Abstract
Bisphenol A (BPA) is an endocrine disrupting, high volume production chemical found in a variety of products. Evidence of prenatal exposure has raised concerns that developmental BPA may disrupt sex-specific brain organization and, consequently, induce lasting changes on neurophysiology and behavior. We and others have shown that exposure to BPA at doses below the no-observed-adverse-effect level can disrupt the sex-specific expression of estrogen-responsive genes in the neonatal rat brain including estrogen receptors (ERs). The present studies, conducted as part of the Consortium Linking Academic and Regulatory Insights of BPA Toxicity program, expanded this work by examining the hippocampal and hypothalamic transcriptome on postnatal day 1 with the hypothesis that genes sensitive to estrogen and/or sexually dimorphic in expression would be altered by prenatal BPA exposure. NCTR Sprague-Dawley dams were gavaged from gestational day 6 until parturition with BPA (0-, 2.5-, 25-, 250-, 2500-, or 25 000-μg/kg body weight [bw]/d). Ethinyl estradiol was used as a reference estrogen (0.05- or 0.5-μg/kg bw/d). Postnatal day 1 brains were microdissected and gene expression was assessed with RNA-sequencing (0-, 2.5-, and 2500-μg/kg bw BPA groups only) and/or quantitative real-time PCR (all exposure groups). BPA-related transcriptional changes were mainly confined to the hypothalamus. Consistent with prior observations, BPA induced sex-specific effects on hypothalamic ERα and ERβ (Esr1 and Esr2) expression and hippocampal and hypothalamic oxytocin (Oxt) expression. These data demonstrate prenatal BPA exposure, even at doses below the current no-observed-adverse-effect level, can alter gene expression in the developing brain.
Bisphenol A (BPA) is a well-known endocrine-disrupting compound (EDC) used in a wide range of products, including food and beverage containers, medical equipment, plastic water pipes, and thermal receipts, from which it can leach, thereby resulting in human exposure (1–3). BPA has been found in human fetal plasma and in placental tissue (1, 2), indicating that maternal BPA is able to cross the placenta. Evidence of prenatal exposure has raised concern that subtle effects of developmental BPA exposure on brain organization and sexual differentiation may have long-term impacts on neurophysiology and behavior (3–5). Over the past decade, the National Toxicology Program (NTP), World Health Organization, Food and Agricultural Organization, and others have expressed concern for effects on the brain and behavior (6–10). The Food and Drug Administration (FDA), however, subsequently departed from this view and in 2014 stated that the “FDA's current perspective, based on its most recent safety assessment, is that BPA is safe at the current levels occurring in foods. Based on FDA's ongoing safety review of scientific evidence, the available information continues to support the safety of BPA for the currently approved uses in food containers and packaging.” Historically, hazard characterization studies used to inform regulatory decision making have not investigated neural endpoints or included human-relevant doses (11, 12), limitations which have contributed to the lack of consensus on the potential health effects of developmental BPA exposure.
The experiments here were conducted as part of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) program (11–13), a collaborative effort coordinated by the National Institute of Environmental Health Sciences (NIEHS)/NTP and the FDA's National Center for Toxicological Research (NCTR) and specifically designed to address these information gaps and validate prior findings. CLARITY-BPA studies incorporate research recommendations from the World Health Organization and others (6–9, 14, 15), including comparing effects in both sexes, testing at multiple ages, use of a low phytoestrogen diet, inclusion of a reference estrogen, and evaluation of multiple BPA doses, particularly doses at or below the no-observed-adverse-effect level (NOAEL) of 5 mg/kg per day. The present study was specifically designed to focus on the neonatal brain and tested the hypothesis that gestational BPA exposure can have sex-specific effects on the brain transcriptome.
It is well established that even transient alterations in gene expression during perinatal brain development can cause irreversible changes in brain organization and, consequently, neuroendocrine physiology and behavior (16–19). Work by us and others has revealed that exposure to low doses (defined here as doses at or below the NOAEL of 50-mg/kg body weight [bw]/d) of BPA early in life impairs spatial memory (20) and alters sociosexual (21, 22) and anxiogenic behaviors in a variety of animal models (23, 24). Although the mechanisms by which BPA induces these and related behavioral outcomes remain unclear (3), developmental BPA exposure induces structural and molecular changes within brain regions essential for the coordination of these behaviors, most notably the hippocampus (25–28) and hypothalamus (29–32). For example, prenatal low-dose exposure has been demonstrated to alter dendritic (26) and synaptic structure and decrease mRNA expression of synaptophysin, spinophilin, and other genes critical for synapse formation and plasticity in the hippocampus of juvenile rodents (25, 26). In the hypothalamus, we and others have found age, region and sex-specific evidence for disrupted estrogen receptor (ER) expression or immunoreactivity after perinatal BPA exposure at or below the NOAEL (27, 29, 32–36).
Although the developing hippocampus and the hypothalamus appear to be vulnerable to BPA and other endocrine disruptors, surprisingly few studies have focused on prenatal exposure specifically, or assessed neural outcomes in the immature brain, particularly during the neonatal period (37). Albeit a relatively small literature, available studies suggest that prenatal BPA exposure can alter ER density in the developing brain, especially the hypothalamus. For example, in utero exposure to low doses of BPA disrupted sexually dimorphic gene expression patterns of ERα and ERβ (Esr1 and Esr2) and estrogen-related receptor-γ (Esrrg) in juvenile mice (27). In a prior study using similar dosing methods and the same strain of rat as the present study, we found expression of Esr1 and Esr2 to be up-regulated by BPA in multiple subregions of the hypothalamus and amygdala in a sex-dependent manner on the day after birth (postnatal day [PND]1) (38). In other prior rat studies, we have also observed evidence of disruption on the expression of the neuropeptide oxytocin (Oxt) (23, 29). The present studies extend this prior work by examining the full transcriptome in the hypothalamus and hippocampus on PND1 with the hypothesis that, in addition to Esr1 and Esr2, gene families sensitive to estrogen and/or sexually dimorphic in expression would be altered by prenatal BPA exposure, including Oxt.
That low doses of BPA have an estrogenic mode of action in vivo has been questioned because BPA has relatively low binding affinities for nuclear ERs (10 000- to 100 000-fold lower than estradiol) (39–41). Disruption of ER expression, particularly during critical windows of hormone-dependent sexual differentiation, may be an alternative mechanism by which BPA alters estrogen-mediated brain organization. Thus, the transcriptomic approach was undertaken to look for evidence that BPA might be active via other modes of action. Quantitative real-time PCR (qRT-PCR) was used to verify prior findings related to ER expression and compare expression of a predetermined set of genes across all available exposure groups and follow-up on applicable RNA-sequencing (RNA-seq) findings (Table 1).
Table 1.
Candidate and Novel Genes Assessed With qRT-PCR
| ABI Assay Number | Gene | Description | NCBI Accession Number | Region of Interest | Rationale for Analysis | References |
|---|---|---|---|---|---|---|
| Rn03928990_g1 | 18s | R. norvegicus 18S ribosomal RNA | NR_046237 | Both | Endogenous control | |
| Rn01640372_m1 | Esr1 | R. norvegicus estrogen receptor1 (ERα) | NM_012689.1 | Both | Coordinate estrogen signaling, can be nuclear or membrane bound, bind BPA | 42–44 |
| Rn00562610_m1 | Esr2 | R. norvegicus estrogen receptor2 (ERβ) | NM_012754.1 | Both | Coordinate estrogen signaling, can be nuclear or membrane bound, bind BPA | 42–44 |
| Rn00564446_g1 | Oxt | R. norvegicus oxytocin/neurophysin 1 prepropeptide | NM_012996.3 | Both | Mediates affiliation, social behavior, and mood, responsive to estrogen, altered by perinatal BPA | 29, 45 |
| Rn00564605_m1 | Ptgds | R. norvegicus prostaglandin D2 synthase (brain) | NM_013015.2 | Both | Plays role in neuroprotection; sexually dimorphic expression in neonates; differential expression observed in RNA-seq | 46–48 |
| Rn00691548_m1 | Slc1a2 | R. norvegicus solute carrier family 1 member 2, transcript variant 2 | NM_001035233.1 | Both | Represents primary excitatory pathway; differential expression observed in RNA-seq | 49 |
| Rn00824654_m1 | Slc32a1 | R. norvegicus solute carrier family 32, member 1 | NM_031782.1 | Both | Represents primary inhibitory signaling pathway; differential expression observed in RNA-seq | 50 |
| Rn01433205_m1 | Lepr | R. norvegicus leptin receptor | NM_012596.1 | Hypothalamus | Signaling altered by BPA in vitro; differential expression observed in RNA-seq | 51 |
Pregnant NCTR Sprague-Dawley (NCTR-SD) rats were treated orally by gavage with vehicle, 5 doses of BPA (2.5-, 25-, 250-, 2500-, and 25 000-μg/kg bw/d), or ethinyl estradiol (EE) (0.05- or 0.5-μg/kg bw/d) from gestational day (GD)6 through parturition. Because it was not economically feasible to assess the transcriptome from all available groups, 3 (vehicle, BPA 2.5, and BPA 2500) were selected for this purpose, and qRT-PCR was used to assess expression levels of candidate genes (Esr1, Esr2, and Oxt) and novel genes of interest identified by RNA-seq in all exposure groups. Data on other endpoints, at other ages, are forthcoming from other CLARITY studies but are beyond the scope of this specific subproject.
Materials and Methods
Animal husbandry and dosing
PND1 pups were obtained from litters generated for the CLARITY-BPA consortium program (11, 12). Detailed descriptions of animal husbandry, diet, breeding, and dose preparation and administration have been published elsewhere (13); therefore, only relevant methods are summarized here. All elements of the experimental design including doses, timing of exposure, and day of euthanizing were developed and agreed upon by the consortium. The program uses sibling NCTR-SD rats from an ongoing guideline-compliant chronic 2-year study, which follows standard protocols and contains classical endpoints typically considered by regulatory agencies in hazard identification and risk assessment (11, 12). Two other CLARITY-BPA studies (one by our own lab and both focused on behavior) have been published before this one (52, 53). Animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility, and the NCTR Institutional Animal Care and Use Committee approved all procedures in advance.
Dams were housed in rooms maintained on a 12-hour light, 12-hour dark cycle (6 am to 6 pm) at 23 ± 3°C with a relative humidity level of 50 ± 20%. A soy- and alfalfa-free (5K96 verified casein diet 10 IF, round pellets, γ-irradiated; catalog 1810069, Purina Mills) diet and Millipore-filtered water in glass water bottles with silicone stoppers (7721 clear; Plasticoid Co) were provided ad libitum. Extracts of diet and other study materials were monitored for BPA and myco/phytoestrogens (genistein, daidzein, zearalenone, and coumestrol) by liquid chromatography and mass spectrometry (54). Each diet lot assayed contained less BPA than the protocol-specified limit of 5 parts per billion (54) and less than 1 parts per million genistein and daidzein and less than 0.5 parts per million zearalenone and coumestrol. Similarly, drinking water, polysulfone cage leachates, and bedding extracts were found to have BPA levels below the level of the average analytical method blanks (13).
Approximately 2 weeks before mating, female Sprague-Dawley rats from the NCTR colony (NCTR-SD) were randomized to 8 exposure groups stratified by bw to produce approximately equal mean bws in each group. Eight dose groups were included in this study (vehicle and BPA 2.5-, 25-, 250-, 2500-, and 25 000-μg/kw bw/d and EE 0.05- and 0.5-μg/kw bw/d). The 2 EE groups were incorporated into the CLARITY design for the purposes of serving as a reference estrogen and to provide directional guidance to establish whether BPA-related effects were consistent with an “estrogenic” effect. Mating pairs were assigned randomly, subject to the constraint that no sibling or first cousin mating was permitted. Aside from mating occurring in solid-bottomed polysulfone caging with hardwood chip bedding, mating was conducted as previously described (54).
Beginning on GD6, dams were gavaged daily with 0.3% carboxymethyl cellulose/kg bw/d (vehicle control group), 2.5-μg BPA/kg bw/d (BPA 2.5 group), 25.0-μg BPA/kg bw/d (BPA 25 group), 250-μg BPA/kg bw/d (BPA 250 group), 2500-μg BPA/kg bw/d (BPA 2500 group), 25 000-μg BPA/kg bw/d (BPA 25 000 group), 0.05-μg EE/kg bw/d (EE 0.05 group), or 0.5-μg EE/kg bw/d (EE 0.5 group). Dose volume was determined immediately after daily bw collection and dosing continued until the day of parturition (PND0). Internal dose levels were not assessed, but information regarding BPA pharmacokinetics in this strain is published elsewhere (55, 56). Dams and pups were left undisturbed during PND0. On PND1, terminal pup weights were collected and pups (no more than 1 per sex per litter) were euthanized by decapitation. Within sex, there were no significant effects of exposure on pup terminal bw (data not shown). Heads were rapidly frozen in dry ice and shipped to the Patisaul laboratory at North Carolina State University (NCSU), where they were archived and stored at −80°C until processing. All tissues were coded, and all testing was done blinded to exposure group.
Tissue collection
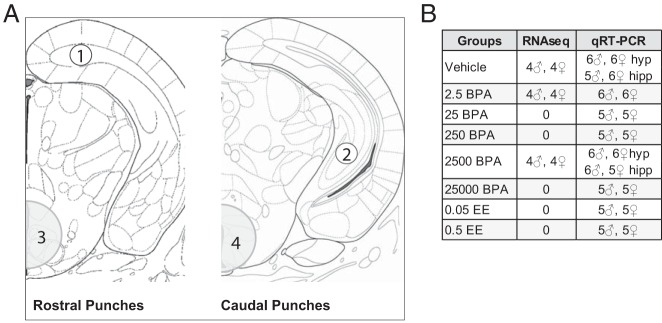
Each whole head was coronally cryosectioned (Leica CM1900) from the caudal end until the caudal borders of the hypothalamus and hippocampus were identified. Anatomical landmarks were located with the assistance of a rat and mouse brain atlas (57, 58). Tissue samples were obtained using a sterilized stainless steel punch that was briefly cooled on dry ice, as previously described (23). For each animal, 2 hypothalamic punches and 4 hippocampal punches were obtained (Figure 1A). The hypothalamic tissue collected consisted of 2 sequential punches, 1 taken anteromedially and 1 caudomedially, with a micropunch 1.25 mm in diameter and 1.00 mm in depth. These punches were combined and collectively comprised the entire hypothalamus sample. Because the hippocampus is irregular in shape, a series of smaller punches were made to obtain sufficient material but also ensure anatomical specificity. For each animal, 4 punches were made, each 0.5 mm in diameter and 1.00 mm in depth: 1 pair of bilateral anterodorsal punches and 1 pair of bilateral caudoventral punches. All 4 samples were combined and collectively comprised the entire hippocampus sample. Each punch sample was expelled out of the stainless steel punch directly into a BPA-free Eppendorf tube on dry ice and stored at −80°C.
Figure 1.
A, Anatomical representation of regions extracted via micropunch (obtained by approaching the regions of interest caudally and punching rostrally) and used for gene expression analysis. For each animal, 1 pair of bilateral anterodorsal hippocampal punches (1, unshaded) and 1 pair of bilateral caudoventral hippocampal punches (2, unshaded) were made, each 0.5 mm in diameter and 1.00 mm in depth. All 4 punches were combined, collectively comprising the whole hippocampus. Hypothalamic tissue consisted of 2 sequential punches (1.25 mm in diameter and 1.00 mm in depth): 1 anteromedial (3, shaded) and 1 caudomedial (4, shaded). B, Sample sizes for RNA-seq and qRT-PCR.
RNA-sequencing
The experimental design for transcriptome sequencing was developed in consultation with the NCSU Genomic Sciences Laboratory and the Bioinformatics Research Core. Transcriptome sequencing was performed by the Genomic Sciences Laboratory on 24 hippocampal (n = 4 per sex per group) and 24 hypothalamic (n = 4 per sex per group) samples (Figure 1B). Three experimental groups were examined: control, BPA 2.5, and BPA 2500. These doses were selected in consultation with other consortium members based on effects observed in other subprojects (52, 53) and prior results in analogous studies using NCTR-SD rats (34, 38). Because the DNA needed to be sent to another CLARITY member for subsequent studies (to be published elsewhere), DNA and RNA were coextracted from frozen tissue samples using the ZR-duet DNA/RNA miniprep copurification kit and treated with an on-column DNase I digestion, per the manufacturer's protocol (Zymo Research). RNA quality was assessed with the Agilent 2100 Bioanalyzer. All hippocampal samples had an RNA integrity number more than or equal to 9 and all hypothalamic samples had an RNA integrity number of 10. To optimize library complexity, all samples used as input material for library preparation had greater than 100 ng of total RNA. Sequencing libraries were prepared with the NEBNext Ultra Directional RNA Library Prep kit for Illumina and the NEBNext Poly (A) mRNA Magnetic Isolation Module (catalogs E7420 and E7490; New England Biolabs). Per manufacturer's instructions, mRNA was isolated, heat fragmented, and primed with random primers. First strand cDNA synthesis was performed with actinomycin D and Protoscript II Reverse Transcriptase. Second strand cDNA synthesis was performed with second strand synthesis reaction buffer containing dUTP, which replaces dTTPs and preserves strand orientation information. After cDNA synthesis, the fragments were purified and size selected using AMPure XP beads (catalog A63881; Beckman Coulter Genomics). The cDNA library fragments were then treated with an End Repair enzyme mix and ligated onto adaptors specifically designed for the Illumina platform. PCR was used to replace the dUTPs in the adaptor sequence and the second strand of the cDNA fragments, enrich adaptor-ligated cDNA, and add 6-nucleotide barcode sequences that allow for pooling of multiple samples for sequencing and sorting of data during analysis. Library clean-up was performed with AMPure XP beads and quality was confirmed on the Agilent 2100 Bioanalyzer. For both experiments (hippocampus and hypothalamus), cDNA libraries were combined into 2 pools of equal molar amounts. Following a balanced block design (59), both pools were multiplexed and run across 3 lanes. Libraries were sequenced using the 125-bp single-end protocol on an Illumina HiSeq2500 sequencer. Approximately 29.9 million reads were generated per hippocampal library and 29.2 million reads per hypothalamic library.
RNA-seq data processing
RNA-seq data analysis was performed by the Bioinformatics Core at the University of Virginia. Quality control of read data was assessed with FastQC before and after adaptor trimming and filtering. The STAR alignment tool (60) was used to align reads to the Rattus norvegicus (rn5) reference genomic sequence, downloaded from UCSC's Genome Browser. After aligning data, the number of reads mapping to GENCODE was calculated using the featureCounts software in the Subread package (61). Count data were normalized for sequencing depth and distortion, and dispersion was estimated using the DESeq2 Bioconductor (62, 63) package in the R statistical computing environment.
Quantitative real-time PCR
Gene expression analysis by qRT-PCR was performed on 8 treatment groups (n = 5–6; sample numbers indicated in Supplemental Figure 1B): vehicle and BPA 2.5-, 25-, 250-, 2500-, and 25 000-μg/kg bw/d and EE 0.05- and 0.5-μg/kg bw/d. A DNA/RNA miniprep copurification kit (catalog D7001; Zymo Research) with the addition of an on-column DNase I digestion (catalog E1007; Zymo Research) was used to extract RNA. Extracted DNA was sent to another CLARITY consortium member for independent analysis. mRNA was reverse transcribed to single-strand complementary cDNA with the high capacity RNA-to-cDNA kit (catalog 4387406; Applied Biosystems). Each RT reaction was incubated for 60 minutes at 37°C, 5 minutes at 95°C, and stored at −20°C until use. Real-time PCR was performed with an ABI StepOnePlus Real-Time PCR System. A TaqMan probe-based protocol was used to detect gene expression, and primers and probes were included in these predesigned assays (Table 1). The PCRs were incubated in 96-well plates and run using the manufacturer's recommended cycling parameters of 50°C for 2 minutes, 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. All reactions consisted of 1-μL hypothalamic RT product or 2-μL hippocampal RT product, 1-μL of 20× TaqMan gene expression assay mix, 10 μL of TaqMan Universal PCR Master Mix, and nuclease-free H2O in a quantity sufficient to make a 20-μL total reaction volume. No-template controls were run for each TaqMan gene expression assay and each PCR was run in triplicate. An inter-run calibrator and the sample maximization approach was followed to avoid technical and run-to-run variation (64). To correct for variation in starting cDNA concentrations, cycle threshold (Ct) values for the gene of interest for each sample were normalized to the Ct for 18s rRNA for each sample. 18s was selected as the normalizing transcript based on preliminary work for these experiments with other candidates showing that 18s was the most reliable and robust. Quantitative mRNA expression data were acquired and analyzed by the Livak ΔΔ-Ct (ΔΔ-Ct) method (65).
Data decoding
All testing and data collection for the RNA-seq was conducted blind to exposure. All individuals had a unique identifier and the samples belonging to each experimental group (grouped by exposure and sex) were designated with a letter (A, B, C, etc). The blinded raw data were submitted to the NTP Chemical Effects in Biological Systems database. It was then independently verified to account for all expected datasets and data points, and “locked” such that data could not be altered. The NCSU researchers were then provided with the exposure code for data analysis. The qRT-PCR experimental design depended, in part, on the RNA-seq results. Thus, the qRT-PCR phase was performed after the RNA-seq data was decoded but blind to the other, remaining, groups.
Genes for the qRT-PCR analyses were selected either a priori, because they were previously shown to be altered by developmental BPA exposure in brain (Esr1, Esr2, and Oxt), sexually dimorphic in PND1 hypothalamus (prostaglandin D2 synthase [Ptgds]) (66), or identified from the RNA-seq results (Lepr, Slc32a1, and Slc1a2). Additionally, Slc32a1 and Slc1a2 were considered gross indicators of altered inhibitory and excitatory neurotransmission. The blinded raw data were then submitted to Chemical Effects in Biological Systems and locked as described for the RNA-seq experiments and analyzed once the data were decoded.
Statistical analysis
The statistical approach was developed to be consistent with previously published transcriptome projects of similar scale (equivalent sample size or smaller) in rat brain (48) and guidelines for low-dose EDC studies with sample sizes in this range (67). Within each exposure group, no same-sex litter mates were included, so potential litter effects did not need to be statistically accounted for.
RNA-seq analysis
For all data, the hippocampus and the hypothalamus were analyzed separately. The DEseq2 package was used to fit a negative binomial model for each gene using an extended model matrix. To identify sex differences in gene expression, the male and female controls were compared independent of other exposure groups. With that exception, all other data were compared within sex. For each comparison, the P value was adjusted for multiple testing using the Benjamini-Hochberg false discovery rate (padj) (68). Some genes could not be corrected because their abundance was too low to justify analysis (padj = N/A). Thus, for all comparisons, statistical significance was defined as padj ≤ .05. The group for which the greatest numbers of genes were significantly altered by BPA was the hypothalamic male BPA 2500 group. Thus, to further query the sex-specificity of these effects, an interaction term in the negative binomial model was fit to examine the nonlinear effect of sex-by-exposure compared with unexposed controls. This approach considerably narrowed the prospective list of significantly altered genes.
qRT-PCR analysis
Statistical analyses were performed and graphed using Prism v6 software (GraphPad Software, Inc). Two samples (1 for hypothalamic Oxt and 1 for hypothalamic Esr2) were excluded due to technical error. Outliers (no more than 1 per group) were identified using a Grubb's test. Final sample sizes are listed in table 3 below. Data from the hippocampus and hypothalamus were analyzed independently and, because gene expression was anticipated to be sexually dimorphic in some cases, analyzed within sex. The study had multiple related but independent hypotheses, each of which was tested independently and included only the relevant groups for addressing that specific hypothesis. Using a 2-tailed Mann-Whitney U test, it was first established whether expected sex differences in gene expression between the male and female controls were present. Also using a 2-tailed Mann-Whitney U test, we next addressed the primary goal of the study: establish whether BPA impacts gene expression in either region of interest at any of the doses employed, in either sex. When BPA-related effects were found, qualitative comparisons with the EE groups were made to see whether directionality was consistent with an estrogenic effect. Finally, EE was used as a reference estrogen and thus expected to masculinize sexually dimorphic gene expression in females. Within sex, differences in mean expression values between the controls and each of the 2 EE groups were identified using a 2-tailed Mann-Whitney U test. In all cases, effects were considered significant at P ≤ .05.
Results
Expression assessment by RNA-seq
Effect of prenatal BPA exposure on hippocampal gene expression
Effects of BPA on the PND1 hippocampal transcriptome were minimal. Expression of 13 genes was altered in the BPA 2.5 exposed females. Of these, endoglin (Eng) and Centers for Disease Control-like kinase 4 (Clk4) exhibited the largest fold change (−1.52 and 1.65, respectively) (Supplemental Table 1a). No significant effects of BPA were observed in the BPA 2500 exposed females. In BPA 2.5 exposed males, only expression of RuvB-like AAA ATPase 2 (Ruvbl2) was decreased (Supplemental Table 1b). In the BPA 2500 exposed males, 10 genes were differentially expressed. Of these, neurotrophin receptor associated death domain (Nradd) and ER degradation enhancing α-mannosidase-like protein 1 (Edem1) showed the largest fold change (−1.75 and 1.70, respectively).
Effect of prenatal BPA exposure on hypothalamic gene expression
BPA-related transcriptional changes were more numerous in the hypothalamus than in the hippocampus but only in males. In the BPA 2.5 exposed females only one gene was altered and only 2 were altered in the BPA 2500 group. Common salivary protein 1 (Csap1) was down-regulated in both the BPA 2.5 and BPA 2500 females (fold changes were −1.31 and −1.32, respectively). In addition, SPARC-like 1 (Sparcl1) was down-regulated in the BPA 2500 females (Supplemental Table 2a). In males, there was a robust effect of prenatal exposure to BPA at both doses (Supplemental Table 2b). In the BPA 2.5 exposed males, 639 genes were affected by BPA compared with same-sex controls. Of these, 360 were down-regulated and 279 were up-regulated. In males exposed to BPA 2500, 1107 genes were significantly altered. Of these, 553 were down-regulated and 554 were up-regulated. A total of 371 genes were significantly altered by BPA in both dose groups (Supplemental Table 3). Because so many more genes were affected in males than females in the BPA 2500 group, a two-way ANOVA with sex and exposure as factors was used to specifically identify genes for which there was a significant sex by exposure interaction. This approach identified 32 differentially expressed genes (28 up-regulated and 4 down-regulated) (Table 2). Of these, aquaporin 1 (Aqp1) exhibited the largest fold change (1.62); 13 of these genes were also significantly affected at the 2.5 dose (Ap5M1, Atad5, Atm, Cd2Ap, Dennd4C, Dusp26, Mat2A, Scn9A, Setx, Slc32A1, Tia1, Ythdc2, and Zdhhc15).
Table 2.
Differentially Expressed Genes for Which There Was a Significant Sex by Exposure Interaction at 2500-μg BPA/kg bw/d in Males
| Gene | Gene Name | Fold Change | log2 (Fold Change) | padj Value |
|---|---|---|---|---|
| Atm | Ataxia telangiectasia mutated homolog | 1.318 | 0.398 | .019 |
| Ddx26b | DEAD/H (Asp-Glu-Ala-Asp/His) box polypeptide 26B | 1.431 | 0.517 | .019 |
| Prp2l1 | Proline-rich protein 2-like 1 | 1.471 | 0.557 | .019 |
| Scn9a | Sodium channel, voltage-gated, type IX, α | 1.389 | 0.474 | .019 |
| Tbc1d4 | TBC1 domain family, member 4 | 1.432 | 0.518 | .019 |
| Tgds | TDP-glucose 4,6-dehydratase | 1.331 | 0.413 | .019 |
| Tia1 | TIA1 cytotoxic granule-associated RNA-binding protein | 1.253 | 0.325 | .019 |
| Ythdc2 | YTH domain containing 2 | 1.290 | 0.367 | .019 |
| Aqp1 | Aquaporin 1 | 1.616 | 0.692 | .020 |
| Cd2ap | CD2-associated protein | 1.320 | 0.400 | .022 |
| Fancd2 | Fanconi anemia, complementation group D2 | 1.331 | 0.413 | .022 |
| Slc32a1 | Solute carrier family 32 (GABA vesicular transporter), member 1 | −1.449 | −0.535 | .022 |
| Trpm3 | Transient receptor potential cation channel, subfamily M, member 3 | 1.253 | 0.325 | .022 |
| Vstm2b | V-set and transmembrane domain containing 2B | −1.316 | −0.396 | .022 |
| Ap5m1 | Adaptor-related protein complex 5, μ-1 subunit | 1.373 | 0.457 | .025 |
| Sparcl1 | SPARC-like 1 (hevin) | 1.366 | 0.450 | .025 |
| Arhgap29 | ρ-GTPase activating protein 29 | 1.388 | 0.473 | .027 |
| Itsn2 | Intersectin 2 | 1.254 | 0.327 | .027 |
| Atad5 | ATPase family, AAA domain containing 5 | 1.282 | 0.358 | .027 |
| Lepr | Leptin receptor | 1.497 | 0.582 | .027 |
| Mrs2 | Magnesium homeostasis factor homolog | 1.330 | 0.411 | .035 |
| Tnc | Tenascin C | 1.365 | 0.449 | .041 |
| Ralgapa2 | Ral GTPase-activating protein, α-subunit 2 | 1.281 | 0.357 | .043 |
| Mat2a | Methionine adenosyltransferase II, α | 1.237 | 0.307 | .044 |
| Supt20 | Suppressor of Ty 20 | 1.266 | 0.340 | .044 |
| Dennd4c | DENN/MADD domain containing 4C | 1.300 | 0.378 | .044 |
| Pycrl | Pyrroline-5-carboxylate reductase like | −1.198 | −0.261 | .044 |
| Cep295 | Centrosomal protein 295 | 1.359 | 0.443 | .046 |
| Adamts12 | ADAM metallopeptidase with thrombospondin type 1 motif, 12 | 1.447 | 0.533 | .047 |
| Dusp26 | Dual specificity phosphatase 26 (putative) | −1.251 | −0.323 | .047 |
| Setx | Senataxin | 1.240 | 0.310 | .047 |
| Zdhhc15 | Zinc finger, DHHC-type containing 15 | 1.312 | 0.392 | .047 |
Sex differences in gene expression levels
For each brain region, the male and female controls were compared independent of other exposure groups to identify genes for which expression differed by sex. This was done to both confirm that our procedures were robust enough to detect known sex differences and to potentially identify novel genes for which expression varies by sex on PND1. As anticipated, the hippocampus had fewer sexually dimorphic transcripts than the hypothalamus. In the hippocampus, 29 genes were expressed at significantly different levels in males and females, with 23 (79.3%) being more highly expressed in males and 6 (20.7%) being more highly expressed in females (Supplemental Table 4a). In the hypothalamus, 210 genes with sexually dimorphic expression patterns were identified, with 147 (74.6%) having higher expression levels in females (Supplemental Table 4b). Of these, hypothetical protein LOC680227 (LOC680227) and pro-α-1 collagen, type 1 (Col1a1) exhibited the largest fold changes (6.98 and 4.94, respectively). Consistent with previous reports, expression of Ptgds and cAMP-regulated phosphoprotein 21 (Arpp21) was higher in females (47, 48). Similarly, as anticipated, Oxt, nuclear receptor subfamily 2, group F, member 2 (Nr2f2), and ribosomal protein L30 (Rpl30) were expressed at higher levels in males (48, 66).
Expression assessment by qRT-PCR
A summary of the significant outcomes in both hippocampus and hypothalamus are listed in Table 3. Data for genes in which there was no significant effect of BPA in either sex are not listed. A full summary of the RNA-seq (including raw and adjusted P values) and qRT-PCR data for all genes assessed by qRT-PCR is provided in Supplemental Table 5. For the RNA-seq data, only comparisons where padj ≤ .05 were considered biologically meaningful and statistically significant.
Table 3.
qRT-PCR Outcomes and Descriptive Statistics for Genes Found to be Significantly Altered by BPA Exposure
| Gene | Comparison (n) | Relative Abundance | U | P Value |
|---|---|---|---|---|
| Hippocampus | ||||
| ERβ (Esr2) | ♂ BPA 25 000 (5) to ♂ Control (4) | 9.451 | 0 | .016 |
| ♂ EE 0.05 (5) to ♂ Control (4) | 8.209 | 0 | .016 | |
| Oxytocin (Oxt) | ♀ BPA 25 (5) to ♀ Control (5) | 3.729 | 2 | .032 |
| ♂ BPA 2500 (6) to ♂ Control (5) | 0.438 | 3 | .030 | |
| ♂ BPA 25 000 (4) to ♂ Control (5) | 0.434 | 1 | .032 | |
| Hypothalamus | ||||
| ERα (Esr1) | ♀ BPA 2.5 (6) to ♀ Control (5) | 6.055 | 0 | .004 |
| ♀ BPA 250 (5) to ♀ Control (5) | 5.254 | 1 | .016 | |
| ♀ BPA 25 000 (5) to ♀ Control (5) | 3.514 | 0 | .008 | |
| ERβ (Esr2) | ♀ BPA 2.5 (6) to ♀ Control (5) | 5.507 | 1 | .009 |
| ♀ BPA 250 (5) to ♀ Control (5) | 3.534 | 2 | .032 | |
| ♀ BPA 25 000 (5) to ♀ Control (5) | 2.810 | 2 | .032 | |
| ♀ Control (4) to ♂ Control (6) | 0.230 | 2 | .038 | |
| Oxytocin (Oxt) | ♀ BPA 2.5 (6) to ♀ Control (5) | 8.795 | 3 | .030 |
| ♀ BPA 250 (5) to ♀ Control (5) | 6.200 | 1 | .016 | |
| ♀ EE 0.05 (5) to ♀ Control (5) | 16.245 | 2 | .032 | |
| ♂ BPA 25 (5) to ♂ Control (5) | 20.757 | 2 | .032 | |
| ♂ Control (5) to ♂ EE 0.5 (5) | 11.965 | 0 | .008 | |
| Prostaglandin D2 synthase (Ptgds) | ♀ EE 0.5 (5) to ♀ Control (6) | 5.927 | 1 | .009 |
| ♂ EE 0.5 (5) to ♂ Control (6) | 7.365 | 2 | .017 | |
| GABA vesicular transporter (Slc32a1) | ♀ BPA 2.5 (6) to ♀ Control (6) | 5.247 | 0 | .004 |
| ♀ BPA 250 (5) to ♀ Control (5) | 4.398 | 0 | .008 | |
| ♀ BPA 25 000 (5) to ♀ Control (5) | 3.761 | 2 | .032 | |
| ♀ EE 0.05 (5) to ♀ Control (5) | 4.329 | 1 | .016 | |
| ♂ EE 0.5 (5) to ♂ Control (5) | 1.878 | 3 | .030 | |
| ♀ Control (4) to ♂ Control (6) | 0.210 | 0 | .010 | |
For each group, the sample size is listed in parentheses.
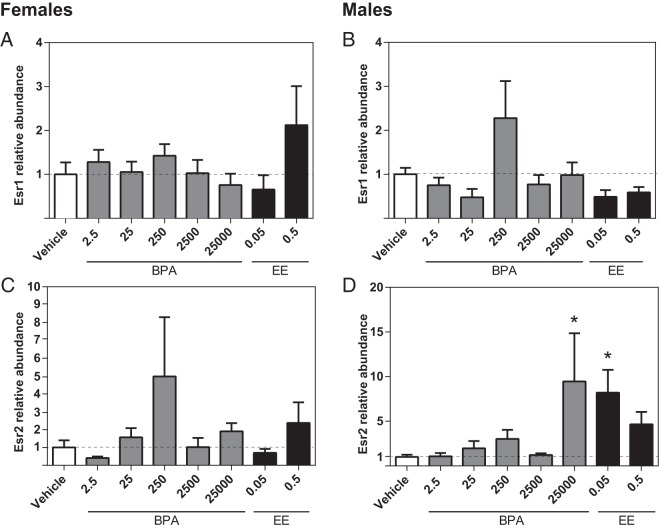
Effect of prenatal BPA or EE exposure on hippocampal gene expression
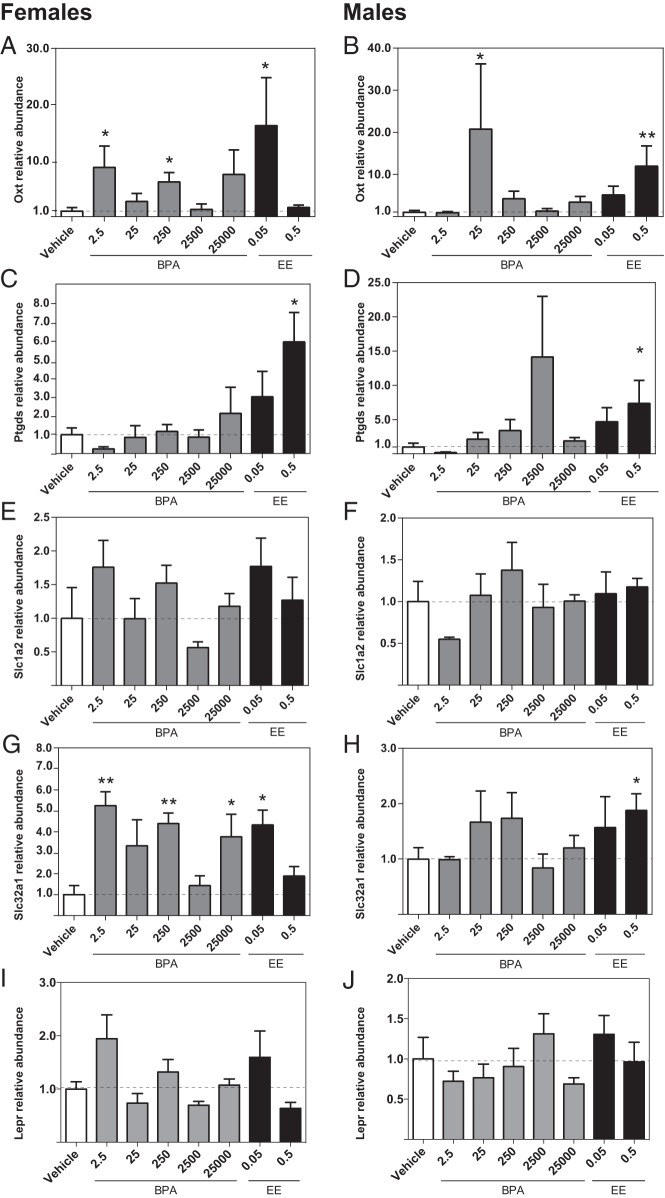
Esr1 expression levels in the hippocampus were not affected by BPA or EE in either sex (Figure 2, A and B). In males, Esr2 expression levels were significantly increased in the BPA 25 000 and EE 0.05 groups (Figure 2D). Within females, however, there were no significant effects of exposure on Esr2 expression (Figure 2C). In males, Oxt expression was significantly decreased in the BPA 2500 and BPA 25 000 groups (Figure 3B), whereas in females, Oxt expression was higher in the BPA 25 group (Figure 3A). No significant effects of BPA or EE on the expression of Ptgds, Slc1a2, or Slc32a1 were identified in either sex (Figure 3, C–H).
Figure 2.
Effects of gestational BPA and EE on neonatal hippocampal ER expression. BPA or EE did not affect Esr1 expression in females (A) or males (B). In females, there was no effect of BPA or EE on Esr2 expression (C). In males, exposure to the highest BPA dose and lowest EE dose resulted in significantly increased Esr2 expression (D). Graphs depict mean ± SEM; *, P ≤ .05.
Figure 3.
Effects of gestational BPA and EE exposure on neonatal hippocampal expression of selected genes. In females, exposure to 25-μg BPA/kg bw/d significantly increased Oxt expression (A). In males, exposure to 2500- and 25 000-μg BPA/kg bw/d decreased expression of Oxt (B). BPA and EE had no significant effect on Ptgds (C and D), Slc1a2 (E and F), or Slc32a1 (G and H) expression in either females or males. Graphs depict mean ± SEM; *, P ≤ .05.
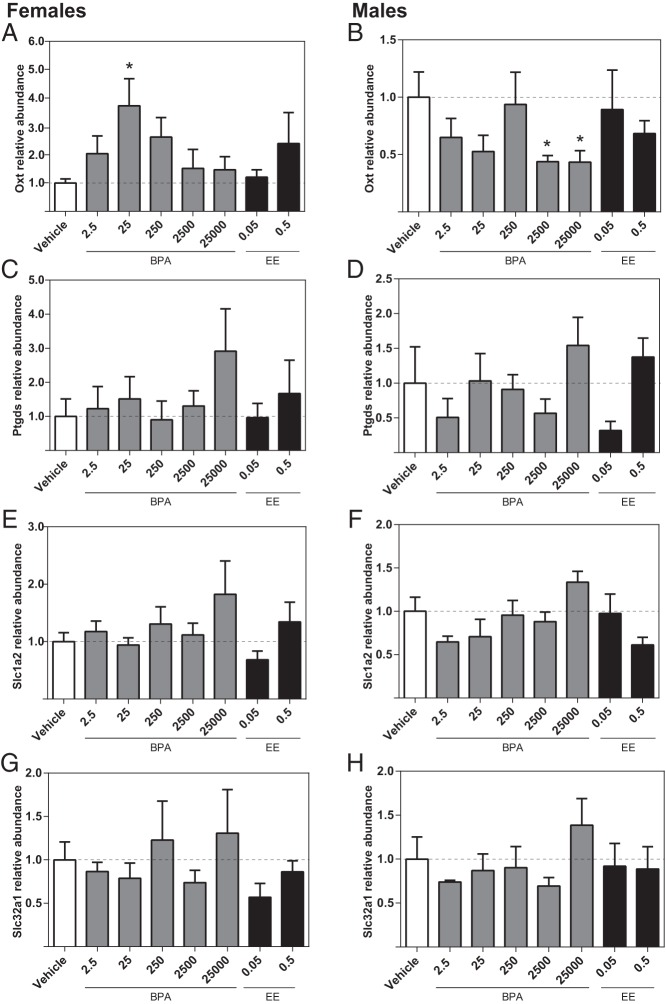
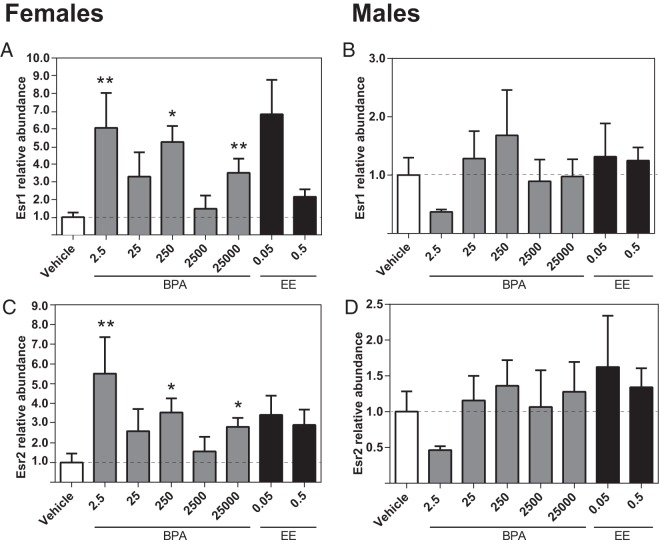
Effect of prenatal BPA or EE exposure on hypothalamic gene expression
Higher expression of Esr1 was identified in the female BPA 2.5, BPA 250, and BPA 25 000 groups (Figure 4A), but BPA had no significant effect on Esr1 expression in the male hypothalamus (Figure 4B). Similarly, expression levels of Esr2 were significantly higher in the female BPA 2.5, BPA 250, and BPA 25 000 groups, but there were no significant effects of BPA or EE on Esr2 in males (Figure 4, C and D). Oxt expression was elevated in the female BPA 2.5, BPA 250, and EE 0.05 groups (Figure 5A) and the male BPA 25 and EE 0.05 groups (Figure 5B). For both males and females, Ptgds expression in the EE 0.5 groups was increased compared with the same-sex control (Figure 5, C and D). In females (Figure 5G), Slc32a1 expression was elevated in the BPA 2.5, BPA 250, BPA 25 000, and EE 0.5 groups. By contrast, within males, elevated levels were only observed in the EE 0.05 group (Figure 5H). For both males and females, there were no significant effects of prenatal BPA or EE on the expression of Slc1a2 or leptin receptor (Lepr) (Figure 5, E, F, I, and J).
Figure 4.
Effects of gestational BPA and EE exposure on neonatal hypothalamic ER expression. Exposure to 2.5-, 250-, and 25 000-μg BPA/kg bw/d increased female expression of Esr1 and Esr2 (A and C). Expression in males was unaffected (B and D). EE had no effect on ER expression in either sex. Graphs depict mean ± SEM; *, P ≤ .05 and **, P ≤ .01.
Figure 5.
Effects of gestational BPA or EE on neonatal hypothalamic expression of selected genes. Exposure to 2.5- and 250-μg BPA/kg bw/d and 0.05-μg EE/kg bw/d increased female expression of Oxt. A, In males, 25-μg BPA/kg bw/d and the highest dose of EE increased Oxt expression (B). Only the highest dose of EE affected Ptgds, increasing expression in both females and males (C and D). Slc1a2 expression levels were not affected by BPA or EE in either sex (E and F). The low dose of EE and 2.5-, 250-, and 25 000-μg BPA/kg bw/d masculinized (increased) female expression of Slc32a1 (G). The high dose of EE increased male expression of Slc32a1 (H). BPA or EE did not affect Lepr expression in either females (I) or males (J). Graphs depict mean ± SEM; *, P ≤ .05 and **, P ≤ .01.
Sex differences in gene expression levels
No significant sex differences were observed between control animals in the hippocampus (Figure 6A). In the hypothalamus, Esr2 and Slc32a1 were expressed at higher levels in males (Figure 6B). EE masculinized hypothalamic Slc32a1 expression but only at the lower dose. In males, the highest dose of EE exhibited a hypermasculinizing effect on Slc32a1, up-regulating expression (Figure 5G). EE did not significantly affect hypothalamic Esr2 expression in either sex (Figure 4, C and D).
Figure 6.
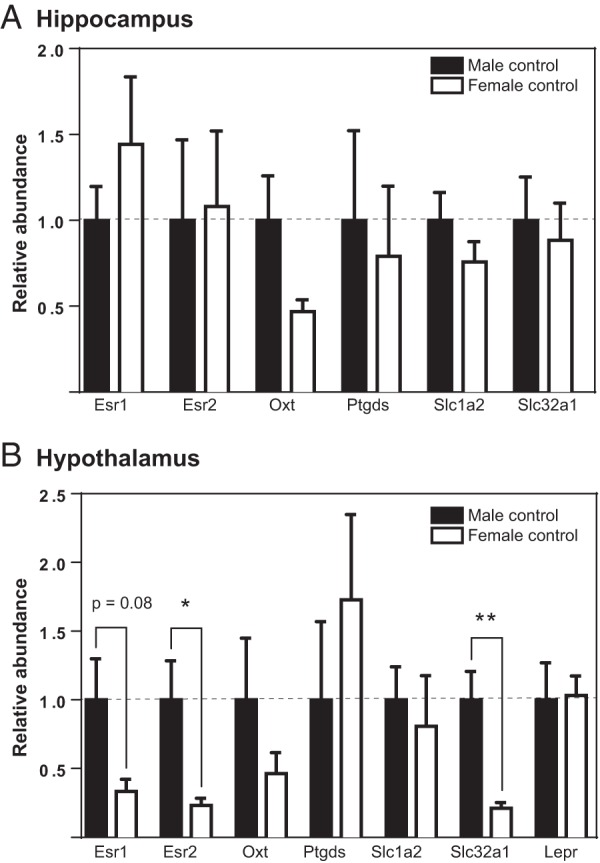
Sex differences in hippocampal and hypothalamic expression of selected genes. Relative differences in gene expression between male and female control (unexposed) groups with male gene expression set as baseline. A, No genes were sexually dimorphic in the neonatal hippocampus. B, Esr2 and Slc32a1 exhibited male biased expression. Graphs depict mean ± SEM; *, P ≤ .05 and **, P ≤ .01.
Discussion
The present study represents the most comprehensive evaluation of gestational BPA exposure on gene expression levels in the neonatal rat hippocampus and hypothalamus to date. Additionally, to our knowledge, this is the only study that has analyzed the full hippocampal transcriptome at PND1 and looked for possible sex differences. Thus, the present studies contribute fundamental new knowledge about sex-specific gene expression in the newborn rat hypothalamus and hippocampus as well as sex-specific sensitivity to gestational BPA exposure at what is generally accepted as a low-dose range.
As anticipated, expression changes induced by BPA were primarily confined to the hypothalamus, a region that coordinates a wide range of neuroendocrine activities including growth, feeding, and reproductive behavior. Hypothalamic transcription changes identified by RNA-seq were overwhelmingly male specific with robust overlap between the 2 dose groups examined (2.5 and 2500). The single gene found by RNA-seq to be altered by BPA at both doses in the female hypothalamus is most abundant in respiratory epithelium but is also in pituitary and has previously been identified as being sensitive to BPA in a seminiferous tubule culture model (69). It is unclear what the functional significance of this might be and was thus not followed up. Detection may indicate that the micropunches contained at least some material from the pituitary, which is not unexpected given that the pituitary is physically connected to the hypothalamus.
Gene Ontology analysis using the functional annotation tool of DAVID 6.7 (70) for the 371 genes found to be altered in the hypothalamus of both the BPA 2.5 and 2500 male groups revealed “regulation of transcription” as the only significantly (adjusted P ≤ .05) enriched pathway (45 genes). Deeper analysis of specific cellular pathways within this general term identified none for which more than only a handful of genes were altered, an outcome interpreted to indicate that BPA is unlikely to be acting in the developing brain via a mode of action not previously identified. A caveat of this conclusion is that the vast majority of available gene annotations (available in DAVID and similar annotation tools) were made in tissues outside of the brain and not in neonatal tissues (or both sexes). Thus, the annotations may not be reasonably applicable to this specific data set. A follow-up analysis was then performed using Ingenuity Pathway Analysis (QIAGEN) with a stringent preanalysis filter restricting input to only neural tissues/cell lines. This revealed the “nervous system development and function” as a significantly enriched physiological system (26 genes) with BPA-sensitive genes identified for 37 subfunctions, but, again, all subfunctions were enriched with only a handful of genes (with significance ranging from P ≤ .05 to P ≤ .002). Interestingly, the single function with the most BPA-sensitive genes (Fox06, Limk1, Mark4, Rhob, and Scn1a; P ≤ .02) was “morphology of dendritic spines.” Disruption of spine morphology has previously been associated with BPA exposure in rodents and nonhuman primates of both sexes (71–73). Annotation analysis for the subset of 32 transcripts identified as significantly affected in BPA 2500 males also identified no significantly enriched pathways (via DAVID or Ingenuity Pathway Analysis).
A limitation of the RNA-seq analysis is that an unsupervised principle components analysis did not clearly delineate RNA-seq data by exposure group or sex in either the hippocampus or the hypothalamus (Supplemental Figure 1B). This outcome is not entirely unexpected for the hippocampus, because there were few effects of BPA and only minimal evidence for sexually dimorphic neonatal gene expression (74). In the neonatal hypothalamus, however, many genes are known to be sexually dimorphic, and BPA-related effects were male biased at both doses examined, thus we expected this data to cluster more robustly. Despite this caveat, the analysis successfully detected transcripts known to be sexually dimorphic. For example, a study analyzing sexually dimorphic gene expression in the rat preoptic area of the hypothalamus at PND2 detected higher expression of Ptgds and Arpp21 in females and higher expression of Oxt (found in the present study to be sensitive to BPA) and Nr2f2 in males (66). Similarly, a microarray analysis of the whole hypothalamus of male and female mice detected female-biased expression of Ptgds on the day of birth (47). Detection of these and other transcriptional sex differences by RNA-seq in the present study was interpreted to signify that our RNA-seq approach had sufficient resolution and statistical power (Supplemental Table 4) to detect group differences (sex and exposure).
Specific genes of interest (selected both a priori based on prior work showing they are sensitive to BPA exposure, and from the RNA-seq analysis) were further assessed by qRT-PCR on all 8 of exposure groups (summarized in Supplemental Table 5). The directionality of the results generally confirmed the RNA-seq data, including the small number of BPA-related effects in the hippocampus. That the highest BPA dose up-regulated of Esr2 in PND1 male hippocampus is consistent with a prior study in BALB/c mice investigating the effects of in utero exposure to 2-μg/kg BPA on juveniles (27). As early as embryonic day 17, ERβ is abundantly expressed in the developing rodent hippocampus (75), but the specific functional role it plays in the organization of the hippocampus has not been fully characterized. Hippocampal Oxt was down-regulated in males exposed to 2500- and 25 000-μg/kg bw/d and up-regulated in females exposed to 25-μg/kg bw/d. Oxytocin (OT) is primarily synthesized in the paraventricular and supraoptic nuclei of the hypothalamus (PVN and SON, respectively) and axonal projections from a subset of these neurons transport OT to the hippocampus and other regions (76–78). OT is normally translated in the perikarya of neurons; however, mRNA has been found in hypothalamic axons, suggesting it may also be locally synthesized in nerve terminals (79, 80). Emerging evidence in adult rodents now indicates OT may play a neuroprotective role in the hippocampus by buffering neuroendocrine and behavioral responses to stress and stimulating neurogenesis (81, 82). We and others have previously reported effects of BPA on OT signaling pathways in the hypothalamus and the amygdala (3, 23, 24, 29, 83), but the present study is the first to suggest gestational exposure to BPA may alter Oxt expression in the hippocampus. Subsequent investigation will be necessary to confirm this observation and delineate its functional significance.
To date, only 4 other animal studies have investigated the effects of gestational BPA exposure on hippocampal gene expression, and, in all of these, the analysis was conducted in juveniles or adults (25–27, 84). A paper published as this study was in preparation found prenatal (GD0–GD19) exposure to 200-μg/kg BPA per day induced sex-specific changes in juvenile and adult mouse expression of brain-derived neurotrophic factor (Bdnf), a gene known to be epigenetically regulated in response to environmental exposures (84). In the present study, differential expression of Bdnf was not detected with RNA-seq, and we had insufficient amounts of RNA to evaluate expression with qRT-PCR.
The hippocampus was selected as a region of interest because it is critically involved in learning and memory and sensitive to stress (85). Some published data suggest BPA-related effects on these behaviors, including a related study conducted as part of the CLARITY-BPA consortium, which reported that females perinatally exposed to 2500-μg/kg BPA completed the Barnes maze, a spatial learning and memory task, more slowly than unexposed controls, but no other decrements (53). Others have not found memory-related effects (eg, Refs. 3, 87). It is possible that few hippocampal effects were detected here because exposure was entirely prenatal, and we looked for effects so early in postnatal development. Rodent hippocampal development begins in midembryogenesis (approximately embryonic d 8.5) but undergoes radical changes in cell acquisition and gross morphology during the first 2 weeks of postnatal life (88–92), making it possible that the critical period of hippocampal susceptibility to endocrine disruption is postnatal (93). Subsequent studies in the CLARITY-BPA program will examine hippocampal endpoints at later ages.
By contrast, the critical period of hypothalamic hormone-mediated sexual differentiation spans perinatal development and peaks a few days after birth (94) and EDC sensitivity during this time is well characterized (95, 96), including age, sex, and subregional impacts of BPA on ER expression (37). A prior study by our group, using a similar exposure paradigm and rats of the same strain and age, detected BPA- and EE-related effects on ER gene transcription across the hypothalamus (38). These effects, as well as sex differences in expression, varied by subregion. Here, expected sex differences were only detected for Esr2. This effect is likely attributable to sex-specific Esr2 expression differences in the anteroventral periventricular nucleus of the hypothalamus (32, 97, 98). Similarly, although evidence of BPA-related impacts on ER expression is consistent with prior work by us and others, the outcomes reported here do not completely recapitulate the observations made in our previous study using the same rat strain (including a sex difference in Esr1 expression in unexposed animals) (38). This result is not entirely unexpected, however, because in the present study, we examined the whole hypothalamus, rather than its individual subregions. Thus, region-specific differences in ER expression (between sexes and/or exposure groups) are homogenized and, consequently, not fully detectable. Additionally, we have previously shown that gestational gavage of the dam can potentially minimize or obfuscate sex differences in newborn rats (32). That gestational BPA exposure increased Esr1 and Esr2 expression in females but not in proportion to the dose administered suggests a possible nonmonotonic dose response worthy of follow-up study. Finally, effects of BPA and EE exposure were not concordant. Although BPA masculinized expression of Esr2, EE had no effect. This result was unexpected as endogenous estrogens are well established to induce brain masculinization in rodents (95, 96).
Evidence of disrupted Oxt was also found in the hypothalamus. Sexually dimorphic expression of Oxt has previously been reported in the preoptic area of the prenatal hypothalamus (66), and both the RNA-seq and qRT-PCR analyses in the present study indicated a similar pattern of expression (higher in males than females), but the difference failed to reach statistical significance. Increased Oxt expression in females at 2.5- and 250-μg/kg BPA, is indicative of masculinization and concordant with prior work from our lab demonstrating the ability of neonatal BPA to increase the number of OT neurons in the PVN of adult females (29). OT has been shown to regulate and induce ERα expression during development, and manipulations of OT can alter the expression of ERα in both juveniles and adults (99). Thus, evidence for concomitant disruption of Esr1 and OT by BPA in females of the present study suggests these outcomes may be related. EE masculinized expression in females but only at the lower dose suggesting some sort of compensation for endocrine disruption at higher doses (of EE or BPA).
The RNA-seq analysis revealed possible effects on hypothalamic Lepr. Identified changes in Lepr expression did not survive false discovery rate correction for any comparisons except the BPA 2500 male exposure group in the exposure by sex analysis (up-regulated by BPA). We thus followed up with qRT-PCR across all exposure groups, but Lepr was not found to be significantly altered in any group. Thus, it remains unclear whether Lepr is vulnerable to gestational BPA exposure. A more robustly powered analysis of the arcuate nucleus, where Lepr is most heavily concentrated, would likely be more revealing.
Prenatal BPA (2.5, 250, and 25 000 μg/kg·d) exposure enhanced expression of the sexually dimorphic gene Slc32a1 in the female hypothalamus. Slc32a1 encodes the vesicular inhibitory amino acid transporter, which is essential for inhibitory synaptic transmission (100) and responsible for the reuptake and storage of glycine and γ-aminobutyric acid (GABA) into synaptic vesicles. Data related to BPA effects on neurotransmitters are sparse, but evidence for heightened GABA levels in the female adult mouse brain after perinatal exposure to 500-μg/kg bw/d BPA by gavage of the dam has been reported (101). Similarly, GABA release from hypothalamic fragments collected from perinatally exposed rats was elevated, as were serum GABA levels (102). Disruption of inhibitory signaling in the hypothalamus could be a mechanism by which BPA and other EDCs impact the developing brain but further studies are necessary to fully explore this possibility.
Notably, it is not unexpected that qRT-PCR, which is more targeted and sensitive, identified effects of BPA that were not identified as statistically significant with RNA-seq, particularly after accounting for false discovery rate (hypothalamic Slc32a1 is a notable example for some dose levels; see Supplemental Table 5). Imperfect quantitative concordance between different methodologies, particularly for low abundance transcripts such as the ERs, is not unexpected given that these approaches have different levels of sensitivity and different technical limitations (103). The capacity for RNA-seq to detect expression differences is contingent on both the number of read counts per gene and the magnitude of expression change. For low expression genes, RNA-seq may not achieve sufficient transcript sampling to adequately resolve group differences in mRNA abundance (104, 105). Additionally, a critical goal of the present study was to maximize anatomical specificity while simultaneously ensuring we obtained enough tissue to extract sufficient quantities of RNA for sequencing. Incorporating a preamplification step before RNA-seq could have allowed us to use less tissue and/or increase our capacity to detect rare transcripts but doing so can result in the preferential amplification of some transcripts (typically the most abundant) resulting in decreased representation of the remaining transcripts. Thus, we opted not to take this approach.
Summary and Conclusions
The present studies found limited effects of BPA on the PND1 rat hypothalamus and hippocampus but provide further evidence that developmental BPA exposure, at levels below the NOAEL, can alter brain mRNA levels of ERs and OT. Altered Slc32a1 levels in the hypothalamus suggest that BPA may also alter aspects of GABA signaling. These data are consistent with the 2 CLARITY-BPA studies published to date, both of which found some but minimal evidence of BPA-related effects on hypothalamic and hippocampal-mediated behaviors (52, 53). That EE was not consistently masculinizing for all sexually dimorphic endpoints is inconsistent with decades of prior literature showing that estradiol is masculinizing in the rodent hypothalamus (95, 96), possibly indicating that EE does not fully recapitulate the neural actions of endogenous estrogen. Most basic neuroendocrine research into ER-mediated brain development has historically used either 17β-estradiol or estradiol benzoate via injection, because it is rapidly converted to estradiol (106). By contrast, toxicological studies preferentially use EE, because it can be orally administered and thus mimics human exposure routes to BPA and other EDCs. Alternatively, the lack of EE effects could be interpreted to indicate that neither dose was sufficient to induce full masculinization, or that the SD rat is insensitive to exogenously administered estrogens (as has been purported but debated for decades) (86, 107–109). Emerging data from the CLARITY-BPA studies will be informative for distinguishing between these possibilities and establishing the degree to which BPA impacts the development of other tissue targets and organ systems. Collectively, these data support prior conclusions that prenatal BPA exposure, even at doses below the current NOAEL, alters ER and OT gene expression in the developing brain.
Acknowledgments
We thank Dr K. Barry Delclos, Luisa Camacho, and their colleagues at National Center for Toxicological Research/Food and Drug Administration for their assistance with the conception, organization, and execution of the CLARITY-BPA projects; Thaddeus Schug and Retha Newbold of NIEHS for their leadership, guidance, and sage advice throughout the duration of this project; Jennifer Schaff, David (Andy) Baltzegar, and the staff of the North Carolina State University (NCSU) Genomic Sciences Laboratory for their assistance with cDNA library preparation and sequencing; and Dereje Jima of the NCSU Center for Human Health and the Environment at NCSU for his careful validation of the RNA-seq bioinformatics. The work of Keith Gonzales for data storage and preliminary analysis via Stirplate was invaluable and innovative as was the statistical and experimental design guidance provided by our terrific colleagues David Aylor and David Reif.
This study is part of the NIEHS CLARITY-BPA Consortium supported by NIEHS Grant U011ES020929 (to H.B.P.). The animal portion of this study is supported by NIEHS Interagency Agreement AES12013 (FDA IAG 224–12-0003).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- bw
- body weight
- CLARITY-BPA
- Consortium Linking Academic and Regulatory Insights on BPA Toxicity
- Ct
- cycle threshold
- EDC
- endocrine-disrupting compound
- EE
- ethinyl estradiol
- ER
- estrogen receptor
- FDA
- Food and Drug Administration
- GABA
- γ-aminobutyric acid
- GD
- gestational day
- Lepr
- leptin receptor
- NCSU
- North Carolina State University
- NCTR
- National Center for Toxicological Research
- NCTR-SD
- NCTR Sprague-Dawley
- NOAEL
- no-observed-adverse-effect level
- NTP
- National Toxicology Program
- OT
- oxytocin
- padj
- P value was adjusted for multiple testing using the Benjamini-Hochberg false discovery rate
- PND
- postnatal day
- Ptgds
- prostaglandin D2 synthase
- qRT-PCR
- quantitative real-time PCR
- RNA-seq
- RNA-sequencing.
References
- 1. Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–2841. [DOI] [PubMed] [Google Scholar]
- 3. Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gioiosa L, Palanza P, Parmigiani S, Vom Saal FS. Risk evaluation of endocrine-disrupting chemicals: effects of developmental exposure to low doses of bisphenol A on behavior and physiology in mice (Mus musculus). Dose Response. 2015;13:1559325815610760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mustieles V, Pérez-Lobato R, Olea N, Fernández MF. Bisphenol A: human exposure and neurobehavior. Neurotoxicology. 2015;49:174–184. [DOI] [PubMed] [Google Scholar]
- 6. Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol. 2008;83:157–395. [DOI] [PubMed] [Google Scholar]
- 7. FAO/WHO. Toxicological and Health Aspects of Bisphenol A: Report of Joint FAO/WHO Expert Meeting and Report of Stakeholder Meeting on Bisphenol A. http://whqlibdoc.who.int/publications/2011/97892141564274_eng.pdf: World Health Organization; 2011. [Google Scholar]
- 8. NTP. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. http://cerhr.niehs.nih.gov: National Institutes of Health No. 08-5994; 2008. [PubMed]
- 9. Food and Drug Administration. Bisphenol A (BPA): Use in Food Contact Application. http://www.fda.gov/NewsEvents/PublicHealthFocus/ucm064437.htm: Food and Drug Administration; 2012. [Google Scholar]
- 10. Beronius A, Rudén C, Håkansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of bisphenol A. Reprod Toxicol. 2010;29:132–146. [DOI] [PubMed] [Google Scholar]
- 11. Birnbaum LS, Bucher JR, Collman GW, et al. Consortium-based science: the NIEHS's multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ Health Perspect. 2012;120:1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schug TT, Heindel JJ, Camacho L, et al. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod Toxicol. 2013;40:35–40. [DOI] [PubMed] [Google Scholar]
- 13. Heindel JJ, Newbold RR, Bucher JR, et al. NIEHS/FDA CLARITY-BPA research program update. Reprod Toxicol. 2015;58:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schug TT, Blawas AM, Gray K, Heindel JJ, Lawler CP. Elucidating the links between endocrine disruptors and neurodevelopment. Endocrinology. 2015;156:1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. EFSA. Panel on food contact materials, enzymes, flavourings and processing acids (CEF). EFSA J. 2015;13:3978. [Google Scholar]
- 16. Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–1425. [DOI] [PubMed] [Google Scholar]
- 17. McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. [DOI] [PubMed] [Google Scholar]
- 19. Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–362. [DOI] [PubMed] [Google Scholar]
- 20. Kumar D, Thakur MK. Perinatal exposure to bisphenol-A impairs spatial memory through upregulation of neurexin1 and neuroligin3 expression in male mouse brain. PLoS One. 2014;9:e110482. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Farabollini F, Porrini S, Della Seta D, Bianchi F, Dessi-Fulgheri F. Effects of perinatal exposure to bisphenol A on sociosexual behavior of female and male rats. Environ Health Perspect. 2002;110(suppl 3):409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patisaul HB, Sullivan AW, Radford ME, et al. Anxiogenic effects of developmental bisphenol a exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PLoS One. 2012;7:e43890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sullivan AW, Beach EC, Stetzik LA, et al. A novel model for neuroendocrine toxicology: neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster). Endocrinology. 2014;155:3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Niu R, Zhu Y, et al. Changes in memory and synaptic plasticity induced in male rats after maternal exposure to bisphenol A. Toxicology. 2014;322:51–60. [DOI] [PubMed] [Google Scholar]
- 26. Kimura E, Matsuyoshi C, Miyazaki W, et al. Prenatal exposure to bisphenol A impacts neuronal morphology in the hippocampal CA1 region in developing and aged mice. Arch Toxicol. 2016;90:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110:9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tiwari SK, Agarwal S, Chauhan LK, Mishra VN, Chaturvedi RK. Bisphenol-A impairs myelination potential during development in the hippocampus of the rat brain. Mol Neurobiol. 2015;51:1395–1416. [DOI] [PubMed] [Google Scholar]
- 29. Adewale HB, Todd KL, Mickens JA, Patisaul HB. The impact of neonatal bisphenol-A exposure on sexually dimorphic hypothalamic nuclei in the female rat. Neurotoxicology. 2011;32:38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. [DOI] [PubMed] [Google Scholar]
- 31. Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. [DOI] [PubMed] [Google Scholar]
- 32. Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cao J, Joyner L, Mickens JA, Leyrer SM, Patisaul HB. Sex-specific Esr2 mRNA expression in the rat hypothalamus and amygdala is altered by neonatal bisphenol A exposure. Reproduction. 2014;147:537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rebuli ME, Cao J, Sluzas E, et al. Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus. Toxicol Sci. 2014;140:190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monje L, Varayoud J, Luque EH, Ramos JG. Neonatal exposure to bisphenol A modifies the abundance of estrogen receptor α transcripts with alternative 5′-untranslated regions in the female rat preoptic area. J Endocrinol. 2007;194:201–212. [DOI] [PubMed] [Google Scholar]
- 36. Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor α expression in nuclei controlling estrous cyclicity. Reprod Toxicol. 2010;30:625–634. [DOI] [PubMed] [Google Scholar]
- 37. Rebuli ME, Patisaul HB. Assessment of sex specific endocrine disrupting effects in the prenatal and pre-pubertal rodent brain. J Steroid Biochem Mol Biol. 2016;160:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cao J, Rebuli ME, Rogers J, et al. Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci. 2013;133:157–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 40. Andersen ME, Conolly RB, Faustman EM, et al. Quantitative mechanistically based dose-response modeling with endocrine-active compounds. Environ Health Perspect. 1999;4(suppl 107):631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol. 1998;54:105–112. [DOI] [PubMed] [Google Scholar]
- 42. Richter CA, Taylor JA, Ruhlen RL, Welshons WV, Vom Saal FS. Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007;115:902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145:592–603. [DOI] [PubMed] [Google Scholar]
- 44. Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H. Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol. 2000;73:1–10. [DOI] [PubMed] [Google Scholar]
- 45. Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor β in the female mouse hypothalamus. J Neuroendocrinol. 2003;15:787–793. [DOI] [PubMed] [Google Scholar]
- 46. Liang X, Wu L, Hand T, Andreasson K. Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem. 2005;92:477–486. [DOI] [PubMed] [Google Scholar]
- 47. Sakakibara M, Uenoyama Y, Minabe S, et al. Microarray analysis of perinatal-estrogen-induced changes in gene expression related to brain sexual differentiation in mice. PLoS One. 2013;8:e79437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCarthy MM, Auger AP, Bale TL, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479:237–247. [DOI] [PubMed] [Google Scholar]
- 50. Gasnier B. The SLC32 transporter, a key protein for the synaptic release of inhibitory amino acids. Pflugers Arch. 2004;447:756–759. [DOI] [PubMed] [Google Scholar]
- 51. Ptak A, Gregoraszczuk EL. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol Lett. 2012;210:332–337. [DOI] [PubMed] [Google Scholar]
- 52. Rebuli ME, Camacho L, Adonay ME, Reif DM, Aylor DL, Patisaul HB. Impact of low dose oral exposure to bisphenol A (BPA) on juvenile and adult rat exploratory and anxiety behavior: a CLARITY-BPA Consortium Study. Toxicol Sci. 2015;148:341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnson SA, Javurek AB, Painter MS, et al. Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: a CLARITY-BPA study. Horm Behav. 2016;80:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delclos KB, Camacho L, Lewis SM, et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol Sci. 2014;139:174–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Churchwell MI, Camacho L, Vanlandingham MM, et al. Comparison of life-stage-dependent internal dosimetry for bisphenol A, ethinyl estradiol, a reference estrogen, and endogenous estradiol to test an estrogenic mode of action in Sprague Dawley rats. Toxicol Sci. 2014;139:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255:261–270. [DOI] [PubMed] [Google Scholar]
- 57. Paxinos G. Atlas of the Developing Mouse Brain at E17.5, P0 and P6. 1st ed Amsterdam, Boston: Elsevier; 2007. [Google Scholar]
- 58. Paxinos G. Atlas of the Developing Rat Brain. San Diego, CA: Academic Press; 1991. [Google Scholar]
- 59. Auer PL, Doerge RW. Statistical design and analysis of RNA sequencing data. Genetics. 2010;185:405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. [DOI] [PubMed] [Google Scholar]
- 62. Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Derveaux S, Vandesompele J, Hellemans J. How to do successful gene expression analysis using real-time PCR. Methods. 2010;50:227–230. [DOI] [PubMed] [Google Scholar]
- 65. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 66. Nugent BM, Wright CL, Shetty AC, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61:201–210. [DOI] [PubMed] [Google Scholar]
- 68. Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 69. Ali S, Steinmetz G, Montillet G, et al. Exposure to low-dose bisphenol A impairs meiosis in the rat seminiferous tubule culture model: a physiotoxicogenomic approach. PLoS One. 2014;9:e106245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. [DOI] [PubMed] [Google Scholar]
- 71. Bowman RE, Luine V, Diaz Weinstein S, Khandaker H, DeWolf S, Frankfurt M. Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Horm Behav. 2015;69:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008;105:14187–14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Armoskus C, Moreira D, Bollinger K, Jimenez O, Taniguchi S, Tsai HW. Identification of sexually dimorphic genes in the neonatal mouse cortex and hippocampus. Brain Res. 2014;1562:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ivanova T, Beyer C. Ontogenetic expression and sex differences of aromatase and estrogen receptor-α/β mRNA in the mouse hippocampus. Cell Tissue Res. 2000;300:231–237. [DOI] [PubMed] [Google Scholar]
- 76. Buijs RM, Swaab DF. Immuno-electron microscopical demonstration of vasopressin and oxytocin synapses in the limbic system of the rat. Cell Tissue Res. 1979;204:355–365. [DOI] [PubMed] [Google Scholar]
- 77. Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. [DOI] [PubMed] [Google Scholar]
- 78. Sofroniew MV. Projections from vasopressin, oxytocin, and neurophysin neurons to neural targets in the rat and human. J Histochem Cytochem. 1980;28:475–478. [DOI] [PubMed] [Google Scholar]
- 79. Jirikowski GF, Sanna PP, Bloom FE. mRNA coding for oxytocin is present in axons of the hypothalamo-neurohypophysial tract. Proc Natl Acad Sci USA. 1990;87:7400–7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cohen H, Kaplan Z, Kozlovsky N, Gidron Y, Matar MA, Zohar J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J Neuroendocrinol. 2010;22:889–904. [DOI] [PubMed] [Google Scholar]
- 82. Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wolstenholme JT, Edwards M, Shetty SR, et al. Gestational exposure to bisphenol A produces transgenerational changes in behaviors and gene expression. Endocrinology. 2012;153:3828–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kundakovic M, Gudsnuk K, Herbstman JB, Tang D, Perera FP, Champagne FA. DNA methylation of BDNF as a biomarker of early-life adversity. Proc Natl Acad Sci USA. 2015;112:6807–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. [DOI] [PubMed] [Google Scholar]
- 86. Putz O, Schwartz CB, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat: II. Effects on male puberty and the reproductive tract. Biol Reprod. 2001;65:1506–1517. [DOI] [PubMed] [Google Scholar]
- 87. Sadowski RN, Park P, Neese SL, Ferguson DC, Schantz SL, Juraska JM. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicol Teratol. 2014;42:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Tyzio R, Represa A, Jorquera I, Ben-Ari Y, Gozlan H, Aniksztejn L. The establishment of GABAergic and glutamatergic synapses on CA1 pyramidal neurons is sequential and correlates with the development of the apical dendrite. J Neurosci. 1999;19:10372–10382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol. 1974;158:55–79. [DOI] [PubMed] [Google Scholar]
- 90. Khalaf-Nazzal R, Francis F. Hippocampal development - old and new findings. Neuroscience. 2013;248:225–242. [DOI] [PubMed] [Google Scholar]
- 91. Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. 2005;15:562–578. [DOI] [PubMed] [Google Scholar]
- 92. Maciejewska B, Lipowska M, Kowianski P, Domaradzka-Pytel B, Morys J. Postnatal development of the rat striatum–a study using in situ DNA end labeling technique. Acta Neurobiol Exp (Wars). 1998;58:23–28. [DOI] [PubMed] [Google Scholar]
- 93. Bourguignon JP, Franssen D, Gérard A, et al. Early neuroendocrine disruption in hypothalamus and hippocampus: developmental effects including female sexual maturation and implications for endocrine disrupting chemical screening. J Neuroendocrinol. 2013;25:1079–1087. [DOI] [PubMed] [Google Scholar]
- 94. Döhler KD. The pre- and postnatal influence of hormones and neurotransmitters on sexual differentiation of the mammalian hypothalamus. Int Rev Cytol. 1991;131:1–57. [DOI] [PubMed] [Google Scholar]
- 95. Wright CL, Schwarz JS, Dean SL, McCarthy MM. Cellular mechanisms of estradiol-mediated sexual differentiation of the brain. Trends Endocrinol Metab. 2010;21:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. [DOI] [PubMed] [Google Scholar]
- 97. Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors α and β and kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wolfe CA, Van Doren M, Walker HJ, Seney ML, McClellan KM, Tobet SA. Sex differences in the location of immunochemically defined cell populations in the mouse preoptic area/anterior hypothalamus. Brain Res Dev Brain Res. 2005;157:34–41. [DOI] [PubMed] [Google Scholar]
- 99. Kramer KM, Yoshida S, Papademetriou E, Cushing BS. The organizational effects of oxytocin on the central expression of estrogen receptor α and oxytocin in adulthood. BMC Neurosci. 2007;8:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wojcik SM, Katsurabayashi S, Guillemin I, et al. A shared vesicular carrier allows synaptic corelease of GABA and glycine. Neuron. 2006;50:575–587. [DOI] [PubMed] [Google Scholar]
- 101. Ogi H, Itoh K, Ikegaya H, Fushiki S. Alterations of neurotransmitter norepinephrine and γ-aminobutyric acid correlate with murine behavioral perturbations related to bisphenol A exposure. Brain Dev. 2015;37:739–746. [DOI] [PubMed] [Google Scholar]
- 102. Cardoso N, Pandolfi M, Lavalle J, et al. Probable γ-aminobutyric acid involvement in bisphenol A effect at the hypothalamic level in adult male rats. J Physiol Biochem. 2011;67:559–567. [DOI] [PubMed] [Google Scholar]
- 103. Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology. 2014;155:3853–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kanitz A, Gypas F, Gruber AJ, Gruber AR, Martin G, Zavolan M. Comparative assessment of methods for the computational inference of transcript isoform abundance from RNA-seq data. Genome Biol. 2015;16:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Liu S, Lin L, Jiang P, Wang D, Xing Y. A comparison of RNA-Seq and high-density exon array for detecting differential gene expression between closely related species. Nucleic Acids Res. 2011;39:578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cox NM, Ramirez JL, Matamoros IA, Bennett WA, Britt JH. Influence of season on estrous and luteinizing hormone responses to estradiol benzoate in ovariectomized sows. Theriogenology. 1987;27:395–405. [DOI] [PubMed] [Google Scholar]
- 107. Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–1786. [DOI] [PubMed] [Google Scholar]
- 108. Delclos KB, Weis CC, Bucci TJ, et al. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Putz O, Schwartz CB, Kim S, LeBlanc GA, Cooper RL, Prins GS. Neonatal low- and high-dose exposure to estradiol benzoate in the male rat: I. Effects on the prostate gland. Biol Reprod. 2001;65:1496–1505. [DOI] [PubMed] [Google Scholar]