Abstract
Pituitary somatotropes perform the key function of coordinating organismic growth and body composition with metabolic signals. However, the mechanism by which they sense and respond to metabolic signals via the adipokine leptin is unknown. The complex interplay between the heterogeneous cell types of the pituitary confounds the identification of somatotrope-specific mechanisms. Somatotropes represent 30%–40% of the anterior pituitary population and are derived from a lineage of cells that are activated by the Pit-Oct-Unc domain family domain class 1 transcription factor 1 (POU1F1) to produce GH, prolactin (PRL). and TSH. To determine the mechanism by which leptin controls somatotrope function, we used Cre-LoxP technology and fluorescence-activated cell sorting to purify and study control or leptin receptor-deleted (Lepr null) somatotropes. We report that Lepr-null somatotropes show significant reductions in GH protein (GH) and Gh mRNA. By contrast, enzyme immunoassays detected no changes in ACTH, LH, and FSH levels in mutants, indicating that the control of these hormones is independent of leptin signaling to somatotropes. Reduced TSH and PRL levels were also observed, but interestingly, this reduction occurred only in in Lepr-null somatotropes from mutant females and not from males. Consistent with the sex-specific reduction in Gh mRNA, TSH, and PRL, enzyme immunoassays detected a sex-specific reduction in POU1F1 protein levels in adult female Lepr-null somatotropes. Collectively, this study of purified Lepr-null somatotropes has uncovered an unexpected tropic role for leptin in the control of POU1F1 and all POU1F1-dependent hormones. This supports a broader role for somatotropes as metabolic sensors including sex-specific responses to leptin.
Anterior pituitary somatotropes are the most abundant of the pituitary cell types (1, 2). Their development, maturation, and function are vital for optimized growth and body composition (3, 4). To perform their function, somatotropes also have to be metabolic sensors, receiving signals from the metabolome about nutrition and fat stores (5). One important signal comes from the adipokine leptin, which is well known for its role in regulating appetite and metabolism (6).
Most somatotropes have leptin receptors (LEPRs) (7, 8), and we have recently reported studies of the significance of LEPR to somatotropes after their selective ablation with Cre-LoxP technology (7, 8). We reported that the selective loss of LEPR in somatotropes resulted in GH deficiency (7, 8), significant metabolic dysfunction (7, 9), and adult-onset obesity (7, 8). Our in vitro studies showed that the Lepr-null somatotropes had reduced receptors for GHRH and were poorly responsive to GHRH (10).
Thus, these studies (7–10) demonstrated the importance of leptin to somatotrope functions. However, the underlying mechanisms behind leptin actions were unknown. Such mechanistic studies require analyses of signaling pathways and regulatory factors, but these may also be shared by other pituitary cell types, This task is thus made difficult by the fact that the somatotropes are part of a mixed cell population. Indeed, for such mechanistic studies, pituitary cytophysiologists have long sought strategies to enrich or purify each hormone-bearing cell with approaches as diverse as unit gravity separation (11–16), counterflow centrifugation (17–20), electrophoretic mobility in the microgravity environment of space flight (13, 21), flow cytometry based on light-scattering properties (22, 23), or fluorescence-activated cell sorting (FACS) (24, 25). These purified populations could be grown in culture and were responsive to regulators of secretion (19, 26, 27), cell proliferation (28, 29), and ion channel activity (30–35).
In evaluating the many choices open to us for studies of our mutant Lepr-null somatotropes, we recognized that we were limited by their deficiencies. Indeed, our previous counterflow centrifugation studies required that the cells be responsive to GHRH (18), and, as reported above, the mutant somatotropes had reduced GHRH receptors (GHRHRs) (10). Therefore, we used a FACS approach after introducing a Cre-reporter transgene that rendered somatotropes fluorescent. The changes in the fluorescent mutant somatotropes were then compared with those seen in the nonfluorescent mutants (7, 8) to determine whether the recombination efficiency was reduced in the mutants because of the additional floxed Cre-reporter alleles (36).
Our original objective for this report was to describe the successful FACS purification protocol, showing that the somatotropes had high-enough yield and purity for assays of gene products. However, we also discovered novel findings for these purified populations. Our analysis of the products in the control somatotropes revealed that Gh mRNA, TSH and prolactin (PRL) were reduced in pure Lepr-null somatotropes from females. We also discovered a reduction in the Pit-Oct-Unc domain family domain class 1 transcription factor 1 (POU1F1) in the mutants, which provides an important clue to leptin functions because POU1F1 specifies the somatotrope, thyrotrope, and lactotrope lineage (37, 38). The report thus highlights a novel sex-dependent tropic role for leptin in the regulation of the somatotrope contribution to the POU1F1 lineage.
Materials and Methods
Animal models
Mutants lacking LEPR in somatotropes were produced by breeding mice bearing Cre recombinase driven by the rat GH promoter (rGHp-cre; from Rhonda Kineman, PhD [39]) with mice bearing floxed Lepr exon 1 (from Jeffery Friedman, PhD [40]) as detailed in our recent report (7). We introduced the Cre-reporter transgene by crossing control or mutant mice bearing rGhp-cre with mice bearing floxed tandem dimer Tomato (tdTomato)/enhanced green fluorescent protein (eGFP) driven by a promoter that targets membrane-bound tdTomato or eGFP (Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J; stock 007576; The Jackson Laboratory). In the absence of Cre recombinase, all cells express the red fluorescent tdTomato in their membranes. In cells producing Cre recombinase, floxed tdTomato is deleted, and the rGHp-cre-bearing cells exhibit green fluorescence (eGFP). To produce the deletion mutants, we developed a colony of double-floxed mice by breeding mice bearing two floxed alleles of the Cre reporter with mice bearing two floxed alleles of Lepr-exon 1. Their progeny were backcrossed to homogeneity, which was detected by tail snips and genotyping. Primers and genotyping for floxed LEPR are described in our recent study (7). The primers and genotyping protocol for floxed tdTomato-eGFP are found at the Jackson Laboratories web site.
The use of animals was approved by the University of Arkansas for Medical Sciences Animal Care and Use Committee annually. In all experiments, animals were killed before 9:00 am to avoid diurnal variations. Mixed-cycling females were used, although we tended to choose females in metestrus or diestrus. Trials showed that there were no differences in serum gonadotropins with the stage of the cycle in females taken in the morning. Controls included the following: 1) littermate controls bearing two floxed alleles of LEPR exon 1 and two floxed alleles of the Cre reporter but no Cre recombinase and 2) controls bearing rGhp-cre and two floxed alleles of the Cre reporter but wild-type LEPR.
The full validation of the cell specificity of our somatotrope Lepr-null deletion mutant model was reported previously (7, 8). Organ genotyping showed that excision was limited to the pituitary. Immunolabeling showed the reduction of LEPR proteins only in somatotropes and not in other pituitary cell types. Lepr-null somatotropes failed to respond to leptin by increasing phosphorylated signal transducer and activator of transcription 3.
Cell dispersion and FACS purification
Pituitary cells were dispersed with a technique that was modified from that used for rat or mouse pituitaries (8) to allow the production of single-cell populations. The modified protocol is as follows: individual mouse pituitaries are placed in 300 μL DMEM containing trypsin (stock 15 mg per 5 mL) and deoxyribonuclease (DNase; stock 1 mg per 0.5 mL), and the pituitary is broken up by moving it through a 26-gauge needle with a tuberculin syringe two times. The pieces are then incubated at 37°C for 20 minutes followed by centrifugation for 10 minutes at 1400 rpm. The trypsin-DNase solution is then removed without disturbing the pellet and replaced with 300 μL of dispersion solution (stock is 4 mL of DMEM + 200 μL trypsin inhibitor + 200 μL DNase). The pituitary pieces are dispersed through the syringe 15 times to produce single-cell suspensions. The cells are then centrifuged for 10 minutes, and the dispersion solution is replaced with DMEM for culture or FACS sorting solution if the cells are being separated.
For FACS separation, cells were dispersed as described in the above section and resuspended in FACS running buffer (PBS containing 15 mM HEPES, 1 mM EDTA, and 1% BSA). Samples are then placed on ice until the time of sorting (usually < 20 min). They are dispersed again just before sorting and then placed through a filter to screen out remaining cell clumps (BD Falcon; number 352235). The pituitary cells are sorted with a FACSAria machine (BD Biosciences) into 5-mL polystyrene tubes (coated with 4% BSA in 1× PBS) containing 200 μL of DMEM + ITS supplement (insulin, transferrin, and sodium selenite; Sigma). Immediately after sorting, each fraction is transferred to a 1.5-mL microcentrifuge tube and placed on ice. All samples are then centrifuged at 1400 rpm for 20 minutes at 4°C. The buffer is removed, and the samples are processed for protein or RNA or resuspended in the media to be plated and fixed. Cell counts from all FACSAria runs were recorded for future normalization purposes.
The FACSAria instrument was gated to sort tdTomato (emission wavelength range 600–620 nm) and eGFP-positive (emission wavelength of 505–525) cells. Control populations that contained no fluorescent cells were used to control for basal autofluorescence. Cre-negative tdTomato-bearing cells were used to set the gate for tdTomato fluorescence. Enrichment of somatotropes in the eGFP fraction was confirmed by immunolabeling 1-day cultures, enzyme immunoassays (EIAs) of protein extracts, and quantitative PCR (qPCR) assays of RNA extracts.
Validation of the eGFP population: immunocytochemistry, EIA, and qPCR
To analyze the purity of the FACS population in the paraformaldehyde-fixed cells, we used fluorescence immunocytochemistry and in situ hybridization for GH protein and mRNA. After overnight culture, cells from control and mutant mice were fixed in paraformaldehyde and immunolabeled for GH with antirat GH ([1:10 000 to 1:30 000] biotinylated goat antirabbit IgG [1:100] and streptavidin Alexa Fluor 350 (Life Technologies/ThermoFisher Scientific) detected the primary antibody [1:100]). After prehybridization protocols (10), streptavidin Alexa Fluor 350 (1:100) detected the biotinylated Gh-mRNA oligoprobes.
To validate the loss of LEPR in the somatotrope Lepr exon 1-null mutants, we detected leptin receptors on eGFP cells from both control and mutant (Lepr exon 1-null) mice with 1:1000 anti-LEPR (extracellular domain), followed by detection with biotinylated anti-IgG and streptavidin Dylight 405 (ThermoFisher Scientific) or Alexa Fluor 350. We had previously reported that labeling with this anti-LEPR was neutralized by preabsorption with the protein that made the antibody (8, 41).
To detect GH and GHRHRs, purified somatotropes were plated overnight and GH was detected as described in the previous section; only streptavidin Dylight 549 (red fluorescence) was used to detect the second antibody. To detect GHRHRs, the somatotropes were stimulated with biotinylated GHRH for 10 minutes followed by fixation in 4% paraformaldehyde. After four buffer washes, 1:100 streptavidin Dylight 549 was applied to detect the biotinylated GHRH. All cell counts were done either directly with the microscope or on photographs taken with an Olympus fluorescence microscope and digitized by Metamorph software. We counted a minimum of 100 cells/field and multiple (at least three) fields per coverslip. We averaged these counts from three coverslips per sample to get the final average. Our counts routinely evaluated 1200–2000 cells/group.
To detect pituitary hormones and other proteins, we modified our protein-extraction protocol as follows to produce higher protein yields from tdTomato and eGFP fractions taken from single pituitaries. Pelleted cells from each fraction were resuspended in 130 μL of RIA precipitation buffer containing protease inhibitors. The samples were homogenized with a handheld homogenizer and incubated overnight at 4°C. The next day, the samples were centrifuged at 4°C and 14 000 rpm for 20 minutes, and the protein concentrations were assayed by the DC protein assay (Bio-Rad Laboratories; catalog number 5000112). The protein extracts were either rapidly frozen as aliquots or used immediately in an EIA. Protein extracts of each fraction were assayed for all pituitary hormones with the Luminex platform and the Millipore Multiplex mouse pituitary hormone kit with a sample dilution of 1:2.5, as recommended by the manufacturer (7, 42). We assayed protein fractions for POU1F1 by EIA (MyBiosource.com; number MBS7207807).
To detect mRNAs, RNA extracts from tdTomato or eGFP fractions from individual pituitaries were prepared and assayed for mRNAs of all pituitary hormones by qPCR. The protocol has been published (42, 43), but we will repeat it briefly. For mRNA quantification, we used the Promega simplyRNA tissue kit for the Maxwell 16 platform to extract the RNA, and we used the iQ Supermix (Bio-Rad Laboratories) to make the cDNA. qPCR is performed with Power SYBR green master mix (Applied Biosystems) on the QuantStudio 12k Flex system (Applied Biosystems) with a 96-well block. Reported values are based on the δδcycle threshold method or relative quantitation as in references (42, 43). The sequence of all primer sets are reported in the tables in recent publications (41, 42).
Statistical analysis
Statistical analyses of mRNA and pituitary hormone levels were done on extracts from individual fractions from at least five mice. We used an ANOVA followed by Newman-Keul's and Bonferroni's post hoc tests (P < .05 was considered significant). Experiments with only two variables used a Student's t test.
We ran the cytochemical tests on two to three coverslips/fraction × five mice, counting 300 cells/coverslip or five photographic fields of 50 cells each. Numbers were determined by a post hoc power analysis. Our analysis of changes in the percentages of GH cells with a given treatment predicts that a significant increase from 26% of mixed pituitary cells in the presence of GHRH to 41% of pituitary cells with an SD of 8 and three replicate experiments will achieve a power of 0.946, with an α error level of .05 and a two-tailed t test.
Results
Cytochemical validation of the use of the Cre reporter
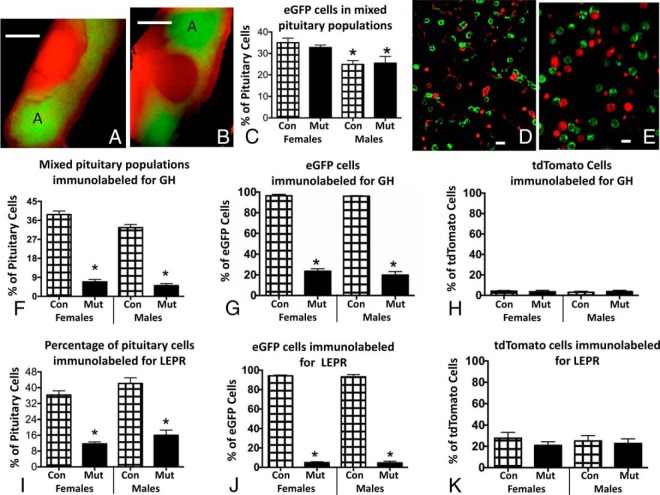
The first sets of experiments were designed to validate the use of the Cre reporter transgene. Figure 1 shows fluorescent images of whole pituitaries from control (Figure 1A) or somatotrope Lepr exon 1-null mutant (Figure 1B) mice. As expected, the green fluorescent eGFP cells were abundant in the anterior pituitary and not present in the neurointermediate lobes. The eGFP labeling in wild-type and somatotrope Lepr exon 1-null mutant pituitaries was similar; there was no apparent qualitative loss in eGFP cells from mutants. This qualitative assessment was confirmed by counts of eGFP cells in dispersed mixed pituitary cells in control and mutant male and female pituitaries. Figure 1C shows that both mutant and control females have higher percentages of eGFP cells than males. However, there were no differences when mutants and controls of either sex were compared. The images in Figure 1, D and E, depict these findings in female populations and show that the tdTomato or eGFP fluorescence is mainly confined to the cell periphery, as would be expected because the promoter drives expression of a membrane-bound tdTomato or eGFP.
Figure 1.
A and B, Whole female pituitaries from a control (A) and somatotrope Lepr-null mutant (B) showing expected concentration of eGFP cells (green fluorescence) in anterior pituitary lateral lobes (region A). The remaining cells are tdTomato positive. Note there are no eGFP cells in the neurointermediate lobe. Also, there is no loss of signal for eGFP in the pituitary from the mutant. Bar, 500 μm. C, Counts of eGFP-labeled cells in dispersed mixed anterior pituitary cells show no differences when controls and somatotrope Lepr-null mutants are compared. However, females have more eGFP cells than males. *, Lowest values (ANOVA, P = .04; n = 9 averages or 2000 cells/group). D and E, Images of dispersed pituitary cells from female control (D) or somatotrope Lepr-null mutant (E) show no differences between these groups in numbers of eGFP cells. Note fluorescence is concentrated at periphery of the cells. Bar, 10 μm. F, Counts of the overall percentages of immunopositive GH cells in mixed pituitary populations from control or mutant male or female mice show significant reductions in GH cells in mutants. *, Lowest values (ANOVA, P < .0001; Tukey's post hoc test; n = 9 averages or 1500–2000 cells/group). G, Analyses of the percentages of eGFP cells that are immunolabeled for GH in control or mutant pituitary cells show that 99% of eGFP cells are immunolabeled in controls but not mutant populations. *, Lowest values (ANOVA, P < .0001; Newman-Keul's; n = 9 averages or 1500–2000 cells/group). H, Counts of tdTomato cells with immunolabeling for GH show 3%–4% store GH and there are no differences seen among any of the experimental groups (ANOVA/Tukey's, P = .9; n = 9 averages or 1500–2000 cells/group). I, Counts of mixed pituitary cells immunolabeled for LEPR show significant reductions in the somatotrope Lepr-null mutants. *, Lowest values, ANOVA/Tukey's;. P < .0001; n = 3–9 averages or 1200–1500 cells/group). J and K, Analysis of percentages of eGFP cells immunolabeled for LEPR show a dramatic reduction in LEPR proteins in eGFP cells (J) but not in tdTomato cells (K). *, Lowest values (ANOVA/Tukey's, P < .0001; n = 3–9 averages or 1200–1500 cells/group). Con, control; Mut, mutant.
Counts of cells immunolabeled for GH in these mixed populations of pituitary cells showed that somatotropes are 30%–40% of the pituitary cell populations, which confirms percentages in our previous reports (8) (Figure 1F). These studies show similar percentages of GH cells in males and females. Most importantly, the counts also confirm previous studies that show a significant reduction in percentage of GH-immunopositive cells in somatotrope Lepr exon 1-null mutants, with a decrement in both males and females, similar to that seen in our previous studies of nonfluorescent mutants (7, 8). This reduction is illustrated in multiple images in previous publications (7, 8, 10).
When we expressed the counts as percentages of eGFP or tdTomato cells, nearly all control eGFP cells but only 3%–4% of tdTomato cells contain immunoreactive GH (Figure 1, G and H). These same figures show that the reduction in the percentages of GH cells seen overall in the somatotrope Lepr-null mutant mice (Figure 1G) is found only in the eGFP cells. tdTomato GH cells appear unaffected by the loss of LEPR. There are no sex differences in the expression of GH in eGFP or tdTomato cells.
We also immunolabeled these populations for LEPR, and the counts show that numbers of immunolabeled LEPR cells in the control male and female mixed pituitary populations were as reported in our previous studies of nonfluorescent mutants (Figure 1I) (7, 8). Also, as reported previously (8), the percentages of LEPR cells were reduced significantly in the somatotrope Lepr-null mutants by the expected decrement (Figure 1I). Images showing the loss in LEPR in somatotrope Lepr-null pituitaries are published (7, 8). Collectively these findings confirm our previous reports and show that the presence of the two additional floxed tdTomato alleles has not compromised the activity of Cre recombinase (7, 8).
When we expressed these counts as percentages of eGFP cells, they showed that most eGFP cells express LEPR in controls (Figure 1J). Furthermore, the reduction in LEPR was limited to the eGFP (somatotrope) fraction (Figure 1J). Percentages of LEPR cells in the tdTomato fraction were unaffected by the loss of LEPR in somatotropes (Figure 1K).
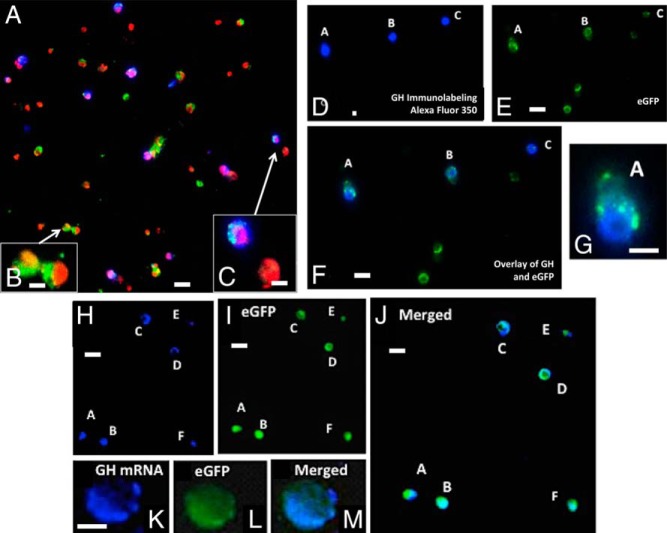
Figure 2 illustrates the labeling for GH proteins or Gh mRNA in mixed pituitary populations from the mutants. Whereas we illustrated the loss of GH stores in homozygous mutant Lepr-null somatotropes in three previous publications (7, 8, 10), in this report we deliberately chose fields from a heterozygous somatotrope Lepr-null mutant because our previous counts showed that GH stores are reduced by a lower amount (8). This strategy allowed us to show the reduction in GH cells and the variety of labeling patterns at a lower magnification in Figure 2, A–C. We were essentially able to include more cells in the fields with GH stores. Figure 2, A–F, also shows that a field containing eGFP cells in these heterozygous mutant animals include a mixture of cells with and without GH stores. Finally, in agreement with our previous study (10), Figure 2, H–M, show that most eGFP cells have Gh mRNA as detected by in situ hybridization. Counts of more than 1500 eGFP cells showed Gh mRNA labeling in 95% ± 2% of eGFP cells in mutants and controls.
Figure 2.
A, Immunolabeling for GH with Alexa Fluor 350 on a field of mixed pituitary cells from a heterozygous female mouse bearing rGHp-Cre, one floxed allele of Lepr exon 1, and the floxed tdTomato-eGfp Cre-reporter. After immunolabeling, the cells on the coverslip were mounted in media containing propidium iodide (red fluorescence; Vector Laboratories) to detect nuclei. Thus, the following color combinations can be seen: green, eGFP only; blue, GH only; cyan, GH and eGFP; orange/red, all nuclei and tdTomato cytoplasm; purple, GH labeling in cells with red nuclei. B, Cells bearing eGFP without GH labeling fluoresced green with orange/red nuclei. These two cells were not labeled for GH, as expected for mutant somatotrope Lepr-null populations. C, One of these cells shows that GH immunolabeling introduced blue fluorescence, which turned cyan, when overlaid with green. An overlay of blue GH immunolabeling also rendered the nuclei purple. The other cell is a red tdTomato cell that contains no GH. D–F, A field showing five eGFP cells from a mutant mouse illustrating the lack of GH stores in a subset of these cells, as reported previously (8). Three of the cells in this field show immunolabeling for GH (cells A, B, and C in field D or the overlay in field F). G, A higher magnification of cell A from this field. H–J, A field containing six eGFP cells from these mutants, which have been labeled for Gh mRNA with in situ hybridization. As reported previously, most of the somatotropes that are Lepr null have some labeling for Gh mRNA (10). This figure confirms these findings, showing Gh mRNA (blue fluorescence, Alexa-fluor 350; panel H) in all of the eGFP cells (panel I). The overlay of the two fields turns the labeling cyan (panel J). K–M, A higher magnification of a cell labeled for Gh mRNA, eGFP, and the overlay of both labels. Bar, 30 μm (panels A, D, E, F, H, I, and J); 10 μm (panels B, C, G, and K).
GH Content of FACS-separated eGFP fractions
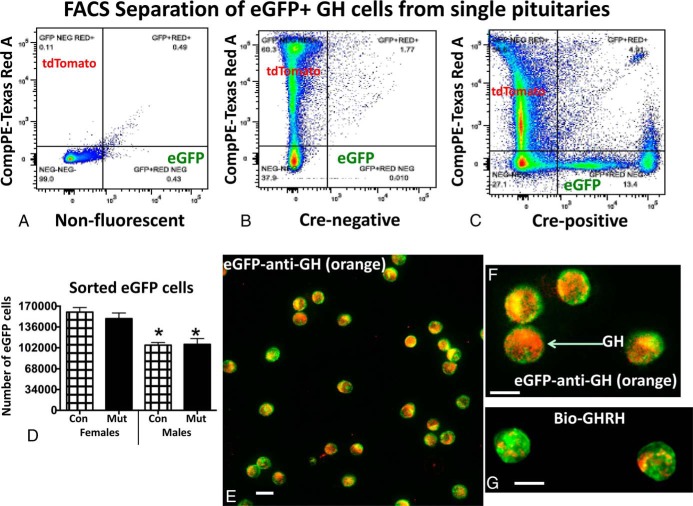
Once the eGFP cells were confirmed as somatotropes, we began FACS trials to purify them. Figure 3, A–C, compares the FACS output from the nonfluorescent pituitary (Figure 3A) with that from the non-Cre recombinase-bearing pituitary (Figure 3B), the latter showing gating for tdTomato. These figures are compared with the output from the Cre-bearing pituitary cells, which shows the sorting of the eGFP fraction and a clear separation between cells bearing eGFP and tdTomato (Figure 3C). Figure 3D graphs the average number of eGFP cells recovered from a single pituitary, which is 160 232 ± 7599 (n = 38 control females) or 106 383 ± 4168 (n = 28 control males). The sex differences are significant. In 66 different FACS runs, the yield from a single pituitary ranged from 42 000 to 250 000 cells. We consistently collected higher numbers of eGFP cells from control or mutant females compared with males. This correlates well with the sex differences seen in the overall counts of eGFP cells reported in Figure 1C. However, Figure 3C shows that there were no differences in numbers of eGFP cells collected when controls and mutants of either sex were compared (which also agrees with the counts shown in Figure 1C). Analysis of FACS fractions from 29 female pituitaries revealed that eGFP-bearing cells were 24.7% ± 1% (18 controls) or 24.9% ± 1.8% (11 mutants) of the total sorted cells. In 17 individually sorted male pituitaries, eGFP-bearing cells were 17% ± 0.9% (10 controls) or 17.8% ± 1% (seven mutants) of the total sorted cells.
Figure 3.
A–C, FACS report of the sorting of cells from nonfluorescent (A), Cre-negative fluorescent (B), and Cre-positive (C) fluorescent reporter mice. FACS is gated to separate tdTomato (532 nm, green laser) and eGFP (488 nm, blue laser) cells. Fluorescence strength is shown along the y- and x-axis for each of the fluorophores. D, Graph of average numbers of eGFP cells collected after FACS and show that females have a higher yield than males. Con, control; Mut, somatotrope Lepr-null mutant. *, Lowest values showing only a sex difference (ANOVA/Tukey's multiple comparisons test; n = individual fractions from 38 control females, 23 mutant females; 28 control males, 16 mutant males). E and F, eGFP fraction immunolabeled for GH with 1:30 000 anti-GH detected by biotinylated goat antirabbit IgG and streptavidin Dylight 549 (red-orange fluorescence). GH labeling is detected in most eGFP cells. G, eGFP cells were treated with biotinylated GHRH for 10 minutes and then fixed and labeled with streptavidin Dylight 549. Most eGFP cells show binding in peripheral patches at the periphery. Bar, 12 μm.
FACS sorting retrieves only single cells that have fluorescence above the threshold for detection by the machine. The overall final yield of FACS-sorted cells/pituitary (including both tdTomato and eGFP cells) ranged from 325 000 to 1.12 million cells/mouse pituitary. Total cells from control and mutant females were not different (controls yielded 888 098 ± 41 614 cells; n = 16 and mutants yielded 725 714 ± 56 747 cells; n = 16). However, the total average yield from control and mutant females was greater than that from control males (659 653 ± 43 427 cells, n = 17) (ANOVA/Tukey's, P = .01) but not from mutant males (748 759 ± 58 049 cells, n = 16).
To confirm the identity of the cells in the eGFP fraction, we immunolabeled fixed, plated eGFP cells for GH and labeled plated living cells for biotinylated GHRH. Most eGFP cells (1200–2000 cells/group) contain GH immunolabeling (Figure 3, E and F), which confirms data shown in the mixed cultures in Figure 1G. In addition, most of the 1200 eGFP cells counted (95%) bound biotinylated GHRH (illustrated in Figure 3G), indicating that they were fully differentiated. The biotinylated GHRH labeling may be seen in patches at the cell periphery in the controls.
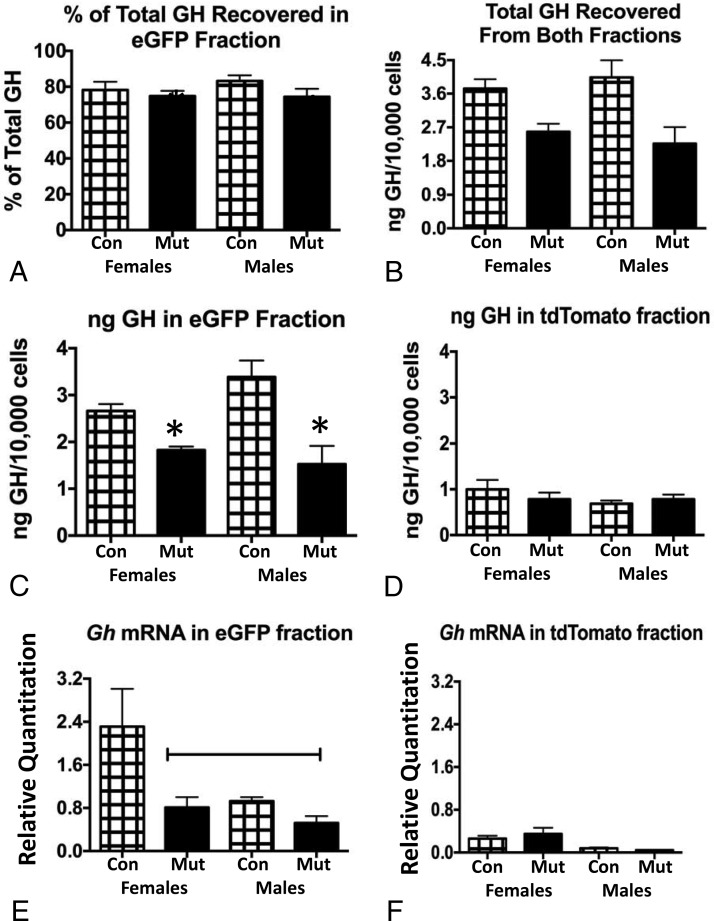
Protein extracts of each fraction taken from single pituitaries (n = 5/experimental group) were then used in multiplex EIAs to assay GH content. We first determined the total amount of GH recovered in both fractions by adding the values from eGFP and tdTomato fractions together. Figure 4A shows that there was a significant overall reduction in total GH in deletion mutant (somatotrope Lepr-null) females and males. This correlates well with the reduced serum GH reported for these mutants in our previous studies (7, 8). Similarly, it compares favorably with our previous report of reduced secreted GH in mutant Lepr-null somatotropes (10) and the reports showing reduced percentages of GH cells in the deletion mutants (7, 8, 10). Figure 4B shows the percentage of total GH that was found in eGFP fractions. Despite the reduction in the mutant fractions, the percentage of total GH in eGFP fractions from all groups was similar (∼80% of the total GH). When the average amount of GH recovered in the eGFP fractions (Figure 4C) was compared with that from tdTomato fractions (Figure 4D), the reduction in GH seen overall (Figure 4A) was evident only in eGFP fractions. GH protein levels were reduced in the somatotrope Lepr-null mutant eGFP fraction by 35% in females and by 50% in males. Although the data from Figure 4, C and D, are displayed on separate graphs, they were analyzed by an ANOVA in the same spreadsheet. Values shown in Figure 4D are lower than all other values, including those from the eGFP mutant fractions (ANOVA/Newman-Keuls; P < .0001; n = 4–5).
Figure 4.
A, Total GH recovered in both eGFP and tdTomato fractions showed that GH proteins are reduced significantly in both male and female somatotrope Lepr-null mutants (Mut) compared with controls (Con). *, Lowest values (ANOVA/Newman-Keuls, P = .008; n = 5/group). B, The percentage of the total GH recovered in eGFP fractions is similar in all experimental groups (ANOVA), ranging from 74% to 83% of the total GH. C, Reduced GH protein levels in eGFP fractions from the Lepr-null somatotrope fractions (Mut) compared with controls. *, Lowest values (ANOVA/Newman-Keuls, P = .001; n = 5/group). D, No changes in GH proteins recovered from tdTomato fractions when comparing males, females, or controls and mutants. E, Gh mRNA is highest in eGFP fractions from females, significantly different from mutants and both groups of males (line indicates lowest values, ANOVA/Newman-Keuls, P < .0001, n = 5–8/group). F, Gh mRNA is low in tdTomato fractions. An ANOVA shows no significant difference between males, females, or controls and mutants.
mRNA was extracted from additional groups of fractions for qPCR assays, each representing a single pituitary. eGFP fractions from control females have the highest Gh mRNA levels when compared with mutant females or both groups of males (Figure 4E). Levels of Gh mRNA were not different when control and mutant males were compared. Gh mRNA in tdTomato fractions is relatively low (Figure 4F), and tdTomato fractions showed no differences in the expression of Gh mRNA comparing males and females or controls and mutants (Figure 4F). The analyses from Figure 4, E and F, were run on the same spreadsheet, and all values for Figure 4F were lower than all other values (ANOVA/Newman-Keuls; P < .0001; n = 5–11).
Assays of all pituitary hormones show relatively high expression of PRL and TSH by FACS-purified somatotropes
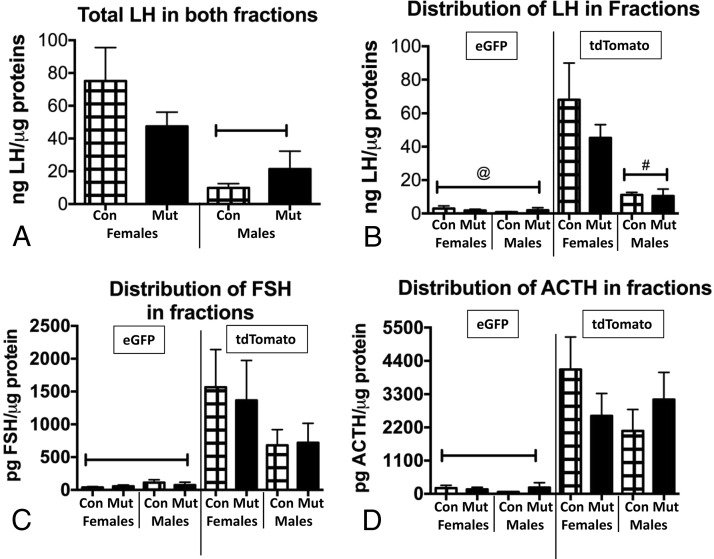
Multiplex EIAs detected the distribution of all pituitary hormones in the same protein extracts reported in Figure 4. The LH assays showed sex differences in the total LH content assayed in both fractions (Figure 5A) but no significant differences between controls and mutants. The distribution of LH in eGFP and tdTomato fractions showed that it was barely detectable in eGFP fractions and that most LH was found in the tdTomato fraction, with higher levels in females than males (Figure 5B). There were no differences in LH in either fraction when controls and mutants were compared. With respect to FSH and ACTH, levels were also barely detectable in eGFP fractions (Figure 5, C and D), and there were no differences between males and females or controls and mutants in the distribution of FSH or ACTH in either fraction. The content assays of LH, FSH, and ACTH correlate well with previously reported assays of serum levels of these three hormones, which show no differences when controls and mutants were compared (7).
Figure 5.
A, Multiplex EIAs of LH show sex differences in total LH in both fractions, with females having higher LH levels. Capped bar, lowest values (ANOVA/Newman-Keuls, P = .01; n = 4–5). B, Distribution of LH across eGFP and tdTomato fractions of controls (Con) and somatotrope Lepr-null mutants (Mut) showing no difference in fractions from mutants. LH is barely detectable in eGFP fractions, and the sex difference is seen in the tdTomato fractions. @, Values significantly lower than all others; #, values lower than control and mutant females (ANOVA/Newman-Keuls, P < .0001; n = 4–5). C, Distribution of FSH in eGFP vs tdTomato fractions shows no sex differences or differences between controls (Con) and mutants (Mut). FSH is barely detectable in eGFP fractions. Capped bar, Lowest values by ANOVA/Newman-Keuls (P = .0059; n = 4–5). D, ACTH distribution is similar to that of FSH with no sex differences or differences between controls and mutants. It is also barely detectable in the eGFP fractions. Capped bar, Lowest values by ANOVA/Newman-Keuls (P = .0002; n = 4–5).
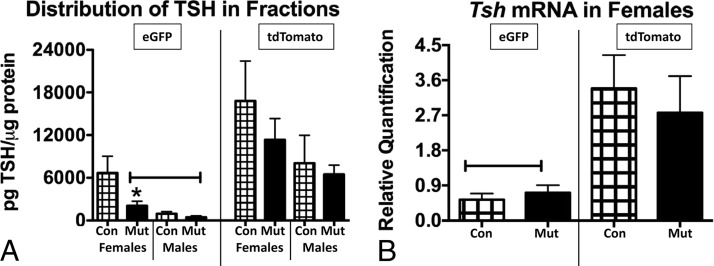
Assays of total TSH protein levels (obtained from adding values from eGFP and tdTomato fractions) showed no significant differences between males and females or controls and mutants. This correlates with the serum TSH levels showing no changes in the mutants (7), although serum TSH levels in males are greater than those in females. When the distribution of TSH in the two fractions was compared, Figure 6A showed greater recovery overall in the tdTomato fractions from both males and females when compared with eGFP fractions, as detected by an ANOVA/Newman-Keuls. The analysis showed that 24% of the total TSH proteins was recovered in control female eGFP fractions. Furthermore, TSH content levels in female mutant eGFP fractions were significantly reduced when compared with control female eGFP fractions. This change prompted qPCR assays of mRNA extracts of female eGFP and tdTomato fractions. The assays showed that Tsh mRNA levels are significantly higher in tdTomato fractions when compared with eGFP fractions (Figure 6B). However, eGFP fractions do contain Tsh mRNA. There are no differences in Tsh mRNA levels when controls and mutants from either fraction are compared.
Figure 6.
A, Distribution of TSH across the two fractions (eGFP and tdTomato) shows that TSH is lowest in eGFP fractions from mutant females or both groups of males. There are no differences when the remaining groups are compared. Capped bar, Lowest values, different from all others (ANOVA/Newman-Keuls, P = .0037; n = 4–5). *, Significantly different from control eGFP fraction (Student's t test, P = .047). B, Analysis of Tsh mRNA distribution in females shows significantly higher expression in tdTomato fractions and no differences between controls and mutants. Capped line, Lowest values by ANOVA/Newman-Keuls (P < .0001; n = 10–15). Con, control; Mut, somatotrope Lepr-null mutant.
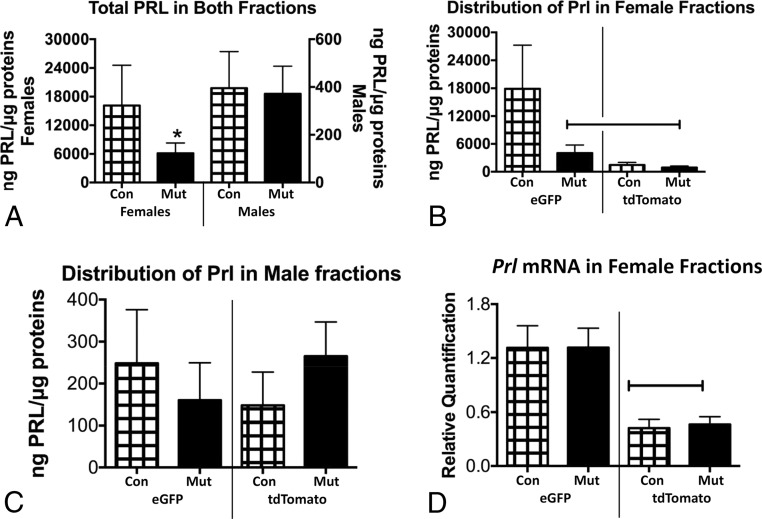
When PRL levels from eGFP and tdTomato fractions were assayed, the levels of total PRL were much higher in females compared with males (note the different y-axes ranges in Figure 7A). Because of the sex difference in overall PRL content levels, we plotted the distribution of PRL on separate graphs for males and females, although we analyzed all groups in the same spreadsheet by an ANOVA. Figure 7B shows that eGFP fractions from female somatotrope Lepr-null mutants contained lower levels of PRL than controls. Levels in female tdTomato fractions were also lower than control eGFP levels. In contrast, Figure 7C shows that, in males, there were no differences in PRL levels between the eGFP and tdTomato fractions or between the controls and mutants. Finally, the reduced PRL in female mutant eGFP fractions stimulated qPCR assays of Prl mRNA. Figure 7D shows that ablation of LEPR in female somatotropes did not affect Prl mRNA levels in either eGFP or tdTomato fractions.
Figure 7.
A, EIAs of total PRL show significant sex difference in overall levels, comparing females (left, y-axis) with males (right, y-axis) and an overall reduction of PRL only in somatotrope Lepr-null females. *, Lowest value by ANOVA/Newman-Keuls (P = .04; n = 4–5). B, Distribution of Prl across eGFP and tdTomato fractions shows that eGFP fractions from control females have significantly more Prl than tdTomato fractions. Mutant eGFP fractions store significantly less Prl, however. Capped line, Lowest values by ANOVA/Newman-Keuls (P = .038; n = 4–5). C, Distribution of Prl in male fractions shows no differences between controls and mutants or between eGFP and tdTomato fractions (ANOVA, P = .75). D, Distribution of Prl mRNA in female fractions shows that eGFP fractions from mutants have the highest levels compared with tdTomato fractions. There are no differences between controls and mutants for either fraction. Capped line, Lowest values by ANOVA/Newman-Keuls (P = .0002; n = 4–5). Con, control; Mut, somatotrope Lepr-null mutant.
POU1f1: a target of leptin in the pituitary
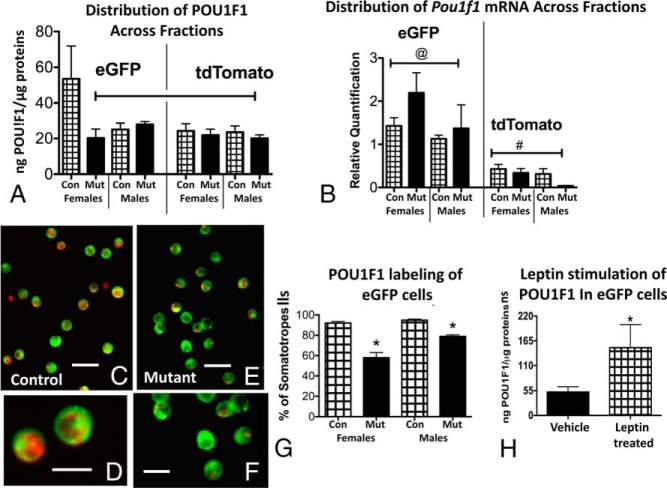
The findings for TSH and PRL in females suggested that the eGFP cells included multihormonal cells from the lineage that expresses the POU1F1 transcription factor. These cells may function in paradoxical secretion, may represent cells undergoing transdifferentiation, or may represent a more primitive progenitor cell population (1, 37, 38, 44–46). Therefore, we applied three independent tests to determine whether POU1F1 was present in the eGFP fraction and whether it could be a leptin target. If it is a leptin target, we reasoned that this transcription factor might be reduced in mutant somatotropes lacking LEPRs. Figure 8A shows that POU1F1 is expressed by the eGFP cells as well as tdTomato cells, with the highest expression in control females. Expression was reduced only in eGFP cells from female mice lacking LEPR in somatotropes. Expression in eGFP cells from control and mutant males was similar to that of tdTomato cells. Figure 8B shows that Pou1f1 mRNA expression was highest in eGFP cells when compared with tdTomato cells, but it appeared unaffected by the ablation of LEPR in somatotropes. There are also no sex differences in the expression of Pou1f1 mRNA.
Figure 8.
A, POU1F1 EIAs were applied to protein fractions from all experimental groups. The highest values were seen in eGFP fractions from control (Con) females. eGFP fractions from somatotrope Lepr-null females showed reduced POU1F1 levels. eGFP fractions from control and mutant (Mut) males were not different from one another. Similarly, POU1F1 levels in tdTomato fractions were not different when controls and mutants or males and females were compared. Capped line, Lowest values, not different from one another but different from control female eGFP levels (ANOVA/Newman-Keuls, P = .02; n = 4–5). B, Distribution of Pou1f1 mRNA in all experimental groups showing highest levels in all eGFP fractions. There are no differences between males and females, nor are there sex differences. @, Highest values, not different from one another; #, lowest values (ANOVA/Newman-Keuls, P < .0001; n = 4–5). C and D, Immunolabeling for POU1F1 in control eGFP cells showing red fluorescent labeling in nearly 100% of the cells. E and F, eGFP cells from somatotrope Lepr-null mutants showing reduced red fluorescent POU1F1 labeling. Bar, 30 μm (panels C and E); bar, 15 μm (panels D and F). G, Counts of eGFP cells labeled with POU1F1 (1500 cells) show a significant 40% reduction in eGFP cells from females and 16% in eGFP cells from males. *, Lowest values (ANOVA/Newman-Keuls test, P < .01; n = 9). H, eGFP cells from females were stimulated for 3 hours with leptin (10 nM) and extracts assayed for POU1F1. EIAs showed a 3-fold increase in POU1F1 expression in eGFP cells. *, Significantly different from vehicle control (P = .037; n = 4–5; Student's t test).
The second group of tests involved immunolabeling for POU1F1 in paraformaldehyde-fixed populations of eGFP cells. Figure 8, C and D, show the red immunofluorescence for POU1F1 found in nearly all eGFP cells. However, in contrast, Figure 8, E and F, show that the fluorescent labeling for POU1F1 is reduced in eGFP cells from somatotrope Lepr-null mutants. Figure 8G graphs the counts of 1500 cells showing that nearly 100% of eGFP cells in males or females contain labeling for POU1F1. This immunolabeling is reduced in somatotrope Lepr-null mutants by 40% in cells from females and only 16% in males. Finally, our third group of tests involved stimulation of eGFP cells from control females with leptin for 3 hours (10 nM). We would thus also demonstrate that pure somatotropes were responsive to leptin, which would further validate the cellular model. Protein extracts from vehicle and leptin-stimulated cells were then assayed by an EIA for POU1F1. Figure 8H shows that POU1F1 levels were three times higher in extracts from leptin-treated cells.
Discussion
This study met its original objective, which was to develop and validate a purification strategy for somatotropes to allow us to study leptin-modulated regulatory mechanisms. However, when we combined this efficient purification protocol with state-of-the-art multiplex assays, we opened the door to new discoveries about leptin regulation of somatotropes, especially in females. These findings identified sex differences in the expression of new targets for leptin (Gh mRNA, TSH, PRL, and POU1F1), which can now be integrated into the information about the phenotype of the somatotrope Lepr-null mouse model, which was first presented in 2011 (8). This animal model is distinguished by obesity (7, 8), GH deficiency (7, 8), lack of responses to GHRH (10), and metabolic dysfunction (7, 9). The reduction in the POU1F1, TSH, and PRL content of somatotropes may contribute more broadly to the metabolic dysfunction.
In our purification of somatotropes, we used an efficient, robust transgenic model that exploits the fact that these mutant cells continue to express the rGh promoter, which drives Cre recombinase. Somatotropes are thereby identified and can be sorted by their eGFP fluorescence. We used multiple cytochemical approaches in mixed and pure populations to confirm that greater than 98% of the eGFP population were GH cells. We thus confirmed the fidelity of the rGHp-cre transgene, which was originally validated by Luque et al (39). In addition, this Cre reporter produced more eGFP cells in females than males. This was unexpected because most reports show that males have slightly more GH cells than females by routine immunolabeling. In the present study, the percentages of GH cells in the pituitaries of control males and females were not different, however. Also, there are no differences in the non-eGFP GH cells when males and females are compared. This may reflect the fact that males have fewer cells in the pituitary overall.
Additional findings validated the mutant model further. We showed that the presence of four floxed alleles in the mutants did not alter the efficiency of Cre recombinase because the mutants showed reductions in GH and LEPR that were similar to those reported in our previous studies of nonfluorescent mutants (7, 8, 10). Also, the reduction in LEPR is limited to eGFP cells, which confirms the cell type specificity reported by our previous studies (7, 8, 10).
The approach used in the present study, although not new (24, 25), was essential to overcome the GHRHR deficit (10) that would normally facilitate GHRH-dependent purification (18). Our approach was particularly advantageous because it allowed the rapid sorting of cells from single pituitaries (10 min/pituitary), resulting in cell yields that were sufficient for multiplex assays of protein extracts. Our previous counterflow centrifugation protocol for purified somatotropes was an all-day, two-step protocol that required cells from at least five rats or 10 mice (5 million to 10 million cells) (18). In two hours we can obtain somatotropes from individual mice, comparing five controls and five mutants in the same run, which saves both time and animal costs.
A small subset of GH cells did not express eGFP and remained with the tdTomato fraction. When we applied the percentages of GH cells expressing only tdTomato (Figure 1) to the average number of tdTomato cells recovered by FACS, the resulting cell numbers were 2.0%–2.4% of the total number of cells sorted or 12%–13% of total somatotropes recovered by FACS. These non-eGFP-expressing GH cells may be a progenitor or reserve population, although their content of GH as determined by EIA of tdTomato fractions suggest that they contain a potentially significant source of GH. They could be quiescent cells, which store GH but do not have sufficient receptivity to release their stores. If they are quiescent cells, future studies may determine whether they could be sorted with their somatotrope counterparts after stimulation by GHRH or ghrelin. Pioneering studies of secretion from individual somatotropes in reverse hemolytic plaque assays show significant heterogeneity in the amount of GH secreted, indicating different states of readiness (47, 48).
Alternatively, these non-eGFP-containing somatotropes could be somatogonadotropes, which do not express significant amounts of GHRHR except during proestrus (49). Luque et al (39) also reported that a small percentage (<1%) of their immunolabeled GH cells did not express the Cre reporter and suggested that either expression was below the detection of the assay system or that select somatotropes can inactivate the Cre- or floxed Cre-reporter transgenes. Similarly, Behringer et al (50) reported that less than 1% of GH cells survived in mice bearing a diphtheria-toxin transgene that was activated by Cre-GH and hypothesized that this diphtheria-toxin-resistant subset of GH cells may have inactivated the transgene.
Our use of the state-of-the-art multiplex assays with these fractions provided opportunities to detect multiple gene products from the same protein or mRNA sample. As expected, FACS-purified somatotrope fractions from either males or mixed cycling females had barely detectable LH, FSH, and ACTH, (Figure 5, A–F) and males had relatively low TSH (Figure 6). Furthermore, there were no changes in these hormones in either the tdTomato or eGFP fractions from the somatotrope Lepr-null mutants, which shows that the loss of LEPR in somatotropes has not affected these other cell types.
In addition, the analysis of gene products in the purified somatotropes from Lepr-null mutants expanded our understanding of leptin targets and leptin signaling pathways in somatotropes. Our previous in situ hybridization studies of mixed mutant pituitary populations had shown no reduction in percentages of GH cells labeled for Gh mRNA (10), which led to the hypothesis that leptin signaling pathways mostly involved posttranscriptional regulation. However, in the present study, the qPCR assays showed clearly that Gh mRNA in the eGFP fraction from female somatotrope Lepr-null mutants was reduced. The counts of in situ hybridization-labeled cells (10) did not account for net changes overall caused by reduced levels of expression/cell, which were obviously detected by the sensitive qPCR assays. Thus, we have altered our hypothesis to state that leptin may directly or indirectly activate transcriptional pathways in female somatotropes. Collectively the lower GH and Gh mRNA in eGFP fractions correlate well with the reduced serum GH and lower responses to GHRH reported in previous studies of these mice (7, 8, 10).
This hypothesis is now supported further in females by our discovery that POU1F1 is reduced in pure Lepr-null somatotropes. POU1F1 is a critical transcriptional activator of Gh mRNA. The data from the mutants suggest that POU1F1 is a leptin target, which was supported when we detected a 3-fold rise in POU1F1 proteins after leptin stimulation. This strengthens the hypothesis and suggests that leptin may regulate Gh mRNA through the stimulation of POU1F1. However, in males, the data showing leptin regulation of POU1f1 is mixed because we showed a slight (16%) reduction in immunolabeled POU1F1 in Lepr-null somatotropes but no changes in POU1F1 proteins by an EIA. This may reflect sex differences in leptin signaling pathways leading to Gh mRNA transcription or translation.
The fact that POU1F1 protein levels are reduced in deletion-mutant females but Pou1f1 mRNA levels are not provides further clues about leptin-regulated pathways and suggests that leptin actions on POU1F1 protein production may be posttranscriptional. Further tests of leptin regulation of POU1F1 and the identification of mediators in this pathway are beyond the purview of this report, which was designed to present and validate the model. However, future studies of this regulatory pathway will certainly benefit from the new cellular model described in the present report.
The discoveries related to POU1F1 in female somatotropes may correlate with the reduced TSH and PRL in the Lepr-null somatotropes because POU1F1 is an important transactivation factor for both Tsh and Prl mRNAs (37, 38). However, at this point, it is not clear whether the reduced POU1F1 causes the reduction in TSH and PRL because neither Tsh nor Prl mRNA levels are reduced in female Lepr-null somatotropes. These data suggest that leptin regulation of TSH and PRL in somatotropes may be posttranscriptional; however, the pathway has yet to be discovered.
The discovery of TSH and PRL in somatotropes is not a new finding and may relate to early studies of somatotrope transdifferentiation. As early as 1990, studies of rodents and humans have reported that somatotropes could transdifferentiate into thyroidectomy cells (thyrotropes) to support the thyroid in a hypothyroid condition (51–53). Thus, the relatively small amount of TSH in pure somatotropes from females may reflect this potential. Early studies have also described a subset of mammosomatotropes, especially in females (45, 54–56). A number of other workers have reported the presence of multihormonal somatotropes on the basis of their expression of other pituitary hormones or receptors for multiple neuropeptides (reviewed in references 1, 44, and 45).
Our studies agree with Strathmann et al (57), who reported that, even when the protein extracts came formalin-fixed tissues, the multiplex EIA detected hormones better than immunocytochemistry. When eGFP content measurements and serum assays (7) were compared, reduced GH content in Lepr-null somatotropes correlated with reduced serum GH (7, 8, 10). Similarly, except for TSH and PRL, content and serum levels of other hormones were comparable (7). It is interesting to note that the reduced PRL and TSH levels in eGFP cells and the reduced PRL in tdTomato cells has not compromised normal serum levels of PRL or TSH (7).
To summarize, this study reports a successful, efficient purification strategy for somatotropes because it highlights important benefits of using purified pituitary cell populations to test gene products. The study reminds us of the fact that somatotropes are heterogeneous (1, 44, 45), which is a caveat for those who seek to purify only monohormonal somatotropes.
Furthermore, the purification of mutant Lepr-null somatotropes has uncovered new and unexpected molecular targets for leptin signaling, opening the door to future tests of leptin transcriptional and translational regulatory pathways. We propose that the production of purified somatotropes will provide an excellent cellular model for future studies of the integrated regulatory circuits that govern somatotrope function and multihormonal expression.
Acknowledgments
We thank the Hormone Distribution Program and Dr A. F. Parlow for the antirat GH antiserum and Andrea Harris and the Flow Cytometry Laboratory for their outstanding help and service.
The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported in part by National Institutes of Health (NIH) Grant R01 HD059056 (to G.V.C.); NIH Grant R03HD082793 (to G.V.C. and A.M.M.); NIH Grant R01HD087057 (to G.V.C. and A.M.M.); Sturgis Charitable Trust; pilot grants from the National Institute of General Medical Sciences (NIGMS) IDeA Program Award P30 GM110702 (to G.V.C. and A.M.M.); the Arkansas Breast Cancer Research Program (to A.M.M.); and intramural funding support from the University of Arkansas for Medical Sciences College of Medicine Research Council (to A.M.M.). The FACS Core was supported in part by NIH-National Center for Research Resources Grant UL1TR000039; NIH-NIGMS Grant P30GM11070; and the Center for Microbial Pathogenesis and Host Inflammatory Responses NIH-NIGMS Grant P20GM103625 through the NIH NIGMS-Centers of Biomedical Research Excellence.
Disclosure Summary: The authors have nothing to disclose.
Appendix
See Table 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| LEPR | Extracellular domain | Anti-LEPR | Affinity BioReagents | Rabbit, polyclonal | 1:1000 |
| GH | Rat GH | A. F. Parlow, Hormone Distribution Program | Rabbit, polyclonal | 1:10 000–1:30 000 | |
| Pit-1 | Pit-1 antibody (X-7) | Santa Cruz Biotechnology | Rabbit, polyclonal | 1:1000 |
Footnotes
- DNase
- deoxyribonuclease
- eGFP
- enhanced green fluorescent protein
- EIA
- enzyme immunoassay
- FACS
- fluorescence-activated cell sorting
- GHRHR
- GHRH receptor
- LEPR
- leptin receptor
- POU1F1
- Pit-Oct-Unc domain family domain class 1 transcription factor 1
- PRL
- prolactin
- qPCR
- quantitative PCR
- tdTomato
- tandem dimer Tomato.
References
- 1. Childs GV. Growth hormone cells as co-gonadotropes: partners in the regulation of the reproductive system. Trends Endocrinol Metab. 2000;11:168–175. [DOI] [PubMed] [Google Scholar]
- 2. Childs GV, Unabia G, Wu P. Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. [DOI] [PubMed] [Google Scholar]
- 3. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev. 2006;27:101–140. [DOI] [PubMed] [Google Scholar]
- 4. Veldhuis JD, Roemmich JN, Richmond EJ, et al. Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev. 2005;26:114–146. [DOI] [PubMed] [Google Scholar]
- 5. Luque RM, Gahete MD, Cordoba-Chacon J, Childs GV, Kineman RD. Does the pituitary somatotrope play a primary role in regulating GH output in metabolic extremes? Ann NY Acad Sci. 2011;1220:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. [DOI] [PubMed] [Google Scholar]
- 7. Allensworth-James M, Odle AK, Haney A, Childs GV. Sex differences in somatotrope dependency on leptin receptors in young mice: ablation of LEPR causes severe growth hormone deficiency and abdominal obesity in males. Endocrinology. 2015;156:3253–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Childs GV, Akhter N, Haney A, et al. The somatotrope as a metabolic sensor: deletion of leptin receptors causes obesity. Endocrinology. 2011;152:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Akhter N, Odle AK, Allensworth-James ML, et al. Ablation of leptin signaling to somatotropes: changes in metabolic factors that cause obesity. Endocrinology. 2012;153:4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Syed M, Cozart M, Haney AC, et al. Ghrelin restoration of function in vitro in somatotropes from male mice lacking the Janus kinase (JAK)-binding site of the leptin receptor. Endocrinology. 2013;154:1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Denef C, Hautekeete E. Parallel changes in the plasma concentration of follicle-stimulating hormone and pituitary 5alpha-dihydrotestosterone formation after cryptorchidectomy in the rat. J Endocrinol. 1978;77:279–280. [DOI] [PubMed] [Google Scholar]
- 12. Denef C, Follebouckt JJ. Differential effects of dopamine antagonists on prolactin secretion from cultured rat pituitary cells. Life Sci. 1978;23:431–435. [DOI] [PubMed] [Google Scholar]
- 13. Hymer WC, Barlow GH, Blaisdell SJ, et al. Continuous flow electrophoretic separation of proteins and cells from mammalian tissues. Cell Biophysics. 1987;10:61–85. [DOI] [PubMed] [Google Scholar]
- 14. Hymer WC, Evans WH, Kraicer J, Mastro A, Davis J, Griswold E. Enrichment of cell types from the rat adenohypophysis by sedimentation at unit gravity. Endocrinology. 1973;92:275–287. [DOI] [PubMed] [Google Scholar]
- 15. Leuschen MP, Tobin RB, Moriarty CM. Enriched populations of rat pituitary thyrotrophs in monolayer culture. Endocrinology. 1978;102:509–518. [DOI] [PubMed] [Google Scholar]
- 16. Scheikl-Lenz B, Markert C, Sandow J, Trager L, Kuhl H. Functional integrity of anterior pituitary cells separated by a density gradient. Acta Endocrinol (Copenh). 1985;109:25–31. [DOI] [PubMed] [Google Scholar]
- 17. Lindahl PE. Principle of a counter-streaming centrifuge for the separation of particles of different sizes. Nature. 1948;161:648. [DOI] [PubMed] [Google Scholar]
- 18. Childs GV, Unabia G. The use of counterflow centrifugation to enrich gonadotropes and somatotropes. J Histochem Cytochem. 2001;49:663–664. [DOI] [PubMed] [Google Scholar]
- 19. Childs GV, Lloyd J, Unabia G, Rougeau D. Growth and secretory responses of enriched populations of corticotropes. Endocrinology. 1989;125:2540–2549. [DOI] [PubMed] [Google Scholar]
- 20. Childs GV, Lloyd JM, Rougeau D, Unabia G. Enrichment of corticotropes by counterflow centrifugation. Endocrinology. 1988;123:2885–2895. [DOI] [PubMed] [Google Scholar]
- 21. Grindeland R, Hymer WC, Farrington M, et al. Changes in pituitary growth hormone cells prepared from rats flown on Spacelab 3. Am J Physiol. 1987;252:R209–R215. [DOI] [PubMed] [Google Scholar]
- 22. Hatfield JM, Hymer WC. Flow cytometric analysis and sorting of live male rat anterior pituitary cell types by forward angle and perpendicular light scatter. Endocrinology. 1986;119:2670–2682. [DOI] [PubMed] [Google Scholar]
- 23. Hatfield JM, Hymer WC. Flow cytometric analysis and sorting of live female rat anterior pituitary cell types by forward angle and perpendicular light scatter: effect of 17β-estradiol. Endocrinology. 1986;119:2683–2694. [DOI] [PubMed] [Google Scholar]
- 24. Wynick D, Critchley R, Venetikou MS, Burrin JM, Bloom SR. Purification of functional lactotrophs and somatotrophs from female rats using fluorescence-activated cell sorting. J Endocrinol. 1990;126:269–274. [DOI] [PubMed] [Google Scholar]
- 25. Park S, Park H, Lee M, et al. Modulation of pituitary somatostatin receptor subtype (sst1–5) mRNA levels by growth hormone (GH)-releasing hormone in purified somatotropes. Korean J Physiol Pharmacol. 2003;7:79–84. [Google Scholar]
- 26. Xie J, Nagle GT, Ritchie AK, Collins TJ, Childs GV. Cold stress and corticotropin-releasing hormone induced changes in messenger ribonucleic acid for the α(1)-subunit of the L-type Ca(2+) channel in the rat anterior pituitary and enriched populations of corticotropes. Neuroendocrinology. 1999;70:10–19. [DOI] [PubMed] [Google Scholar]
- 27. Childs GV, Unabia G. Rapid corticosterone inhibition of corticotropin-releasing hormone binding and adrenocorticotropin release by enriched populations of corticotropes: counteractions by arginine vasopressin and its second messengers. Endocrinology. 1990;126:1967–1975. [DOI] [PubMed] [Google Scholar]
- 28. Childs GV, Unabia G. Epidermal growth factor and gonadotropin-releasing hormone stimulate proliferation of enriched population of gonadotropes. Endocrinology. 2001;142:847–853. [DOI] [PubMed] [Google Scholar]
- 29. Childs GV, Rougeau D, Unabia G. Corticotropin-releasing hormone and epidermal growth factor: mitogens for anterior pituitary corticotropes. Endocrinology. 1995;136:1595–1602. [DOI] [PubMed] [Google Scholar]
- 30. Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996;137:2269–2277. [DOI] [PubMed] [Google Scholar]
- 31. Kuryshev YA, Childs GV, Ritchie AK. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology. 1995;136:3925–3935. [DOI] [PubMed] [Google Scholar]
- 32. Kuryshev YA, Childs GV, Ritchie AK. Three high threshold calcium channel subtypes in rat corticotropes. Endocrinology. 1995;136:3916–3924. [DOI] [PubMed] [Google Scholar]
- 33. Kuryshev YA, Haak L, Childs GV, Ritchie AK. Corticotropin releasing hormone inhibits an inwardly rectifying potassium current in rat corticotropes. J Physiol. 1997;502(Pt 2):265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marchetti C, Childs GV, Brown AM. Voltage-dependent calcium currents in rat gonadotropes separated by centrifugal elutriation. Am J Physiol. 1990;258:E589–E596. [DOI] [PubMed] [Google Scholar]
- 35. Ritchie AK, Kuryshev YA, Childs GV. Corticotropin-releasing hormone and calcium signaling in corticotropes. Trends Endocrinol Metab. 1996;7:365–369. [DOI] [PubMed] [Google Scholar]
- 36. Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 37. Li S, Crenshaw EB, 3rd, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990;347:528–533. [DOI] [PubMed] [Google Scholar]
- 38. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. [DOI] [PubMed] [Google Scholar]
- 39. Luque RM, Amargo G, Ishii S, et al. Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148:1946–1953. [DOI] [PubMed] [Google Scholar]
- 40. Cohen P, Zhao C, Cai X, et al. Selective deletion of leptin receptor in neurons leads to obesity. J Clin Invest. 2001;108:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Akhter N, CarlLee T, Syed MM, et al. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology. 2014;155:4027–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Odle AK, Haney A, Allensworth-James M, Akhter N, Childs GV. Adipocyte vs pituitary leptin in the regulation of pituitary hormones: Somatotropes develop normally in the absence of circulating leptin. Endocrinology. 2014;155:4316–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Odle AK, Allensworth-James M, Haney A, Akhter N, Syed M, Childs GV. Adipocyte versus somatotrope leptin: regulation of metabolic functions in the mouse. Endocrinology. 2016;157(4):1443–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nunez L, Villalobos C, Senovilla L, Garcia-Sancho J. Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J Physiol. 2003;549:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Villalobos C, Nunez L, Garcia-Sancho J. Phenotypic characterization of multi-functional somatotropes, mammotropes and gonadotropes of the mouse anterior pituitary. Pflugers Arch. 2004;449:257–264. [DOI] [PubMed] [Google Scholar]
- 46. Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 1998;14:284–290. [DOI] [PubMed] [Google Scholar]
- 47. Hoeffler JP, Frawley LS. Capacity of individual somatotropes to release growth hormone varies according to sex: analysis by reverse hemolytic plaque assay. Endocrinology. 1986;119:1037–1041. [DOI] [PubMed] [Google Scholar]
- 48. Frawley LS, Neill JD. A reverse hemolytic plaque assay for microscopic visualization of growth hormone release from individual cells: evidence for somatotrope heterogeneity. Neuroendocrinology. 1984;39:484–487. [DOI] [PubMed] [Google Scholar]
- 49. Childs GV, Unabia G, Rougeau D. Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) β-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH β, FSH β, and/or growth hormone. Endocrinology. 1994;134:990–997. [DOI] [PubMed] [Google Scholar]
- 50. Behringer RR, Mathews LS, Palmiter RD, Brinster RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev. 1988;2:453–461. [DOI] [PubMed] [Google Scholar]
- 51. Vidal S, Horvath E, Kovacs K, Cohen SM, Lloyd RV, Scheithauer BW. Transdifferentiation of somatotrophs to thyrotrophs in the pituitary of patients with protracted primary hypothyroidism. Virchows Arch. 2000;436:43–51. [DOI] [PubMed] [Google Scholar]
- 52. Vidal S, Horvath E, Kovacs K, Lloyd RV, Smyth HS. Reversible transdifferentiation: interconversion of somatotrophs and lactotrophs in pituitary hyperplasia. Mod Pathol. 2001;14:20–28. [DOI] [PubMed] [Google Scholar]
- 53. Horvath E, Lloyd RV, Kovacs K. Propylthiouracyl-induced hypothyroidism results in reversible transdifferentiation of somatotrophs into thyroidectomy cells. A morphologic study of the rat pituitary including immunoelectron microscopy. Lab Invest. 1990;63:511–520. [PubMed] [Google Scholar]
- 54. Frawley LS, Boockfor FR. Mammosomatotropes: presence and functions in normal and neoplastic pituitary tissue. Endocr Rev. 1991;12:337–355. [DOI] [PubMed] [Google Scholar]
- 55. Frohman LA, Kineman RD, Kamegai J, et al. Secretagogues and the somatotrope: signaling and proliferation. Recent Prog Horm Res. 2000;55:269–291. [PubMed] [Google Scholar]
- 56. Kineman RD, Faught WJ, Frawley LS. The ontogenic and functional relationships between growth hormone- and prolactin-releasing cells during the development of the bovine pituitary. J Endocrinol. 1992;134:91–96. [DOI] [PubMed] [Google Scholar]
- 57. Strathmann FG, Borlee G, Born DE, Gonzalez-Cuyar LF, Huber BR, Baird GS. Multiplex immunoassays of peptide hormones extracted from formalin-fixed, paraffin-embedded tissue accurately subclassify pituitary adenomas. Clin Chem. 2012;58:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]