Abstract
Heart disease is the leading global cause of death. The risk for this disease is significantly increased in populations exposed to ionizing radiation, but the mechanisms are not fully elucidated yet. This review aims to gather and discuss the latest data about pathological and biological consequences in the radiation-exposed heart in a comprehensive manner. A better understanding of the molecular and cellular mechanisms underlying radiation-induced damage in heart tissue and cardiac vasculature will provide novel targets for therapeutic interventions. These may be valuable for individuals clinically or occupationally exposed to varying doses of ionizing radiation.
Keywords: ionizing radiation, cardiac disease, myocardium, endothelial cell, heart
INTRODUCTION
Cardiac disease (CD) is the leading cause of morbidity and mortality in the Western world [1]. It accounts for nearly one-third of all deaths worldwide [2].
There are multiple contributory risk factors for heart disease. Some are of a controllable nature, such as life-style and dietary factors, and metabolic disorders such as high cholesterol levels or hypertension. Others are non-controllable fixed risk factors, such as gender, age, and genetic predisposition [3, 4].
In addition, there are environmental factors affecting the risk of CD, ionizing radiation being one such factor. It has been known for a long time that high doses of radiation, such as those given during radiotherapy, cause damage to the heart and vasculature and thus increase the risk of CD [5]. The data from animal experiments have strongly supported this observation [6–8].
The earliest data indicating radiation-induced heart disease originated from studies of long-term outcomes after radiotherapy treatment of malignant disease such as breast cancer [9] or Hodgkin´s disease [10]. Here, the local doses to some regions of the heart could exceed 40 Gy [11]. Observed pathologies of high-dose radiation on the heart in clinical studies include direct damage to the coronary arteries, fibrosis of the pericardium and myocardium, pericardial adhesions, microvascular damage, and stenosis of the valves [12, 13].
The recent case–control study among women who underwent radiotherapy for breast cancer indicates that the rate of ischemic heart disease is significantly increased, even with doses <2 Gy [14]. Especially in clinical settings when high local doses are used, radiation-induced heart damage depends not only on the heart dose but also on the dose to the lung [15, 16]. This further complicates the estimation of radiation response in the low-dose range. Similarly, data on accidentally or occupationally exposed populations, especially the Life Span Study of the A-bomb survivors in Japan and epidemiological studies on Mayak nuclear facility workers in Russia, show that doses much lower than previously assumed may increase the risk of myocardial infarction and stroke [17–21].
The majority of epidemiological data seem to support linear no-threshold (LNT) radiation-dose response. However, only acute or cumulative doses of ~0.5 Gy and above have been shown to significantly elevate CD risk, while the magnitude of the risk below this dose is uncertain and a threshold cannot be excluded.
Although the risk for CD at low and moderate doses has been extensively analyzed [22–24], the combination of multiple contributory risk factors for CD make it difficult for epidemiological studies to detect increased risk at doses <0.5 Gy. Experimental animal and cellular studies are necessary, not only for a correct extrapolation of risk estimations, but also for the elucidation of biological mechanisms and the development of therapeutic countermeasures.
PATHOLOGY OF RADIATION-INDUCED CD
Radiation-induced heart complications are related to total radiation dose volume, younger age at the exposure, a greater time elapsed since exposure, and concomitant use of cardiotoxic chemotherapeutic agents such as anthracyclines [25]. Cardiac complications in human normally appear late—years to decades following the irradiation of the heart [26]; the first signs of cardiac toxicity typically appear with a median of >10–15 years of follow-up [12].
Based on clinical data, radiation-induced CD may be categorized into the following pathological conditions: pericarditis, pericardial fibrosis, diffuse myocardial fibrosis, coronary artery disease, microvascular damage, and valvular stenosis [12, 13, 27]. None of these conditions is restricted to radiation exposure, but there seems to be a significant acceleration in the pathogenesis of these symptoms by irradiation. These various pathologies probably result in different cardiac outcomes, e.g. coronary artery atherosclerosis leading to myocardial infarct, versus microvascular damage and fibrosis leading to congestive heart failure [28].
Radiation-induced changes in the heart tissue
Radiation-induced pericarditis in radiotherapy patients is characterized by exudation of protein-rich fluid within the pericardial sac and fibrin accumulation on the mesothelial lining between pericardium and epicardium [27]. Acute pericarditis is still occasionally seen within weeks after cardiac irradiation, but symptoms usually resolve quickly without consequences if non-steroidal anti-inflammatory drug therapy is used [27]. However, some patients develop chronic pericarditis up to 10 years later, which can be difficult to treat effectively [27]. Similar to clinical observations, preclinical animal studies indicate that pericardial fibrosis consists of collagen deposition in the parietal region, but also in the interstitial areas of the thickened pericardium [27, 29].
Radiation-related myocardial fibrosis is often asymptomatic but can be diagnosed late, at least 10 years after radiation therapy [30]. It is characterized by diffuse collagen bands criss-crossing the cardiac tissue, separating and replacing cardiomyocytes [27, 29]. In animal models, myocardial fibrosis and loss of cardiac function develop several months after irradiation [27]. Using a rat model, Boerma et al. investigated the effect of pentoxifylline (a xanthine derivate used to treat muscle pain in patients with peripheral artery disease) and alpha-tocopherol (vitamin E) on radiation-induced fibrosis [31]. A daily dose of 9 Gy was administered for five consecutive days, resulting in a considerable increase in cardiac fibrosis 6 months after irradiation that could be significantly reduced by both agents. Fibroblasts isolated from irradiated rat hearts showed distinguished actin filaments, consistent with the formation of stress fibers and cytoskeletal remodeling. The formation of cytoplasmic actin stress fibers is considered to be associated with the formation of myofibroblasts [32], cells that show increased collagen synthesis. Moreover, the irradiated animals showed decreased heart weight and heart/body weight ratios and increased left ventricular diastolic pressure [31]. Irradiation induced a significant increase in left ventricular anterior wall thickness, both in systole and in diastole, that was accompanied by reduced left ventricular inner diameter in irradiated hearts. Both ejection fraction and fractional shortening were significantly increased in irradiated hearts, but stroke volume was not altered. Pentoxifylline and alpha-tocopherol treatment significantly reduced radiation-induced increases in left ventricular diastolic pressure and deposition of collagen, whereas radiation-induced alterations in heart/body weight ratios, myocardial degeneration, left ventricular mast cell densities, and most echocardiographic parameters were not significantly altered by the treatment. This suggests that this treatment could be used to specifically target radiation-induced cardiac fibrosis and collagen deposition formation in human.
Seemann et al. demonstrated a decrease in both systolic and diastolic volumes and increased ejection fractions in C57BL/6 mice 40 weeks after local heart irradiation with 16 Gy [33]. Increased collagen deposition after irradiation may have contributed to impaired myocardial contractility [34]. In addition, cardiomyocytes are known to react to stress signals directly by initiating an inflammatory response through activation of macrophages [35]. This leads to decreased myocyte contractility in vitro and in vivo, resulting in a decrease in systolic and diastolic filling [36].
Radiation-induced changes in the cardiac vasculature
The progression of radiation-induced coronary artery disease follows that of normal atherosclerotic process caused by other factors. The initial event is endothelial damage, infiltration of monocytes into the intima [37, 38], and subsequent incorporation of low-density lipoproteins and the formation of fatty streaks [38, 39]. Pre-clinical animal models have long been used to study these initial events [40–44]. Mice lacking the apolipoprotein E (ApoE) are a good model for studying atherosclerotic plaque formation because this mutant (in contrast to the wild-type counterpart) is specifically prone to developing this pathology [45]. As ApoE is necessary for the normal catabolism of triglyceride-rich lipoprotein constituents, the defective mutants develop hypercholesterolemia. Local irradiation of the neck has been shown to accelerate the development of carotid artery atherosclerosis in the ApoE mutant of C57BL/6 mice 30 weeks after single doses (8 or 14 Gy) and fractionated exposures (20 × 2.0 Gy in 4 weeks) [46, 47]. Thirty weeks after irradiation, the formation of inflammatory, macrophage-rich atherosclerotic plaques with high levels of metalloproteinases and other proteolytic enzymes, intraplaque hemorrhage and reduced fibrous caps was observed. Such plaques are very vulnerable to rupture, causing thrombosis [47]. Lipidemia at the time of irradiation has been shown to stimulate the formation of fatty streaks [48].
Evidence for the radiation-induced microvascular damage comes mainly from animal experiments. However, regional cardiac perfusion defects have been observed in non-symptomatic breast cancer patients only 6 months after radiotherapy [49–51]. These symptoms persist and progress for several years [52]. Radiation-induced cardiac microvascular damage is characterized by decreased capillary density and has been shown in both rats [53, 54] and mice [33, 55] after high (≥8 Gy) local heart doses. In an experiment with rabbits, capillaries showed considerably more morphological changes in response to radiation than did larger venules and arterioles [56]. The damage to the microvascular network seems to be progressive, depending on the dose and time, suggesting a role in the underlying cause of ischemic injury [33, 52].
In general, studies using rodents to investigate radiation-induced coronary artery disease are limited in number [47, 53, 55]. On the other hand, many laboratory animals have been used successfully as models of radiation-induced cardiomyopathy [33, 34, 44, 53, 57, 58]. It is reasonable to believe that radiation exposure to the heart will induce both macro- and microvascular damage and that the two mechanisms may act together to produce radiation-induced CD in human populations [27]. In addition to that, direct damage of the myocardium by ionizing radiation is probable [59–61].
BIOLOGY OF RADIATION-INDUCED CD
The human and animal data indicate the important role of vascular injury and endothelial dysfunction [25], but also of myocardial remodeling, degeneration and dysfunction [31, 61–63] in the pathogenesis of radiation-induced CD. Endothelial dysfunction (loss of thromboresistance and increased expression of adhesion molecules and cytokines) contributes to pro-fibrotic and pro-inflammatory environments, which are common aspects of radiation-induced tissue injury [64, 65].
Oxidative stress, increased levels of endothelial adhesion molecules, vascular inflammation and cellular senescence are all consequences of the normal aging process [66], but are observed early in irradiated tissues [67], including heart [68], suggesting an intensification and acceleration of these molecular processes.
Oxidative stress and macromolecular damage
An immediate (24 h) increase in the amount of protein oxidation and lipid peroxidation, observed as enhanced protein carbonylation and malodialdehyde levels, was observed in mice after total body irradiation at 3 Gy [69]. Both indicated a rapid increase in oxidative stress corresponding to tissue damage. Persistent increase in oxidative stress was found in the heart tissue of total-body-irradiated (5 and 7 Gy) rats [70, 71]. Signs of increased oxidative stress, such as increases in malondialdehyde levels and xanthine oxidase and adenosine deaminase activities, and significant decreases in total nitrate/nitrite levels and activities of antioxidant enzymes (glutathione peroxidase, superoxide dismutase and catalase) have all been observed in irradiated heart tissue [70, 72].
The membrane structures of cardiomyocytes are abundant in phospholipids that are particularly sensitive to oxidative stress. Cardiomyocyte membrane lipid peroxidation leads to structural and functional damage [73]. Heart muscle is especially vulnerable to the oxidative activity of free radicals generated by ionizing radiation because of its low antioxidant defense [73, 74]. Depressed contractile function, impaired energy production, increased resting tension and enhanced levels of lipid peroxidation have been reported in various cardiac preparations exposed to free radicals [75].
Oxidative stress and inflammation are implicated independently in the development and progression of heart failure. Their interaction, however, is also evident throughout the process from initial injury to cardiac remodeling and failure [76].
Cellular adhesion and inflammation
Cellular studies show that upregulation of endothelial cell adhesion molecules and chemokines is the first step in both normal and radiation-induced atherosclerosis, leading to monocyte attachment, transmigration and finally foam cell formation [77–79]. Foam cells are fat-laden macrophages containing low-density lipoprotein cholesterol (‘bad’ cholesterol) and are typically observed in atherosclerotic plaque formation. In general, inflammation and oxidative damage play a role in radiation-induced atherosclerosis, as the response can be mitigated by the overexpression of CuZn-superoxide dismutase (SOD1) [48, 80], an enzyme responsible for destroying free superoxide radicals in the body.
The levels of adhesion molecules such as intracellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin and platelet endothelial cell adhesion molecule (PECAM-1) all increase after high doses of ionizing radiation [77, 81], probably due to the activation of nuclear factor kappa-B (NF-κB) [82, 83]. However, the changes in the adhesion molecule expression are tissue-specific because ICAM-1 is primarily expressed in the microvasculature, E-selectin in the endothelium of large blood vessels and P-selectin, another pro-inflammatory adhesion molecule, only in the Weibel–Palade bodies of the endothelium, never in the microvasculature [68, 79]. Furthermore, latest data indicate that the large arteries respond somewhat differently to radiation than the small vessels, showing decreased ICAM-1 levels after radiation exposure [84]. Radiation causes loss of thromboresistance by reducing the level of thrombomodulin and increasing the level of pro-inflammatory tissue factor [85–87]. An increased release of von Willebrand factor (vWF), a blood glycoprotein involved in hemostasis, has been demonstrated in endothelial cells after high single-dose irradiation in vitro and in vivo [88]. Increased plasma levels of this factor are presumed to arise from adverse alterations in the endothelium, and may contribute to an increased risk of thrombosis [89]. Sievert et al. quantified the expression of adhesive markers in cardiac microvascular endothelial cells isolated from mice that had received a local heart dose of 8 Gy [90]. Interestingly, inflammatory adhesion markers (PECAM-1, ICAM-1, ICAM-2 and VCAM-1) started to increase 10 weeks after irradiation, and ICAM-1 and VCAM-1 remained upregulated for at least 20 weeks.
Experimental studies have shown increased levels of endogenous inflammatory factors within hours to weeks after irradiation of a wide range of organs and tissues [91, 92]. In particular, endothelial cells release excessive amounts of inflammatory eicosanoids as a response to ionizing radiation. These include prostaglandins, prostacyclin, thromboxanes and leukotriens, which are endogenous mediators of inflammation through vasodilation, vasoconstriction, vascular permeability, microthrombus formation and extravasation of leukocytes [91, 92].
Upregulation of several cytokines, including IL-6 and IL-8, has been observed after endothelial cell high-dose irradiation in a time- and dose-dependent manner [93]. In addition, ionizing radiation has been shown to lead to increased permeability of endothelial cells and, in the presence of hypercholesterolemia, to accumulation of lipids [48].
Increased vascular adhesiveness, permeability and inflammation are characteristic hallmarks of endothelial dysfunction. One of the central modulators of radiation-induced endothelial dysfunction is transforming growth factor beta (TGF-β) [94, 95]. It was induced after local irradiation of rat heart with 20 Gy or five fractions of 9 Gy [31, 96, 97]. TGF-β, together with Rho/ROCK signaling [98], plays a central role in radiation-induced fibrosis [25].
Nitric oxide (NO), a powerful vasodilator, is an atheroprotective compound commonly used as a surrogate index of endothelial function [99]. Heat shock proteins are produced by cells in response to exposure to stressful conditions, and many of them function as chaperones to facilitate proper protein folding [100]. In particular, heat shock proteins heme oxygenase-1 (HO-1) and inducible heat shock protein 70 (HSP70) have been increasingly attributed a role as potential negative regulators of inducible NO synthase (iNOS) and as endogenous mediators of inflammation [101]. In addition, reactive oxygen species (ROS) have been shown to scavenge NO directly [102].
Azimzadeh et al. used 10-week-old C57Bl/6N mice that had received local X-ray heart doses of 8 or 16 Gy and were sacrificed after 16 weeks to isolate cardiac microvascular endothelial cells, using streptavidin-CD31 coated micro-beads [103]. Irradiated endothelial cells showed premature senescence, increased oxidative stress, decreased NO availability and enhanced inflammation compared with the sham-irradiated control cells.
The role of the kallikrein–kinin system (KKS) in radiation-induced cardiac inflammation was investigated using the kininogen-deficient Brown Norway Katholiek (BN/Ka) rat model [104]. BN/Ka rats and wild-type Brown Norway (BN) rats were exposed to local heart irradiation with a single dose of 18 Gy or 24 Gy and were observed for 3–6 months. BN rats, but not BN/Ka rats, showed a 56% reduction in cardiac numbers of CD2-positive cells, and a 57% increase in CD68-positive cells, specific for monocytes and macrophages, together with a 52% increase in phosphorylation of extracellular signal-regulated kinase 1/2 (Erk1/2). These results suggest that the KKS plays a role in the recruitment of inflammatory cells, presumably by altering the Erk1/2 signalling.
Further, blocking the kinin B2 receptor by the selective antagonist HOE-140 in male BN rats, starting 5 days before and continuing 4 weeks after the local heart irradiation (21 Gy), enhanced CD68-positive cells at four weeks in irradiated hearts [105].
In contrast to high doses, acute lower doses in the range of 0.1–1 Gy seem to result in reduced monocyte adhesion and thus have an anti-inflammatory effect [106–108]. These cellular observations have also been confirmed in animal models [108–110]. However, reduction in the level of NO is seen for all radiation doses [6, 109, 111].
Cellular apoptosis and senescence
The mammalian myocardium contains several cell types, of which the cardiomyocytes make up most of the heart's mass. Although a small proportion of cardiomyocytes in the adult myocardium remain mitotic, most of them lose the capacity to undergo cell division shortly after birth [112]. In the adult heart, ~70% of the cells are represented by non-myocytes, most of which belong to the fibroblast compartment. Irradiation of the heart may induce apoptosis and necrosis of all cells of the cardiac tissue, irrespective of type, including cardiomyocytes, cardiac fibroblasts and conducting tissue, but also of the cardiac vasculature, consisting of capillaries and epicardial vessels [68].
Long-term effects in the myocardium have been reported after local heart irradiation ranging from 0.2 Gy to 16 Gy using both wild-type C57BL/6 and ApoE-defective mice [72, 113, 114]. The results show, in agreement with other data [33, 55], that wild-type mice are more susceptible to radiation-induced cardiac impairment than the ApoE-/- mutant. This was manifested in the decreased mitochondrial respiration, increased ROS production, and increased oxidation of mitochondrial proteins in the wild-type but not in the mutant mouse [114]. An inactivation of the transcription factor peroxisome proliferator-activated receptor (PPAR) alpha was observed in the wild-type mouse after local heart irradiation (16 Gy) [72]. PPAR alpha is a key regulator of lipid metabolism in heart tissue. The reduced activity of PPAR alpha possibly contributed to the sudden death by amyloidosis of these mice at the age of 40 weeks [33], whereas this dose did not lead to sudden death in the ApoE-/- mice [55].
In contrast to cardiomyocytes, endothelial cells do divide, albeit with a slow turnover rate of about once every three years in humans [67], and they may thus be more sensitive to radiation than non-proliferating cells. Ionizing radiation induces morphological changes in the endothelium, whereas cardiomyocytes do not show altered morphology, in spite of mechanical impairment [6].
Cardiac endothelial cells in culture undergo apoptosis after high and moderate radiation doses [115]. However, endothelial cells isolated from mouse heart showed resistance to apoptosis after in vitro irradiation [116]. Similarly, in in vitro–irradiated (10 Gy) pulmonary artery endothelial cells, only a low population (8%) underwent extrinsic and intrinsic apoptosis, as indicated by the activation of caspases 3, 8 and 9, as well as by the neutral comet assay. A majority of the endothelial cells underwent premature senescence [117].
Senescent endothelial cells do not proliferate but they stay metabolically active [118]. They have a greatly increased capacity for monocyte attachment and their recruitment into the intima, two essential initiating steps in atherosclerosis development. Not surprisingly, senescent endothelial cells have been found in both ‘normal’ [119, 120] and radiation-associated atherosclerotic plaques appearing after radiotherapy [121].
Compared with normal endothelial cells, senescent cells have altered morphology, with a characteristically large size that could be described as ‘fried egg’ instead of the more normal ‘cobblestone’ appearance [122]. In addition, senescent cells show altered gene expression and function [67]. They express senescence-associated β-galactosidase [123], show increased production of ROS [124] and superoxide [125], downregulation of CDK2 [126], accumulation of cell cycle arrest proteins p16, p21 and p27 [127], alterations in ICAM-1 level and function [128], reduction of NO production [129] and lower expression of NO synthase [130]. Some of these characteristic traits for senescent cell cultures have also been found in senescent vasculature in vivo [131].
High single doses of ionizing radiation have been shown to induce a senescence-like phenotype in endothelial cells, with a significant decrease in angiogenic activity in vitro [132, 133] and in vivo [134], and increased expression of adhesion molecules ICAM-1, VCAM-1 and E-selectin [135]. Chronic low-dose-rate radiation exposure has been shown to lead to premature endothelial senescence in vitro by inactivating PI3K and MAP kinase signaling pathways [136, 137].
In studies using Wistar and Sprague-Dawley rat models, local high-dose (20 Gy) heart irradiation induced myocardial degeneration preceded by a focal loss of capillary number and alkaline phosphatase (AP) activity, presumably a marker of functional endothelium. Within AP-negative areas, there was an increased number of enlarged endothelial cells and enhanced lymphocyte adherence to endothelial cells [138], both characteristic features of a senescent endothelium [122]. In phosphatase-positive areas, endothelial cell proliferation was unchanged, indicating that the decrease in capillary density was not due to mitotic death of proliferating cells, as is commonly seen in other tissues [139]. Around the AP-negative foci, progressive signs of cardiomyocyte necrosis became evident, the lag time depending on the rat strain. In Wistar rats, enzyme loss started at 25 days after 20 Gy and reached its maximum extent by 90 days. In Sprague-Dawley rats, which show significantly higher enzyme activity before irradiation, the onset of alkaline phosphatase loss and associated alterations was delayed by ~30 days and was significantly less extensive [138].
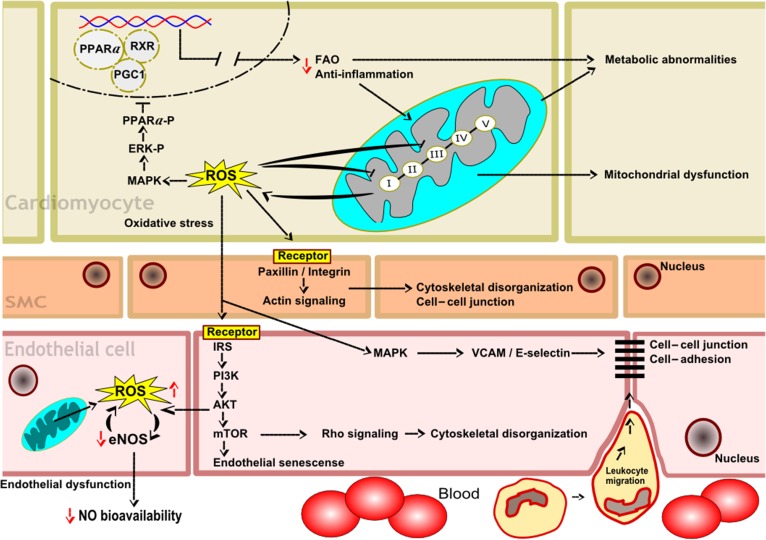
Some of the biological pathways described in this section are illustrated in Fig. 1.
Fig. 1.
Proposed model of the biological pathways in cardiomyocytes, smooth muscle cells (SMCs) and endothelial cells involved in radiation-induced heart disease. In cardiomyocytes, the inactivation of PPAR alpha, reduced fatty acid oxidation (FAO), increased inflammatory response, and enhanced production of mitochondrial ROS are indicated. In SMCs, the adverse effects on paxillin/integrin and actin signaling, cytoskeletal organization, and cell–cell junctions are shown. In endothelial cells, the inactivation of PI3K, MAP kinase and Rho signaling pathways, increased cytoskeletal disorganization, decreased NO production and bioavailability, and enhanced leukocyte migration due to increased cell adhesion and loosening of cell–cell junctions are indicated.
CONCLUSIONS
A better understanding of biological mechanisms underlying radiation-induced damage in heart tissue and cardiac vasculature is essential for preventing cardiac damage in clinical and occupational settings. A promising novel target for such interventions could be PPAR alpha. Since the PPAR ligands are essential for the transcriptional activity of the PPAR family members, they are attractive candidates for designing novel therapeutic countermeasures in CD. Several clinical and preclinical studies have already demonstrated the beneficial effect of PPAR ligands on various CD risk factors [140–142]. In addition, pentoxifylline and alpha-tocopherol treatment could be used to reduce cardiac fibrosis in humans, but more studies using animal models are needed. Clearly, patients given thoracic radiotherapy would benefit from increased surveillance of known risk factors of heart disease, such as hypertension and hypercholesterolemia [27]. Screening echocardiography could be considered for diagnosing asymptomatic myocardial fibrosis [30].
Large amounts of epidemiological, pathological and biological data are available on the cardiac effects of high-dose radiation levels. The known pathological responses of cardiac vasculature to high doses of ionizing radiation, such as increased adhesiveness, inflammation and permeability, may also be used as endpoints when considering possible biological effects at low doses. However, the low-dose effects may be completely different from the high-dose effects, as indicated by the pro-inflammatory response of the vasculature after high-dose exposures, but anti-inflammatory effect after low-dose exposures. Further, the question of whether radiation-induced CD is a deterministic phenomenon, i.e. whether there is a threshold below which biological effects are not present, remains unanswered. Moreover, in addition to studies of dose effects, the cellular and tissue response to various dose rates (acute vs fractionated or chronic exposures) needs more investigation.
FUNDING
This work was supported by a grant from the European Community’s Seventh Framework Program (EURATOM), Contract No. 295823 (PROCARDIO).
CONFLICT OF INTEREST
The author declares that there are no conflicts of interest.
REFERENCES
- 1.Rosamond W, Flegal K, Friday G, et al.. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2007;115:e69–171. [DOI] [PubMed] [Google Scholar]
- 2.Cannon B. Cardiovascular disease: biochemistry to behaviour. Nature 2013;493:S2–3. [DOI] [PubMed] [Google Scholar]
- 3.Burns DM. Epidemiology of smoking-induced cardiovascular disease. Prog Cardiovasc Dis 2003;46:11–29. [DOI] [PubMed] [Google Scholar]
- 4.Wilson PW, D'Agostino RB, Levy D, et al.. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97:1837–47. [DOI] [PubMed] [Google Scholar]
- 5.Adams J, Palombella VJ, Elliott PJ.. Proteasome inhibition: a new strategy in cancer treatment. Invest New Drugs 2000;18:109–21. [DOI] [PubMed] [Google Scholar]
- 6.Baker JE, Fish BL, Su J, et al.. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol 2009;85:1089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boerma M. Experimental radiation-induced heart disease: past, present, and future. Radiat Res 2012;178:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JE, Moulder JE, Hopewell JW.. Radiation as a risk factor for cardiovascular disease. Antioxid Redox Signal 2011;15:1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby S, McGale P, Peto R, et al.. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003;326:256–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock SL, Donaldson SS, Hoppe RT.. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol 1993;11:1208–15. [DOI] [PubMed] [Google Scholar]
- 11.McGale P, Darby SC.. Low doses of ionizing radiation and circulatory diseases: a systematic review of the published epidemiological evidence. Radiat Res 2005;163:247–57. [DOI] [PubMed] [Google Scholar]
- 12.Demirci S, Nam J, Hubbs JL, et al.. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys 2009;73:980–7. [DOI] [PubMed] [Google Scholar]
- 13.Adams MJ, Hardenbergh PH, Constine LS, et al.. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 2003;45:55–75. [DOI] [PubMed] [Google Scholar]
- 14.Darby SC, Ewertz M, McGale P, et al.. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 15.Ghobadi G, van der Veen S, Bartelds B, et al.. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 2012;84:e639–46. [DOI] [PubMed] [Google Scholar]
- 16.Cella L, Liuzzi R, Conson M, et al.. Multivariate normal tissue complication probability modeling of heart valve dysfunction in Hodgkin lymphoma survivors. Int J Radiat Oncol Biol Phys 2013;87:304–10. [DOI] [PubMed] [Google Scholar]
- 17.Yamada M, Wong FL, Fujiwara S, et al.. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res 2004;161:622–32. [DOI] [PubMed] [Google Scholar]
- 18.Shimizu Y, Kodama K, Nishi N, et al.. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ 2010;340:b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azizova TV, Muirhead CR, Druzhinina MB, et al.. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res 2010;174:155–68. [DOI] [PubMed] [Google Scholar]
- 20.Azizova TV, Muirhead CR, Druzhinina MB, et al.. Cerebrovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res 2010;174:851–64. [DOI] [PubMed] [Google Scholar]
- 21.Azizova TV, Muirhead CR, Moseeva MB, et al.. Cerebrovascular diseases in nuclear workers first employed at the Mayak PA in 1948–1972. Radiat Environ Biophys 2011;50:539–52. [DOI] [PubMed] [Google Scholar]
- 22.Little MP, Tawn EJ, Tzoulaki I, et al.. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res 2008;169:99–109. [DOI] [PubMed] [Google Scholar]
- 23.Little MP, Tawn EJ, Tzoulaki I, et al.. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys 2010;49:139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little MP, Azizova TV, Bazyka D, et al.. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect 2012;120:1503–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerma M, Hauer-Jensen M.. Preclinical research into basic mechanisms of radiation-induced heart disease. Cardiol Res Pract 4 October 2010; 10.4061/2011/858262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giraud P, Cosset JM.. Radiation toxicity to the heart: physiopathology and clinical data. Bull Cancer 2004;91 Suppl 3:147–53. [PubMed] [Google Scholar]
- 27.Darby SC, Cutter DJ, Boerma M, et al.. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart FA, Seemann I, Hoving S, et al.. Understanding radiation-induced cardiovascular damage and strategies for intervention. Clin Oncol (R Coll Radiol) 2013;25:617–24. [DOI] [PubMed] [Google Scholar]
- 29.Fajardo LF, Stewart JR.. Experimental radiation-induced heart disease. I. Light microscopic studies. Am J Pathol 1970;59:299–316. [PMC free article] [PubMed] [Google Scholar]
- 30.Heidenreich PA, Hancock SL, Lee BK, et al.. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol 2003;42:743–9. [DOI] [PubMed] [Google Scholar]
- 31.Boerma M, Roberto KA, Hauer-Jensen M.. Prevention and treatment of functional and structural radiation injury in the rat heart by pentoxifylline and alpha-tocopherol. Int J Radiat Oncol Biol Phys 2008;72:170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hinz B, Gabbiani G.. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol 2003;14:538–46. [DOI] [PubMed] [Google Scholar]
- 33.Seemann I, Gabriels K, Visser NL, et al.. Irradiation induced modest changes in murine cardiac function despite progressive structural damage to the myocardium and microvasculature. Radiother Oncol 2012;103:143–50. [DOI] [PubMed] [Google Scholar]
- 34.Kruse JJ, Zurcher C, Strootman EG, et al.. Structural changes in the auricles of the rat heart after local ionizing irradiation. Radiother Oncol 2001;58:303–11. [DOI] [PubMed] [Google Scholar]
- 35.Boyd JH, Kan B, Roberts H, et al.. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res 2008;102:1239–46. [DOI] [PubMed] [Google Scholar]
- 36.Simms MG, Walley KR.. Activated macrophages decrease rat cardiac myocyte contractility: importance of ICAM-1-dependent adhesion. Am J Physiol 1999;277:H253–60. [DOI] [PubMed] [Google Scholar]
- 37.Hendry JH, Akahoshi M, Wang LS, et al.. Radiation-induced cardiovascular injury. Radiat Environ Biophys 2008;47:189–93. [DOI] [PubMed] [Google Scholar]
- 38.Vos J, Aarnoudse MW, Dijk F, et al.. On the cellular origin and development of atheromatous plaques. A light and electron microscopic study of combined X-ray and hypercholesterolemia-induced atheromatosis in the carotid artery of the rabbit. Virchows Arch B Cell Pathol Incl Mol Pathol 1983;43:1–16. [DOI] [PubMed] [Google Scholar]
- 39.Konings AW, Smit Sibinga CT, Aarnoudse MW, et al.. Initial events in radiation-induced atheromatosis. II. Damage to intimal cells. Strahlentherapie 1978;154:795–800. [PubMed] [Google Scholar]
- 40.Labudova O, Hardmeier R, Rink H, et al.. The transcription of the XRCC1 gene in the heart of radiation-resistant and radiation-sensitive mice after ionizing irradiation. Pediatr Res 1997;41:435–9. [DOI] [PubMed] [Google Scholar]
- 41.McChesney SL, Gillette EL, Orton EC.. Canine cardiomyopathy after whole heart and partial lung irradiation. Int J Radiat Oncol Biol Phys 1988;14:1169–74. [DOI] [PubMed] [Google Scholar]
- 42.McChesney SL, Gillette EL, Powers BE.. Radiation-induced cardiomyopathy in the dog. Radiat Res 1988;113:120–32. [PubMed] [Google Scholar]
- 43.Yang VV, Stearner SP, Tyler SA.. Radiation-induced changes in the fine structure of the heart: comparison of fission neutrons and 60Co gamma rays in the mouse. Radiat Res 1976;67:344–60. [PubMed] [Google Scholar]
- 44.Eltringham JR, Fajardo LF, Stewart JR.. Adriamycin cardiomyopathy: enhanced cardiac damage in rabbits with combined drug and cardiac irradiation. Radiology 1975;115:471–2. [DOI] [PubMed] [Google Scholar]
- 45.Ellulu MS, Patimah I, Khaza'ai H, et al.. Atherosclerotic cardiovascular disease: a review of initiators and protective factors. Inflammopharmacology 2016;24:1–10. [DOI] [PubMed] [Google Scholar]
- 46.Hoving S, Heeneman S, Gijbels MJ, et al.. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE-/- mice. Int J Radiat Oncol Biol Phys 2008;71:848–57. [DOI] [PubMed] [Google Scholar]
- 47.Stewart FA, Heeneman S, Te Poele J, et al.. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tribble DL, Barcellos-Hoff MH, Chu BM, et al.. Ionizing radiation accelerates aortic lesion formation in fat-fed mice via SOD-inhibitable processes. Arterioscler Thromb Vasc Biol 1999;19:1387–92. [DOI] [PubMed] [Google Scholar]
- 49.Gyenes G, Fornander T, Carlens P, et al.. Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur J Nucl Med 1997;24:286–92. [DOI] [PubMed] [Google Scholar]
- 50.Seddon B, Cook A, Gothard L, et al.. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol 2002;64:53–63. [DOI] [PubMed] [Google Scholar]
- 51.Marks LB, Yu X, Prosnitz RG, et al.. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 2005;63:214–23. [DOI] [PubMed] [Google Scholar]
- 52.Prosnitz RG, Hubbs JL, Evans ES, et al.. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer 2007;110:1840–50. [DOI] [PubMed] [Google Scholar]
- 53.Lauk S, Kiszel Z, Buschmann J, et al.. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys 1985;11:801–8. [DOI] [PubMed] [Google Scholar]
- 54.Schultz-Hector S, Sund M, Thames HD.. Fractionation response and repair kinetics of radiation-induced heart failure in the rat. Radiother Oncol 1992;23:33–40. [DOI] [PubMed] [Google Scholar]
- 55.Gabriels K, Hoving S, Seemann I, et al.. Local heart irradiation of ApoE-/- mice induces microvascular and endocardial damage and accelerates coronary atherosclerosis. Radiother Oncol 2012;105:358–64. [DOI] [PubMed] [Google Scholar]
- 56.Dimitrievich GS, Fischer-Dzoga K, Griem ML.. Radiosensitivity of vascular tissue. I. Differential radiosensitivity of capillaries: a quantitative in vivo study. Radiat Res 1984;99:511–35. [PubMed] [Google Scholar]
- 57.Hu S, Chen Y, Li L, et al.. Effects of adenovirus-mediated delivery of the human hepatocyte growth factor gene in experimental radiation-induced heart disease. Int J Radiat Oncol Biol Phys 2009;75:1537–44. [DOI] [PubMed] [Google Scholar]
- 58.Fajardo LF, Stewart JR.. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest 1973;29:244–57. [PubMed] [Google Scholar]
- 59.Monceau V, Meziani L, Strup-Perrot C, et al.. Enhanced sensitivity to low dose irradiation of ApoE-/- mice mediated by early pro-inflammatory profile and delayed activation of the TGFβ1 cascade involved in fibrogenesis. PLoS One 2013;8:e57052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chae HJ, Kim HR, Lee WG, et al.. Radiation protects adriamycin-induced apoptosis. Immunopharmacol Immunotoxicol 2005;27:211–32. [DOI] [PubMed] [Google Scholar]
- 61.Boerma M, van der Wees CG, Vrieling H, et al.. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 2005;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qian L, Cao F, Cui J, et al.. The potential cardioprotective effects of hydrogen in irradiated mice. J Radiat Res 2010;51:741–7. [DOI] [PubMed] [Google Scholar]
- 63.Kruse JJ, Strootman EG, Bart CI, et al.. Radiation-induced changes in gene expression and distribution of atrial natriuretic peptide (ANP) in different anatomical regions of the rat heart. Int J Radiat Biol 2002;78:297–304. [DOI] [PubMed] [Google Scholar]
- 64.Hopewell JW, Calvo W, Jaenke R, et al.. Microvasculature and radiation damage. Recent Results Cancer Res 1993;130:1–16. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Boerma M, Fu Q, et al.. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J Gastroenterol 2007;13:3047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.El Assar M, Angulo J, Rodriguez-Manas L.. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med 2013;65:380–401. [DOI] [PubMed] [Google Scholar]
- 67.Schofield PN, Garcia-Bernardo J.. Radiation, oxidative stress and senescence; the vascular endothelial cell as a common target? In: Mothersill C, Mosse I, Seymour C (eds) Multiple Stressors: A Challenge for the Future. Netherlands: Springer, 2007, 325–34. [Google Scholar]
- 68.AGIR Circulatory Disease Risk Report on the Independent Advisory Group on Ionising Radiation. Health Protection Agency, 2010. https://www.gov.uk/government/publications/radiation-circulatory-disease-risk.
- 69.Azimzadeh O, Scherthan H, Sarioglu H, et al.. Rapid proteomic remodeling of cardiac tissue caused by total body ionizing radiation. Proteomics 2011;11:3299–311. [DOI] [PubMed] [Google Scholar]
- 70.Mansour HH, Tawfik SS.. Early treatment of radiation-induced heart damage in rats by caffeic acid phenethyl ester. Eur J Pharmacol 2012;692:46–51. [DOI] [PubMed] [Google Scholar]
- 71.Pradeep K, Ko KC, Choi MH, et al.. Protective effect of hesperidin, a citrus flavanoglycone, against gamma-radiation-induced tissue damage in Sprague-Dawley rats. J Med Food 2012;15:419–27. [DOI] [PubMed] [Google Scholar]
- 72.Azimzadeh O, Sievert W, Sarioglu H, et al.. PPAR alpha: a novel radiation target in locally exposed Mus musculus heart revealed by quantitative proteomics. J Proteome Res 2013;12:2700–14. [DOI] [PubMed] [Google Scholar]
- 73.Przybyszewski WM, Widel M, Rzeszowska-Wolny J.. Cardiotoxic consequences of ionizing radiation and anthracyclines. Postepy Hig Med Dosw 2006;60:397–405. [PubMed] [Google Scholar]
- 74.Antunes F, Han D, Cadenas E.. Relative contributions of heart mitochondria glutathione peroxidase and catalase to H2O2 detoxification in in vivo conditions. Free Radic Biol Med 2002;33:1260–7. [DOI] [PubMed] [Google Scholar]
- 75.Singal PK, Khaper N, Palace V, et al.. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res 1998;40:426–32. [DOI] [PubMed] [Google Scholar]
- 76.Khaper N, Bryan S, Dhingra S, et al.. Targeting the vicious inflammation–oxidative stress cycle for the management of heart failure. Antioxid Redox Signal 2010;13:1033–49. [DOI] [PubMed] [Google Scholar]
- 77.Baluna RG, Eng TY, Thomas CR.. Adhesion molecules in radiotherapy. Radiat Res 2006;166:819–31. [DOI] [PubMed] [Google Scholar]
- 78.Hallahan D, Kuchibhotla J, Wyble C.. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res 1996;56:5150–5. [PubMed] [Google Scholar]
- 79.Hallahan DE, Virudachalam S.. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res 1999;152:6–13. [PubMed] [Google Scholar]
- 80.Tribble DL, Krauss RM, Chu BM, et al.. Increased low density lipoprotein degradation in aorta of irradiated mice is inhibited by preenrichment of low density lipoprotein with α-tocopherol. J Lipid Res 2000;41:1666–72. [PubMed] [Google Scholar]
- 81.Wondergem J, Wedekind LE, Bart CI, et al.. Irradiation of mechanically-injured human arterial endothelial cells leads to increased gene expression and secretion of inflammatory and growth promoting cytokines. Atherosclerosis 2004;175:59–67. [DOI] [PubMed] [Google Scholar]
- 82.Halle M, Hall P, Tornvall P.. Cardiovascular disease associated with radiotherapy: activation of nuclear factor kappa-B. J Intern Med 2011;269:469–77. [DOI] [PubMed] [Google Scholar]
- 83.Chou CH, Chen SU, Cheng JC.. Radiation-induced interleukin-6 expression through MAPK/p38/NF-κB signaling pathway and the resultant antiapoptotic effect on endothelial cells through Mcl-1 expression with sIL6-Rα. Int J Radiat Oncol Biol Phys 2009;75:1553–61. [DOI] [PubMed] [Google Scholar]
- 84.Hoving S, Heeneman S, Gijbels MJ, et al.. Irradiation induces different inflammatory and thrombotic responses in carotid arteries of wildtype C57BL/6J and atherosclerosis-prone ApoE-/- mice. Radiother Oncol 2012;105:365–70. [DOI] [PubMed] [Google Scholar]
- 85.Richter KK, Fink LM, Hughes BM, et al.. Is the loss of endothelial thrombomodulin involved in the mechanism of chronicity in late radiation enteropathy. Radiother Oncol 1997;44:65–71. [DOI] [PubMed] [Google Scholar]
- 86.Van der Meeren A, Mouthon MA, Vandamme M, et al.. Combinations of cytokines promote survival of mice and limit acute radiation damage in concert with amelioration of vascular damage. Radiat Res 2004;161:549–59. [DOI] [PubMed] [Google Scholar]
- 87.Wang J, Zheng H, Ou X, et al.. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol 2002;160:2063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Kleef E, Verheij M, te Poele H, et al.. In vitro and in vivo expression of endothelial von Willebrand factor and leukocyte accumulation after fractionated irradiation. Radiat Res 2000;154:375–81. [DOI] [PubMed] [Google Scholar]
- 89.Franchini M, Lippi G.. Von Willebrand factor and thrombosis. Ann Hematol 2006;85:415–23. [DOI] [PubMed] [Google Scholar]
- 90.Sievert W, Trott KR, Azimzadeh O, et al.. Late proliferating and inflammatory effects on murine microvascular heart and lung endothelial cells after irradiation. Radiother Oncol 2015;117:376–81. [DOI] [PubMed] [Google Scholar]
- 91.Stewart FA, Hoving S, Russell NS.. Vascular damage as an underlying mechanism of cardiac and cerebral toxicity in irradiated cancer patients. Radiat Res 2010;174:865–9. [DOI] [PubMed] [Google Scholar]
- 92.Michalowski AS. On radiation damage to normal tissues and its treatment. II. Anti-inflammatory drugs. Acta Oncol 1994;33:139–57. [DOI] [PubMed] [Google Scholar]
- 93.Meeren AV, Bertho JM, Vandamme M, et al.. Ionizing radiation enhances IL-6 and IL-8 production by human endothelial cells. Mediators Inflamm 1997;6:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Scharpfenecker M, Kruse JJ, Sprong D, et al.. Ionizing radiation shifts the PAI-1/ID-1 balance and activates notch signaling in endothelial cells. Int J Radiat Oncol Biol Phys 2009;73:506–13. [DOI] [PubMed] [Google Scholar]
- 95.Kruse JJ, Floot BG, te Poele JA, et al.. Radiation-induced activation of TGF-beta signaling pathways in relation to vascular damage in mouse kidneys. Radiat Res 2009;171:188–97. [DOI] [PubMed] [Google Scholar]
- 96.Kruse JJ, Bart CI, Visser A, et al.. Changes in transforming growth factor-beta (TGF-beta 1), procollagen types I and II mRNA in the rat heart after irradiation. Int J Radiat Biol 1999;75:1429–36. [DOI] [PubMed] [Google Scholar]
- 97.Liu H, Xiong M, Xia YF, et al.. Studies on pentoxifylline and tocopherol combination for radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys 2009;73:1552–9. [DOI] [PubMed] [Google Scholar]
- 98.Monceau V, Pasinetti N, Schupp C, et al.. Modulation of the Rho/ROCK pathway in heart and lung after thorax irradiation reveals targets to improve normal tissue toxicity. Curr Drug Targets 2010;11:1395–404. [DOI] [PubMed] [Google Scholar]
- 99.Laroux FS, Lefer DJ, Kawachi S, et al.. Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid Redox Signal 2000;2:391–6. [DOI] [PubMed] [Google Scholar]
- 100.Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci 2001;2:205–25. [DOI] [PubMed] [Google Scholar]
- 101.Schaue D, Jahns J, Hildebrandt G, et al.. Radiation treatment of acute inflammation in mice. Int J Radiat Biol 2005;81:657–67. [DOI] [PubMed] [Google Scholar]
- 102.Stamler JS, Singel DJ, Loscalzo J.. Biochemistry of nitric oxide and its redox-activated forms. Science 1992;258:1898–902. [DOI] [PubMed] [Google Scholar]
- 103.Azimzadeh O, Sievert W, Sarioglu H, et al.. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res 2015;14:1203–19. [DOI] [PubMed] [Google Scholar]
- 104.Sridharan V, Tripathi P, Sharma SK, et al.. Cardiac inflammation after local irradiation is influenced by the kallikrein–kinin system. Cancer Res 2012;72:4984–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lieblong BJ, Sridharan V, Srivastava AK, et al.. Role of the bradykinin B2 receptor in a rat model of local heart irradiation. Int J Radiat Biol 2015;91:634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hosoi Y, Miyachi H, Matsumoto Y, et al.. Induction of interleukin-1β and interleukin-6 mRNA by low doses of ionizing radiation in macrophages. Int J Cancer 2001;96:270–6. [DOI] [PubMed] [Google Scholar]
- 107.Kern PM, Keilholz L, Forster C, et al.. Low-dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiother Oncol 2000;54:273–82. [DOI] [PubMed] [Google Scholar]
- 108.Roedel F, Kley N, Beuscher HU, et al.. Anti-inflammatory effect of low-dose X-irradiation and the involvement of a TGF-beta1-induced down-regulation of leukocyte/endothelial cell adhesion. Int J Radiat Biol 2002;78:711–9. [DOI] [PubMed] [Google Scholar]
- 109.Hildebrandt G, Seed MP, Freemantle CN, et al.. Mechanisms of the anti-inflammatory activity of low-dose radiation therapy. Int J Radiat Biol 1998;74:367–78. [DOI] [PubMed] [Google Scholar]
- 110.Mitchel RE, Hasu M, Bugden M, et al.. Low-dose radiation exposure and atherosclerosis in ApoE-/- mice. Radiat Res 2011;175:665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rodel F, Frey B, Gaipl U, et al.. Modulation of inflammatory immune reactions by low-dose ionizing radiation: molecular mechanisms and clinical application. Curr Med Chem 2012;19:1741–50. [DOI] [PubMed] [Google Scholar]
- 112.Tamamori-Adachi M, Ito H, Sumrejkanchanakij P, et al.. Critical role of cyclin D1 nuclear import in cardiomyocyte proliferation. Circ Res 2003;92:e12–9. [DOI] [PubMed] [Google Scholar]
- 113.Barjaktarovic Z, Schmaltz D, Shyla A, et al.. Radiation–induced Signaling results in mitochondrial impairment in mouse heart at 4 weeks after exposure to X-rays. PLoS One 2011;6:e27811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barjaktarovic Z, Shyla A, Azimzadeh O, et al.. Ionising radiation induces persistent alterations in the cardiac mitochondrial function of C57BL/6 mice 40 weeks after local heart exposure. Radiother Oncol 2013;106:404–10. [DOI] [PubMed] [Google Scholar]
- 115.Rousseau M, Gaugler MH, Rodallec A, et al.. RhoA GTPase regulates radiation-induced alterations in endothelial cell adhesion and migration. Biochem Biophys Res Commun 2011;414:750–5. [DOI] [PubMed] [Google Scholar]
- 116.Jelonek K, Walaszczyk A, Gabrys D, et al.. Cardiac endothelial cells isolated from mouse heart – a novel model for radiobiology. Acta Biochim Pol 2011;58:397–404. [PubMed] [Google Scholar]
- 117.Panganiban RA, Mungunsukh O, Day RM.. X-irradiation induces ER stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int J Radiat Biol 2013;89:656–67. [DOI] [PubMed] [Google Scholar]
- 118.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Minamino T, Miyauchi H, Yoshida T, et al.. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation 2002;105:1541–4. [DOI] [PubMed] [Google Scholar]
- 120.Vasile E, Tomita Y, Brown LF, et al.. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J 2001;15:458–66. [DOI] [PubMed] [Google Scholar]
- 121.Suzuki K, Mori I, Nakayama Y, et al.. Radiation-induced senescence-like growth arrest requires TP53 function but not telomere shortening. Radiat Res 2001;155:248–53. [DOI] [PubMed] [Google Scholar]
- 122.Tokunaga O, Fan JL, Watanabe T.. Atherosclerosis- and age-related multinucleated variant endothelial cells in primary culture from human aorta. Am J Pathol 1989;135:967–76. [PMC free article] [PubMed] [Google Scholar]
- 123.Kurz DJ, Decary S, Hong Y, et al.. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 2000;113:3613–22. [DOI] [PubMed] [Google Scholar]
- 124.Haendeler J, Hoffmann J, Diehl JF, et al.. Antioxidants inhibit nuclear export of telomerase reverse transcriptase and delay replicative senescence of endothelial cells. Circ Res 2004;94:768–75. [DOI] [PubMed] [Google Scholar]
- 125.Oudot A, Martin C, Busseuil D, et al.. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med 2006;40:2214–22. [DOI] [PubMed] [Google Scholar]
- 126.Freedman DA, Folkman J.. CDK2 translational down-regulation during endothelial senescence. Exp Cell Res 2005;307:118–30. [DOI] [PubMed] [Google Scholar]
- 127.Hyland P, Barnett C, Pawelec G, et al.. Age-related accumulation of oxidative DNA damage and alterations in levels of p16INK4a/CDKN2a, p21WAF1/CIP1/SDI1 and p27KIP1 in human CD4+ T cell clones in vitro. Mech Ageing Dev 2001;122:1151–67. [DOI] [PubMed] [Google Scholar]
- 128.Zhou X, Perez F, Han K, et al.. Clonal senescence alters endothelial ICAM-1 function. Mech Ageing Dev 2006;127:779–85. [DOI] [PubMed] [Google Scholar]
- 129.Sato I, Morita I, Kaji K, et al.. Reduction of nitric oxide producing activity associated with in vitro aging in cultured human umbilical vein endothelial cell. Biochem Biophys Res Commun 1993;195:1070–6. [DOI] [PubMed] [Google Scholar]
- 130.Matsushita H, Chang E, Glassford AJ, et al.. eNOS activity is reduced in senescent human endothelial cells: preservation by hTERT immortalization. Circ Res 2001;89:793–8. [DOI] [PubMed] [Google Scholar]
- 131.Erusalimsky JD. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol 2009;106:326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oh CW, Bump EA, Kim JS, et al.. Induction of a senescence-like phenotype in bovine aortic endothelial cells by ionizing radiation. Radiat Res 2001;156:232–40. [DOI] [PubMed] [Google Scholar]
- 133.Igarashi K, Sakimoto I, Kataoka K, et al.. Radiation-induced senescence-like phenotype in proliferating and plateau-phase vascular endothelial cells. Exp Cell Res 2007;313:3326–36. [DOI] [PubMed] [Google Scholar]
- 134.Imaizumi N, Monnier Y, Hegi M, et al.. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS One 2010;5:e11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sermsathanasawadi N, Ishii H, Igarashi K, et al.. Enhanced adhesion of early endothelial progenitor cells to radiation-induced senescence-like vascular endothelial cells in vitro. J Radiat Res 2009;50:469–75. [DOI] [PubMed] [Google Scholar]
- 136.Yentrapalli R, Azimzadeh O, Barjaktarovic Z, et al.. Quantitative proteomic analysis reveals induction of premature senescence in human umbilical vein endothelial cells exposed to chronic low-dose rate gamma radiation. Proteomics 2013;13:1096–107. [DOI] [PubMed] [Google Scholar]
- 137.Yentrapalli R, Azimzadeh O, Sriharshan A, et al.. The PI3K/Akt/mTOR pathway is implicated in the premature senescence of primary human endothelial cells exposed to chronic radiation. PLoS One 2013;8:e70024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schultz-Hector S, Balz K.. Radiation-induced loss of endothelial alkaline phosphatase activity and development of myocardial degeneration. An ultrastructural study. Lab Invest 1994;71:252–60. [PubMed] [Google Scholar]
- 139.Lauk S, Trott KR.. Endothelial cell proliferation in the rat heart following local heart irradiation. Int J Radiat Biol 1990;57:1017–30. [DOI] [PubMed] [Google Scholar]
- 140.Fernandez AZ. PPARs as targets for the modulation of cardiovascular risk factors associated with the metabolic syndrome. Curr Opin Investig Drugs 2004;5:936–40. [PubMed] [Google Scholar]
- 141.Chinetti-Gbaguidi G, Fruchart JC, Staels B.. Role of the PPAR family of nuclear receptors in the regulation of metabolic and cardiovascular homeostasis: new approaches to therapy. Curr Opin Pharmacol 2005;5:177–83. [DOI] [PubMed] [Google Scholar]
- 142.Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutr J 2014;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]