Abstract
Fibrocytes are monocyte progenitor cells that have been implicated in normal and pathological tissue remodeling. Among the prominent chemokine receptors expressed by these cells is CXC motif receptor 4 (CXCR4), which, with its cognate ligand CXCL motif ligand 12 (CXCL-12), directs fibrocytes to sites of fibrosis. Fibrocytes have been implicated in the pathogenesis of thyroid-associated ophthalmopathy, the ocular manifestation of Graves' disease (GD), by virtue of their unique accumulation as CD34+ orbital fibroblasts (OFs). Fibrocytes also express high levels of functional TSH receptor (TSHR). Here, we determined CXCL-12 and CXCR4 expression in fibrocytes and GD-OF and whether that pathway interacts with TSHR. CXCL-12 is highly expressed in GD-OF, whereas CXCR4 levels are dramatically higher in fibrocytes. Levels of these proteins are differentially regulated by TSH in a cell type-specific manner. Further, CXCL-12 enhances the induction by TSH of IL-6 in fibrocytes but attenuates this induction in GD-OF. In contrast, in pure CD34+ OF, the interplay between TSH and CXCL-12 reverts to that observed in fibrocytes. Our results indicate that CXCL-12 enhances TSH actions in fibrocytes but inhibits them in GD-OF, a dichotomy imposed by factors emanating from CD34− OF. They also suggest a potentially important modulatory role for CD34− OF in determining the factors that influence pathological TSHR signaling in the TAO orbit.
CD34+ fibrocytes are progenitor cells that derive from the monocyte lineage and play important roles in wound repair and fibrosis (1). They are important participants in tissue remodeling and apparently serve complex roles in immune responses by virtue of their ability to present antigens, express coactivation factors, and generate a diverse array of cytokines (2–6). They infiltrate tissues where they can be identified as exhibiting phenotypes distinct from fibroblasts and other similarly appearing cells (7). An important and recently identified attribute of fibrocytes is their high level of functional TSH receptor (TSHR) expression (2). Fibrocytes activated through TSHR express a number of genes encoding proteins that participate in inflammation (3, 4, 8).
CXCL-12, also known as stromal cell-derived factor 1 (SDF1), forms an important, widely distributed chemokine network involved in T-cell and macrophage trafficking (9), angiogenesis (10), and tumor progression (11). It belongs to the CXC family of chemokines (12). Its expression is induced by several factors, including other cytokines and growth factors (13–15). CXCL-12 binds to its cognate receptor, CXCR4 (16). The CXCL-12/CXCR4 pathway is involved in orchestrating the tissue targeting of fibrocytes (17, 18). Fibrocytes uniformly express CXCR4 (7). In mouse models of bleomycin-induced lung fibrosis, interrupting the CXCL-12/CXCR4 pathway spares the lung of fibrocyte infiltration and abrogates tissue activation. Besides chemoattraction, the impact of the CXCL-12/CXCR4 pathway on other aspects of fibrocyte function has not been examined previously.
Thyroid-associated ophthalmopathy (TAO) is the disfiguring ocular manifestation of Graves' disease (GD) that can culminate in visually debilitating fibrosis (19, 20). GD results from the loss of immune tolerance to the TSHR. Activating antibodies targeting TSHR underlie the disregulation of thyroid function in GD; however, their role in the pathogenesis of TAO is less clear. At the center of the orbital manifestations of GD are orbital fibroblasts (OFs), which exhibit heterogeneous phenotypes (21). We have reported previously that circulating fibrocytes become more abundant in individuals with GD and accumulate in orbital connective tissues in TAO (2). Further, OFs from donors with TAO (GD-OF) comprise a mixture of CD34+ and CD34− cells, and we surmise that the CD34+ OFs derive from fibrocytes on the basis of their retention of distinguishing cellular markers (7). CD34+ OF are uniquely detected in TAO orbital tissues (2). We postulate that CD34+ OFs help orchestrate the characteristic immune responses occurring within the TAO orbit. A key aspect of the phenotypic repertoire common to both fibrocytes and CD34+ OF is the expression of functional TSHR as well as other thyroid-associated autoantigens (2, 22, 23). When provoked by TSH or GD-specific, activating anti-TSHR stimulating immunoglobulins (TSIs), these cells express several cytokines that have been widely implicated in the pathogenesis of TAO, including IL-6, IL-1β, and TNF-α (3). The counterparts of CD34+ OF, namely CD34− OF, represent the fibroblasts uniformly populating healthy orbits (23). We have provided evidence that they generate as yet unidentified molecular signal(s) involved in suppressing the inflammatory phenotype the fibrocytes exhibit as they infiltrate the TAO orbit (22, 23).
The current studies focused on comparing CXCL-12 and CXCR4 expression in fibrocytes and GD-OF and determining whether this chemokine pathway might cross talk with that of TSHR. We report that although CXCL-12 is expressed abundantly in GD-OF, it is undetectable in fibrocytes. In contrast, although CXCR4 is expressed by fibrocytes from healthy control donors and individuals with GD, it cannot be detected in GD-OF. However, once mixed parental GD-OF, comprising an admixture of CD34+ and CD34− cells, are sorted into pure subsets, CD34+ OF express CXCR4, whereas pure CD34− OF express CXCL-12. CXCL-12 conditions responses to TSH and TSI in fibrocytes by enhancing their induction of IL-6. In contrast, the chemokine attenuates this same action in GD-OF. These findings implicate the CXCL-12/CXCR4 pathway as a mechanism through which residential TAO orbital connective tissues might both promote fibrocyte infiltration and amplify disease-related signaling mediated through TSHR. The interplay between the TSHR/CXCR4 signaling pathways could be therapeutically targeted as a means of dampening the TSI-provoked signaling associated with TAO.
Materials and Methods
Materials
DMEM (catalog 11965–092), fetal bovine serum (catalog 16000–044) and penicillin-streptomycin mixture (catalog 15140–122) were from Life Technology. Bovine TSH (bTSH) (catalog 609385) and 5,6-dichloro-β-D-ribofuranosylbenzimidazole (catalog 10010302) came from Cayman Chemical. AMD3100 was supplied by Selleckchem (catalog S3013). Mouse anti-CD34 fluorescein isothiocyanate (catalog 555822) and its isotype control (catalog 555749) were from BD. M22 activating anti-TSHR mAb was from Kronus (catalog M22–5c/00–690). SDF1-α human ELISA kit (catalog ab100637) was from Abcam. Recombinant human (rh)CXCL-12/SDF1-α (catalog 350-NS), rhCCL-21 (catalog 366–6C), and phycoerythrin-conjugated antihuman CXCR4 mAb (catalog FAB173P) were from R&D Systems. Dexamethasone (Dex) (catalog D4902) was from Sigma-Aldrich.
Cell culture
Fibrocytes were cultivated as described previously (2) from individuals with GD or from healthy donors. Subject participation occurred after obtaining informed consent following the practices approved by the Institutional Review Board of the University of Michigan health system. Briefly, peripheral blood mononuclear cells were isolated from blood by centrifugation over Ficoll-Paque Plus (catalog 17–1440-03; GE); 107 peripheral blood mononuclear cells were plated in each well of a 6-well array. Cells were covered with DMEM containing 10% fetal bovine serum, 2mM glutamine, sodium pyruvate (110 mg/mL), and penicillin/streptomycin. Medium was changed every 3–4 days. Unattached cells were discarded by gentle aspiration after 7 days, whereas adherent monolayers were incubated for another 3–5 days. They were maintained in a 37°C, humidified, 5% CO2 environment. Culture purity was verified to be more than 90% fibrocytes by flow cytometry for their surface markers.
GD-OFs were generated from surgical waste generated from orbital decompression surgery for severe TAO (21). None of the donors had undergone orbital irradiation or were receiving systemic corticosteroids at the time of study participation. Orbital fibroblasts from healthy tissues (H-OF) derived from healthy orbital tissues or from periorbital tissues obtained during cosmetic surgery. OFs were allowed to proliferate as previously described (21), and monolayers were covered with DMEM as described above. Culture strains were used between passages 3 and 8, an interval during which cell phenotypes remain constant.
RNA isolation and quantitative RT-PCR
RNA was extracted with the Aurum total RNA mini kit (catalog 732–6820; Bio-Rad). RNA was reverse-transcribed using a QuantiTec Reverse Transcription kit (catalog 205314; QIAGEN). Quantitative RT-PCR was conducted with iQ SYBR Green supermix (catalog 170–8882; Bio-Rad) in a Bio-Rad CFX96 thermocycler. PCRs were performed in triplicate and glyceraldehyde-3-phosphate dehydrogenase was used as the housekeeping gene control. The primers used were as follows: CXCL-12, forward 5′-ATGAACGCCAAGGTCGTG-3′ and reverse 5′-TTCGAAGAATCGGCATGG-3′; IL-6, forward 5′-TGAGAAAGGAGACATGTAACAAGAGT-3′ and reverse 5′-TTGTTCCTCACTACTCTCAAATCTGT-3′. TaqMan gene expression assays for C-C chemokine receptor (CCR)2 (catalog Hs01560352), CCR3 (catalog Hs00266213), CCR5 (catalog Hs00152917), CCR7 (catalog Hs01013469), and glyceraldehyde-3-phosphate dehydrogenase (catalog Hs02758991) were synthesized by Applied Biosystems. CXCR4 custom made primers (catalog PPH00621A) were from QIAGEN.
Flow cytometry and cell sorting
Dispersed OF and fibrocytes were washed with staining buffer containing PBS with 0.1% sodium azide and 1% BSA. Cell layers were incubated with heat-inactivated mouse serum for 5 minutes and then Peridinin Chlorophyll Protein Complex-conjugated antihuman CXCR4 monoclonal antibody for 30 minutes at 4°C. Following extensive rinsing, cells were resuspended in staining buffer at 4°C and subjected to flow cytometric analysis on an LSR II instrument (BD Biosciences) (2). Mean fluorescent intensity was calculated as a ratio of mean fluorescence sample/isotype control fluorescence. Viable cells were gated on the basis of forward light scatter, and data were analyzed with the FCS Express software program (De Novo Software). All studies were performed at least 3 times.
Cell sorting was accomplished with confluent cultures of early passage GD-OF parental strains comprising a mixture of CD34+ and CD34− OF from donors with stable TAO (2). Detached cells were stained for 30 minutes at 4°C with fluorescein isothiocynate-conjugated antimouse CD34 or its isotype control. Washed cells were sorted under sterile conditions with a FACSAria III (BD Biosciences). Parental, CD34+ and CD34− OF populations were recultured for 48 hours, and nothing (controls) or bTSH was added 6 hours before RNA extraction.
Statistics
Statistical analysis was performed using a 2-tailed Student's t test. Data are reported as the mean ± SD. All experiments were conducted in triplicate and performed at least 3 times.
Results
CXCL-12 and CXCR4 expression in OFs and fibrocytes
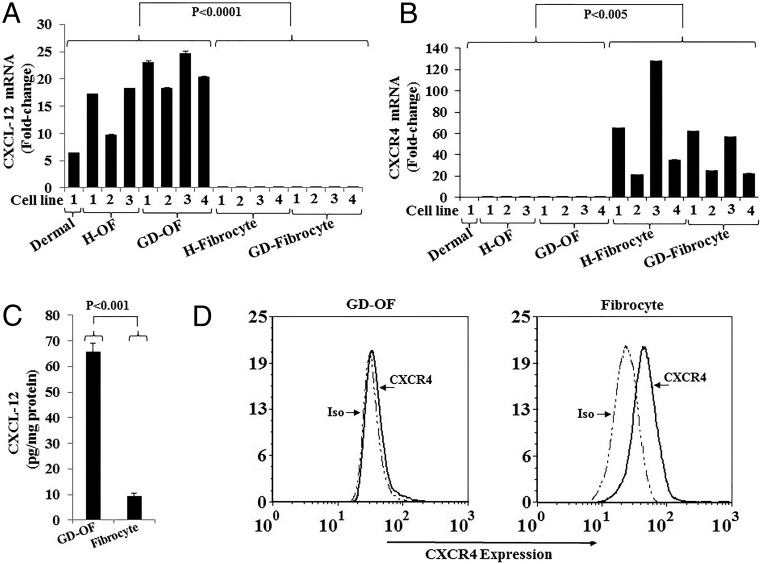
Initial studies were conducted to characterize the expression patterns of CXCL-12 and CXCR4 mRNAs in OFs and fibrocytes. The respective transcripts were quantified in 4 strains of GD-OF, 3 H-OF strains, dermal fibroblasts (as control), 4 GD-fibrocyte strains, and 4 H-fibrocyte strains, each from a separate donor. As the data in Figure 1A demonstrate, CXCL-12 mRNA is relatively abundant in all fibroblast strains examined and no obvious differences could be detected between GD-OF and H-OF. In contrast, CXCL-12 mRNA is either undetectable or is expressed at extremely low levels in all 8 fibrocyte strains tested. CXCR4 mRNA expression exhibits a distinctly different pattern from that of CXCL-12 in fibrocytes. The receptor-encoding transcript is very rare in all OF strains and in dermal fibroblasts but is abundant in the fibrocyte strains examined (Figure 1B). As with CXCL-12, no apparent differences could be detected in strains from healthy donors and those with GD. The divergent pattern of relative CXCL-12 and CXCR4 mRNA levels in GD-OF and fibrocytes results in similar cell type-specific patterns of the respective proteins (Figure 1, C and D). Levels of CXCL-12 protein in the medium of untreated GD-OFs were 65.84 ± 3.20-pg/mg protein, whereas those of fibrocytes were 9.46 ± 0.91-pg/mg protein (mean ± SD, n = 3, P < .001). In contrast, cell-surface display of CXCR4, as determined by flow cytometry, was dramatically greater in fibrocytes than GD-OF (Figure 1D).
Figure 1.
Expression of CXCL-12 and CXCR4 in orbital fibroblasts (OFs) and fibrocytes. Multiple strains of OF from healthy donors (H-OF), individuals with GD (GD-OF), and fibrocytes from healthy controls and patients with GD were cultured as described in Materials and Methods, cell layers harvested, RNA extracted, reverse transcribed, and subjected to real-time PCR for (A) CXCL-12 mRNA and (B) CXCR4 mRNA. C, Comparison between CXCL-12 production in GD-OF and fibrocytes. Medium was collected from confluent cultures and subjected to a specific CXCL-12 ELISA. Cytokine concentration was normalized to the protein content in the respective cell layers. Data are expressed as the mean ± SD of 3 independent replicates. D, Gating strategy of the cytometric analysis of CXCR4 displayed on the cell surface of GD-OF (left) and fibrocytes (right).
Cell sorting GD-OF into pure subsets reveals distinct patterns of CXCL-12 and CXCR4 expression
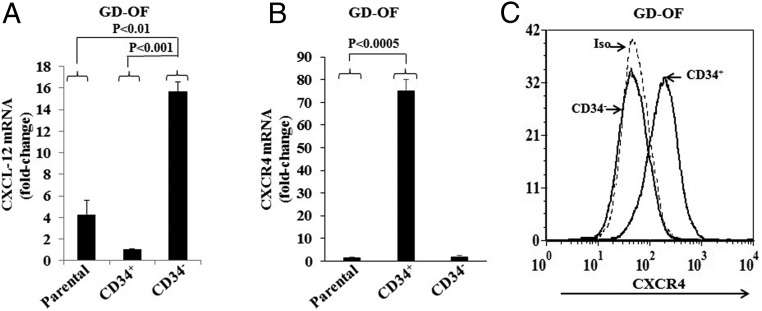
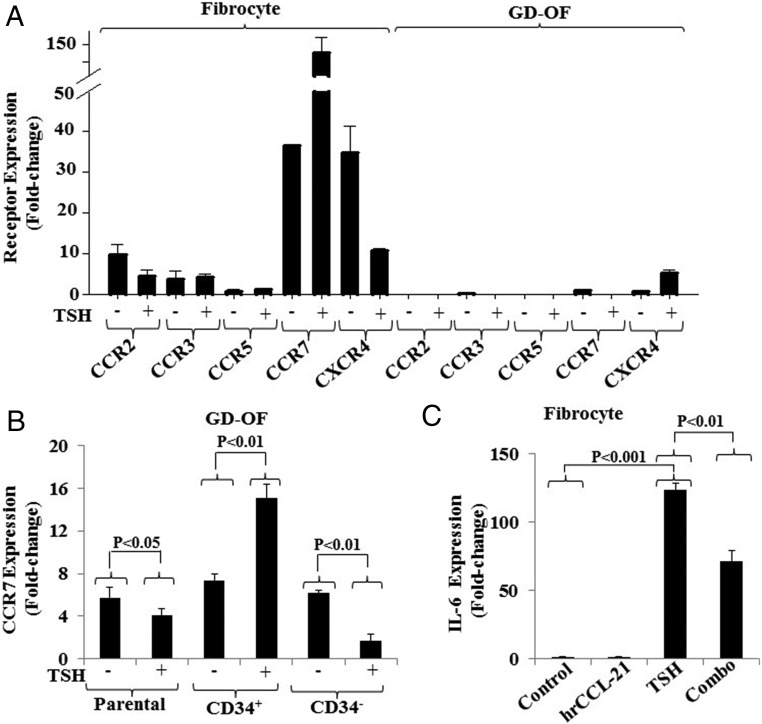
GD-OF cultures contain a mixture of CD34+ and CD34− fibroblasts (2). Substantial evidence indicates that CD34+ cells in GD-OF cultures derive from circulating fibrocytes, based on their phenotypic characteristics, including retention of several distinguishing markers (2). Similarities between fibrocytes and CD34+ OF more fully emerge when mixed populations of GD-OF are separated into pure subsets (22, 23). Thus, patterns of CXCL-12 and CXCR4 expression were next examined in sorted cultures. CD34− OF express dramatically higher levels of CXCL-12 than do mixed GD-OF parental strains or pure CD34+ OF (Figure 2A). CXCL-12 mRNA levels in CD34− OF are 72.71 ± 7.46-fold above those in mixed cultures (P < .01) and 93.33 ± 0.57-fold above those in pure CD34+ OF (P < .001). In contrast, CXCR4 mRNA levels were extremely low in parental GD-OF (1.43 ± 0.37-fold change) but are increased to 75.04 ± 5.05-fold change (P < .001) in pure CD34+ OF (Figure 2B). The low-level CXCR4 transcript abundance in CD34− OF (1.81 ± 0.53-fold change) resembles that in the mixed parental cultures (P < .0005). Flow-cytometric analysis of surface CXCR4 display reveals a consistent pattern in that CD34+ OF abundantly express the receptor, whereas CD34− OF do not (Figure 2C). Thus a clear-cut divergent pattern of CXCL-12 and CXCR4 expression emerges in the 2 populations comprising GD-OF. Further, the expression patterns in CD34+ OF resemble those in circulating fibrocytes.
Figure 2.
Expression patterns of CXCL-12 and CXCR4 in GD-OF subjected to cell sorting into pure CD34+OF and CD34−OF subsets. Monolayers were harvested, RNA extracted, and mRNA subjected to PCR for (A) CXCL-12 mRNA and (B) CXCR4 mRNA. Data are expressed as the mean ± SD of 3 independent replicates. C, Flow cytometric analysis of CXCR4 display on CD34+ and CD34− OF.
The influence of TSHR activation on CXCL-12 and CXCR4 expression in GD-OF and fibrocytes
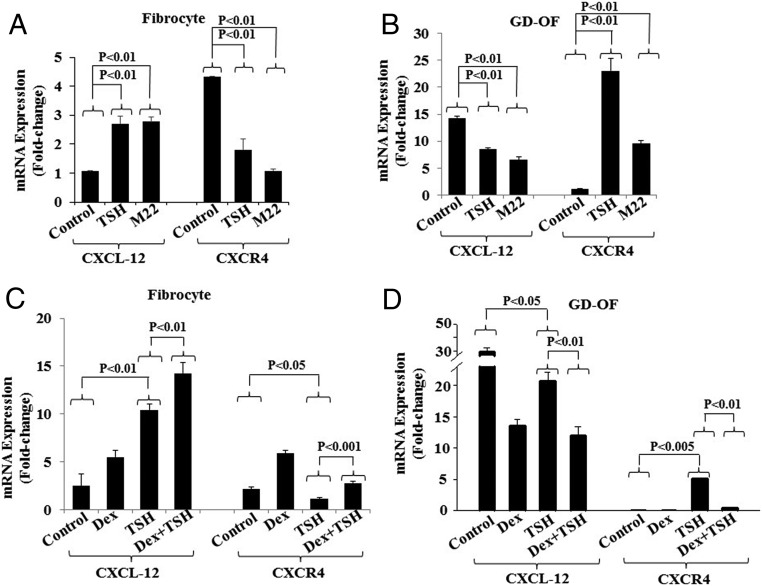
TSHR has been implicated in the pathogenesis of TAO (19, 24) and is detected at relatively low levels in GD-OF (25). In contrast, the receptor is considerably more abundant in fibrocytes (2, 22, 23). Further, TSH and TSIs have been shown to alter cytokine gene expression in these cells (3, 4). Thus, we next determined what impact bTSH and the monoclonal TSI, designated M22, might exert on CXCL-12 and CXCR4 expression in fibrocytes and GD-OF. Both bTSH (5 mIU/mL) and M22 (1 μg/mL) up-regulate CXCL-12 mRNA expression in GD-OF from a relatively low baseline of 1.06 ± 0.02 to 2.70 ± 0.26 after 6 hours, and to 2.78 ± 0.17, respectively (both P < .01) (Figure 3A). In contrast to their inductions in fibrocytes, both attenuated CXCL-12 expression in GD-OF (both P < .01) (Figure 3B). The effects of the 2 agents on CXCR4 were inversely related to their effects on CXCL-12 in each cell type. Both attenuated expression of CXCR4 in fibrocytes (Figure 3A) where levels are reduced to 1.80 ± 0.38, P < .05 and 1.08 ± 0.07 (P < .01) compared with controls (4.34 ± 0.78). But both induced CXCR4 expression in GD-OF (Figure 3B). Levels in untreated controls were 1.09 ± 0.15-fold change and increased in to 23.04 ± 2.31-fold change (P < .01) following treatment with bTSH and to 9.56 ± 0.63 (P < .01) in those treated with M22 after 6 hours (Figure 3B). Impact of glucocorticoids on fibrocytes has not been explored previously. Cultures were thus treated with the potent pure corticosteroid, Dex (10nM). Both expression and induction by bTSH of CXCL-12 and CXCR4 were enhanced in fibrocytes (Figure 3C) but attenuated in GD-OF (Figure 3D).
Figure 3.
Effects of bTSH, M22, and Dex on CXCL-12 and CXCR4 expression in fibrocytes (A and C) and GD-OF (B and D). Confluent cultures were treated with nothing, bTSH (5 mIU/mL), M22 (1 μg/mL), and/or Dex (10nM) for 6 hours, alone or in the combination indicated. Cell layers were harvested and RNA extracted and subjected to real-time PCR for CXCL-12 and CXCR4 mRNA. Data are expressed as the mean ± SD of 3 independent replicates.
CXCL-12 can both induce IL-6 and enhance the induction of IL-6 by bTSH in fibrocytes
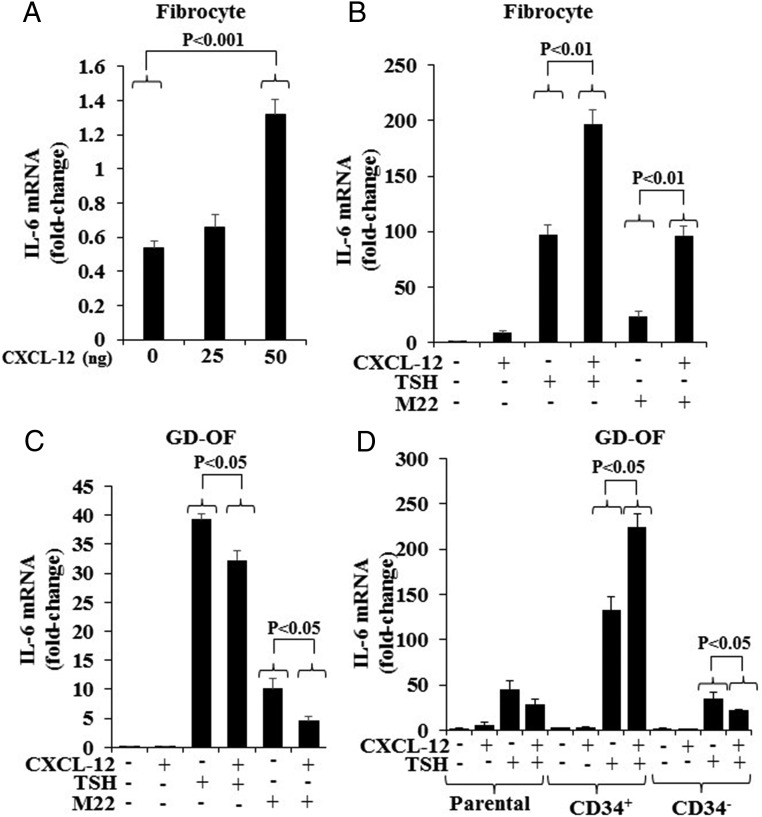
rhCXCL-12 was examined for its potential to influence the previously established induction by TSH of IL-6. As a single agent, CXCL-12 modestly induces IL-6 when compared with the effect of bTSH (Figure 4). The effect is concentration dependent (Figure 4A). IL-6 induction following treatment with both rhCXCL-12 (50 ng/mL) and bTSH (5 mIU/mL) is considerable greater than that observed with either compound alone (control, 0.64 ± 0.11; CXCL-12, 8.23 ± 2.19; bTSH, 97.42 ± 8.84; CXCL-12 plus bTSH, 197.23 ± 12.44 [bTSH alone vs combination, P < .01]; rhCXCL-12 also enhanced the induction of M22 in fibrocytes [P < .01]) (Figure 4B). In contrast to its effects in fibrocytes, CXCL-12 substantially attenuated the induction by bTSH and M22 of IL-6 in GD-OF (both P < .01) (Figure 4C).
Figure 4.
Effects of rhCXCL-12 on the induction by bTSH and M22 of IL-6 in fibrocytes and GD-OF. A, Graded concentrations of rhCXCL-12 were added to confluent fibrocyte cultures for 6 hours, monolayers harvested, and RNA extracted and subjected to real-time PCR for IL-6 mRNA. rhCXCL-12 (50 ng/mL), bTSH (5 mIU/mL), and M22 (1 μg/mL) were added for 6 hours in the combinations indicated along the abscissas to (B) fibrocytes and (C) GD-OF. D, Parental GD-OFs were subjected to cytometric cell sorting into pure CD34+ OF and CD34− OF. These were then treated as in B and C with the indicated combinations for 6 hours, and then RNA was processed for real-time PCR targeting IL-6 mRNA. Data are expressed as the mean ± SD of 3 independent replicates.
Given the very different impact of rhCXCL-12 in fibrocytes and GD-OF, its effects were next determined in pure CD34+ OF, thus testing the hypothesis that CD34− OF influences the phenotype displayed by CD34+ OF. As the data in Figure 4D demonstrate, whereas rhCXCL-12 suppresses the induction by bTSH of IL-6 in a (mixed) parental GD-OF by 37% and pure CD34− OF by 40% (both P < .05), it enhances the induction in pure CD34+ OF by 40% (P < .05). Thus the cellular context of GD-OF subsets and the presence of CD34− OF represent potentially important determinants of the impact that CXCL-12 exerts on CD34+ OF.
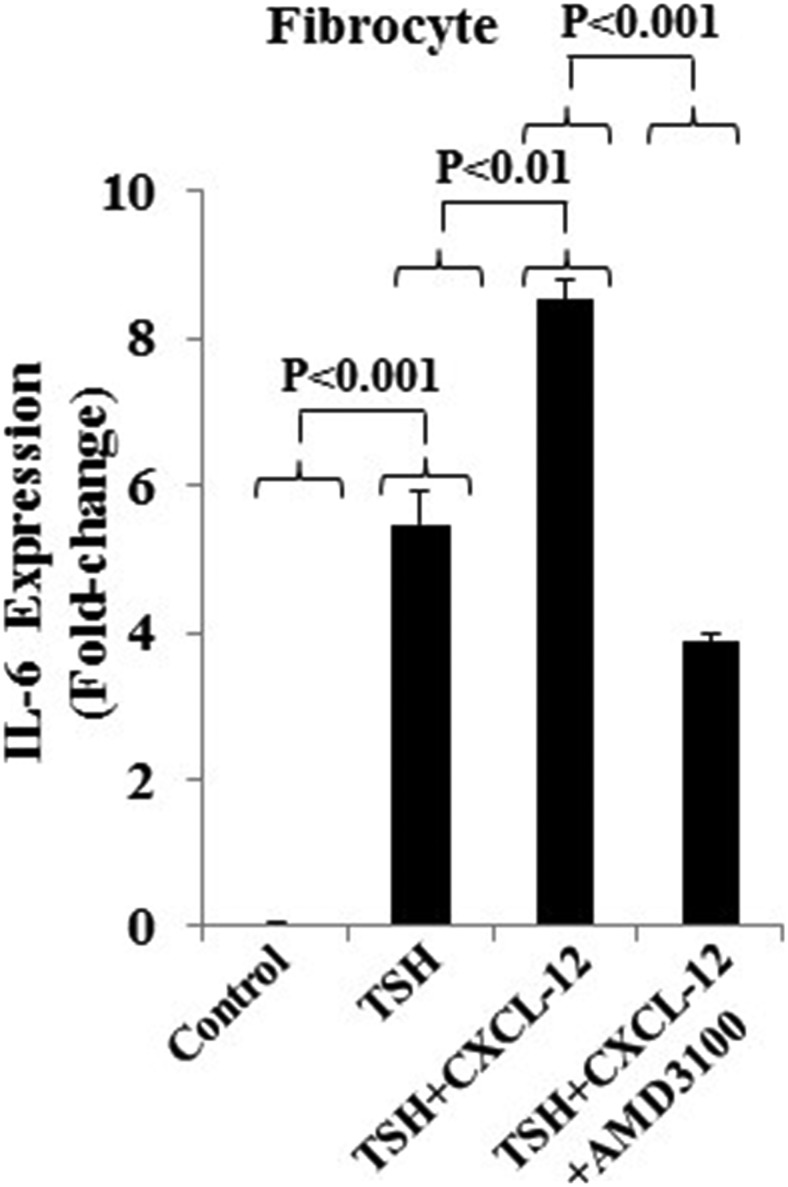
To prove that these effects of CXCL-12 are mediated by CXCR4, the specific CXCR4 antagonist, AMD3100 (5μM) was utilized. The reagent could block the actions of CXCL-12 in enhancing the induction of IL-6 by bTSH by 50% (P < .01) (Figure 5).
Figure 5.
The effects of AMD3100 on the induction of IL-6 mRNA by rhCXCL-12 and bTSH. Confluent cultures of fibrocytes were treated with AMD3100 (5μM), bTSH (5 mIU/mL), and rhCXCL-12 (50 ng/mL) in the combinations indicated for 6 hours. RNA was extracted from monolayers and processed for real-time PCR for IL-6 mRNA. Data are expressed as the mean ± SD of 3 independent replicates.
Fibrocytes express other chemokine receptors
The expression of several other receptor proteins was inventoried as potentially relevant to fibrocyte trafficking and influencing actions mediated through TSHR. As the findings in Figure 6A demonstrate, CCR2, CCR3, and CCR5 are expressed at extremely low levels in fibrocytes and are undetectable in GD-OF. Their levels of expression are essentially uninfluenced by bTSH. In contrast, CCR7 is detected at relatively high levels in fibrocytes and is greatly induced by bTSH (control, 36.49 ± 0.07 and bTSH, 128.01 ± 44.02; P < .001). The baseline expression of CCR7 is slightly higher in pure CD34+ OF than in the CD34− subset but these levels are substantially increased following treatment with bTSH in the former while reduced in the latter (Figure 6B). Fibrocytes were next treated with the CCR7-activating ligand, CCL-21 (0.2 μg/mL). Unlike CXCL-12, rhCCL-21 fails to influence IL-6 mRNA expression as a single agent but attenuates the induction by bTSH of IL-6 (Figure 6C). Thus, CXCL-12 and CCL-21 have opposing influences on the induction by TSH of IL-6 in fibrocytes. The profile of chemokines acting on fibrocytes in their microenvironmental context may condition the responses of these cells to TSH.
Figure 6.
A, Survey of chemokine receptor expression in fibrocytes and GD-OF. Fibrocytes were cultivated to confluence and then were treated with nothing or bTSH (5 mIU/mL) for 6 hours. Cell layers were harvested, RNA extracted, and subjected to real-time PCR for the targets indicated. B, Confluent GD-OFs were subjected to cell sorting into pure CD34+ and CD34− fibroblasts, recultured for 2 days and incubated without or with bTSH for the final 6 hours. Cell layers were harvested and RNA extracted and subjected to real-time PCR for CCR7 mRNA. C, Confluent fibrocytes were treated with rhCCL-21 (50 ng/mL) and bTSH (5 mIU/mL) alone or in combination for 6 hours. Monolayers were harvested, RNA extracted and processed for real-time PCR targeting IL-6 mRNA quantification. Data are expressed as the mean ± SD of 3 independent replicates.
Discussion
Chemokines and their receptors play essential roles in cell trafficking and have been scrutinized for their participation in disease processes. They have emerged as attractive therapeutic candidates in autoimmune diseases and cancer (26). Besides their participation in tissue infiltration, these cytokine/receptor cognates can provoke responses in target cells not directly related to locomotion and trafficking. The importance of the CXCL-12/CXCR4 pathway in fibrocyte targeting of fibrotic lesions was demonstrated initially by Phillips et al (18) who utilized bleomycin-treated mice that were administered a CXCL-12 neutralizing antibody. Recruitment to the lung of CD45+ColI+CXCR4+ fibrocytes, deposition of collagen, and fibrosis were attenuated in animals receiving the antibody. In a follow-up study, the same group found that levels of CXCL-12 were higher in both lung tissues and plasma from individuals with pulmonary fibrosis (27). Song et al (28) demonstrated that AMD3100 could inhibit lung fibrosis. CCL-21/CCR7 is involved in a fibrocyte-dependent renal fibrosis model (29), whereas circulating levels of 3 CCR2 ligands become elevated following fluorescein isothiocyanate-induced lung fibrosis (30), whereas CCR2-null mice are protected from disease (31).
We have focused here on CXCL-12/CXCR4 and its previously unexplored interactions with TSHR because both pathways exert dominant influences on fibrocytes (2, 3, 12, 17, 27). Insinuation of fibrocytes in the pathogenesis of TAO was based on the identification of these cells within the diseased orbit and their high level expression of functional TSHR (2). Several earlier studies have quantified serum and tissue levels of chemokines and related cytokines in GD and TAO. For instance, CC chemokines were found to be highly expressed in the thyroid in GD (32). Expression of the chemokine, regulated on activation, normal T cells expressed and secreted, correlated with T-cell infiltration of the thyroid in GD (33), whereas serum and thyroid CXCL-9 and CXCL-10 levels were elevated (34, 35). Thyroid epithelium exposed to inflammatory cytokines produces substantial CXCL-12 (36). Intrathyroidal CXCR3+ and CCR5+ T cells, primarily within the CD45RO+ subset, were more abundant than in peripheral T cells (37). Interferon-γ induces CXCL-9, CXCL-10, CXCL-11, and CCL-2 in GD-OF and extraocular muscle cells, whereas PPARα and PPARγ agonists modulate these inductions, but in a dissimilar pattern (38–41). Regulated on activation, normal T cells expressed and secreted- and IL-16-dependent T-cell migration could be induced in GD-OF by IL-1β (42) and GD-IgG (43). However, no previous studies had examined the potential role of CXCL-12/CXCR4 in TAO, yet inferences to potential involvement of this pathway can be made from experimental models of other diseases.
The repertoire of cytokines and related molecules expressed by fibrocytes depends in large part on their molecular environment. This changes as they transition from the circulation to orbital tissue-infiltrating cells which are recognized as CD34+ OF. We postulate that several factors generated by their neighbors within the orbit exert influence on their function and the direction in which they differentiate. The cellular neighborhood within the orbit comprises several types including CD34− OF, endothelial cells, vascular smooth muscle, and extraocular muscle cells. Substantial differences have been identified in the phenotypes of CD34+ and CD34− OF, including the expression of genes encoding thyroid-associated proteins (22, 23). Divergent expression of CXCL-12 and CXCR4 in these fibrocyte subsets must now be added to the list of phenotypic attributes that differ dramatically in CD34+ and CD34− OF (Figure 2). It is therefore possible that CXCL-12 might represent a molecular bridge between CD34+ and CD34− OFs.
The current studies characterize the expression pattern of CXCL-12/CXCR4 in fibrocytes and GD-OF. They identify a previously unrecognized enhancing influence that this pathway can exert on TSH and TSI actions in these cells. In contrast, another chemokine cognate pair, CCL-21/CCR7, can attenuate the actions mediated through TSHR (Figure 6C). Thus, it may be possible to therapeutically manipulate cross talk occurring between these and related pathways, either by altering activating ligand concentrations with neutralizing antibodies or by down-regulating receptor expression. Alternatively, chemokine pathways might be altered pharmacologically with newly developed drugs or those repurposed from other diseases. An example is rapamycin which can down-regulate both CXCR4 and CXCL-12 levels (17).
Acknowledgments
This work was supported by National Institutes of Health Grants EY008976 and 5UM1AI110557, a Center for Vision Grant EY007003 from the National Eye Institute, an unrestricted grant from Research to Prevent Blindness, and by the Bell Charitable Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- bTSH
- bovine TSH
- CCR
- C-C chemokine receptor
- CXCL
- CXC motif chemokine
- CXCR
- CXC motif chemokine receptor
- Dex
- dexamethasone
- GD
- Graves' disease
- HO-F
- orbital fibroblast from healthy tissue
- OF
- orbital fibroblast
- rh
- recombinant human
- TAO
- thyroid-associated ophthalmopathy
- TSHR
- TSH receptor
- TSI
- TSHR stimulating immunoglobulin.
References
- 1. Bucala R. Fibrocytes at 20 years. Mol Med. 2015;21(suppl 1):S3–S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Douglas RS, Afifiyan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gillespie EF, Papageorgiou KI, Fernando R, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Endocrinol Metab. 2012;97:E740–E746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: clear delineation of its cAMP-independent actions. PLoS One. 2013;8:e75100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niedermeier M, Reich B, Rodriguez Gomez M, et al. CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci USA. 2009;106:17892–17897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:6307–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li B, Smith TJ. Regulation of IL-1 receptor antagonist by TSH in fibrocytes and orbital fibroblasts. J Clin Endocrinol Metab. 2014;99:E625–E633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradfield PF, Amft N, Vernon-Wilson E, et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–2482. [DOI] [PubMed] [Google Scholar]
- 10. Rempel SA, Dudas S, Ge S, Gutiérrez JA. Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res. 2000;6:102–111. [PubMed] [Google Scholar]
- 11. Koshiba T, Hosotani R, Miyamoto Y, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–3535. [PubMed] [Google Scholar]
- 12. Strieter RM, Gomperts BN, Keane MP. The role of CXC chemokines in pulmonary fibrosis. J Clin Invest. 2007;117:549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jourdan P, Vendrell JP, Huguet MF, et al. Cytokines and cell surface molecules independently induce CXCR4 expression on CD4+ CCR7+ human memory T cells. J Immunol. 2000;165:716–724. [DOI] [PubMed] [Google Scholar]
- 14. Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. [DOI] [PubMed] [Google Scholar]
- 15. McCandless EE, Budde M, Lees JR, Dorsey D, Lyng E, Klein RS. IL-1R signaling within the central nervous system regulates CXCL12 expression at the blood-brain barrier and disease severity during experimental autoimmune encephalomyelitis. J Immunol. 2009;183:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–185. [DOI] [PubMed] [Google Scholar]
- 17. Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol Vis Sci. 2014;55:1735–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy. Exp Eye Res. 2016;142:83–91. [DOI] [PubMed] [Google Scholar]
- 21. Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392. [DOI] [PubMed] [Google Scholar]
- 22. Fernando R, Atkins S, Raychaudhuri N, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernando R, Lu Y, Atkins SJ, Mester T, Branham K, Smith TJ. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99:E1236–E1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 protein levels may underlie differential expression of UDP-glucose dehydrogenase by fibroblasts: role in susceptibility to orbital Graves disease. J Biol Chem. 2011;286:24487–24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bahn RS, Dutton CM, Natt N, Joba W, Spitzweg C, Heufelder AE. Thyrotropin receptor expression in Graves' orbital adipose/connective tissues: potential autoantigen in Graves' ophthalmopathy. J Clin Endocrinol Metab. 1998;83:998–1002. [DOI] [PubMed] [Google Scholar]
- 26. Ingold B, Simon E, Ungethüm U, et al. Vascular CXCR4 expression - a novel antiangiogenic target in gastric cancer? PLoS One. 2010;5:e10087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. [DOI] [PubMed] [Google Scholar]
- 28. Song JS, Kang CM, Kang HH, et al. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp Mol Med. 2010;42:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakai N, Wada T, Yokoyama H, et al. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore BB, Paine R, 3rd, Christensen PJ, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 32. Ashhab Y, Dominguez O, Sospedra M, Roura-Mir C, Lucas-Martín A, Pujol-Borrell R. A one-tube polymerase chain reaction protocol demonstrates CC chemokine overexpression in Graves' disease glands. J Clin Endocrinol Metab. 1999;84:2873–2882. [DOI] [PubMed] [Google Scholar]
- 33. Simchen C, Lehmann I, Sittig D, Steinert M, Aust G. Expression and regulation of regulated on activation, normal T cells expressed and secreted in thyroid tissue of patients with Graves' disease and thyroid autonomy and in thyroid-derived cell populations. J Clin Endocrinol Metab. 2000;85:4758–4764. [DOI] [PubMed] [Google Scholar]
- 34. Antonelli A, Rotondi M, Fallahi P, et al. Increase of interferon-γ-inducible CXC chemokine CXCL10 serum levels in patients with active Graves' disease, and modulation by methimazole therapy. Clin Endocrinol (Oxf). 2006;64:189–195. [DOI] [PubMed] [Google Scholar]
- 35. Romagnani P, Rotondi M, Lazzeri E, et al. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves' disease. Am J Pathol. 2002;161:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Armengol MP, Cardoso-Schmidt CB, Fernández M, Ferrer X, Pujol-Borrell R, Juan M. Chemokines determine local lymphoneogensis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune disease. J Immunol. 2003;170:6320–6328. [DOI] [PubMed] [Google Scholar]
- 37. Aust G, Sittig D, Steinert M, Lamesch P, Lohmann T. Graves' disease is associated with an altered CXCR3 and CCR5 expression in thyroid-derived compared to peripheral blood lymphocytes. Clin Exp Immunol. 2002;127:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Antonelli A, Ferrari SM, Frascerra S, et al. Peroxisome proliferator-activated receptor-α agonists modulate CXCL9 and CXCL11 chemokines in Graves' ophthalmopathy fibroblasts and preadipocytes. Mol Cell Endocrinol. 2012;349:255–261. [DOI] [PubMed] [Google Scholar]
- 39. Antonelli A, Ferrari SM, Frascerra S, et al. β (CCL2) and α (CXCL10) chemokine modulations by cytokines and peroxisome proliferator-activated receptor-α agonists in Graves' ophthalmopathy. J Endocrinol. 2012;213:183–191. [DOI] [PubMed] [Google Scholar]
- 40. Antonelli A, Ferrari SM, Fallahi P, et al. Monokine induced by interferon γ (INFγ) (CXCL9) and INFγ inducible T cell α-chemoattractant (CXCL11) involvement in Graves' disease and ophthalmopathy: modulation by peroxisome proliferator-activated receptor-γ agonists. J Cin Endocrinol Metab. 2009;94:1803–1809. [DOI] [PubMed] [Google Scholar]
- 41. Antonelli A, Ferrari SM, Corrado A, et al. Extra-ocular muscle cells from patients with Graves' ophthalmopathy secrete α (CXCL10) and β (CCL2) chemokines under the influence of cytokines that are modulated by PPARγ. Autoimmun Rev. 2014;13:1160–1166. [DOI] [PubMed] [Google Scholar]
- 42. Sciaky D, Brazer W, Center DM, Cruikshank WW, Smith TJ. Cultured human fibroblasts express constitutive IL-16 mRNA: cytokine induction of active IL-16 protein synthesis through a caspase-3 dependent mechanism. J Immunol. 2000;164:3806–3814. [DOI] [PubMed] [Google Scholar]
- 43. Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950. [DOI] [PubMed] [Google Scholar]