Abstract
Exposure of primary cultures of neonatal rat cortical astrocytes to bacterial lipopolysaccharide (LPS) results in the appearance of nitric oxide synthase (NOS) activity. The induction of NOS, which is blocked by actinomycin D, is directly related to the duration of exposure and dose of LPS, and a 2-hr pulse can induce enzyme activity. Cytosol from LPS-treated astrocyte cultures, but not from control cultures, produces a Ca(2+)-independent conversion of L-arginine to L-citrulline that can be completely blocked by the specific NOS inhibitor NG-monomethyl-L-arginine. The induced NOS activity exhibits an apparent Km of 16.5 microM for L-arginine and is dependent on NADPH, FAD, and tetrahydrobiopterin. LPS also induces NOS in C6 glioma cells and microglial cultures but not in cultured cortical neurons. The expression of NOS in astrocytes and microglial cells has been confirmed by immunocytochemical staining using an antibody to the inducible NOS of mouse macrophages and by histochemical staining for NADPH diaphorase activity. We conclude that glial cells of the central nervous system can express an inducible form of NOS similar to the inducible NOS of macrophages. Inducible NOS in glia may, by generating nitric oxide, contribute to the neuronal damage associated with cerebral ischemia and/or demyelinating diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agulló L., García A. Norepinephrine increases cyclic GMP in astrocytes by a mechanism dependent on nitric oxide synthesis. Eur J Pharmacol. 1991 Apr 25;206(4):343–346. doi: 10.1016/0922-4106(91)90120-7. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Chung I. Y., Norris J. G., Benveniste E. N. Differential tumor necrosis factor alpha expression by astrocytes from experimental allergic encephalomyelitis-susceptible and -resistant rat strains. J Exp Med. 1991 Apr 1;173(4):801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Feinstein D. L., Durand M., Milner R. J. Expression of myosin regulatory light chains in rat brain: characterization of a novel isoform. Brain Res Mol Brain Res. 1991 May;10(2):97–105. doi: 10.1016/0169-328x(91)90099-j. [DOI] [PubMed] [Google Scholar]
- Freeman M. R., Beckmann S. L., Sueoka N. Regulation of the S100 protein and GFAP genes is mediated by two common mechanisms in RT4 neuro-glial cell lines. Exp Cell Res. 1989 Jun;182(2):370–383. doi: 10.1016/0014-4827(89)90242-5. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Giulian D. Ameboid microglia as effectors of inflammation in the central nervous system. J Neurosci Res. 1987;18(1):155-71, 132-3. doi: 10.1002/jnr.490180123. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Iacovitti L., Lee J., Joh T. H., Reis D. J. Expression of tyrosine hydroxylase in neurons of cultured cerebral cortex: evidence for phenotypic plasticity in neurons of the CNS. J Neurosci. 1987 Apr;7(4):1264–1270. doi: 10.1523/JNEUROSCI.07-04-01264.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982 May 6;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannoji H., Yeger H., Becker L. E. A specific histochemical marker (lectin Ricinus communis agglutinin-1) for normal human microglia, and application to routine histopathology. Acta Neuropathol. 1986;71(3-4):341–343. doi: 10.1007/BF00688060. [DOI] [PubMed] [Google Scholar]
- McCarthy K. D., de Vellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980 Jun;85(3):890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollace V., Salvemini D., Anggård E., Vane J. Cultured astrocytoma cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Biochem Biophys Res Commun. 1990 Oct 30;172(2):564–569. doi: 10.1016/0006-291x(90)90710-5. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Murphy S., Minor R. L., Jr, Welk G., Harrison D. G. Evidence for an astrocyte-derived vasorelaxing factor with properties similar to nitric oxide. J Neurochem. 1990 Jul;55(1):349–351. doi: 10.1111/j.1471-4159.1990.tb08860.x. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Pollock J. S., Förstermann U., Mitchell J. A., Warner T. D., Schmidt H. H., Nakane M., Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins D. S., Shirazi Y., Drysdale B. E., Lieberman A., Shin H. S., Shin M. L. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987 Oct 15;139(8):2593–2597. [PubMed] [Google Scholar]
- Salter M., Knowles R. G., Moncada S. Widespread tissue distribution, species distribution and changes in activity of Ca(2+)-dependent and Ca(2+)-independent nitric oxide synthases. FEBS Lett. 1991 Oct 7;291(1):145–149. doi: 10.1016/0014-5793(91)81123-p. [DOI] [PubMed] [Google Scholar]
- Scherer-Singler U., Vincent S. R., Kimura H., McGeer E. G. Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods. 1983 Nov;9(3):229–234. doi: 10.1016/0165-0270(83)90085-7. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Seifert R., Böhme E. Formation and release of nitric oxide from human neutrophils and HL-60 cells induced by a chemotactic peptide, platelet activating factor and leukotriene B4. FEBS Lett. 1989 Feb 27;244(2):357–360. doi: 10.1016/0014-5793(89)80562-9. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Wilke P., Evers B., Böhme E. Enzymatic formation of nitrogen oxides from L-arginine in bovine brain cytosol. Biochem Biophys Res Commun. 1989 Nov 30;165(1):284–291. doi: 10.1016/0006-291x(89)91067-x. [DOI] [PubMed] [Google Scholar]
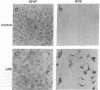
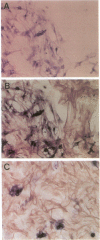
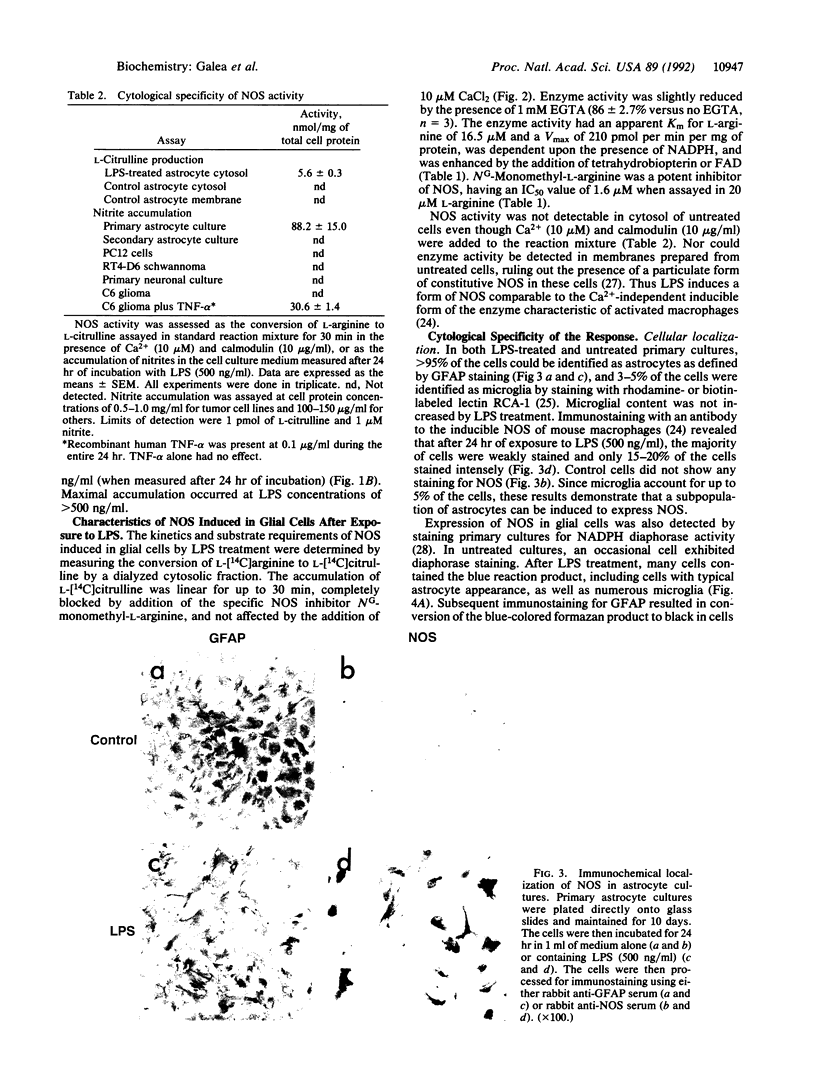
- Simmons M. L., Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992 Sep;59(3):897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Cho H. J., Kwon N. S., Weise M. F., Nathan C. F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7773–7777. doi: 10.1073/pnas.88.17.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Bartlett P. F., Clark-Lewis I., Battye F., Schrader J. W. Inducible expression of H-2 and Ia antigens on brain cells. Nature. 1984 Aug 23;310(5979):688–691. doi: 10.1038/310688a0. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Mülsch A., Busse R., Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989 Apr 28;160(2):813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]