Abstract
We examined the effect of mild (Mi; ∼25%) and moderate (Mo; ∼50%) maternal calorie restriction (MCR) vs ad libitum-fed controls on placental glucose and leucine transport impacting fetal growth potential. We observed in MiMCR a compensatory increase in transplacental (TP) glucose transport due to increased placental glucose transporter isoform (GLUT)-3 but no change in GLUT1 protein concentrations. This change was paralleled by increased glut3 mRNA and 5-hydroxymethylated cytosines with enhanced recruitment of histone 3 lysine demethylase to the glut3 gene locus. To assess the biologic relevance of placental GLUT1, we also examined glut1 heterozygous null vs wild-type mice and observed no difference in placental GLUT3 and TP or intraplacental glucose and leucine transport. Both MCR states led to a graded decrease in TP and intraplacental leucine transport, with a decline in placental L amino acid transporter isoform 2 (LAT2) concentrations and increased microRNA-149 (targets LAT2) and microRNA-122 (targets GLUT3) expression in MoMCR alone. These changes were accompanied by a step-wise reduction in uterine and umbilical artery Doppler blood flow with decreased fetal left ventricular ejection fraction and fractional shortening. We conclude that MiMCR transactivates placental GLUT3 toward preserving TP glucose transport in the face of reduced leucine transport. This contrasts MoMCR in which a reduction in placental GLUT3 mediated glucose transport with a reciprocal increase in miR-122 expression was encountered. A posttranscriptional reduction in LAT2-mediated leucine transport also occurred with enhanced miR-149 expression. Both MCR states, although not affecting placental GLUT1, resulted in uteroplacental insufficiency and fetal growth restriction with compromised cardiovascular health.
Maternal malnutrition is a worldwide problem resulting in fetal growth restriction (1, 2). In poorly resourced countries, undernutrition due to inadequate intake of sufficient calories during pregnancy is associated with the birth of growth restricted small babies with either high perinatal mortality or survival with vulnerability for developing chronic diseases subsequently (3–5). Although it is easy to state that mothers should be given adequate calories during pregnancy and lactation, access to sufficient food intake is marred by governmental policies, war-stricken conditions, educational status, cultural beliefs, and no access to proper and timely health care, and most of these situations are not controlled by pregnant women (1, 6). In contrast, in well-resourced Western countries, a majority of growth-restricted babies are the product of a multifactorial condition termed placental insufficiency (7–9). Placental insufficiency or uteroplacental insufficiency is a chronic condition that consists of gradually increasing insufficiency of blood flow to the uteroplacental unit during pregnancy due to maternal disorders such as hypertension and preeclampsia, in which vascular resistance builds in the low-resistance placental vascular bed (10, 11). It has been believed that these two conditions of maternal undernutrition and placental insufficiency are distinctly different from each other, even though the end result is one and the same, namely intrauterine growth restriction (IUGR) and associated consequences (1, 12).
Hence, multiple animal models have been created to mimic maternal undernutrition or placental insufficiency. In the former, manipulation of food intake during pregnancy was encountered (13, 14), whereas in the latter, restriction of uterine blood flow was achieved surgically (15–17) or by drug induction (18), yet in both cases the impact on the fetus and subsequently on the offspring was one and the same, with the outcome directly proportional to the severity of the maternal intervention (9, 19). More importantly, what remains unknown is whether maternal undernutrition causes placental insufficiency and thereby results in IUGR. In addition, the impact of varying severity of maternal undernutrition on uterine blood flow and associated molecular mechanisms that define placental macronutrient transfer remains undefined.
We have previously demonstrated that a moderate amount (∼50%) of calorie restriction during mid- to late gestation in a mouse caused fetal growth restriction (vs a severe amount of calorie restriction [∼75%] ending in intrauterine demise and resorption) by interfering with transplacental glucose and leucine transport (14). The mechanism responsible for reduced glucose transport was determined not to be due to placental glucose transporter isoform 1 (GLUT1) but rather due to reduced placental glucose transporter isoform 3 (GLUT3) (14), related to disabled transcriptional mechanisms reliant on enhanced DNA methylation of the promoter region (20). This lack of a change in placental GLUT1 as opposed to GLUT3 raised doubt regarding the biological significance of GLUT1 vs that of GLUT3 in mediating transplacental and intraplacental glucose transport. The moderate maternal calorie-restricted fetoplacental phenotype was mimicked to some extent by the glut3 heterozygous (+/−) null mice (21). However, similar investigations in the glut1 heterozygous (+/−) null mice affirming GLUT1's biological role in this context do not exist. In contrast to the changes encountered in placental glut3 gene transcription-reducing glucose transport, reduced leucine transport was perhaps related to posttranscriptional mechanisms involving DNA methylation of a specific microRNA (miR-149) gene body (22) that regulates system L amino acid transporter isoform 2 (LAT2) (14).
However, lack of information regarding a milder state of maternal calorie restriction (∼25%) described previously in other investigational contexts (23–27) and often encountered in the human situation (28) fueled our current hypothesis. We hypothesized that mild maternal calorie restriction will recruit differing placental mechanisms that will reflect compensation toward preserving the fetoplacental well-being, whereas moderate maternal calorie restriction reflects a decompensated state associated with ensuing placental insufficiency. To test this hypothesis, we used two states of murine maternal calorie restriction, approximately 25% (mild) and approximately 50% (moderate) restriction of daily food availability from gestational day 10 to 19 and examined at gestational day 19 Doppler blood flow and molecular mechanisms responsible for changes in transplacental and intraplacental glucose and leucine transport. In addition, we used the glut1 heterozygous (+/−) null mice to decipher the biological significance of placental GLUT1 in mediating late-gestation transplacental and intraplacental glucose transport.
Materials and Methods
Animal model
Maternal calorie restriction (MCR)
We created a mild MCR (MiMCR) model by reducing daily chow intake by approximately 25% of previously estimated daily intake of pregnant 8- to 10-week-old C57BL/6 mice, beginning from gestational day 10 to 19 (25–27). The moderate maternal calorie restriction group (MoMCR) included in this study was created as previously described, with approximately 50% restriction in daily food availability during the same gestational period (14). Control mice (CON) had daily ad libitum access to chow (Pico Lab Rodent Diet 20 [5053 irradiated; Lab Diet]: protein percentage by weight is 21% and percentage by kilocalories is 24.49%, fat percentage by weight is 5% and percentage by kilocalories is 13.1%, and carbohydrates percentage by weight is 53.5% and percentage by kilocalories is 62.3%). Both groups had ad libitum access to water daily. All mice were maintained in 12-hour light, 12-hour dark cycles in a 24-hour period. The presence of a vaginal plug detected soon after being housed with a male mouse was considered gestational day 1. At gestational day (GD) 19, animals were euthanized by 100 mg/kg of ip phenobarbital after completion of the studies. Protocols for the care and use of mice were approved by the Animal Research Committee of the University of California, Los Angeles, in accordance with guidelines set by the National Institutes of Health.
Doppler assessment of blood flow
Real-time high-frequency ultrasound biomicroscopy
Recording was performed on timed pregnant female mice at GD19 using a Vevo2100 imaging station (Vevo2100 Visual Sonics). A total of 12 dams were studied in these experiments, including three dams from the control group, five dams from the MiMCR group, and four from the MoMCR group. No other experimental procedures were performed during the ultrasound recording. In total, 58 fetuses (males and females) were imaged; of these, data from 49 fetuses were obtained and used for subsequent analysis.
Preparation of pregnant mice for imaging
The dams were anesthetized using inhalational isoflurane (isoflurane 2%–3%) mixed with 100% oxygen (100% O2) at a flow rate of 1 L/min in the induction chamber, and then the sedated animals were placed in a supine position on a heating pad to maintain body temperature within a range of 37.0°C ± 0.5°C. The limbs were gently taped to the embedded electrocardiographic electrodes after application of the electrode gel. Maternal cardiac and respiratory rates were constantly monitored. Steady-state sedation was maintained throughout the procedure using a face mask connected to the anesthesia system delivering isoflurane (1.0%–1.5%) mixed with 100% O2 at 1 L/min. Leakage of the anesthetic was scavenged using a ventilation system equipped with a charcoal filter containing canister. The level of isoflurane was adjusted to maintain a target heart rate of 400 ± 50 beats/min (bpm). Fur was removed from the midchest level to lower limbs with a depilatory cream (Nair cream) to minimize ultrasound attenuation. Nair cream was removed 15–20 seconds after its application with alternating wet and dry gauzes to prevent damage to the skin. The image acquisition times were minimized to less than 30 minutes per dam.
Evaluation of heart function
A Vevo200 high-frequency (30/45 MHz) probe was used. The ultrasound probe was fixed and mobilized with a mechanical holder. Using the mother's bladder as a landmark, fetuses on the left and right uterine horns were labeled as L1, 2, 3, etc (left side) and R1, 2, 3, etc (right side). Scan planes were modified by changing the orientation of the mouse with respect to the scan planes. Images were obtained in the transverse and sagittal planes for each fetus. Nonoptimal, oblique images were excluded from the final analysis. Scanning B-mode images were used to identify basic cardiac structures such as the atria, interventricular septum, ventricular chambers, and left and right outflow tracts. First, the right and left orientation of the individual fetus in real time was identified by moving the imaging platform in the horizontal plane and scanning from head to tail to annotate the snout, limbs and spine as landmarks. Next, left and right ventricular chambers were annotated using the longitudinal four-chamber view. M-mode cardiac scanning was performed using the 30-MHz transducer. M-mode images were obtained from the short axis view and were further analyzed to measure ventricular wall thickness and chamber dimensions and to calculate percentile ejection fraction. Temporal changes between left ventricular end-systolic dimension and left ventricular end-diastolic dimension (LVEDD) throughout the cardiac cycle were used for the calculation of percentile fractional shortening as follows: percentile fractional shortening = ([LVEDD − left ventricular end systolic dimension]/LVEDD) × 100. Pulsed-wave Doppler measurements were obtained using the 45-MHz transducer at angle of acquisition of less than 60º. Visualizing the bifurcation of the pulmonary artery facilitated the identification of the right outflow tract. Pulmonary outflow measurements including peak systolic velocity (PkV), acceleration time (AT), and ejection time (ET) were obtained, and the AT to ET ratio was calculated. Heart rate was calculated from the measurement of one flow cycle to the following flow cycle.
Assessment of uterine, placental, and fetal blood flow
The 45-MHz transducer was used to perform uterine, fetal and umbilical artery scans. The uterine arteries were visualized in the maternal pelvis on each side of the maternal bladder, and the umbilical artery and vein were identified in the intraamniotic segment of the umbilical cord, just after the cord exits from the fetal abdomen. The fetal carotid artery was visualized in a lateral sagittal view of the fetal thorax and neck. Uteroplacental and fetoplacental vascular trees for individual fetus were imaged using the color Doppler, and placental minimal and maximal diameters were measured. Pulsed wave interrogation was used to measure vascular flow velocities. Three to five consecutive waveforms in each vessel in the absence of fetal movements and without maternal respiratory movements were obtained. Peak velocities of each vessel were measured.
Postimaging animal monitoring
After the completion of imaging, the dam was returned to the appropriate housing and monitored according to standard institutional postprocedure protocol. Full resumption of normal activity was achieved within five minutes.
GLUT1 heterozygous mice
Glut1 homozygous null mice created by Wang et al (29) faced embryonic lethality at embryonic day (e) 13. The glut1 heterozygous null mice were described to be small-sized adults that successfully procreated. Pregnant glut1 heterozygous mice with ad libitum access to chow and water yielded both glut1 heterozygous null and wild-type GD19 placentas and e19 embryos (males and females).
Transport studies
Glucose transport
Ten-week-old pregnant female mice (GD19) belonging to four groups, namely MiMCR, MoMCR, and two respective CON, or two groups of glut1 (+/−) and wild type (wt; +/+) were ip injected with a bolus of 12 μCi of 2-deoxy-d-[1-14C] glucose (specific activity = 45–60 mCi/mmol; PerkinElmer; catalog number-NEC 495A). An hour later, maternal blood was collected from the jugular vein and glucose concentration and radioactivity were assessed. Soon afterward the mice were euthanized with pentobarbital sodium (100 mg/kg, ip), and the fetuses and placentas were separated and collected, and placentas and whole fetuses were hydrolyzed separately in 1M NaOH at 60°C for 45 minutes and then neutralized with 1 M HCl. One aliquot (200 μL) of the neutralized lysate was added to 1 mL of HClO4, and another aliquot (200 μL) was added to Ba(OH)2/ZnSO4. After centrifugation, the supernatants (800 μL) were used to assess radioactivity in a liquid scintillation counter. Protein and glucose concentrations were also estimated in placental/fetal lysates. Glucose uptake by the placenta and fetus was calculated as a ratio between specific activities of glucose in the placenta/fetus and maternal blood (30, 31). Placental and fetal total glucose uptake (HClO4 precipitated supernatant) reflected both the intracellular glucose transport (Ba[OH]2/ZnSO4 precipitated supernatant) and phosphorylation (Ba[OH]2/ZnSO4 precipitate), whereas glucose uptake (transport+phosphorylation) by the fetus as a ratio to placental weight reflected transplacental glucose transport and phosphorylation (14, 32).
Leucine transport
Five microcuries per 100 μL L-[U-14C]leucine (GE Healthcare Life Sciences; CFB67, specific activity 318 mCi/mmol) in sterile saline were administered via tail vein at GD19 to 10-week-old pregnant mice of all four groups of the calorie-restricted study and two groups of the GLUT1 study. After the administration of isotope, the mice were placed in cages to allow free ambulation for 5 minutes when a venous blood sample was withdrawn for determination of plasma radioactivity. Mice were euthanized with sodium pentobarbital (100 mg/kg, ip), and placentas and fetuses were collected separately and weighed individually and then lysed overnight at 55°C in 4 mL of Biosol for the fetus and 0.5 mL volume for the placenta. Radioactivity in samples was assessed and expressed per gram (wet weight) of tissue (33).
Placental glucose and leucine transporters
The placentas were separated from the respective fetuses and collected. After accurate weighing of the placentas in a Mettler AB104 precision balance (0.01 mg sensitivity), they were snap frozen immediately and stored at −80°C until further analyses. Solubulized protein homogenates (30 μg) were subjected to electrophoresis on 10% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes (Transblot; Bio-Rad Laboratories). The primary antibody consisted of an affinity-purified rabbit antimouse GLUT3 or GLUT1 IgG that was generated against a keyhole limpet-linked terminal 17 amino acids of the mouse GLUT3 or GLUT1 peptide, the isoform specificity of which has been previously characterized by us (34, 35). The primary antimouse GLUT3 and the antimouse GLUT1 antibodies were used at a 1:1000 dilution for 2 hours at room temperature. The secondary antibody consisted of a horseradish peroxidase-conjugated antibody (1:2500) that allowed detection of the immunoreactive protein bands by enhanced chemiluminescence. For the LAT2 protein, solubulized protein homogenates (50 μg) were subjected to electrophoresis on 10% SDS-polyacrylamide gels and transferred to same nitrocellulose membranes. Membranes were blocked in 5% bovine milk for an hour and then probed overnight at 4°C with antibodies against LAT2 (sc27581, 1:500 dilution). The secondary antibody consisted of a horseradish peroxidase-conjugated antibody (1:2500) that allowed the detection of the immunoreactive protein bands by enhanced chemiluminescence. Proteins were normalized to vinculin and quantification was performed using the image Quant 5.2 software (GE Healthcare Biosciences).
Glut3 mRNA was assessed by reverse transcription-quantitative real-time PCR as previously described using the same primers (Mm 00441483_m1) and PCR cycling conditions (20).
Placental sodium-coupled neutral amino acid transporters (SNATs)
Solubulized protein homogenates (30 μg) were subjected to electrophoresis on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked in 5% bovine milk for an hour and then probed overnight at 4°C with antibodies against SNAT1 (sc 67080, 1:500 dilution), SNAT2 (sc 166366, 1:500), and SNAT4 (sc 67086, 1:500). The secondary antibody consisted of a horseradish peroxidase-conjugated antibody (1:2500), which allowed detection of the immunoreactive protein bands by enhanced chemiluminescence. Proteins were normalized to vinculin and quantification was performed using the image Quant 5.2 software (GE Healthcare Biosciences).
Epigenetic studies
DNA methylation and hydroxymethylation
Genomic DNA was isolated from placentas using a DNeasy blood and tissue kit (QIAGEN). The 5-methylated cytosines and 5-hydroxymethylated cytosines (5-hmC) were measured by methylated and hydroxymethylated DNA quantification kits (Epigentek). Briefly, 200 ng of genomic DNA, the quality and concentration of which were assessed by the NanoDrop 1000 spectrophotometer (ThermoScientific), was bound to a 96-well plate. Next, the methylated and the hydroxymethylated DNA fractions were detected using respective capture and detection antibodies and colorimetrically quantified by reading the absorbance at 450 nm by a microplate spectrophotometer (Molecular Devices; model S/N 6291) following the manufacturer's directions.
Chromatin immunoprecipitation (ChIP) assay quantifying histone 3 lysine demethylase (KDM3A)
ChIP was performed according to ChIP-IT Express kit (Active Motif) with some modifications. Frozen placental tissues were homogenized and then fixed in 1% formaldehyde at room temperature for 10 minutes, and then 1 mL of 10% Glycine Stop Fix Solution was added followed by centrifugation at 720 relative centrifugal force for 5 minutes. After washing twice with cold PBS, cells were resuspended in cell lysis buffer supplemented with 7.5 μL protease inhibitor cocktail and phenylmethylsulfonyl fluoride and homogenized with a tissue homogenizer. Cells were then centrifuged, and the nuclear pellet was separated and sonicated on ice in 0.5 mL of complete shearing buffer using a Fisher Scientific Sonic Dismembrator 100 with 20 pulses of 20 seconds each with 20 second intervals to obtain chromatin fragments of 300–500 bp size as determined by 2% agarose gel electrophoresis. The sample was then centrifuged at high speed (15 000 rpm for 15 min) to remove cell debris from the crude chromatin lysate. Ten microliters of the chromatin lysate was used as the input control for PCR and 50 μL of sheared chromatin was added to a final volume of 100 μL in preparation for the ChIP assay. The ChIP assay was next initiated by adding protein G magnetic beads (25 μL), ChIP buffer (10 μL), sheared chromatin (50 μL), protease inhibitor cocktail (1 μL), and 2 μg of antibody against KDM3A (sc 292213; Santa Cruz Biotechnology Inc) and incubating overnight at 4°C. The magnetic beads were separated from antibody-antigen-DNA binding and the antibody-protein-DNA complex was eluted with an elution buffer from the protein A agarose beads. This was followed by reverse cross-linking of this complex by using 50 μL of reverse cross-link buffer to elute the chromatin. The eluted chromatin was then treated with 5 M NaCl at 95°C for 15 minutes in a thermocycler followed by incubating with 2 μL Proteinase K at 37°C for 1 hour. The supernatant that contained the DNA was then centrifuged and used as the template for PCR.
Real-time quantitative PCR (qPCR)
Real-time PCR amplification was performed in triplicate using SYBR green on a Step One qPCR thermocycler (Applied Biosystems). ChIP-isolated genomic DNA was used as the template and lamin A was used as the internal control. The amplification cycles consisted of a hot start at 95°C for 10 minutes, followed by 40 cycles at 95°C for 15 seconds (denaturation) and 56°C for more than 60 seconds (annealing), using primers specific for mouse glut3 (forward: 5′ACTGGCCTTTGGGCTTTACT, reverse: 5′-AGCAAACCTGGTCCCTTTCT) that encompass the CpG island, and mouse lamin A (forward: 5′-AGCCTCTGTCCTTCTGTCCA, reverse: 5′-TGAACTCCTCACGCACTTTG). The fold change in the MiMCR group relative to control samples was determined by the comparative cycle threshold method described by Livak and Schmittgen (36).
MicroRNA analysis
A panel of miRs was selected based on potential mRNA targets being GLUT3, LAT2, or SNATs based on Targetscan analysis. These include miR-15b, -122, -16, -195 (target being glut3), -149 (target being LAT2), -363 (target being SNAT1 and SNAT2) and -10b (target being multiple factors responsible for modulating angiogenesis and vasculogenesis). These miRs were assessed in the three groups of the maternal calorie restricted study. Total RNA was extracted using Trizol reagent (Invitrogen), and cDNA synthesis was performed using the Taqman microRNA reverse transcription kit (Applied Biosystems) with special reverse transcriptase LNA-based primer set for miR-149 (assay identification 002255), miR-10b (assay identification 002218), miR-363 (assay identification 001271), miR-122 (assay identification 002245), miR-15b (assay identification 000390), miR-16 (assay identification 000391), and miR-195 (assay identification 002107). Briefly, reverse transcription reactions were performed with 10 ng of total RNA using Multiscribe reverse transcription enzyme (50 U/μL) along with specific miR primers for all miRs obtained from Applied Biosystems by placing them in a thermal cycler at 16°C for 30 minutes and 42°C for 30 minutes followed by denaturation at 85°C for 5 minutes. The reverse transcription reaction for the endogenous control (U6 and miR -429) was done exactly the same way as for all other microRNAs. The subsequent PCR mixture consisted of 10 μL TaqMan Universal PCR master Mix II (no uracil N-glycosylase) with forward and universal reverse primers and cDNA obtained by reverse transcription of total RNA (10 ng). The samples were initially heated at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. The same conditions were used for endogenous control, miR-429 and U6. Each RT-qPCR assay was performed in triplicate and the relative miR expression quantified by subtracting the average of quantification cycle (Cq) values from the triplicate reaction of reference control (miR-429 and U6). The difference between the target (miR-122, miR-149, miR-10b, and miR-363 and others) and the internal control provided the difference in Cq values (δCq).
Statistical analysis
For blood flow studies, statistics were performed with the GraphPad Software. Data are expressed as means ± SEM. Comparisons were made by a Kruskal-Wallis one-way ANOVA. If statistical significance was shown by the ANOVA, then Dunn's multiple comparison post hoc analysis was performed between groups to infer intergroup comparisons. A value of P ≤ .05 was considered statistically significant. For the remaining studies, statistical analyses were performed using Sigmastat 3.5 software (Systat). Data are presented as means ± SEM. Because the MiMCR and MoMCR studies were conducted at different times, MiMCR was compared with its respective CON, and MoMCR was compared with its respective CON group, which was set up simultaneously with the MCR concerned. Two groups were compared by a Student's t test with normal distribution, whereas the Mann-Whitney rank sum test was used in the absence of a normal distribution. Significance was assigned at a P < .05.
Results
Blood flow studies
Whereas maternal heart rate remained unchanged, MoMCR reduced uterine artery blood flow to placenta by approximately 39% during systole and approximately 49% during diastole vs CON, whereas MiMCR decreased 24% during systole and 44% during diastole vs CON (Table 1). In response to these changes, fetal echocardiography revealed no change in MoMCR but a 27% reduction (P < .001) in fetal heart rate in MiMCR vs CON (Table 1). The MoMCR group demonstrated some intragroup variability, ie, in a total of 15 MoMCR fetuses, we encountered a slight decrease in heart rate (mean ± SEM 107 ± 4.5; range 100–112 bpm; P < .001) in 11 of them (∼75% of the fetuses), whereas four of them (∼25%) revealed tachycardia (mean ± SEM 232 ± 20, range 201–290; P < .001). Similarly, in the MiMCR group, some variability was also detected, namely in a total of 16 fetuses, bradycardia (mean ± SEM 88 ± 6; range 78–96 bpm; P < .001) was noted in six of them (37.5% of the fetuses) with no change (mean ± SEM 116 ± 4; range 107–132 bpm) seen in 10 of them (62.5%). Doppler ultrasound revealed a significant reduction in fetal left ventricular ejection fraction and fractional shortening vs CON in both MCR groups (P < .001). This in turn revealed a reduction in umbilical artery blood flow during systole (PkV; mm/second) by approximately 38% (P < .001) and during diastole (PkV, mm/second) by 57% P < .01) in MoMCR, whereas reduced umbilical artery systolic pressure by 30% and diastolic pressure by 47% (P < .05) vs CON was evident in MiMCR. In addition, MoMCR significantly reduced the umbilical artery AT to ET ratio by approximately 25% (P < .01) compared with CON. Overall the umbilical venoarterial delay was almost 2-fold in MiMCR and 3-fold in MoMCR vs CON (Table 1).
Table 1.
Uteroplacental and Fetal Blood Flow Studies: Characterization of Uteroplacental-Fetal Circulation in Maternal and Fetal Mice in Caloric Restriction (CR) Models Using Ultrasound (30/45 MHZ) Biomicroscopy
| Parameter (Unit) | Control (Range, Mean ± SEM) | MiMCR (Range, Mean ± SEM) | MoMCR (Range, Mean ± SEM) | ANOVA |
|---|---|---|---|---|
| Number of fetuses | 18 | 16 | 15 | |
| Fetal heart rate, bpm | 108–158 (133 ± 4) | 78–132 (97 ± 5)a | 100–290 (142 ± 20) | P < .0002 |
| Left ventricle EF, % | 68–84 (77.2 ± 1) | 58–80 (66 ± 1.4)a | 53–70 (61.7 ± 1.2)a | P < .0001 |
| Left ventricle FS, % | 53.8–50 (44.6 ± 1) | 27.3–45 (34.7 ± 1.3)b | 25–32 (28.2 ± 0.6)a,c | P < .0001 |
| Left ventricle vol, d, μL | 1.1–2.1 (1.8 ± 0.05) | 1–2.11 (1.75 ± 0.07) | 1.6–2.4 (1.9 ± 0.05) | 0.1 (NS) |
| Left ventricle vol, s, μL | 0.24–0.52 (0.44 ± 0.01) | 0.34–0.62 (0.8 ± 0.04)d | 0.6–0.75 (0.69 ± 0.02)b | P = .03 |
| Pulmonary artery PkV, mm/sec | 87–109 (94 ± 2.7) | 61–142 (106 ± 6.6) | 83–258 (136 ± 2.2) | P = .08 |
| Pulmonary artery AT to ET ratio | 0.29-.56 (0.41 ± 0.03) | 0.26–0.55 (0.37 ± 0.02) | 0.32–0.4 (0.32 ± 0.01)d | P = .04 |
| Umbilical artery PkV, s, mm/sec | 49–75 (59 ± 1.9) | 32–50 (41 ± 1.3)a | 28–52 (36 ± 1.8)a | P < .0001 |
| Umbilical artery AT/ET ratio | 0.45–0.58 (0.51 ± 0.02) | 0.32–0.52 (0.45 ± 0.02) | 0.33–0.43 (0.38 ± 0.02)b | P = .004 |
| Umbilical artery PkV, d, mm/sec | 13–27 (18.9 ± 3.3) | 6–17 (10 ± 1.4)d | 5–12 (8 ± 0.8)b | P = .0029 |
| Umbilical vein PkV, s, mm/sec | 10–18 (13 ± 1.03) | 10–26 (19.3 ± 2.2) | 9.5–29 (17 ± 3.8) | P = .21 (NS) |
| Umbilical vein PkV, d, mm/sec | 9–13 (12.2 ± 0.8) | 9.5–13.5 (11 ± 1.2) | 4.4–7.4 (6 ± 0.9)d | P = .043 |
| Umbilical arterial-venous delay, msec | 110–140 (120 ± 4) | 160–300 (245 ± 16)d | 270–355 (308 ± 20)a | P = .003 |
| Uterine artery PKv, s, mm/sec | 74–132 (93 ± 4) | 57–86 (70.6 ± 2.5)d | 34–82 (57 ± 3.3)a,c | P < .0001 |
| Uterine artery PKv, d, mm/sec | 40–65 (61 ± 3.7) | 30–42 (34 ± 1.5)d | 26–36 (31 ± 0.8)a | P < .0003 |
| Maternal heart rate, bpm | 350–497 (430 ± 26) | 350–465 (417 ± 19) | 352–455 (390 ± 21) | P = .2 (NS) |
| Placenta surface area, mm2 | 2.3–2.72 (2.52 ± 0.07) | 2.01–2.5 (2.3 ± 0.07) | 1.96–2.3 (2.1 ± 0.06)d | P = .004 |
| Placenta maximum diameter, mm | 1.87–2.9 (2.55 ± 0.15) | 2.4–2.8 (2.52 ± 0.1) | 2.3–2.5 (2.42 ± 0.08) | NS |
Abbreviations: EF, ejection fraction; FS, fractional shortening; NA, not available; NS, not significant; PkV, peak velocity; PkV, d, peak velocity during diastole; PkV, s, peak velocity during systole; Vol d, end diastolic volume; Vol s, end systolic volume. Hemodynamic parameters and structural heart measurements of CON, MiMCR, and MoMCR maternal and fetal mice at GD19 are summarized. Statistical analysis is as follows: one-way ANOVA is shown in the last column. Dunn's multiple comparison posttest was performed between pairs of groups to infer intergroup differences.
P ≤ .001.
P ≤ .01.
P < 0.05 represents a significant difference in inter-group comparisons among MiMCR and MoMCR. Nonsignificance was left blank.
P ≤ 0.5.
Placental glucose and leucine transport
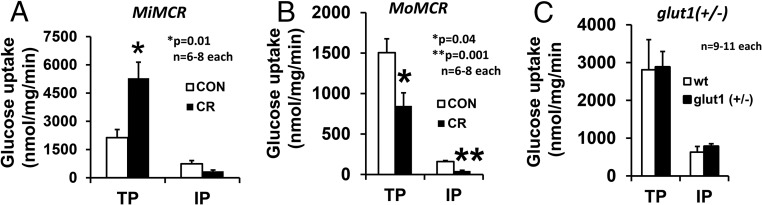
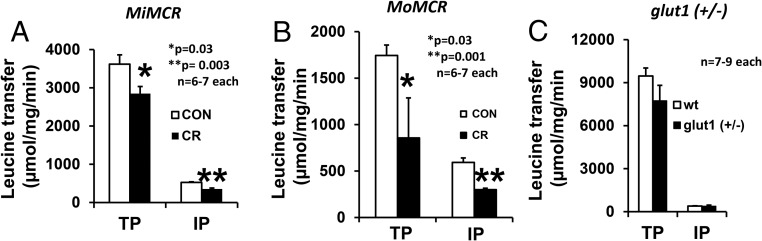
Transplacental (TP) glucose transport increased by 2-fold (P = .01) in MiMCR, but, interestingly, intraplacental (IP) glucose transport was unchanged (P = .12, not significant) (Figure 1A). In contrast, TP and IP were reduced by approximately 51.5% (P = .04) and 72.5% (P = .001), respectively, in MoMCR vs CON (Figure 1B). When placental GLUT1 protein was reduced by approximately 50% in the glut1 (+/−) genotype, no difference in TP or IP glucose transport was evident in glut1 (+/−) vs wt (+/+) mice (Figure 1C). TP and IP leucine transport was reduced by 21% (P = .03) and 37% (P = .003), respectively, in MiMCR (Figure 2A) and 50.9% (P = .03) and 49.5% (P = .001), respectively, in MoMCR (Figure 2B). In addition, assessment of TP and IP leucine transport failed to reveal significant changes between glut1 (+/−) and wt genotypes (Figure 2C).
Figure 1.
TP and IP glucose transport in GD19 placenta (IP) and fetus (TP) are shown in MiMCR vs CON. *, P = .01 (A), MoMCR vs CON; *, P = .04, **, P = .001 (B); glut1 (+/−) vs wild type (wt; +/+), not significant (C). CR, respective maternal calorie restriction.
Figure 2.
TP and IP leucine transfer in GD19 placenta (IP) and fetus (TP) are shown. *, P = .03, **, P = .003 (A), MiMCR vs control (CON); *, P = .03, **, P = .001 (B), MoMCR vs CON; and glut1 (+/−) vs wt (+/+) (C), not significant. CR, respective maternal calorie restriction.
Placental and embryonic size
MiMCR reduced embryo weight by 37.8% (P < .001) with no change in placental weight (Table 2), whereas MoMCR significantly reduced embryo weight by 47.9% (P < .001) and placental weight by 37.5% (P < .001) at GD19 when compared with the gestational age-matched controls (Table 2). These placental weight changes paralleled the changes in ultrasound-detected placenta surface area (Table 1). Whereas the embryo weights were not significantly different between MiMCR and MoMCR (Table 2), the percentage change from the respective CON groups revealed approximately 10% greater reduction in MoMCR (∼48%) vs the reduction seen in MiMCR (∼38%) (Table 2). This is despite a trend toward an approximately 15% reduction in litter size in MiMCR (5.75 ± 0.22; not significant) and an approximately 21% reduction in MoMCR (5.4 ± 0.24; P < .02) vs the combined respective CON groups (6.8 ± 0.44). However, no change in either GD19 placental or fetal weights was seen in glut1 (+/-) vs wt (+/+) mice (Table 2) along with no change in litter size (glut1[+/−] = 6.75 ± 0.85; wt = 5.66 ± 0.71, not significant).
Table 2.
Placental and Fetal Weights
| Group | Fetal Weight, g | Placental Weight, g |
|---|---|---|
| MiMCR versus CON | ||
| CON (n = 33) | 1.07 ± 0.02 | 0.08 ± 0.001 |
| MiMCR (n = 23) | 0.67 ± 0.003a | 0.08 ± 0.0005 |
| MoMCR versus CON | ||
| CON (n = 22) | 1.15 ± 0.02 | 0.08 ± 0.002 |
| MoMCR (n = 27) | 0.6 ± 0.02a | 0.05 ± 0.002a |
| glut1 (+/−) versus wt (+/+) | ||
| wt (+/+) (n = 33) | 1.1 ± 0.02 | 0.07 ± 0.001 |
| glut1 (+/−) (n = 26) | 1.08 ± 0.03 | 0.07 ± 0.003 |
P = .001 vs CON.
Data shown as mean ± sem.
Placental glucose and leucine transporters
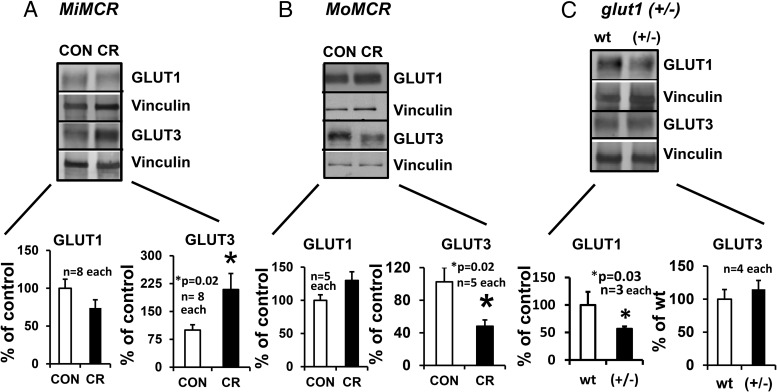
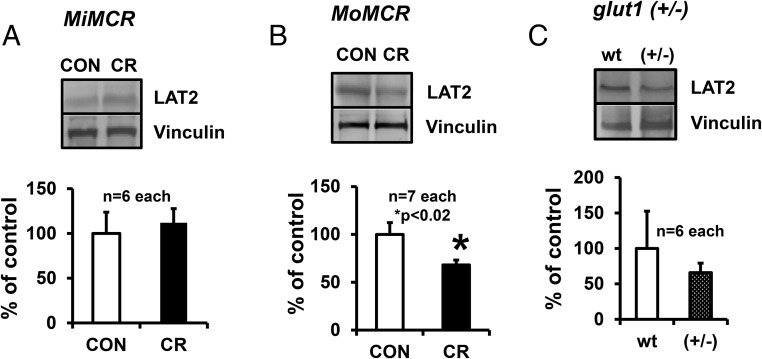
Placental GLUT3 protein increased by approximately 2-fold (P = .02) vs CON in MiMCR (Figure 3A). In contrast, MoMCR decreased GLUT3 protein by approximately 51.88% vs CON (P = .02) (Figure 3B), paralleled previously by decreased glut3 mRNA (20). Placental GLUT1 protein concentrations remained unchanged in MiMCR (P = .13) and MoMCR (P = .22) vs the respective CON group (Figure 3, A and B). Given that GLUT1 is an important glucose transporter that is abundantly expressed in placenta, our studies using glut1 (+/−) vs wt mice revealed a 49% reduction in placental GLUT1 protein concentrations, as expected, but with no change in placental GLUT3 protein concentrations (Figure 3C). Assessment of placental GLUT8 concentrations revealed rather low amounts in both genotypes, making detection and accurate quantification difficult. In the case of LAT2, an approximately 32.7% reduction was seen in MoMCR (P = .02), with no change in MiMCR vs CON (Figure 4, A and B) or in glut1 (+/−) vs wt (+/+) genotypes (Figure 4C). LAT1 protein could not be measured accurately because multiple commercial antibodies tested by us proved not to be specific in quality.
Figure 3.
Placental GLUT1 and GLUT3 proteins were quantified by Western blot analysis at GD19 in MiMCR vs CON (A), MoMCR vs CON (B), and glut1 (+/−) vs wt (+/+) (C). Upper panels demonstrate representative Western blots depicting the 45-kDa GLUT1 and 50-kDa GLUT3 protein bands with their corresponding vinculin protein bands, which served as the internal control in CON and CR GD19 placentas. Lower panels demonstrate the densitometric quantification of GLUT1 and GLUT3/vinculin protein depicted as a percentage of CON/wt values. *, P = .02 vs CON/wt. CR, respective maternal calorie restriction.
Figure 4.
Placental LAT2 protein was quantified by Western blot analysis at GD19 in MiMCR vs CON (A), MoMCR vs CON (B), and glut1 (+/−) vs wt (+/+) (C). Upper panels demonstrate representative Western blots depicting the LAT2 protein bands with the corresponding vinculin protein bands, which served as the internal control in CON and CR GD19 placentas. Lower panels demonstrate the densitometric quantification of LAT2/vinculin protein, depicted as a percentage of CON/wt values. *, P = .02 vs CON. CR, respective maternal calorie restriction.
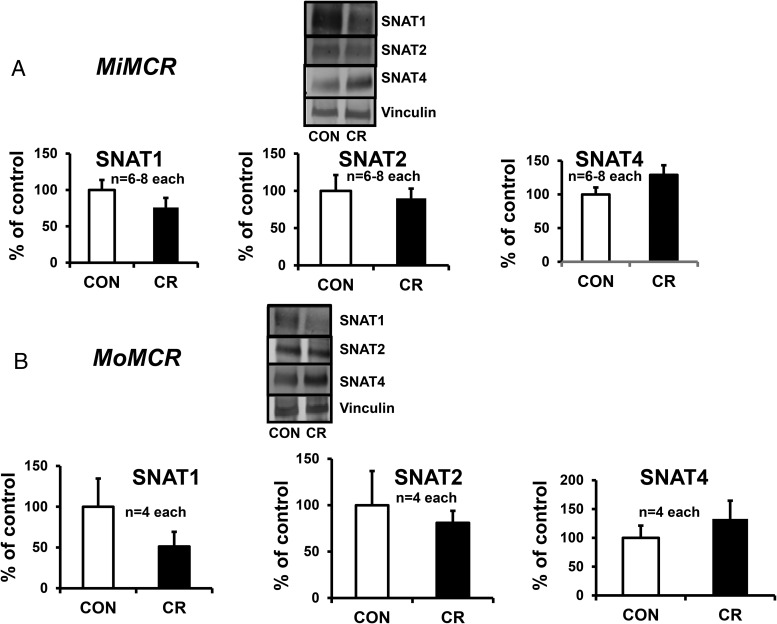
Placental sodium-coupled neutral amino acid transporters in MiMCR and MoMCR
We assessed placental SNAT1, SNAT2, and SNAT4 in MiMCR vs CON and found no significant differences (Figure 5A). Similarly, no significant differences in SNAT1, SNAT2, and SNAT4 were seen in MoMCR vs CON (Figure 5B). Given these observations, we did not pursue measuring TP or IP system A amino acid transport. For the same reason, we did not assess placental SNATs in the glut1 (+/−) vs wt (+/+) mice.
Figure 5.
Placental SNATs in MiMCR and MoMCR vs CON were quantified by Western blot analysis at GD19 in MiMCR vs CON (A) and MoMCR vs CON (B). Upper panels demonstrate representative Western blots depicting SNAT1, SNAT2, SNAT4, and vinculin (internal control) protein bands in CON and CR GD19 placentas. Lower panels demonstrate the densitometric quantification of SNAT proteins/vinculin protein, depicted as a percentage of corresponding CON values. No differences are seen. CR, respective maternal calorie restriction.
Mechanisms responsible for changes in placental GLUT3 and LAT2 in MiMCR and MoMCR
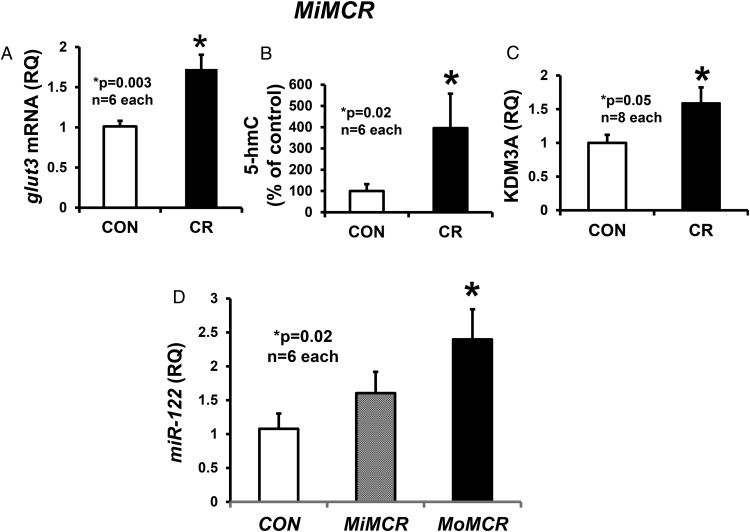
To examine mechanisms responsible for changes in perturbed placental GLUT3 concentrations in MiMCR and LAT2 concentrations in MoMCR, we first examined glut3 mRNA concentrations and observed an increase (P < .05) in MiMCR (1.72 ± 0.18) vs CON (1.01 ± 0.069) (Figure 6A), paralleling that seen with GLUT3 protein concentrations (Figure 3A). We next assessed hydroxymethylation in MiMCR, the first step in oxidation of methylated cytosines, which correlates with increased gene expression (37). We observed an increase in 5-hmC in MiMCR vs CON (Figure 6B). This contrasted no difference in total DNA methylation (5-methylated cytosines) in MiMCR vs CON (negative data not shown). We did not examine MoMCR because previously we reported hypermethylation of the CpG island associated with the glut3 promoter region along with reduced glut3 mRNA (20). Furthermore, we assessed the recruitment of a Jumonji domain-containing KDM3A to the glut3 gene locus and observed an increase in MiMCR (Figure 6C) vs CON. In contrast, previously we had observed recruitment of methyl CpG binding protein 2 (MeCP2) and histone deacetylase 2 to the glut3 gene locus in MoMCR vs CON (20).
Figure 6.
Placental glut3 mRNA, 5-hydroxymethylation, and glut3-KDM3A quantification. GLUT3 mRNA quantified by reverse transcription and real-time qPCR (A), DNA 5-hmC by antibody capture and detection assay (B), recruitment of KDM3A to the glut3 gene locus by ChIP assay and PCR (C), and RT-qPCR of miR-122 (D) are shown in CON and CR GD19 MiMCR and MoMCR (only miR-122) placentas. P values are shown in the graphs. CR, respective maternal calorie restriction.
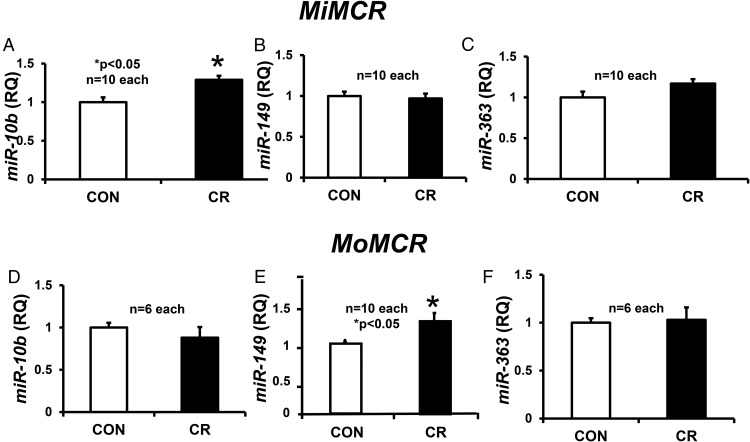
Assessment of the posttranscriptional role of specific miRs identified by genome-wide and TargetScan analyses revealed no change in the placental expression of miRs, miR-15b, miR-16, and miR-195, which target glut3 mRNA, in MiMCR or MoMCR vs CON (data not shown). In contrast, although no change in miR-122 (target glut3) was seen in MiMCR, a 2.4-fold increase was evident in MoMCR vs CON (Figure 6D). This increase in miR-122 opposes the decrease in placental glut3 mRNA observed previously in MoMCR vs CON (20). Unlike miR-122, miR-10b (which modulates angiogenesis and vasculogenesis) increased in MiMCR, with no change in MoMCR vs CON (Figure 7, A and D). In contrast, no change in miR-149 (targets LAT2) and miR-363 (targets SNAT1 and SNAT2) was observed in MiMCR vs CON (Figure 7, B and C). However, when comparing MoMCR with CON, an increase in miR-149 with no change in miR-363 was observed (Figure 7, E and F). The increase in miR-149 in MoMCR was reciprocal to the decrease in LAT2 protein concentrations (Figure 4B), whereas a lack of change in miR-363 reflected the lack of change in placental SNAT1 or SNAT2 protein concentrations (Figure 5B).
Figure 7.
Placental microRNAs miR-10b (A and D), miR-149 (B and E), and miR-363 (C and F) were quantified by reverse transcription and real-time qPCR at GD19 in MiMCR vs CON (A–C) and MoMCR vs CON (B–D). The graphs demonstrate RQ in CON and CR GD19 placentas. *, P < .05 vs CON. CR, respective maternal calorie restriction; RQ, relative quantification.
Discussion
We have for the first time established the impact of mild and moderate maternal calorie restriction on uterine blood flow, fetal cardiac function, and umbilical venoarterial blood flow. Whereas calorie restriction at first appears to affect only placentofetal nutrient transfer and thereby affect the fetal growth potential, we show that maternal calorie restriction that encompasses mid- and late gestational periods results in a graded reduction in uterine blood flow, which in turn has a detrimental effect on the fetal cardiac function and thereby the umbilical arterial blood flow. Although we demonstrate no differences in umbilical venous blood flow, this is related to difficulties encountered in accurately assessing the fetal venous system with high capacitance and low pressures as opposed to the pressures in the arterial system. Hence, the umbilical arterial system is monitored by Doppler ultrasound in human IUGR pregnancies (38). Thus, both mild and moderate MCR cause murine uteroplacental insufficiency during late gestation.
In response to reduced uteroplacental blood flow, despite some intragroup variability, a quarter of MoMCR fetuses expressed tachycardia, whereas three-quarters of these fetuses were mildly bradycardic. These fetal heart rate changes may signal compensatory mechanisms targeting survival under more dire conditions of nutrient supply restriction. A similar need to garner compensation may not have occurred under mild nutrient restriction in which MiMCR fetuses were either bradycardic (only ∼38%) or normal (∼63%). Unlike the human in which fetal heart rate (∼140 bpm) is much higher than the adult (72 bpm), murine fetal heart rate (∼120 bpm) is lower than the adult heart rate (∼400 bpm). Thus, fetal cardiac compensatory mechanisms encountered in the human cannot be completely extrapolated to the murine fetus, supporting the premise that mild reduction in uteroplacental blood flow causes no change or fetal bradycardia, whereas moderate reduction causes both bradycardia and/or tachycardia.
In contrast to the uteroplacental blood flow changes, which get more severe with increasing severity of the maternal calorie restriction imposed, perturbations in transplacental glucose transport are diametrically opposite under the two MCR circumstances. Whereas MiMCR increased placental GLUT3 concentrations, which mediated the observed increase in transplacental glucose transport, MoMCR in turn reduced placental GLUT3 concentrations and thereby the transplacental glucose transport. In the former circumstance in which milder uteroplacental insufficiency is encountered, whereas transplacental glucose transport increased, there was no change in intraplacental glucose transport. This may signify conservation of maternal glucose supply for the growth compromised fetus, which is incapable of endogenous glucose production (39) while simply maintaining fueling of the placenta.
Reflecting this observation, placental size was uncompromised, again a sign of compensation targeting protection of the fetus. This preservation of placental size is similar to calorie restriction imposed during early pregnancy as opposed to that in late pregnancy (40, 41). Thus, in addition to the gestational timing during pregnancy, the severity of MCR has a differential effect on not only the placental size but also on placental GLUT3 mediated transplacental glucose transport. Most importantly, the effect of MiMCR on placental GLUT3 concentrations parallel that seen in human placentas procured from late gestation IUGR pregnancies (28). In contrast, MoMCR may reflect a decompensated state as uteroplacental insufficiency gets worse, in which placental GLUT3-mediated transplacental glucose transport diminishes with a reduction in both the placental and fetal weights. Regardless of these differential changes in glucose transport, TP and IP leucine (branched chain amino acid) transport was reduced in a graded fashion in proportion to the severity of MCR imposed. In fact, in MiMCR the reduction in leucine transport is not related to a change in placental LAT2 concentrations but perhaps to its Michaelis constant. In contrast, in MoMCR, a reduction in LAT2 concentrations reflects the diminution in leucine transport.
The most intriguing observation was the fact that although placental GLUT3 changed, placental GLUT1, which is found in abundance and considered the main placental glucose transporter isoform in the third trimester of human pregnancy (42), demonstrated no change in MiMCR or MoMCR. Previous investigation has revealed that in MoMCR there were no cis-acting perturbations, ie, DNA methylation of the promoter region, in keeping with the absence of a change in placental GLUT1 expression (20). This lack of change in placental GLUT1 expression reflects that reported in human placentas from late-gestation IUGR pregnancies (28).
To understand the biological significance of the murine placental GLUT1 during late gestation, we engaged the glut1 heterozygous null mice. Previously, glut1 heterozygous adult mice have been described to be smaller in size than their wt counterparts (29); however, there are no studies demonstrating whether this is related to a reduction in transplacental glucose transport culminating in intrauterine growth restriction, which subsequently results in adult stunting. Our present fetal studies demonstrated that a 50% reduction in placental GLUT1 failed to affect the late gestation transplacental or intraplacental glucose transport. This is despite no compensatory increase in placental GLUT3 concentrations. Furthermore, there is no associated change in placental LAT2-mediated leucine transport either, with resultant absence of change in placental or fetal weights. Thus, fetal growth restriction does not occur in response to reduced placental GLUT1. These findings suggest a dose dependency because antisense glut1 transgenic mice revealed fetal malformations and growth restriction encountered earlier in pregnancy, perhaps related to the biological importance of embryonic GLUT1 rather than placental GLUT1 (43). A complete loss of glut1 in the homozygote null mice led to fetal demise by e13, again supporting an important role for embryonic GLUT1 rather than placental GLUT1 (29) because the latter in the trophectoderm would have led to a much earlier demise at e6.5, similar to that seen with lack of GLUT3 (21).
Our glut1 heterozygous null placenta-fetal observations are highly significant, given that most human mutations are carried on a single allele and result in the birth of a normally grown baby with a normal phenotype (44). Symptoms and growth are affected only during infancy and beyond (45, 46). In contrast to the glut1 heterozygous null mice, our previous studies have demonstrated that a 50% reduction in placental GLUT3 concentrations as encountered in glut3 heterozygous null mice caused a diminution in transplacental and intraplacental glucose transport, with slowing of fetal growth, despite a compensatory increase in transplacental leucine transfer (21). Thus, our present studies demonstrate that placental GLUT3 at a 50% reduced dosage plays a greater role in mediating transplacental and intraplacental glucose transport during late gestation than reduction of placental GLUT1. Further MCR perturbations of placental GLUT3 are biologically significant rather than the changes in placental GLUT1. Given that GLUT3 has a higher affinity for glucose when compared with GLUT1, the impact of a change in GLUT3 concentrations may be far greater than simply that expected by a linear relationship to the dose.
Our focus next shifted to determining the molecular mechanisms responsible for the changes seen in MiMCR induced placental GLUT3- and MoMCR-induced placental LAT2 concentrations. In the case of GLUT3, we have previously demonstrated DNA methyltransferase 3b mediated hypermethylation of the CpG island noted in the 5′-flanking region of the glut3 gene, which recruited MeCP2 that contributed toward transcriptionally reducing gene expression in MoMCR (20). This in turn led to additional recruitment of histone deacetylase-2, which interfered with sufficient recruitment of specificity protein-1, a transactivator of the glut3 gene (20, 34). In contrast to these findings, our present study demonstrated that MiMCR increased 5-hydroxymethylation of cytosines in placental genomic DNA. Furthermore, enhanced recruitment of histone 3 lysine demethylase to the same glut3 gene locus was apparent. This recruitment parallels the observation in human umbilical vascular endothelial cells in which hypoxic exposure led to enhanced KDM3A recruitment, which in turn increased glut3 gene expression (47). Thus, it is possible that the mild uteroplacental insufficiency encountered in MiMCR may have triggered this response, which in turn increased placental GLUT3 expression (mRNA). Given this transcriptional regulatory observation, we also examined the posttranscriptional mechanism related to placental microRNA expression in response to MiMCR. We specifically examined miR-10b, which has been described to play a role in vasculogenesis and angiogenesis, and observed an increase in response to MiMCR but not with MoMCR. miR-10b has previously been shown to target transcripts that mediate cellular proliferation (48), vasculogenesis, and angiogenesis (49, 50).
Whereas MoMCR has previously been shown to transcriptionally regulate placental glut3, the role of miRs in posttranscriptionally contributing toward the ultimate gene expression has not been systematically examined. Our present observation suggests that the increase in miR-122 observed in MoMCR may play a role in further regulating placental glut3 gene expression. Although a reciprocal relationship between placental miR-122 and glut3 mRNA was observed, future biological cause-and-effect confirmation is necessary. Along the same lines, the effect of MoMCR on placental LAT2 appears to be posttranscriptional (14). This is because MoMCR fails to affect LAT2 mRNA but reduces the LAT2 protein (14). In this case, miR-149, which we previously noted to exhibit differential methylation (22), demonstrated increased placental expression. However, in the case of MiMCR, no change in placental miR-149 expression was seen, in keeping with a lack of change in placental LAT2 concentrations. miR-149 is known to target LAT2 mRNA, perhaps suppressing mRNA translation with resultant decrease in the corresponding protein concentrations. This aspect requires future biological confirmation as well. miR-149 has also been described in maternal human circulation, precipitously disappearing with delivery of the placenta (51). In contrast, no differences in miR-363 or the different SNAT protein isoforms were seen in response to MiMCR or MoMCR.
We conclude that varying severity of maternal calorie restriction has a proportional effect on uteroplacental blood flow, resulting in an uteroplacental insufficient state. This is associated with diametrically opposing effects on placental GLUT3-mediated transplacental glucose transport, which is transcriptionally regulated. Whereas the mild state demonstrates a compensatory increase in GLUT3, which is regulated by enhanced DNA 5-hydroxymethylation and demethylation of histone 3 lysine, the moderate state reduces GLUT3 related to DNA hypermethylation and recruitment of two silencers, MeCP2 and histone deacetylase 2 (20). In addition, posttranscriptional miR-122 may target and regulate glut3 expression as well. In contrast, no change in placental GLUT1 was seen in both states of maternal calorie restriction. However, a proportional diminution in transplacental leucine transport is mediated by reduced placental LAT2 with increasing severity of the condition. This reduction in LAT2 is perhaps posttranscriptionally regulated by miR-149. The next step would be to determine the role of these posttranscriptional microRNA mechanisms in the human placenta associated with intrauterine growth restriction. Our present murine observations are novel and provide the basis for considering future biological confirmation in vitro toward development of targeted drug therapeutics for reversing fetal growth restriction before fetal compromise becomes incompatible with life or results in an unhealthy childhood and adult life related to the subsequent development of chronic disorders.
Acknowledgments
This work was supported by Grants HD-41230 and HD-081206 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Appendix
See Table 3.
Table 3.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| GLUT1 | GLUT1 antibody | Dr Sherin Devaskar provided this antibody | Rabbit, polyclonal | 1:1000 | |
| GLUT3 | GLUT3 antibody | Dr Sherin Devaskar provided this antibody | Rabbit, polyclonal | 1:1000 | |
| LAT2 | LAT2 antibody (G19) | Santa Cruz Biotechnology, sc-27581 | Goat polyclonal | 1:500 | |
| SNAT1 | SNAT1 antibody (H-60) | Santa Cruz Biotechnology, sc-67080 | Rabbit, polyclonal | 1:500 | |
| SNAT2 | SNAT2 antibody (G-8) | Santa Cruz Biotechnology, sc-166366 | Mouse monoclonal | 1:500 | |
| SNAT4 | SNAT4 antibody (R-60) | Santa Cruz Biotechnology, sc-67086 | Rabbit, polyclonal | 1:500 | |
| KDM3A | JMJD1A antibody (H-300) | Santa Cruz Biotechnology, sc-292213X | Rabbit polyclonal | 1:50 | |
| Vinculin | Monoclonal antivinculin antibody | Sigma-Aldrich Co | Mouse monoclonal | 1:5000 |
Footnotes
- AT
- acceleration time
- ChIP
- chromatin immunoprecipitation
- Cq
- quantification cycle
- e
- embryonic day
- ET
- ejection time
- GD
- gestational day
- GLUT1
- glucose transporter isoform 1
- GLUT3
- glucose transporter isoform 3
- 5-hmC
- 5-hydroxymethylated cytosine
- IP
- intraplacental
- IUGR
- intrauterine growth restriction
- KDM3A
- histone 3 lysine demethylase
- LAT2
- L amino acid transporter isoform 2
- LVEDD
- left ventricular end-diastolic dimension
- MCR
- maternal calorie restriction
- MeCP2
- methyl CpG binding protein 2
- MiMCR
- mild MCR
- MoMCR
- moderate MCR
- PkV
- peak systolic velocity
- qPCR
- quantitative PCR
- SDS
- sodium dodecyl sulfate
- SNAT
- sodium-coupled neutral amino acid transporter
- TP
- transplacental
- wt
- wild type.
References
- 1. Triunfo S, Lanzone A. Impact of maternal under nutrition on obstetric outcomes. J Endocrinol Invest. 2015;38:31–38. [DOI] [PubMed] [Google Scholar]
- 2. Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010;83:325–331. [DOI] [PubMed] [Google Scholar]
- 3. Barker DJ. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Biol Sci. 1995;262:37–43. [DOI] [PubMed] [Google Scholar]
- 4. Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. [DOI] [PubMed] [Google Scholar]
- 5. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. [DOI] [PubMed] [Google Scholar]
- 6. Victora CG, Adair L, Fall C, et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371:340–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–230. [DOI] [PubMed] [Google Scholar]
- 8. Allison RD, Hale SA, Harvey BJ, et al. The American College of Preventive Medicine Position Statement on Hepatitis C Virus Infection. Am J Prev Med. 2016;50:419–426. [DOI] [PubMed] [Google Scholar]
- 9. Devaskar SU, Chu A. Intrauterine growth restriction: hungry for an answer. Physiology (Bethesda). 2016;31:131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagnon R. Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol. 2003;110(suppl 1):S99–S107. [DOI] [PubMed] [Google Scholar]
- 11. Rozance PJ, Brown LD, Thorn SR, Anderson MS, Hay WW., Jr Intrauterine growth restriction and small-for-gestational age infants. Avery's Neonatology: Pathophysiology: Management of the Newborn. MacDonald MG, Seshia MMK. (Eds.) 7th ed Kluwer, Wolters; 2016. [Google Scholar]
- 12. Henriksen T, Clausen T. The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations. Acta Obstet Gynecol Scand. 2002;81:112–114. [DOI] [PubMed] [Google Scholar]
- 13. Dimasuay KG, Boeuf P, Powell TL, Jansson T. Placental responses to changes in the maternal environment determine fetal growth. Front Physiol. 2016;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut3 null heterozygous mice. Endocrinology. 2012;153:3995–4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadiq HF, Das UG, Tracy TF, Devaskar SU. Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res. 1999;823:96–103. [DOI] [PubMed] [Google Scholar]
- 16. Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001;50:2279–2286. [DOI] [PubMed] [Google Scholar]
- 17. Fung C, Ke X, Brown AS, Yu X, McKnight RA, Lane RH. Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr Res. 2012;72:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fung CM, White JR, Brown AS, et al. Intrauterine growth restriction alters mouse intestinal architecture during development. PLoS One. 2016;11:e0146542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Janot M, Cortes-Dubly ML, Rodriguez S, Huynh-Do U. Bilateral uterine vessel ligation as a model of intrauterine growth restriction in mice. Reprod Biol Endocrinol. 2014;12:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganguly A, Chen Y, Shin BC, Devaskar SU. Prenatal caloric restriction enhances DNA methylation and MeCP2 recruitment with reduced murine placental glucose transporter isoform 3 expression. J Nutr Biochem. 2014;25:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ganguly A, McKnight RA, Raychaudhuri S, Shin BC, Ma Z, Moley K, Devaskar SU. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab. 2007;292:E1241–E1255. [DOI] [PubMed] [Google Scholar]
- 22. Chen PY, Ganguly A, Rubbi L, et al. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics. 2013;45:565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia AP, Priego T, Palou M, Sanchez J, Palou A, Pico C. Early alterations in plasma ghrelin levels in offspring of calorie-restricted rats during gestation may be linked to lower sympathetic drive to the stomach. Peptides. 2013;39:59–63. [DOI] [PubMed] [Google Scholar]
- 24. Konieczna J, Palou M, Sanchez J, Pico C, Palou A. Leptin intake in suckling rats restores altered T3 levels and markers of adipose tissue sympathetic drive and function caused by gestational calorie restriction. Int J Obes (Lond). 2015;39:959–966. [DOI] [PubMed] [Google Scholar]
- 25. MacDonald L, Radler M, Paolini AG, Kent S. Calorie restriction attenuates LPS-induced sickness behavior and shifts hypothalamic signaling pathways to an anti-inflammatory bias. Am J Physiol Regul Integr Comp Physiol. 2011;301:R172–R184. [DOI] [PubMed] [Google Scholar]
- 26. Renaud HJ, Klaassen CD, Csanaky IL. Calorie restriction increases P-glycoprotein and decreases intestinal absorption of digoxin in mice. Drug Metab Dispos. 2016;44:366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varady KA, Allister CA, Roohk DJ, Hellerstein MK. Improvements in body fat distribution and circulating adiponectin by alternate-day fasting versus calorie restriction. J Nutr Biochem. 2010;21:188–195. [DOI] [PubMed] [Google Scholar]
- 28. Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta. 2013;34:1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang D, Pascual JM, Yang H, et al. A mouse model for Glut-1 haploinsufficiency. Hum Mol Genet. 2006;15:1169–1179. [DOI] [PubMed] [Google Scholar]
- 30. Ferre P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985;228:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thomas CR, Eriksson GL, Eriksson UJ. Effects of maternal diabetes on placental transfer of glucose in rats. Diabetes. 1990;39:276–282. [DOI] [PubMed] [Google Scholar]
- 32. Middleton JE, Griffiths WJ. Rapid colorimetric micro-method for estimating glucose in blood and C.S.F. using glucose oxidase. Br Med J. 1957;2:1525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernard JR, Liao YH, Hara D, et al. An amino acid mixture improves glucose tolerance and insulin signaling in Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2011;300:E752–E760. [DOI] [PubMed] [Google Scholar]
- 34. Rajakumar RA, Thamotharan S, Menon RK, Devaskar SU. Sp1 and Sp3 regulate transcriptional activity of the facilitative glucose transporter isoform-3 gene in mammalian neuroblasts and trophoblasts. J Biol Chem. 1998;273:27474–27483. [DOI] [PubMed] [Google Scholar]
- 35. Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75:835–844. [DOI] [PubMed] [Google Scholar]
- 36. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δδ C[T]) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 37. Chen H, Dzitoyeva S, Manev H. Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus. Restor Neurol Neurosci. 2012;30:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Berkley E, Chauhan SP, Abuhamad A. Doppler assessment of the fetus with intrauterine growth restriction. Am J Obstet Gynecol. 2012;206:300–308. [DOI] [PubMed] [Google Scholar]
- 39. Wesolowski SR, Hay WW., Jr Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production. Mol Cell Endocrinol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol. 2008;586:4567–4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harper JL, Caesar GA, Pennington KA, Davis JW, Schulz LC. Placental changes caused by food restriction during early pregnancy in mice are reversible. Reproduction. 2015;150(3):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77:1554–1562. [DOI] [PubMed] [Google Scholar]
- 43. Heilig CW, Saunders T, Brosius FC, 3rd, et al. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc Natl Acad Sci USA. 2003;100:15613–15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Graham JM., Jr GLUT1 deficiency syndrome as a cause of encephalopathy that includes cognitive disability, treatment-resistant infantile epilepsy and a complex movement disorder. Eur J Med Genet. 2012;55:332–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Vivo DC, Trifiletti RR, Jacobson RI, Ronen GM, Behmand RA, Harik SI. Defective glucose transport across the blood-brain barrier as a cause of persistent hypoglycorrhachia, seizures, and developmental delay. N Engl J Med. 1991;325:703–709. [DOI] [PubMed] [Google Scholar]
- 46. Wang D, Pascual JM, Yang H, et al. Glut-1 deficiency syndrome: clinical, genetic, and therapeutic aspects. Ann Neurol. 2005;57:111–118. [DOI] [PubMed] [Google Scholar]
- 47. Mimura I, Nangaku M, Kanki Y, et al. Dynamic change of chromatin conformation in response to hypoxia enhances the expression of GLUT3 (SLC2A3) by cooperative interaction of hypoxia-inducible factor 1 and KDM3A. Mol Cell Biol. 2012;32:3018–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang J, Sun C, Wang S, He Q, Li D. microRNA miR-10b inhibition reduces cell proliferation and promotes apoptosis in non-small cell lung cancer (NSCLC) cells. Mol Biosyst. 2015;11:2051–2059. [DOI] [PubMed] [Google Scholar]
- 49. Plummer PN, Freeman R, Taft RJ, et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. 2013;73:341–352. [DOI] [PubMed] [Google Scholar]
- 50. Wang X, Ling CC, Li L, et al. MicroRNA-10a/10b represses a novel target gene mib1 to regulate angiogenesis. Cardiovasc Res. 2016;110(1):140–150. [DOI] [PubMed] [Google Scholar]
- 51. Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482–490. [DOI] [PubMed] [Google Scholar]