Abstract
Global change is causing drastic changes in the pollinator communities of the Arctic. While arctic flowers are visited by a wide range of insects, flies in family Muscidae have been proposed as a pollinator group of particular importance. To understand the functional outcome of current changes in pollinator community composition, we examined the role of muscids in the pollination of a key plant species, the mountain avens (Dryas). We monitored the seed set of Dryas across 15 sites at Zackenberg, northeast Greenland, and used sticky flower mimics and DNA barcoding to describe the flower-visiting community at each site. To evaluate the consequences of shifts in pollinator phenology under climate change, we compared the flower visitors between the early and the late season. Our approach revealed a diverse community of insects visiting Dryas, including two-thirds of all insect species known from the area. Even against this diverse background, the abundance of muscid flies emerged as a key predictor for seed set in Dryas, whereas overall insect abundance and species richness had little or no effect. With muscid flies as the main drivers of the pollinating function in the High Arctic, a recently observed decline in their abundances offers cause for concern.
Keywords: Dryas, pollination, arctic ecology, DNA barcoding, ecosystem functioning
1. Introduction
The environment of the Arctic is changing fast [1,2]. This is causing challenges for local plants and animals, as well as for the biological interactions between them [1,3,4]. Overall, the species-poor communities of the Arctic can be expected to be less robust to environmental change than more diverse communities [5,6]. Imprints of global warming are already detectable in the phenology, abundance and distribution of Arctic species [3,7–9]. The lengthening of growing seasons has resulted in a documented increase in plant biomass and net productivity [10]. Altogether, this will cause both plants and animals adapted to longer seasons to become more common in the Arctic [11]. In the long run, the distributions of species will also shift northwards, potentially leading to the demise of the most northern species [1,12]. Thus, overall, we may be bound for major changes in the arctic flora and fauna, and in the ecological functioning of these communities.
Functions stemming from the interaction of different organisms, such as plants and their pollinators, are of particular interest. Even in the harsh environment of the High Arctic, a large proportion of plants are highly dependent on insects for seed set [13]. At a global level, most of the interactions between plants and their pollinators are rather weak [14–16], and as a result it has been suggested that few plant or pollinator species are actual specialists [14–18] (but see [19]). In this context, the pollinator communities of the Artic have been proposed to be particularly generalistic [20,21] (but see [22]).
Yet not all flower visitors may be equally efficient in terms of their functional contribution to pollination. While past work has emphasized the role of bees, hoverflies and butterflies as pollinators, recent studies identified non-syrphid flies as key pollinators across a range of systems [23]. In many alpine and arctic areas, muscid flies (family Muscidae) have been identified as the most common flower visitors [13,20,23–28], and the efficiency of muscid flies in pollen transport has been proposed to be higher than other taxa such as midges [4,13,25]. Among muscid flies, particular species have been advanced as being very functionally efficient: based on visitation rates and on the percentage of individuals carrying pollen, Kevan [13] singled out the fly Spilogona sanctipauli as one of the 11 species or groups of pollinators accounting for most of the Dryas pollination at Lake Hazen (Ellesmere Island, Nunavut, Canada).
With the arctic climate warming year by year, the abundance of muscid flies has been observed to decline [3], whereas another abundant and diverse flower-visiting group, chironomids (Chironomidae), tend to increase [29]. Thus, changes in the availability of muscids as efficient pollinators could harm insect-pollinated plants [4,25]. Nonetheless, the evidence for muscids as key pollinators is largely circumstantial, and we are not aware of any previous studies directly relating seed set to muscid abundance in natural systems.
To predict the functional outcome of current changes in arctic pollinator community composition [3], we examined the role of muscids in the pollination of mountain avens (Dryas), a key plant species in arctic and alpine pollination networks [27,30,31]. First, we describe local variation in the flower-visiting insect community (i.e. which flower visitors are present when and where, and in what numbers). Second, we measure the net pollination function (across all its steps [32]) by measuring the seed set of Dryas. Third, we describe how the flower-visiting insect community—and specifically its muscids—is connected to the ecosystem service it provides. Finally, to derive further insights into the effects of climate change on the pollination of Dryas, we compare the differences in the flower-visiting insect community—including its muscid component—between the early and late season. Through this last approach, we aimed to establish how the flowering peak of Dryas is attuned to the current phenology of its most important pollinators.
Based on previous information on Dryas biology [13], and on the proposal of muscids as abundant and efficient pollinators [13,20,23–27], we tested the following a priori hypotheses: (1) the composition of the community of flower visitors varies in time and in space; (2) Dryas is insect-pollinated, with spatial variation in the composition of the community of flower visitors yielding spatial variation in seed set [13]; (3) following a general association between biodiversity and functioning [33,34], more diverse insect communities will lead to higher seed set; (4) regardless of flower-visitor identity, more visitors will result in higher seed set [35]; (5) given the presumed functional efficiency of Muscidae, an increased abundance of these flies will result in a disproportionate increase in seed set; (6) among muscids, S. sanctipauli will account for a major part of the group's pollination efficiency [13]; and (7) the availability of pollinators will change over the season [27,31], with total pollinator abundance peaking around the flowering peak early in the season [36], and with the abundance of muscids following this pattern.
2. Methods
To study the connection between the composition of the flower visitor community and pollination efficiency, we sampled the flower visitors and monitored the success of seed set in Dryas at 15 locations in the Zackenberg valley, northeast Greenland. To include variation in environmental conditions, thus generating variation in flower visitor community structure, these locations were distributed across an elevational gradient (figure 1a). To separate pollination induced by insects from that owing to wind- and autogamy, we precluded insects from visiting some flowers at each site by small mesh tents. Additionally, to get a perspective on how climate change and earlier snowmelt may impact pollinator availability through the season, we sampled the flower visitors of Dryas during the early-season flowering peak and later in the season—thus examining phenological changes in the pollinator set available to plants flowering early versus late in the season.
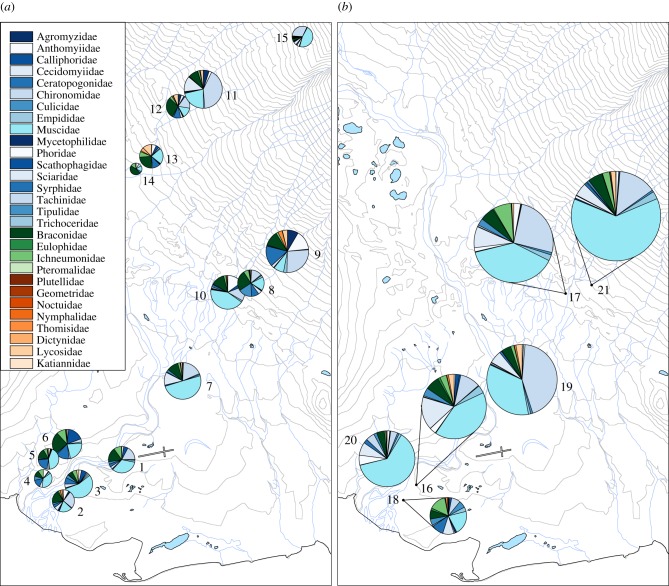
Figure 1.
The location of sampling sites at Zackenberg, with family-level composition of local flower-visiting communities in the (a) early and (b) late season as shown by pie charts. The families belonging to Diptera, Hymenoptera and other orders are represented in different shades of blue, green and brown, respectively (for family-specific interpretation, see colour key in figure; within pies, families run clockwise with Agromyzidae at 12 o'clock; note that darker and lighter hues alternate, and that two dark slices next to each other will signal the lack or low abundance of the light taxon in between). The size of each circle represents the number of individuals caught, whereas sectors indicate the relative proportion of each taxon. Contour lines show elevations at a 10 m interval. For a complete list of taxa recorded at each site, see electronic supplementary material, appendix C.
(a). Study system
(i). Study area
The Zackenberg valley (74°30′ N, 21°00′ W) is characterized by a High Arctic climate, with monthly average temperatures ranging from −20 to +7°C and annual precipitation being around 260 mm [37]. The valley faces south and is characterized by limestone-rich soil. The vegetation is relatively rich and diverse, with the landscape mainly covered by small shrubs [38]. During the growing season from early June to the end of August, the slow melting of snow keeps numerous small streams running down the hillsides, with fens occurring in areas of standing water. Erosion by water and ice creates many vegetation-free patches of mineral soil. Owing to this erosion and to substantial spatial variation in snowmelt patterns, the vegetation of the valley is a diverse mosaic [39], creating pronounced spatial variation in assemblage structure of the local flower visitors.
(ii). Target plant
Dryas is a circumarctic, perennial plant, common and abundant in many arctic and alpine areas. Individuals can reach the age of 100 years [13]. The two dominant species of Dryas crossbreed, with most of those in northeastern Greenland being hybrids between the European and North American species, Dryas octopetala × integrifolia [40,41]. Dryas flowering starts shortly after snowmelt with most individuals flowering within a month. Pollination in Dryas is assumed to be insect-dependent, with only a low level of wind pollination and autogamy [13,42]. As an abundant and highly pollinator-dependent plant with an open flower structure, Dryas is a well-connected key species of pollination networks in many regions of the Arctic [27,30,31]. In previous pollination studies conducted in the Zackenberg valley, at least 30 species of insects were observed to visit its flowers [27]. This involves more than 10% of all insect species known from the area [43].
(iii). Sampling sites
To achieve sufficient sample size for recording seed set success, we chose sites with abundant flowering Dryas (i.e. more than 50 buds m−2). The sampling sites ranged across an elevation gradient from 5 to 400 m above sea level (figure 1). To quantify small- and large-scale variation in seed set and in the flower visitor community, we placed five 1 × 1 m study squares within each of the 15 study sites (i.e. 5 × 15 = 75 study squares in total).
(b). Responses scored
(i). Success of seed set
At each study site, we recorded seed set success of Dryas. To establish the original number of flowers, we counted all flower heads at the start of insect sampling. At this point, we also estimated the number of flowers damaged by insects, subtracting these numbers from later estimates. At the end of the season, we counted all seed heads of Dryas in the study squares and classified them according to whether they had generated viable seeds or not (for illustrations of categories, see electronic supplementary material, appendix A and figure S2). Potential wind pollination and autogamy of Dryas were examined by recording seed set under pollinator exclusion. For this purpose, we constructed small mesh tents of light and neutrally coloured fabric (mesh size, 0.3 × 0.3 mm, Eurokangas, Marley T300; for illustrations, see electronic supplementary material, appendix A and figure S3), chosen not to affect the growth of the plants, stop the wind or attract flower visitors to nearby plants. Two tents were placed in each study square, whereas the buds were still closed. At the end of the season, the seed heads inside the tents were counted and divided into the same categories as outlined above (electronic supplementary material, appendix A and figure S2). As an estimate of the role of insects in Dryas pollination (hypothesis 2), we compared seed set success inside the pollinator exclusions to seed set success outside these tents.
(ii). Sampling of pollinators
To establish how the composition of the community of flower visitors varies in time and in space (hypothesis 1), we sampled flower visitors using sticky mimics of Dryas flowers. This trap design was originally tested in 2013 [44], with insect visitation rates not detectably different from that of real Dryas flowers. Each trap was made of two circular pieces of sticky paper: a white piece (Ø 30 mm; made of Sticky Roll, Barrettine Environmental Health, Bristol, UK) to represent the petals and a yellow piece (Ø 8 mm; Yellow Sticky Board, Barrettine Environmental Health, Bristol, UK) to represent the stamen. To attach the traps to the soil, and to expose them level with natural flowers, we equipped each flower with a short stem made out of iron wire and stuck it into the soil. Within each study square (n = 75), 20 such mimics were placed in Dryas tussocks among the real flowers (for illustrations, see electronic supplementary material, appendix A and figure S4). Sampling of flower visitors was implemented during peak flowering of Dryas (20 June 2014 to 5 July 2014). The flowering peak at each location was scored when half of the flower heads were open. Owing to great variation in weather conditions between days, the traps were kept in the field until 3 days (72 h) of decent weather conditions had accumulated, with ‘decent’ defined as no rain, at least 30% of the sky being cloudless and wind velocity below 5 m s−1 (with all conditions met simultaneously). One sampling site was omitted owing to a very low catch resulting from poor weather. To test whether the availability of pollinators changed over the season [27,31], whether total pollinator abundance peaked around the flowering peak early in the season [36] and whether the abundance of muscids followed this pattern (hypothesis 7), six of the 15 study locations were resampled later in the season (10–21 July 2014). In a paired design, these six locations were chosen close to the original set of sites with late-flowering Dryas (figure 1), maintaining similar flower densities in the early and late season.
(iii). Identification of flower visitors
To resolve the structure of the flower-visiting insect communities, we used DNA barcoding to identify all specimens of flower visitor to the species level. This was done by sequencing the standard barcode region [45] of the cytochrome c oxidase 1 (CO1) mitochondrial gene of the flower visitors, then comparing the sequences acquired to a reference library in The Barcode of Life Data Systems (BOLD; www.barcodinglife.org) [46]. For DNA barcode analysis, DNA was extracted from a small piece of tissue from every insect specimen that was sampled, and the barcode region was PCR amplified and sequenced following standard protocols in the Canadian Center for DNA Barcoding (www.ccdb.ca/resources.php; i.e. as Arthropod samples in [43]). The sampled flower visitors were individually labelled and are now stored by the Spatial Foodweb Ecology Group, University of Helsinki. The sequence information was then imported to BOLD for further processing. Here, the species were identified using barcode index numbers (BINs) as taxonomical units [47]. With some exceptions, a BIN equals a morphologically identifiable species [43], and for simplicity, we henceforth refer to them as ‘species’.
(c). Statistical models
(i). The role of insects in the pollination of Dryas
To test for spatial variation in the composition of flower-visiting communities (hypothesis 1), we compared the pollinator abundances and species numbers between and within study locations. The test was performed by ANOVA in SAS for Windows (v. 9.4, proc anova, SAS Institute Inc., Cary, NC). To test for a general role of insects in the pollination of Dryas (hypothesis 2), we compared the success of seed set in the presence versus absence of flower visitors. First, the proportion of flower heads successfully generating seeds was calculated in each study square (n = 75) and in each pollinator exclusion cage (n = 150). To distinguish the role of flower visitors on seed set, we then used a generalized linear mixed-effect model (GLMM) of seed set success as a function of treatment (tent/no tent). To account for site-to-site differences in seed set success (as caused by e.g. environmental variation), we included the study site and study square (nested within study site) as random effects. To account for the effects of elevation, we included meters above sea level as a continuous, fixed effect. To identify the possible interaction between elevation and the presence or absence of pollinators on seed set, the interaction of elevation and treatment (tent/no tent) was included in the model. This test was performed in SAS for Windows (version 9.4, proc glimmix). Because the dependent variable was a proportion of events, we assumed a logit-link function and binomially distributed errors.
(ii). Effects of flower-visitor diversity and abundance on the pollination of Dryas
To examine the effects of flower visitors on seed set (i.e. the fraction of flowers producing seeds), we started from a model adjusting for background variation in local seed set, which considered the effects of elevation, seed set inside the pollinator exclusions (reflecting possible genetic and abiotic impacts on seed set) and the interaction between the two. These parameters were included as a baseline in all further models. To then address the general effect of diversity (hypothesis 3) and abundance (hypothesis 4) of flower visitors on the seed set of Dryas, we first pooled the counts of all flower-visiting individuals, and counted all species at each site. These counts were log-transformed to account for the fact that high densities of flower visitors may result in pollen saturation on the stigmas [48–50]. We then modelled the fraction of flowers producing seeds as separate functions of total visitor abundance and total species richness, respectively, using the change in Akaike's information criterion (ΔAIC, compared with the null model including environmental parameters only) as a metric of explanatory power. To determine the specific effect of muscids, and of individual species within this family (hypothesis 5), we modelled the fraction of flowers producing seeds as a function of total muscid abundance. To explore the functional contribution of S. sanctipauli as a taxon of presumed particular importance (hypothesis 6), we added its abundance to the model, examining the change in the AIC as a metric of improved model fit. To control for an effect of the abundance of taxa other than those tested, the abundance of all other species (with the target taxon excluded) was included as a covariate. All tests were performed in SAS for Windows (v. 9.4, proc genmod). Because the dependent variable was a proportion of events, we assumed a logit-link function and binomially distributed errors. Electronic supplementary material, appendix B provides a more data-driven approach, exploring the contribution of individual pollinator taxa and arrives at the same qualitative result as the hypothesis-driven approach presented above.
(iii). Phenological change in the flower-visiting community
To resolve temporal changes in the composition of the flower-visiting community (hypothesis 7), we used a paired t-test to compare sites within site-pairs sampled in the early versus late season, respectively (figure 1), in terms of overall species richness, overall flower visitor abundance and the abundance of dominating muscid species, respectively. These tests were performed in SAS for Windows (v. 9.4, proc ttest).
3. Results
(a). Spatial variation in insect community structure
We sampled a total of 8504 flower visitors, using sticky flower mimics. Of these, we successfully sequenced and identified 7997 individuals (94%), detecting a total of 177 different BINs (for a complete list, see electronic supplementary material, appendix C). Even though the study area was relatively small, the insect communities visiting the flowers significantly differed among sites in terms of both species richness and flower visitor abundance (hypothesis 1; F13,56 = 7.68, p < 0.0001 and F13,56 = 9.89, p < 0.0001, respectively). These overall differences reflected substantial variation in community composition and species abundances between sites (figure 1).
(b). Spatial variation in Dryas seed set success
Of 9500 flower heads counted across the 75 study squares, 8292 grew outside and 1208 inside the pollinator exclusions. Seed set by Dryas was significantly greater in the presence of pollinators (hypothesis 2; 33.3 ± 3.2% versus 8.6 ± 2.7% outside and inside of the tents, respectively; F1,148 = 45.3, p < 0.0001), providing clear-cut evidence that Dryas primarily relies on insect pollination (hypothesis 2). Variation in seed set among both free-growing and caged flowers decreased slightly with elevation (F1,148 = 3.59, p = 0.06; figure 2), with no indication of differential effects of elevation in the two treatment groups, as indicated by the insignificant interaction between the exclusion treatment and elevation (F1,148 = 0.01, p = 0.94; figure 2).
Figure 2.
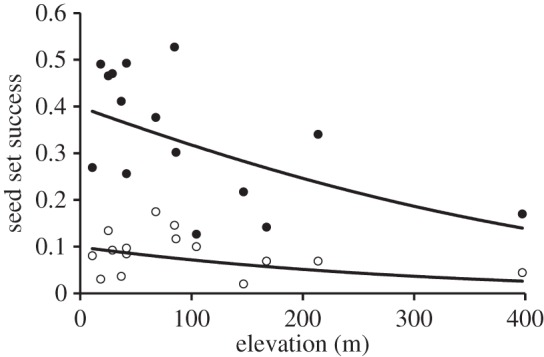
Seed set of Dryas at individual study sites. The y-axis shows the proportion of successful seed set in Dryas, whereas the x-axis is elevation in metres above sea level. Black and open circles represent seed set with pollinators present versus excluded, respectively. In both the cases, seed set decreased with increasing altitude (fitted lines from a logistic model of seed set as a function of elevation and treatment; see text for details).
(c). Effects of flower-visitor diversity and abundance on Dryas seed set success
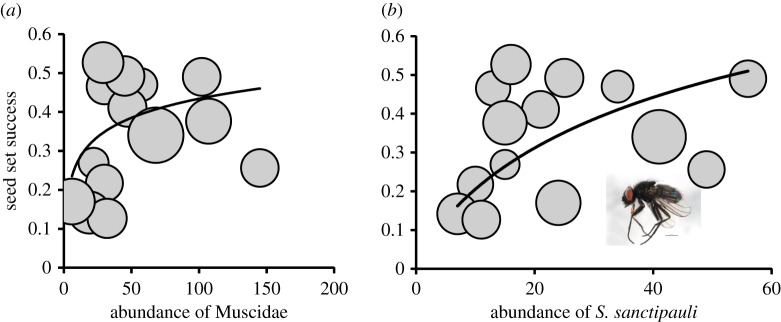
Although the overall abundance of flower-visiting arthropods had a minor impact on the success of seed set in Dryas (hypothesis 4; ΔAIC = 0.8, p = 0.094), the overall species richness explained a significant part of the seed set (hypothesis 3; ΔAIC = 23.5, p < 0.0001). In this context, the overall abundance of muscid flies had a significant positive impact on seed set (hypothesis 5; ΔAIC = 50.5; figure 3a), when compared with a marginally negative effect of all other flower-visiting taxa (coefficient estimate at the logit scale −0.11 ± 0.05). As expected a priori, an increasing abundance of S. sanctipauli (accounting for 36.5% of all sequenced muscids and 14.9% of all sequenced flower visitors) resulted in a detectable increase in Dryas seed set success (hypothesis 6; ΔAIC = 222.56; figure 3b). By contrast, the abundance of another, almost equally abundant muscid fly Drymeia segnis (accounting for 35.4% of all muscids and 10.3% of all individuals) had a much weaker association with seed set (ΔAIC = 47.01).
Figure 3.
Seed set in Dryas as a function of the abundance of (a) family Muscidae (counts pooled across species) and (b) Spilogona sanctipauli. The area of each circle is scaled to the number of flower heads on which the observation is based. Fitted curves represent estimates from a logistic model of seed set as a function of the variable shown while accounting for effects of elevation, of seed set success inside pollinator exclusions, and of the interaction of these two (see text for details). The inset photograph shows an individual of S. sanctipauli (photo credit: BIO Photography Group, Biodiversity Institute of Ontario). (Online version in colour.)
(d). Phenological changes in the flower-visiting community
The overall abundance of flower visitors increased dramatically over the season: during the early season, we observed a total of 2831 flower-visiting individuals and 111 species across 15 study locations. By contrast, in the late season, we observed 5673 individuals and 163 species across only six sites sampled. When comparison was restricted to the relevant subset of six sites sampled in both early and late season, the difference was even more striking, as just 1169 individuals representing 80 species were encountered in the early season. At the same time, both species richness and individual abundance increased between matched pairs of sites visited early versus late in the season (hypothesis 7; t5 = −6.81, p = 0.001 and t5 = −4.57, p = 0.006, respectively). As the overall abundance of Diptera and muscid flies increased in time, so did the abundance of the four most abundant muscid species (including S. sanctipauli; figure 4).
Figure 4.
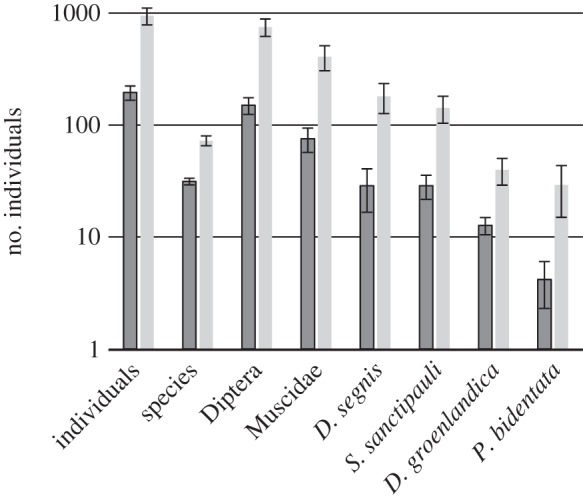
Average pollinator abundances at study sites visited in the early (dark columns) versus late (light columns) season, respectively, with error bars showing the standard errors of the mean. ‘Individuals’ refer to total counts of flower visitors, ‘species’ to species richness, and ‘Diptera’ to pooled counts of all individuals attributed to this order, including Drymeia segnis, Spilogona sanctipauli, Drymeia groenlandica and Phaonia bidentata (singled out as separate columns).
4. Discussion
In this study, we identify muscid flies as the key pollinators of Dryas in the High Arctic—moving from previous suppositions based on their abundance or pollen load [13,23–27] to patterns of realized functioning. The success in seed set of Dryas as a key species in the arctic plant–pollinator network [13,42,44] proved highly dependent on flower-visiting insects in general and on muscids in particular. Where species richness and overall insect abundance had only a weak effect on the success of seed set, the abundance of a single, regionally common muscid species, S. sanctipauli, left a detectable positive imprint on the reproductive output of this plant. By comparison, the most species-rich group of flower-visiting insects (family Chironomidae with 57 species detected versus 15 species of Muscidae) showed no detectable association with the seed set of Dryas (see electronic supplementary material, appendix B). Given a recently observed decline in High Arctic muscid abundances [3], these findings offer fuel for concern.
The current results emerge from the high resolution of pollinator communities achieved by new methodological solutions. Using sticky flower mimics to sample local insect communities visiting Dryas flowers and applying DNA barcoding for species identification, we obtained a comprehensive description of the insect community visiting the target plant species. As evidence of the power achieved, previous studies on pollination in the Zackenberg area have recorded 28 and 30 insect species visiting the flowers of Dryas [27,31], respectively, whereas we found 177 Dryas-visiting insect species. This is two-thirds of all insect species known to occur in this thoroughly studied area (269 species in total [43]). It is worth emphasizing that the few discordances between morphologically distinguishable species and the BIN concept of species identity applied here account for only the tiniest fraction of overall pollinator richness recorded in this study (cf. [43]). Thus, the methods used for trapping and for insect identification appeared well suited to generate highly resolved insights into on community structure and species associations [51].
Because our inference was based on the testing of seven a priori hypotheses, we briefly examine the support for each and synthesize the conclusions below.
First, we expected the composition of the community of flower visitors to vary in time and space, thus providing variation in species content that could be linked with variation in functioning (i.e. seed set). Our methods revealed high spatial variation in both the success of seed set and in the composition of the flower-visiting insect community at Zackenberg. In particular, spatial variation in pollinator community structure was pronounced. In addition to variation in species numbers and in the total abundance of pollinators, differences in species composition and in species-specific abundances accentuated the difference in the communities between study sites (cf. figure 1). Thus, overall the flower-visiting insect community changed markedly across a relatively small spatial scale, causing significant differences in the pollination services locally available. This fine-scale variation allowed us to pinpoint the effect of local variation in insect community structure on the seed set success of Dryas.
Second, we assumed Dryas to be predominantly insect-pollinated, with spatial variation in composition of the community of flower visitors yielding spatial variation in seed set [13]. For this hypothesis, we found clear support: at each of the 15 study locations, the seed set for Dryas was greater in the presence than in the absence of flower visitors (i.e. when flower visitors had free access to the flowers versus were excluded, respectively). This suggests that Dryas relies heavily on insect pollination for reproduction [13,42,44] (but see [52,53]).
Third, following the general association between biodiversity and functioning [33,34], we expected more diverse insect communities to lead to higher success in seed set. For this assumption, we found limited support. Even though an increase in overall species richness slightly increased seed set, the main contribution to pollination stemmed from a few species and from their local abundances. Thus, for providing pollination services, species identity seems far more important than species richness. This supports the notion of a few particularly efficient keystone species as the main driving force behind ecosystem functioning [5,54], and proves the value of using species-poor arctic communities for testing general theory on community structure versus functioning—as given the limited set of species, we can actually pinpoint their specific effects.
Fourth, beyond species richness, we expected more flower visitors to result in higher seed set [35]. In fact, overall insect abundance had only a weak effect on the success of seed set in Dryas. We attribute this finding to the fact that different arctic insects come with very different pollen-carrying capacities [4,13] and behaviour, making simple counts of insects a poor predictor of the function that they provide.
Fifth, we expected an increased abundance of the functionally efficient Muscidae to result in a disproportionate increase in seed set—which proved to be true. At the family level, the abundance of muscids offered the best predictor of seed set success, both when tested by our hypothesis-driven approach (see Results) and when examined by data-driven model selection (cf. electronic supplementary material, appendix B). This pattern supports the recent contention that groups of insects beyond hoverflies, bees and butterflies make key contributions to global pollination services—with non-syrphid flies as a group of particular importance [23]. The high efficiency of muscid flies in pollination [13,20,23–28] can, in part, be attributed to their morphology, abundance and behaviour: these flies are numerous, large and hairy, and actively visit flowers, leading to a potentially great pollen transport rate [4,13,25]. However, there is a general paucity of studies relating the arctic pollinator community to the actual ecosystem service that it provides. Thus, this study adds critical evidence for the role of muscid flies in arctic ecosystems.
Sixth, among muscids, we expected that S. sanctipauli would account for much of the group's pollination efficiency [13]—which it proved to do. This finding supports the inference of Kevan [13], who used visitation rates and pollen loads to propose that this species might be among the most important pollinators of Dryas. Importantly, this role of muscids as a group and of S. sanctipauli as a species emerged against a background of high species richness—we reiterate that no fewer than 177 different insect species were found to visit the flowers of Dryas (see above). One might have expected the effects of single taxa to be diluted across the backdrop of this massively complex interaction network. Yet, within this web, we found evidence that only a few insect species drive a major part of all pollination. This finding contrasts starkly with the usual assumption that arctic pollinator communities are characterized by high degrees of generalism and high functional redundancy [20,21]. Thus, despite being visited by diverse insect taxa, a generalist flower may depend on one or a few pollinator species for its seed set.
Finally, we expected the abundance and diversity of pollinators to change seasonally, peaking around maximal flowering in the early season [36], and with muscids following this general pattern. The pollinator assemblage at Zackenberg did indeed change between the early and late season, but in a fashion opposite to that expected—most early-season pollinators were present and also more abundant after the main flowering season (see also [4]). This pattern included the most important pollinators, the muscids in general and S. sanctipauli in particular, both of which were more abundant later in the season. In addition, many new species emerged towards autumn. Thus, the overall pollination services probably improved over the season. This causes specific concern in a warming Arctic where the flowering season seems to shift towards the early season, with no or little concomitant shift in pollinator flight times [4]. From a plant perspective, this implies that a major part of the pollination function may be left unused—and from an insect perspective that many late-season flower visitors may be deprived of their nectar and pollen resources [4]. Indeed, the overlap between insects and flowers in one year emerges as a key predictor of population densities in the next [3].
Taken together, the functional importance of muscid flies uncovered by our study provides cause for concern. The diversity and abundance of muscid flies at Zackenberg are decreasing [3], perhaps owing to an increasing mismatch with their flower resources [3,4]. If this temporal rift expands and muscid populations dwindle further, then this may threaten arctic pollination.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
Aarhus University is thanked for providing access to the Zackenberg Research Station, and the Logistics team and the Biobasis team created an excellent working environment. Bess Hardwick and Helena Wirta offered help and valuable advice at all stages of the project, while Heidi Viljanen and Anna Ojala contributed to the tissue sampling of insect specimens. Multiple volunteers from the Helsinki Biology Student's Association Symbioosi ry assisted in cutting the sticky paper, and M.T.'s mother, Ulla Tiusanen, kindly sewed the pollinator exclusion tents.
Data accessibility
Site-specific metadata are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.66f6m [55].
Authors' contributions
T.R. and M.T. conceived the study, and M.T., T.R. and N.M.S. planned the field design. M.T. and T.R. performed the fieldwork, and P.D.N.H. led the DNA sequencing work. M.T. and T.R. performed the analyses and wrote the first draft of the manuscript, and all authors contributed substantially to revisions.
Competing interests
We have no competing interests.
Funding
T.R. and M.T. were supported by the Academy of Finland (grant no. 276909), by the Ella & Georg Ehrnrooth Foundation, and by the International Network for Terrestrial Research and Monitoring in the Arctic under the European Community's Seventh Framework Programme. M.T. was also supporeted by Societas pro Fauna et Flora Fennica, by the Finnish-Danish Cultural Foundation, by Societas Entomologica Helsingforsiensis, and by the Faculty of Biological and Environmental Sciences, University of Helsinki. The Aage V. Jensen Charity Foundation provided financial support for the DNA barcoding. Sequence analysis was supported by funding from the Government of Canada through Genome Canada and Ontario Genomics Institute in support of the Internal Barcode of Life Project. We are also grateful to the Ontario Ministry of Research and Innovation and to NSERC for their support of BOLD.
References
- 1.Post E, et al. 2009. Ecological dynamics across the Arctic associated with recent climate change. Science 325, 1355–1358. ( 10.1126/science.1173113) [DOI] [PubMed] [Google Scholar]
- 2.Kattsov VM, et al. 2015. Future climate change: modeling and scenarios for the Arctic. In ACIA 2005: Arctic climate impact assessment, pp. 99–150. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Høye TT, Post E, Schmidt NM, Trøjelsgaard K, Forchhammer MC. 2013. Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nat. Clim. Change 3, 759–763. ( 10.1038/nclimate1909) [DOI] [Google Scholar]
- 4.Schmidt NM, Mosbacher JB, Nielsen PS, Rasmussen C, Høye TT, Roslin T. In press. An ecological function in crisis?—shrinking temporal overlap between plant flowering and pollinator function in a warming Arctic. Ecography. ( 10.1111/ecog.02261) [DOI] [Google Scholar]
- 5.Hooper DU, Chapin FS III, Ewel JJ. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 6.Thébault E, Fontaine C. 2010. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329, 853–856. ( 10.1126/science.1188321) [DOI] [PubMed] [Google Scholar]
- 7.Høye TT, Post E, Meltofte H, Schmidt NM, Forchhammer MC. 2007. Rapid advancement of spring in the High Arctic. Curr. Biol. 17, R449–R451. ( 10.1016/j.cub.2007.04.047) [DOI] [PubMed] [Google Scholar]
- 8.Hegland SJ, Nielsen A, Lázaro A, Bjerknes A-L, Totland Ø. 2009. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 12, 184–195. ( 10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- 9.Wheeler HC, Høye TT, Schmidt NM, Svenning J-C, Forchhammer MC. 2015. Phenological mismatch with abiotic conditions: implications for flowering in Arctic plants. Ecology 96, 775–787. ( 10.1890/14-0338.1) [DOI] [PubMed] [Google Scholar]
- 10.Hill GB, Henry GHR. 2011. Responses of High Arctic wet sedge tundra to climate warming since 1980. Glob. Chang. Biol. 17, 276–287. ( 10.1111/j.1365-2486.2010.02244.x) [DOI] [Google Scholar]
- 11.Sturm M, Racine C, Tape K, Cronin TW, Caldwell RL, Marshall J. 2001. Increasing shrub abundances in the Arctic. Nature 411, 2001–2002. [DOI] [PubMed] [Google Scholar]
- 12.Hodkinson ID, Webb NR, Bale JS, Block W, Coulson SJ, Strathdee AT. 1998. Global change and Arctic ecosystems: conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arct. Alp. Res. 30, 306–313. ( 10.2307/1551978) [DOI] [Google Scholar]
- 13.Kevan PG. 1972. Insect pollination of high Arctic flowers. J. Ecol. 60, 831–847. ( 10.2307/2258569) [DOI] [Google Scholar]
- 14.Jordano P. 1987. Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am. Nat. 129, 657–677. ( 10.1086/284665) [DOI] [Google Scholar]
- 15.Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77, 1043–1060. ( 10.2307/2265575) [DOI] [Google Scholar]
- 16.Memmott J. 1999. The structure of a plant-pollination food web. Ecol. Lett. 2, 276–280. ( 10.1046/j.1461-0248.1999.00087.x) [DOI] [PubMed] [Google Scholar]
- 17.Bluthgen N, Menzel F, Hovestadt T, Fiala B, Blu N. 2007. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346. ( 10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- 18.Olesen JM, Bascompte J, Dupont YL, Jordano P. 2007. The modularity of pollination networks. Proc. Natl Acad. Sci. USA 104, 19 891–19 896. ( 10.1073/pnas.0706375104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenster CB, et al. 2004. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403. ( 10.1146/annurev.ecolsys.34.011802.132347) [DOI] [Google Scholar]
- 20.Elberling H, Olesen J. 1999. The structure of a high latitude plant-flower visitor system: the dominance of flies. Ecography 22, 314–323. ( 10.1111/j.1600-0587.1999.tb00507.x) [DOI] [Google Scholar]
- 21.Olesen JM, Jordano P. 2002. Georaphic patterns in plant–pollinator mutualistic networks. Ecology 83, 2416–2424. [Google Scholar]
- 22.Schleuning M, et al. 2012. Specialization of mutualistic interaction networks decreases toward tropical latitudes. Curr. Biol. 22, 1925–1931. ( 10.1016/j.cub.2012.08.015) [DOI] [PubMed] [Google Scholar]
- 23.Orford KA, Vaughan IP, Memmott J. 2015. The forgotten flies: the importance of non-syrphid Diptera as pollinators. Proc. R. Soc. B 282, 20142934 ( 10.1098/rspb.2014.2934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McAlpine JF. 1965. Insects and related terrestrial invertebrates of Ellef Ringnes Island. Arctic 18, 73–103. ( 10.14430/arctic3455) [DOI] [Google Scholar]
- 25.Kearns AC. 1992. Anthophilous fly distribution across an elevation gradient. Am. Midl. Nat. 127, 172–182. ( 10.2307/2426332) [DOI] [Google Scholar]
- 26.Pont AC. 1993. Observations on anthophilous Muscidae and other Diptera (Insecta) in Abisko National Park, Sweden. J. Nat. Hist. 27, 631–643. ( 10.1080/00222939300770361) [DOI] [Google Scholar]
- 27.Olesen JM, Bascompte J, Elberling H, Jordano P. 2008. Temporal dynamics in a pollination network. Ecology 89, 1573–1582. ( 10.1890/07-0451.1) [DOI] [PubMed] [Google Scholar]
- 28.Wagner J, Lechleitner M, Hosp D. 2016. Pollen limitation is not the rule in nival plants: a study from the European Central Alps. Am. J. Bot. 103, 375–387. ( 10.3732/ajb.1500214) [DOI] [PubMed] [Google Scholar]
- 29.Smol JP, et al. 2005. Climate-driven regime shifts in the biological communities of arctic lakes. Proc. Natl Acad. Sci. USA 102, 4397–4402. ( 10.1073/pnas.0500245102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundgren R, Olesen JM. 2005. The dense and highly connected world of Greenland's plants and their pollinators. Arct. Antarct. Alp. Res. 37, 514–520. ( 10.1657/1523-0430(2005)037%5B0514:TDAHCW%5D2.0.CO;2) [DOI] [Google Scholar]
- 31.Rasmussen C, Dupont YL, Mosbacher JB, Trøjelsgaard K, Olesen JM. 2013. Strong impact of temporal resolution on the structure of an ecological network. PLoS ONE 8, e81694 ( 10.1371/journal.pone.0081694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ne'Eman G, Jürgens A, Newstrom-Lloyd L, Potts SG, Dafni A. 2010. A framework for comparing pollinator performance: effectiveness and efficiency. Biol. Rev. 85, 435–451. ( 10.1111/j.1469-185X.2009.00108.x) [DOI] [PubMed] [Google Scholar]
- 33.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 34.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 35.Rader R, et al. 2016. Non-bee insects are important contributors to global crop pollination. Proc. Natl Acad. Sci. USA 113, 146–151. ( 10.1073/pnas.1517092112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Totland Ø. 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arct. Alp. Res. 26, 66–71. ( 10.2307/1551879) [DOI] [Google Scholar]
- 37.Sigsgaard C, Rasmussen L, Cappelen J, Hinkler J, Mernild SH, Petersen D, Tamstorf MP, Rasch M, Hosholt B. 2008. Present-day climate at Zackenberg. Adv. Ecol. Res. 40, 111–149. ( 10.1016/S0065-2504(07)00006-2) [DOI] [Google Scholar]
- 38.Bersier L-F, Banašek-Richter C, Cattin M-F. 2002. Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407. ( 10.1890/0012-9658(2002)083%5B2394:QDOFWM%5D2.0.CO;2) [DOI] [Google Scholar]
- 39.Bay C. 1998. Vegetation mapping of Zackenberg valley in Northeast Greenland. Copenhagen, Denmark: Danish Polar Centre and Botanical Museum, University of Copenhagen.
- 40.Philipp M, Siegismund HR. 2003. What can morphology and isozymes tell us about the history of the Dryas integrifolia–octopetala complex? Mol. Ecol. 12, 2231–2242. ( 10.1046/j.1365-294X.2003.01875.x) [DOI] [PubMed] [Google Scholar]
- 41.Elkington TT. 1965. Studies on the variation of the genus Dryas in Greenland. Meddelelser Om Grønl. 178, 1–56. [Google Scholar]
- 42.Hocking B, Sharplin CD. 1965. Flower basking by Arctic insects. Nature 206, 215 ( 10.1038/206215b0) [DOI] [Google Scholar]
- 43.Wirta H, et al. 2016. Establishing a community-wide DNA barcode library as a new tool for arctic research. Mol. Ecol. Resour. 16, 809–822. ( 10.1111/1755-0998.12489) [DOI] [PubMed] [Google Scholar]
- 44.Visakorpi K, Ek M, Várkonyi G, Wirta H, Hardwick B, Hambäck P, Roslin T. 2014. Dissecting the interaction web of Zackenberg: targeting pollinators. In Zackenberg Ecological Research Operations 19th Annual Report 2013, pp. 99–101. Aarhus, Denmark: Aarhus University.
- 45.Hebert PDN, Cywinska A, Ball SL, DeWaard JR. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 270, 313–321. ( 10.1098/rspb.2002.2218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratnasingham S, Hebert PDN. 2007. BOLD: the barcode of life data system (www.barcodinglife.org). Mol. Ecol. Notes 7, 355–364. ( 10.1111/j.1471-8286.2006.01678.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratnasingham S, Hebert PDN. 2013. A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS ONE 8, e66213 ( 10.1371/journal.pone.0066213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrie E, Lang G. 1992. Self-and cross-pollination affect stigmatic pollen saturation in blueberry. HortScience 27, 1105–1107. [Google Scholar]
- 49.Rodet G, Vaissiere BE, Brevault T, Grossa J-PT. 1998. Status of self-pollen in bee pollination efficiency of white clover (Trifolium repens L.). Oecologia 114, 93–99. ( 10.1007/s004420050424) [DOI] [PubMed] [Google Scholar]
- 50.Morris WF, Vázquez DP, Chacoff NP. 2010. Benefit and cost curves for typical pollination mutualisms. Ecology 91, 1276–1285. ( 10.1890/08-2278.1) [DOI] [PubMed] [Google Scholar]
- 51.Roslin T, Majaneva S. 2016. The use of DNA barcodes in food web construction—terrestrial and aquatic ecologists unite! Genome 59, 603–628. ( 10.1139/gen-2015-0229) [DOI] [PubMed] [Google Scholar]
- 52.Wada N. 1999. Factors affecting the seed-setting success of Dryas octopetala in front of Broggerbreen (Brogger Glacier) in the high Arctic, Ny-Alesund, Svalbard. Polar Res. 18, 261–268. ( 10.1111/j.1751-8369.1999.tb00302.x) [DOI] [Google Scholar]
- 53.Lundemo S, Totland Ø. 2007. Within-population spatial variation in pollinator visitation rates, pollen limitation on seed set, and flower longevity in an alpine species. Acta Oecol. 32, 262–268. ( 10.1016/j.actao.2007.05.007) [DOI] [Google Scholar]
- 54.Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. 2001. Diversity and productivity in a long-term grassland experiment. Science 294, 843–845. ( 10.1126/science.1060391) [DOI] [PubMed] [Google Scholar]
- 55.Tiusanen M, Hebert PDN, Schmidt NM, Roslin T. 2016. Data from: One fly to rule them all—muscid flies are the key pollinators in the Arctic. Dryad Digital Repository. 10.10561/dryad.66f6m [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Site-specific metadata are available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.66f6m [55].