivity and age-related alterations in mitochondrial synthesis and degradation (Carter et al., 2015), indicating a complex pathophysiology involving both structural changes to the muscle fibers, as well as the enzymatic machinery that controls glucose and lipid metabolism. The mitochondrial defect most likely to impact aging muscle is an increased susceptibility to permeability transition, a likely cause of the increased recruitment of mitochondrial-mediated pathways of apoptosis (Hepple, 2016).
Metabolic dysfunction and skeletal atrophy of aging are insidious, as they develop over time and they slowly, but surely, compromise the quality of life. Moreover, these conditions place a major burden on society. The International Diabetes Federation (IDF) estimates there are 415 million adults living with diabetes, mainly in low and middle income countries, and projects this will rise to 642 million people by 2040 (IDF, 2015). With no cure in sight, efforts are clearly needed to drive these numbers down. Aging-related loss of skeletal muscle mass is also exacerbated indirectly by obesity, with a vicious cycle between an increased fat mass and the concomitant decrease in skeletal muscle mass, giving rise to “sarcopenic obesity” (Heber et al., 1996). Individuals with sarcopenic obesity have increased risk of adverse health events over and above those who are obese or sarcopenic alone (Cleasby et al., 2016). Thus, skeletal muscle insulin resistance resides at the confluence of age-related metabolic dysfunction, fat accumulation and muscle atrophy.
Loss of skeletal muscle mass and strength with aging is also influenced by sex and hormonal status. For example, the age-related decline in muscle force is greater for peri-menopausal and post-menopausal women and this decline is prevented by hormone replacement therapy (Phillips et al., 1993). While women may experience earlier strength losses than men, the age-related decreases in strength are similar between sexes when controlling for muscle mass (Goodpaster et al., 2006). Moreover this strength decline is more rapid than the concomitant loss of muscle mass, suggesting a decline in muscle quality (Goodpaster et al., 2006). From a public health perspective, loss of muscle mass not only reduces mobility and functional capacity, but also accelerates obesity and progression toward type 2 diabetes. As the proportion of older inactive adults rises and the incidence of obesity escalates, this constellation of conditions will collide to have a dramatic impact on the lives of an increasing number of the world’s citizens.
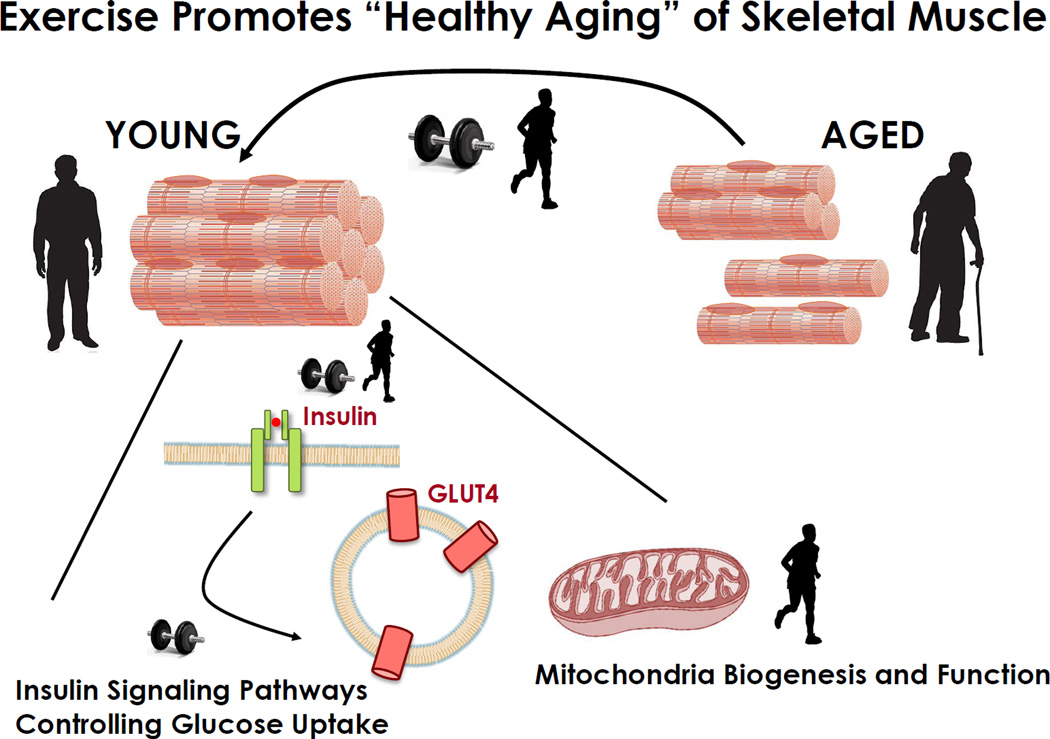
Today, there is growing appreciation that type 2 diabetes can be avoided or at least delayed, and aging-related loss of skeletal muscle mass can be attenuated by lifestyle intervention strategies. Regular exercise counteracts these aging-related diseases. Endurance exercise incorporating repetitive, low-resistance load performed 3–5 times weekly for several weeks improves insulin sensitivity and reduces body fat. Resistance exercise incorporating short-duration activity at high or maximal exercise intensities and high resistance or exercises of a single or relatively few repetitions performed 3–5 times weekly for several weeks slows the decline in skeletal muscle mass and strength. Thus, efforts to maintain insulin sensitivity and normal body weight, as well as maintaining functional skeletal muscle mass should be at the forefront of any intervention strategy to achieve “healthy aging”. This review will focus on the effects of exercise to promote “healthy aging” through the maintenance of insulin sensitivity and functional skeletal muscle mass (Figure 1). We will highlight the current understanding of the key components of pathways controlling glucose homeostasis, mitochondrial content/function, as well as mechanism governing the control of skeletal muscle mass. Unless otherwise stated, the clinical studies cited have evaluated the effects of aging and exercise in non-trained (sedentary) individuals.
Figure 1. Exercise Is a Potent Countermeasure against Secondary Aging.
Endurance exercise enhances muscle insulin sensitivity in older individuals and prevents declines in mitochondrial respiratory capacity with aging. Resistance exercise induces remarkable gains in strength and power in older adults.
Insulin Sensitivity and Aging
Because normal glucose homeostasis is essential for health, it is troubling that recent analysis of adults in the U.S. revealed the combined prevalence of diabetes (mostly type 2 diabetes), abnormal fasting glycemia and IGT increased from 20.9% (at 20–39 years) to 46.9% (at 40–59 years) to 67.4% (at 60–74 years) and 75.6% (at ≥75 years) (Cowie et al., 2009). Insulin resistance is linked to many of the most prevalent and devastating age-related pathologies, including cardiovascular disease, some cancers and cognitive dysfunction (Facchini et al., 2001; Haffner, 1999; Kumari et al., 2000). Whole body insulin sensitivity is determined by the integrated actions of multiple tissues, but skeletal muscle primarily accounts for insulin-mediated blood glucose clearance (DeFronzo et al., 1981).
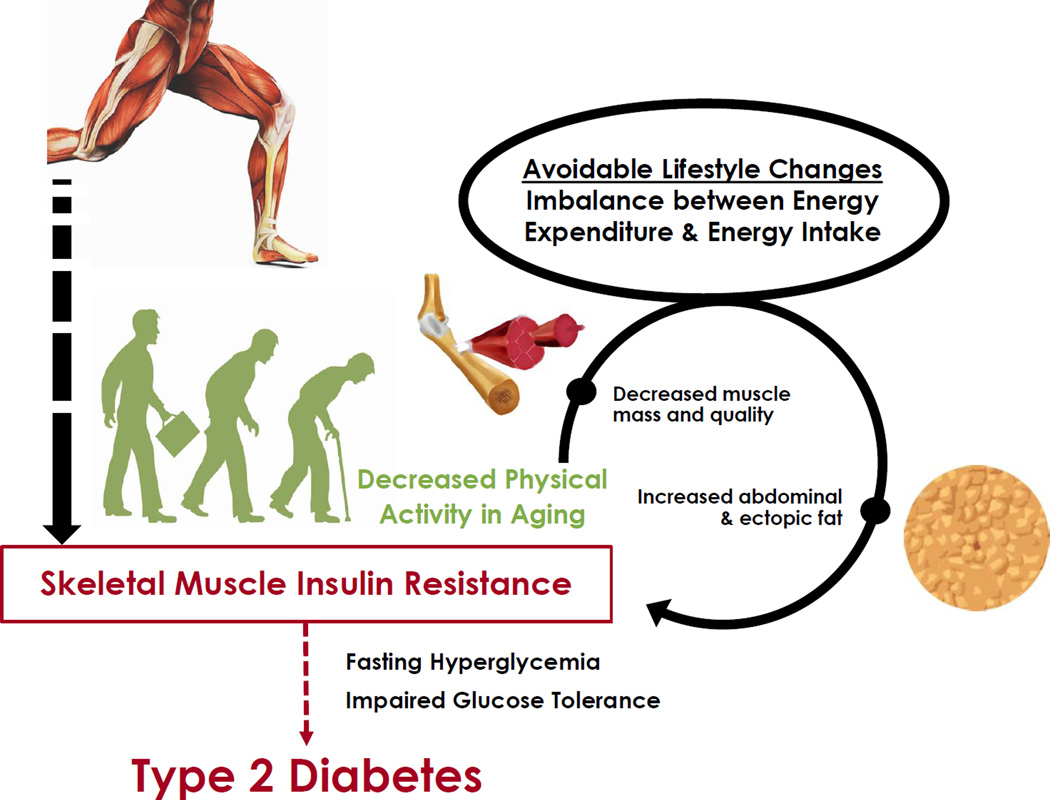
Age-related changes in body composition contribute to insulin resistance observed in older individuals. The accumulation of abdominal and ectopic fat is associated with the deterioration in glucoregulation with advancing age (Huffman and Barzilai, 2009; Kohrt et al., 1993). Age-associated muscle atrophy and changes in muscle quality further contribute to metabolic dysfunction. Modestly reduced whole body glucose disposal has been reported for healthy, older (∼65–70 years-old) versus younger (∼30 years-old) men, even when values are expressed relative to lean body mass (Fink et al., 1986; Meneilly et al., 1996; Rowe et al., 1983). Age-related insulin resistance has been suggested to be independent of primary aging. For example, similar insulin sensitivity was reported for younger (35 years-old) compared to older (66 years-old) individuals who were matched for exercise training status and body composition (Amati et al., 2009). Although there is not a consensus regarding the notion that primary aging induces modest insulin resistance, it is clear that primary aging per se, independent of age-related changes in body composition and physical activity, does not account for the extremely high prevalence of abnormal glucose homeostasis with advancing age in the United States and many developed countries. This disturbing situation is largely attributable to an avoidable condition that is not unique to aging, and arises from an imbalance between energy expenditure and energy intake (Figure 2).
Figure 2. Age-Related Changes in Body Composition and Insulin Sensitivity in Older Individuals Are Influenced by Physical Activity and Exercise Training.
Advancing age is typically characterized by altered body composition (increased abdominal and ectopic fat accumulation and attenuated mass and metabolic quality of skeletal muscle) together with reduced physical activity, leading to insulin resistance in skeletal muscle. These age-related changes can be exacerbated by lifestyle behaviors that produce a major imbalance between energy expenditure and energy intake, leading to further dysregulation of glucose metabolism and increasing the likelihood of type 2 diabetes.
Insulin Signaling and Glucose Uptake
Understanding skeletal muscle insulin resistance requires consideration of the insulin signaling pathway regulating glucose transport, beginning with insulin binding to its receptor, resulting in receptor autophosphorylation and greater tyrosine-phosphorylation of insulin receptor substrate (IRS) proteins (Boucher et al., 2014; Leto and Saltiel, 2012). Tyrosine-phosphorylated IRS binds phosphatidylinositol 3-kinase (PI3K) facilitating Akt2 phosphorylation, which phosphorylates the Rab GTPase TBC1D4 (also called Akt Substrate of 160 kDa or AS160) on multiple Akt Ser/Thr phosphomotifs, including Thr642 and Ser588 that control the rate of GLUT4 translocation to the plasma membrane, resulting an increase in glucose uptake.
GLUT4 protein abundance in human muscle has been reported to decline with aging, in some, but not all studies. Vastus lateralis GLUT4 density in fast-twitch fibers was lower for older (64 years-old) versus younger (29 years-old) women and men, without age-related differences in slow-twitch fibers (Gaster et al., 2000). Another study including women and men from 18 to 80 years-old found a modest negative correlation between age and vastus lateralis GLUT4 abundance, but not gastrocnemius GLUT4 (Houmard et al., 1995). In contrast, other research found no age-related differences for GLUT4 protein abundance in the vastus lateralis of young (22–23 years-old) versus old (∼60 years-old) humans (Cox et al., 1999; Dela et al., 1994). GLUT4 abundance in various muscles with diverse fiber type profiles did not differ for rats at 6–12 versus 24–31 months-old (Cartee and Bohn, 1995; Gulve et al., 1993; Sharma et al., 2010). Thus, reduced GLUT4 abundance seems unlikely to fully account for age-related insulin resistance.
Defective insulin signaling may contribute to skeletal muscle insulin resistance. Older (69 years-old) versus younger (27 years-old) healthy and normal weight humans were characterized by insulin resistance concomitant with reduced insulin-stimulated Akt2 activity in skeletal muscle (Petersen et al., 2015). Older women and men (69 years-old) versus young (24 years-old) controls were modestly insulin resistant, and age was negatively correlated to TBC1D4 phosphorylation on Ser588 and Thr642 in insulin-stimulated muscle, but not correlated with Akt Ser473 phosphorylation (Consitt et al., 2013). These findings are consistent with the notion impairments in the insulin signaling cascade are associated with insulin resistance in aged skeletal muscle.
In rats, there is evidence suggesting muscle-type and/or fiber-type selective effects of aging on insulin sensitivity. Insulin-stimulated glucose uptake by the predominantly slow-twitch soleus was lower for 24 months-old versus 8 months-old rats, but there was no age-related insulin resistance in the predominantly fast-twitch quadriceps muscle from the same rats (Escriva et al., 2007). In isolated soleus, there was age-related insulin resistance (25 versus 9 months-old), but not in the predominantly fast-twitch epitrochlearis (Sharma et al., 2010). In the soleus, but not the epitrochlearis, there was an age-related decrease in Thr308 phosphorylation of Akt2. In neither muscle was there an age-related difference for phosphorylation of TBC1D4 Thr642. The causes for the apparent fiber type and/or muscle differences in insulin signaling and insulin sensitivity in rats remain to be determined, and similar comparisons are lacking for human aging.
Exercise Counteracts Age-related Changes in Insulin Sensitivity
One exercise session can induce subsequently greater whole body insulin-stimulated glucose disposal in young humans or rats secondary to greater insulin-stimulated glucose uptake by muscle (Cartee, 2015; Cartee et al., 1989; Nagasawa et al., 1991; Perseghin et al., 1996; Wojtaszewski et al., 2000; Wojtaszewski et al., 1997). Improved insulin sensitivity is observed ∼1–7 hours post-exercise and can persist up to 1–2 days post-exercise in both species. The improved muscle insulin sensitivity is localized primarily, if not exclusively, in the previously active muscle. Research using one-legged kicking endurance exercise and comparing the exercised leg to the unexercised leg demonstrate that exercise has a local effect on the active muscle (Wojtaszewski et al., 2000). Furthermore, insulin-stimulated leg glucose uptake on the day after cycling exercise was accompanied by no exercise effect on the inactive forearm muscle (Annuzzi et al., 1991). Prior exercise can produce markedly elevated insulin-stimulated uptake of glucose by isolated muscles from rats (Cartee and Holloszy, 1990; Hansen et al., 1998; Wallberg-Henriksson et al., 1988), indicating a persistent effect that is inherent to skeletal muscle. However, the inherent muscle effects may be accompanied by altered muscle blood flow in vivo that contributes to elevated glucose uptake.
In older (67 years-old) women, one hour of brisk walking produced elevated insulin sensitivity on the following day (Wang et al., 2013). Whole body insulin sensitivity was also increased the day after swim-exercise by 27 months-old rats (Pauli et al., 2010). At 3 hours post-exercise by 24–30 months-old rats, insulin-stimulated glucose uptake by isolated epitrochlearis muscle was increased (Cartee et al., 1993; Sharma et al., 2015; Xiao et al., 2013). Thus, even with aging, skeletal muscle retains the capacity for metabolic adaptation to exercise.
The mechanism by which acute exercise enhances insulin sensitivity has mainly been elucidated in studies of young humans or rats. Several hours after acute exercise by young rats, insulin-stimulated glucose uptake is elevated because of greater GLUT4 translocation (Hansen et al., 1998). In young rats and humans, this improvement is not attributable to elevated insulin signaling at proximal steps ranging from insulin receptor binding to Akt activity (Castorena et al., 2014; Hansen et al., 1998; Wojtaszewski et al., 2000; Wojtaszewski et al., 1999). Several lines of evidence suggest that greater TBC1D4 phosphorylation accompanies the improved insulin-simulated glucose uptake noted 3–27 hours after acute exercise (Arias et al., 2007; Cartee, 2015; Castorena et al., 2014; Funai et al., 2010; Funai et al., 2009; Pehmoller et al., 2012; Schweitzer et al., 2012; Treebak et al., 2009). Therefore, elevated TBC1D4 phosphorylation is an attractive candidate for the enhanced insulin-stimulated glucose uptake in skeletal muscle of young humans or rats after acute exercise, but a causal link remains to be established.
The effects of acute exercise on GLUT4 abundance or insulin signaling in muscles from older humans is unknown, but studies performed in animal models reveal molecular insight. For example, insulin-stimulated glucose uptake by the epitrochlearis of 24 months-old rats was increased 3 hours post-exercise with unaltered GLUT4 protein abundance (Xiao et al., 2013). In contrast, the increased insulin-stimulated glucose uptake in the epitrochlearis of 30 months-old rats at 3 hours post-exercise was accompanied by a small increase in GLUT4 abundance (Sharma et al., 2015). In the insulin-stimulated epitrochlearis from both 24 and 30 months-old rats at 3 hours post-exercise, insulin-stimulated Akt phosphorylation was increased on both Thr308 and Ser473, but only in 24 months-old rats was insulin-stimulated TBC1D4 Thr642 phosphorylation increased post-exercise (Sharma et al., 2015; Xiao et al., 2013). Studies of young rats have consistently found greater TBC1D4 phosphorylation with little or no change in insulin-stimulated Akt activation post-exercise, whereas in old rats, insulin-stimulated Akt activation was consistently altered, but TBC1D4 phosphorylation was not always increased. It is notable that the exercise protocols for young rats (2–4 × 30 minute swim-exercise bouts with 5 minute rest intervals) differed from the protocols for old rats (8–9 × 10–20 minute swim-exercise bouts with 10 minute rest intervals) and therefore the different exercise protocols between studies cannot be excluded.
Although acute exercise has impressive effects on insulin sensitivity, the regular and repeated performance of exercise for multiple days, weeks or months has additional effects. Insulin-stimulated glucose disposal was determined in a group of initially untrained young men under 3 conditions: without exercise, 48 hours after 1 exercise bout, and 48 hours after the final session of a 6 week training period (Perseghin et al., 1996). One exercise bout increased subsequent insulin-mediated glucose disposal above resting values, and there appeared to be a further increase after 6 weeks of training, although the difference between acute and chronic exercise was not statistically tested. Similar to the results for young humans, various modes of chronic exercise (cycling, running, walking, resistance exercise) can lead to subsequently elevated insulin sensitivity in older humans (60–87 years–old) (Consitt et al., 2013; Evans et al., 2005; Prior et al., 2015). These studies did not include comparison of the acute versus chronic exercise effects on insulin sensitivity in older people.
A hallmark adaptation of chronic exercise by young humans and rats is increased muscle GLUT4 abundance (Holloszy et al., 1998; Richter and Hargreaves, 2013). Chronic exercise training can also elevate GLUT4 abundance in muscles of older (∼60–65 years-old) humans (Bienso et al., 2015; Cox et al., 1999; Hughes et al., 1993; Prior et al., 2015). Middle-aged (16 months-old) rats (Kern et al., 1992) and older (24 months-old) mice (Willis et al., 1998) had increased muscle GLUT4 abundance after chronic exercise. However, other studies did not detect a training-induced increase in muscle GLUT4 of 25–28 months-old rats (Gulve et al., 1993; Han et al., 1998; Kern et al., 1992). Insulin-stimulated glucose uptake in old rats was elevated by training despite unaltered GLUT4 abundance (Han et al., 1998), suggesting a role for other mechanisms.
There is evidence supporting several other possible mechanisms by exercise training enhances glucose metabolism in older people. Endurance training by 69 years-old women and men produced increased glucose disposal concomitant with elevated TBC1D4 Ser588 and Thr642 phosphorylation in insulin-stimulated muscle (Consitt et al., 2013). Chronic strength training also elevated glucose disposal in older women and men together with greater TBC1D4 phosphorylation on Thr642, but not Ser588. Other adaptations in muscle after endurance training by older people (∼60–80 years-old) include increased hexokinase II and glycogen synthase protein abundance (Bienso et al., 2015), greater glycogen concentration (Hughes et al., 1993) and elevated capillarization (Prior et al., 2015). Chronic resistance exercise can increase muscle mass, but the magnitude of these changes is relatively modest with the exercise protocols commonly used by older people (Marcus et al., 2013). Accordingly, changes in muscle metabolic quality, potentially together with adaptations in other tissues (e.g., adipose and vascular tissues) are likely more important for training-induced improvement in insulin sensitivity. Clearly, older people retain the capacity for exercise-induced modifications in the metabolic qualities of their muscle, although the robustness of the response may well decline with advancing age, much like many other exercise-induced adaptations.
Mitochondrial Involvement and Impact in Aging Skeletal Muscle
Mitochondria play a pivotal role in skeletal muscle homeostasis and bioenergetics. Accordingly, age-related changes in mitochondria will have a crucial impact on skeletal muscle mass and function. Skeletal muscle mitochondria are highly adaptable and can be increased or decreased in content according to the metabolic demands of the tissue. As such, the level of habitual physical activity is a crucial determinant of skeletal muscle mitochondrial content irrespective of age. Furthermore, mitochondrial morphology is remarkably dynamic, and changes in response to various conditions within the cell. Finally, mitochondria are continually turned over to maintain their fidelity, and thus mitochondrial content and morphology also need to be considered in context with mitochondrial function. In the following sections, current knowledge concerning mitochondrial content, morphology, and function, and their plasticity across the continuum of aging is reviewed. Note that the issues discussed herein cannot represent all of the seminal works in the area and several recent reviews provide a more in-depth treatment of this topic (Carter et al., 2015; Dai et al., 2014).
Mitochondrial Content and Morphology
One of the most inconsistent findings in the literature concerns the impact of ageing on skeletal muscle mitochondrial content, with some studies suggesting no change (Gouspillou et al., 2014b; Konopka et al., 2014) and others a decrease (Chabi et al., 2008; Conley et al., 2000; Hebert et al., 2015). This controversy is due in part to the many different measures used to represent mitochondrial content, including enzyme activities, mitochondrial protein levels, mitochondrial DNA levels (mtDNA), and mitochondrial volume density. In mouse skeletal muscle, individual proteins within a mitochondrion appear to have distinct turnover rates (Karunadharma et al., 2015), suggesting that there is strong potential for altered protein stoichiometries in aging mitochondria, a factor that likely contributes to the disparities noted above between different markers of mitochondrial content. Notably, even within a given study comparing the same indices of mitochondrial content, individual muscles can exhibit different changes with aging in rats (Lyons et al., 2006), such that some muscles may show declines, while others may show increases (Picard et al., 2011a).
Mitochondrial content is dynamically regulated according to the metabolic needs of the tissue, and therefore changes in physical activity patterns with aging can also contribute to the inconsistencies between studies. Thus, isolating the effects of primary aging on mitochondrial content from the effects of the decline in physical activity is a challenge, since these are intertwined events (e.g., a decline in mitochondrial content due to primary aging can contribute to reduced physical activity levels). Moreover, the changes in mitochondrial content may vary at different points along the aging continuum and depend upon fiber type (Mathieu-Costello et al., 2005), and they may to some extent be sex-specific based upon recent evidence suggesting women are more susceptible to a decline in mitochondrial content with aging (Callahan et al., 2014). Although some studies have addressed various indices of skeletal muscle mitochondrial content across a broad age range in humans (Short et al., 2005), limited data is available in individuals with very advanced age (>75 y). A more thorough review of this issue can be found elsewhere (Hepple, 2014).
The diversity of mitochondrial structure that exists in skeletal muscle (Kuznetsov et al., 2006; Ogata and Yamasaki, 1997), its dynamic plasticity in response to different myocellular conditions (Pham et al., 2012; Romanello et al., 2010; Toyama et al., 2016), and the mechanisms responsible for the regulation of this structure (Chan, 2012; Mishra et al., 2014; Toyama et al., 2016) are being increasingly understood. In the context of aging muscle atrophy, a variety of muscle atrophy conditions (e.g., immobilization, fasting, denervation) result in mitochondrial fission, which in turn results in activation of muscle proteolytic pathways (Cannavino et al., 2015; Romanello et al., 2010). Whereas one might logically hypothesize, therefore, that mitochondria should be more fissioned in aging muscle due to their atrophy, the evidence addressing this to date is inconclusive. Some evidence suggests mitochondria are more fragmented in aging skeletal muscle (Iqbal et al., 2013), whereas other evidence suggests mitochondria are hyper-fused in aging skeletal muscle (Beregi et al., 1988; Leduc-Gaudet et al., 2015). Part of this disparity likely relates to the heterogeneity of fiber affect in aging muscle, particularly in very advanced age when severely atrophied, denervated fibers are intermingled with relatively normal-looking fibers (Rowan et al., 2012). For example, since denervation is one of the stimuli that induce mitochondrial fission in muscle (Iqbal et al., 2013; Romanello et al., 2010), studies examining muscles in advanced age are more likely to sample regions from denervated fibers and are thus more likely to observe mitochondria with highly fissioned morphology. This notion is consistent with the fact that the studies finding more fused mitochondria with aging examined younger animals (Beregi et al., 1988; Leduc-Gaudet et al., 2015) than the study reporting more fissioned mitochondria (Iqbal et al., 2013). Whereas the appearance of fissioned mitochondria in advanced age is reasonable given the impact of denervation, the tendency towards hyper-fused mitochondria in aging muscle at younger ages is consistent with observations of so-called giant mitochondria in aging heart (Coleman et al., 1987), a finding which has been ascribed to impaired autophagy (Navratil et al., 2008). This latter idea is consistent with recent evidence for impaired mitochondrial quality control mechanisms (Joseph et al., 2013) and a reduction in the mitochondrial-targeted ubiquitin ligase, Parkin, in aging human muscle (Drummond et al., 2014; Gouspillou et al., 2014b).
Mitochondrial Function
Mitochondria serve a wide variety of roles in skeletal muscle including synthesis of ATP, reactive oxygen species (ROS) signaling, and regulation of intrinsic pathways of apoptosis. As was the case for mitochondrial content, a wide variety of approaches have been taken in evaluating changes in mitochondrial function with ageing. These include in vivo measures of phosphocreatine synthesis (Gouspillou et al., 2014a; Kent-Braun and Ng, 2000), in vitro measures of biochemical activities of various enzymes in muscle homogenates (Houmard et al., 1998) and various aspects of function in mechanically isolated mitochondria (Chabi et al., 2008; Gouspillou et al., 2010; Picard et al., 2010), and finally ex vivo measures of various aspects of function in saponin-permeabilized myofibers (Gouspillou et al., 2014b; Hutter et al., 2007; Tonkonogi et al., 2003). Measurements in isolated organelles or saponin-permeabilized myofibers provide for a more in-depth assessment of mitochondrial functions, including respiration/bioenergetics, ROS signaling, and sensitivity to permeability transition, than the other approaches (Picard et al., 2011b).
While mitochondrial respiratory capacity may decline with aging in physically inactive humans (Conley et al., 2000) or rodents (Chabi et al., 2008), it is maintained in physically active humans (65–75 years-old) at least up to old age (75 y) (Gouspillou et al., 2014b; Lanza et al., 2005). However this issue has not yet been examined in very advanced age (>75 y), where the impact of muscle aging is more clinically meaningful. Conversely, numerous studies find reduced coupling (e.g., reduced ratio of respiration under non-phosphorylating [state 2 or state 4 respiration] versus maximally phosphorylating conditions [state 3 conditions]) (Amara et al., 2007; Gouspillou et al., 2014b; Marcinek et al., 2005; Picard et al., 2010). Whether this represents an adaptive response to reduce ROS (Amara et al., 2007; Speakman et al., 2004) or dysfunction that results in a less efficient harnessing of the potential energy created by proton pumping of the electron transport system, remains inconclusive.
Mitochondrial ROS are increasingly being understood in terms of their importance to cellular signaling and in promoting beneficial cellular adaptations (Lapointe and Hekimi, 2008; Schaar et al., 2015). Conversely, conceptually it seems logical to suggest that elevated mitochondrial ROS would at some point become deleterious, and certainly there has been suggestion that oxidative stress promotes muscle atrophy with aging (Jang et al., 2010). However, despite many years of interrogating this issue, the role of mitochondrial ROS in aging muscle remains a point of contention. Changes in mitochondrial ROS generation in aging skeletal muscle are remarkably variable between studies, although the majority report an increase with aging in both humans and animal models (Capel et al., 2004; Capel et al., 2005; Mansouri et al., 2006). In contrast to this view however, a high reliance on isolated organelles, a procedure that potentiates muscle mitochondrial ROS (Picard et al., 2011c) and which exaggerates the impact of aging (Picard et al., 2010), has skewed perception of the changes in mitochondrial ROS in aging skeletal muscle. Indeed, across four muscles of contrasting fiber type and atrophy susceptibility the increase in mitochondrial ROS in aging skeletal muscle, even in advanced age, is quite small when using methods that preserve mitochondrial structure (Picard et al., 2011a). Furthermore, in physically active humans mitochondrial ROS is not increased despite significant muscle atrophy (Gouspillou et al., 2014b), suggesting the role of mitochondrial ROS in driving muscle atrophy with aging has likely been over-estimated. Consistent with this view, mice that lack superoxide dismutase 2 (the primary mitochondrial isoform) do not exhibit a muscle atrophy phenotype (Lustgarten et al., 2011), even if the SOD2 knockout is restricted to skeletal muscle (Kuwahara et al., 2010), but do exhibit evidence of contractile dysfunction. Thus, an increase in mitochondrial ROS in aging skeletal muscle could contribute to impaired contractility, but it is unlikely to be the proximate cause of muscle atrophy. The fact that no increase in mitochondrial ROS is seen in physically active elderly individuals (Gouspillou et al., 2014b), whereas muscle immobilization induces an increase in mitochondrial ROS in otherwise healthy subjects (Gram et al., 2015), suggests that physical activity is likely an effective way of limiting an increase in mitochondrial ROS in aging skeletal muscle.
Besides ATP production and ROS signaling, mitochondria are also key regulators of the intrinsic pathway of apoptosis. Specifically, under stressful conditions such as high Ca2+ load, mitochondria can undergo a process called permeability transition wherein a pore across the inner mitochondrial membrane is formed (the so-called mitochondrial permeability transition pore; mPTP) that permits the passage of proteins up to 1500 Da in size (Bernardi et al., 2015). Notably, there are several mitochondrial-localized proteins that, once released through mPTP opening, can induce apoptosis, including endonuclease G (EndoG), apoptosis inducing factor (AIF), and others (Marzetti et al., 2010). In this respect, and in marked contrast to the highly variable changes in respiration and ROS in aging skeletal muscle reviewed above, an increased susceptibility to mPTP opening is consistently reported in aging rodents (Chabi et al., 2008; Picard et al., 2011a), but it appears it cannot be prevented through maintaining a physically active lifestyle in humans (Gouspillou et al., 2014b). The functional impact of an increased sensitivity of mitochondria to mPTP opening in aging skeletal muscle remains unclear, but it is noteworthy that a sensitization to mPTP opening occurs only in aging muscles that undergo atrophy (Hepple, 2014).
Mitochondrial Plasticity in Aging Skeletal Muscle
In view of the potent role that physical activity can play in limiting mitochondrial changes in aging skeletal muscle, and the well-established role that exercise training plays in inducing mitochondrial biogenesis, the ability to increase or maintain organelle biosynthesis with aging is important to consider. An early seminal study from Holloszy’s group provides evidence that the activity of several mitochondrial enzymes is maintained in response to nearly life-long swim training in aging rats (Young et al., 1983). However, at more advanced and thus clinically relevant ages, exercise responsiveness declines as reviewed below.
Key to understanding the declining mitochondrial plasticity in advanced age is the signal transducing machinery that regulates mitochondrial biogenesis. Although many over-lapping pathways are now implicated in mitochondrial biogenesis, a key player is peroxisome proliferator activated receptor gamma 1 (PGC-1), particularly the alpha isoform (PGC-1α). Upstream of PGC-1α, adenosine monophosphate kinase (AMPK) (Irrcher et al., 2008) and sirtuin 1 (Sirt1) (Menzies et al., 2013), have emerged as key elements determining the induction of PGC-1α in response to muscle contractile activity. In this respect, whereas exercise training and chronic muscle contractile activity with electrical stimulation are both potent inducers of mitochondrial biogenesis and muscle aerobic performance in aging muscle (Betik et al., 2008; Cartee and Farrar, 1987; Skorjanc et al., 2001), in advanced age the response becomes blunted (Betik et al., 2009; Chekanov et al., 2000; Ljubicic and Hood, 2009a). We have noted a robust increase in muscle mitochondrial enzyme activities in old rat muscle following 8 weeks of endurance-focused treadmill exercise training (Betik et al., 2008), however, continuing the exercise training for a further 4 months until very old age resulted in no elevation of mitochondrial enzymes or aerobic contractile performance in the muscles from the exercise trained versus sedentary animals (Betik et al., 2009). The implications of this latter result are that following the initial benefit, the muscles of very old animals undergoing exercise training exhibited an accelerated decline in muscle aerobic capacity relative to the sedentary animals (since the trained animals gained an advantage that was lost in very advanced age). The causes of this remarkably diminished plasticity of skeletal muscle mitochondria in advanced age likely relate to a failure to upregulate PGC-1α (Betik et al., 2009) and other signaling pathways necessary for inducing an adaptive response (Ljubicic and Hood, 2009a, b). Examining whether this blunted response can be ascribed to gene silencing subsequent to changes in DNA methylation and acetylation status (or other epigenetic changes) would seem a logical next step.
Aging Muscle Atrophy
For the past three decades, there has been a flurry of research into the etiology of aging muscle atrophy, its functional sequelae, and treatment efforts. The research has grown concurrent with (1) a steep rise in the number of adults over 65 years-old and an exponential increase in the number of octagenarians, and (2) epidemiological findings pointing toward loss of muscle mass as a major contributor to functional decline, disability, and dependence (Janssen et al., 2004a; Janssen et al., 2002). However, the mechanistic underpinnings remain poorly understood and at best aging muscle atrophy can be described as a multifactorial degenerative process impacted by cellular aging biology (primary aging) and environmental/behavioral factors along with disease (secondary aging). Among the potential mechanisms, oxidative stress has received significant attention (Fulle et al., 2004; Marzetti et al., 2013), and there may be an emerging role for local muscle inflammation susceptibility with aging that also manifests in human primary myoblasts and myotubes in vitro (Merritt et al., 2013).
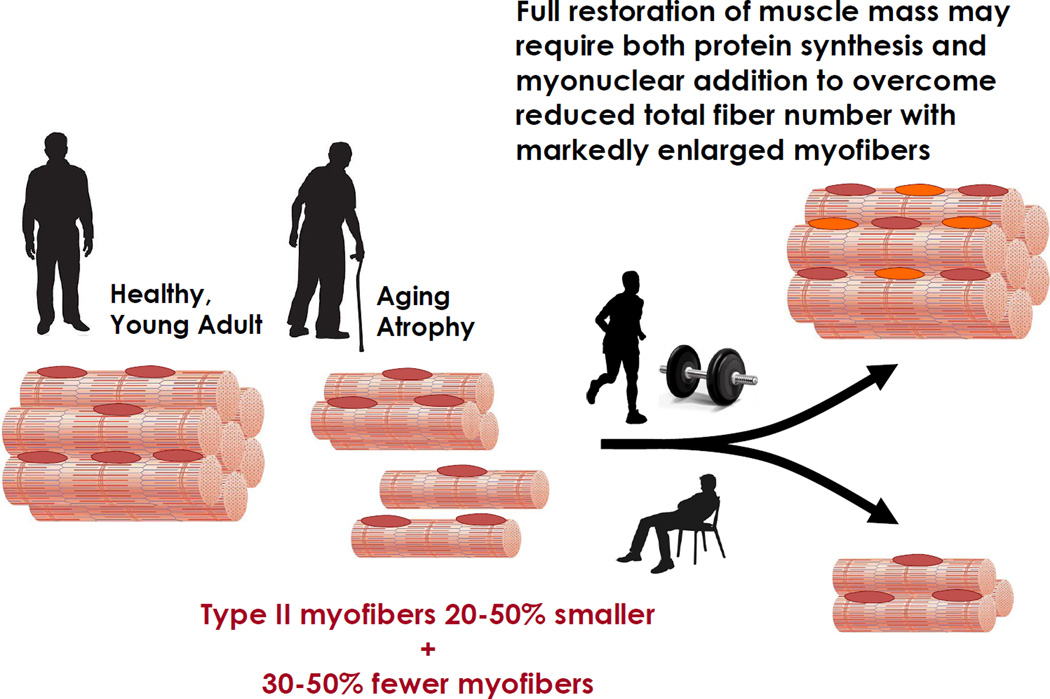
At its root, aging muscle atrophy results from atrophy of type II myofibers (Merritt et al., 2013) and loss of both type I and type II myofibers (Figure 3). These were seminal findings in the early studies of aging human limb muscles (Lexell et al., 1986; Lexell et al., 1983). Decreased myofiber number is presumed to result from alpha motor neuron death or peripheral denervation at the neuromuscular junction. Whether central or peripheral denervation in origin (or both), one indicator of this neurodegenerative process is an increase in myofiber type grouping often noted in aging human muscle (Lexell, 1995; Lexell et al., 1986), as some denervated myofibers survive via reinnervation by a sprouting axon of a different motor unit type (e.g. type I neuron innervating denervated type II myofibers). Whether evaluated at the myofiber level histologically, or by gross measures such as dual-energy x-ray absorptiometry to assess regional limb lean mass (e.g., upper limbs, lower limbs, thighs), age group differences are typically noted when young adults (e.g., third to fourth decade) are compared to older adults in the sixth or seventh decade and beyond. The rate of whole muscle atrophy appears to be similar in women and men; although, the amount attributable to type II myofiber atrophy vs. myofiber loss may differ by gender, as type II atrophy among older women appears to exceed that of age-matched men, particularly among the type IIx myofiber population (Kim et al., 2005; Kosek et al., 2006).
Figure 3. Conceptual Model of Aging Muscle Atrophy and the Impact of Progressive Resistance Exercise Training.
In addition to muscle atrophy, a hallmark of aging is an obligatory decline in neuromuscular performance. Loss of strength appears to be greater in lower than upper limbs (Landers et al., 2001), and a decline in muscle power far exceeds that of strength (Petrella et al., 2005; Young and Skelton, 1994) and appears to occur more precipitously (Reid and Fielding, 2011). Specific strength (force per unit muscle) clearly decreases with advancing age (Goodpaster et al., 2006; Petrella et al., 2005), indicating that in addition to muscle atrophy, the decline in strength is influenced by factors that reduce muscle quality, such as muscle lipid content (Goodpaster et al., 2001). Furthermore, the remarkable loss of muscle power even after adjusting for muscle mass (Petrella et al., 2005), suggests age-related changes in excitation-contraction coupling and/or other subcellular mechanisms responsible for rapid force generation.
Impact of Exercise Countermeasures for Muscle Atrophy
Favorable neuromuscular adaptations have been observed in older adults undergoing resistance training, including muscle and myofiber hypertrophy and remarkable gains in strength and power (Figure 3). Progressive resistance exercise training is the most widely accepted strategy to promote muscle regrowth in atrophied older adults, with more proven efficacy than pharmacologic or nutritional alternatives. However, the hypertrophic adaptation to progressive resistance exercise training varies widely among individuals (Bamman et al., 2007) and is generally blunted in older adults (e.g., 60–75 years-old) compared to young (Bickel et al., 2011). The oldest old (e.g., octagenarians) experience very limited hypertrophy and little to no improvement in single myofiber function (Raue et al., 2009; Slivka et al., 2008). This blunted response is not due to a difference in the ability to perform resistance training at the intensity effective for muscle hypertrophy, as we have prescribed standardized doses and reported nearly identical exercise training intensities among participants ranging from non-responders to extreme responders (Bamman et al., 2007).
To more fully appreciate the benefits and limitations of attempts to restore lost muscle mass in older adults, it is noteworthy that adult myofibers are multinucleated, terminally differentiated cells. Therefore, the only option is to induce hypertrophy of existing myofibers via mechanisms leading to cellular hypertrophy (Adams and Bamman, 2012), which will limit overall muscle hypertrophy in an aging muscle that may have lost 30–50% of its original myofibers (Lexell et al., 1988). The two primary mechanisms for myofiber hypertrophy in humans involve (1) accretion of protein in the various cellular protein compartments (e.g., myofibrillar pool) via mechanosensitive signaling pathways that drive translation, and (2) the activation and recruitment of resident muscle stem (satellite) cells that differentiate to fusion competent myoblasts – each one donating its nucleus to a growing myofiber via a fusion process. Both processes are important, as the effectiveness of each appears prognostic for the magnitude of myofiber hypertrophy achieved (Mayhew et al., 2011; Petrella et al., 2008). In fact, in addition to aging differences in resistance exercise-induced translational efficiency (i.e. translation initiation signaling) (Fry et al., 2011), recent evidence suggests translational capacity (i.e. de novo ribosome biogenesis) may be a key determinant of human myofiber hypertrophy potential (Figueiredo et al., 2015), as noted in older adults both in a progressive resistance exercise training model and in myotubes in vitro (Stec et al., 2016). A blunted induction of ribosome biogenesis among old vs. younger adults in response to each resistance exercise bout (Stec et al., 2015) may be one mechanism responsible for the lesser progressive resistance exercise training hypertrophy commonly seen with advancing age. Thus, ribosome biogenesis may be central to both mechanisms – that is, a myofiber undergoing substantial cytoplasmic volume expansion (i.e. hypertrophy) via net protein accretion may be in need of myonuclear addition to provide more rDNA template to facilitate ribosome biogenesis. Aging myofibers deficient in either mechanism may suffer limited regrowth potential; whereas aging myofibers with a viable stem cell pool and mechanosensitive translational signaling would be expected to benefit greatly from progressive resistance exercise training.
Future Directions
Physical inactivity has profound deleterious effects on health (Booth et al., 2011). Conversely, the decline in the age-related decrease in maximal oxygen consumption is markedly reduced (up to 50%) in individuals who engage in long-term vigorous endurance exercise training as compared to sedentary people (Rogers et al., 1990a). Fortunately, even quite late in life, an increase in physical activity is richly rewarded with health benefits. The Diabetes Prevention Program (DPP) demonstrated that for people 25–85 years-old with a high risk for developing type 2 diabetes, the oldest group (60–85 years-old) exceeded the younger group for achieving both the 7% weight loss and 150 minute/week physical activity goals, and the oldest group was most successful for preventing diabetes (Diabetes Prevention Program Research et al., 2006; Wing et al., 2004). Because the DPP combined diet modifications with increased physical activity, these results were not solely attributable to exercise. However, for women and men 60–85 years-old, increasing physical activity alone can improve insulin sensitivity and glucose metabolism (Evans et al., 2005; Kirwan et al., 1993; Short et al., 2003), as well as mitigate age-related muscle loss (Hunter et al., 2004). There are currently no interventions with a higher therapeutic index than either physical exercise and/or diet manipulation to reduce the risk of virtually all chronic diseases simultaneously, with little or no adverse side effects. Consequently future research should be directed towards deciphering optimal exercise and nutrition programs to achieve healthy aging.
Future efforts should be focused on mechanisms by which different modes of exercise enhance whole body metabolism and mitigate declines in strength across the aging population. To achieve compliance, it may be more realistic to incorporate short bouts of exercise to be undertaken throughout the day that are aligned with activities of daily living. For example, in insulin resistant men and women age 18–55 years old (48±6 years), undertaking an “exercise snacking” routine of six 1 min exercise bouts, consisting of walking at 90% maximal heart rate with 1 min recovery (slow walking) between each, 30 min before breakfast, lunch, and dinner, markedly improved glycemic control compared with a single 30 min bout of moderate continuous exercise undertaken before the evening meal (Francois et al., 2014). Similar improvements in glucose homeostasis were achieved when the exercise bouts were alternated between aerobic-based exercise and resistance-based exercise using resistance bands (as many reps as possible within 60 s) (Francois et al., 2014). These findings suggest that the timing and intensity of exercise may be important for optimizing glucose control. Further exercise training studies of different endurance and strength modalities that are conducted over weeks or months are warranted, particularly in the elderly, to evaluate the effects on insulin sensitivity, long-term blood glucose control, and muscle strength. This type of program is relatively easy to monitor and can be performed in the home setting and in conjunction with activities of daily living, making compliance easier to achieve.
Despite the compelling evidence that exercise is medicine, several questions remain. From a mechanistic perspective, greater insight into the specific cellular and molecular events during and after exercise that account for the enhanced insulin sensitivity and preservation of muscle mass with aging is required. Such efforts may advance the discovery of unique molecular biometrics to identify individuals with early onset of skeletal muscle insulin resistance, mitochondrial dysfunction, or aging-induced muscle atrophy. Stratification of individuals based on their response to different endurance- or resistance-based exercise training programs in terms of enhanced insulin sensitivity and mitochondrial function, as well as the hypertrophic adaptation of skeletal muscle, may also identify people at risk for metabolic disease and secondary aging. In such cases, specialized exercise prescription may be beneficial to ward off secondary aging.
Integrated approaches to identify molecular signatures of skeletal muscle and the adaptive response to diverse modes of exercise training programs (both endurance-based and resistance-based) across the spectrum of aging may be valuable to stratify different response-patterns for personalized treatment. For example, biomarkers derived from a composite of an individual’s genomic, transcriptomic/proteomic, and metabolomic profile, compared against physiological measures of insulin sensitivity and functional muscle mass/strength, at rest or after exercise training, may identify people at risk for accelerated muscle wasting, diminished work/exercise capacity or rapid progression into type 2 diabetes. The future will advance research into how these parameters can be altered to promote healthy aging. Several efforts are now underway to identify and validate molecular signatures of skeletal muscle that are associated with aging-related disorders including overt type 2 diabetes and the adaptive response to exercise training in humans using multiple omics-bases approaches (Neufer et al., 2015; Zierath and Wallberg-Henriksson, 2015). Initiatives to uncover individual variability that will influence risk assessment, diagnostic categories, and therapeutic strategies are ongoing, heralding in a new era of precision medicine (Collins and Varmus, 2015). However, much distance needs to be covered before this personalized approach can be translated into targeted clinical care. Future efforts to integrate physiological and molecular data from clinical cohorts, cell-based systems, and animal models, as well as measures of behavioral, physiological and environmental factors will be required before the spectrum of primary and secondary age-related changes on human physiology are fully realized.
From a translational perspective, clinical insight into key aspects of specific exercise protocols designed to improve insulin sensitivity, mitochondrial function, muscle mass and overall physical capacity are needed. Moreover, it is important to consider how such protocols can be implemented to achieve optimal benefits while accommodating the physical limitations of very old and frail individuals to achieve “healthy aging”. Accordingly, the interactions between lifestyle interventions (nutrition and physical activity) in treating, and managing insulin resistance, obesity and aging muscle atrophy are important to determine. By implementing strategies to maintain insulin sensitivity and mitigate aging muscle atrophy before the onset of frailty to maintain glucose homeostasis and physical function, many of the deleterious effects of unhealthy ageing can be markedly attenuated. Such efforts have the potential to dramatically reduce the associated health care costs of unhealthy aging. Given the evidence that modifiable lifestyle factors have the potential to limit secondary aging, the proverbial “fountain of youth” may be just down the street at your nearest neighborhood gym.
REFERENCES
- Adams GR, Bamman MM. Characterization and regulation of mechanical loading-induced compensatory muscle hypertrophy. Compr Physiol. 2012;2:2829–2870. doi: 10.1002/cphy.c110066. [DOI] [PubMed] [Google Scholar]
- Amara CE, Shankland EG, Jubrias SA, Marcinek DJ, Kushmerick MJ, Conley KE. Mild mitochondrial uncoupling impacts cellular aging in human muscles in vivo. Proc.Natl.Acad.Sci U.S.A. 2007;104:1057–1062. doi: 10.1073/pnas.0610131104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amati F, Dube JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care. 2009;32:1547–1549. doi: 10.2337/dc09-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annuzzi G, Riccardi G, Capaldo B, Kaijser L. Increased insulin-stimulated glucose uptake by exercised human muscles one day after prolonged physical exercise. Eur J Clin Invest. 1991;21:6–12. doi: 10.1111/j.1365-2362.1991.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2007;292:E1191–E1200. doi: 10.1152/ajpendo.00602.2006. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol. 2007;102:2232–2239. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Beaudart C, Reginster JY, Slomian J, Buckinx F, Dardenne N, Quabron A, Slangen C, Gillain S, Petermans J, Bruyere O. Estimation of sarcopenia prevalence using various assessment tools. Exp Gerontol. 2015;61:31–37. doi: 10.1016/j.exger.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Beaudart C, Reginster JY, Slomian J, Buckinx F, Locquet M, Bruyere O. Prevalence of sarcopenia: the impact of different diagnostic cut-off limits. J Musculoskelet Neuronal Interact. 2014a;14:425–431. [PubMed] [Google Scholar]
- Beaudart C, Rizzoli R, Bruyere O, Reginster JY, Biver E. Sarcopenia: burden and challenges for public health. Arch Public Health. 2014b;72:45. doi: 10.1186/2049-3258-72-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beregi E, Regius O, Huttl T, Gobl Z. Age-related changes in the skeletal muscle cells. Z Gerontol. 1988;21:83–86. [PubMed] [Google Scholar]
- Bernardi P, Rasola A, Forte M, Lippe G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betik AC, Baker DJ, Krause DJ, McConkey MJ, Hepple RT. Exercise training in late middle-aged male Fischer 344 x Brown Norway F1-hybrid rats improves skeletal muscle aerobic function. Exp Physiol. 2008;93:863–871. doi: 10.1113/expphysiol.2008.042069. [DOI] [PubMed] [Google Scholar]
- Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol. 2009;297:R744–R755. doi: 10.1152/ajpregu.90959.2008. [DOI] [PubMed] [Google Scholar]
- Bickel CS, Cross JM, Bamman MM. Exercise dosing to retain resistance training adaptations in young and older adults. Medicine & Science in Sports & Exercise. 2011;43:1177–1187. doi: 10.1249/MSS.0b013e318207c15d. [DOI] [PubMed] [Google Scholar]
- Bienso RS, Olesen J, Gliemann L, Schmidt JF, Matzen MS, Wojtaszewski JF, Hellsten Y, Pilegaard H. Effects of Exercise Training on Regulation of Skeletal Muscle Glucose Metabolism in Elderly Men. J Gerontol A Biol Sci Med Sci. 2015;70:866–872. doi: 10.1093/gerona/glv012. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol (1985) 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. Journal of applied physiology. 2011;111:1497–1504. doi: 10.1152/japplphysiol.00420.2011. [DOI] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2:1143–1211. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90:1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a009191. http://dx.doi.org/10.1101/cshperspect.a009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan DM, Bedrin NG, Subramanian M, Berking J, Ades PA, Toth MJ, Miller MS. Age-related structural alterations in human skeletal muscle fibers and mitochondria are sex specific: relationship to single-fiber function. J Appl Physiol (1985) 2014;116:1582–1592. doi: 10.1152/japplphysiol.01362.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol. 2015;593:1981–1995. doi: 10.1113/jphysiol.2014.286740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel F, Buffiere C, Patureau MP, Mosoni L. Differential variation of mitochondrial H2O2 release during aging in oxidative and glycolytic muscles in rats. Mech.Ageing Dev. 2004;125:367–373. doi: 10.1016/j.mad.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Capel F, Rimbert V, Lioger D, Diot A, Rousset P, Mirand PP, Boirie Y, Morio B, Mosoni L. Due to reverse electron transfer, mitochondrial H2O2 release increases with age in human vastus lateralis muscle although oxidative capacity is preserved. Mech.Ageing Dev. 2005;126:505–511. doi: 10.1016/j.mad.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Cartee GD. Mechanisms for greater insulin-stimulated glucose uptake in normal and insulin-resistant skeletal muscle after acute exercise. Am J Physiol Endocrinol Metab. 2015;309:E949–E959. doi: 10.1152/ajpendo.00416.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee GD, Bohn EE. Growth hormone reduces glucose transport but not GLUT-1 or GLUT-4 in adult and old rats. Am J Physiol. 1995;268:E902–E909. doi: 10.1152/ajpendo.1995.268.5.E902. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Briggs-Tung C, Kietzke EW. Persistent effects of exercise on skeletal muscle glucose transport across the life-span of rats. Journal of applied physiology. 1993;75:972–978. doi: 10.1152/jappl.1993.75.2.972. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Farrar RP. Muscle respiratory capacity and VO 2max in identically trained young and old rats. Journal of Applied Physiology. 1987;63:257–261. doi: 10.1152/jappl.1987.63.1.257. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J Physiol. 1990;258:E390–E393. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- Cartee GD, Young DA, Sleeper MD, Zierath J, Wallberg-Henriksson H, Holloszy JO. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256:E494–E499. doi: 10.1152/ajpendo.1989.256.4.E494. [DOI] [PubMed] [Google Scholar]
- Carter HN, Chen CC, Hood DA. Mitochondria, muscle health, and exercise with advancing age. Physiology (Bethesda) 2015;30:208–223. doi: 10.1152/physiol.00039.2014. [DOI] [PubMed] [Google Scholar]
- Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. [PMC free article] [PubMed] [Google Scholar]
- Castorena CM, Arias EB, Sharma N, Cartee GD. Postexercise improvement in insulin-stimulated glucose uptake occurs concomitant with greater AS160 phosphorylation in muscle from normal and insulin-resistant rats. Diabetes. 2014;63:2297–2308. doi: 10.2337/db13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabi B, Ljubicic V, Menzies KJ, Huang JH, Saleem A, Hood DA. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell. 2008;7:2–12. doi: 10.1111/j.1474-9726.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- Chekanov VS, Karakozov P, Rieder M, Zander G. Age related skeletal muscle response to electrical stimulation. ASAIO J. 2000;46:474–481. doi: 10.1097/00002480-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Cleasby ME, Jamieson P, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016 doi: 10.1530/JOE-15-0533. Published online March 1, 2016. http://dx.doi.org/10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- Coleman R, Silbermann M, Gershon D, Reznick AZ. Giant mitochondria in the myocardium of aging and endurance-trained mice. Gerontology. 1987;33:34–39. doi: 10.1159/000212851. [DOI] [PubMed] [Google Scholar]
- Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and aging in human muscle. Journal of Physiology 526. 2000;1:203–210. doi: 10.1111/j.1469-7793.2000.t01-1-00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consitt LA, Van Meter J, Newton CA, Collier DN, Dar MS, Wojtaszewski JF, Treebak JT, Tanner CJ, Houmard JA. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes. 2013;62:3437–3447. doi: 10.2337/db13-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Ford ES, Eberhardt MS, Byrd-Holt DD, Li C, Williams DE, Gregg EW, Bainbridge KE, Saydah SH, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Cortright RN, Dohm GL, Houmard JA. Effect of aging on response to exercise training in humans: skeletal muscle GLUT-4 and insulin sensitivity. J Appl Physiol. 1999;86:2019–2025. doi: 10.1152/jappl.1999.86.6.2019. [DOI] [PubMed] [Google Scholar]
- Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- Demakakos P, Hamer M, Stamatakis E, Steptoe A. Low-intensity physical activity is associated with reduced risk of incident type 2 diabetes in older adults: evidence from the English longitudinal study of ageing. DiabetologiA. 2010;53:1877–1885. doi: 10.1007/s00125-010-1785-x. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research, G. Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, Dagogo-Jack S, Andres R. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–1081. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond MJ, Addison O, Brunker L, Hopkins PN, McClain DA, LaStayo PC, Marcus RL. Downregulation of e3 ubiquitin ligases and mitophagy-related genes in skeletal muscle of physically inactive, frail older women: a cross-sectional comparison. J Gerontol A Biol Sci Med Sci. 2014;69:1040–1048. doi: 10.1093/gerona/glu004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escriva F, Gavete ML, Fermin Y, Perez C, Gallardo N, Alvarez C, Andres A, Ros M, Carrascosa JM. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194:131–141. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- Evans EM, Racette SB, Peterson LR, Villareal DT, Greiwe JS, Holloszy JO. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77–87 yr olds. Journal of applied physiology. 2005;98:40–45. doi: 10.1152/japplphysiol.00928.2004. [DOI] [PubMed] [Google Scholar]
- Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, Caldow MK, Massie V, Markworth JF, Cameron-Smith D, Blazevich AJ. Ribosome biogenesis adaptation in resistance training-induced human skeletal muscle hypertrophy. Am J Physiol Endocrinol Metab. 2015;309:E72–E83. doi: 10.1152/ajpendo.00050.2015. [DOI] [PubMed] [Google Scholar]
- Fink RI, Wallace P, Olefsky JM. Effects of aging on glucose-mediated glucose disposal and glucose transport. J Clin Invest. 1986;77:2034–2041. doi: 10.1172/JCI112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois ME, Baldi JC, Manning PJ, Lucas SJ, Hawley JA, Williams MJ, Cotter JD. ‘Exercise snacks’ before meals: a novel strategy to improve glycaemic control in individuals with insulin resistance. DiabetologiA. 2014;57:1437–1445. doi: 10.1007/s00125-014-3244-6. [DOI] [PubMed] [Google Scholar]
- Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skeletal Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Castorena CM, Kanzaki M, Cartee GD. In vivo exercise followed by in vitro contraction additively elevates subsequent insulin-stimulated glucose transport by rat skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E999–E1010. doi: 10.1152/ajpendo.00758.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E242–E251. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaster M, Poulsen P, Handberg A, Schroder HD, Beck-Nielsen H. Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E910–E916. doi: 10.1152/ajpendo.2000.278.5.E910. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Biran M, Deschodt-Arsac V, Miraux S, Thiaudiere E, Pasdois P, Detaille D, et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell. 2014a;13:39–48. doi: 10.1111/acel.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Franconi JM, Deschodt-Arsac V, Diolez P. Alteration of mitochondrial oxidative phosphorylation in aged skeletal muscle involves modification of adenine nucleotide translocator. Biochim Biophys ActA. 2010;1797:143–151. doi: 10.1016/j.bbabio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA, et al. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J. 2014b;28:1621–1633. doi: 10.1096/fj.13-242750. [DOI] [PubMed] [Google Scholar]
- Gram M, Vigelso A, Yokota T, Helge JW, Dela F, Hey-Mogensen M. Skeletal muscle mitochondrial H2 O2 emission increases with immobilization and decreases after aerobic training in young and older men. J Physiol. 2015;593:4011–4027. doi: 10.1113/JP270211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulve EA, Rodnick KJ, Henriksen EJ, Holloszy JO. Effects of wheel running on glucose transporter (GLUT4) concentration in skeletal muscle of young adult and old rats. Mech Ageing Dev. 1993;67:187–200. doi: 10.1016/0047-6374(93)90122-8. [DOI] [PubMed] [Google Scholar]
- Haffner SM. Epidemiology of insulin resistance and its relation to coronary artery disease. Am J Cardiol. 1999;84:11J–14J. doi: 10.1016/s0002-9149(99)00351-3. [DOI] [PubMed] [Google Scholar]
- Han DH, Hansen PA, Chen MM, Holloszy JO. DHEA treatment reduces fat accumulation and protects against insulin resistance in male rats. J Gerontol A Biol Sci Med Sci. 1998;53:B19–E24. doi: 10.1093/gerona/53a.1.b19. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Nolte LA, Chen MM, Holloszy JO. Increased GLUT-4 translocation mediates enhanced insulin sensitivity of muscle glucose transport after exercise. J Appl Physiol. 1998;85:1218–1222. doi: 10.1152/jappl.1998.85.4.1218. [DOI] [PubMed] [Google Scholar]
- Heber D, Ingles S, Ashley JM, Maxwell MH, Lyons RF, Elashoff RM. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am J Clin Nutr. 1996;64:472S–477S. doi: 10.1093/ajcn/64.3.472S. [DOI] [PubMed] [Google Scholar]
- Hebert SL, Marquet-de Rouge P, Lanza IR, McCrady-Spitzer SK, Levine JA, Middha S, Carter RE, Klaus KA, Therneau TM, Highsmith EW, et al. Mitochondrial Aging and Physical Decline: Insights From Three Generations of Women. J Gerontol A Biol Sci Med Sci. 2015;70:1409–1417. doi: 10.1093/gerona/glv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT. Mitochondrial Involvement and Impact in Aging Skeletal Muscle. Frontiers in Aging Neuroscience. 2014;6:211. doi: 10.3389/fnagi.2014.00211. http://dx.doi.org/10.1016/j.freeradbiomed.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT. Impact of aging on mitochondrial function in cardiac and skeletal muscle. Free Radic Biol Med. 2016 doi: 10.1016/j.freeradbiomed.2016.03.017. Published online March 24, 2016. http://dx.doi.org/10.1016/j.freeradbiomed.2016.03.017. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. The biology of aging. Mayo Clin Proc. 2000;75(Suppl):S3–S8. discussion S8–9. [PubMed] [Google Scholar]
- Holloszy JO, Kohrt WM, Hansen PA. The regulation of carbohydrate and fat metabolism during and after exercise. Front BioSci. 1998;3:D1011–D1027. doi: 10.2741/a342. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner MD, Dolan PL, Leggett-Frazier N, Gavigan KE, Hickey MS, Tyndall GL, Zheng D, Alshami A, Dohm GL. Skeletal muscle GLUT4 protein concentration and aging in humans. Diabetes. 1995;44:555–560. doi: 10.2337/diab.44.5.555. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Weidner ML, Gavigan KE, Tyndall GL, Hickey MS, Alshami A. Fiber type and citrate synthase activity in the human gastrocnemius and vastus lateralis with aging. J Appl Physiol. 1998;85:1337–1341. doi: 10.1152/jappl.1998.85.4.1337. [DOI] [PubMed] [Google Scholar]
- Huffman DM, Barzilai N. Role of visceral adipose tissue in aging. Biochim Biophys ActA. 2009;1790:1117–1123. doi: 10.1016/j.bbagen.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes VA, Fiatarone MA, Fielding RA, Kahn BB, Ferrara CM, Shepherd P, Fisher EC, Wolfe RR, Elahi D, Evans WJ. Exercise increases muscle GLUT-4 levels and insulin action in subjects with impaired glucose tolerance. Am J Physiol. 1993;264:E855–E862. doi: 10.1152/ajpendo.1993.264.6.E855. [DOI] [PubMed] [Google Scholar]
- Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- Hutter E, Skovbro M, Lener B, Prats C, Rabol R, Dela F, Jansen-Durr P. Oxidative stress and mitochondrial impairment can be separated from lipofuscin accumulation in aged human skeletal muscle. Aging Cell. 2007;6:245–256. doi: 10.1111/j.1474-9726.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA. Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve. 2013;48:963–970. doi: 10.1002/mus.23838. [DOI] [PubMed] [Google Scholar]
- Irrcher I, Ljubicic V, Kirwan AF, Hood DA. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS ONE. 2008;3:e3614. doi: 10.1371/journal.pone.0003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010;24:1376–1390. doi: 10.1096/fj.09-146308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004a;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004b;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Adhihetty PJ, Wawrzyniak NR, Wohlgemuth SE, Picca A, Kujoth GC, Prolla TA, Leeuwenburgh C. Dysregulation of mitochondrial quality control processes contribute to sarcopenia in a mouse model of premature aging. PLoS One. 2013;8:e69327. doi: 10.1371/journal.pone.0069327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunadharma PP, Basisty N, Chiao YA, Dai DF, Drake R, Levy N, Koh WJ, Emond MJ, Kruse S, Marcinek D, et al. Respiratory chain protein turnover rates in mice are highly heterogeneous but strikingly conserved across tissues, ages, and treatments. FASEB J. 2015;29:3582–3592. doi: 10.1096/fj.15-272666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. Journal of Applied Physiology. 2000;89:1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- Kern M, Dolan PL, Mazzeo RS, Wells JA, Dohm GL. Effect of aging and exercise on GLUT-4 glucose transporters in muscle. Am J Physiol. 1992;263:E362–E367. doi: 10.1152/ajpendo.1992.263.2.E362. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol. 2005;99:2149–2158. doi: 10.1152/japplphysiol.00513.2005. [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol. 1993;48:M84–M90. doi: 10.1093/geronj/48.3.m84. [DOI] [PubMed] [Google Scholar]
- Kohrt WM, Kirwan JP, Staten MA, Bourey RE, King DS, Holloszy JO. Insulin resistance in aging is related to abdominal obesity. Diabetes. 1993;42:273–281. [PubMed] [Google Scholar]
- Konopka AR, Suer MK, Wolff CA, Harber MP. Markers of human skeletal muscle mitochondrial biogenesis and quality control: effects of age and aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2014;69:371–378. doi: 10.1093/gerona/glt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- Kumari M, Brunner E, Fuhrer R. Minireview: mechanisms by which the metabolic syndrome and diabetes impair memory. J Gerontol A Biol Sci Med Sci. 2000;55:B228–B232. doi: 10.1093/gerona/55.5.b228. [DOI] [PubMed] [Google Scholar]
- Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M, et al. Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radic Biol Med. 2010;48:1252–1262. doi: 10.1016/j.freeradbiomed.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Troppmair J, Sucher R, Hermann M, Saks V, Margreiter R. Mitochondrial subpopulations and heterogeneity revealed by confocal imaging: possible physiological role? Biochim Biophys ActA. 2006;1757:686–691. doi: 10.1016/j.bbabio.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56:B443–B448. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Befroy DE, Kent-Braun JA. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. Journal of Applied Physiology. 2005;99:1736–1744. doi: 10.1152/japplphysiol.00566.2005. [DOI] [PubMed] [Google Scholar]
- Lapointe J, Hekimi S. Early mitochondrial dysfunction in long-lived Mclk1+/− mice. J Biol Chem. 2008;283:26217–26227. doi: 10.1074/jbc.M803287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc-Gaudet JP, Picard M, St-Jean Pelletier F, Sgarioto N, Auger MJ, Vallee J, Robitaille R, St-Pierre DH, Gouspillou G. Mitochondrial morphology is altered in atrophied skeletal muscle of aged mice. Oncotarget. 2015;6:17923–17937. doi: 10.18632/oncotarget.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. Spec No. [DOI] [PubMed] [Google Scholar]
- Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m. vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci. 1986;72:211–222. doi: 10.1016/0022-510x(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Lexell J, Henriksson-Larsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell. 2009a;8:394–404. doi: 10.1111/j.1474-9726.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Specific attenuation of protein kinase phosphorylation in muscle with a high mitochondrial content. Am J Physiol Endocrinol Metab. 2009b;297:E749–E758. doi: 10.1152/ajpendo.00130.2009. [DOI] [PubMed] [Google Scholar]
- Lustgarten MS, Jang YC, Liu Y, Qi W, Qin Y, Dahia PL, Shi Y, Bhattacharya A, Muller FL, Shimizu T, et al. MnSOD deficiency results in elevated oxidative stress and decreased mitochondrial function but does not lead to muscle atrophy during aging. Aging Cell. 2011;10:493–505. doi: 10.1111/j.1474-9726.2011.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons CN, Mathieu-Costello O, Moyes CD. Regulation of skeletal muscle mitochondrial content during aging. J Gerontol A Biol Sci Med Sci. 2006;61:3–13. doi: 10.1093/gerona/61.1.3. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van RH. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech.Ageing Dev. 2006;127:298–306. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–473. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus RL, Addison O, LaStayo PC, Hungerford R, Wende AR, Hoffman JM, Abel ED, McClain DA. Regional muscle glucose uptake remains elevated one week after cessation of resistance training independent of altered insulin sensitivity response in older adults with type 2 diabetes. J Endocrinol Invest. 2013;36:111–117. doi: 10.3275/8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: From signaling pathways to clinical trials. International Journal of Biochemistry & Cell Biology. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS, Bernabei R, Leeuwenburgh C. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochimica et biophysica actA. 2010;1800:235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Costello O, Ju Y, Trejo-Morales M, Cui L. Greater capillary-fiber interface per fiber mitochondrial volume in skeletal muscles of old rats. Journal of Applied Physiology. 2005;99:281–289. doi: 10.1152/japplphysiol.00750.2004. [DOI] [PubMed] [Google Scholar]
- Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol. 2011;589:3023–3037. doi: 10.1113/jphysiol.2010.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneilly GS, Elliott T, Tessier D, Hards L, Tildesley H. NIDDM in the elderly. Diabetes Care. 1996;19:1320–1325. doi: 10.2337/diacare.19.12.1320. [DOI] [PubMed] [Google Scholar]
- Menzies KJ, Singh K, Saleem A, Hood DA. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J Biol Chem. 2013;288:6968–6979. doi: 10.1074/jbc.M112.431155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt EK, Stec MJ, Thalacker-Mercer A, Windham ST, Cross JM, Shelley DP, Craig Tuggle S, Kosek DJ, Kim JS, Bamman MM. Heightened muscle inflammation susceptibility may impair regenerative capacity in aging humans. J Appl Physiol (1985) 2013;115:937–948. doi: 10.1152/japplphysiol.00019.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra P, Carelli V, Manfredi G, Chan DC. Proteolytic cleavage of Opa1 stimulates mitochondrial inner membrane fusion and couples fusion to oxidative phosphorylation. Cell Metab. 2014;19:630–641. doi: 10.1016/j.cmet.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa J, Sato Y, Ishiko T. Time course of in vivo insulin sensitivity after a single bout of exercise in rats. Int J Sports Med. 1991;12:399–402. doi: 10.1055/s-2007-1024701. [DOI] [PubMed] [Google Scholar]
- Navratil M, Terman A, Arriaga EA. Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Exp Cell Res. 2008;314:164–172. doi: 10.1016/j.yexcr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Neufer PD, Bamman MM, Muoio DM, Bouchard C, Cooper DM, Goodpaster BH, Booth FW, Kohrt WM, Gerszten RE, Mattson MP, et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22:4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat.Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pauli JR, Ropelle ER, Cintra DE, De Souza CT, da Silva AS, Moraes JC, Prada PO, de Almeida Leme JA, Luciano E, Velloso LA, et al. Acute exercise reverses aged-induced impairments in insulin signaling in rodent skeletal muscle. Mech Ageing Dev. 2010;131:323–329. doi: 10.1016/j.mad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Pehmoller C, Brandt N, Birk JB, Hoeg LD, Sjoberg KA, Goodyear LJ, Kiens B, Richter EA, Wojtaszewski JF. Exercise alleviates lipid-induced insulin resistance in human skeletal muscle-signaling interaction at the level of TBC1 domain family member 4. Diabetes. 2012;61:2743–2752. doi: 10.2337/db11-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perseghin G, Price TB, Petersen KF, Roden M, Cline GW, Gerow K, Rothman DL, Shulman GI. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Morino K, Alves TC, Kibbey RG, Dufour S, Sono S, Yoo PS, Cline GW, Shulman GI. Effect of aging on muscle mitochondrial substrate utilization in humans. Proc Natl Acad Sci U S A. 2015;112:11330–11334. doi: 10.1073/pnas.1514844112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol. 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Tuggle SC, Hall SR, Bamman MM. Age differences in knee extension power, contractile velocity, and fatigability. J Appl Physiol. 2005;98:211–220. doi: 10.1152/japplphysiol.00294.2004. [DOI] [PubMed] [Google Scholar]
- Pham AH, McCaffery JM, Chan DC. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis. 2012;50:833–843. doi: 10.1002/dvg.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SK, Rook KM, Siddle NC, Bruce SA, Woledge RC. Muscle weakness in women occurs at an earlier age than in men, but strength is preserved by hormone replacement therapy. Clin Sci (Lond) 1993;84:95–98. doi: 10.1042/cs0840095. [DOI] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Thomas MM, Wright KJ, Hepple RT. Alterations in intrinsic mitochondrial function with aging are fiber type-specific and do not explain differential atrophy between muscles. Aging cell. 2011a;10:1047–1055. doi: 10.1111/j.1474-9726.2011.00745.x. [DOI] [PubMed] [Google Scholar]
- Picard M, Ritchie D, Wright KJ, Romestaing C, Thomas MM, Rowan SL, Taivassalo T, Hepple RT. Mitochondrial functional impairment with aging is exaggerated in isolated mitochondria compared to permeabilized myofibers. Aging Cell. 2010;9:1032–1046. doi: 10.1111/j.1474-9726.2010.00628.x. [DOI] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Gouspillou G, Hepple RT. Mitochondria: Isolation, Structure and Function. The Journal of physiology 589. 2011b;18:4413–4421. doi: 10.1113/jphysiol.2011.212712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 2011c;6:e18317. doi: 10.1371/journal.pone.0018317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior SJ, Goldberg AP, Ortmeyer HK, Chin ER, Chen D, Blumenthal JB, Ryan AS. Increased Skeletal Muscle Capillarization Independently Enhances Insulin Sensitivity in Older Adults After Exercise Training and Detraining. Diabetes. 2015;64:3386–3395. doi: 10.2337/db14-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue U, Slivka D, Minchev K, Trappe S. Improvements in whole muscle and myocellular function are limited with high-intensity resistance training in octogenarian women. J Appl Physiol (1985) 2009;106:1611–1617. doi: 10.1152/japplphysiol.91587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev. 2011;40:4–12. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Hagberg JM, Martin WH, 3rd, Ehsani AA, Holloszy JO. Decline in VO2max with aging in master athletes and sedentary men. J Appl Physiol (1985) 1990a;68:2195–2199. doi: 10.1152/jappl.1990.68.5.2195. [DOI] [PubMed] [Google Scholar]
- Rogers MA, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Effect of 10 days of physical inactivity on glucose tolerance in master athletes. J Appl Physiol (1985) 1990b;68:1833–1837. doi: 10.1152/jappl.1990.68.5.1833. [DOI] [PubMed] [Google Scholar]
- Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, Milan G, Masiero E, Del Piccolo P, Foretz M, et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29:1774–1785. doi: 10.1038/emboj.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and Myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Minaker KL, Pallotta JA, Flier JS. Characterization of the insulin resistance of aging. J Clin Invest. 1983;71:1581–1587. doi: 10.1172/JCI110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar CE, Dues DJ, Spielbauer KK, Machiela E, Cooper JF, Senchuk M, Hekimi S, Van Raamsdonk JM. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet. 2015;11:e1004972. doi: 10.1371/journal.pgen.1004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer GG, Arias EB, Cartee GD. Sustained postexercise increases in AS160 Thr642 and Ser588 phosphorylation in skeletal muscle without sustained increases in kinase phosphorylation. Journal of applied physiology. 2012;113:1852–1861. doi: 10.1152/japplphysiol.00619.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Arias EB, Sajan MP, MacKrell JG, Bhat AD, Farese RV, Cartee GD. Insulin resistance for glucose uptake and Akt2 phosphorylation in the soleus, but not epitrochlearis, muscles of old vs. adult rats. J Appl Physiol. 2010;108:1631–1640. doi: 10.1152/japplphysiol.01412.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Wang H, Arias EB, Castorena CM, Cartee GD. Mechanisms for independent and combined effects of calorie restriction and acute exercise on insulin-stimulated glucose uptake by skeletal muscle of old rats. Am J Physiol Endocrinol Metab. 2015;308:E603–E612. doi: 10.1152/ajpendo.00618.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896. doi: 10.2337/diabetes.52.8.1888. [DOI] [PubMed] [Google Scholar]
- Skorjanc D, Dunstl G, Pette D. Mitochondrial Enzyme Defects in Normal and Low-Frequency-Stimulated Muscles of Young and Aging Rats. Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56:B503–B509. doi: 10.1093/gerona/56.12.b503. [DOI] [PubMed] [Google Scholar]
- Slivka D, Raue U, Hollon C, Minchev K, Trappe S. Single muscle fiber adaptations to resistance training in old (>80 yr) men: evidence for limited skeletal muscle plasticity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R273–R280. doi: 10.1152/ajpregu.00093.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Stec MJ, Kelly NA, Many GM, Windham ST, Tuggle SC, Bamman MM. Ribosome biogenesis may augment resistance training-induced myofiber hypertrophy and is required for myotube growth in vitro. Am J Physiol Endocrinol Metab. 2016 doi: 10.1152/ajpendo.00486.2015. ajpendo 00486 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec MJ, Mayhew DL, Bamman MM. The effects of age and resistance loading on skeletal muscle ribosome biogenesis. J Appl Physiol (1985) 2015;119:851–857. doi: 10.1152/japplphysiol.00489.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkonogi M, Fernstrom M, Walsh B, Ji LL, Rooyackers O, Hammarqvist F, Wernerman J, Sahlin K. Reduced oxidative power but unchanged antioxidative capacity in skeletal muscle from aged humans. Pflugers Arch. 2003;446:261–269. doi: 10.1007/s00424-003-1044-9. [DOI] [PubMed] [Google Scholar]
- Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science. 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Frosig C, Pehmoller C, Chen S, Maarbjerg SJ, Brandt N, MacKintosh C, Zierath JR, Hardie DG, Kiens B, et al. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. DiabetologiA. 2009;52:891–900. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Berg JD, Stehouwer CD, Bosma H, van der Velde JH, Willems PJ, Savelberg HH, Schram MT, Sep SJ, van der Kallen CJ, Henry RM, et al. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: The Maastricht Study. DiabetologiA. 2016;59:709–718. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallberg-Henriksson H, Constable SH, Young DA, Holloszy JO. Glucose transport into rat skeletal muscle: interaction between exercise and insulin. J Appl Physiol. 1988;65:909–913. doi: 10.1152/jappl.1988.65.2.909. [DOI] [PubMed] [Google Scholar]
- Wang X, Patterson BW, Smith GI, Kampelman J, Reeds DN, Sullivan SA, Mittendorfer B. A ∼60-min brisk walk increases insulin-stimulated glucose disposal but has no effect on hepatic and adipose tissue insulin sensitivity in older women. Journal of applied physiology. 2013;114:1563–1568. doi: 10.1152/japplphysiol.01364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis PE, Chadan SG, Baracos V, Parkhouse WS. Restoration of insulin-like growth factor I action in skeletal muscle of old mice. Am J Physiol. 1998;275:E525–E530. doi: 10.1152/ajpendo.1998.275.3.E525. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Higaki Y, Hirshman MF, Michael MD, Dufresne SD, Kahn CR, Goodyear LJ. Exercise modulates postreceptor insulin signaling and glucose transport in muscle-specific insulin receptor knockout mice. J Clin Invest. 1999;104:1257–1264. doi: 10.1172/JCI7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Sharma N, Arias EB, Castorena CM, Cartee GD. A persistent increase in insulin-stimulated glucose uptake by both fast-twitch and slow-twitch skeletal muscles after a single exercise session by old rats. Age (Dordr) 2013;35:573–582. doi: 10.1007/s11357-012-9383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med. 1994;15:149–151. doi: 10.1055/s-2007-1021037. [DOI] [PubMed] [Google Scholar]
- Young JC, Chen M, Holloszy JO. Maintenance of the adaptation of skeletal muscle mitochondria to exercise in old rats. Med Sci Sports Exerc. 1983;15:243–246. [PubMed] [Google Scholar]
- Zierath JR, Wallberg-Henriksson H. Looking Ahead Perspective: Where Will the Future of Exercise Biology Take Us? Cell Metab. 2015;22:25–30. doi: 10.1016/j.cmet.2015.06.015. [DOI] [PubMed] [Google Scholar]