Abstract
BACKGROUND
Neonates with necrotizing enterocolitis (NEC) have higher calprotectin levels in stool than do healthy neonates. However, it is not known whether high stool calprotectin at the onset of bowel symptoms identifies neonates who truly have NEC vs. other bowel disorders.
STUDY DESIGN
Neonates were eligible for this study when an x-ray was ordered to “rule-out NEC”. Stool calprotectin was quantified at that time and in a follow-up stool. Each episode was later categorized as NEC or not NEC. The location of calprotectin in the bowel was determined by immunohistochemistry.
RESULTS
Neonates with NEC had higher initial and follow-up stool calprotectin levels than did neonates without NEC. Calprotectin in bowel from neonates with NEC was within neutrophil extracellular traps (NETs).
CONCLUSION
At the onset of signs concerning for NEC, fecal calprotectin is likely to be higher in neonates with NEC. Calprotectin in their stools is exported from neutrophils via NETs.
Keywords: neonate, NICU, necrotizing enterocolitis, feeding intolerance, hematochezia, calprotectin, neutrophil extracellular traps
INTRODUCTION
Necrotizing enterocolitis (NEC) can be a life-threatening disorder and we need more accurate ways to recognize cases during its earliest stages (1-4). When the initial x-rays are inconclusive, we typically stop the feedings, initiate antibiotics and parenteral nutrition, and order serial abdominal x-rays (5). If a reliable way were available to confirm the diagnosis of NEC at the onset of abdominal distention when x-rays are not diagnostic, that information could help guide the course of treatment (5).
Calprotectin is an antibacterial protein found in neutrophils, monocytes, and macrophages. It constitutes approximately 60% of the cytosolic proteins of neutrophils (6-8). Calprotectin binds to calcium, stabilizing it and preventing degradation in the intestine (9). Adults with inflammatory bowel disorders have high stool calprotectin concentrations (6-12). High levels of calprotectin in stool is likely from migration of activated neutrophils across gastrointestinal epithelial membranes (11). Previous studies report higher calprotectin levels in stools of neonates with NEC, compared with stools of healthy control neonates (8, 10, 13-25). However, it is not clear whether measuring stool calprotectin at the onset of intestinal signs would discriminate between cases that are eventually going to be diagnosed as NEC vs. more benign forms of feeding intolerance. We prospectively tested this possibility.
As part of this study, we also investigated the source of calprotectin in the intestine of neonates with NEC. Calprotectin is located within neutrophils. As part of the innate immune response, neutrophils can eject nuclear chromatin and bactericidal proteins known as neutrophil extracellular traps (NETs) to trap and kill microorganisms (26, 27). NETs contain calprotectin (28). We sought to further define the relationship between calprotectin and NETs in bowel tissue from neonates with NEC.
METHODS
Study Design and Populations
This was a prospective pilot study conducted in two Intermountain Healthcare NICUs; McKay-Dee Hospital, Ogden, UT and Utah Valley Regional Medical Center, Provo, UT. The Intermountain Healthcare Institutional Review Board approved the protocol and an informed consent document was signed by the parents of all participating neonates. Intermountain Healthcare is a not-for-profit system that owns and operates 22 hospitals in Utah and Idaho. IRB approval for the calprotectin immunohistochemical and immunocytochemical experiments with analysis of NETosis was obtained through the University of Utah. The University of Utah IRB classified this study as exempt from requiring signed consent because of the deidentified status of the tissues.
When a clinician ordered an abdominal x-ray to “rule out NEC” the neonate was considered eligible for this study. Any stools at the time of the x-ray, or preceding the x-ray by <2 hours, or within the 12 hour period following the x-ray, were placed in a special stool-collection vial (minimum one gram) for calprotectin analysis as a research study. Parents were contacted within several hours of the qualifying x-ray and told of the study. If the parents consented, the stool was submitted to ARUP laboratories for fecal calprotectin assay. If the parents refused, the stool was discarded. For consented patients, a repeat stool sample was also sent for calprotectin assay within 72 hours of the qualifying x-ray. If a second stool was not passed by 72 hours, the next-passed stool was used and the time recorded. The fecal calprotectin tests were not billed to families or third-party payers, but were paid by a research grant. The calprotectin levels were not placed in the medical record or reported to the clinicians.
This was a convenience sample of 30 episodes of “rule-out NEC”. Thirty episodes was selected on the basis of the funding available for the study. Patients were only entered into the protocol when a study nurse or study neonatologist was available to explain the study and provide informed consent for parents. The research did not involve ordering any other laboratory tests or x-rays; however clinically indicated blood tests and x-rays, physical examination findings, and clinical decisions on the study patients were available to the research team and included in the study analysis.
During the study period the two NICUs had a consistent clinical approach which included: feeding mother's own milk or pasteurized human milk (29); using restrictive erythrocyte transfusion guidelines (30); using delayed cord clamping or cord milking for preterm delivery (31); obtaining the initial laboratory blood work from otherwise discarded fetal blood in the umbilical cord (32); and not providing enteral feedings during blood transfusions.
One week or more following the study entry, study staff assembled all relevant clinical and research data, including the diagnosis arrived at by the clinicians regarding the cause of the abdominal distention. Causes were categorized by the research staff, as shown in Table 1, as either; not NEC (with hematochezia or without hematochezia), Medical NEC (Stage II), or Surgical NEC (Stage III). Cases were evaluated for the possibility of misdiagnosis, i.e. for spontaneous intestinal perforation as a potential confounding diagnosis.
Table 1.
Each of 30 episodes of “rule-out NEC” were judged, one week or more after the onset of the qualifying x-ray, as having been due to one of the four categories below.
| Category | Final Diagnosis | Radiological Signs | Typical Intestinal Signs | Typical Systemic Signs | Treatment |
|---|---|---|---|---|---|
| 1 | NOT NEC - Feeding intolerance no hematochezia | Normal or dilated loops, no pneumatosis or portal air | Abdominal distention and visible bowel loops | Apnea, bradycardia, lethargy | Antibiotics and NPO period ≤ 2 days |
| 2 | NOT NEC - Feeding intolerance with hematochezia | Normal or dilated loops, no pneumatosis or portal air | Abdominal distention and visible bowel loops and hematochezia | Apnea, bradycardia, lethargy | Antibiotics and NPO period ≤ 2 days |
| 3 | Medical NEC stage II (surgical procedure not performed) | Dilated loops, pneumatosis or portal air | Abdominal distention, visible bowel loops, apparent abdominal tenderness, ileus ± hematochezia | Apnea, bradycardia, lethargy, ± acidosis, hypotension, neutropenia, thrombocytopenia | Antibiotics and NPO period > 5 days |
| 4 | Surgical NEC stage III (surgical procedure performed or death from fulminant NEC) | Dilated loops, pneumatosis or portal air | Signs of peritonitis, marked tenderness, abdominal distention, ileus ± hematochezia | Apnea, bradycardia, lethargy, with acidosis, hypotension, neutropenia, thrombocytopenia | Antibiotics and NPO period > 5 days |
Calprotectin assay
The PhiCal fecal calprotectin immunoassay (Genova Diagnostics, Inc. Ashville, NC) is an enzyme-linked immunoassay system with colorimetric detection, and was performed at ARUP laboratories (Salt Lake City, UT) according to the manufactures instructions. The test uses a polyclonal antibody against calprotectin. Calprotectin present in the diluted sample is bound by the antibody absorbed to the surface of the plastic well. The enzyme conjugated antibody binds to the captured antigen and subsequently the enzyme catalyzes the conversion of the substrate to a colored product. The intensity of the color is proportional to the amount of conjugate bound, and thus to the amount of captured calprotectin. Concentration of calprotectin in the samples is calculated using a standard, provided in the test kit.
Localization of calprotectin to neutrophil extracellular traps
PMNs were isolated from ACD or EDTA anticoagulated venous blood from healthy adults under protocols approved by the University of Utah IRB. PMN suspensions (> 96% pure) were prepared by positive immunoselection using anti-CD15-coated microbeads and an auto-MACS cell sorter (Miltenyi Biotec, Inc.) and were resuspended at 2 × 106 cells/mL concentration in serum-free M-199 media at 37° C in 5% CO2/95% air. Qualitative assessment of NET formation was performed as previously reported (26, 33). Briefly, primary PMNs isolated from healthy adults (2 × 106 cells/mL) were incubated with control buffer or stimulated with LPS (100 ng/mL) for 1 hour at 37°C in 5% CO2/95% air on glass coverslips coated with poly-L-lysine. After stimulation, PMNs were gently washed with PBS and fixed with 4% p-FA for 10 minutes followed by permeabilization with Triton-X100 for 10 minutes. Calprotectin protein expression was detected via immunocytochemistry using a FITC-conjugated anti-human calprotectin primary antibody (LifeSpan BioSciences, 1:100 dilution). Confocal microscopy was accomplished using a FV1000 1X81 confocal Microscope and FluoView software (Olympus). Both 20X and 60X objectives were used. Z-series images were obtained at a step size 1μm over a range of 20μm for each field. Olympus FluoView and Adobe Photoshop CS software were used for image processing.
Localization of calprotectin within the bowel of neonates with NEC
Gastrointestinal tissue samples from surgically treated prematurely born neonates with NEC (n=5) were compared using immunohistochemical techniques to gastrointestinal tissue samples processed in parallel from control patients operated on for indications other than NEC (n=6). Immunohistochemistry on paraffin sections followed established procedures, including de-waxing, rehydration, and high temperature antigen retrieval (Vector Unmasking Solution, Vector Laboratories, Burlingame, CA) prior to blocking. Primary antibodies utilized in this study were as follows: anti-human Calprotectin (LifeSpan Biosciences, 1:100 dilution); anti-human Neutrophil Elastase (Hycult, 1:100 dilution). An Alexa-568 goat anti-rabbit secondary antibody (Molecular Probes, 1:1000 dilution) was used. Prior to imaging via confocal microscopy, tissue sections were incubated with DRAQ5 (Cell Signaling, 1:1000 dilution) as a DNA counterstain. Confocal microscopy was accomplished as described previously for immunocytochemistry (26, 33).
Statistical Analysis
The program used for data collection was a modified subsystem of Clinical Workstation. The 3M Company (Minneapolis, MN, USA) approved the structure and definitions of all data points for use within the program. Data were managed and accessed by authorized data analysts. Means and standard deviations were used to express values in groups that were normally distributed, and medians and interquartile ranges to express values in groups that were not. Differences in categorical variables were assessed using the Fisher exact test or chi-square for normally distributed data and Tukey's bi-weight estimator for groups that were not. Statistical analysis used Statit (Midas, Tucson, AZ). Statistical significance was set as p <0.05. The statistical performance of fecal calprotectin was also assessed by receiver operating characteristic (ROC) curves (IBM SPSS Statistics, Version 23.0, Armonk, NY).
RESULTS
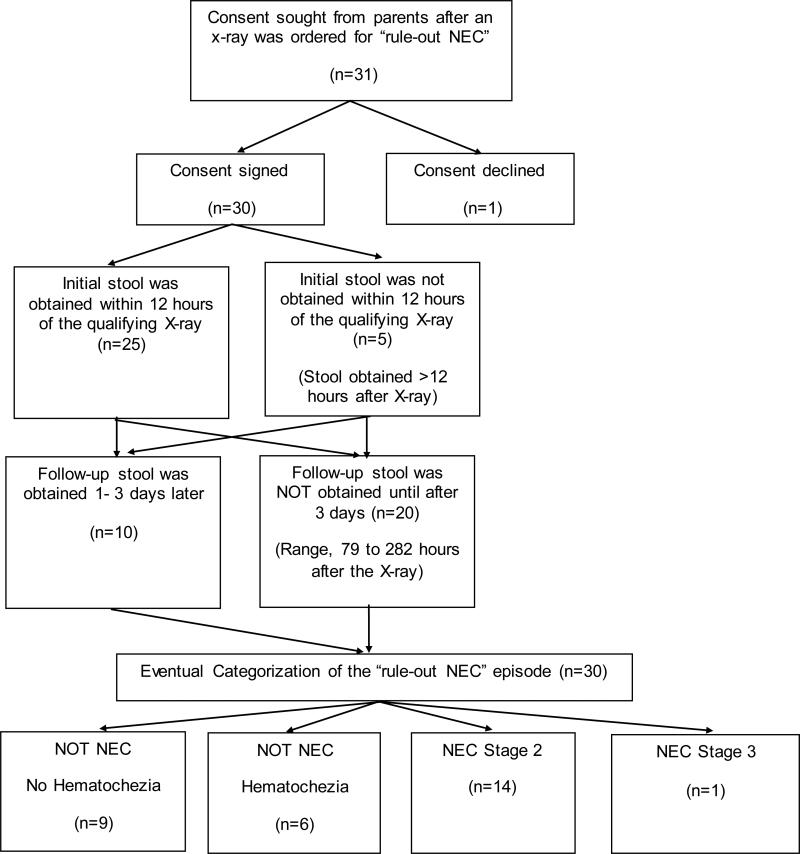
We initiated study enrollment at McKay-Dee Hospital, Ogden, Utah, in May 2014 and at Utah Valley Regional Medical Center, Provo, Utah, in June 2014, and concluded in July 2015. As diagramed in Figure 1, 31 episodes qualified for the study at a time when study-personnel were available to ask parents for study consent. Parental consent was obtained for 30. Figure 1 shows study enrollment, procedures, and the eventual clinical diagnosis made for each of the 30 “rule-out NEC” episodes. There were no cases of spontaneous intestinal perforation.
Figure 1.
Flow diagram for the study of episodes of “rule-out NEC”.
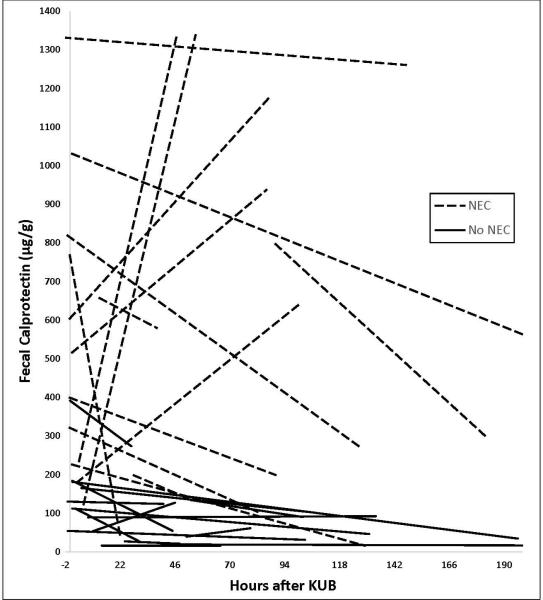
The paired (initial and follow-up) fecal calprotectin levels of the 30 episodes are shown in Figure 2. Calprotectin levels were higher in the first-sample stools of the 15 with NEC (median 516 μg/g stool; 1st quartile 226 μg/g; 3rd quartile 797 μg/g) than in the 15 who turned out not to have NEC (median 110 μg/g; 1st quartile 39 μg/g; 3rd quartile 165 μg/g; p<0.00001). Calprotectin levels were also higher in the second-sample stools of the 15 with NEC (372 μg/g stool, 1st quartile 135 μg/g; 3rd quartile 1175 μg/g) than in the 15 who did not have NEC (54 μg/g; 1st quartile 28 μg/g; 3rd quartile 103 μg/g, p<0.0001). The time to passage of the second stool was similar in the 15 who did not have NEC (92±62 hours) and in the 15 who did (100±66 hours, p<0.665).
Figure 2.
Stool calprotectin levels in 30 episodes of “Rule-out NEC”. Time “0” is the time the x-ray was obtained to “rule-out NEC”. Paired follow-up levels of stools passed after the qualifying x-ray are connected by dashed (NEC) or solid (Not NEC) lines. One week or more after the qualifying “rule out NEC” x-ray, each of the 30 episodes was assigned as most likely having been due NEC or Not NEC.
Clinical and laboratory findings during the week following the qualifying X-ray are shown in Table 2. All 15 who were retrospectively judged as having had NEC received antibiotics for ≥ 5 days and were labeled as having NEC in the medical record. None of the 15 judged as not having had NEC had an x-ray showing pneumatosis, portal venous gas, or perforation, and none had thrombocytopenia or neutropenia, or antibiotics for ≥5 days, and none were labeled as having NEC in the medical record.
Table 2.
At the onset of each of 30 “rule-out NEC” episodes, x-rays were not diagnostic of NEC (no pneumatosis or portal air or perforation), because this study focused only on cases where NEC was possible but not definite at the onset. One week after the episode began, each case was judged retrospectively as having been due to NEC, or not having been due to NEC, on the basis of the elements in table 1 (note: fecal calprotectin level was not used in this judgement, as it was not available to clinicians).
| Findings following the “rule-out NEC” episode | NEC (n=15) | Not NEC (n=15) |
|---|---|---|
| Pneumatosis intestinalis* | 8/15 | 0/15 |
| Portal venous gas* | 1/15 | 0/15 |
| Peritoneal gas* | 1/15 | 0/15 |
| Post-NEC bowel stricture** | 1/15 | 0/15 |
| Thrombocytopenia (<150,000/μL) | 4/15 | 0/15 |
| Neutropenia (<1000/μL) | 3/15 | 0/15 |
| Duration of antibiotics ≥5 days | 15/15 | 0/15 |
| Diagnosis of NEC by attending neonatologist | 15/15 | 0/15 |
Pneumatosis was first observed on a repeat x-ray taken in 8 neonates at the following times (hours after the initial qualifying x-ray); 2h, 3h, 6h, 24h, 24h, 24h, 24h, and 72h. Portal venous gas was diagnosed in one neonate 24 hours after the qualifying x-ray. Free intraperitoneal air was found in one neonate 24 hours after the qualifying x-ray.
A post-NEC ileal stricture was identified 4 weeks after the qualifying “rule-out NEC” episode.
Ten of the 15 neonates with NEC had a fall in fecal calprotectin between their first-studied stool (655±369 pg/g) and their second-studied stool (327±368 pg/g). The other five had an increase, from (331±214 pg/g in the first stool to 1086±299 pg/g in the second; figure 2, dashed lines). The 10 where it fell and the 5 where it increased were of similar gestational age at birth (p=0.55), birth weight (p=0.39), corrected age at the time the “rule-out NEC” x-ray was taken (p=0.49), and length of time between the initial and follow-up stool (p=0.42). However in all six with the highest initial calprotectin, the subsequent level fell. The five where the level went up had a lower initial value than those where the level fell (p<0.0001).
In the 15 that did not have NEC, bloody stools did not significantly elevate the fecal calprotectin level. Hematochezia (n=6) (median 101 μg/g stool; 1st quartile 39 μg/g, 3rd quartile 168 μg/g); no hematochezia (n=9) (median 54 μg/g stool; 1st quartile 21 μg/g; 3rd quartile 116 μg/g; p = 0.074).
Demographic, clinical, and laboratory information describing the neonates at the onset of their “rule-out NEC” episode are shown in Table 2. None of the 15 episodes of NEC, and none of the 15 episodes that were not NEC, had a blood transfusion in the preceding 48 hours. The group of 15 with NEC were of similar birth weight, gestational age, gender, race, and postnatal age as the 15 who turned out not to have NEC. CBC values obtained with the qualifying x-ray as part of the “rule out NEC” evaluation, and any CBC value obtained within 24 hours before that evaluation, are included in Table 2. In the 15 who had NEC the I/T neutrophil ratios were slightly higher (p=0.046) and stool calprotectin levels significantly higher (p<0.0001).
Table 3 shows laboratory information within the 4-days following the “rule out NEC” xray. The one patient with surgical NEC had the highest and also the lowest leukocyte counts of the group, the highest I/T neutrophil ratio, and the lowest monocyte and platelet counts. The 15 with NEC had higher follow-up stool calprotectin levels than did the 15 who did not have NEC (p<0.0001).
Table 3.
AT THE BEGINNING OF THE “RULE OUT NEC” EPISODE. Demographic features, selected laboratory values, and fecal calprotectin levels at the onset of 30 episodes of “rule out NEC” evaluations.
| EVENTUAL DIAGNOSIS OF THE “RULE-OUT NEC” EPISODE | |||||
|---|---|---|---|---|---|
| Feeding Intolerance •Not NEC •No hematochezia (n=9) | Feeding Intolerance •Not NEC •Hematochezia (n=6) | NEC Stage II (Medical) (n=14) | NEC Stage III (Surgical) (n=1) | p value No NEC vs NEC | |
| Birth weight (grams) | 1239 ± 932 | 1699 ± 417 | 1289 ± 555 | 3550 | 0.924 |
| Gestational age at birth (weeks ± days) | 28 ± 5 | 31 ± 3 | 29 ± 3.8 | 36 | 0.693 |
| Gender (% male) | 7/9 (78%) | 5/6 (83%) | 9/14 (64%) | 1/1 (100%) | 0.410 |
| Race (% non-white) | 3/9 (33%) | 0/6 (0%) | 1/14 (7%) | 0 (0%) | 0.283 |
| Postnatal age at “R/O NEC” (weeks) | 32 ± 3 | 33 ± 4 | 31 ± 9 | 36 | 0.384 |
| Leukocyte count (X103/μL) | 11.9 ± 6.4 | 14.8 ± 6.2 | 11.9 ± 6.2 | 9.8 ± 5.9 | 0.480 |
| I/T neutrophil ratio | 0.10 ± 0.07 | 0.16 ± 0.05 | 0.18 ± 0.17 | 0.67 ± 0.26 | 0.046 |
| Monocyte count (X103/μL) | 1.3 ± 0.8 | 1.9 ± 0.9 | 1.7 ± 0.9 | 1.6 ± 1.3 | 0.987 |
| Eosinophil count (X103/μL) | 0.6 ± 1.0 | 0.7 ± 0.3 | 0.5 ± 0.4 | 0.6 | 0.620 |
| Platelet count (X103/μL) | 232 ± 78 | 284 ± 157 | 341 ± 82 | 184 ± 85 | 0.005 |
| Stool Calprotectin (μg/g stool) | 83 ± 66 | 159 ± 121 | 567 ± 371 | 235 | 0.000 |
Values are either shown as (mean±SD or median with 1st and 3rd quartile)
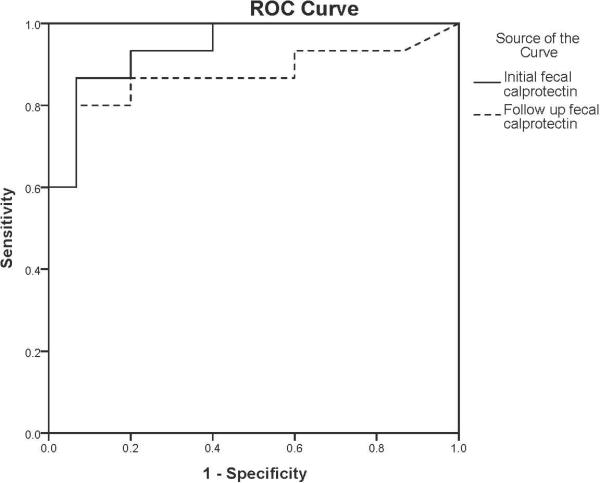
Figure 3 shows ROC analysis, showing curves for both the initial and the follow-up stool levels of calprotectin.
Figure 3.
ROC curves for initial and follow-up fecal calprotectin levels. The initial fecal calprotectin levels are represented by the solid line, and the follow-up calprotectin levels are represented by the dashed line. Area under the curve: 0.942 (95% confidence interval 0.864-1.000) for the initial fecal calprotectin and 0.871 (95% confidence interval 0.729-1.000) for the follow-up level.
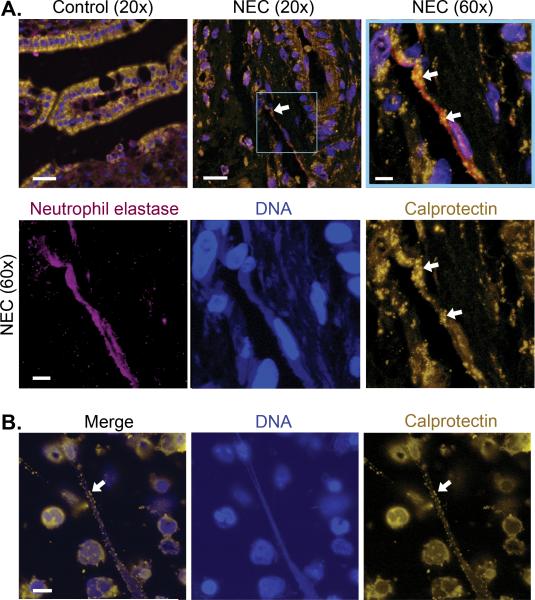
Using immunohistochemistry, calprotectin in bowel excised from neonates with NEC was associated with activated neutrophils in neutrophil extracellular traps. Figure 4A demonstrates NET-associated calprotectin in intestinal tissue obtained from neonates treated surgically for NEC (n= 5 different neonates). Parallel analysis of intestinal tissue samples from control newborn infants who underwent surgical treatment for issues other than NEC (n=6 different neonates) showed no NET formation and no increase in calprotectin protein expression. Immunocytochemical analysis of LPS-stimulated PMNs isolated from healthy adults also demonstrated robust NET formation in vitro with a clear association of calprotectin with NETs (Figure 4B).
Figure 4. NETs contribute to intestinal injury associated with NEC and are decorated with calprotectin protein.
(A) We used immunohistochemical techniques to compare calprotectin protein expression in gastrointestinal tissue samples collected from 5 different infants who underwent surgical treatment for NEC and 6 control infants operated on for indications other than NEC. Representative images of each are shown. Calprotectin is seen in gold fluorescence. Neutrophil elastase, a protein known to be expressed on NETs, is seen in magenta fluorescence. Nuclear and NET-associated DNA is shown in blue fluorescence. A NET is seen in the intestinal tissue of an infant with NEC (arrows), while no NETs or neutrophil elastase staining were seen in the control tissues. The magnified image of the highlighted box, complete with the three separate fluorescent channel images, demonstrate neutrophil elastase, DNA, and calprotectin staining consistent with NET formation. The images highlighting DNA and neutrophil elastase have been brought up to brighter exposures for the purpose of clearer demonstration. (B) NET formation was detected using immunocytochemistry with the same primary antibodies used to detect calprotectin and DNA employed in panel A. Freshly isolated adult PMNs were treated with LPS (100 ng/mL) for 1 hour to stimulate NET formation. Again, gold fluorescence denotes calprotectin protein expression and blue fluorescence denotes DNA. These images are representative of 6 different replicates using PMNs isolated from 6 different healthy adults. Bar, 20μm on A row 1, images 1 and 2, 5μm on A row 1, image 3 and row 2, and 10μm on B.
DISCUSSION
When a NICU patient has abdominal distention or other findings that could suggest a diagnosis of NEC, it is sometimes difficult to know if the condition is indeed NEC or is a less pernicious variety of bowel dysfunction. If the initial x-ray shows pneumatosis or portal venous air, a diagnosis of NEC can be straight forward. However, many times the initial radiograph is not definitive and clinicians must devise a plan for treatment without certainty as to whether or not the patient has NEC.
Certain CBC changes can help identify the condition as NEC (34). These include thrombocytopenia, disturbance in coagulation, anemia, neutrophilia or neutropenia, and a leukocyte “left shift” (34-36). However, we observed relatively minimal CBC changes early in the course of NEC, consistent with a recent report of Maheshwari et al. who reported that inflammatory cytokines did not generally rise until after the onset of NEC (37). Thuijls et al. also found that at the onset of suspicion for NEC, the platelet count and CRP were not different between those who were eventually diagnosed with NEC vs those with other diagnoses (15).
In 12 of 13 previous studies, neonates with established NEC had higher stool calprotectin levels than healthy controls [8, 13-25 and Supplemental Material]. We designed the present study as distinctly different than all previous reports. Specifically, we did not study neonates with well-established NEC, nor did we study healthy controls. Rather, we examined NICU patients at the onset of intestinal pathology sufficient to warrant an abdominal x-ray to “rule-out NEC”, and we asked whether the level of calprotectin in the stool at that time could differentiate between those who would go on to a diagnosis of NEC from those who would have a less severe form of feeding intolerance.
Calprotectin is present within neutrophils and is likely to be associated with activated macrophages in the bowel, as suggested by the work of Maheshwari (37, 37, 39). Neutrophils use several antibacterial modalities including degranulation and phagocytosis. In 2004, Brinkmann et al. reported a different mechanism; the ejection of nuclear chromatin and bactericidal proteins in a structure known as neutrophil extracellular traps (NET) to ensnare and kill microorganisms (40). The process has been termed NETosis (41). We found that calprotectin in excised NEC-affected bowel is one of the proteins released by neutrophils in association with NETs. Thus, at least some of the calprotectin in stools of neonates with NEC is derived from activated neutrophils migrating to the bowel mucosa and lumen and exporting antimicrobial calprotectin by way of NETosis.
We found that at the onset of abdominal distension that raised a concern for NEC, a higher calprotectin level was indicative of NEC. Hematochezia did not significantly increase the calprotectin level. This is consistent with the observation in adult patients that gastrointestinal bleeding of as much as 100 mL per day increases the fecal calprotectin concentration by only 15 μg/g [ARUP Laboratories http://ltd.aruplab.com/Tests/Pub/0092303]. Thus, perhaps a fecal calprotectin level can be of value, in questionable cases, in initially planning the length of NPO, TPN, and antibiotics, and in discussions with the family and staff.
We recognize limitations of our study. It is a pilot report involving only 30 episodes. We investigated infants with gastrointestinal signs that prompted a “r/o NEC” x-ray, and therefore we cannot determine a normal fecal calprotectin reference interval from our data. Fecal calprotectin levels are generally higher in neonates than in older children and adults 6, and the levels have inter and intra-individual variability based on gestational age, postnatal age, and time of day collected (11, 12, 14, 16). Thus, creating reference intervals for fecal calprotectin in neonates will be more complex than in older children or adults. Due to a low rate of surgical NEC in the patients in this study, the stool samples measured for calprotectin and the tissue samples assayed for NETs were obtained from different patients. The one case of surgical NEC occurred prior to our investigation into the source of fecal calprotectin which prevented us from using that patient's tissue sample. We realize that our evidence that calprotectin in stool originates from activated neutrophils is limited and correlative. However it is the first such evidence in neonates with NEC.
In conclusion, we present pilot data that a fecal calprotectin level at the time of concern for NEC could help distinguish NEC from more benign forms of feeding intolerance. Larger studies are needed to confirm this, and gestational-age and postnatal-age reference ranges for fecal calprotectin are also needed.
Supplementary Material
Table 4.
DURING THE THREE-DAY PERIOD FOLLOWING THE ONSET OF THE “RULE OUT NEC” EPISODE. Selected laboratory values, and fecal calprotectin levels following 30 episodes of “rule out NEC”.
| EVENTUAL DIAGNOSIS OF THE “RULE-OUT NEC” EPISODE | |||||
|---|---|---|---|---|---|
| Feeding Intolerance •Not NEC •No hematochezia (n=9) | Feeding Intolerance •Not NEC •Hematochezia (n=6) | NEC Stage II (Medical) (n=14) | NEC Stage III (Surgical)\(n=1) | P value No NEC vs NEC | |
| Highest leukocyte count (X103/μL) | 12.1 ± 6.1 | 20.0 ±0.3 | 13.8 ±5.8 | 27.4 | 0.812 |
| Lowest leukocyte count (X103/μL) | 10.0 ± 3.3 | 15.37± 3.9 | 13.3 ± 5.3 | 1.1 | 0.921 |
| Highest I/T neutrophil ratio | 0.05 ± 0.06 | 0.07 ± 0.06 | 0.16 ± 0.07 | 0.69 | 0.055 |
| Lowest monocyte count (X103/μL) | 0.9 ± 0.3 | 2.4 ± 1.1 | 1.3 ± 1.1 | 0.3 | 0.331 |
| Highest eosinophil count (X103/μL) | 0.5 ± 0.6 | 0.5 ± 0.4 | 0.8 ± 0.3 | 0.8 | 0.279 |
| Lowest platelet count (X103/μL) | 224 + 96 | 311 ± 190 | 310 ± 51 | 76 | 0.818 |
| Highest Calprotectin (μg/g stool) | 59 ± 40 | 97 ± 95 | 526 ± 471 | 1342 | 0.0001 |
Acknowledgements
The authors thank Diana Lim from the University of Utah Molecular Medicine Program for assistance with graphics.
Funding Source: Research grants from the McKay-Dee Hospital Foundation and from Sigma Theta Tau International Honor Society of Nursing provided partial funding for the project. ARUP Laboratories, Salt Lake City, UT, provided partial funding for the calprotectin assays. This work was supported in part by the US National Institutes of Health (K08HD049699 to C. Yost)
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this study to disclose. Dr. Robert Schlaberg and Jonathan Lowe are employees of ARUP laboratories where the calprotectin assays were run.
REFERENCES
- 1.Neu J. Necrotizing enterocolitis: the mystery goes on. Neonatology. 2014;106(4):289–295. doi: 10.1159/000365130. [DOI] [PubMed] [Google Scholar]
- 2.Gordon PV, Swanson JR. Necrotizing enterocolitis is one disease with many origins and potential means of prevention. Pathophysiology. 2014;21(1):13–19. doi: 10.1016/j.pathophys.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Lambert DK, Christensen RD, Baer VL, Henry E, Gordon PV, Besner GE, et al. Fulminant necrotizing enterocolitis in a multihospital healthcare system. J Perinatol. 2012;32(3):194–199. doi: 10.1038/jp.2011.61. [DOI] [PubMed] [Google Scholar]
- 4.Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol. 2013;40(1):27–51. doi: 10.1016/j.clp.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng PC, Chan KY, Poon TC. Biomarkers for prediction and diagnosis of necrotizing enterocolitis. Clin Perinatol. 2013;40(1):149–59. doi: 10.1016/j.clp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Alibrahim B, Aljasser MI, Salh B. Fecal calprotectin use in inflammatory bowel disease and beyond: A mini-review. Can J Gastroenterol Hepatol. 2015;29(3):157–163. doi: 10.1155/2015/950286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menees SB, Powell C, Kurlander J, Goel A, Chey WD. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol. 2015;110(3):444–454. doi: 10.1038/ajg.2015.6. [DOI] [PubMed] [Google Scholar]
- 8.Bin-Nun A, Booms C, Sabag N, Mevorach R, Algur N, Hammerman C. Rapid fecal calprotectin (FC) analysis: point of care testing for diagnosing early necrotizing enterocolitis. Am J Perinatol. 2015;32(4):337–342. doi: 10.1055/s-0034-1384640. [DOI] [PubMed] [Google Scholar]
- 9.Waugh N, Cummins E, Royle P, Kandala NB, Shyangdan D, Arasaradnam R, et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17(55):xvxix, 1–211. doi: 10.3310/hta17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll D, Corfield A, Spicer R, Cairns P. Faecal calprotectin concentrations and diagnosis of necrotising enterocolitis. Lancet. 2003;361(9354):310–311. doi: 10.1016/S0140-6736(03)12333-1. [DOI] [PubMed] [Google Scholar]
- 11.Wright EK, De Cruz P, Gearry R, Day AS, Kamm MA. Fecal biomarkers in the diagnosis and monitoring of Crohn's disease. Inflamm Bowel Dis. 2014;20(9):1668–1677. doi: 10.1097/MIB.0000000000000087. [DOI] [PubMed] [Google Scholar]
- 12.D'Inca R, Caccaro R. Measuring disease activity in Crohn's disease: what is currently available to the clinician. Clin Exp Gastroenterol. 2014;7:151–161. doi: 10.2147/CEG.S41413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josefsson S, Bunn SK, Domellof M. Fecal calprotectin in very low birth weight infants. J Pediatr Gastroenterol Nutr. 2007;44(4):407–413. doi: 10.1097/MPG.0b013e3180320643. [DOI] [PubMed] [Google Scholar]
- 14.Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94(4):267–271. doi: 10.1159/000151645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Noninvasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251(6):1174–1180. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 16.Aydemir O, Aydemir C, Sarikabadayi YU, Emre Canpolat F, Erdeve O, Biyikli Z, et al. Fecal calprotectin levels are increased in infants with necrotizing enterocolitis. J Matern Fetal Neonatal Med. 2012;25(11):2237–2241. doi: 10.3109/14767058.2012.684172. [DOI] [PubMed] [Google Scholar]
- 17.Dabritz J, Jenke A, Wirth S, Foell D. Fecal phagocyte-specific S100A12 for diagnosing necrotizing enterocolitis. J Pediatr. 2012;161(6):1059–1064. doi: 10.1016/j.jpeds.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Zoppelli L, Guttel C, Bittrich HJ, Andree C, Wirth S, Jenke A. Fecal calprotectin concentrations in premature infants have a lower limit and show postnatal and gestational age dependence. Neonatology. 2012;102(1):68–74. doi: 10.1159/000337841. [DOI] [PubMed] [Google Scholar]
- 19.Selimoglu MA, Temel I, Yildirim C, Ozyaln F, Aktas M, Karabiber H. The role of fecal calprotectin and lactoferrin in the diagnosis of necrotizing enterocolitis. Pediatr Crit Care Med. 2012;13(4):452–454. doi: 10.1097/PCC.0b013e3182388ae9. [DOI] [PubMed] [Google Scholar]
- 20.Aydemir G, Cekmez F, Tanju IA, Canpolat FE, Genc FA, Yildirim S, et al. Increased fecal calprotectin in preterm infants with necrotizing enterocolitis. Clin Lab. 2012;58(7-8):841–844. [PubMed] [Google Scholar]
- 21.Reisinger KW, Van der Zee DC, Brouwers HA, Kramer BW, van Heurn LW, Buurman WA, et al. Noninvasive measurement of fecal calprotectin and serum amyloid A combined with intestinal fatty acid-binding protein in necrotizing enterocolitis. J Pediatr Surg. 2012;47(9):1640–1645. doi: 10.1016/j.jpedsurg.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 22.Jenke AC, Postberg J, Mariel B, Hensel K, Foell D, Dabritz J, et al. S100A12 and hBD2 correlate with the composition of the fecal microflora in ELBW infants and expansion of E. coli is associated with NEC. Biomed Res Int. 2013;2013:150372. doi: 10.1155/2013/150372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reisinger KW, Kramer BW, Van der Zee DC, Brouwers HA, Buurman WA, van Heurn E, et al. Non-invasive serum amyloid A (SAA) measurement and plasma platelets for accurate prediction of surgical intervention in severe necrotizing enterocolitis (NEC). PLoS One. 2014;9(6):e90834. doi: 10.1371/journal.pone.0090834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon JM, Park JY, Ko KO, Lim JW, Cheon EJ, Kim HJ. Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Korean J Pediatr. 2014;57(8):351–356. doi: 10.3345/kjp.2014.57.8.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albanna EA, Ahmed HS, Awad HA. Stool calprotectin in necrotizing enterocolitis. J Clin Neonatol. 2014;3(1):16–19. doi: 10.4103/2249-4847.128721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yost CC, Cody MJ, Harris ES, Thornton NL, McInturff AM, Martinez ML, et al. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113(25):6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yost CC. Toward the “ideal” inhibitor of NETs. Blood. 2014;123(16):2439–40. doi: 10.1182/blood-2013-12-545400. [DOI] [PubMed] [Google Scholar]
- 28.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009;5(10):e1000639. doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel AL, Trivedi S, Bhandari NP, Ruf A, Scala CM, Witowitch G, et al. Reducing necrotizing enterocolitis in very low birth weight infants using quality-improvement methods. J Perinatol. 2014;34(11):850–7. doi: 10.1038/jp.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry E, Christensen RD, Sheffield MJ, Eggert LD, Carroll PD, Minton SD, et al. Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates? J Perinatol. 2015;35(2):132–6. doi: 10.1038/jp.2014.171. [DOI] [PubMed] [Google Scholar]
- 31.Christensen RD, Carroll PD, Josephson CD. Evidence-based advances in transfusion practice in neonatal intensive care units. Neonatology. 2014;106(3):245–53. doi: 10.1159/000365135. [DOI] [PubMed] [Google Scholar]
- 32.Baer VL, Lambert DK, Carroll PD, Gerday E, Christensen RD. Using umbilical cord blood for the initial blood tests of VLBW neonates results in higher hemoglobin and fewer RBC transfusions. J Perinatol. 2013;33(5):363–5. doi: 10.1038/jp.2012.127. [DOI] [PubMed] [Google Scholar]
- 33.McInturff AM, Cody MJ, Elliott EA, Glenn JW, Rowley JW, Rondina MT, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxiainducible factor 1 α. Blood. 2012;120(15):3118–25. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutter JJ, Jr., Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr. 1976;88(6):1026–31. doi: 10.1016/s0022-3476(76)81069-4. [DOI] [PubMed] [Google Scholar]
- 35.Kling PJ, Hutter JJ. Hematologic abnormalities in severe neonatal necrotizing enterocolitis: 25 years later. J Perinatol. 2003;23(7):523–530. doi: 10.1038/sj.jp.7210983. [DOI] [PubMed] [Google Scholar]
- 36.Remon J, Kampanatkosol R, Kaul RR, Muraskas JK, Christensen RD, Maheshwari A. Acute drop in blood monocyte count differentiates NEC from other causes of feeding intolerance. J Perinatol. 2014;34(7):549–554. doi: 10.1038/jp.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maheshwari A, Schelonka RL, Dimmitt RA, Carlo WA, Munoz-Hernandez B, Das A, et al. Cytokines associated with necrotizing enterocolitis in extremely-low-birth-weight infants. Pediatr Res. 2014;76(1):100–108. doi: 10.1038/pr.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho TT, Groer MW, Luciano AA, Schwartz A, Ji M, Miladinovic BS, et al. Red blood cell transfusions increase fecal calprotectin levels in premature infants. J Perinatol. 2015;35(10):837–841. doi: 10.1038/jp.2015.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gordon PV, Swanson JR, Clark R, Spitzer A. The complete blood cell count in a refined cohort of preterm NEC: the improtance of gestational age and day of diagnosis when using the CBC to estimate mortality. J Perinatol. 2015 Nov 12; doi: 10.1038/jp.2015.162. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;2007(379):pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.