Abstract
Background
Abnormal proliferation of vascular smooth muscle cells (VSMC) is a major cause of cardiovascular diseases (CVDs). Many studies suggest that vascular injury triggers VSMC dedifferentiation, which results in VSMC changes from a contractile to a synthetic phenotype; however, the underlying molecular mechanisms are still unclear.
Methods
In this study, we examined how VSMC responds under mechanical stress by using time-course microarray data. A three-phase study was proposed to investigate the stress-induced differentially expressed genes (DEGs) in VSMC. First, DEGs were identified by using the moderated t-statistics test. Second, more DEGs were inferred by using the Gaussian Graphical Model (GGM). Finally, the topological parameters-based method and cluster analysis approach were employed to predict the last batch of DEGs. To identify the potential drugs for vascular diseases involve VSMC proliferation, the drug-gene interaction database, Connectivity Map (cMap) was employed. Success of the predictions were determined using in-vitro data, i.e. MTT and clonogenic assay.
Results
Based on the differential expression calculation, at least 23 DEGs were found, and the findings were qualified by previous studies on VSMC. The results of gene set enrichment analysis indicated that the most often found enriched biological processes are cell-cycle-related processes. Furthermore, more stress-induced genes, well supported by literature, were found by applying graph theory to the gene association network (GAN). Finally, we showed that by processing the cMap input queries with a cluster algorithm, we achieved a substantial increase in the number of potential drugs with experimental IC50 measurements. With this novel approach, we have not only successfully identified the DEGs, but also improved the DEGs prediction by performing the topological and cluster analysis. Moreover, the findings are remarkably validated and in line with the literature. Furthermore, the cMap and DrugBank resources were used to identify potential drugs and targeted genes for vascular diseases involve VSMC proliferation. Our findings are supported by in-vitro experimental IC50, binding activity data and clinical trials.
Conclusion
This study provides a systematic strategy to discover potential drugs and target genes, by which we hope to shed light on the treatments of VSMC proliferation associated diseases.
Keywords: Drug repositioning, Cardiovascular disease, Gaussian graphical model, Vascular smooth muscle cell, Gene set enrichment analysis, Topological parameters, Time-course microarray, Mechanical stress
Background
Cardiovascular diseases (CVDs), such as coronary heart attacks, arrhythmia, and cerebrovascular diseases (strokes), are the leading causes of death in many countries. According to the World Health Organization (WHO) report, CVDs affect tens of millions of human beings each year. Therefore, how to improve the diagnosis, treatment, and prevention of CVDs is an urgent and important issue. Vascular smooth muscle cells (VSMC) comprise the majority of the wall of the blood vessel and play an important role in the pathogenesis of CVD. It has been known that when the vessel is exposed to high pressure such as mechanical stretch, this injurious forces will lead to differential gene expression, and then induce VSMC hypertrophy and hyperplasia as well as changes in phenotype from the contractile state to the synthetic state and matrix proteins (Anwar et al., 2012; Intengan & Schiffrin, 2001; Levy et al., 1988). This type of transition between different phenotypes is referred to as “phenotypic modulation” or “vascular remodeling.” Contractile VSMC are elongated, spindle-shaped cells, whereas synthetic VSMC are less elongated and have a cobblestone morphology (Rensen, Doevendans & van Eys, 2007).
Vascular injury triggers VSMC dedifferentiation and then results in phenotypic modulation (Campbell & Campbell, 1990) which is the major cause of restenosis (Haudenschild, 1993), atherosclerosis (Rivard & Andres, 2000) and hypertension (Lemarié, Tharaux & Lehoux, 2010). However, to date, the intracellular molecular mechanisms that regulate the VSMC phenotype have not been well understood. In this study, we identified and characterized the mechanical stress-induced genes that resulted in an abnormal expression (namely, differentially expressed genes (DEGs)), as well as the inter-molecular interaction network in the VSMC.
Time-course gene expression experiments have been extensively used for studying a wide range of biological processes (BPs). Due to the use of the temporal structure embedded in the time-course data, we may be able to capture the dynamical behavior of the gene expression. In particular, time series data allow us to infer the gene association network (GAN).
To study how VSMC react in response to mechanical stress, time-course microarray data were used to identify stress-induced genes, and further to understand the role of gene regulatory networks under mechanical stress in the VSMC. First, we identified DEGs and then conducted the gene set enrichment analysis to highlight the most relevant biological process terms associated with a given gene list. Next, the Gaussian Graphical Model (GGM) was employed to infer the GAN by considering the partial correlation coefficient based on the previous study proposed by Schafer & Strimmer (2005). More studies had also reported the use of GGM in biological systems, such as, inferring the gene dependency network (Schafer & Strimmer, 2006), circadian rhythm regulatory network construction (Liu et al., 2012), and integration of multiple sources of genomics data to infer gene regulatory network (Chun et al., 2013). After that, the GAN derived from the GGM can be characterized by certain topological parameters. Finally, graph theory approach was introduced to analyze the GAN. Furthermore, we also validated that our prediction of stress-induced genes corresponds to previous publications; hence, we can demonstrate the effectiveness of the present approach.
Materials and Methods
Datasets
To examine how mechanical stress contributes to the response of VSMC and its underlying signal transduction pathways, we made use of the microarray experiment, E-MEXP-569, downloaded from the ArrayExpress database (Brazma et al., 2003). The experiment compared the gene expression profiles of the VSMC in response to a cyclical mechanical strain over a time-course of 0, 2, 4 and 24 h. Each sample consisted of two replicates prepared from human aortic VSMC purchased from Cambrex Bioscience.
Differentially expressed gene identification
A gene which has an observed score that deviates significantly from the expected score is considered as a DEG. There are many statistical methods available for identifying DEGs by microarray data analysis. The use of false discovery rates (FDR) is a common approach to discover significant genes (Efron & Tibshirani, 2002). Analysis of variance (ANOVA) is another approach to investigate the impact of microarray gene expression values within a single factor (Kerr et al., 2002). Among the many statistical methods, Significance Analysis of Microarray (SAM) (Tusher, Tibshirani & Chu, 2001; Zhang, 2007), Empirical Bayes Analysis of Microarrays (EBAM) (Efron et al., 2001), and empirical Bayes statistics (EBAYES) (Efron, 2003) are three commonly employed approaches to identify DEGs.
SAM is a statistical method for identifying DEGs by comparing two or more groups of samples. It uses repeated permutations of the data to estimate FDR based on observed versus expected scores obtained from randomized data. SAM is able to handle time-course data, by considering different time points as distinct groups (Mutarelli et al., 2008).
EBAM performs one and two class analyses using either a modified t-statistic or standardized Wilcoxon rank statistic, and a multiclass analysis using a modified F-statistic. Moreover, this function provides an EBAM procedure for categorical data such as SNP data and the possibility of employing a user-written score function. EBAM can be applied to a differential time-course experiment to determine a subset of cDNAs that have the largest probability of being differentially expressed with respect to treatment conditions across a set of unequally spaced time points (Eckel et al., 2004).
The EBAYES algorithm computes moderated t-statistics, moderated F-statistics, and log-odds of differential expression by empirical Bayes shrinkage of the standard errors towards a common value. The moderated t-statistics is defined by
| (1) |
where g and k denote the gth gene and the kth time respectively, the contrast estimator denotes the difference between two classes, stands for the shrinkage estimation of the standard deviation of the gth gene expression level, and represents the kth diagonal element of the covariance matrix.
The EBAYES algorithm overcomes the possibility of identifying genes with small fold-change that might be significant from statistical analysis. In our previous study (Chen, Wu & Ng, 2012), we suggested that EBAYES, SAM, and EBAM achieve a similar level of cancer gene prediction accuracy, i.e. around 20%; therefore, without any bias EBAYES is adopted in the present analysis.
The microarray data were pre-processed by using the Bioconductor (Efron, 2003; Irizarry, 2005) R package ‘limma’ before passed to EBAYES for performing moderate F-test. The pEBAYES-value reported in the manuscript is the p-value for moderate F-test, i.e. F.p.value < 0.01, this value sets the threshold (Efron & Tibshirani, 2002) used to determine the DEGs.
Gene set enrichment analysis
Functional annotation of the DEGs is given by implementing the Database for Annotation, Visualization and Integrated Discovery, DAVID (Huang, Sherman & Lempicki, 2009). DAVID accepts batch annotation and conducts GO term enrichment analysis to highlight the most relevant GO terms associated with a given gene list. The gene identifiers used in DAVID is the microarray probe ID, i.e. AFFYMETRIX_3PRIME_IVT_ID.
Gaussian graphical model (GGM)
Inferring gene regulatory networks from microarray data is an important issue in systems biology. GGM is a graphical model, which was developed by Dempster (1972) to study the dependencies among a set of variables. In principle, the GGM infers GAN by considering the partial correlation coefficient instead of the Pearson correlation coefficient (PCC). The simple method of inferring GAN based on the PCC is not valid in most case studies because the high PCC of two variables does not imply a direct relationship. The GGM solves such a problem by using partial correlations to measure the independence of two genes. In partial correlation calculation, one introduces a third variable that has a relationship between the other two variables, and then calculates the correlation between two variables while excluding the impact of the third variable. Therefore, GGM allows us to distinguish between direct and indirect gene-gene interactions.
Within the GGM framework, the presence of an edge between two genes, gi and gk, is determined by the value of the partial correlation, pcor(i,k). Given the covariance matrix of all genes, Σ, it can be shown that full order pcor(i,k) is given by (Schafer & Strimmer, 2005),
| (2) |
where , which denotes the covariance between the i-th and j-th genes in the inverse of the covariance matrix. If an entry in the inverse covariance matrix is close to zero, then genes gi and gk are condition independent given all remaining genes. Since the number of microarray samples is much smaller than the number of genes considered, we employed a technique called shrinkage to improve the estimation of the sample covariance matrix. In actual implementation, we used the R package, GeneNet (Schäfer, Opgen-Rhein & Strimmer, 2006) to infer the GAN from microarray data.
Topological graph theory
In this work, we introduce the graph theory approach to analyze the GAN. Many studies indicated that there are underlying global and local topological structures of biological networks. The GAN derived from the GGM may have a complex topology. A complex network can be characterized by certain topological parameters; these parameters can be computed by using the SBEToolbox (Konganti et al., 2013). The 11 computed topological parameter values have been normalized between −1 and 1, a larger topological parameter value implies stronger topological effect. Three global topological parameters (average graph distance, diameter and network efficiency) and eight local topological parameters; i.e. the topological parameters of a node in the network (closeness centrality (CC), degree centrality (DC), eccentricity centrality (EC), betweenness centrality (BC), bridging centrality (BRC), clustering coefficient (CLC), brokering coefficient (BROC), local average connectivity (LAC)) are defined in the Section 1 of the Supplemental Information.
In the previous study, we have proposed a method to identify the important nodes in a network by topological parameter-based classification (Huang, Chen & Ng, 2016). Given a GAN, we propose that nodes have a higher degree, betweenness, centrality and densely connected which may play an important role in VSMC under mechanical stress. To test and demonstrate the presented concept, we classified eight of the 11 parameters into five groups based on their topological properties. Our classification considers the local parameters only. Classification of the eight parameters is shown in Table 1. Global topological parameters are not included, i.e. diameter, average graph distance and network efficiency.
Table 1. Classification of the local topological parameters.
| Group | Topological parameter | Abbreviation |
|---|---|---|
| 1 | Degree centrality | DC |
| 2 | Betweenness centrality | BC |
| 3 | Bridging centrality | BRC |
| 4 | Closeness centrality, eccentricity centrality | CC, EC |
| 5 | Clustering coefficient, brokering coefficient, local average connectivity | CLC, BROC, LAC |
In order to further our understanding of the GAN network property, we sorted the results of the eight local parameters’ values in descending order. For each group, if a node is ranked in the top 10%, a score value (SV) of one is assigned to this node. Since there are five groups, a node can have a maximum score of five. Since groups 4 and 5 consist of more than one topological parameters, a node will receive an SV of one if it ranks in the top 10% in any one of the parameters. The same analysis is repeated with the 15 and 20% thresholds.
Cluster analysis by using CFinder
In order to deepen our understanding of the GAN, we proposed that genes do not highly interact with others were assumed less important and consequently removed before the enrichment analysis.
The clustering tool, CFinder (Palla et al., 2005) which is based on the clique percolation clustering approach, was employed to perform the cluster analysis. The CFinder program identifies interacting clusters, which are called k-communities. A 3-community is set as k being equal to three (complete subgraphs of size 3). Any two k-communities are adjacent if they share k−1 common nodes. A k-community is constructed by merging all possible adjacent (k−1)-communities.
Drug repositioning
The idea of drug repositioning is a recently developed approach in the pharmaceutical industry that endeavors to identify new uses for existing drugs; and has achieved certain successes (Ashburn & Thor, 2004). This approach has the potential to reduce the development time required for drug discovery, as well as reducing side-effects. There are many works on identifying repositioned drugs which are based on various methods: the graph-based inference method (Iorio et al., 2010; Wu, Wang & Chen, 2013), the microarray expression method (Wu, Wang & Chen, 2012), and the differential expressed correlation method (Sun et al., 2013).
We made use of the drug-gene interaction databases, Connectivity Map (cMap) (Lamb, 2007), to find potential drugs for the treatments of VSMC proliferation associated diseases. Although the cMap resource is aimed at identifying drug treatment for cancer diseases, it is hypothesized that some of the known drugs may be repositioned for treating VSMC proliferation associated diseases.
Both CVDs, including atherosclerosis, as well as cancers lead to the cause of death worldwide, and they are characterized by a local increase in tissue mass. Many studies have suggested that atherosclerosis and cancer formation involve similar cellular processes i.e. cell proliferation, inflammation, and genomic instability. Both types of diseases possess a significantly common role in the pathogenesis and progression of atherosclerosis and cancer, especially in molecular pathways (Li & Gao, 2005; Ross et al., 2001a; Ross et al., 2001b). Common pathways or signal transduction networks, such as PI3k/Akt, can mediate several functional and morphological alterations of VSMC after being activated to develop vascular diseases (Jung et al., 2000), as well as affect the growth, apoptosis and cell cycle regulation of various cell types to induce cancer progression (Arcaro & Guerreiro, 2007). It is also known that the MAPK pathway, involved in VSMC proliferation, hypertrophy, and migration, central to the pathogenesis of vascular diseases (Jacob et al., 2005), possessed the same effects similar to PI3k/Akt in cancer occurrence (Sebolt-Leopold & Herrera, 2004).
Based on the above observations, we put forward the hypothesis for further investigation. It is conjectured that a drug molecule may potentially reverse the CVDs signature if the molecule induced signature is significantly negatively correlated with the disease-induced signature found in the cMap.
Since the time-course expression profile of DEG may exhibit an oscillatory pattern, therefore, we filtered out up- and down-regulated DEGs with at least two consecutive time points are up and down regulated respectively.
This set of DEGs were used to query the cMap database, where potential drugs with adjusted pcMAP-value less than 0.05 are retained. The adjusted pcMAP-value (named permutation p-value) is an estimate of the likelihood that the enrichment of a set of potential drugs in the list of all cMap drugs in given result/prediction would be observed by chance. This permutation p-value is determined by computing the enrichment of 100,000 sets of potential drugs selected at random from the set of all cMap drugs in the result/prediction.
There may be concern that the times points 0, 2, 4, 24 h are not consecutive. As Qiu et al. (2014a) noted in their review, mechanical stress regulates the functions of VSMC within 24 h in most of the studies (for details of the study, see the Table 1 (12 studies) and Table 2 (21 studies) of the review article by (Qiu et al., 2014a). For instance, Feng et al. (1999) reported that the gene expression fold changes of mechanically induced genes at 12 and 24 h were remarkably similar except only for three genes out of 3,160 DEGs.
Table 2. The 23 DEGs, their functional description and supporting study.
| Gene | Full gene name | Functional description | Study |
|---|---|---|---|
| CCL2 | Chemokine ligand 2 | Aged VSMC exhibited upregulation of CCL2 gene | Mascall et al. (2012) and Song et al. (2012) |
| CDC | Cell division cycle proteins | Involved in controlling cell cycle phase transition, such G2/M transition in VSMC | Su et al. (2007) |
| CDH6 | Cadherin 6 | A VSM cell-cell adhesion molecule, it is important for tissue integrity and cell proliferation | Sun et al. (2014) |
| CDK | Cyclin-dependent kinase | Differential effects on VSMC proliferation | Tanner et al. (2000) |
| CEBPD/GADD | Growth arrest and DNA damage-inducible gene | Cyclic stretch enhanced GADD153 expression in cultured rat VSMC | Oyadomari & Mori (2004) and Cheng et al. (2008) |
| COL | Collagen | Is expressed by VSMC in response to vascular injury | Sibinga et al. (1997) |
| CTGF | Connective tissue growth factor | Stimulates VSMC growth and migration | Fan & Karnovsky (2002) and Fan, Pech & Karnovsky (2000) |
| CXCL2 | Chemokine (C-X-C Motif) ligand 2 | Aged VSMC exhibited upregulation of CXCL2 gene | Song et al. (2012) |
| HSP | Heat shock protein | Cyclic strain-induced expression of HSP in human endothelial cells | Hurley et al. (2010) |
| IGF1R | Insulin-like growth factor type 1 receptor | Mechanical stretch simulates proliferation through activation of IGF1R | Cheng & Du (2007), Li et al. (2008), Okura et al. (2001) and Qiu et al. (2014b) |
| IL | Interleukin-1 | Plays a role in the migration of VSMC into the neointima following acute injury | Dardik et al. (2005), Wójtowicz et al. (2010) and Zampetaki et al. (2005) |
| JUNB/AP-1 | Jun B proto-oncogene | Is rapidly activated in a balloon-injured artery in rat | Yasumoto et al. (2001) |
| MAPK | Mitogen-activated protein kinase | Mechanical stress-initiated MAPK signal transduction in VSMC | Li et al. (2000) and Li & Xu (2000) |
| MCM3AP | Minichromosome maintenance complex component 3 | Expression of minichromosome maintenance proteins in VSMC | Bruemmer et al. (2003a) and Bruemmer et al. (2003b) |
| MMP | Matrix metalloproteinase | Upregulation of MMP-1 plays a critical role in the flow-enhanced motility in VSMC | Asanuma et al. (2003), Chung et al. (2005), Feng et al. (1999), Pascarella et al. (2008) and Shi et al. (2009) |
| PCNA | Proliferating cell nuclear antigen | Cyclic strain-induced expression of PCNA in human endothelial cells | Hurley et al. (2010) and Richard et al. (2007) |
| PDGF | Platelet-derived growth factor | Plays a role in the migration of VSMC into the neointima following acute injury | Dardik et al. (2005) and Raines (2004) |
| SGK1 | Serum-glucocorticoid-regulated kinase1 | Mechanical stress-activated SGK1 contributes to neointima formation in vein graft | Cheng et al. (2010) |
| TENM | Tenascin | Mechanically induced genes in human VSMC | Feng et al. (1999) |
| TGF | Transforming growth factor | Involved in controlling cell cycle phase transition, such G2/M transition in VSMC | Su et al. (2007), Mata-Greenwood et al. (2005), Reddy & Howe (1993) and Ueba, Kawakami & Yaginuma (1997) |
| TIMP | TIMP metallopeptidase inhibitor 3 | Mechanical strain induces TIMP-1 and TIMP-2 synthesis. Inhibition of angiogenesis in human artery endothelial cells is mediated by TIMP-2 from VSMC | Chung et al. (2005) and Mascall et al. (2012) |
| TNF | Tumor necrosis factor | Induction of MMP-14 and 2 by cyclical mechanical stretch is mediated by TNF | Wang et al. (2003) |
| TP53 | p53 protein | Plays a role in VSMC proliferation and atherosclerosis | Mercer & Bennett (2006) and Yonemitsu et al. (1998) |
We further noted that the use of non-consecutive time points for gene expression measurements are rather common; for example, Morrow et al. (2005) examined the effect of strain at the 6, 10 and 24 h, whereas Schad et al. (2011) investigated the strain effect at the 1, 6, 18 and 48 h.
Cell viability was determined using the MTT and clonogenic assay. Cancer cell lines, A549 and H460 were used in both experiments. The protocols were described in our previous works (Huang et al., 2014; Lan et al., 2010). Furthermore, we also submitted the predicted DEGs to NCBI PubChem database and the DrugBank databases (Wishart et al., 2008) to identify potential drugs for VSMC proliferation associated diseases. The results of the potential drugs identified by cMap are reported in the following ‘Results’ section.
IC50 is the drug concentration inducing 50% inhibition of the cell viability. Cell viability was determined using the MTT and clonogenic assay. Non-small cell lung cancer cell lines were used in both experiments. In this paper we retrieved two IC50 measurements from two different resources to validate our predictions, for clarity, “IC50” and “ICdb50” represent experimentally determined IC50 activities obtained from in-house measurement (MTT and clonogenic assay) and the NCBI PubChem database, respectively.
Results
To determine the DEGs, we made use of the “Linear Models for Microarray Data” (limma) package, which is available from the Bioconductor service. Details are described in the following sections.
Differentially expressed gene identification
In this study, the Robust Multi-array Average (RMA) was used for gene expression normalization. After that, a model matrix (use the function, model.matrix) was created with rows and columns denoting the replicates (with the four time points information) and the time points respectively. Then, we seek a linear model to describe each probe/gene using the lmFit function provided by the limma package (Smyth, 2004; Smyth, 2005). DEGs are determined by first constructing the contrast matrix (use the function, cont.matrix), which made a pairwise comparison of two consecutive time points between the two replicates (use the function contrast.fit). EBayes analysis was subsequently conducted on the previous results, and the DEGs were selected by setting a pEBAYES-value threshold of 0.01 corresponding to the moderated F-statistics.
Through a manual literature search, we have collected a list of genes that are involved in VSMC phenotypic modification. Among the 473 DEGs, there are at least 23 DEGs are found in the literature. Table 2 summarized the results of those 23 genes, their biological functions, and references. These results of the DEGs can be accessed at http://ppi.bioinfo.asia.edu.tw/vsmc, which provides several important types of genetic information, such as the chromosomal locations, PubMed IDs, GO annotations, pathways, and pEBAYES-values.
The results of gene set enrichment analysis
A total of 473 DEGs were submitted to DAVID for clustering, and thus enriched BPs related gene groups were obtained, included KEGG, BioCarta and REACTOME, in which not only the most often found enriched BPs are cell cycle-related processes, but also the enriched pathways are associated with cancer- or cell cycle-related events. These results show that many DEGs might involve in the cell proliferation or apoptosis regulatory pathways to induce cell proliferation or apoptosis which lead to cardiovascular disease or cancer.
Details of the top three clusters (with enrichment scores 13.84, 8.59, and 5.93 given by DAVID) of enriched BPs information were presented in Table 3. Obviously, the most often found enriched BPs are cell-cycle-related processes, such as, M phase, regulation of mitotic cell cycle and cytoskeleton organization. For the cluster with the highest enrichment score, 64 genes were included in the cell cycle process.
Table 3. The Results of the top three clusters’ enriched BPs.
| Enrichment score: 13.84 | Count | pDAVID-value | Benjamini |
|---|---|---|---|
| Cell cycle | 64 | 6.90E-17 | 2.60E-13 |
| M phase | 41 | 9.30E-17 | 1.30E-13 |
| Organelle organization | 86 | 4.00E-16 | 3.40E-13 |
| Cell cycle process | 52 | 1.60E-15 | 9.70E-13 |
| Cell cycle phase | 44 | 2.40E-15 | 1.10E-12 |
Note:
The ‘Count’ denotes the number of DEGs found in the BPs. The ‘pDAVID-value’ and ‘Benjamini’ columns denote the p-value and Benjamini p-value given by DAVID, respectively.
Many studies have noted that hemodynamic factors, including shear stress, cyclic strain, and hydrostatic pressure, can (i) regulate the proliferation rate of VSMC (Boehm & Nabel, 2001; Sterpetti et al., 1993; Sterpetti et al., 1992), (ii) and thus trigger many cell-cycle-related molecules (Qiu et al., 2014a; Shi & Tarbell, 2011), and then (iii) decrease proliferation and increase apoptosis, mediated by the Akt pathway (Fitzgerald et al., 2008).
For the cluster with the highest enrichment scores, 64 genes were included in the cell cycle process. It has been reported that cell cycle and cell migration proteins regulate multiple biological functions in the cardiovascular systems (Boehm & Nabel, 2001).
For the M phase process, there are 41 genes. A number of studies have shown that the transition of VSMC from G2 phase into the M (mitosis) phase of the cell cycle is a tightly controlled process (Grainger et al., 1993; Somoza et al., 2004; Su et al., 2007). Furthermore, much evidence (de la Cuesta et al., 2013; Hellstrand & Albinsson, 2005; Martinez-Lemus, 2014; Sarkar et al., 2005; Zheng et al., 2010) also suggested that cytoskeleton deregulation may explain how VSMC switch from a contractile to a synthetic phenotype.
Topological analysis of the gene association network (GAN)
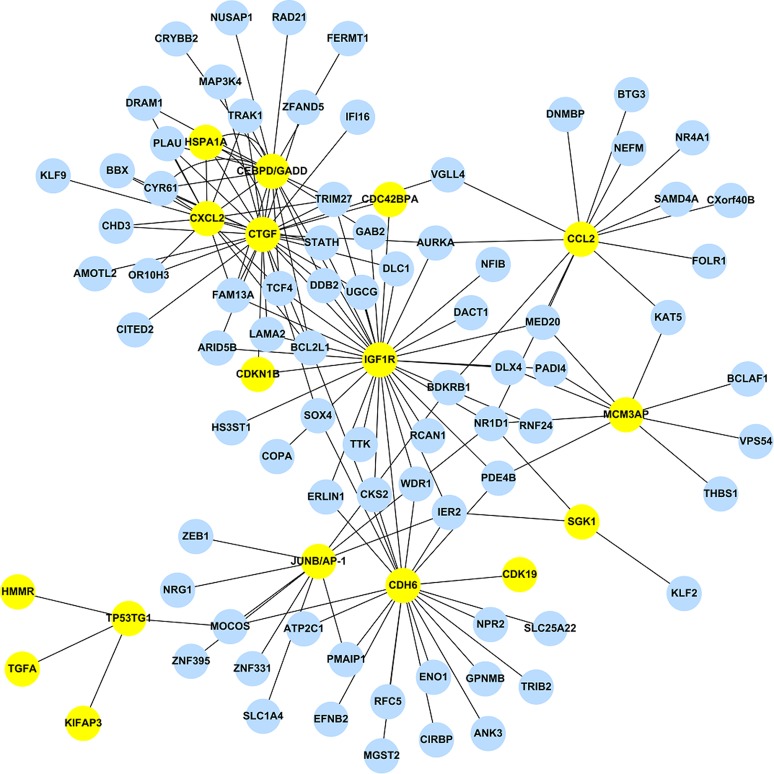
Using the 473 DEGs as input, we performed the GGM analysis, and ranked the partial correlation coefficients according to their absolute values, i.e. both activation and suppression events were kept. After ranking the results obtained from GGM, only the top 1% correlation events were selected to construct the GAN. GGM analysis reduced the total number of DEGs from 473 to 243. This network consists of the 1,442 significant links (adjusted pGGM-value < 0.01) among the 243 genes. Among those 243 genes, 17 genes are the DEGs listed in Table 2, while the corresponding GAN is shown in Fig. 1 using Cytoscape (http://www.cytoscape.org/), a useful tool for visualizing a molecular interaction network. The 17 genes were highlighted and their linked genes (each link has an adjusted pGGM-value < 0.01) were shown. The R package, network.test.edges, was used for assessing the significance of edges in the GGM. A mixture model is fitted to the partial correlations using the R language false discover rate tool, fdrtool. This results in two-sided p-values for the test of non-zero correlation.
Figure 1. The stress-induced GAN for the 17 DEGs are shown by the yellow circles.
Blue circles denoted DEGs, which were predicted by EBayes analysis.
The top 1% correlation events obtained from the GGM analysis has a modest percentage of overlapping genes, i.e. 73.9% (17/23), where the rest of the 226 genes are DEGs but not included in Table 2.
Given the GAN (1,442 links among 243 genes), we analyzed the network by using the topological graph theory approach. The results of the 11 parameters of the GAN were summarized in Table 4.
Table 4. The results of global and local topological parameters of the GAN.
| Average | Standard deviation | |
|---|---|---|
| Graph distance | 2.68 | – |
| Graph diameter | 6.00 | – |
| Network efficiency | 0.41 | – |
| Degree centrality | 0.05 | 0.049 |
| Betweenness centrality | 0.007 | 0.015 |
| Bridging centrality | 0.0002 | 0.00027 |
| Closeness centrality | 0.38 | 0.054 |
| Eccentricity centrality | 0.23 | 0.0285 |
| Clustering coefficient | 0.50 | 0.331 |
| Local average connectivity | 0.372 | 0.281 |
| Brokering coefficient | −0.33 | 0.241 |
In Table 4, it was found that the GAN had an average node graph distance of 2.68, and a network diameter of 6. A small average node graph distance value means that any two genes may have indirect interactions via less successive gene-gene interaction.
Using 0.05 as a threshold, the VSMC GAN had a relatively small DC value, i.e. 0.05. This means that nodes of GAN were much less interaction directly with each other. Here ‘direct’ refers to the fact that DC characterized a node’s neighborhood connections. However, the GAN had a rather small BC (0.007) and BRC (0.0002) values. A higher BC value indicated that nodes of the GAN were in between many other nodes. Also, a higher BRC value suggested that nodes of GAN can reach a higher portion of nodes, which is termed ‘reachability’ in some literature. In addition, it is noted that the GAN had a negative BROC which means the GAN inclined to form a cluster.
In order to further our understanding of the GAN network property, we sorted the eight local parameters’ values in descending order, and counted the number of genes ranked in the top 10, 15 and 20%. Table 5 summarized the results of the rank statistics of the GAN. As seen in Table 5, there is no gene with an SV of five ranked in the top 10 and 15%. However, there are three/eight genes which have an SV of five/four at the top 20/10%, respectively.
Table 5. The number of top-ranked genes by using graph topological analysis.
| %\score | 5 | 4 | 3 | 2 | 1 |
|---|---|---|---|---|---|
| 10% | 0 | 8 | 18 | 38 | 99 |
| 15% | 0 | 19 | 23 | 43 | 81 |
| 20% | 3 | 30 | 25 | 41 | 76 |
As shown in Table 5, at the 10 and 15% thresholds, the highest score is four, while a closer examination revealed that most of the nodes’ group 3 values, i.e. BRC, were ranked beyond the top 20% threshold. This means information flow or node connecting densely connected components was less likely.
For an SV of four and 10% threshold, the eight genes are: AURKA, CYR61, DUSP10, HSPA1A, MED20, NFIB, NR1D1 and TCF4, their roles and related studies were depicted in Table 6. These results provide substantial evidence that top ranked nodes play a role during the stress-inducing process.
Table 6. Top-ranked genes, their functional description and supporting study.
| Gene | Functional description | Study |
|---|---|---|
| AURKA | Cell cycle-related genes induced by PDGF-DD in VSMC | Alexander et al. (2012) |
| CYR61 | Supports VSMC adhesion and induces VSMC chemotaxis associated with post-angioplasty restenosis | Grzeszkiewicz et al. (2002) and Lee et al. (2007) |
| DUSP10 | DUSP10 encodes a dual-specificity MAPK phosphatase that has a principal function in both innate and adaptive immune responses | Weinsheimer et al. (2007) |
| HSPA1A | In VSMC, low-density lipoproteins (LDL) modulate HSP phosphorylation and subcellular localization, affecting action polymerization and cytoskeleton dynamics in rats, increases heart tissue hsp70i expression results in protection of the heart against inadequate blood supply injury | García-Arguinzonis et al. (2010) and Marber et al. (1995) |
| MED20 | A repressor of smooth muscle cell differentiation | Beyer et al. (2007) |
| NFIB | A transcriptional inhibitor of p21(CIP), and CDC25A | Dellago et al. (2013) |
| NR1D1 | NR1D1 also known as Rev-ErbA, upregulates NF-κB-responsive genes in VSMC | Migita, Morser & Kawai (2004) |
| TCF4 | Plays a dual role in vascular remodeling by inhibiting VSMC apoptosis and promoting proliferation | Wang et al. (2002) |
Among the eight genes, only HSPA1A belongs to the HSP protein family and is also identified as a DEGs, whereas the other seven genes are not DEGs.
If we relaxed the threshold to the 20% level, there is a gene with a SV of five, i.e. IGF1-receptor (IGF1R), which plays an important role in migration, cell cycle progression and survival of VSMC (Cheng & Du, 2007; Li et al., 2008; Okura et al., 2001; Qiu et al., 2014b).
Thus, the above results suggested a useful scenario to identify stress-induced genes by using time series data, and elaborate the network structure by using graphical and cluster analysis. Also, the findings support our suggestion that if the genes have higher degree, betweenness, centrality and densely connected, they may play important roles in VSMC under mechanical stress.
The results of cluster analysis
For a given k, CFinder may return more than one cluster; therefore, we selected the largest k-community for cluster analysis. Table 7 summarized the results of the sizes and numbers of clusters for the k-communities identified by CFinder. For example, CFinder identified two largest clusters with a size of 56 for the 6-communities.
Table 7. Sizes and number of clusters for the k-communities identified by CFinder.
| k | Size (number of clusters) |
|---|---|
| 3 | 204, 3 |
| 4 | 176, 5, 4 |
| 5 | 136, 7, 5 |
| 6 | 56(2), 11, 7(2), 6(5) |
| 7 | 43, 42, 9 |
| 8 | 16(2), 12(2), 10, 9, 8 |
| 9 | 14, 11, 10(3) |
| 10 | 11, 10 |
Note:
The parenthesis denotes the number of clusters identified by CFinder, else only one cluster was found.
Enriched biological pathways’ annotations of communities were given by implementing DAVID. According to the REACTOME (Croft et al., 2011) and KEGG (Kanehisa et al., 2004), pathways with their pDAVID-values less than 0.05 and ranked among the top ten were reported.
Table 8 summarized the results of the pathway (pDAVID-value less than 0.05) or disease information and references for the k-communities with k equals to 7–10. As we noted from Table 8, cluster analysis allowed us to infer more VSMC-related genes (denoted by bold-faced letters) that were not identified by differential expression or topological analysis. We performed the gene set enrichment analysis for communities with higher connectivity only, the same analysis can be applied for k equals to 3–6 if necessary.
Table 8. Enriched pathway or disease returned by DAVID.
| k | Gene name | Pathway or disease |
|---|---|---|
| 10 | DUSP10 | MAPK (Mascall et al., 2012) |
| SH2D4A | Angiogenesis, VEGF (Mascall et al., 2012; Qiu et al., 2014b) | |
| 8, 9 | BCL2L1 | Ras (Lan et al., 2010) |
| CTGF | Cardiovascular disease | |
| HSPA1A | Restenosis | |
| PLAU | Blood coagulation | |
| 7 | BCL2L1 | Ras (Lan et al., 2010; Mascall et al., 2012) |
| BDKRB1 | Cardiovascular disease | |
| CCL2 | Cardiovascular disease | |
| CTGF | Cardiovascular disease | |
| CDKN1B | Cardiovascular disease | |
| DDB2 | p53 (Dardik et al., 2005; Wójtowicz et al., 2010) | |
| DLC1 | PDGF (Hurley et al., 2010; Smyth, 2005) | |
| FAM13A | Angiogenesis | |
| HSPA1A | Restenosis | |
| IGF1R | IGF1R (Arcaro & Guerreiro, 2007; Huang et al., 2014; Jacob et al., 2005; Sebolt-Leopold & Herrera, 2004) | |
| MED20 | Gene expression (Boehm & Nabel, 2001) | |
| NFIB | Cellular process (Qiu et al., 2014a) | |
| NR1D1 | NFkB (Qiu et al., 2014a) | |
| NR4A1 | MAPK (Hinze et al., 2013) | |
| PMAIP1 | p53 (Dardik et al., 2005; Wójtowicz et al., 2010) | |
| PLAU | Blood coagulation | |
| TCF4 | Cellular process (Fitzgerald et al., 2008) |
Note:
Bold-faced gene name denote new putative genes identified by cluster analysis; they were not found by differential expression or topological analysis.
The present approach suggested that a combination of the DEGs, topological and cluster analyses may be more beneficial in terms of identifying VSMC-related genes, i.e. narrowed down to a total of 43 (not for a specific k-community). In Table 2, there are 23 DEGs obtained from literature. Through topological analysis of the GGM result (with a SV of four and listed in the top 10%) there are eight genes found (listed in Table 6, but one gene is the same in Table 2), so seven genes were counted. Using the same network from GGM analysis, through the Cfinder calculation and followed by enrichment analysis (DAVID), 13 more genes are found to be relevant to VSMC based on the pathway or disease annotations given by DAVID. Therefore, when we added up 23, 7 and 13, 43 DEGs were found.
A possible explanation for this lies in the fact that different methods of analysis obtained different aspects of the microarray data. For instance, the DEG analysis is based on the fold changes of gene expression, the GGM is rested on partial correlation calculation, whereas the cluster analysis made of PPI and biological functional analysis.
The results of drug repositioning for VSMC proliferation associated diseases
Using a pEBAYES-value < 0.01, 473 DEGs were identified. The use of these genes to query against cMAP did not return any potential drugs. Therefore, we used a less stringent p-value to estimate the association of the DEGs with drugs. A total of 208 and 394 up- and down-regulated DEGs respectively (pEBAYES-value < 0.05 and cMap enrichment score < 0, which is shown in Section 2 of the Supplemental Information), with at least two consecutive up- and down-regulated time points, were submitted to cMap, where 30 drugs were returned as shown in Table 9. Among these drugs, three of them, i.e. thioridazine, niclosamide, and pyrvinium have in-house experimental determined IC50 activities.
Table 9. The potential drugs returned by cMap under pcMAP-value < 0.05 and cMap enrichment score < 0.
| 5224221 | 5707885 | Alimemazine | Arachidonic acid | Ascorbic acid |
| Benzamil | Bromopride | Bucladesine | Calmidazolium | Cicloheximide |
| Clomifene | Fluspirilene | Gossypol | LM-1685 | Loperamide |
| Lovastatin | LY-294002 | Maprotiline | Methylbenzethonium chloride | Niclosamide |
| Pentamidine | Pimozide | Puromycin | Pyrvinium | Rottlerin |
| Syrosingopine | Thioridazine | Trolox C | Valinomycin | Wortmannin |
For thioridazine, its association with CVD is its deadly adverse effect (Eder et al., 2014), namely, torsades des pointes arrhythmia, a very severe type of irregular heart beating. One research proposed that there was a significant dose–response relationship for increasing heart rate and increasing drug-induced long Q-T syndrome but not other cardiovascular changes (Strachan, Kelly & Bateman, 2004). Although it took a relatively high dose of thioridazine to cause adverse cardiovascular effects, thioridazine is less likely to be a potential drug for VSMC proliferation associated diseases.
Niclosamide has been found to inhibit rapidly mTORC1 signaling which is required for embryonic cardiovascular development and for postnatal maintenance of cardiac structure and function. Also, mTORC1 is necessary for cardiac adaptation to pressure overload and development of compensatory hypertrophy (Balgi et al., 2009; Sciarretta, Volpe & Sadoshima, 2014). Therefore, it may be less possible to be beneficial for VSMC proliferation associated diseases. Another study suggested niclosamide and L-4F co-administered orally in a mouse model would reduce the atherosclerotic lesion, but not niclosamide alone. Thus, niclosamide may interact with L-4F to protect the effective peptide from trypsin digestion; therefore, allowing its absorption (Navab et al., 2009).
For pyrvinium, multiple ex vivo experiments have suggested it can heal a scarring heart (Murakoshi et al., 2013; Saraswati et al., 2010). Moreover, pyrvinium reduced adverse cardiac remodeling (Saraswati et al., 2010).
By use of the NCBI PubChem database, we found that among the 442 DEGs (after excluding genes which do not have identifiable GeneID) there are 31 DEGs, targeted by 73 drugs. Among the 31 DEGs, seven of them, i.e. CCL, CDK, CDKN1B, IGF1R, MMP, PLAU and TP53, matched with our 43 DEGs list, i.e. 16.3%.
Among these six families of genes (CDK and CDKN1B belong to the same family), the ICdb50 information for five of them, except IGF1R, are given in Table 10. We noted that all the drugs in Table 10 had ICdb50 values, in particular, the ICdb50 records are available for two families of DEGs, i.e. the CDK and MMP families. Given the fact that the NCBI PubChem resource is not designed specifically for recording CVD drugs; hence, this may limit the number of matching events. For the moment, the findings listed in Table 10 should be important in VSMC proliferation associated disease treatments.
Table 10. The ICdb50 information for CCL, CDK, MMP, PLAU and TP53.
| Drug name | DEG | ICdb50 (μM) | Study |
|---|---|---|---|
| Acetylsalicylic acid | TP53 | Redondo et al. (2003) | |
| Amiloride | PLAU | Chan et al. (2011) and Liu et al. (2014) | |
| AT7519 | CDK family | 3 ICdb50 records were found | Wyatt et al. (2008) |
| Danazol | CCL3 | – | |
| Flavopiridol | CDK family | 31 ICdb50 records were found | Andres (2004), Dzau, Braun-Dullaeus & Sedding (2002) and Ruef et al. (1999) |
| Marimastat | MMP family | 28 ICdb50 records were found | Peterson et al. (2000) |
Note:
‘-’ denotes not available.
In summary, given the 30 potential drugs, we had identified the drug targets which are also the DEGs input in cMAP. With the drug target’s ICdb50 also found in NCBI, the results imply “very” specific drug action mechanism.
Acetylsalicylic acid (ASA) is under clinical trials (clinical trial NCT00501059) for cardiovascular disease treatment and has been demonstrated to inhibit VSMC proliferation (Redondo et al., 2003).
For amiloride, this drug may reduce the increased VMSC hypertrophy by mediating ion transport mechanism which is induced by angiotensin II. Besides, it has been shown that pre-treatment with 5-N,N-hexamethylene amiloride attenuates angiotensin II-induced VSMC proliferation (Liu et al., 2014). Also, the amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the Bone Morphogenetic Protein signaling pathway (Chan et al., 2011). Amiloride is also under clinical trials (clinical trial NCT01195805) for cardiovascular disease treatment.
AT7519, a novel small-molecule multi-cyclin dependent kinase inhibitor, has been evaluated in clinical trials for the treatment of cancers (Dolman et al., 2015; Wyatt et al., 2008).
For AT7519 and flavopiridol, the corresponding clinical trials (clinical trials NCT01183949 and NCT00112723) for myeloma are ongoing. Furthermore, for flavopiridol, it has been reported that it inhibits VSMC growth in vitro and in vivo. Its oral availability and selectivity for CDKs make it a potential therapeutic tool in the treatment of vascular lesions (Andres, 2004; Dzau, Braun-Dullaeus & Sedding, 2002; Ruef et al., 1999).
For marimastat, the clinical trial (clinical trial NCT00261391) for vascular anomalies has completed, and it has been reported that marimastat (a selective but non-specific MMP inhibitor) can prevent intimal hyperplasia in a cultured human internal mammary artery (Peterson et al., 2000). Interestingly, it is known that MMP inhibitors (MMPIs) could suppress the effects of MMPs on VSMC proliferation, perhaps it is the most promising clinical application of MMPIs in vascular medicine.
Taken together, with our finding and the literature support, these above-mentioned five drugs might have chances to be potential drugs for CVDs.
Targeted genes for VSMC proliferation
Drug-target relationships have been used for drug repositioning. For example, Huang et al. (2015) constructed a weighted and integrated drug-target interactome (WinDTome) in drug repurposing for schizophrenia. Wu, Wang & Chen (2013) merged disease-gene and drug-target relations for drug repositioning. We are motivated to derive the targeted genes for the potential drugs.
The potential drugs in Table 9 were submitted to NCBI to search for their corresponding targeted genes. Finally yielding a total of 38 targeted genes for diseases associated with VSMC proliferation, as shown in Table 11, which are the potential therapeutic targets for future VSMC proliferation diseases clinical trials. For each targeted gene in Table 11, the number in parentheses is the number of associated cMap drugs, and could be regarded as a metric for prioritizing the genes in the list. The TP53 and ADRB2 genes ranked top of the lists.
Table 11. The genes targeted by the 30 potential drugs.
| NPC1 (20) U | IL1B (14) U | PPP3CA (10) U | HSPA1A (4) U | PTBP1 (3) U |
| CYP51A1 (2) U | RNASEH1 (1) U | CDK19 (1) U | AKT3 (1) U | INSR (1) U |
| TP53 (23) D | ADRB2 (23) D | RGS4 (22) D | SMPD1 (17) D | POLI (17) D |
| BAZ2B (15) D | MMP14 (15) D | BCL2L1 (15) D | NR2F2 (14) D | SUMO1 (14) D |
| VIPR1 (10) D | EGFR (10) D | ID4 (9) D | RXRA (7) D | PLEC (5) D |
| NLRP1 (5) D | STAT1 (4) D | WEE1 (4) D | STK3 (3) D | SRPK1 (3) D |
| SGK1 (3) D | MKNK2 (3) D | DUSP1 (2) D | PLAU (1) D | CDC42BPA (1) D |
| CDK2 (1) D | KDR (1) D | SIK1 (1) D |
Note:
The parentheses represent the number of associated cMap drugs, U and D represent up and down-regulated respectively.
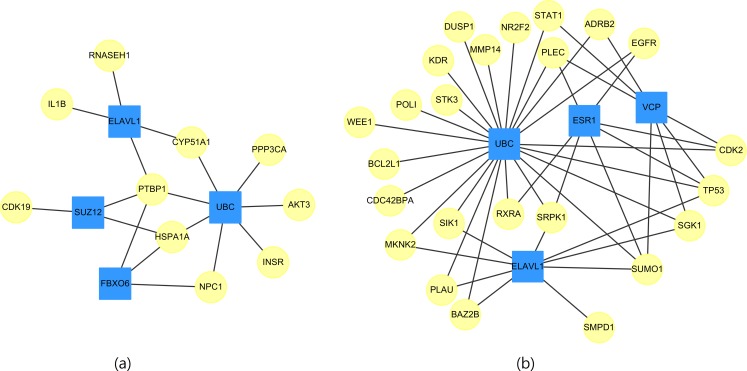
Whether a particular gene is related to most of the targeted genes in Table 11 is of interest. Therefore, networks of the targeted genes and their adjacent genes in CVDs PPI were constructed. Figure 2 displays the top four genes that exhibit the largest, the second largest, the third largest and the fourth largest degree in CVDs networks. The UBC gene directly interacts with 7(23) targeted genes in up(down) regulated CVDs networks, while the other genes, such as ELAVL1, connect to no more than 4(9) targeted genes.
Figure 2. The top four genes (squares) which connect to the largest, the second largest, the third largest and the fourth largest degree of targeted genes (circles) for (A) up-regulated and (B) down-regulated CVDs network.
We noted that the ubiquitin-conjugating gene (UBC) is not a DEG. However, as early as 1997, it has been reported by Adam et al. (1997) that UBC is involved in the function or maintenance of the contractile phenotype of VMSC. UBC is responsible for catalyzing the transfer of ubiquitin to substrate proteins is a component of the ubiquitin–proteasome system (UPS).
It was known that the UPS, regulates the degradation of oxidized, misfolded or damaged proteins, plays a significant role in the control of VSMC phenotype and survival signaling (Demasi & Laurindo, 2012). It has been suggested that the UPS may be a therapeutic target in vascular diseases (Willis et al., 2010). Pagan et al. (2013) also postulated that the UPS is a promising potential therapeutic target in ischemia-reperfusion injury. We expect the development of novel therapeutic drugs are capable of modulating the UPS for CVD treatments.
Improving drug prediction accuracy by using clustering approach
In the above analysis, a total of 208 and 394 up- and down-regulated DEGs were used to identify 30 potential drugs (as listed in Table 9) for CVDs, and three of them, i.e. thioridazine, niclosamide and pyrvinium have in-house experimental determined IC50 activities. In an attempt to improve the drug prediction accuracy according to IC50 measurements, we further applied graph clustering to extract densely connected clusters of DEGs. A well-known algorithm–ClusterONE (Nepusz, Yu & Paccanaro, 2012) was used for clustering, and the top two largest up- (down-) regulated clusters with sizes 7 and 6 (22 and 8) were selected for analysis (listed in Section 3 of the Supplemental Information). There are four options formed by combining the two up-regulated clusters and the two down-regulated clusters. After submitted to cMap, among the four combinations, we found that by combining the up-regulated cluster of size 6 with the down-regulated cluster with size 22 achieve the highest prediction accuracy according to IC50 measurements, 30.0% (15/50). That is, it predicts 50 drugs, of which 15 drugs were validated as effective by IC50 experiments. Furthermore, combining the up-regulated cluster of size 6 with the down-regulated cluster with size 8 achieve the second highest prediction accuracy according to IC50 measurements, 21.5% (11/51). In contrast, combining the up-regulated cluster of size 7 with the down-regulated cluster with sizes 22 and 8 achieves none and one drug (thapsigargin) is verified by IC50 experiments, respectively.
Out of the 15 predicted potential CVD compounds mentioned in above, 11 of them (73% success rate) have been found to act on the cardiovascular system in the literature. Among them are compounds from various notable class of mechanism including peroxisome-proliferator-activated receptors gamma (PPAR-Ƴ) agonist (15-delta prostaglandin J2), heat shock protein 90 (HSP90) inhibitors (monorden, also known as radicicol; and tanespimycin (17AAG)), histone deacetylase (HDAC) inhibitor (trichostatin A) and anti-fibrotic agent (withaferin a). 15-delta prostaglandin J2 acts as a PPAR agonist which has been shown to elicit protection against myocardial injury through the remote ischemic preconditioning in an in vivo model (Lotz et al., 2011). Mefloquine, an anti-malarial drug, has been demonstrated in multiple studies to possess cardiotoxicity exerting a negative inotropic effect on the heart and certain cardiac arrhythmia (Coker et al., 2000). The HSP90 inhibitor, monorden, was shown to induce heat shock protein expression in neonatal rat cardiomyocytes which ultimately conferred cardioprotection to these cardiomyocytes (Griffin, Valdez & Mestril, 2004). Tanespimycin (17AAG), another HSP90 inhibitor, may also possess similar cardioprotective effects. Parthenolide, isolated from a herb extract, has recently been demonstrated to be able to inhibit the VSMC proliferation by inducing G0/G1 phase cell cycle arrest (Weng et al., 2009). Phenoxybenzamine has been used in open heart surgery, coronary artery bypass grafting (CABG) with a radial artery, and in one study, treatment of the radial artery grafts with phenoxybenzamine was associated with a reduction in perioperative myocardial injury (Kulik et al., 2007). Piperlongumine is an alkaloid extracted from the long pepper, Piper Longum L., which was found to have anti-atherosclerotic action suppressing atherosclerosis plaque formation in vivo (Son et al., 2012). Trichostatin A, an HDAC inhibitor, has been shown to be able to confer cardioprotection against myocardial ischemia/reperfusion injury in vivo in one animal study (Yu et al., 2012). Trifluoperazine is a calmodulin inhibitor which ranks high in the order of effectiveness amongst its same class compounds in protecting the calcium-overloaded myocardium (Hong et al., 2014). Zhao et al. (2012) has reported the use of vorinostat in pulmonary arterial hypertension. Finally in the list is withaferin A, an anti-fibrotic compound against diseases such as cardiac interstitial fibrosis (Challa et al., 2012).
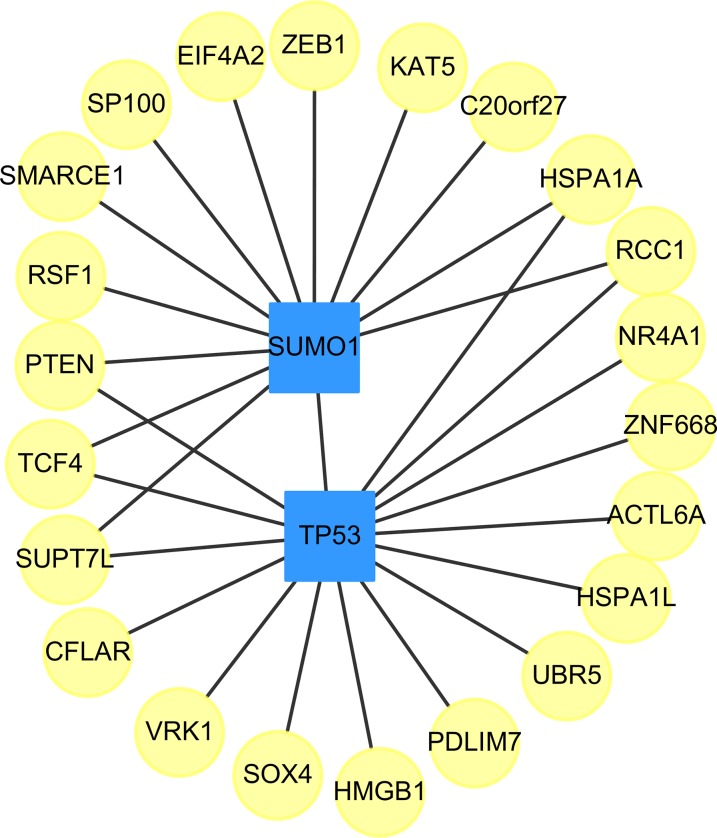
We found two drug-targeted genes, TP53, and SUMO1, which are appeared in our prediction with and without the use of the clustering algorithm, ClusterONE. The networks of targeted genes and their adjacent genes in CVDs PPI were shown in Fig. 3. If drugs could indirectly affect down-stream PPI of the TP53 and SUMO1 genes, the therapeutic role of these two genes is worth further exploration.
Figure 3. The top two genes (squares) which connect to the largest and the second largest degree of targeted genes (circles) in the down-regulated CVDs PPI after cluster analysis.
The small ubiquitin-related modifier (SUMO) family of proteins play an important role in post-translational modification (Mahajan et al., 1997). There are four SUMO genes (SUMO1-4), which encode proteins that are similar to ubiquitin. SUMO plays an important role in PPARgamma Peroxisome proliferator-activated receptor gamma (PPARgamma) activity. PPARgamma is a nuclear receptor regulating inflammation, atherosclerosis, insulin sensitivity and adipogenesis (Lim et al., 2009).
In Table 12, we provided the references for potential cardiovascular-acting mechanisms, which do not have any clinical trial IDs, those are new potential drugs which were not studied in themes of CVD. In other words, our approach is able to identify potential drugs for the treatment of stress-induced CVD. Table 12 lists the drug names, the IC50 values, the drugs’ target genes, i.e. SUMO1 (Gene ID: 7341) and TP53 (Gene ID: 7157), their ICdb50 values and the supporting studies. In addition, because TP53 is frequently found in liver cancer patients, we further analyzed the ICliver50 of these prioritized drugs in three different liver cancer cell lines, including Mahlavu, Huh7, and PLC5 cells. In Section 4 of the Supplemental Information, Table S4 summarized the ICliver50 values of those prioritized drugs.
Table 12. The MTT and clonogenic assay values (IC50), target gene (DEG) and ICdb50 information for the cMAP drugs.
| Drug name | MTT/Clonogenic IC50 (μM) | DEG | ICdb50 (μM) | Study* |
|---|---|---|---|---|
| Lomustine | −/< 10 | SUMO1 | – | – |
| Parthenolide | > 5/− | TP53 | 15.5 | Kurdi & Booz (2007) and Weng et al. (2009) |
| Phenoxybenzamine | > 5/− | SUMO1 | – | Corvera et al. (2003) and Kulik et al. (2007) |
| Piperlongumine | > 5/− | TP53 | 3.16 | Son et al. (2012) |
| SUMO1 | – | |||
| Securinine | > 5/− | TP53 | 29.8 | – |
| Sulconazole | −/< 10 | SUMO1 | – | – |
| Tanespimycin | < 1/− | SUMO1 | – | Kim et al. (2014) |
| Thiostrepton | < 5/− | TP53 | 7.38 | – |
| Trifluoperazine | < 10/− | TP53 | 19.95 | Hong et al. (2014) |
| SUMO1 | – | |||
| Vorinostat | < 1/− | TP53 | 0.708 | Zhao et al. (2012) |
| SUMO1 | – |
Note:
Denotes studies shown evidence related to CVDs.
‘-’ denotes not determined.
In total, there are ten common drugs inferred by both the highest and the second highest predictions. Four drugs are specific to the highest prediction, and one drug is specific to the second highest prediction.
For the rest of the 35 drugs (without IC50), only 21 drugs have identifiable targeted DEGs, in which 11 drugs, i.e. 52%, have potential cardiovascular-acting mechanisms, where seven of them have the ICdb50 values (see Section 5 of the Supplemental Information).
Discussion
Abnormal expression of VSMC is a major cause of CVDs. To examine how VSMC react in response to mechanical stress, a three-phase study was proposed to examine this problem by employing time-course microarray data. First, the DEGs were identified by using the moderated t-statistics test package, EBayes. Second, the GAN for VSMC was inferred by using GGM. Finally, graph theory and cluster analysis were employed to predict the last batch of DEGs by analyzing the GAN.
A total of 23 genes are found relevant to VSMC phenotypic modification, which is summarized in Table 2. Those genes are differential expressed. Then, by constructing the GAN, one obtained the dependence among the DEGs, in which this approach allows us to infer more DEGs based on their topological properties of the interaction network. To further explore stress-induced DEGs, we made use of the assumption that highly interact DEGs are assumed playing an important role in VSMC phenotype changes, in which dense interaction regions were identified by using the clustering approach.
Our research has suggested that nodes with top-ranked local topological values and densely interacting regions (modulus) represent stress-induced genes in VSMC, where the results were well supported by the literature. The three phases, complement each other well, each phase emphasizes a different aspect of the GAN.
To identify the potential drugs for vascular diseases involve VSMC proliferation, we made use of the drug-gene interaction databases, cMap. Use of up- and down-regulated DEGs query predict 30 drugs, where three of them have in-house IC50 activities. In other words, the success of the predictions was determined using in-vitro data, i.e. MTT and clonogenic assay. We note that more IC50 hits are expected.
The overall drug prediction can be improved if one applies cluster analysis for the input gene sets before making a query against the cMap database. We performed a gene set enrichment analysis on the input sets, and found that the up-regulated cluster of size 6 and down-regulated clusters of size 22 and 8 are enriched in apoptosis and cellular metabolism processes, where the up-regulated cluster of size 7 is enriched in both non-coding RNA and RNA metabolic process. In other words, using up- and down- regulated gene sets involve with similar BPs result in identifying more IC50 hits; hence, provide more potential therapeutic drugs for treating VSMC related diseases.
As we stated in the ‘Drug repositioning’ section, both cancers and the VSMC proliferation process involved the same signal transduction pathways; therefore, it is hypotheized that cancer drug molecules may be repositioned for treating VSMC-associated CVDs. Table 12 summarized the IC50 (lung cancer cells) activities for the ten prioritized drugs. We further analyzed the ICliver50 of the prioritized drugs in three different liver cancer cell lines and found that eight drugs shown IC50 assay activity (Table S5). Although the present study has yielded promising findings, we note that further experiments should be pursued for VSMC before the hypothesis is accepted.
Conclusions
In conclusion, biological networks are composed of functional related modules, which play an essential role in many BPs. This research proposed a computational framework; i.e. use of GGM, multiple topological parameters and cluster analysis, to dissect network structures, which may shed light on the mechanism of CVDs. Furthermore, potential drugs and their targeted genes were identified from cMap and NCBI PubChem, and certain potential drugs have been tested for effectiveness by in vitro anti-tumor effects and clinical trials. Interestingly, the UBC gene dominates most of the targeted genes associated with CVDs network, so its role in the cancer pathway warrants further investigation. Finally, by applying cluster algorithm to the GAN, we achieved a substantial increase in the number of predicted potential drugs for CVDs according to the IC50 measurements. Consequently, we have been able to assess the potential existing drugs to identify novel indications, which may be helpful in drug repositioning discovery for CVDs.
Supplemental Information
Acknowledgments
Our gratitude goes to Michael Burton, Center for the Development of Language Teaching and Research, Asia University, for his assistance in proof-reading the manuscript. Jin-Shuei Ciou, Shun-Tsung Chen, Victor C. Kok are the second co-authors and these authors contributed equally to this work.
Funding Statement
The work of Chien-Hung Huang was supported by the Ministry of Science and Technology of Taiwan (MOST) under the grant of MOST 104-2221-e-150-039 and MOST 105-2221-E-150-063. The work of Ka-Lok Ng, Jin-Shuei Ciou and Jeffrey J. P. Tsai were supported by MOST under the grant of MOST 102-2632-e-468-001-MY3 and MOST 105-2632-E-468-002. The work of Ka-Lok Ng is also supported by the grant of MOST 104-2221-e-468-012, MOST 105-2221-E-468-016, and the Asia University grants 104-asia-03. The work of Chi-Ying F Huang is supported by the grant of MOST 104-2627-b-010-001. The work of Nilubon Kurubanjerdjit is supported by the Mae Fah Luang University, Thailand. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Chi-Ying F. Huang, Email: bpshuang@gmail.com.
Ka-Lok Ng, Email: ppiddi@gmail.com.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Chien-Hung Huang conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Jin-Shuei Ciou analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper, help using Endnote to manage all the references.
Shun-Tsung Chen analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
Victor C. Kok analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Yi Chung analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
Jeffrey J. P. Tsai analyzed the data, reviewed drafts of the paper.
Nilubon Kurubanjerdjit analyzed the data, contributed reagents/materials/analysis tools.
Chi-Ying F. Huang performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Ka-Lok Ng conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Data Deposition
The following information was supplied regarding data availability:
The research in this article did not generate any raw data.
References
- Adam et al. (1997).Adam PJ, Weissberg PL, Cary NR, Shanahan CM. Polyubiquitin is a new phenotypic marker of contractile vascular smooth muscle cells. Cardiovascular Research. 1997;33(2):416–421. doi: 10.1016/S0008-6363(96)00220-9. [DOI] [PubMed] [Google Scholar]
- Alexander et al. (2012).Alexander MR, Murgai M, Moehle CW, Owens GK. Interleukin-1β modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-κB-dependent mechanisms. Physiological Genomics. 2012;44(7):417–429. doi: 10.1152/physiolgenomics.00160.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres (2004).Andres V. Control of vascular cell proliferation and migration by cyclin-dependent kinase signalling: new perspectives and therapeutic potential. Cardiovascular Research. 2004;63(1):11–21. doi: 10.1016/j.cardiores.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Anwar et al. (2012).Anwar MA, Shalhoub J, Lim CS, Gohel MS, Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. Journal of Vascular Research. 2012;49(6):463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- Arcaro & Guerreiro (2007).Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Current Genomics. 2007;8(5):271–306. doi: 10.2174/138920207782446160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma et al. (2003).Asanuma K, Magid R, Johnson C, Nerem RM, Galis ZS. Uniaxial strain upregulates matrix-degrading enzymes produced by human vascular smooth muscle cells. American Journal of Physiology–Heart and Circulatory Physiology. 2003;284(5):H1778–H1784. doi: 10.1152/ajpheart.00494.2002. [DOI] [PubMed] [Google Scholar]
- Ashburn & Thor (2004).Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nature Reviews Drug Discovery. 2004;3(8):673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Balgi et al. (2009).Balgi AD, Fonseca BD, Donohue E, Tsang TCF, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS ONE. 2009;4(9):e2478. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer et al. (2007).Beyer KS, Beauchamp RL, Lee M-F, Gusella JF, Näär AM, Ramesh V. Mediator subunit MED28 (Magicin) is a repressor of smooth muscle cell differentiation. Journal of Biological Chemistry. 2007;282(44):32152–32157. doi: 10.1074/jbc.M706592200. [DOI] [PubMed] [Google Scholar]
- Boehm & Nabel (2001).Boehm M, Nabel EG. Cell cycle and cell migration: new pieces to the puzzle. Circulation. 2001;103(24):2879–2881. doi: 10.1161/01.CIR.103.24.2879. [DOI] [PubMed] [Google Scholar]
- Brazma et al. (2003).Brazma A, Parkinson H, Sarkans U, Shojatalab M, Vilo J, Abeygunawardena N, Holloway E, Kapushesky M, Kemmeren P, Lara GG, Oezcimen A, Rocca-Serra P, Sansone S-A. ArrayExpress–a public repository for microarray gene expression data at the EBI. Nucleic Acids Research. 2003;31(1):68–71. doi: 10.1093/nar/gkg091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruemmer et al. (2003a).Bruemmer D, Yin F, Liu J, Kiyono T, Fleck E, Van Herle A, Graf K, Law RE. Atorvastatin inhibits expression of minichromosome maintenance proteins in vascular smooth muscle cells. European Journal of Pharmacology. 2003a;462(1–3):15–23. doi: 10.1016/S0014-2999(03)01317-7. [DOI] [PubMed] [Google Scholar]
- Bruemmer et al. (2003b).Bruemmer D, Yin F, Liu J, Kiyono T, Fleck E, Van Herle AJ, Law RE. Expression of minichromosome maintenance proteins in vascular smooth muscle cells is ERK/MAPK dependent. Experimental Cell Research. 2003b;290(1):28–37. doi: 10.1016/S0014-4827(03)00311-2. [DOI] [PubMed] [Google Scholar]
- Campbell & Campbell (1990).Campbell GR, Campbell JH. The phenotypes of smooth muscle expressed in human atheroma. Annals of the New York Academy of Sciences. 1990;598(1):143–158. doi: 10.1111/j.1749-6632.1990.tb42286.x. [DOI] [PubMed] [Google Scholar]
- Challa et al. (2012).Challa AA, Vukmirovic M, Blackmon J, Stefanovic B. Withaferin-A reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS ONE. 2012;7(8):e2478. doi: 10.1371/journal.pone.0042989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan et al. (2011).Chan MC, Weisman AS, Kang H, Nguyen PH, Hickman T, Mecker SV, Hill NS, Lagna G, Hata A. The amiloride derivative phenamil attenuates pulmonary vascular remodeling by activating NFAT and the bone morphogenetic protein signaling pathway. Molecular and Cellular Biology. 2011;31(3):517–530. doi: 10.1128/MCB.00884-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Wu & Ng (2012).Chen ST, Wu HF, Ng KL. A platform for querying breast and prostate cancer-related microRNA genes. Proceedings of the International Conference on Bioinformatics and Biomedical Engineering (ICBBE ’12); Shanghai. 2012. pp. 271–274. [Google Scholar]
- Cheng & Du (2007).Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(8):1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2010).Cheng J, Wang Y, Ma Y, Chan BT, Yang M, Liang A, Zhang L, Li H, Du J. The mechanical stress-activated serum-, glucocorticoid-regulated kinase 1 contributes to neointima formation in vein grafts. Circulation Research. 2010;107(10):1265–1274. doi: 10.1161/CIRCRESAHA.110.222588. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2008).Cheng W-P, Hung H-F, Wang B-W, Shyu K-G. The molecular regulation of GADD153 in apoptosis of cultured vascular smooth muscle cells by cyclic mechanical stretch. Cardiovascular Research. 2008;77(3):551–559. doi: 10.1093/cvr/cvm057. [DOI] [PubMed] [Google Scholar]
- Chun et al. (2013).Chun H, Chen M, Li B, Zhao H. Joint conditional Gaussian graphical models with multiple sources of genomic data. Frontiers in Genetics. 2013;4:294. doi: 10.3389/fgene.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung et al. (2005).Chung AWY, Rauniyar P, Luo H, Hsiang YN, van Breemen C, Okon EB. Pressure distention compared with pharmacologic relaxation in vein grafting upregulates matrix metalloproteinase-2 and -9. Journal of Vascular Surgery. 2005;42(4):747–756. doi: 10.1016/j.jvs.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Coker et al. (2000).Coker SJ, Batey AJ, Lightbown ID, Díaz ME, Eisner DA. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. British Journal of Pharmacology. 2000;129(2):323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera et al. (2003).Corvera JS, Morris CD, Budde JM, Velez DA, Puskas JD, Lattouf OM, Cooper WA, Guyton RA, Vinten-Johansen J. Pretreatment with phenoxybenzamine attenuates the radial artery’s vasoconstrictor response to α-adrenergic stimuli. Journal of Thoracic and Cardiovascular Surgery. 2003;126(5):1549–1554. doi: 10.1016/S0022-5223(03)01190-5. [DOI] [PubMed] [Google Scholar]
- Croft et al. (2011).Croft D, O’Kelly G, Wu G, Haw R, Gillespie M, Matthews L, Caudy M, Garapati P, Gopinath G, Jassal B, Jupe S, Kalatskaya I, Mahajan S, May B, Ndegwa N, Schmidt E, Shamovsky V, Yung C, Birney E, Hermjakob H, D’Eustachio P, Stein L. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Research. 2011;39(Suppl 1):D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardik et al. (2005).Dardik A, Yamashita A, Aziz F, Asada H, Sumpio BE. Shear stress-stimulated endothelial cells induce smooth muscle cell chemotaxis via platelet-derived growth factor-BB and interleukin-1α. Journal of Vascular Surgery. 2005;41(2):321–331. doi: 10.1016/j.jvs.2004.11.016. [DOI] [PubMed] [Google Scholar]
- de la Cuesta et al. (2013).de la Cuesta F, Zubiri I, Maroto AS, Posada M, Padial LR, Vivanco F, Alvarez-Llamas G, Barderas MG. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. Journal of Proteomics. 2013;82:155–165. doi: 10.1016/j.jprot.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Dellago et al. (2013).Dellago H, Preschitz-Kammerhofer B, Terlecki-Zaniewicz L, Schreiner C, Fortschegger K, Chang MWF, Hackl M, Monteforte R, Kühnel H, Schosserer M, Gruber F, Tschachler E, Scheideler M, Grillari-Voglauer R, Grillari J, Wieser M. High levels of oncomiR-21 contribute to the senescence-induced growth arrest in normal human cells and its knock-down increases the replicative lifespan. Aging Cell. 2013;12(3):446–458. doi: 10.1111/acel.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demasi & Laurindo (2012).Demasi M, Laurindo FRM. Physiological and pathological role of the ubiquitin-proteasome system in the vascular smooth muscle cell. Cardiovascular Research. 2012;95(2):183–193. doi: 10.1093/cvr/cvs128. [DOI] [PubMed] [Google Scholar]
- Dempster (1972).Dempster AP. Covariance selection. Biometrics. 1972;28(1):157–175. doi: 10.2307/2528966. [DOI] [Google Scholar]
- Dolman et al. (2015).Dolman MEM, Poon E, Ebus MEM, den Hartog IJM, van Noesel CJM, Jamin Y, Hallsworth A, Robinson SP, Petrie K, Sparidans RW, Kok RJ, Versteeg R, Caron HN, Chesler L, Molenaar JJ. Cyclin-dependent kinase inhibitor AT7519 as a potential drug for MYCN-dependent neuroblastoma. Clinical Cancer Research. 2015;21(22):5100–5109. doi: 10.1158/1078-0432.ccr-15-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau, Braun-Dullaeus & Sedding (2002).Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nature Medicine. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- Eckel et al. (2004).Eckel JE, Gennings C, Chinchilli VM, Burgoon LD, Zacharewski TR. Empirical bayes gene screening tool for time-course or dose-response microarray data. Journal of Biopharmaceutical Statistics. 2004;14(3):647–670. doi: 10.1081/BIP-200025656. [DOI] [PubMed] [Google Scholar]
- Eder et al. (2014).Eder A, Hansen A, Uebeler J, Schulze T, Neuber C, Schaaf S, Yuan L, Christ T, Vos MA, Eschenhagen T. Effects of proarrhythmic drugs on relaxation time and beating pattern in rat engineered heart tissue. Basic Research in Cardiology. 2014;109(6):436. doi: 10.1007/s00395-014-0436-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron (2003).Efron B. Robbins, empirical Bayes and microarrays. The Annals of Statistics. 2003;31(2):366–378. doi: 10.1214/aos/1051027871. [DOI] [Google Scholar]
- Efron & Tibshirani (2002).Efron B, Tibshirani R. Empirical bayes methods and false discovery rates for microarrays. Genetic Epidemiology. 2002;23(1):70–86. doi: 10.1002/gepi.1124. [DOI] [PubMed] [Google Scholar]
- Efron et al. (2001).Efron B, Tibshirani R, Storey JD, Tusher V. Empirical bayes analysis of a microarray experiment. Journal of the American Statistical Association. 2001;96(456):1151–1160. doi: 10.1198/016214501753382129. [DOI] [Google Scholar]
- Fan & Karnovsky (2002).Fan W-H, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. Journal of Biological Chemistry. 2002;277(12):9800–9805. doi: 10.1074/jbc.M111213200. [DOI] [PubMed] [Google Scholar]
- Fan, Pech & Karnovsky (2000).Fan W-H, Pech M, Karnovsky MJ. Connective tissue growth factor (CTGF) stimulates vascular smooth muscle cell growth and migration in vitro. European Journal of Cell Biology. 2000;79(12):915–923. doi: 10.1078/0171-9335-00122. [DOI] [PubMed] [Google Scholar]
- Feng et al. (1999).Feng Y, Yang J-H, Huang H, Kennedy SP, Turi TG, Thompson JF, Libby P, Lee RT. Transcriptional profile of mechanically induced genes in human vascular smooth muscle cells. Circulation Research. 1999;85(12):1118–1123. doi: 10.1161/01.RES.85.12.1118. [DOI] [PubMed] [Google Scholar]
- Fitzgerald et al. (2008).Fitzgerald TN, Shepherd BR, Asada H, Teso D, Muto A, Fancher T, Pimiento JM, Maloney SP, Dardik A. Laminar shear stress stimulates vascular smooth muscle cell apoptosis via the Akt pathway. Journal of Cellular Physiology. 2008;216(2):389–395. doi: 10.1002/jcp.21404. [DOI] [PubMed] [Google Scholar]
- García-Arguinzonis et al. (2010).García-Arguinzonis M, Padró T, Lugano R, Llorente-Cortes V, Badimon L. Low-density lipoproteins induce heat shock protein 27 dephosphorylation, oligomerization, and subcellular relocalization in human vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(6):1212–1219. doi: 10.1161/ATVBAHA.109.198440. [DOI] [PubMed] [Google Scholar]
- Grainger et al. (1993).Grainger DJ, Witchell CM, Watson JV, Metcalfe JC, Weissberg PL. Heparin decreases the rate of proliferation of rat vascular smooth muscle cells by releasing transforming growth factor beta-like activity from serum. Cardiovascular Research. 1993;27(12):2238–2247. doi: 10.1093/cvr/27.12.2238. [DOI] [PubMed] [Google Scholar]
- Griffin, Valdez & Mestril (2004).Griffin TM, Valdez TV, Mestril R. Radicicol activates heat shock protein expression and cardioprotection in neonatal rat cardiomyocytes. American Journal of Physiology: Heart and Circulatory Physiology. 2004;287(3):H1081–H1088. doi: 10.1152/ajpheart.00921.2003. [DOI] [PubMed] [Google Scholar]
- Grzeszkiewicz et al. (2002).Grzeszkiewicz TM, Lindner V, Chen N, Lam SCT, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin α6β1 and cell surface heparan sulfate proteoglycans. Endocrinology. 2002;143(4):1441–1450. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- Haudenschild (1993).Haudenschild CC. Pathobiology of restenosis after angioplasty. The American Journal of Medicine. 1993;94(4A):40S–44S. [PubMed] [Google Scholar]
- Hellstrand & Albinsson (2005).Hellstrand P, Albinsson S. Stretch-dependent growth and differentiation in vascular smooth muscle: role of the actin cytoskeleton. Canadian Journal of Physiology and Pharmacology. 2005;83(10):869–875. doi: 10.1139/y05-061. [DOI] [PubMed] [Google Scholar]
- Hinze et al. (2013).Hinze AV, Mayer P, Harst A, von Kügelgen I. P2X1 receptor-mediated inhibition of the proliferation of human coronary smooth muscle cells involving the transcription factor NR4A1. Purinergic Signalling. 2013;9(4):677–686. doi: 10.1007/s11302-013-9380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong et al. (2014).Hong DH, Son YK, Li H, Jung ID, Park Y-M, Jung W-K, Kim HS, Choi I-W, Park WS. The calmodulin inhibitor and antipsychotic drug trifluoperazine inhibits voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells. Biochemical and Biophysical Research Communications. 2014;443(1):321–325. doi: 10.1016/j.bbrc.2013.11.115. [DOI] [PubMed] [Google Scholar]
- Huang, Chen & Ng (2016).Huang C-H, Chen T-H, Ng K-L. Graph theory and stability analysis of protein complex interaction networks. IET Systems Biology. 2016;10(2):64–75. doi: 10.1049/iet-syb.2015.0007.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2014).Huang C-H, Wu M-Y, Chang PMH, Huang C-Y, Ng K-L. In silico identification of potential targets and drugs for non-small cell lung cancer. IET Systems Biology. 2014;8(2):56–66. doi: 10.1049/iet-syb.2013.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Sherman & Lempicki (2009).Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2015).Huang L-C, Soysal E, Zheng W, Zhao Z, Xu H, Sun J. A weighted and integrated drug-target interactome: drug repurposing for schizophrenia as a use case. BMC Systems Biology. 2015;9(Suppl 4):S2. doi: 10.1186/1752-0509-9-S4-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley et al. (2010).Hurley NE, Schildmeyer LA, Bosworth KA, Sakurai Y, Eskin SG, Hurley LH, McIntire LV. Modulating the functional contributions of c-Myc to the human endothelial cell cyclic strain response. Journal of Vascular Research. 2010;47(1):80–90. doi: 10.1159/000235928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intengan & Schiffrin (2001).Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Iorio et al. (2010).Iorio F, Bosotti R, Scacheri E, Belcastro V, Mithbaokar P, Ferriero R, Murino L, Tagliaferri R, Brunetti-Pierri N, Isacchi A, di Bernardo D. Discovery of drug mode of action and drug repositioning from transcriptional responses. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14621–14626. doi: 10.1073/pnas.1000138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry (2005).Irizarry RA. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. From CEL files to annotated lists of interesting genes; pp. 431–442. [Google Scholar]
- Jacob et al. (2005).Jacob T, Ascher E, Alapat D, Olevskaia Y, Hingorani A. Activation of P38MAPK signaling cascade in a VSMC injury model: role of P38MAPK inhibitors in limiting VSMC proliferation. European Journal of Vascular and Endovascular Surgery. 2005;29(5):470–478. doi: 10.1016/j.ejvs.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Jung et al. (2000).Jung F, Haendeler J, Goebel C, Zeiher AM, Dimmeler S. Growth factor-induced phosphoinositide 3-OH kinase/Akt phosphorylation in smooth muscle cells: induction of cell proliferation and inhibition of cell death. Cardiovascular Research. 2000;48(1):148–157. doi: 10.1016/S0008-6363(00)00152-8. [DOI] [PubMed] [Google Scholar]
- Kanehisa et al. (2004).Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Research. 2004;32(Suppl 1):D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr et al. (2002).Kerr MK, Afshari CA, Bennett L, Bushel P, Martinez J, Walker NJ, Churchill GA. Statistical analysis of a gene expression microarray experiment with replication. Statistica Sinica. 2002;12(1):203–217. [Google Scholar]
- Kim et al. (2014).Kim C, Yang H, Fukushima Y, Saw PE, Lee J, Park J-S, Park I, Jung J, Kataoka H, Lee D, Heo WD, Kim I, Jon S-I, Adams RH, Nishikawa S-I, Uemura A, Koh GY. Vascular RhoJ is an effective and selective target for tumor angiogenesis and vascular disruption. Cancer Cell. 2014;25(1):102–117. doi: 10.1016/j.ccr.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Konganti et al. (2013).Konganti K, Wang G, Yang E, Cai JJ. SBEToolbox: a Matlab toolbox for biological network analysis. Evolutionary Bioinformatics. 2013;9:355–362. doi: 10.4137/EBO.S12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik et al. (2007).Kulik A, Rubens FD, Gunning D, Bourke ME, Mesana TG, Ruel M. Radial artery graft treatment with phenoxybenzamine is clinically safe and may reduce perioperative myocardial injury. The Annals of Thoracic Surgery. 2007;83(2):502–509. doi: 10.1016/j.athoracsur.2006.09.089. [DOI] [PubMed] [Google Scholar]
- Kurdi & Booz (2007).Kurdi M, Booz GW. Evidence that IL-6-type cytokine signaling in cardiomyocytes is inhibited by oxidative stress: parthenolide targets JAK1 activation by generating ROS. Journal of Cellular Physiology. 2007;212(2):424–431. doi: 10.1002/jcp.21033. [DOI] [PubMed] [Google Scholar]
- Lamb (2007).Lamb J. The connectivity map: a new tool for biomedical research. Nature Reviews Cancer. 2007;7(1):54–60. doi: 10.1038/nrc2044. [DOI] [PubMed] [Google Scholar]
- Lan et al. (2010).Lan M-Y, Chen C-L, Lin K-T, Lee S-A, Yang WLR, Hsu C-N, Wu J-C, Ho C-Y, Lin J-C, Huang CYF. From NPC therapeutic target identification to potential treatment strategy. Molecular Cancer Therapeutics. 2010;9(9):2511–2523. doi: 10.1158/1535-7163.MCT-09-0966. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2007).Lee H-Y, Chung J-W, Youn S-W, Kim J-Y, Park K-W, Koo B-K, Oh B-H, Park Y-B, Chaqour B, Walsh K, Kim H-S. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circulation Research. 2007;100(3):372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- Lemarié, Tharaux & Lehoux (2010).Lemarié CA, Tharaux P-L, Lehoux S. Extracellular matrix alterations in hypertensive vascular remodeling. Journal of Molecular and Cellular Cardiology. 2010;48(3):433–439. doi: 10.1016/j.yjmcc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- Levy et al. (1988).Levy BI, Michel JB, Salzmann JL, Azizi M, Poitevin P, Safar M, Camilleri JP. Effects of chronic inhibition of converting enzyme on mechanical and structural properties of arteries in rat renovascular hypertension. Circulation Research. 1988;63(1):227–239. doi: 10.1161/01.RES.63.1.227. [DOI] [PubMed] [Google Scholar]
- Li et al. (2000).Li C, Hu Y, Sturm G, Wick G, Xu Q. Ras/Rac-dependent activation of p38 mitogen-activated protein kinases in smooth muscle cells stimulated by cyclic strain stress. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(3):e1–e9. doi: 10.1161/01.ATV.20.3.e1. [DOI] [PubMed] [Google Scholar]
- Li & Xu (2000).Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cellular Signalling. 2000;12(7):435–445. doi: 10.1016/S0898-6568(00)00096-6. [DOI] [PubMed] [Google Scholar]
- Li & Gao (2005).Li J-J, Gao R-L. Should atherosclerosis be considered a cancer of the vascular wall? Medical Hypotheses. 2005;64(4):694–698. doi: 10.1016/j.mehy.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Li et al. (2008).Li M, Chiu J-F, Gagne J, Fukagawa NK. Age-related differences in insulin-like growth factor-1 receptor signaling regulates Akt/FOXO3a and ERK/Fos pathways in vascular smooth muscle cells. Journal of Cellular Physiology. 2008;217(2):377–387. doi: 10.1002/jcp.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim et al. (2009).Lim S, Ahn BY, Chung SS, Park HS, Cho BJ, Kim M, Choi SH, Lee IK, Lee S-W, Choi SJ, Chung CH, Cho YM, Lee HK, Park KS. Effect of a peroxisome proliferator-activated receptor γ sumoylation mutant on neointimal formation after balloon injury in rats. Atherosclerosis. 2009;206(2):411–417. doi: 10.1016/j.atherosclerosis.2009.02.031. [DOI] [PubMed] [Google Scholar]
- Liu et al. (2014).Liu G, Hitomi H, Rahman A, Nakano D, Mori H, Masaki T, Ma H, Iwamoto T, Kobori H, Nishiyama A. High sodium augments angiotensin II-induced vascular smooth muscle cell proliferation through the ERK 1/2-dependent pathway. Hypertension Research. 2014;37(1):13–18. doi: 10.1038/hr.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2012).Liu Z-P, Zhang W, Horimoto K, Chen L. A Gaussian graphical model for identifying significantly responsive regulatory networks from time series gene expression data. 2012 IEEE 6th International Conference on Systems Biology (ISB); Piscataway: IEEE; 2012. pp. 142–147. [Google Scholar]
- Lotz et al. (2011).Lotz C, Lazariotto M, Redel A, Smul TM, Stumpner J, Blomeyer C, Tischer-Zeitz T, Schmidt J, Pociej J, Roewer N, Kehl F, Lange M. Activation of peroxisome-proliferator-activated receptors α and γ mediates remote ischemic preconditioning against myocardial infarction in vivo. Experimental Biology and Medicine. 2011;236(1):113–122. doi: 10.1258/ebm.2010.010210. [DOI] [PubMed] [Google Scholar]
- Mahajan et al. (1997).Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88(1):97–107. doi: 10.1016/S0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Marber et al. (1995).Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. Journal of Clinical Investigation. 1995;95(4):1446–1456. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lemus (2014).Martinez-Lemus LA. Current opinions on the control and role of vascular smooth muscle cell adhesion, calcium sensitization, and the cytoskeleton in vascular structure and function. Microcirculation. 2014;21(3):197–200. doi: 10.1111/micc.12130. [DOI] [PubMed] [Google Scholar]
- Mascall et al. (2012).Mascall KS, Small GR, Gibson G, Nixon GF. Sphingosine-1-phosphate-induced release of TIMP-2 from vascular smooth muscle cells inhibits angiogenesis. Journal of Cell Science. 2012;125(9):2267–2275. doi: 10.1242/jcs.099044. [DOI] [PubMed] [Google Scholar]
- Mata-Greenwood et al. (2005).Mata-Greenwood E, Grobe A, Kumar S, Noskina Y, Black SM. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-β1 and reactive oxygen species: a requirement for NAD(P)H oxidase. American Journal of Physiology: Lung Cellular and Molecular Physiology. 2005;289(2):L288–L289. doi: 10.1152/ajplung.00417.2004. [DOI] [PubMed] [Google Scholar]
- Mercer & Bennett (2006).Mercer J, Bennett M. The role of p53 in atherosclerosis. Cell Cycle. 2006;5(17):1907–1909. doi: 10.4161/cc.5.17.3166. [DOI] [PubMed] [Google Scholar]
- Migita, Morser & Kawai (2004).Migita H, Morser J, Kawai K. Rev-erbα upregulates NF-κB-responsive genes in vascular smooth muscle cells. FEBS Letters. 2004;561(1–3):69–74. doi: 10.1016/S0014-5793(04)00118-8. [DOI] [PubMed] [Google Scholar]
- Morrow et al. (2005).Morrow D, Sweeney C, Birney YA, Cummins PM, Walls D, Redmond EM, Cahill PA. Cyclic strain inhibits Notch receptor signaling in vascular smooth muscle cells in vitro. Circulation Research. 2005;96(5):567–575. doi: 10.1161/01.RES.0000159182.98874.43. [DOI] [PubMed] [Google Scholar]
- Murakoshi et al. (2013).Murakoshi M, Saiki K, Urayama K, Sato TN. An anthelmintic drug, pyrvinium pamoate, thwarts fibrosis and ameliorates myocardial contractile dysfunction in a mouse model of myocardial infarction. PLoS ONE. 2013;8(11):e2478. doi: 10.1371/journal.pone.0079374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutarelli et al. (2008).Mutarelli M, Cicatiello L, Ferraro L, Grober OM, Ravo M, Facchiano AM, Angelini C, Weisz A. Time-course analysis of genome-wide gene expression data from hormone-responsive human breast cancer cells. BMC Bioinformatics. 2008;9(Suppl 2):S12. doi: 10.1186/1471-2105-9-S2-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navab et al. (2009).Navab M, Ruchala P, Waring AJ, Lehrer RI, Hama S, Hough G, Palgunachari MN, Anantharamaiah GM, Fogelman AM. A novel method for oral delivery of apolipoprotein mimetic peptides synthesized from all L-amino acids. Journal of Lipid Research. 2009;50(8):1538–1547. doi: 10.1194/jlr.M800539-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepusz, Yu & Paccanaro (2012).Nepusz T, Yu H, Paccanaro A. Detecting overlapping protein complexes in protein-protein interaction networks. Nature Methods. 2012;9(5):471–472. doi: 10.1038/nmeth.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura et al. (2001).Okura Y, Brink M, Zahid AA, Anwar A, Delafontaine P. Decreased expression of insulin-like growth factor-1 and apoptosis of vascular smooth muscle cells in human atherosclerotic plaque. Journal of Molecular and Cellular Cardiology. 2001;33(10):1777–1789. doi: 10.1006/jmcc.2001.1441. [DOI] [PubMed] [Google Scholar]
- Oyadomari & Mori (2004).Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death and Differentiation. 2004;11(4):381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Pagan et al. (2013).Pagan J, Seto T, Pagano M, Cittadini A. Role of the ubiquitin proteasome system in the heart. Circulation Research. 2013;112(7):1046–1058. doi: 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- Palla et al. (2005).Palla G, Derényi I, Farkas I, Vicsek T. Uncovering the overlapping community structure of complex networks in nature and society. Nature. 2005;435(7043):814–818. doi: 10.1038/nature03607. [DOI] [PubMed] [Google Scholar]
- Pascarella et al. (2008).Pascarella L, Lulic D, Penn AH, Alsaigh T, Lee J, Shin H, Kapur V, Bergan JJ, Schmid-Schönbein GW. Mechanisms in experimental venous valve failure and their modification by Daflon© 500 mg. European Journal of Vascular and Endovascular Surgery. 2008;35(1):102–110. doi: 10.1016/j.ejvs.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Peterson et al. (2000).Peterson M, Porter KE, Loftus IM, Thompson MM, London NJM. Marimastat inhibits neointimal thickening in a model of human arterial intimal hyperplasia. European Journal of Vascular and Endovascular Surgery. 2000;19(5):461–467. doi: 10.1053/ejvs.1999.1041. [DOI] [PubMed] [Google Scholar]
- Qiu et al. (2014a).Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. Journal of The Royal Society Interface. 2014a;11(90):20130852. doi: 10.1098/rsif.2013.0852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu et al. (2014b).Qiu Y, Wang L, Han Y, Jiang Z. Endothelial insulin-like growth factor-1 induced proliferation of vascular smooth muscle cells in response to low shear stress (544.4) The FASEB Journal. 2014b;28(1 Supplement):544-4. [Google Scholar]
- Raines (2004).Raines EW. PDGF and cardiovascular disease. Cytokine & Growth Factor Reviews. 2004;15(4):237–254. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Reddy & Howe (1993).Reddy KB, Howe PH. Transforming growth factor β1-mediated inhibition of smooth muscle cell proliferation is associated with a late G1 cell cycle arrest. Journal of Cellular Physiology. 1993;156(1):48–55. doi: 10.1002/jcp.1041560108. [DOI] [PubMed] [Google Scholar]
- Redondo et al. (2003).Redondo S, Santos-Gallego CG, Ganado P, Garcia M, Rico L, Del Rio M, Tejerina T. Acetylsalicylic acid inhibits cell proliferation by involving transforming growth factor-β. Circulation. 2003;107(4):626–629. doi: 10.1161/01.CIR.0000045664.75269.A5. [DOI] [PubMed] [Google Scholar]
- Rensen, Doevendans & van Eys (2007).Rensen SSM, Doevendans PAFM, van Eys GJJM. Regulation and characteristics of vascular smooth muscle cell phenotypic diversity. Netherlands Heart Journal. 2007;15(3):100–108. doi: 10.1007/BF03085963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard et al. (2007).Richard MN, Deniset JF, Kneesh AL, Blackwood D, Pierce GN. Mechanical stretching stimulates smooth muscle cell growth, nuclear protein import, and nuclear pore expression through mitogen-activated protein kinase activation. Journal of Biological Chemistry. 2007;282(32):23081–23088. doi: 10.1074/jbc.M703602200. [DOI] [PubMed] [Google Scholar]
- Rivard & Andres (2000).Rivard A, Andres V. Vascular smooth muscle cell proliferation in the pathogenesis of atherosclerotic cardiovascular diseases. Histology and Histopathology. 2000;15(2):557–571. doi: 10.14670/HH-15.557. [DOI] [PubMed] [Google Scholar]
- Ross et al. (2001a).Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Annals of the New York Academy of Sciences. 2001a;947(1):271–293. doi: 10.1111/j.1749-6632.2001.tb03949.x. [DOI] [PubMed] [Google Scholar]
- Ross et al. (2001b).Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis: a cancer of the blood vessels? American Journal of Clinical Pathology. 2001b;116(Supp 1):S97–S107. doi: 10.1309/YNCK-9R19-5JA3-K2K9. [DOI] [PubMed] [Google Scholar]
- Ruef et al. (1999).Ruef J, Meshel AS, Hu Z, Horaist C, Ballinger CA, Thompson LJ, Subbarao VD, Dumont JA, Patterson C. Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation In vivo after carotid injury in the rat. Circulation. 1999;100(6):659–665. doi: 10.1161/01.CIR.100.6.659. [DOI] [PubMed] [Google Scholar]
- Saraswati et al. (2010).Saraswati S, Alfaro MP, Thorne CA, Atkinson J, Lee E, Young PP. Pyrvinium, a potent small molecule Wnt inhibitor, promotes wound repair and post-MI cardiac remodeling. PLoS ONE. 2010;5(11):e2478. doi: 10.1371/journal.pone.0015521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar et al. (2005).Sarkar S, Dadhania M, Rourke P, Desai TA, Wong JY. Vascular tissue engineering: microtextured scaffold templates to control organization of vascular smooth muscle cells and extracellular matrix. Acta Biomaterialia. 2005;1(1):93–100. doi: 10.1016/j.actbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Schad et al. (2011).Schad JF, Meltzer KR, Hicks MR, Beutler DS, Cao TV, Standley PR. Cyclic strain upregulates VEGF and attenuates proliferation of vascular smooth muscle cells. Vascular Cell. 2011;3(1):21. doi: 10.1186/2045-824X-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, Opgen-Rhein & Strimmer (2006).Schäfer J, Opgen-Rhein R, Strimmer K. Reverse engineering genetic networks using the GeneNet package. R News. 2006;6(5):50–53. [Google Scholar]
- Schafer & Strimmer (2005).Schafer J, Strimmer K. An empirical Bayes approach to inferring large-scale gene association networks. Bioinformatics. 2005;21(6):754–764. doi: 10.1093/bioinformatics/bti062. [DOI] [PubMed] [Google Scholar]
- Schafer & Strimmer (2006).Schafer J, Strimmer K. Inferring gene dependency networks from genomic longitudinal data: a functional data approach. RevStat. 2006;4(1):53–65. [Google Scholar]
- Sciarretta, Volpe & Sadoshima (2014).Sciarretta S, Volpe M, Sadoshima J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circulation Research. 2014;114(3):549–564. doi: 10.1161/CIRCRESAHA.114.302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebolt-Leopold & Herrera (2004).Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nature Reviews Cancer. 2004;4(12):937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- Shi et al. (2009).Shi Z-D, Ji X-Y, Qazi H, Tarbell JM. Interstitial flow promotes vascular fibroblast, myofibroblast, and smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. American Journal of Physiology: Heart and Circulatory Physiology. 2009;297(4):H1225–H1234. doi: 10.1152/ajpheart.00369.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi & Tarbell (2011).Shi Z-D, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Annals of Biomedical Engineering. 2011;39(6):1608–1619. doi: 10.1007/s10439-011-0309-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibinga et al. (1997).Sibinga NES, Foster LC, Hsieh C-M, Perrella MA, Lee W-S, Endege WO, Sage EH, Lee M-E, Haber E. Collagen VIII is expressed by vascular smooth muscle cells in response to vascular injury. Circulation Research. 1997;80(4):532–541. doi: 10.1161/01.RES.80.4.532. [DOI] [PubMed] [Google Scholar]
- Smyth (2004).Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical Applications in Genetics and Molecular Biology. 2004;3(1):1–25. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Smyth (2005).Smyth GK. Bioinformatics and computational biology solutions using R and Bioconductor. New York: Springer; 2005. Limma: linear models for microarray data; pp. 397–420. [Google Scholar]
- Somoza et al. (2004).Somoza B, González C, Cachofeiro V, Lahera V, Fernández-Alfonso MS. Chronic l-arginine treatment reduces vascular smooth muscle cell hypertrophy through cell cycle modifications in spontaneously hypertensive rats. Journal of Hypertension. 2004;22(4):751–758. doi: 10.1097/00004872-200404000-00018. [DOI] [PubMed] [Google Scholar]
- Son et al. (2012).Son DJ, Kim SY, Han SS, Kim CW, Kumar S, Park BS, Lee SE, Yun YP, Jo H, Park YH. Piperlongumine inhibits atherosclerotic plaque formation and vascular smooth muscle cell proliferation by suppressing PDGF receptor signaling. Biochemical and Biophysical Research Communications. 2012;427(2):349–354. doi: 10.1016/j.bbrc.2012.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song et al. (2012).Song Y, Shen H, Schenten D, Shan P, Lee PJ, Goldstein DR. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(1):103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterpetti et al. (1993).Sterpetti AV, Cucina A, D’Angelo LS, Cardillo B, Cavallaro A. Shear stress modulates the proliferation rate, protein synthesis, and mitogenic activity of arterial smooth muscle cells. Surgery. 1993;113(6):691–699. [PubMed] [Google Scholar]
- Sterpetti et al. (1992).Sterpetti AV, Cucina A, Santoro L, Cardillo B, Cavallaro A. Modulation of arterial smooth muscle cell growth by haemodynamic forces. European Journal of Vascular Surgery. 1992;6(1):16–20. doi: 10.1016/S0950-821X(05)80088-X. [DOI] [PubMed] [Google Scholar]
- Strachan, Kelly & Bateman (2004).Strachan EM, Kelly CA, Bateman DN. Electrocardiogram and cardiovascular changes in thioridazine and chlorpromazine poisoning. European Journal of Clinical Pharmacology. 2004;60(8):541–545. doi: 10.1007/s00228-004-0811-7. [DOI] [PubMed] [Google Scholar]
- Su et al. (2007).Su C, Deaton RA, Iglewsky MA, Valencia TG, Grant SR. PKN activation via transforming growth factor-β1 (TGF-β1) receptor signaling delays G2/M phase transition in vascular smooth muscle cells. Cell Cycle. 2007;6(6):739–749. doi: 10.4161/cc.6.6.3985. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2013).Sun S-Y, Liu Z-P, Zeng T, Wang Y, Chen L. Spatio-temporal analysis of type 2 diabetes mellitus based on differential expression networks. Scientific Reports. 2013;3:2268. doi: 10.1038/srep02268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2014).Sun Z, Parrish AR, Hill MA, Meininger GA. N-cadherin, a vascular smooth muscle cell-cell adhesion molecule: function and signaling for vasomotor control. Microcirculation. 2014;21(3):208–218. doi: 10.1111/micc.12123. [DOI] [PubMed] [Google Scholar]
- Tanner et al. (2000).Tanner FC, Boehm M, Akyurek LM, San H, Yang Z-Y, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101(17):2022–2025. doi: 10.1161/01.CIR.101.17.2022. [DOI] [PubMed] [Google Scholar]
- Tusher, Tibshirani & Chu (2001).Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueba, Kawakami & Yaginuma (1997).Ueba H, Kawakami M, Yaginuma T. Shear stress as an inhibitor of vascular smooth muscle cell proliferation: role of transforming growth factor-β1 and tissue-type plasminogen activator. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(8):1512–1516. doi: 10.1161/01.ATV.17.8.1512. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2003).Wang BW, Chang H, Lin S, Kuan P, Shyu KG. Induction of matrix metalloproteinases-14 and -2 by cyclical mechanical stretch is mediated by tumor necrosis factor-α in cultured human umbilical vein endothelial cells. Cardiovascular Research. 2003;59(2):460–469. doi: 10.1016/S0008-6363(03)00428-0. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2002).Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the β-catenin/T-cell factor signaling cascade in vascular remodeling. Circulation Research. 2002;90(3):340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- Weinsheimer et al. (2007).Weinsheimer S, Lenk GM, van der Voet M, Land S, Ronkainen A, Alafuzoff I, Kuivaniemi H, Tromp G. Integration of expression profiles and genetic mapping data to identify candidate genes in intracranial aneurysm. Physiological Genomics. 2007;32(1):45–57. doi: 10.1152/physiolgenomics.00015.2007. [DOI] [PubMed] [Google Scholar]
- Weng et al. (2009).Weng S-X, Sui M-H, Chen S, Wang J-A, Xu G, Ma J, Shan J, Fang L. Parthenolide inhibits proliferation of vascular smooth muscle cells through induction of G0/G1 phase cell cycle arrest. Journal of Zhejiang University Science B. 2009;10(7):528–535. doi: 10.1631/jzus.B0820351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis et al. (2010).Willis MS, Townley-Tilson WHD, Kang EY, Homeister JW, Patterson C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circulation Research. 2010;106(3):463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart et al. (2008).Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Research. 2008;36(Suppl 1):D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójtowicz et al. (2010).Wójtowicz A, Babu SS, Li L, Gretz N, Hecker M, Cattaruzza M. Zyxin mediation of stretch-induced gene expression in human endothelial cells. Circulation Research. 2010;107(7):898–902. doi: 10.1161/CIRCRESAHA.110.227850. [DOI] [PubMed] [Google Scholar]
- Wu, Wang & Chen (2012).Wu Z, Wang Y, Chen L. A new method to identify repositioned drugs for prostate cancer. 2012 IEEE 6th International Conference on Systems Biology (ISB); Piscataway: IEEE; 2012. pp. 280–284. [Google Scholar]
- Wu, Wang & Chen (2013).Wu Z, Wang Y, Chen L. Network-based drug repositioning. Molecular BioSystems. 2013;9(6):1268–1281. doi: 10.1039/c3mb25382a. [DOI] [PubMed] [Google Scholar]
- Wyatt et al. (2008).Wyatt PG, Woodhead AJ, Berdini V, Boulstridge JA, Carr MG, Cross D-M, Davis DJ, Devine LA, Early TR, Feltell RE, Lewis EJ, McMenamin RL, Navarro EF, O’Brien MA, O’Reilly M, Reule M, Saxty G, Seavers LCA, Smith D-M, Squires MS, Trewartha G, Walker MT, Woolford AJA. Identification of N-(4-Piperidinyl)-4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxamide (AT7519), a novel cyclin dependent kinase inhibitor using fragment-based X-ray crystallography and structure based drug design. Journal of Medicinal Chemistry. 2008;51(16):4986–4999. doi: 10.1021/jm800382h. [DOI] [PubMed] [Google Scholar]
- Yasumoto et al. (2001).Yasumoto H, Kim S, Zhan Y, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Iwao H. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Therapy. 2001;8(22):1682–1689. doi: 10.1038/sj.gt.3301590. [DOI] [PubMed] [Google Scholar]
- Yonemitsu et al. (1998).Yonemitsu Y, Kaneda Y, Tanaka S, Nakashima Y, Komori K, Sugimachi K, Sueishi K. Transfer of wild-type p53 gene effectively inhibits vascular smooth muscle cell proliferation in vitro and in vivo. Circulation Research. 1998;82(2):147–156. doi: 10.1161/01.RES.82.2.147. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2012).Yu L, Lu M, Wang P, Chen X. Trichostatin A ameliorates myocardial ischemia/reperfusion injury through inhibition of endoplasmic reticulum stress-induced apoptosis. Archives of Medical Research. 2012;43(3):190–196. doi: 10.1016/j.arcmed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Zampetaki et al. (2005).Zampetaki A, Zhang Z, Hu Y, Xu Q. Biomechanical stress induces IL-6 expression in smooth muscle cells via Ras/Rac1-p38 MAPK-NF-κB signaling pathways. American Journal of Physiology: Heart and Circulatory Physiology. 2005;288(6):H2946–H2954. doi: 10.1152/ajpheart.00919.2004. [DOI] [PubMed] [Google Scholar]
- Zhang (2007).Zhang S. A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics. 2007;8(1):230. doi: 10.1186/1471-2105-8-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2012).Zhao L, Chen C-N, Hajji N, Oliver E, Cotroneo E, Wharton J, Wang D, Li M, McKinsey TA, Stenmark KR, Wilkins MR. Histone deacetylation inhibition in pulmonary hypertension: therapeutic potential of valproic acid and suberoylanilide hydroxamic acid. Circulation. 2012;126(4):455–467. doi: 10.1161/CIRCULATIONAHA.112.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2010).Zheng J-P, Ju D, Shen J, Yang M, Li L. Disruption of actin cytoskeleton mediates loss of tensile stress induced early phenotypic modulation of vascular smooth muscle cells in organ culture. Experimental and Molecular Pathology. 2010;88(1):52–57. doi: 10.1016/j.yexmp.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.