Abstract
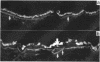
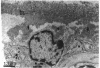
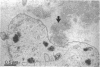
In subretinal neovascularisation capillaries originating from the choriocapillaris must cross Bruch's membrane to reach the subretinal pigment epithelial space. Thus gaps in Bruch's membrane have to be formed before subretinal neovascularisation. Histological examination of eyes with subretinal neovascularisation or disciform scars has shown macrophages adjacent to thin areas and ruptures in Bruch's membrane. This has been interpreted as phagocytosis of Bruch's membrane. The purpose of this study was to investigate whether immune complex depositions can be detected in maculae with early stages of age-related macular degeneration and to explain the macrophage reaction before the disciform reaction. A series of 20 human maculae were examined by direct immunofluorescence light microscopy to detect the presence of immune complexes with antibodies directed against immunoglobulins, fibrinogen, and complement factors. Transmission electron microscopy on several maculae was performed to identify the macrophages. Macrophages were observed in close relation to the readily recognisable long spacing collagen, which suggested that long spacing collagen was selectively internalised by these cells. Definite immune complex depositions were not found in basal laminar deposits or drusen. Linear deposits of fibrinogen and complement were frequently found in the outer collagenous zone of Bruch's membrane. However, because of the absence of immunoglobulins, it seems unlikely that these non-specific deposits might cause chemoattraction of macrophages and play a role in the initial phase of the development of subretinal neovascularisation and disciform macular degeneration.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird A. C. Doyne Lecture. Pathogenesis of retinal pigment epithelial detachment in the elderly; the relevance of Bruch's membrane change. Eye (Lond) 1991;5(Pt 1):1–12. doi: 10.1038/eye.1991.2. [DOI] [PubMed] [Google Scholar]
- Campochiaro P. A., Glaser B. M. Endothelial cells release a chemoattractant for retinal pigment epithelial cells in vitro. Arch Ophthalmol. 1985 Dec;103(12):1876–1880. doi: 10.1001/archopht.1985.01050120110030. [DOI] [PubMed] [Google Scholar]
- Coffey A. J., Brownstein S. The prevalence of macular drusen in postmortem eyes. Am J Ophthalmol. 1986 Aug 15;102(2):164–171. doi: 10.1016/0002-9394(86)90138-8. [DOI] [PubMed] [Google Scholar]
- Glaser B. M., Campochiaro P. A., Davis J. L., Jr, Sato M. Retinal pigment epithelial cells release an inhibitor of neovascularization. Arch Ophthalmol. 1985 Dec;103(12):1870–1875. doi: 10.1001/archopht.1985.01050120104029. [DOI] [PubMed] [Google Scholar]
- Green W. R., McDonnell P. J., Yeo J. H. Pathologic features of senile macular degeneration. Ophthalmology. 1985 May;92(5):615–627. [PubMed] [Google Scholar]
- Grindle C. F., Marshall J. Ageing changes in Bruch's membrane and their functional implications. Trans Ophthalmol Soc U K. 1978 Apr;98(1):172–175. [PubMed] [Google Scholar]
- Heriot W. J., Henkind P., Bellhorn R. W., Burns M. S. Choroidal neovascularization can digest Bruch's membrane. A prior break is not essential. Ophthalmology. 1984 Dec;91(12):1603–1608. doi: 10.1016/s0161-6420(84)34112-4. [DOI] [PubMed] [Google Scholar]
- Killingsworth M. C. Age-related components of Bruch's membrane in the human eye. Graefes Arch Clin Exp Ophthalmol. 1987;225(6):406–412. doi: 10.1007/BF02334166. [DOI] [PubMed] [Google Scholar]
- Killingsworth M. C., Sarks J. P., Sarks S. H. Macrophages related to Bruch's membrane in age-related macular degeneration. Eye (Lond) 1990;4(Pt 4):613–621. doi: 10.1038/eye.1990.86. [DOI] [PubMed] [Google Scholar]
- Leibowitz H. M., Krueger D. E., Maunder L. R., Milton R. C., Kini M. M., Kahn H. A., Nickerson R. J., Pool J., Colton T. L., Ganley J. P. The Framingham Eye Study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973-1975. Surv Ophthalmol. 1980 May-Jun;24(Suppl):335–610. [PubMed] [Google Scholar]
- Löffler K. U., Lee W. R. Basal linear deposit in the human macula. Graefes Arch Clin Exp Ophthalmol. 1986;224(6):493–501. doi: 10.1007/BF02154735. [DOI] [PubMed] [Google Scholar]
- Nishimura T., Goodnight R., Prendergast R. A., Ryan S. J. Activated macrophages in experimental subretinal neovascularization. Ophthalmologica. 1990;200(1):39–44. doi: 10.1159/000310075. [DOI] [PubMed] [Google Scholar]
- Pauleikhoff D., Harper C. A., Marshall J., Bird A. C. Aging changes in Bruch's membrane. A histochemical and morphologic study. Ophthalmology. 1990 Feb;97(2):171–178. [PubMed] [Google Scholar]
- Penfold P. L., Provis J. M., Billson F. A. Age-related macular degeneration: ultrastructural studies of the relationship of leucocytes to angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1987;225(1):70–76. doi: 10.1007/BF02155808. [DOI] [PubMed] [Google Scholar]
- Penfold P. L., Provis J. M., Madigan M. C., van Driel D., Billson F. A. Angiogenesis in normal human retinal development: the involvement of astrocytes and macrophages. Graefes Arch Clin Exp Ophthalmol. 1990;228(3):255–263. doi: 10.1007/BF00920031. [DOI] [PubMed] [Google Scholar]
- Penfold P., Killingsworth M., Sarks S. An ultrastructural study of the role of leucocytes and fibroblasts in the breakdown of Bruch's membrane. Aust J Ophthalmol. 1984 Feb;12(1):23–31. [PubMed] [Google Scholar]
- Pollack A., Korte G. E., Heriot W. J., Henkind P. Ultrastructure of Bruch's membrane after krypton laser photocoagulation. II. Repair of Bruch's membrane and the role of macrophages. Arch Ophthalmol. 1986 Sep;104(9):1377–1382. doi: 10.1001/archopht.1986.01050210131040. [DOI] [PubMed] [Google Scholar]
- Pollack A., Korte G. E., Weitzner A. L., Henkind P. Ultrastructure of Bruch's membrane after krypton laser photocoagulation. I. Breakdown of Bruch's membrane. Arch Ophthalmol. 1986 Sep;104(9):1372–1376. doi: 10.1001/archopht.1986.01050210126039. [DOI] [PubMed] [Google Scholar]
- Sarks S. H. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976 May;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff H. H., Maciejewski W., Scherer R., Braun-Falco O. Immunoelectronmicroscopic examination of early lesions in histamine induced immune complex vasculitis in man. Br J Dermatol. 1978 Jul;99(1):13–24. doi: 10.1111/j.1365-2133.1978.tb01955.x. [DOI] [PubMed] [Google Scholar]
- van der Schaft T. L., Mooy C. M., de Bruijn W. C., Oron F. G., Mulder P. G., de Jong P. T. Histologic features of the early stages of age-related macular degeneration. A statistical analysis. Ophthalmology. 1992 Feb;99(2):278–286. doi: 10.1016/s0161-6420(92)31982-7. [DOI] [PubMed] [Google Scholar]
- van der Schaft T. L., de Bruijn W. C., Mooy C. M., Ketelaars D. A., de Jong P. T. Is basal laminar deposit unique for age-related macular degeneration? Arch Ophthalmol. 1991 Mar;109(3):420–425. doi: 10.1001/archopht.1991.01080030122052. [DOI] [PubMed] [Google Scholar]