Abstract
Zwitterionic polysaccharide antigens such as polysaccharide A (PSA) from Bacteroides fragilis have been shown to activate CD4+ T cells upon presentation by class II major histocompatibility complex (MHCII) on professional antigen presenting cells. For T cell recognition and activation, high affinity binding between MHCII and PSA is required, and complex N-glycans on conserved MHCII asparagine residues play a central role in controlling this interaction. By truncating these glycans in a myeloid-specific knockout of Mgat2, created using the LyzM-CRE mouse (M-cKO), we previously reported defects in PSA responses in vivo. Unfortunately, the M-cKO also showed a propensity to develop common variable immunodeficiency with autoimmune hemolytic anemia features. Here, we describe a novel murine model in which Mgat2 was targeted for ablation using the dendritic cell (DC)-specific CD11c-CRE-GFP strain in order to develop a more specific and robust in vivo model of PSA presentation defects (DC-cKO). This study shows that Mgat2 deficient DCs from DC-cKO mice show ablation of PSA presentation and downstream T cell activation in vitro. However, the CD11c promoter was unexpectedly active and triggered Mgat2 deletion within multiple hematopoietic lineages, showed remarkably poor penetrance within native DC populations, and produced almost undetectable levels of green fluorescent protein signal. These findings show that the CD11c promoter is not DC-specific, and extreme care should be taken in the interpretation of data using any mouse created using the CD11c-CRE model.
Keywords: antigen presentation, CRE-LoxP, dendritic cells, glycoantigen, Mgat2
Introduction
PSA is the founding member of a family of microbial polysaccharide antigens carrying both positive and negative charges within each repeating unit (Tzianabos et al. 1991, 1992, 1993), which have the unexpected ability to stimulate T cell responses (Brubaker et al. 1999; Gibson et al. 1998; Stingele et al. 2004). In much the same fashion as conventional protein antigens, polysaccharide A (PSA) and other glycoantigens are endocytosed into professional antigen presenting cells (APCs) such as dendritic cells (DCs), macrophages and B cells (Cobb et al. 2004). Once internalized, nitric oxide-mediated oxidation cleaves the molecules into small fragments (Cobb et al. 2004; Lewis and Cobb 2011; Velez et al. 2009) that then associate with class II major histocompatibility complex (MHCII) proteins (Cobb and Kasper 2008; Kreisman et al. 2007; Velez et al. 2009). Once bound, the glycoantigen–MHCII complex is shuttled to the cell surface where it is ultimately recognized by canonical αβ T cell receptors (Cobb et al. 2004), leading to activation and clonal expansion of the specific T cell (Johnson et al. 2015b).
The interaction between PSA and MHCII is relatively high affinity (Kd = 315 nM) for a MHCII antigen and is competitive with traditional peptide antigens (Cobb and Kasper 2008), suggesting that these two classes of molecules at least share portions of the binding domain on MHCII. However, we discovered that high affinity binding depends upon the presence of complex N-linked glycans on MHCII. Limiting the complexity of these glycans using glycosylation inhibitors like kifunensine (Ryan et al. 2011) or genetic ablation of Mgat2 (Ryan et al. 2013) in the APC essentially eliminates PSA interactions with MHCII and prevents T cell activation.
Using the CRE-LoxP system for cell-specific gene ablation, we previously created a mouse lacking Mgat2 within the myeloid lineage (M-cKO) (Ryan et al. 2013). This fairly broad knockout showed defects in PSA presentation and T cell responses in vitro and in vivo, but many of these mice also developed common variable immunodeficiency characterized by a loss of antibody responses to vaccination and an autoimmune-mediated destruction of naïve CD4+ helper T cells (Ryan et al. 2014). Moreover, these mice were often anemic, caused by an autoantibody and complement-mediated destruction of circulating erythrocytes (Ryan et al. 2014). These confounding factors led us to create a second murine model.
DCs are considered to be the primary APC for a majority of T cell responses due to their robust ability to process and present antigen (Mellman and Steinman 2001), with their discovery leading to a Nobel Prize for Dr. Ralph Steinman in 2011. CD11c is an integrin widely accepted as the canonical DC marker (O'Doherty et al. 1994), a point of view that is reflected in numerous commercial products that utilize CD11c as the tag for DC purification and characterization. Moreover, DC-specific gene ablation in mice has been performed using the CRE-LoxP system in which the Causes Recombination (CRE) recombinase and green fluorescent protein (GFP) is expressed downstream of the CD11c promoter (Stranges et al. 2007). To date, dozens of published studies have utilized this method to study a gene of interest in the DC population.
We created a CD11c-specific knockout of Mgat2 by crossing the CD11c-CRE-GFP strain with a strain carrying Mgat2 with flanking LoxP sites (Mgat2fl/fl) to create a more specific model in which to evaluate the in vivo impact of PSA T cell recognition on immune regulation. While purified Mgat2-null bone marrow-derived DCs (BMDCs) abrogated presentation of PSA to T cells for activation in vitro, we found that in vivo, PSA responses were normal. The normal PSA response was directly attributable to the observation that Mgat2 ablation was poorly penetrant in vivo. More troubling, however, was that Mgat2 deletion was readily seen within other hematopoietic lineages, including erythrocytes, B cells, CD11b+ myeloid cells and CD4+ T cells. These findings reveal the incomplete deletion in DCs and nonspecific hematopoietic ablation in the CD11c-CRE system, and eliminate the ability to use this model for in depth in vivo analysis of antigen presentation. Moreover, the non-DC gene excision pattern raises concerns about data interpretation in models exclusively using CD11c-CRE to understand DC function in vivo.
Results
Ablation of Mgat2 prevents PSA-mediated T cell activation
Previous findings have demonstrated that complex N-glycans on MHCII molecules are required for high affinity interactions with T cell-dependent glycoantigens such as PSA from the commensal bacterium Bacteroides fragilis (Ryan et al. 2011, 2013). In order to understand the in vivo ramifications of this lack of commensal antigen recognition on immune homeostasis, the mannosyl-α-1,6-glycoprotein β-1,2-GlcNAc transferase encoded by the Mgat2 locus was ablated in DCs by crossing the CD11c-CRE-GFP and Mgat2fl/fl strains. Conditional knockout mice homozygote for both loci (DC-cKO) was confirmed by PCR of genomic DNA isolated from tail snips (data not shown).
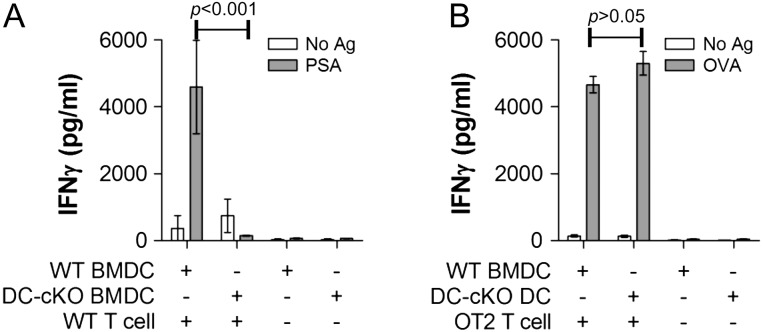
Bone marrow from CD11c-CRE-GFP (wild type, WT) and cKO mice were harvested from both male and female mice at least 10 weeks of age and differentiated into BMDCs using GM-CSF for 10 d as we have described previously (Ryan et al. 2011, 2013). The resulting DCs were then sorted based on Phaseolus vulgaris leucoagglutinin (PHA-L) lectin staining to removing any contaminating cells with intact complex N-glycans, and then cultured with or without WT CD4+ T cells and stimulated with PSA for 4 d. As a control, WT and DC-cKO DCs were also cultured with and without ovalbumin (OVA)-specific CD4+ T cells from OT2 transgenic mice and stimulated with OVA for 3 d. Using interferon-γ (IFNγ) as a measure of CD4+ T cell stimulation, we found that PSA induced robust activation when presented by WT DCs, but not when given to DC-cKO DCs (Figure 1A). In contrast, the OVA response was unaltered by Mgat2 ablation in the DC (Figure 1B), thereby ensuring general cell viability and function. These data support our previous findings that complex N-glycans are required for glycoantigen but not peptide antigen presentation and T cell responses, while confirming the efficacy of Mgat2 ablation as a model for defective PSA presentation.
Fig. 1.
BMDCs from DC-cKO mice fail to present PSA to T cells. BMDCs were cultured from WT (CD11c-CRE-GFP) or DC-cKO mice, purified by PHA-L, cultured with and without WT or OT2 CD4+ T cells, and stimulated with (A) PSA or (B) OVA, respectively. WT BMDCs present both PSA and OVA to generate T cell activation, but DC-cKO BMDCs fail to present PSA, resulting in a failure to activate T cells (n = 3 for all bars).
DC-cKO mice show normal PSA responses in vivo
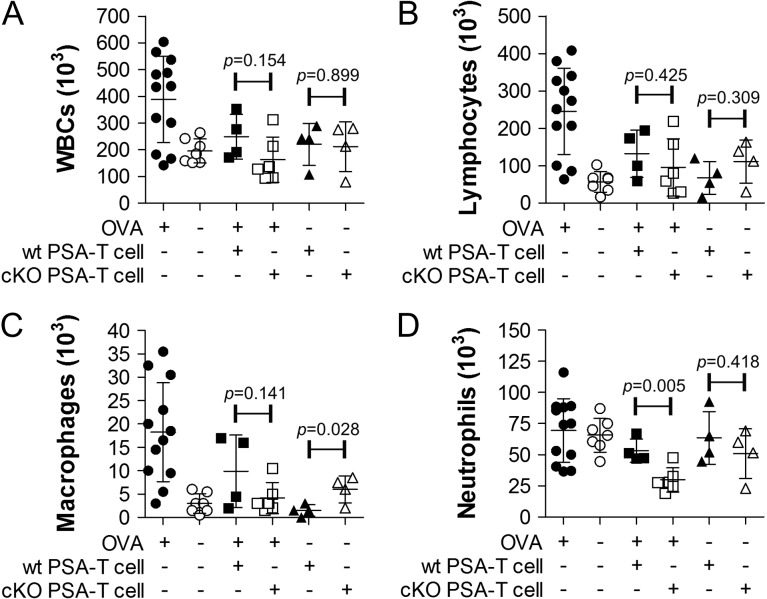
PSA has been shown to be a potent immunomodulatory antigen, which can dramatically reduce the susceptibility of an exposed mouse to the induction of asthma (Johnson et al. 2015a). This protective response is driven by PSA-responding T cells (Johnson et al. 2015a), thus unlike a WT mouse, an animal with defective PSA presentation should remain susceptible to asthma due to a lack of T cell engagement (Figure 1A). Using an OVA-based model of asthma as we previously described, PSA was given to both WT and DC-cKO mice via oral gavage over a 2-week period. On day 15, CD4+ T cells from treated DC-cKO animals were harvested and adoptively transferred into OVA-sensitive WT recipients, which were then challenged with intranasal OVA. On day 7, lungs were lavaged to measure leukocyte infiltration into the airways. We found that CD4+ T cells from DC-cKO PSA-treated mice were able to protect the recipient mice from asthma to an extent equal to that of WT cell (Figure 2). This result strongly suggests that PSA presentation to T cells by DCs remains intact within DC-cKO mice.
Fig. 2.
T cells from PSA-treated DC-cKO protect recipients from asthma. DC-cKO and WT mice were treated with PSA over 12 d, and CD4+ T cells were harvested on day 15. Recipients, all WT, received 2 × 106 each of OT2 T cells and T cells from PSA-treated DC-cKO or WT mice, then challenged intranasally for 1 week. On day 7, lungs were lavaged and analyzed for cellularity. The transfer of PSA-exposed T cells from both mice reduced leukocyte (A), lymphocyte (B) and macrophage (C) infiltration equally. Neutrophil (D) infiltration was essentially unchanged in positive and negative controls, although DC-cKO transfer did show a reduction compared to WT. Statistical comparisons are made between WT and DC-cKO T cell transfers, generally showing no difference (P > 0.05).
DC-cKO mice do not develop autoimmune anemia
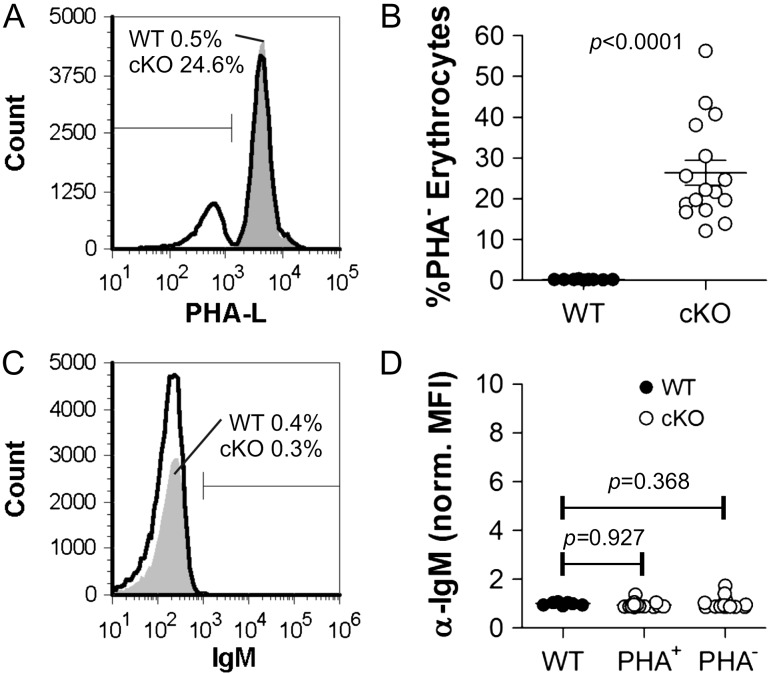
A central confounding problem with the myeloid-specific M-cKO mice we previously developed was the presence of autoantibodies, which bound to Mgat2-ablated erythrocytes that seemingly caused an autoimmune phenotype (Ryan et al. 2014). This is evidence that cell surface glycans modulate the immune system in ways not well understood, thus we sought to confirm the specificity of Mgat2 ablation in the DC-cKO mice. We began by analyzing the circulating erythrocytes for changes in glycosylation in the DC-cKO strain and found that nearly 30% of the erythrocytes were PHA-L-negative, indicating that Mgat2 has been ablated in this cell population (Figure 3A,B). Despite the lack of change in total number of erythrocytes (not shown), we performed a direct Coombs test in order to determine whether this change leads to autoantibody deposition. In contrast to the M-cKO strain (Ryan et al. 2014), no detectable antibody was found on either PHA-L+ or PHA-L− cells from DC-cKO mice (Figure 3C,D).
Fig. 3.
DC-cKO erythrocytes (cKO) show Mgat2 ablation but not autoantibody deposition. Erythrocytes were collected from WT (CD11c-CRE-GFP) or cKO mice and stained with PHA-L and anti-mouse IgM. (A, B) Approximately 30% of erythrocytes from cKO mice show a lack of complex N-glycans (n = 16; P < 0.0001). (C, D) None of the erythrocytes for either WT or cKO mice showed detectable auto-IgM antibody deposition, in contrast to Mgat2−/− erythrocytes from M-cKO mice previously reported (Ryan et al. 2013) (n = 16; P = 0.368, 0.927).
DCs from DC-cKO mice show low GFP expression and low ablation penetrance
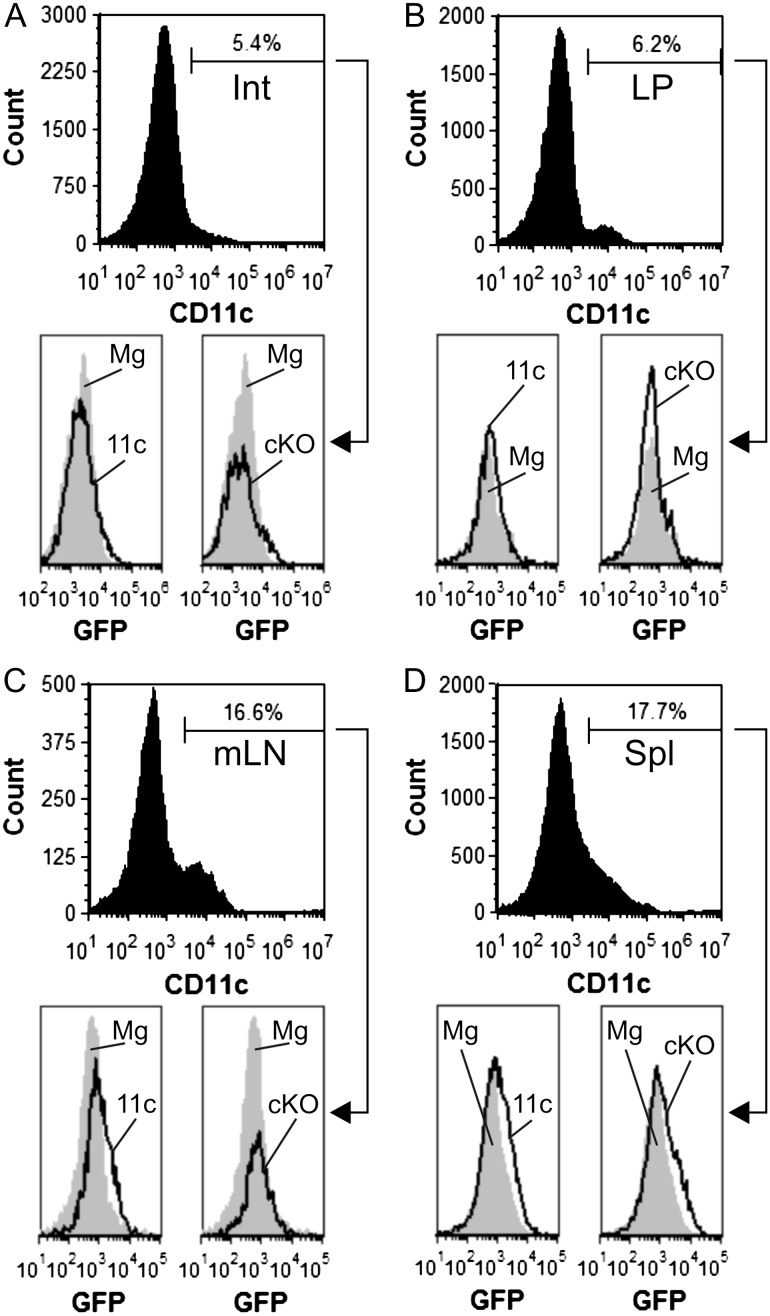
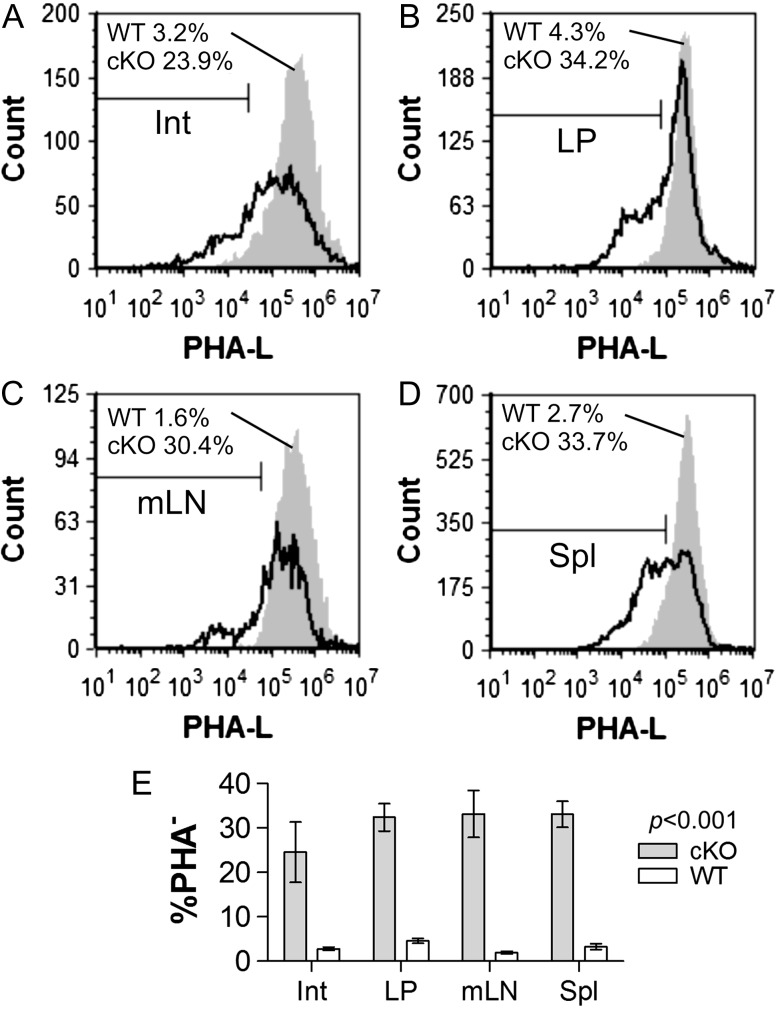
Based on the asthma (Figure 2) and erythrocyte (Figure 3) data, we became concerned about the quality and specificity of the Mgat2 ablation in the DC-cKO mouse. Since the parental CD11c-CRE strain also expresses GFP separated from the CRE gene by an internal ribosome entry sequence (Stranges et al. 2007), we first quantified the GFP fluorescence within DC populations from the intestine (Int; Figure 4A), lamina propria (LP; Figure 4B), mesenteric lymph nodes (mLN; Figure 4C) and the spleen (Spl; Figure 4D) from both homozygous parental lines and the DC-cKO. We found that among CD11c+ cells from each tissue, GFP fluorescence ranged from undetectable to extremely low compared to the Mgat2fl/fl strain, which served as a negative control (Figure 4). Second, we analyzed the penetrance of the knockout using PHA-L staining as before (Ryan et al. 2013). Among the CD11c+ cells from each tissue, the knockout was apparent in only 24–34% of the cells (Figure 5), with the majority of cells showing normal glycosylation. These data provide a solid rationale for the lack of change in PSA-mediated protection from asthma in vivo (Figure 2) despite the clear loss of PSA presentation in purified Mgat2− BMDCs in vitro from the same strain (Figure 1).
Fig. 4.
Primary DCs from DC-cKO mice lack GFP fluorescence. Single cells were recovered from (A) Int, (B) LP, (C) mLNs and (D) Spl from both parental mouse strains, CD11c-CRE-GFP (11c) and Mgat2fl/fl (Mg), and DC-cKO (cKO) mice, and stained for CD11c. Flow cytometric analysis of these cells by GFP fluorescence revealed undetectable to low signal above nonfluorescent cell controls (Mgat2fl/fl) (representative plots of n = 3 mice are shown).
Fig. 5.
Varied tissue DCs show modest Mgat2 ablation penetrance. Single cells were recovered from (A) Int, (B) LP, (C) mLNs and (D) Spl from WT parental mice (CD11c-CRE-GFP) and DC-cKO (cKO) mice, and stained for CD11c and complex N-glycans with PHA-L. Representative histograms of CD11c+ gated cells and replicates (E) show the percentage of PHA-L− cells ranging from 23.9% to 34.2% DC-cKO mice compared to 1.6% to 4.3% in WT mice (n = 3; P < 0.001 compared to WT cells).
Most Mgat2-ablated cells are not DCs
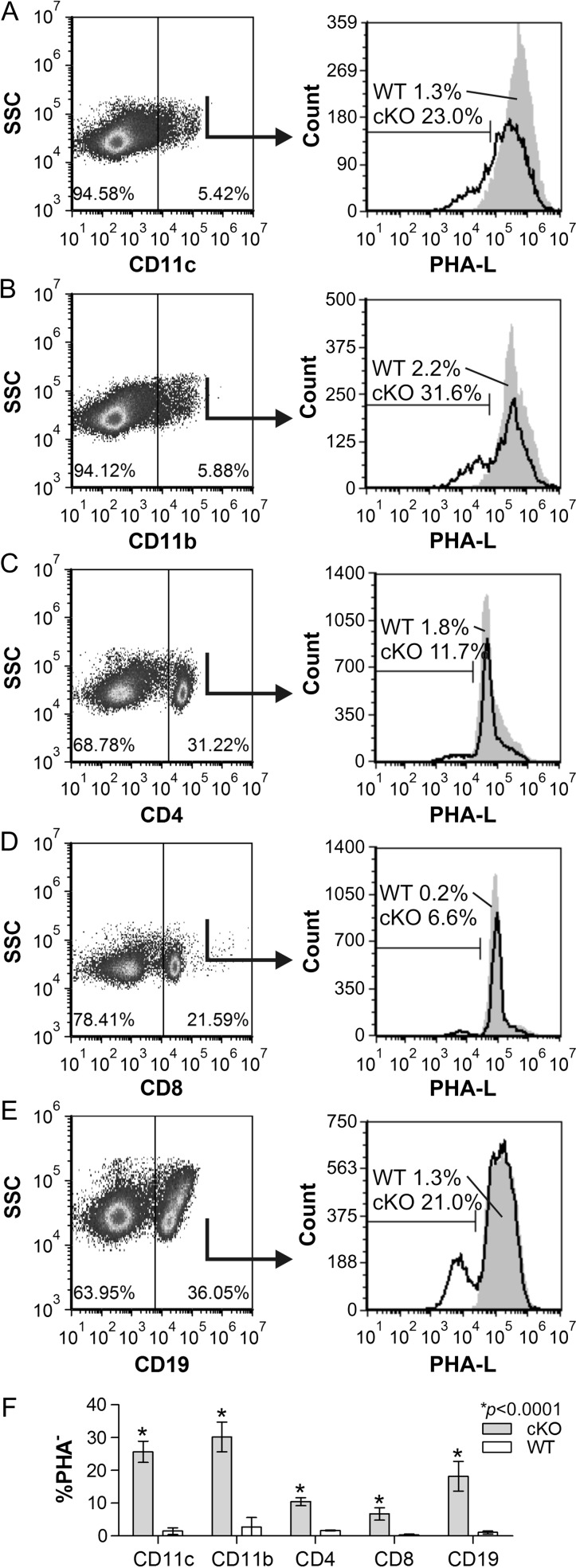
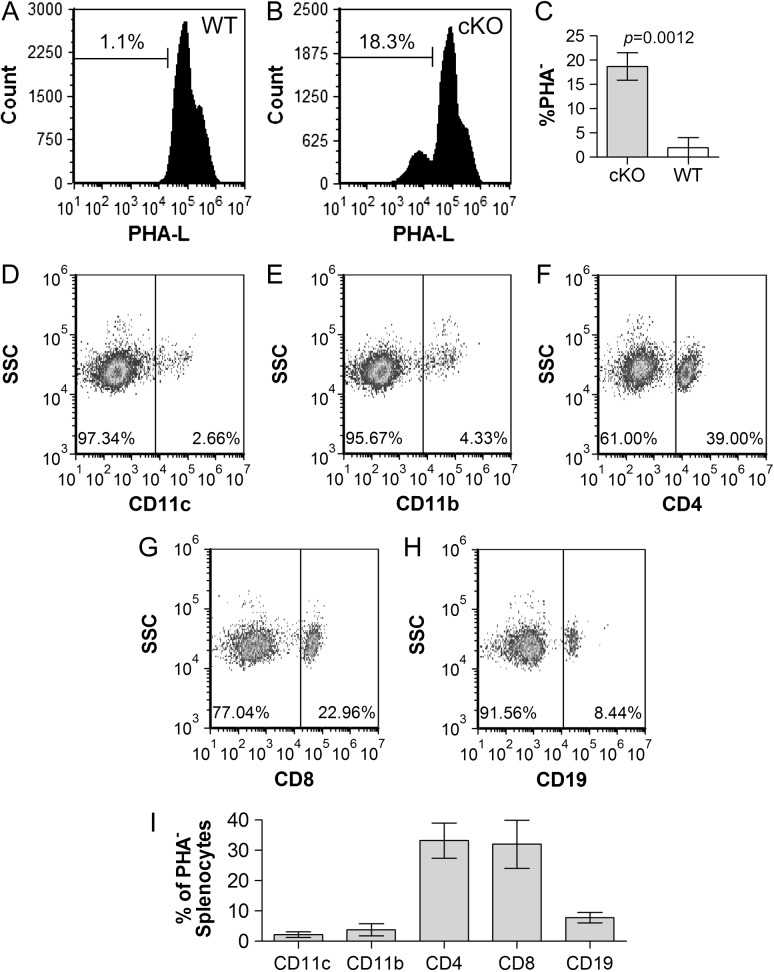
With modest Mgat2 deletion among the CD11c+ population coupled with ablation in erythrocytes, which are not known to express CD11c, we next focused on other hematopoietic cell lineages. Using the Spl as a source of cells, we found a lack of complex N-glycans in approximately 23% among CD11c+ DCs (Figure 6A), 32% of CD11b+ myeloid cells (Figure 6B), 12% of CD4+ T cells (Figure 6C), 7% of CD8+ T cells (Figure 6D) and 21.0% of CD19+ B cells (Figure 6E) compared to <2% in all WT populations (Figure 6F). In a separate analysis, we also found that among all of the PHA-L− cells in the Spl (Figure 7I), which is only seen in the DC-cKO strain (Figure 7A,C), approximately 3% were CD11c+ (Figure 7D), 4% were CD11b+ (Figure 7E), 39% were CD4+ (Figure 7F), 23% were CD8+ (Figure 7G) and 8% were CD19+ (Figure 7H). These results demonstrate the ablation of Mgat2 in all major hematopoietic lineages to significant degrees.
Fig. 6.
Splenocytes show varied Mgat2 ablation. DC-cKO (cKO) Spls were harvested and single cells stained with PHA-L and antibodies against CD11c (A), CD11b (B), CD4 (C), CD8 (D) or CD19 (E), plotted with side-scatter and compared to WT splenocytes (F). We found that 23% of CD11c+ cells, 31.6% CD11b+, 11.7% CD4+, 6.6% of CD8+ and 21.0% of CD19+ B cells lacked complex N-glycans (PHA-L low) in DC-cKO mice compared to <3% in each WT population (n = 3; P < 0.0001 compared to WT cells).
Fig. 7.
T cells are the most abundant splenic Mgat2-null cells. WT (A) and DC-cKO (B) Spls were harvested and single cells stained with PHA-L. PHA-L− cells from DC-cKO mice (C) were also stained with antibodies against CD11c (D), CD11b (E), CD4 (F), CD8 (G) or CD19 (H) and plotted against side-scatter. Data from replicates are also shown (I). In the Spl, over 60% of all Mgat2-ablated cells were T cells (CD4+ and CD8+), with CD11c+ cells accounting for only 2.6% of the total PHA-L− cells (n = 3).
Hematopoietic stem cells show no obvious defect
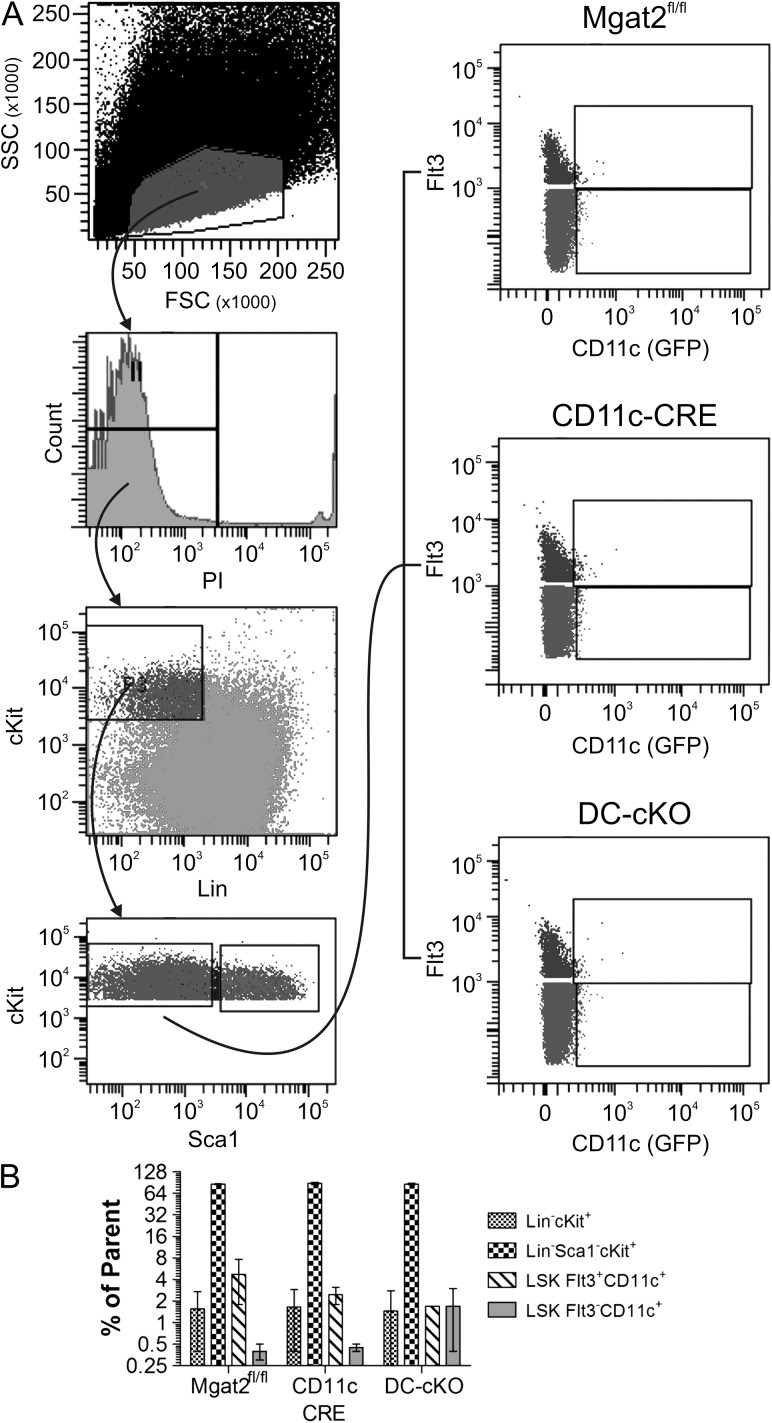
The penetrance of Mgat2 ablation across multiple hematopoietic lineages suggested that CD11c is expressed during hematopoiesis. Using GFP as an indicator of CRE expression, we analyzed the hematopoietic stem cell (HSC) compartment from the marrow of both homozygous parental strains as well as the DC-cKO mouse (Figure 8A). We found no change in the number of Lin−cKit+, Lin−Sca1−cKit+, LSK Flt3+CD11c+ or LSK Flt3−CD11c+ cells (Figure 8B). Likewise, no CD11c expression, as measured by GFP, was readily detectable in any of the cell populations. These results show that while the DC-cKO has a normal proportion of hematopoietic stem and progenitor cell, transient expression of CD11c must occur in minor populations of progenitors to give rise to PHA-L− differentiated cells (Figure 7).
Fig. 8.
HSCs show no defects or CD11c expression. Bone marrow from both parental mouse strains (CD11c-CRE-GFP and Mgat2fl/fl) and DC-cKO mice was harvested and stained with propidium iodide (PI) and antibodies against lineage markers (Lin), cKit, Sca1 and Flt3. GFP fluorescence was used as a marker of CD11c expression. (A) The gating scheme for analysis is shown, which revealed a lack of GFP signal in the HSC compartment. (B) Quantitation of each HSC compartment is shown, indicating a lack of HSC compartment changes in cell proportion associated with loss of Mgat2 (n = 3; P > 0.05 between genotypes for each cell lineage).
Discussion
We have created a novel mouse strain carrying a CD11c-specific deletion of the glycosylation locus Mgat2, which is necessary to synthesize traditional complex N-glycans (Wang et al. 2001). This was done on the widely held assumption that CD11c-powered CRE generates a DC-specific conditional knockout mouse. In these animals, protection from asthma remained intact, whereas erythrocytes, CD11b+ myeloid cells, CD4+ and CD8+ T cells, and B cells all showed deletion of Mgat2. This was coupled with the observation that CD11c+ penetrance of Mgat2 deletion was modest in vivo. While our in vitro results with sorted PHA-L− BMDCs confirm the importance of complex N-glycans for PSA presentation by MHCII, it is clear that this animal model is not useful for in vivo analysis of this phenomenon, and raises serious doubts about the feasibility of using CD11c-powered CRE for the analysis of any DC-specific function within a living mouse.
CD11c, or integrin alpha X (Itgax), is the primary and nearly ubiquitously used marker for DCs. A brief perusal of the data stored at the Immunological Genome Project (www.immgen.org) shows that CD11c is expressed primarily on various DCs, monocytes and natural killer cells in WT mice, and it is essentially absent in T and B cell populations. However, there is a significant difference in the expression level of any chosen gene in differentiated cells versus immature progenitors within the context of the CRE-LoxP system since any transient expression of CRE during development could result in genetic ablation of the target locus in downstream lineages. In fact, the similarity of Mgat2 deletion penetrance in erythrocytes, B and T cells, myeloid cells and DCs (10–30%) suggests that CD11c is expressed at some point in a common HSC progenitor, despite the inability to detect this expression in the HSC compartment.
Interestingly, this phenomenon would not have been obvious if it were not for the gene target—a glycosyltransferase. By deleting Mgat2, or nearly any key glycosylation pathway enzyme, the cell becomes permanently marked as a mutant long after the expression of CRE and its associated marker (i.e., CD11c in the present study) has waned. The characteristic change in surface glycosylation tags the cell as having expressed CRE at some point during the cell's development and differentiation, thereby revealing common lineages. If done carefully, these data suggest that the glycome could be harnessed genetically to study cellular origins and developmental pathways.
These findings suggest that the C57BL/6J-Tg(Itgax-cre,-EGFP)4097Ach/J CD11c-CRE-GFP mouse cannot be used to understand DC-specific function in vivo; however, it is important to note that ex vivo studies of primary cells may still be possible. Our data show that Mgat2− DCs from this mouse behave as previous studies predicted, and it is likely that other similar studies of these DCs may prove useful. Nevertheless, our findings report more on the expression of CD11c and the potential in vivo use of glycosyltransferases as a tool to permanently mark cells that transiently express CRE, than expanding our understanding of glycoantigen presentation and its T cell-dependent role in immune homeostasis.
Materials and methods
Antigens
PSA was expressed by a B. fragilis variant that expresses only PSA in the capsule (Krinos et al. 2001) and purified to homogeneity essentially as previously described (Tzianabos et al. 1992). OVA protein was purchased from Sigma (St. Louis, MO, USA).
Mice
Animals were maintained in a specific pathogen-free environment at Case Western Reserve University and were treated under IACUC-approved guidelines in accordance with approved protocols. DC-cKO mice were generated by crossing the Mgat2 (B6.129-Mgat2tm1Jxm/J; stock 006892) and CD11c-CRE-GFP (C57Bl/6J-Tg(Itgax-cre,-EGFP)4097Ach/J; stock 007567) parental strains, which were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). WT C57BL/6 J mice (stock 000664) and OT-II mice (B6.Cg-Tg(TcraTcrb)425 Cbn/J; stock 004194) were purchased from The Jackson Laboratory. Mouse genotypes were confirmed using Jackson Laboratory PCR protocols. All studies utilized both male and female mice at 10–12 weeks of age.
Flow cytometry
Cells were analyzed by flow cytometry as described previously (Ryan et al. 2011). Briefly, cells were stained with fluorescein-conjugated PHA-L lectin (Vector Laboratories, Burlingame, CA, USA) and/or the indicated antibodies for 30 min at 4°C. Analyses were performed using an Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Analyses of FACS data were performed using FCS Express (De Novo Software, Los Angeles, CA, USA).
T cell activation
T cell activation assays were performed as described previously (Ryan et al., 2011, 2013). Bone marrow cells were differentiated into BMDCs in culture using 10 ng/mL GM-CSF (Life Technologies, Carlsbad, CA, USA) for 10 d. Cells lacking Mgat2 were selected and purified based on PHA-L staining. Briefly, DC-cKO BMDCs were labeled with 20 μg/mL biotinylated PHA-L and separated using anti-biotin magnetic microbeads (Miltenyi Biotec Cologne, Germany). CD4+ T cells were isolated from the Spls of either OT2 or WT mice by CD4+ magnetic bead positive selection (Miltenyi Biotec) and verified by flow cytometry. About 1.5 × 105 CD4+ T cells were co-cultured with 1.5 × 104 BMDCs and incubated for 3 or 4 d with 50 µg/mL OVA or PSA, respectively. Culture supernatants were analyzed for murine IFNγ by sandwich ELISA according to the manufacturer's protocol (BioLegend, San Diego, CA, USA).
Coombs assay
Direct Coombs tests were performed as before (Ryan et al. 2014), cells were collected from blood or Spl and probed directly with biotinylated anti-mouse IgM (Jackson ImmunoResearch) for 30 min at 4°C. Cells were then washed and probed with AlexaFluor-488 conjugated streptavidin (Jackson ImmunoResearch, West Grove, PA, USA) followed by analysis by flow cytometry.
Asthma
Asthma was induced and analyzed as described previously (Johnson et al. 2015a). Briefly, mice were orally gavaged with PSA over 12 d (100 μg/dose in saline every 3 d). Negative controls utilized saline vehicle alone. CD4+ splenocytes from treated mice and from OVA-specific OT2 mice were purified by magnetic bead positive selection (Miltenyi). About 2 × 106 OT2 and 2 × 106 PSA or PBS-treated donor T cells were transferred i.v. into naïve recipient mice. Beginning 24 h later, the mice received intranasal OVA (40 μg/dose in PBS; Sigma) for six consecutive days before being sacrificed on day 7. For intranasal challenge, mice were anesthetized using a table top anesthesia system (Vet Equip) with 3% isoflurane (Baxter). Euthanasia was performed with a mixture of 8.6% ketamine (Fort Dodge), 1.7% xylazine (Anased) and 2.9% acepromazine (Boehringer Ingelheim) in sterile saline. Mice were dosed at 0.006 cc/g. Mice were given a tracheotomy, and lungs were flushed with 1 mL saline containing 0.6 mM EDTA three times. Cells from these washes were collected, resuspended in 50 μL PBS with 0.6 mM EDTA and automated differentials were performed on a Hemavet 950 Hematology Analyzer.
Statistics
Data are shown as mean ± standard error of the mean. Comparisons were generally performed using a nonpaired, two-tailed t-test for significance with a 95% confidence interval using GraphPad Instat software.
Funding
This work was supported by the National Institutes of Health, National Institute for General Medical Sciences to BAC (GM082916) and the National Institute for Allergy and Infectious Disease to MBJ (AI007024, AI114109).
Acknowledgements
We thank Dr. Lan Zhou for assistance and advice with flow analysis of the hematopoietic stem cell compartment.
Conflict of interest statement
None declared.
Abbreviations
APCs, antigen presenting cells; BMDCs, bone marrow-derived dendritic cells; DC-cKO, dendritic cell-specific CD11c-CRE-GFP-Mgat2fl/fl conditional knockout mouse; DCs, dendritic cells; GFP, green fluorescent protein; HSC, hematopoietic stem cell; HSPCs, hematopoietic stem and progenitor cells; Int, intestine; LP, lamina propria; M-cKO, myeloid-specific lysozyme M-CRE-Mgat2fl/fl conditional knockout mouse; MHCII, class II major histocompatibility complex; mLN, mesenteric lymph node; PSA, polysaccharide A; Spl, spleen.
References
- Brubaker JO, Li Q, Tzianabos AO, Kasper DL, Finberg RW. 1999. Mitogenic activity of purified capsular polysaccharide A from Bacteroides fragilis: Differential stimulatory effect on mouse and rat lymphocytes in vitro. J Immunol. 162:2235–2242. [PubMed] [Google Scholar]
- Cobb BA, Kasper DL. 2008. Characteristics of carbohydrate antigen binding to the presentation protein HLA-DR. Glycobiology. 18:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. 2004. Polysaccharide processing and presentation by the MHCII pathway. Cell. 117:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson FC III, Onderdonk AB, Kasper DL, Tzianabos AO. 1998. Cellular mechanism of intraabdominal abscess formation by Bacteroides fragilis. J Immunol. 160:5000–5006. [PubMed] [Google Scholar]
- Johnson JL, Jones MB, Cobb BA. 2015. a. Bacterial capsular polysaccharide prevents the onset of asthma through T-cell activation. Glycobiology. 25:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Jones MB, Cobb BA. 2015. b. Polysaccharide A from the capsule of Bacteroides fragilis induces clonal CD4+ T cell expansion. J Biol Chem. 290:5007–5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman LS, Friedman JH, Neaga A, Cobb BA. 2007. Structure and function relations with a T-cell-activating polysaccharide antigen using circular dichroism. Glycobiology. 17:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krinos CM, Coyne MJ, Weinacht KG, Tzianabos AO, Kasper DL, Comstock LE. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature. 414:555–558. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Cobb BA. 2011. Adaptive immune defects against glycoantigens in chronic granulomatous disease via dysregulated nitric oxide production. Eur J Immunol. 41:2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. 2001. Dendritic cells: specialized and regulated antigen processing machines. Cell. 106:255–258. [DOI] [PubMed] [Google Scholar]
- O'Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Ryan SO, Abbott DW, Cobb BA. 2014. Myeloid glycosylation defects lead to a spontaneous common variable immunodeficiency-like condition with associated hemolytic anemia and antilymphocyte autoimmunity. J Immunol. 192:5561–5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SO, Bonomo JA, Zhao F, Cobb BA. 2011. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J Exp Med. 208:1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SO, Leal SM Jr, Abbott DW, Pearlman E, Cobb BA. 2013. Mgat2 ablation in the myeloid lineage leads to defective glycoantigen T cell responses. Glycobiology. 24:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingele F, Corthesy B, Kusy N, Porcelli SA, Kasper DL, Tzianabos AO. 2004. Zwitterionic polysaccharides stimulate T cells with no preferential Vbeta usage and promote anergy, resulting in protection against experimental abscess formation. J Immunol. 172:1483–1490. [DOI] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, et al.. 2007. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 26:629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. 1993. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 262:416–419. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Pantosti A, Baumann H, Brisson JR, Jennings HJ, Kasper DL. 1992. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 267:18230–18235. [PubMed] [Google Scholar]
- Tzianabos AO, Pantosti A, Baumann H, Michon F, Brisson JR, Jennings HJ, Kasper DL. 1991. Structural characterization of two surface polysaccharides of Bacteroides fragilis. Trans Assoc Am Physicians. 104:285–295. [PubMed] [Google Scholar]
- Velez CD, Lewis CJ, Kasper DL, Cobb BA. 2009. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 127:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tan J, Sutton-Smith M, Ditto D, Panico M, Campbell RM, Varki NM, Long JM, Jaeken J, Levinson SR, et al.. 2001. Modeling human congenital disorder of glycosylation type IIa in the mouse: Conservation of asparagine-linked glycan-dependent functions in mammalian physiology and insights into disease pathogenesis. Glycobiology. 11:1051–1070. [DOI] [PubMed] [Google Scholar]