Myeloma cells disturb a normally balanced bone remodeling process. This imbalance of bone metabolism may cause osteopenic bones, focal osteolytic lesions and clinical symptoms.
The excess bone resorption resulting in osteolytic lesions has traditionally been perceived as irreversible. We investigated the potential for bone healing in a prospective study of previously untreated multiple myeloma (MM) patients using a five-drug bortezomib-containing treatment regimen.
Thirty-five newly diagnosed MM patients requiring treatment1 were enrolled in a prospective single-center phase-II study to evaluate the safety and efficacy of first-line treatment with a five-drug combination (doxorubicin, cyclophosphamide, bortezomib, dexamethasone and lenalidomide; ACVDL).2 This clinical trial was approved by The Regional Scientific Ethical Committees for Southern Denmark and registered at ClinicalTrials.gov (NCT01481194). EUDRACT number 2011-002751-34. All participants provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
As a secondary endpoint of this clinical trial, the impact of ACVDL on bone involvement in MM was evaluated in patients who completed three or more consecutive bone examinations at pre-specified time points.
Bone status was evaluated using low-dose computed tomography (CT)-scan, bone single photon emission computed tomography (SPECT)/CT-scan and serum markers of bone turnover at baseline, after four cycles of ACVDL (at 12 weeks), at the end-of-treatment assessment (at approximately 28 weeks) and thereafter every 6 months.
Well-defined osteolytic lesions with a diameter of ≥10 mm on CT-scans were identified as target lesions at baseline. Each target lesion was then evaluated in terms of size and development of osteosclerosis in all consecutive CT-scans. The presence of osteosclerosis at the edge of a target lesion was interpreted as an early sign of healing and classified dichotomously as being either present or not present (Figure 1). More pronounced formation of sclerotic bone, together with a simultaneous reduction in the largest diameter of the osteolytic lesion by ≥30%, was interpreted as a more advanced sign of healing (Figure 1).
Figure 1.
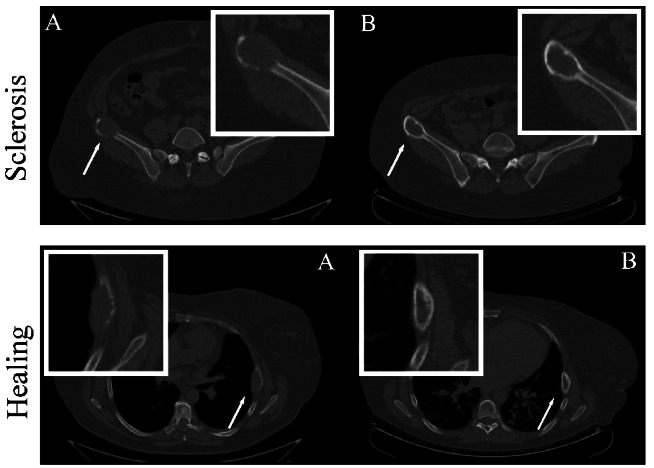
Sclerosis and healing. Illustration of an osteolytic lesion with development of sclerosis (top) and another lesion with development of both sclerosis and size-reduction (bottom). (A) Baseline (B) After treatment.
Tracer uptake by the osteolytic target lesions by bone SPECT was classified as decreased, equal to or increased when compared to uninvolved bone.
The serum bone resorption marker C-terminal telopeptide type-I (CTX) and the serum bone formation marker N-terminal propeptide of procollagen I (P1NP) were measured in fasting blood samples collected in the morning.
Twenty-eight of the 35 enrolled patients completed three or more consecutive bone examinations. Among these, 18 patients showed at least one osteolytic lesion that qualified as a target lesion. (Online Supplementary Table S1).
Fifty-six osteolytic target lesions in 18 patients were followed over time, 34% of which had increased tracer uptake at the baseline bone-SPECT.
Sclerosis appeared in 68% of all target lesions. In 63% of these lesions, sclerosis had already appeared at the interim CT-scan (12 weeks after initiation of treatment). In 24% sclerosis appeared at the end-of-treatment CT-scan (28 weeks after initiation of treatment), while in the remaining 13% of lesions, sclerosis appeared during the follow-up period. Healing (size reduction) was seen in 14% of the osteolytic target lesions (Figure 2).
Figure 2.
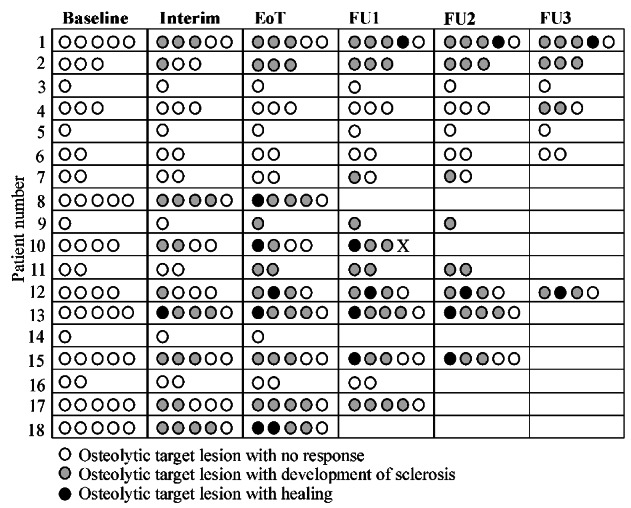
Sclerosis and healing of osteolytic target lesions. Time of development of sclerosis or healing of the 56 osteolytic target lesions observed in 18 patients. The number is the patients’ identification number and each patient had between one and five target lesions. The circles illustrate the target lesions and the color of the circles represent the status at different time points. Each row shows different time points from baseline to the interim assessment, end of treatment and follow-up. Boxes without circles indicate missing scans. Note the variability of responses in individual patients. Open circles: no sclerosis. Gray circles: sclerosis. Black circles: healing. X: osteolytic lesions excluded due to vertebroplasty. EoT: end of treatment; FU1, 2, 3: follow-up assessments 6, 12 and 18 months after end of treatment.
Sclerosis appeared with different hematologic responses; 58% with complete or very good partial remission, 24% at partial response and 18% at less than partial response. Increased baseline bone SPECT uptake had a tendency to be associated with development of sclerosis (P=0.08).
Of the patients with osteolytic target lesions, 72% had at least one lesion with development of sclerosis, while 56% had target lesions both with development of sclerosis and lesions with no response. Thirty-nine percent of the patients with osteolytic target lesions had at least one lesion with healing (size reduction) (Online Supplementary Table S2).
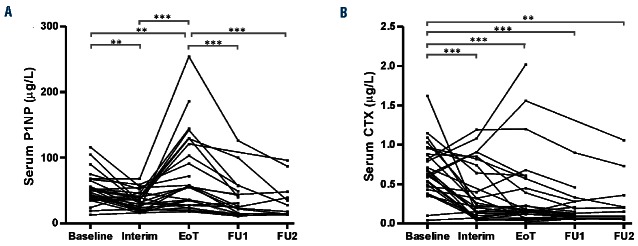
The serum levels of the bone formation marker P1NP showed a significant decrease from baseline to interim assessment (P<0.01), before increasing from the interim to end-of-treatment assessment (P<0.001) (Figure 3A). Serum P1NP levels were significantly higher at end of treatment than at any other time points. The serum level of the bone resorption marker CTX was higher at baseline than at any subsequent measurement in the majority of patients (Figure 3B).
Figure 3.
Effect of treatment on serum markers of bone turnover during treatment and follow-up. (A) The level of the bone formation marker N-terminal propeptide of procollagen I (P1NP) decreased from baseline to the interim assessment and then increased to the highest level at the end of treatment. After treatment completion a decrease was seen. (B) The highest level of the bone resorption marker C-terminal telopeptide (CTX) was seen at baseline with a rapid drop after initiation of treatment. None of the three patients with sustained high serum levels of CTX received treatment with bisphosphonates. EoT: end of treatment; FU1, 2 and 3: follow-up assessments 6, 12 and 18 months after the end of treatment. * = P<0.05, ** = P<0.01, *** = P<0.001.
In recent years, the introduction of several novel agents for the treatment of MM has improved patients’ survival.3 In order to improve quality of life it is also important to control the bone lesions and if possible revert bone damage that has already occurred.
In addressing the impact of a five-drug bortezomib-containing treatment on bone healing in MM, we found development of a rim of sclerosis in the osteolytic lesions of the majority of the patients and that some osteolytic lesions also showed more profound signs of healing. While sclerosis was observed in all the lesions in a small number of patients, a heterogeneous response was found in individual patients in general, with some lesions showing sclerosis or healing and some showing no response.
We found that 72% of the patients with osteolytic target lesions developed bone sclerosis. This is far greater than the 18% reported from a retrospective study of MM patients receiving various bortezomib-containing regimens when also taking into account that the study of Schulze et al. included patients without osteolytic lesions.4 The discrepancy may be due to the fact that only 14% of the patients in the study by Schulze et al. received first-line treatment.4 It is likely that osteolytic lesions that have existed for a long time may lose their potential for healing. Furthermore, only osteolytic lesions with a minimum size of 10 mm were considered in our study. It is unclear whether the size of a lesion plays a role in its healing potential.
We found signs of sclerosis and healing in patients with all levels of responses to anti-myeloma treatment, as previously reported.4 Bone sclerosis often appeared before the best hematologic response was achieved. In some patients sclerosis or healing was seen during follow-up, indicating improved bone metabolism during the period of remission.
Increased tracer uptake by bone SPECT was seen more frequently, albeit not significantly so, in lesions that developed osteosclerosis. This observation suggests that the healing potential of an osteolytic lesion may be dependent on the degree of perturbation of the microenvironment at the time of treatment initiation. Andersen et al. have shown that myeloma cells disrupt a microanatomical structure; the bone remodeling compartment that appears to be of crucial importance for maintaining the integrity of bone.5 The extent of local disruption of the bone remodeling compartments may influence the capacity to initiate new bone formation at a later point in time. The suggested correlation between increased baseline bone SPECT activity and subsequent formation of sclerosis in osteolytic lesions supports the assumption that if the capacity to form new bone locally has been totally eliminated the chance of recovery is poor.6
We found an increase of the bone formation marker P1NP during treatment, with a peak at the end of treatment when the patients had obtained the maximum response to anti-myeloma therapy. The bone formation at this point in time may compensate for the increased bone loss that occurred at the time of diagnosis. Even though we were unable to determine to what extent the increased bone formation was driven by a specific component of the therapy (bortezomib), the result did show that treatment can improve bone formation in MM, as previously reported.7 The levels of CTX, the serum marker of bone resorption, dropped rapidly following initiation of anti-myeloma treatment, which was in agreement with previous studies.8,9 In our study, serum CTX levels remained suppressed following initiation of anti-myeloma treatment, with the inclusion of zoledronate for the majority of patients. Decreased values of bone resorption markers were recently reported in MM patients receiving VTD (bortezomib, thalidomide, dexamethasone) consolidation after autologous stem cell transplantation, but without bisphosphonate treatment.10 This observation supports the view that effective treatment of MM itself is a key factor in the control of excessive bone resorption. Thus, it is reasonable to suggest that both anti-myeloma treatment and bisphosphonates play a role in the control of excessive bone resorption in MM. The combination of specific drugs used to treat myeloma may have significance for the effect on bone, since bortezomib has previously been reported to increase bone formation evaluated by serum bone markers in responding patients,11,12 unlike lenalidomide, which had no influence on the effect.8 The present trial included both bortezomib and lenalidomide in a combined treatment (Online Supplementary Methods) as an attempt to optimize disease control.
This prospective study showed that osteolytic lesions may, at the very least, be partially reversible in the majority of responding patients. Nevertheless, our data also showed intra-patient variation with respect to re-induction of bone formation in the osteolytic lesions. As a consequence, the degree of destruction of the microenvironment within the individual osteolytic lesion may have implications for any possibility of achieving healing of bone lesions.
Acknowledgments
The authors would like to thank the study nurses at the Clinical Research Unit of Hematology, Vejle Hospital for their work regarding the ACVDL-protocol.
Footnotes
Funding: this study was supported by funding from Janssen. A study grant was awarded to MH from the University of Southern Denmark; Lillebaelt Hospital; and The Danish Cancer Society.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–e548. [DOI] [PubMed] [Google Scholar]
- 2.Andersen KT, Hinge M, Segel E, et al. Safety and efficacy of first-line treatment with doxorubicin, cyclophosphamide, bortezomib, dexamethasone and lenalidomide (ACVDL) in newly diagnosed multiple myeloma patients of all ages. IMW 2015, Abstract. Cll Lymph Myeloma Leuk. 2015;(Suppl 3):15. [Google Scholar]
- 3.Pulte D, Jansen L, Castro FA, et al. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171(2):189–196. [DOI] [PubMed] [Google Scholar]
- 4.Schulze M, Weisel K, Grandjean C, et al. Increasing bone sclerosis during bortezomib therapy in multiple myeloma patients: results of a reduced-dose whole-body MDCT study. AJR Am J Roentgenol. 2014;202(1):170–179. [DOI] [PubMed] [Google Scholar]
- 5.Andersen TL, Soe K, Sondergaard TE, Plesner T, Delaisse JM. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br J Haematol. 2010;148(4):551–561. [DOI] [PubMed] [Google Scholar]
- 6.Delaisse JM. The reversal phase of the bone-remodeling cycle: cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014;3:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohty M, Malard F, Mohty B, Savani B, Moreau P, Terpos E. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer. 2014;120(5):618–623. [DOI] [PubMed] [Google Scholar]
- 8.Terpos E, Christoulas D, Kastritis E, et al. The combination of lenalidomide and dexamethasone reduces bone resorption in responding patients with relapsed/refractory multiple myeloma but has no effect on bone formation: final results on 205 patients of the Greek myeloma study group. Am J Hematol. 2014;89(1):34–40. [DOI] [PubMed] [Google Scholar]
- 9.Tosi P, Zamagni E, Cellini C, et al. First-line therapy with thalidomide, dexamethasone and zoledronic acid decreases bone resorption markers in patients with multiple myeloma. Eur J Haematol. 2006;76(5):399–404. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Christoulas D, Kastritis E, et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia. 2014;28(4):928–934. [DOI] [PubMed] [Google Scholar]
- 11.Lund T, Soe K, Abildgaard N, et al. First-line treatment with bortezomib rapidly stimulates both osteoblast activity and bone matrix deposition in patients with multiple myeloma, and stimulates osteoblast proliferation and differentiation in vitro. Eur J Haematol. 2010;85(4):290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zangari M, Yaccoby S, Pappas L, et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2011;96(2):333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]