As in yeast and humans, Arabidopsis cytosolic monothiol glutaredoxins are involved in cytosolic iron-sulfur cluster assembly and the delivery of iron-sulfur clusters to client proteins.
Abstract
Cytosolic monothiol glutaredoxins (GRXs) are required in iron-sulfur (Fe-S) cluster delivery and iron sensing in yeast and mammals. In plants, it is unclear whether they have similar functions. Arabidopsis (Arabidopsis thaliana) has a sole class II cytosolic monothiol GRX encoded by GRXS17. Here, we used tandem affinity purification to establish that Arabidopsis GRXS17 associates with most known cytosolic Fe-S assembly (CIA) components. Similar to mutant plants with defective CIA components, grxs17 loss-of-function mutants showed some degree of hypersensitivity to DNA damage and elevated expression of DNA damage marker genes. We also found that several putative Fe-S client proteins directly bind to GRXS17, such as XANTHINE DEHYDROGENASE1 (XDH1), involved in the purine salvage pathway, and CYTOSOLIC THIOURIDYLASE SUBUNIT1 and CYTOSOLIC THIOURIDYLASE SUBUNIT2, both essential for the 2-thiolation step of 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) modification of tRNAs. Correspondingly, profiling of the grxs17-1 mutant pointed to a perturbed flux through the purine degradation pathway and revealed that it phenocopied mutants in the elongator subunit ELO3, essential for the mcm5 tRNA modification step, although we did not find XDH1 activity or tRNA thiolation to be markedly reduced in the grxs17-1 mutant. Taken together, our data suggest that plant cytosolic monothiol GRXs associate with the CIA complex, as in other eukaryotes, and contribute to, but are not essential for, the correct functioning of client Fe-S proteins in unchallenged conditions.
A large fraction of plant intracellular iron is incorporated in iron-sulfur (Fe-S) prosthetic groups, which are well suited for electron transfer reactions and are often essential for the catalytic function of several enzymes (Balk and Schaedler, 2014). There are three pathways for the assembly of Fe-S clusters in plants: a mitochondrial, a plastidial, and a cytosolic pathway. The cytosolic Fe-S assembly (CIA) pathway is involved in the maturation of [2Fe-2S] clusters into [4Fe-4S] clusters and is capable of providing [4Fe-4S] clusters to cytosolic and nuclear proteins. Because of the absence of a cytosolic Cys desulfurase, the CIA pathway depends on the integrity of the mitochondrial Fe-S cluster machinery and a functional ABC transporter of the mitochondria (Kispal et al., 1999; Gerber et al., 2004). The three pathways share a common mechanism in which the Fe-S is preassembled on a scaffold protein and then transferred to apoproteins by carriers or targeting factors (Fig. 1A; Couturier et al., 2013; Balk and Schaedler, 2014).
Figure 1.
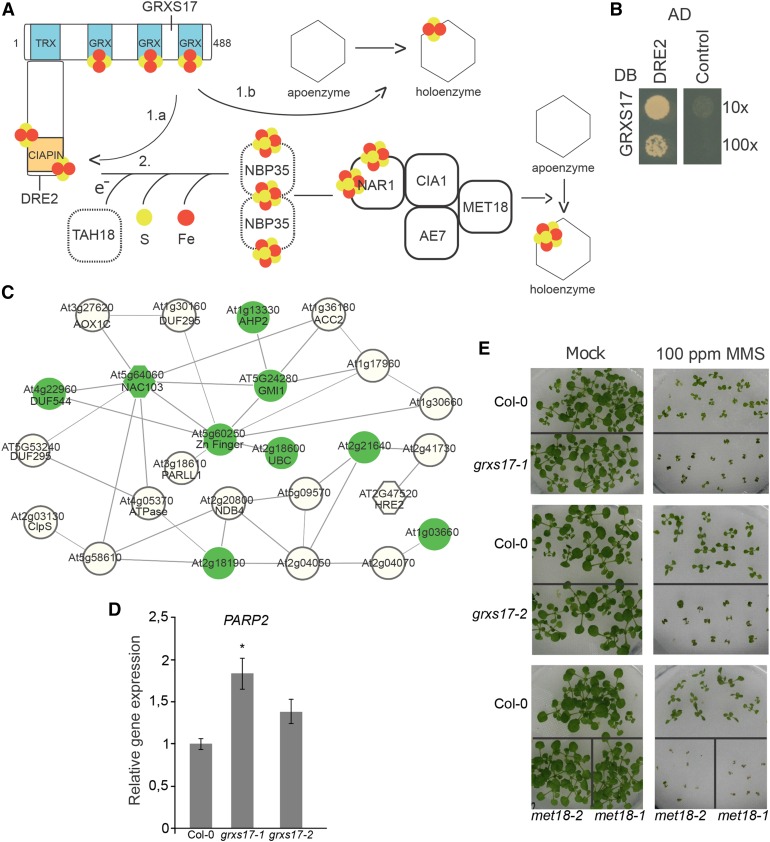
Loss-of-function grxs17 plants display some degree of hypersensitivity to DNA damage. A, Hypothetical model of the CIA pathway and GRXS17 function. GRXS17 contains an N-terminal TRX domain and three GRX domains by which it interacts with three [2Fe-2S] clusters as a homodimer. GRXS17 also forms a complex with DRE2 that can bind two [2Fe-2S] clusters with its CYTOKINE INDUCED APOPTOSIS INHIBITOR1 (CIAPIN1) domain. GRXS17 can transfer a [2Fe-2S] cluster directly to DRE2 (1.a) or to a client apoprotein (1.b). DRE2 functions at the start of the CIA pathway in which a [2Fe-2S] matures to a [4Fe-4S] cluster (2) that can be delivered to client apoproteins. Full and dashed boxes indicate components that copurified or not with GRXS17 by TAP, respectively. B, GRXS17 directly interacts with DRE2. The PJ69-4A yeast strain was cotransformed with GRXS17 in pGBKT7gate and DRE2 in pGADT7gate. The 10× and 100× dilutions of transformed yeasts were dropped on selective medium lacking His. The empty pGADT7gate vector was used as control. One representative from three independent drops is shown. C, Coexpression network of up-regulated genes in grxs17-1. Nodes are genes that are significantly 2-fold up-regulated in grxs17 seedlings; edges represent coexpression in ATTED-II (http://atted.jp/). Green nodes are also significantly and more than 2-fold induced upon genotoxic stress (bleomycin + mitomycin for 12 and 24 h). Hexagon nodes indicate putative transcription factors. D, Relative expression of the DNA-damage marker PARP2 in 14-d-old Col-0, grxs17-1, and grxs17-2 seedlings. Bars represent means ± se of n = 3 (*P < 0.05, Student’s t test). E, Hypersensitivity of grxs17-1, grxs17-2, met18-1, and met18-2 to the DNA-damage agent MMS. Seedlings were grown for 17 d on 0.01% v/v MMS or in control conditions (mock).
Glutaredoxins (GRXs) together with thioredoxins (TRXs) are thiol oxidoreductases that are able to control the redox state of proteins and are present in most organisms (Herrero and de la Torre-Ruiz, 2007). The yeast GRX proteins Grx3/4 and the mammalian ortholog GRX3/PKC-interacting cousin of TRX (PICOT) have been associated with the CIA pathway and contain themselves [2Fe-2S] clusters (Picciocchi et al., 2007; Haunhorst et al., 2010). Deletion of Grx3/4 in yeast leads to defects in cytosolic and mitochondrial Fe-S assembly, deregulation of iron homeostasis, and defects in proteins containing di-iron centers (Mühlenhoff et al., 2010). Yeast Grx3/4 and human GRX3 belong to the PICOT protein family and contain one N-terminal TRX and one (Grx3/4) or two (GRX3) C-terminal GRX domains, also known as PICOT homology domains (Haunhorst et al., 2010). Because they contain only a single Cys residue in their GRX active sites, they are classified as monothiol GRXs. They are conserved and present in a broad range of organisms, including bacteria, yeasts, plants, and mammals (Isakov et al., 2000). Whereas there are other monothiol GRXs present in mitochondria, Grx3/4 and GRX3 are the only nucleocytosolic-localized monothiol GRXs (Herrero and de la Torre-Ruiz, 2007).
The sole class II Arabidopsis (Arabidopsis thaliana) nucleocytosolic monothiol GRX is GRXS17, which contains one N-terminal TRX and three C-terminal GRX domains. GRXS17 dimers are capable of associating with three [2Fe-2S] clusters in vitro (Knuesting et al., 2015). Its physiological and molecular role in plants is not well understood (Couturier et al., 2013). GRXS17 function has been associated with protection against oxidative stress in Arabidopsis and thermotolerance in Arabidopsis and tomato (Cheng et al., 2011; Wu et al., 2012; Knuesting et al., 2015). Arabidopsis GRXS17 loss-of-function plants (grxs17) are hypersensitive to heat stress and show alterations in auxin sensitivity and polar transport (Cheng et al., 2011). The molecular function of an association of cytosolic monothiol GRX with Fe-S clusters and the CIA pathway has been a subject of debate, and a role in Fe, Fe-S, or oxidative signaling has been proposed, in addition to a role in delivery or repair of Fe-S clusters (Couturier et al., 2015). Recently, delivery of an Fe-S cluster by human GRX3 to the CIA pathway component DRE2/Anamorsin has been demonstrated (Banci et al., 2015).
Cytosolic tRNAs in eukaryotes carry several chemical modifications, often at the anticodon loop. Uridines at the first position of the anticodon (U34) of tRNAs tK(UUU), tE(UUC), and tQ(UUG) are modified to 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U) in eukaryotes. Furthermore, this evolutionarily conserved modification (Mehlgarten et al., 2010) is essential for unperturbed translation and cellular signaling (Zinshteyn and Gilbert, 2013; Scheidt et al., 2014; Nedialkova and Leidel, 2015). The 2-thiolation (s2) step of mcm5s2U is catalyzed by the UBIQUITIN-RELATED MODIFIER (URM1) pathway and requires the CIA complex in yeast (Nakai et al., 2007; Leidel et al., 2009). The mcm5 modification is catalyzed by the elongator (ELP) pathway and requires the Elp3/ELO3 catalytic subunit, i.e. a [4Fe-4S] protein (Huang et al., 2005; Paraskevopoulou et al., 2006; Selvadurai et al., 2014), together with the Trm9/Trm112 complex, which is a tRNA methyltransferase necessary for the last step of mcm5 formation (Kalhor and Clarke, 2003; Chen et al., 2011; Leihne et al., 2011). The presence of the mcm5 chain is necessary for an effective thiolation of tRNAs (Leidel et al., 2009; Chen et al., 2011). Although in vivo data are scarce, tRNA modifications might have a regulatory function, because certain open reading frames (ORFs) are enriched in codons recognized by modified tRNAs. In yeast, genes involved in the DNA-damage response are enriched in GAA, AAA, and CAA codons and elongator mutants defective in mcm5s2 modification are hypersensitive to DNA stress (Chen et al., 2011).
Here, we found the Arabidopsis glutaredoxin GRXS17 to associate with members of the CIA pathway machinery and with several putative Fe-S client proteins, such as XANTHINE DEHYDROGENASE1 (XDH1) and the URM1 pathway components CYTOSOLIC THIOURIDYLASE SUBUNIT1 (CTU1) and CTU2. Accordingly, mutants lacking GRXS17 showed similar phenotypes, at molecular, cellular, and/or physiological levels, as mutants in genes encoding CIA components or proteins involved in mcm5s2 tRNA modification. Our results endorse the association of cytosolic monothiol GRXs with the CIA complex, Fe-S cluster metabolism, and tRNA modification in plants.
RESULTS
GRXS17 Associates with the CIA Complex and Fe-S Proteins
To characterize the role of Arabidopsis GRXS17 and uncover potential links with Fe-S cluster assembly or delivery to proteins, we used tandem affinity purification (TAP; Van Leene et al., 2015). GRXS17 was fused both to an N-terminal and a C-terminal TAP-tag and expressed both in Arabidopsis cell cultures and seedlings under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Copurified proteins included nearly all known core components of the CIA pathway, two known Fe-S proteins, i.e. XDH1 and BolA2, and a number of proteins implicated in tRNA metabolism (Table I; Supplemental Dataset S1). Notably, we identified more GRXS17-interacting proteins when using the C-terminal TAP-tag than when using the N-terminal TAP-tag, suggesting that the position of the tag affects protein folding or that different domains of GRXS17 interact with proteins related to different functions. It has recently been suggested that the human GRXS17 ortholog GRX3 establishes protein-protein interactions with its N-terminal TRX domain and that this interaction favors the correct orientation of the Fe-S cluster binding domains (GRX donors) to transfer the cluster to an acceptor protein (Banci et al., 2015).
Table I. GRXS17 associates with the CIA complex and Fe-S proteins in Arabidopsis cells and seedlings.
Proteins identified by mass spectrometry, with at least two significant peptides per identification and background subtracted (as described by Van Leene et al. [2015]). Numbers of independent TAP experiments in which the proteins were copurified with C terminally or N terminally TAP-tagged GRXS17 as bait in each system are presented. Interactions that were confirmed in yeast (Tarassov et al., 2008; Li et al., 2009; Zhang et al., 2011), in humans (Rual et al., 2005; Stehling et al., 2012, 2013; Rolland et al., 2014), or in Arabidopsis (this study; Arabidopsis Interactome Mapping, 2011; Couturier et al., 2014; Duan et al., 2015) are shown in bold. GSrh, GSrhino; Nd, Not determined; *, peptides retrieved cannot discriminate between CTU1 and CTU1L.
| Name |
No. of Affinity Purifications |
|||||||
|---|---|---|---|---|---|---|---|---|
| Arabidopsis | Locus | Orthologs |
C-GSrh |
N-GSrh |
Total | |||
| S. cerevisiae | Homo sapiens | Cells | Seedlings | Cells | Seedlings | |||
| GRXS17 | AT4G04950 | Grx3/4 | GRX3/GRLX3/PICOT | 4 | 2 | 2 | 1 | 9 |
| CIA pathways | ||||||||
| MET18 | AT5G48120 | Met-18/Mms19 | MMS19 | 4 | 2 | 2 | 1 | 9 |
| DRE2/CIAPIN | AT5G18400 | Dre2/Ciapin | Anamorsin/CIAPIN1 | 4 | 2 | 2 | – | 8 |
| NAR1 | AT4G16440 | Nar1 | NARFL/IOP1 | 3 | – | – | – | 3 |
| CIA1 | AT2G26060 | Cia1 | CIAO1 | 2 | – | – | – | 2 |
| AE7/CIA2 | AT1G68310 | Cia2 | CIA2B/MIP18 | 2 | – | – | – | 2 |
| Purine catabolism and salvage | ||||||||
| XDH1 | AT4G34890 | Nd | XDH/XOR/XO | 3 | 2 | 1 | – | 6 |
| URH1 | AT2G36310 | Urh1 | Nd | – | 2 | – | 1 | 3 |
| URH2 | AT1G05620 | Urh1 | Nd | – | 2 | – | 1 | 3 |
| UREG | AT2G34470 | Not present | Not present | – | 2 | – | – | 2 |
| tRNA modification | ||||||||
| CTU1/ROL5 | AT2G44270 | Ncs6 | CTU1 | 2* | – | 1 | – | 1-3 |
| CTU1L | AT1G76170 | Ncs6 | CTU12* | – | – | 0-2 | ||
| CTU2 | AT4G35910 | Ncs2 | CTU2 | 1 | – | 1 | – | 2 |
| Leu-tRNA ligase | AT1G09620 | Cdc60 | LARS | – | 2 | – | 1 | 3 |
| Other known Fe-S protein | ||||||||
| BolA2 | AT5G09830 | Fra2 | BOLA2 | – | 3 | – | – | 3 |
The CIA pathway is responsible for providing Fe-S clusters to respective apoproteins in the cytosol and the nucleus (Bernard et al., 2013). In the current model, electrons provided by NADPH oxidation are transferred by Top1T722A MUTANT HYPERSENSITIVE and DEREPRESSED FOR RIBOSOMAL PROTEIN S14 EXPRESSION (DRE2) to the scaffold protein NUCLEOTIDE BINDING PROTEIN35. Once the [4Fe-4S] clusters have been assembled on the NUCLEOTIDE BINDING PROTEIN35 scaffold, they are transferred to target apoproteins by dedicated proteins forming the CIA targeting complex (Fig. 1A). In Arabidopsis, this complex is composed of NUCLEAR ARCHITECTURE RELATED1 (NAR1), CIA1, AS1/2 ENHANCER7 (AE7/CIA2), and METHIONINE REQUIRING18/METHYL METHANESULFONATE19 (MET18/MMS19) and locates to both the cytosol and the nucleus (Luo et al., 2012). Grx3, the yeast GRXS17 ortholog, has been demonstrated to interact with DRE2 in yeast (Zhang et al., 2011), and recently, it has been shown that the human GRX3 is able to transfer [2Fe-2S] clusters to anamorsin, the human DRE2 ortholog (Banci et al., 2015). We thus hypothesized that GRXS17 might similarly directly interact with DRE2 in Arabidopsis, which was confirmed by yeast two-hybrid analysis (Y2H; Fig. 1B). We confirmed this interaction in planta by bimolecular fluorescent complementation (BiFC). Proteins were fused with N-terminal or C-terminal fragments of GFP (designated nGFP and cGFP, respectively) and coexpressed transiently in leaves of Nicotiana benthamiana (Supplemental Fig. S1A). A cytosolic signal was obtained with the combination of nGFP-GRXS17 and DRE2-cGFP, which corresponded to the localization of GFP-DRE2 (Supplemental Fig. S1C). Thus, we concluded that Arabidopsis GRXS17 associates with the CIA complex as in yeast and humans.
Loss-of-Function grxs17 Plants Show Some Degree of Hypersensitivity to DNA Damage
To obtain insights into the function of GRXS17, we profiled the transcriptome of grxs17 loss-of-function seedlings. A transfer DNA (T-DNA) insertion line (grxs17-1; SALK_021301) described previously (Supplemental Fig. S1; Cheng et al., 2011; Knuesting et al., 2015) was characterized in detail and found to lack both GRXS17 transcripts (Supplemental Fig. S2B) and GRXS17 protein (Supplemental Fig. S2C). Fourteen-day-old seedlings were grown in vitro and used for RNA sequencing (RNA-Seq). One hundred eighty-eight genes were found to be significantly up-regulated, and 209 were down-regulated (Supplemental Table S1). Using ATTED-II (http://atted.jp/), we identified a conserved coexpression network comprising 25 genes up-regulated in grxs17-1 mutants (Fig. 1C; Supplemental Table S1). Many of the genes in this network are known to be induced upon genotoxic stress (Ascencio-Ibáñez et al., 2008). Because Arabidopsis mutant plants compromised in components of the purified CIA complex (Table I), namely AE7/CIA2 (Luo et al., 2012) and MET18/MMS19 (Han et al., 2015), have been reported to have increased expression of DNA-damage response genes (Luo et al., 2012; Han et al., 2015), we analyzed the expression of POLY(ADP-RIBOSE) POLYMERASE2 (PARP2), a frequently used marker gene, in the grxs17-1 mutant, as well as in an antisense line, denominated grxs17-2, that was previously described (Cheng et al., 2011) and which we confirmed to lack GRXS17 protein accumulation (Supplemental Fig. S2C). PARP2 transcript levels were indeed induced in both mutant alleles (Fig. 1D). Therefore, we assessed the sensitivity of the grxs17-1 and grsx17-2 mutant lines to DNA-damage stress. In agreement with the transcriptome data, the grxs17 mutants were found to display some degree of hypersensitivity to the DNA-damage agent MMS (Fig. 1E), though not as marked as the met18 mutants (Fig. 1E; Luo et al., 2012; Han et al., 2015). Together, these data suggest that GRXS17 not only forms part of the CIA complex in Arabidopsis but also may contribute to its function in the DNA-damage response.
Metabolic Profiling of Loss-of-Function grxs17 Plants Points to a Perturbed Flux through the Purine Salvage Pathway
Although human and yeast GRXS17 orthologs are essential for the maturation of cytosolic and nuclear Fe-S proteins (Mühlenhoff et al., 2010; Haunhorst et al., 2013), Arabidopsis GRXS17 has recently been shown to play only a minor role in maintaining the activity of two classes of cytosolic Fe-S enzymes (aconitases and aldehyde oxidases; Knuesting et al., 2015). Nonetheless, we identified another Fe-S enzyme, XDH1, to be associated with GRXS17 (Table I). XDH1 belongs to the family of xanthine oxidoreductases (XORs) that catalyze the oxidation of hypoxanthine and xanthine to uric acid during purine degradation. XDH1 is active as a homodimer in which each monomer has four cofactors: two [2Fe-2S] prosthetic groups, one FAD molecule, and one molybdenum cofactor (Moco), bound to the N-terminal, central, and C-terminal part of the protein, respectively (Zarepour et al., 2010).
Because XDH1 contains two [2Fe-2S] cofactors that are required for electron transfer to reduce the substrates xanthine and hypoxanthine (Zarepour et al., 2010), we investigated the GRXS17-XDH1 interaction in more detail. Interaction between XDH1 and GRXS17 was confirmed in a Y2H assay (Fig. 2A) and by BiFC, showing a cytosolic localization (Supplemental Fig. S1, B and C). To examine the physiological relevance of this interaction, we measured XDH activity in grx17-1 and grxs17-2 seedlings (Fig. 2B) but found no statistically significant difference in XDH activity compared with wild-type seedlings (Fig. 2B). Hence, similar to what was previously found for the enzymes cytosolic aconitase and aldehyde oxidase (Knuesting et al., 2015), our findings indicate that GRXS17 is not essential for XDH1 enzyme activity in plants.
Figure 2.
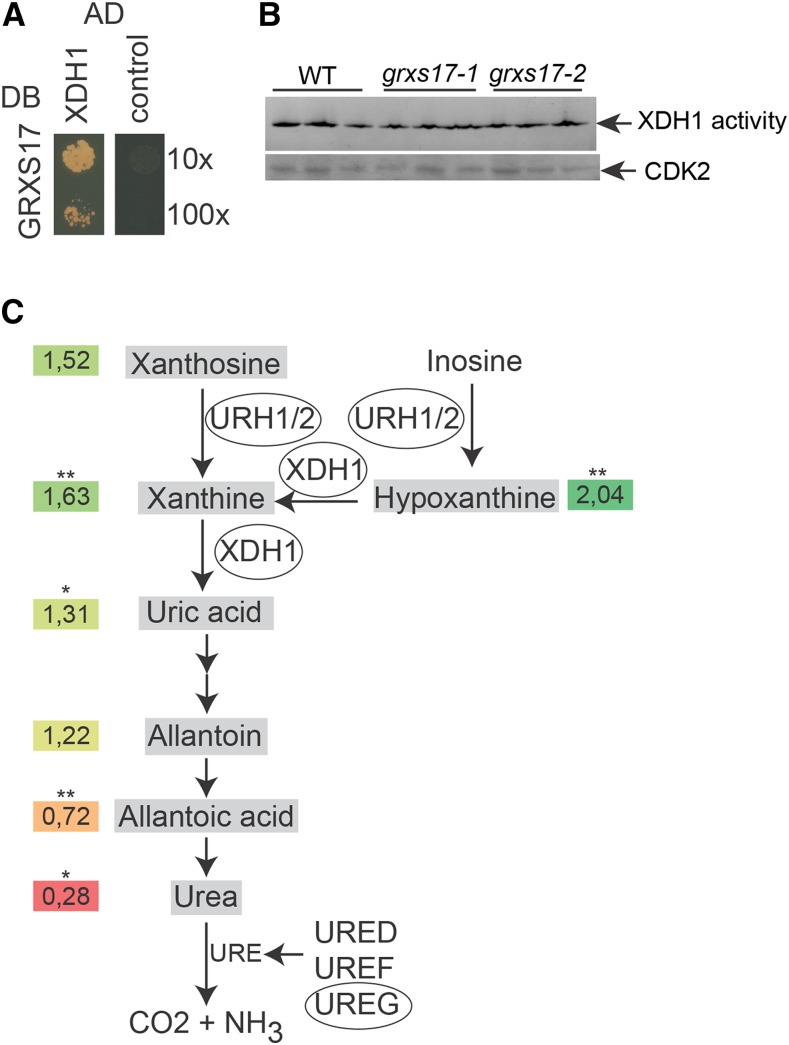
GRXS17 may contribute to the functioning of the purine salvage pathway. A, GRXS17 directly interacts with XDH1. The PJ69-4A yeast strain was cotransformed with GRXS17 in pGBKT7gate and XDH1 in pGADT7gate. The 10× and 100× dilutions of transformed yeasts were dropped on selective medium lacking His. The empty pGADT7gate vector was used as control. One representative from three independent drops is shown. B, XDH1 activity measurements. One hundred micrograms of total protein extract obtained from the indicated plant lines was loaded in each lane (three biological repeats) of a native PAGE and subsequently stained using hypoxanthine as a substrate. C, Purine salvage pathway. Enzymes copurified with GRXS17 in the TAP analysis are encircled. Numbers are averaged relative abundances from five independent samples (grxs17-1/Col-0) of uric acid precursors and ureides mapped on the purine salvage pathway (*P < 0.05; **P < 0.01, Student’s t test; colors: red, decreased; green, increased). The experiments were repeated twice with similar results. WT, Wild type.
In addition to XDH1, three additional proteins required for purine degradation were identified in complex with GRXS17: URIDINE RIBOHYDROLASE1 and 2 (URH1/2) and the urease accessory protein UreG (Table I). URH1 and URH2 are nucleoside ribohydrolases with inosine and xanthosine hydrolytic activity, leading to hypoxanthine and xanthine generation (Riegler et al., 2011). Urease catalyzes the hydrolysis of urea to ammonia and carbon dioxide, which are the final products of purine degradation. In Arabidopsis, urease activation is mediated by the accessory proteins UreD, UreF, and UreG (Witte et al., 2005). To evaluate the impact of loss-of-function of GRXS17 on purine metabolism, we measured the accumulation of uric acid precursors (hypoxanthine, xanthine, and xanthosine) and of ureides (uric acid, allantoin, allantoic acid, and urea) in wild-type and grxs17 seedlings (Fig. 2C; Supplemental Fig. S3). Although the effects were modest, our results are in agreement with the function of XDH1 in the purine salvage pathway and point to a perturbed flux through this pathway in the GRXS17 loss-of-function plants with enhanced accumulation of hypoxanthine, xanthine, and xanthosine and reduced accumulation of allantoic acid and urea. Together, these results suggest that association of GRXS17 with purine metabolism enzymes may be functionally relevant and may contribute to the purine salvage pathway.
GRXS17 Interacts with tRNA Thiolation Proteins
The TAP of GRXS17 protein complexes revealed interactions with several proteins that are linked to tRNA metabolism (Table I). For instance, we found GRXS17 to associate with the Arabidopsis orthologs of CTU1 and CTU2 required for 2-thiolation of U34 of the three cytosolic tRNA species tK(UUU), tE(UUC), and tQ(UUG). These tRNAs carry an mcm5s2U34 modification, which is conserved throughout archae and eukaryotes (Fig. 3A; Leidel et al., 2009; Philipp et al., 2014). Although only the protein encoded by AT2G44270 was described as the Arabidopsis CTU1 ortholog (Leiber et al., 2010), several peptides copurified with GRXS17 could not be distinguished from the product of locus AT1G76170 with 93% sequence similarity to CTU1 (Table I) and was named CTU1-like (CTU1L). To verify the interaction between GRXS17 and CTU1 and CTU2, we performed Y2H and BiFC assays. Y2H indicated only a very weak interaction for both CTU1 and CTU2 (Fig. 3B). Through BiFC, we could confirm a cytosolic-localized GRXS17-CTU2 interaction (Fig. 3C; Supplemental Fig. S4), thus supporting the TAP results and confirming that GRXS17 interacts directly with CTU2. Interaction between GRXS17 and CTU1 could not be detected in BiFC, which may either be a false negative result or an indication of indirect interaction, for instance via heterodimerization with CTU2.
Figure 3.
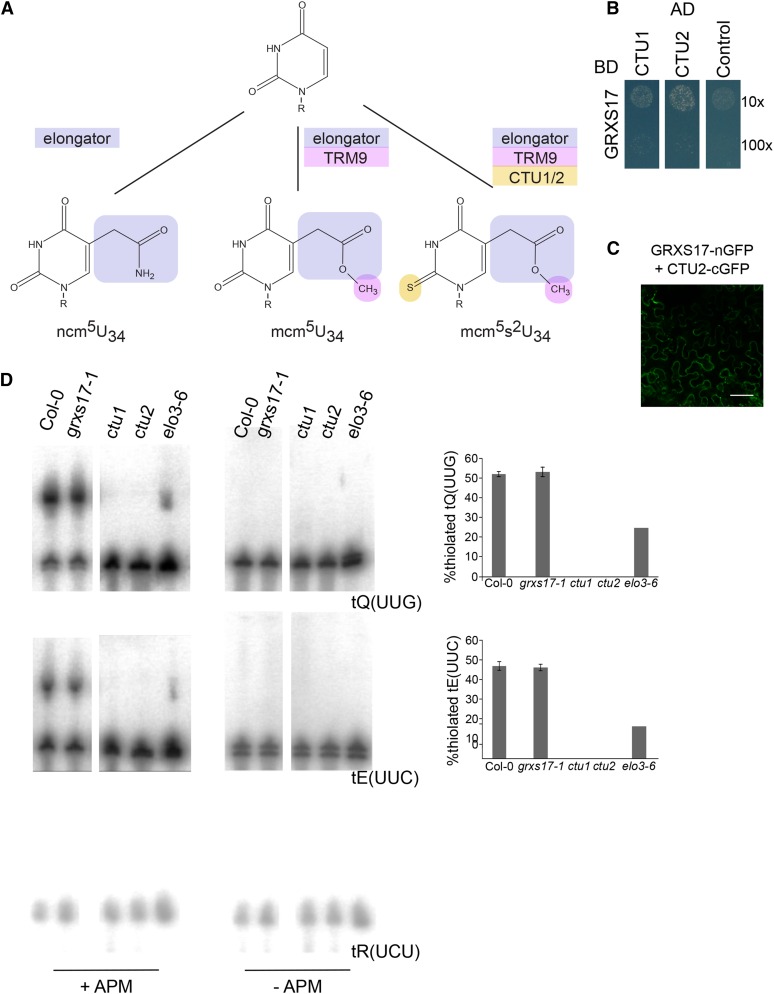
GRXS17 interacts with tRNA thiouridylases. A, Different types of tRNA modifications present in wobble uridines. Modifications by elongator, methylation by TRM9/12, and thiolation by CTUs are indicated in blue, pink, and yellow, respectively. R, Ribose. B, GRXS17 directly interacts with CTU1 and CTU2. The PJ69-4A yeast strain was cotransformed with GRXS17 in pGBKT7gate and CTU1 or CTU2 in pGADT7gate. The 10× and 100× dilutions of transformed yeasts were dropped on selective medium lacking His. The empty pGADT7gate vector was used as control. One representative from three independent drops is shown. C, GRXS17 interaction with CTU2 by BiFC. Fusion proteins with an N-terminal (nGFP) or C-terminal (cGFP) fragment of GFP were transiently coexpressed in N. benthamiana leaves and analyzed by confocal microscopy, 3 d after agro-infiltration. Negative controls are shown in Supplemental Figure S4. D, Total RNA extracted from Col-0, grxs17-1, ctu1, ctu2, or elo3-6 was separated using PAGE in the presence or absence of APM. Subsequently, RNA blots were probed against tQ(UUG), tE(UUC), or control tR(UCU). Quantification of thiolated modified tRNAs (right panels). Bars represent means ± sd of three biological independent samples. No statistical significances were detected between Col-0 and grxs17-1.
To further investigate the link between GRXS17 and CTUs, we tested if GRXS17 was required for tRNA thiolation in Arabidopsis. Thiolated tRNAs were visualized using their retardation in PAGE in the presence of [(N-acryloylamino)phenyl]mercuric chloride (APM), a compound that interacts with 2-thiouridine (Igloi, 1988). As reported previously, ctu1 and ctu2 T-DNA insertion lines did not contain thiolated tRNA (Fig. 3C; Leiber et al., 2010; Philipp et al., 2014). In contrast, in grxs17-1 plants, levels of thiolated total tRNA were similar to wild type (Fig. 3C). Thus, GRXS17 interacts with CTU subunits but is not essential for their tRNA anticodon thiolation function in Arabidopsis.
GRXS17 and Elongator Functions Are Related in Arabidopsis
Whereas CTU1 and CTU2 are essential for 2-thiolation of mcm5-modified tRNA anticodons, the ncm5U34 modification itself is dependent on elongator (Fig. 3A; Huang et al., 2005; Selvadurai et al., 2014), a complex that is structurally and functionally conserved between Saccharomyces cerevisiae and Arabidopsis (Mehlgarten et al., 2010). ELO3, the Arabidopsis ortholog of yeast elongator subunit Elp3, contains histone acetyltransferase and radical sterile alpha motif domains, which were recently shown to be catalytically critical for the tRNA modification function of archaeal Elp3 (Selvadurai et al., 2014).
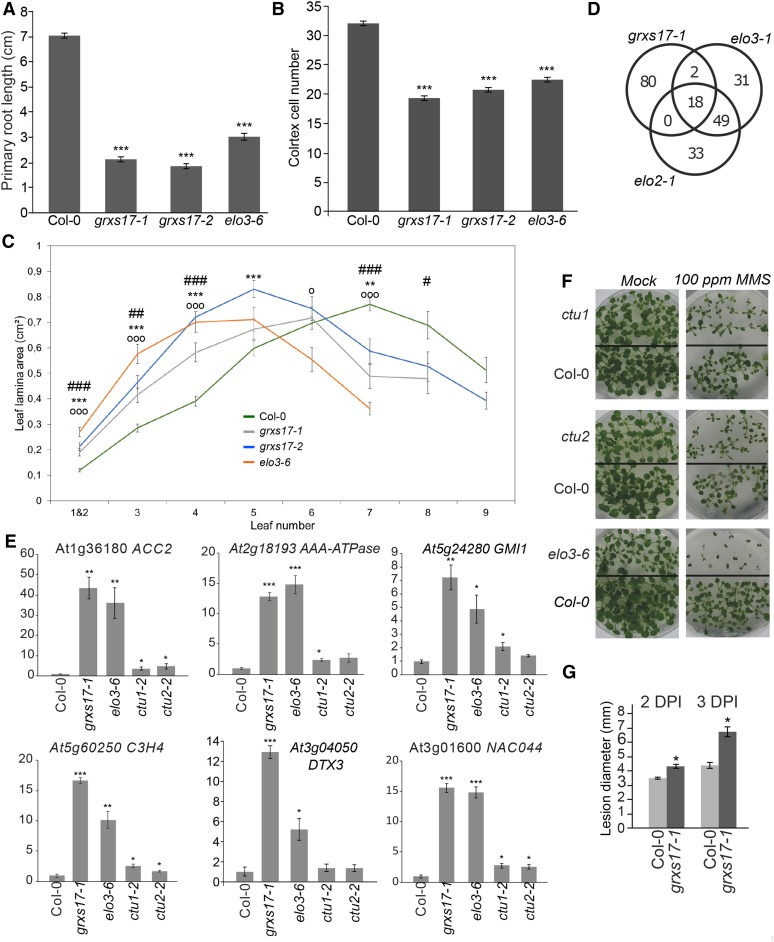
grxs17-1 and grxs17-2 mutant plants exhibit an elongated leaf phenotype with larger leaf length/width ratio as compared to wild type (Supplemental Fig. S5, A and B), which is typical of elongator mutants (Nelissen et al., 2005). Therefore, we investigated the possibility of a link between GRXS17 and elongator. First, we studied the root and leaf phenotypes of grxs17-1, grxs17-2, and elo3-6 in the Columbia-0 (Col-0) background. The elo3-6 and the two grxs17 mutant alleles had a reduced primary root growth (Fig. 4A), which was correlated with a reduced number of cortex cells in the root apical meristem (Fig. 4B), suggesting a faster transition to differentiation. Second, we measured the total leaf area in elo3-6 and the two grxs17 mutant alleles relative to wild-type plants. Notably, all three mutants had a similar growth profile: (1) larger juvenile leaves 1, 2, and 3, which are fully expanded at 24 d after germination (DAG); (2) a larger leaf 4, which is the transition to adult stage; and (3) smaller adult leaves 6, 7, and 8 because of a delay in growth in the mutants (Supplemental Fig. S5A). This is also reflected in the absence of leaf 8 and cauline leaves at the 24 DAG time point in some of the mutant genotypes (Fig. 4C). We examined the cellular basis of the changes in the fully developed leaf 3, of which the total cell number and final cell area are representative of cell proliferation and growth activities during its development. We observed that the cell area in all mutant lines was similar to that of the Col-0 control. However, the calculated number of cells per leaf was significantly higher in the two grxs17 mutant genotypes and in the elo3-6 line as compared to wild type (Supplemental Fig. S5, C and D), indicating that the larger leaf 3 area is the result of more cell proliferation in grxs17 and elo3-6 mutant lines.
Figure 4.
grxs17 and elo3-6 mutants show similar physiological and molecular defects. A–C, Analysis of root and leaf growth. A, Primary root length of 11-d-old Col-0, grxs17-1, grxs17-2, and elo3-6 seedlings grown on Murashige and Skoog (n > 21). B, Number of cortex cells in the root apical meristem of seedlings 5 DAG (n > 34). C, Lamina area of leaf series of 24-d-old plants germinated in soil (n > 10) Bars represent means ± se (*P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test, # comparison between Col-0 and grxs17-1, * for Col-0 and grxs17-2, and o for Col-0 and elo3-6). D and E, Transcriptome analysis. D, Overlap between the top 100 genes up-regulated in grxs17-1 for which a probeset is present on the ATH1 microarray and the top 100 probesets significantly up-regulated in elo2-1 and elo3-1. E, qPCR validation of gene expression in grxs17-1, elo3-6, ctu1-2, and ctu2-2 mutants. The expression ratio relative to that in wild-type Col-0 seedlings is plotted (set at 1). Bars represent means ± se of n = 3 (*P < 0.05; **P < 0.01; ***P < 0.001, Student’s t test). F, Hypersensitivity of ctu1, ctu2, and elo3-6 to MMS. Seedlings were grown for 17 d on 0.01% v/v MMS or under control conditions (mock). G, Hypersensitivity of grxs17-1 mutants to the necrotrophic pathogen B. cinerea. Lesion diameter in grxs17-1 and Col-0 plants infected with B. cinerea, 2 and 3 d postinoculation. Bars represent means ± se with n = 32 (*P < 0.05, Student’s t test).
Next, we compared our RNA-Seq dataset (Supplemental Table S1) with previously published microarray datasets of the elongator mutants elo2-1 and elo3-1 (Nelissen et al., 2005). Although the latter dataset was performed on mutants in the Landsberg erecta ecotype and with a different experimental setup, we observed a large overlap between the top 100 genes induced in grxs17-1, elo2-1, and elo3-1 (Fig. 4D). The overlap comprised the DNA-damage network indicated above (Fig. 1C). Indeed, loss of ELO3 has been reported to lead to a DNA-damage response (Xu et al., 2012). We assessed this observation further by analyzing gene expression in the grxs17 and elo3-6 mutants and in the T-DNA insertion lines of CTU1 and CTU2, called ctu1 and ctu2, all in the Col-0 background (Fig. 4E; Supplemental Fig. S6). Indeed, all genes tested that were up-regulated in grxs17 mutants were also up-regulated in elo3-6. Similarly, also in ctu1 and ctu2 we observed an upregulation of many grxs17-up-regulated genes, although more modest. In agreement with these results, ctu1 and ctu2 also showed some degree of sensitivity to the DNA-damage agent MMS (Fig. 4F).
Finally, previous reports indicated that the elongator subunits ELP2 and ELP3/ELO3 are involved in the salicylic acid signaling pathway (DeFraia et al., 2010, 2013) and that the elongator complex is required for Arabidopsis resistance to necrotrophic fungal pathogens such as Botrytis cinerea and Alternaria brassicicola (Wang et al., 2015). Therefore, we tested the susceptibility of the grxs17-1 mutant to B. cinerea. Compared with wild-type plants, grxs17-1 plants were more susceptible to B. cinerea infection (Fig. 4G), suggesting that GRXS17 plays a role in the defense response against B. cinerea.
Taken together, our data indicate that grxs17 and elo3 mutants have similar phenotypes at the molecular, cellular, and physiological level, both in growth and in defense, and thus support a joined, but not necessarily identical, role in the functioning of particular processes such as tRNA modification.
GRX and Elongator Functions Are Related, Also in Yeast
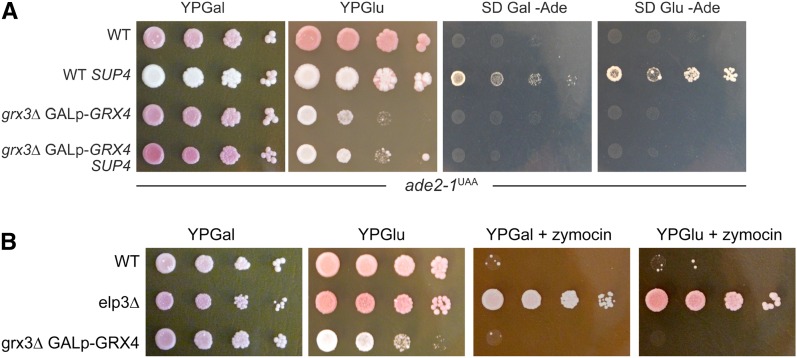
Given the conservation of the GRX proteins in eukaryotes, we wanted to test whether the yeast orthologs of Arabidopsis GRXS17, i.e. Grx3/4 (Herrero and de la Torre-Ruiz, 2007), also have similar related functions in tRNA modification by elongator in S. cerevisiae. Because grx3 grx4 double mutants are barely viable in S288c (Wu et al., 2012) and lethal in the background of yeast strain W303-1A, we used a previously described GRX3 deletion strain of W303 that maintains the GRX4 gene under the control of a GAL1 promoter, which is repressible by Glc and inducible by Gal (Mühlenhoff et al., 2010). W303 contains an ochre stop codon (UAA) mutation in the ADE2 locus (ade2-1ochre), which leads to premature translation termination and causes adenine auxotrophy (Fig. 5A). This phenotype can be suppressed by read-through of SUP4, an ochre suppressor tRNATyr carrying a U34 mutation in its anticodon (Goodman et al., 1977; Huang et al., 2005; Jablonowski et al., 2006; Fig. 5A). Because the SUP4 tRNATyr needs an mcm5-modified U34 to decode the UAA triplet as Tyr (Huang et al., 2005; Jablonowski et al., 2006), it cannot suppress the ade2-1ochre reporter without proper mcm5 formation at U34. SUP4 failed to promote stop codon read-through of ade2-1ochre and hence, conferred adenine auxotrophy in the yeast reporter strain lacking GRX3 function, even if GRX4 was not repressed (Fig. 5A). This indicates that GRX3 gene function in yeast is critical for SUP4 function and thus supports a related role with elongator, as in Arabidopsis.
Figure 5.
GRX and elongator function are related in yeast. A, GRX3 is required for SUP4 suppressor tRNA function in yeast. Serial 10-fold cell dilutions of wild-type (WT) yeast strain W303-1A (ade2-1UAA) and its derivative grx3Δ GALp-GRX4 (Mühlenhoff et al., 2010) both either lacking or carrying the SUP4 ochre tRNA suppressor were grown on rich (YP) medium containing Gal or Glc (Glu) to repress or induce GRX4 expression, respectively. In addition, the indicated yeast strains were cultivated on minimal (SD) medium containing Gal or Glu but lacking Ade to probe for SUP4-mediated adenine prototrophy. B, Same strains as in (A) and including elp3Δ were grown in the presence or absence of zymocin. Both experiments were repeated with similar results.
When we analyzed tRNA thiolation in elo3-6 mutant, we observed, in contrast to grxs17-1, almost no thiolated tRNA (Fig. 3D). These results suggest that grxs17-1 presents no strong defect in mcm5 modification in plants based on the absence of a strong thiolation defect. However, in yeast and plants, the GRXs Grx3/4 and GRXS17 may have a potential influence on elongator tRNA modification function, which we have previously shown to be conserved in and functionally exchangeable between S. cerevisiae and Arabidopsis (Mehlgarten et al., 2010). In the light of these findings, it is interesting to note that the GRX3 gene was identified previously as a high-copy suppressor in yeast of growth inhibition by zymocin, a fungal tRNase ribotoxin complex (Jablonowski et al., 2001; Jablonowski and Schaffrath, 2007). Zymocin activity targets elongator-dependent mcm5s2U34 modifications in tRNA anticodons (Lu et al., 2005; Jablonowski et al., 2006) and eventually kills S. cerevisiae cells. Therefore, loss of tRNA modification in elongator (elp) or tRNA methyltransferase (trm9) mutants protects against zymocin, making the tRNase a useful tool for diagnosing elongator function and, hence, tRNA modification in yeast (Nandakumar et al., 2008) and potentially in Arabidopsis (Mehlgarten et al., 2010; Leitner et al., 2015). We tested zymocin resistance in a yeast ELP3 deletion strain and in a GRX3 deletion strain having the GRX4 gene under control of the GAL1 promoter. As expected, deletion of ELP3 resulted in resistance to zymocin due to loss of mcm5s2U34 modification (Fig. 5B). However, when GRX3/4 were deleted, yeast cells could not grow in the presence of the toxin. Although these results suggest appropriate tRNA modifications, our SUP4 assay above (Fig. 5A) together with previous data showing that high-copy GRX3 confers zymocin resistance (Jablonowski et al., 2001), may indicate that the absence of GRX3/4 function differentially affects mcm5 or mcm5s2 modifications at U34, an option that has to await further elucidation.
DISCUSSION
Cytosolic monothiol GRXs are required in Fe-S cluster delivery in yeast and mammals. In plants, it remained unclear whether they have similar functions. Here, we used interactome analysis and in-depth functional characterization of a loss-of-function mutant to establish that that the sole class II cytosolic monothiol GRX from Arabidopsis associates with CIA components and Fe-S client proteins and contributes to their functioning.
Profiling of Loss-of-Function grxs17 Plants Points to an Elevated DNA-Damage Response
The function of many CIA components in yeast, mammals, and Arabidopsis has been associated with genomic stability, DNA repair, and metabolism, because many proteins necessary for DNA replication and repair are known to contain Fe-S clusters. In yeast and humans, Met-18/MMS19 has been shown to associate not only with several other CIA components, but also with Fe-S target proteins involved in DNA metabolism and to mediate the maturation of certain Fe-S proteins involved in DNA repair and replication (DNA helicases, polymerases, nucleases or glycosylases; Gari et al., 2012; Stehling et al., 2012; van Wietmarschen et al., 2012). The human MMS19 has been shown to be necessary for the maturation of only certain Fe-S proteins, mostly involved in DNA metabolism, but not for the activity of cytosolic aconitase iron regulated protein1 (IRP1) or Gln phosphoribosylpyrophosphate amidotransferase (Stehling et al., 2012). Human GRX3 is able to bind, in addition to [2Fe-2S] clusters, [4Fe-4S] clusters in vitro, which is necessary for the maturation of apo-IRP1 into aconitase (Xia et al., 2015), thus suggesting that GRX3/GRXS17, and not MMS19/MET18, is involved in the transfer of [4Fe-4S] clusters necessary for IRP1 maturation. This hypothesis is in accordance with the decrease in cytosolic aconitase activity observed in the Arabidopsis grxs17-1 mutant (Knuesting et al., 2015).
In Arabidopsis, MET18 also interacts with DNA polymerases and facilitates the maturation of Fe-S clusters on DNA polymerases in the cytosol (Han et al., 2015). Another MET18-interacting protein is the DNA demethylase ROS1, an Fe-S client protein that requires the Fe-S cluster for its activity, linking the CIA pathway with epigenetic modifications (Duan et al., 2015). Accordingly, an elevated DNA-damage response was found in Arabidopsis mutants deficient in CIA complex components, such as ae-7 and met18 (Luo et al., 2012; Han et al., 2015), or in Fe-S cluster containing proteins, such as elo3 (Xu et al., 2012).
In our study, we discovered a network (or regulon) that comprises genes involved in the genotoxic (DNA-damage) stress response to be up-regulated in the grxs17-1 mutant. Accordingly, we found that grxs17 plants present some degree of hypersensitivity to the DNA-alkylating agent MMS. A similar response has previously been reported for the ae-7, met18, and elo3-6 mutants (Luo et al., 2012; Xu et al., 2012; Han et al., 2015). Given that most enzymes implicated in DNA replication and DNA repair contain Fe-S clusters, our data suggest that GRXS17 and its association with the CIA complex may be necessary for their correct functioning and thus in genome integrity maintenance in plants.
GRXS17 Associates with Fe-S Client Proteins
Among the GRXS17-associated proteins that we identified by TAP were CTU1 and CTU2, two proteins essential for the thiolation of uridine at the wobble position of cytosolic tRNA in eukaryotes (Björk et al., 2007; Schlieker et al., 2008; Leidel et al., 2009). CTU1 presents homology with Escherichia coli TtcA, the protein responsible for the thioltransferase activity necessary for s2C32 tRNA thiolation, a tRNA modification not present in eukaryotes (Jäger et al., 2004). E. coli TtcA was shown to bind, through three conserved Cys residues, Fe-S clusters that are essential for its activity (Bouvier et al., 2014). The conserved motifs Cys-X1-X2-Cys present in E. coli TtcA and Arabidopsis CTU1 suggest that Arabidopsis CTU1(L) proteins could be Fe-S client proteins. Accordingly, proteins from the CIA machinery are known to be required for 2-thio modification of cytosolic tRNAs, suggesting that at least one cytosolic or nuclear protein containing Fe-S clusters is necessary for thiolation of cytosolic tRNAs (Nakai et al., 2007). Accordingly, GRXS17 could be involved in the transfer of putative Fe-S clusters to a CTU1(L)/CTU2 complex.
Several proteins already known to bind Fe-S clusters were also found in our GRXS17 TAP interactome, including BolA2 and XDH1. Interactions between GRX and BolA proteins are conserved in yeast, humans, and plants. In all of these eukaryotes, it has been demonstrated that the GRX and BolA domains are bridged by the binding of a [2Fe-2S] cluster (Li et al., 2009, 2012; Couturier et al., 2014). However, GRXS17 can also bind Fe-S clusters independently of BolA2 interaction, through the formation of Fe-S bridged homodimers, and it can contribute to the activity of cytosolic Fe-S enzymes (Knuesting et al., 2015).
In yeast, Grx3/4 are localized in the nucleus, where they regulate the nuclear export of Aft1, a transcription factor that regulates Fe-responsive gene expression under low Fe conditions (Pujol-Carrion et al., 2006). Heterodimerization of Grx3/4 with the BolA-like protein Fra2 leads to nuclear export of Aft1 (Pujol-Carrion et al., 2006; Li et al., 2009). In both yeast and humans, GRXs play an important role in Fe utilization. Grx3/4-deficient yeast cells have defects in de novo synthesis of Fe-S clusters and heme, two of the major Fe-consuming processes in these organisms. The Fe content is not decreased in these yeast cells, but the Fe is not bioavailable due to deficient Fe delivery to mitochondria. A similar defect in Fe bioavailability was observed in zebrafish (Danio rerio) and HeLa cells when HsGRX3 (or the zebrafish ortholog) was depleted (Mühlenhoff et al., 2010; Haunhorst et al., 2013). In plants, BolA2 does not seem to play a role in Fe homeostasis, and the biological output of the GRXS17-BolA2 interaction remains an unanswered question (Couturier et al., 2014; Roret et al., 2014).
XDH1, in turn, belongs to the family of XORs and is a central player in purine catabolism. In Arabidopsis, two XOR-encoding genes are present with a strict XDH activity, i.e. XDH1 and XDH2. Although both genes are located close to each other on the same chromosome, XDH2 is expressed constitutively at basal levels, whereas XDH1 is differentially expressed upon several developmental and environmental stimuli (Hesberg et al., 2004). Plants deficient in XDH1 are affected in growth and development. Seedlings with RNAi-mediated silencing of XDH1 are smaller than wild-type plants with shorter flowering stems, smaller fruit size, and higher sterility rate (Nakagawa et al., 2007). In addition, XDH1-deficient plants also show earlier onset of age-dependent, dark-induced leaf senescence (Nakagawa et al., 2007; Brychkova et al., 2008) and decreased tolerance to drought stress (Watanabe et al., 2010). In accordance with the function of XDH1 in purine catabolism, precursors of uric acid (hypoxanthine and xanthine) are significantly more abundant in XDH1-deficient plants, whereas downstream products (allantoic acid and urea) are less abundant (Nakagawa et al., 2007; Brychkova et al., 2008). Quantification of these metabolites in grxs17-1 plants indicated that these purine catabolism intermediates also accumulate differentially in the absence of GRXS17, reflecting a perturbed flux through the purine salvage pathway. However, it is interesting to note that despite the apparent clarity of these results, certain aspects of the changes in the levels of these metabolites were unexpected, namely the 20% increase in uric acid in the mutant in spite of the reduction of the activity of XDH1. The most likely explanation for this is the upregulation of another, as-yet-undefined, route of uric acid production in an unsuccessful (when viewed from the standpoint of the other downstream metabolites) attempt to compensate for the deficit of XDH1. This aspect suggests additional complexity in the regulation of this pathway and thus warrants further experimental study.
Remarkably, no overlap was found between the interacting partners identified in our study and those in a recent study in which affinity chromatography with HIS-tagged GRXS17 was used to identify GRXS17-interacting proteins (Knuesting et al., 2015). Interactors reported in the latter study were more related to signaling and included the transcriptional regulator Nuclear Factor Y Subunit C11/Negative Cofactor 2α (Knuesting et al., 2015). The discrepancy between these two interactomes could be due to differences in technical procedures and suggests that GRXS17 is able to interact with even more proteins than reported here and that it may be involved in different cellular processes.
GRXS17 Function Is Evolutionarily Conserved
The evolutionary conservation of the processes described in this paper is strong: CIA complex, GRX, XOR, and tRNA-modifying proteins are all conserved in plants and humans. Fe-S proteins are thought to be reminiscent of the origin of life, when Fe and S were readily available under a reducing, anaerobic environment. Metabolic pathways that evolved at these early stages of life became essential to all living organisms, and many of them require Fe-S proteins (Sheftel et al., 2010). Because Arabidopsis grxs17 seedlings actually only show mild developmental defects (Cheng et al., 2011; Knuesting et al., 2015) in contrast to the strong developmental defects associated with GRX depletion in yeast or animal cells, plants such as Arabidopsis may have evolved alternative protein(s) or pathway(s) that can take over these evolutionary conserved tasks.
The role of GRX proteins in the CIA pathway is still not entirely understood. In yeast, incorporation of Fe-S clusters into both cytosolic and mitochondrial proteins is dependent on Grx3/4 (Mühlenhoff et al., 2010). We establish here that, in plants, GRXS17 associates with several components of the CIA complex, including DRE2, NAR1, CIA1, AE7/CIA2, and MET18. GRXS17-DRE2 interaction is conserved in yeast and humans, in which the orthologs also interact directly and in which this close interaction allows the transfer of the [2Fe-2S] clusters from the GRX to DRE2 (Banci et al., 2015; Fig. 1A). In humans, disturbance of the DRE2/Anamorsin-GRX interaction has been proposed as a strategy to reduce cell proliferation in solid tumors in which Anamorsin expression is enhanced (Saito et al., 2011). Possibly, because maturation of DRE2 in humans is dependent on GRX3, GRX3 might, analogously to GRXS17, form the bridge between the mitochondrial and cytosolic Fe-S assembly machineries by providing the initial Fe-S cluster to the essential CIA proteins. Alternatively, GRXS17 might specifically contribute to the maturation of [2Fe-2S] cluster-containing proteins in the cytosol and the nucleus, because the clusters assembled by the CIA are of the [4Fe-4S] type. GRXS17 might thus associate with the CIA-targeting complex and function as a [2Fe-2S] specific adaptor for this complex.
MATERIALS AND METHODS
Plant Material and Growth Conditions
All mutant lines used in this study were in the Col-0 ecotype background. grxs17-1 (SALK_021301), grxs17-2 (antisense line), rol5-2/ctu1-2 (GK-709D04), ctu2-2 (GK-686B10), elo3-6 (GK-555H06), met18-1 (SALK_121963), and met18-2 (SALK_147068) mutants were described previously (Leiber et al., 2010; Nelissen et al., 2010; Chen et al., 2011; Luo et al., 2012; Philipp et al., 2014). Arabidopsis (Arabidopsis thaliana) seeds were sterilized by the chlorine gas method and sown on sterile plates containing Murashige and Skoog medium supplemented with 1% (w/v) Suc, 0.8% (w/v) agarose, pH 5.7. Plates were kept 2 d in the dark for stratification at 4°C before being transferred to a growth room at 21°C with a 16-h light/8-h dark regime, with a light intensity of 80 μmol m−2 s−1, unless mentioned otherwise.
Cloning and Site-Directed Mutagenesis
The coding sequence of GRXS17, DRE2, CTU1, and CTU2 were amplified from cDNA obtained from wild-type Arabidopsis Col-0 seedlings, using specific primers (Supplemental Table S3) containing Gateway recombination attB sites. The amplified ORFs were then recombined with pDONR207 and transferred to the corresponding pDEST Gateway vectors.
Yeast Strains, Media, and SUP4 tRNA Suppressor Assay
Y2H analysis was performed as described previously (Cuéllar Pérez et al., 2013). In brief, the Saccharomyces cerevisiae PJ69-4A strain was cotransformed with pGADT7 and pGBKT7 vectors containing bait and prey, respectively. Transformants were selected on synthetic defined (SD) medium lacking Leu and Trp. Three individual transformants were grown overnight in liquid medium lacking Leu and Trp, and a 10-fold dilution of these cultures was dropped on control and selective solid media additionally lacking His. Empty vectors were used as negative controls and yeast cells were allowed to grow for 2 d at 30°C before interaction was scored.
For the conventional SUP4 tRNA suppressor assay based on ade2-1 ochre read-through (Huang et al., 2005), we used W303-1A strain (MATa ura3-1, ade2-1, trp1-1, his3-11, 15, leu2-3,112; Mortimer and Johnston, 1986) and its pGAL-GRX4/grx3Δ derivative (W303-1A but pGRX4::GAL-l-natNT2; grx3Δ::LEU2; Mühlenhoff et al., 2010). Routine yeast growth was performed in rich yeast extract, peptone dextrose (YPD), or Gal (YPG) media or on SD medium containing 2% (w/v) dextrose (SD Glu) or 2% (w/v) Gal (SD Gal; Sherman, 2002). Both lacked adenine to check for ade2-1 ochre read-through by the tRNA suppressor SUP4 and formation of adenine prototrophic cells (Jablonowski et al., 2006). The latter involved growth for 3 d at 30°C of 10-fold serial dilutions of yeast strains W303-1A and pGAL-GRX4/grx3Δ transformed with SUP4 on a single-copy plasmid pTC3 (Shaw and Olson, 1984; Jablonowski et al., 2009).
TAP
N or C terminally tagged TAP constructs (GS or GSrh tag) were generated as described previously (Van Leene et al., 2015). They were used for the transformation of Arabidopsis PSB-d cell suspension cultures without callus selection and further grown and subcultured as described by Van Leene et al. (2011). Stably transformed cultures were scaled up and harvested 6 d after subculturing. Transgenic Arabidopsis seeds were generated by floral dip (Clough and Bent, 1998), using Col-0 as the background ecotype and the same constructs as for cell culture transformation. Transformants were selected, and purifications were performed as described by Van Leene et al. (2015), with the exception that no benzonase treatment was performed on the cell culture extracts. Expression of TAP-tagged constructs was verified on an aliquot of total protein extract before purification.
BiFC
pCaMV35S:ORF-tag constructs using the N- or C-terminal part of GFP (nGFP and cGFP, respectively) were constructed by triple Gateway reactions using pK7m34GW or pH7m34GW (Karimi et al., 2005) as described previously (Boruc et al., 2010). pCaMV35S::tag-ORF constructs were generated by double Gateway recombination using pH7m24GW2 or pK7m24GW2 (Boruc et al., 2010). The constructs were coexpressed together with a P19-expressing Agrobacterium tumefaciens strain (Voinnet et al., 2003) in Nicotiana benthamiana using Agrobacterium-mediated transient transformation with a modified infiltration buffer (10 mm MgCl2, 10 mm MES, pH 5.7, 100 μm acetosyringone). Interactions were scored by screening the lower epidermal cells for fluorescence using LEICA SP2 confocal microscopy, 3 d after transformation.
RNA-Seq
Seedlings were grown in vertical plates in three biological repeats for 14 d in Murashige and Skoog medium supplemented with 1% (w/v) Suc. Seedlings were frozen in liquid nitrogen, and total RNA was extracted using the RNeasy plant mini kit (Qiagen) and DNase I (Promega) treatment. A Trueseq RNA-Seq library (Illumina) was compiled and sequenced as 50-bp single read using the Illumina HiSEquation 2000 technology at GATC Biotech. Read quality control, filtering, mapping to The Arabidopsis Information Resource 10 version of the Arabidopsis genome, and read counting were carried out using the Galaxy portal running on an internal server (http://galaxyproject.org/). Sequences were filtered and trimmed with the Filter FASTQ v1 and FASTQ Quality Trimmer v1 tools, respectively, with default settings (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were subsequently mapped to The Arabidopsis Information Resource 10 version of the Arabidopsis genome using GSNAPv2 (Wu and Nacu, 2010), allowing a maximum of five mismatches. The concordantly paired reads that uniquely map to the genome were used for quantification on the gene level with HTSeq-count from the HTSeq python package (Anders et al., 2015). Data were normalized using TMM, implemented in edgeR (Robinson et al., 2010), and common dispersion was then estimated using the conditional maximum likelihood method (Robinson and Smyth, 2008). Differentially expressed genes were defined by a 2-fold difference between mutant lines and the wild-type control with P-value < 0.05. The false discovery rate was limited to 5% according to Benjamini and Hochberg (1995).
Gene Expression Analysis
Seedlings were grown in the same conditions described for RNA-Seq, and total RNA was isolated as mentioned above. One microgram of RNA was used for cDNA synthesis using the iScript kit (Bio-Rad). Quantitative reverse transcriptase-PCR was performed on a LightCycler 480 system (Roche) using the Fast Start SYBR Green I PCR mix (Roche). At least three biological repeats and two technical repeats were used for each analysis. Data were analyzed using the second derivative maximum method, and relative expression levels were determined using the comparative cycle threshold method. Primer sequences are provided in Supplemental Table S3.
tRNA Extraction and Analysis
Seedlings were grown for 14 d in half-strength liquid Murashige and Skoog medium supplemented with 1% (w/v) Suc and 100 mg/L myoinositol. Seedlings were frozen in liquid nitrogen, and total RNA was extracted according to Björk et al. (2001). One-half microgram of total RNA was resolved on 8% acrylamide gels containing 0.5× TBE, 7 m urea, and 50 µg/mL APM. Northern-blot analysis was performed essentially as described in Leidel et al. (2009), with the following probes: 5′-TGGCGCCGTCTGTGGGGATCG-3′ to detect tK(UUU), 5′-TGGAGGTTCTACCGGGAGTCGAACC-3′ to detect tQ(UUG), 5′-TGGCTCCATTGCCGGGAATCGAACC-3′ to detect tE(UUC) and as negative control for nonthiolated tRNAs, 5′-TGGCACACCCGGTGGGACTCG-3′ to detect tR(UCU), and 5′-TGGTGCGTCTGCCGGGAGTCG-3′ to detect tG(UCC).
Immunoblot Analysis
After quantification of the protein content as described by Bradford (1976), the indicated protein samples were loaded on a 4% to 15% TGX gel (Bio-Rad) and ran for 20 min at 300 V. Next, proteins were transferred to 0.2-µm polyvinylidene difluoride membranes (Bio-Rad) with the Transblot Turbo (Bio-Rad). Chemiluminescent detection was performed with western Bright ECL (Isogen).
Anti-GRXS17 Antibody
GRXS17 was cloned into the pDEST17 vector (Invitrogen) and expressed in the Escherichia coli BL21(DE3) strain. The 6xHIS-GRX17 recombinant protein was purified by IMAC using Ni-NTA (Qiagen) resin followed by size exclusion chromatography using an Äkta purifier equipped with a Superdex200 column (GE Healthcare). A rabbit was immunized with the purified recombinant 6xHIS-GRX17 in the service facilities of Agro-Bio (France). Total serum was collected 63 d postimmunization. A 1/5,000 dilution of this antibody and a 1/10,000 dilution of anti-rabbit IgG-HRP (GE) were used for immunoblotting.
DNA-Damage Agent
Sterilized seeds were geminated in Murashige and Skoog medium, transferred after 4 d to Murashige and Skoog plates supplemented or not with 0.01% v/v MMS (Sigma) and scored after 17 d. The experiment was performed in triplicate.
Root Phenotype Analysis
Seedlings grown vertically at 21°C under 24-h light conditions (75 µmol m−2 s−1) were used for root analysis. The root meristem size was determined 5 DAG as the number of cells in the cortex cell file from the Quiescent center (QC) to the first elongated cell (Casamitjana-Martínez et al., 2003). The samples were mounted with clearing solution (80 g chloral hydrate, 30 mL glycerol, and 10 mL dH2O) and observed immediately. The main root length was determined 11 DAG using ImageJ software (http://imagej.nih.gov/ij/). At least 23 seedlings of each line were analyzed.
Leaf Phenotype Analysis
Plants, 24 DAG in soil at 21°C under a 16-h light/8-h dark regime with a light intensity of 75 μmol m−2 s−1were used for leaf series on 1% agar plates, picture taking, and image analysis of leaf lamina area, length, and width as described (Cnops et al., 2004). Leaf 3 was chosen for epidermal cell imaging because of its full expansion at 24 DAG (Fig. 4C; Pyke et al., 1991; Medford et al., 1992). Leaves were fixed overnight in 100% ethanol and mounted with 90% lactic acid. The leaf area was measured with the ImageJ software. The epidermal cells on the abaxial side were drawn with a Leica DMLB microscope equipped with a drawing tube and differential interference contrast objectives. The total number of cells per leaf was calculated as described previously (De Veylder et al., 2001). We estimated the total number of cells per leaf by dividing the leaf area by the mean cell area (averaged between the tip and basal positions). Means between samples were compared by a two-tailed Student’s t test.
Enzymatic Activity Measurement (XDH)
XDH1 activity measurements in plant crude extracts were performed as described in Hesberg et al. (2004). Briefly, total protein extract was obtained from 10-d-old Arabidopsis seedlings. Plant tissue was grown in liquid nitrogen, resuspended in two volumes of extraction buffer (100 mm Tris-HCl pH 7.5, 2.5 mm EDTA, 5 mm DTT), and centrifuged. Supernatants were concentrated using Nanosep centrifugal devices (30K Omega, Pall Life Sciences) and 100 μg of total protein quantified by the method of Bradford (1976) were used for activity assays. Four to sixteen percent native polyacrylamide gels under nonreducing conditions were run at 4°C, followed by in-gel staining with 1 mm hypoxanthine, 1 mm 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide, and 0.1 mm phenazine methosulfate in 250 mm Tris-HCl, pH 8.5.
Metabolite Profiling by Gas Chromatography/Time of Flight-Mass Spectrometry
Metabolite analysis by gas chromatography-mass spectrometry was performed essentially as described by Lisec et al. (2006) by injecting metabolite extracts from 1 mg fresh weight of plant material into the gas chromatograph/time of flight-mass spectrometer. Chromatograms and mass spectra were evaluated using Xcalibur 2.1 software (Thermo Fisher Scientific). Specific fragment and expected elution time of target metabolites (urea, allantoic acid, allantoin, hypoxanthine, uric acid, xanthine, and xanthosine) were identified by coelution with authentic standards. Peak areas of the mass (m/z) fragments were normalized to the internal standard (ribitol) and fresh weight of the samples. Identification and annotation of detected peaks are shown in Supplemental Table S2 following recent recommendations for reporting metabolite data (Fernie et al., 2011).
Pathogen Infection
Cultivation and spore harvesting of Botrytis cinerea strain B05.10 (provided by Rudi Aerts, Katholieke Hogeschool Kempen, Belgium) was performed as described previously (Broekaert et al., 1990). Arabidopsis wild-type (Col-0) and mutants plants were grown for 4 weeks in soil (“DCM potgrond voor Zaaien en Stekken”; DCM, Sint-Katelijne-Waver, Belgium) in a growth chamber at 21°C, 75% humidity, and a 12-h day/light cycle with a light intensity of approximately 120 μmol m−2 s−1. A 5-μL drop of a B. cinerea spore suspension (5 × 105/mL in half-strength potato dextrose broth) was inoculated on three leaves per plant. Plants were kept in transparent, sealed boxes to retain almost 100% humidity after inoculation. Disease symptoms were scored by measuring the diameter of the necrotic lesions at 2 and 3 d postinoculation. Thirty-two plants per line were analyzed. Two independent assays with similar results were performed.
Accession Numbers
Accession numbers of the genes used in this study are as follows: GRXS17, AT4G04950; MET18, AT5G48120; DRE2, AT5G18400; NAR1, AT4G16440; CIA1, AT2G26060; AE7/CIA2, AT1G68310; XDH1, AT4G34890; URH1, AT2G36310; URH2, AT1G05620; UREG, AT2G34470; CTU1, AT2G44270; CTU1L, AT1G76170; CTU2, AT4G35910; ELO3, AT5G50320; Leu-tRNA ligase, AT1G09620; PARP2, AT4G02390; ACC2, AT1G36180; GMI, AT5G24280; F4P13.14, AT3G01600; AAA-ATPase, AT2G18193; and DTX3, AT2G04050 and AT5G60250.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. GRXS17 interacts with DRE2 and XDH1 in planta.
Supplemental Figure S2. Characterization of the grxs17 mutants used in this study.
Supplemental Figure S3. Analysis of the functioning of the purine salvage pathway in grxs17-1 seedlings.
Supplemental Figure S4. BIFC controls.
Supplemental Figure S5. grxs17 and elo3-6 mutants have similar developmental defects.
Supplemental Figure S6. grxs17 and elo3-6 mutants show similar molecular defects.
Supplemental Table S1. RNA-Seq analysis of the grxs17-1 mutant.
Supplemental Table S2. Identification and annotation of detected purine metabolite peaks.
Supplemental Table S3. Primers used in this study.
Supplemental Dataset S1. Mass spectrometry data of TAP experiments.
Supplementary Material
Acknowledgments
We thank Florian Bittner for providing the XDH1 clone, Frederik Coppens for support with the RNA-Seq analysis, Pia Neyt for technical support, and Annick Bleys for help in preparing the manuscript.
Glossary
- GRX
glutaredoxin
- CIA
cytosolic Fe-S assembly
- TRX
thioredoxin
- TAP
tandem affinity purification
Footnotes
This work was supported by the Research Foundation Flanders through the projects G005212N and G005312N and a postdoctoral fellowship to L.P.; the Belgian Science Policy Organization for postdoctoral fellowships to S.I. and A.R.; the European Commission Marie Curie Research Training Network (CHIP-ET, FP7-PEOPLE-2013-ITN607880) to M.V.L. and S.L.G.; the Max Planck Society to S.A.L.; the Deutsche Forschungsgemeinschaft SPP1784 “Chemical Biology of Native Nucleic Acid Modifications” to R.K. (KL2937/1-1), R.S. (SCHA750/20-1), and S.A.L. (LE 3260/2-1); and SPP1927 “Iron-Sulfur for Life: Cooperative Function of Iron-Sulfur Centers in Assembly, Biosynthesis, Catalysis and Disease” to R.S. (SCHA750/21-1).
Articles can be viewed without a subscription.
References
- Anders S, Pyl PT, Huber W (2015) HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabidopsis Interactome Mapping Consortium (2011) Evidence for network evolution in an Arabidopsis interactome map. Science 333: 601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascencio-Ibáñez JT, Sozzani R, Lee TJ, Chu TM, Wolfinger RD, Cella R, Hanley-Bowdoin L (2008) Global analysis of Arabidopsis gene expression uncovers a complex array of changes impacting pathogen response and cell cycle during geminivirus infection. Plant Physiol 148: 436–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk J, Schaedler TA (2014) Iron cofactor assembly in plants. Annu Rev Plant Biol 65: 125–153 [DOI] [PubMed] [Google Scholar]
- Banci L, Ciofi-Baffoni S, Gajda K, Muzzioli R, Peruzzini R, Winkelmann J (2015) N-terminal domains mediate [2Fe-2S] cluster transfer from glutaredoxin-3 to anamorsin. Nat Chem Biol 11: 772–778 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 289–300 [Google Scholar]
- Bernard DG, Netz DJ, Lagny TJ, Pierik AJ, Balk J (2013) Requirements of the cytosolic iron-sulfur cluster assembly pathway in Arabidopsis. Philos Trans R Soc Lond B Biol Sci 368: 20120259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk GR, Huang B, Persson OP, Byström AS (2007) A conserved modified wobble nucleoside (mcm5s2U) in lysyl-tRNA is required for viability in yeast. RNA 13: 1245–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk GR, Jacobsson K, Nilsson K, Johansson MJ, Byström AS, Persson OP (2001) A primordial tRNA modification required for the evolution of life? EMBO J 20: 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boruc J, Van den Daele H, Hollunder J, Rombauts S, Mylle E, Hilson P, Inzé D, De Veylder L, Russinova E (2010) Functional modules in the Arabidopsis core cell cycle binary protein-protein interaction network. Plant Cell 22: 1264–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier D, Labessan N, Clémancey M, Latour JM, Ravanat JL, Fontecave M, Atta M (2014) TtcA a new tRNA-thioltransferase with an Fe-S cluster. Nucleic Acids Res 42: 7960–7970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Terras FRG, Cammue BPA, Vanderleyden J (1990) An automated quantitative assay for fungal growth inhibition. FEMS Microbiol Lett 69: 55–59 [Google Scholar]
- Brychkova G, Alikulov Z, Fluhr R, Sagi M (2008) A critical role for ureides in dark and senescence-induced purine remobilization is unmasked in the Atxdh1 Arabidopsis mutant. Plant J 54: 496–509 [DOI] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Scheres B (2003) Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Curr Biol 13: 1435–1441 [DOI] [PubMed] [Google Scholar]
- Chen C, Huang B, Eliasson M, Rydén P, Byström AS (2011) Elongator complex influences telomeric gene silencing and DNA damage response by its role in wobble uridine tRNA modification. PLoS Genet 7: e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng NH, Liu JZ, Liu X, Wu Q, Thompson SM, Lin J, Chang J, Whitham SA, Park S, Cohen JD, et al. (2011) Arabidopsis monothiol glutaredoxin, AtGRXS17, is critical for temperature-dependent postembryonic growth and development via modulating auxin response. J Biol Chem 286: 20398–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circelli P, Donini M, Villani ME, Benvenuto E, Marusic C (2010) Efficient Agrobacterium-based transient expression system for the production of biopharmaceuticals in plants. Bioeng Bugs 1: 221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cnops G, Jover-Gil S, Peters JL, Neyt P, De Block S, Robles P, Ponce MR, Gerats T, Micol JL, Van Lijsebettens M (2004) The rotunda2 mutants identify a role for the LEUNIG gene in vegetative leaf morphogenesis. J Exp Bot 55: 1529–1539 [DOI] [PubMed] [Google Scholar]
- Couturier J, Przybyla-Toscano J, Roret T, Didierjean C, Rouhier N (2015) The roles of glutaredoxins ligating Fe-S clusters: Sensing, transfer or repair functions? Biochim Biophys Acta 1853: 1513–1527 [DOI] [PubMed] [Google Scholar]
- Couturier J, Touraine B, Briat JF, Gaymard F, Rouhier N (2013) The iron-sulfur cluster assembly machineries in plants: current knowledge and open questions. Front Plant Sci 4: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Wu HC, Dhalleine T, Pégeot H, Sudre D, Gualberto JM, Jacquot JP, Gaymard F, Vignols F, Rouhier N (2014) Monothiol glutaredoxin-BolA interactions: redox control of Arabidopsis thaliana BolA2 and SufE1. Mol Plant 7: 187–205 [DOI] [PubMed] [Google Scholar]
- Cuéllar Pérez A, Pauwels L, De Clercq R, Goossens A (2013) Yeast two-hybrid analysis of jasmonate signaling proteins. Methods Mol Bio. 1011: 173–186 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inzé D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFraia CT, Wang Y, Yao J, Mou Z (2013) Elongator subunit 3 positively regulates plant immunity through its histone acetyltransferase and radical S-adenosylmethionine domains. BMC Plant Biol 13: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFraia CT, Zhang X, Mou Z (2010) Elongator subunit 2 is an accelerator of immune responses in Arabidopsis thaliana. Plant J 64: 511–523 [DOI] [PubMed] [Google Scholar]
- Duan CG, Wang X, Tang K, Zhang H, Mangrauthia SK, Lei M, Hsu CC, Hou YJ, Wang C, Li Y, et al. (2015) MET18 connects the cytosolic iron-sulfur cluster assembly pathway to active DNA demethylation in arabidopsis. PLoS Genet 11: e1005559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Aharoni A, Willmitzer L, Stitt M, Tohge T, Kopka J, Carroll AJ, Saito K, Fraser PD, DeLuca V (2011) Recommendations for reporting metabolite data. Plant Cell 23: 2477–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gari K, León Ortiz AM, Borel V, Flynn H, Skehel JM, Boulton SJ (2012) MMS19 links cytoplasmic iron-sulfur cluster assembly to DNA metabolism. Science 337: 243–245 [DOI] [PubMed] [Google Scholar]
- Gerber J, Neumann K, Prohl C, Mühlenhoff U, Lill R (2004) The yeast scaffold proteins Isu1p and Isu2p are required inside mitochondria for maturation of cytosolic Fe/S proteins. Mol Cell Biol 24: 4848–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman HM, Olson MV, Hall BD (1977) Nucleotide sequence of a mutant eukaryotic gene: the yeast tyrosine-inserting ochre suppressor SUP4-o. Proc Natl Acad Sci USA 74: 5453–5457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YF, Huang HW, Li L, Cai T, Chen S, He XJ (2015) The cytosolic iron-sulfur cluster assembly protein MMS19 regulates transcriptional gene silencing, DNA repair, and flowering time in Arabidopsis. PLoS One 10: e0129137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunhorst P, Berndt C, Eitner S, Godoy JR, Lillig CH (2010) Characterization of the human monothiol glutaredoxin 3 (PICOT) as iron-sulfur protein. Biochem Biophys Res Commun 394: 372–376 [DOI] [PubMed] [Google Scholar]
- Haunhorst P, Hanschmann EM, Bräutigam L, Stehling O, Hoffmann B, Mühlenhoff U, Lill R, Berndt C, Lillig CH (2013) Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol Biol Cell 24: 1895–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E, de la Torre-Ruiz MA (2007) Monothiol glutaredoxins: a common domain for multiple functions. Cell Mol Life Sci 64: 1518–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesberg C, Hänsch R, Mendel RR, Bittner F (2004) Tandem orientation of duplicated xanthine dehydrogenase genes from Arabidopsis thaliana: differential gene expression and enzyme activities. J Biol Chem 279: 13547–13554 [DOI] [PubMed] [Google Scholar]
- Huang B, Johansson MJ, Byström AS (2005) An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11: 424–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igloi GL. (1988) Interaction of tRNAs and of phosphorothioate-substituted nucleic acids with an organomercurial. Probing the chemical environment of thiolated residues by affinity electrophoresis. Biochemistry 27: 3842–3849 [DOI] [PubMed] [Google Scholar]
- Isakov N, Witte S, Altman A (2000) PICOT-HD: a highly conserved protein domain that is often associated with thioredoxin and glutaredoxin modules. Trends Biochem Sci 25: 537–539 [DOI] [PubMed] [Google Scholar]
- Jablonowski D, Frohloff F, Fichtner L, Stark MJ, Schaffrath R (2001) Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol Microbiol 42: 1095–1105 [DOI] [PubMed] [Google Scholar]
- Jablonowski D, Schaffrath R (2007) Zymocin, a composite chitinase and tRNase killer toxin from yeast. Biochem Soc Trans 35: 1533–1537 [DOI] [PubMed] [Google Scholar]
- Jablonowski D, Täubert JE, Bär C, Stark MJ, Schaffrath R (2009) Distinct subsets of Sit4 holophosphatases are required for inhibition of Saccharomyces cerevisiae growth by rapamycin and zymocin. Eukaryot Cell 8: 1637–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski D, Zink S, Mehlgarten C, Daum G, Schaffrath R (2006) tRNAGlu wobble uridine methylation by Trm9 identifies Elongator’s key role for zymocin-induced cell death in yeast. Mol Microbiol 59: 677–688 [DOI] [PubMed] [Google Scholar]
- Jäger G, Leipuviene R, Pollard MG, Qian Q, Björk GR (2004) The conserved Cys-X1-X2-Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. J Bacteriol 186: 750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor HR, Clarke S (2003) Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol Cell Biol 23: 9283–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, De Meyer B, Hilson P (2005) Modular cloning in plant cells. Trends Plant Sci 10: 103–105 [DOI] [PubMed] [Google Scholar]
- Kispal G, Csere P, Prohl C, Lill R (1999) The mitochondrial proteins Atm1p and Nfs1p are essential for biogenesis of cytosolic Fe/S proteins. EMBO J 18: 3981–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuesting J, Riondet C, Maria C, Kruse I, Bécuwe N, König N, Berndt C, Tourrette S, Guilleminot-Montoya J, Herrero E, et al. (2015) Arabidopsis glutaredoxin S17 and its partner, the nuclear factor Y subunit C11/negative cofactor 2α, contribute to maintenance of the shoot apical meristem under long-day photoperiod. Plant Physiol 167: 1643–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber RM, John F, Verhertbruggen Y, Diet A, Knox JP, Ringli C (2010) The TOR pathway modulates the structure of cell walls in Arabidopsis. Plant Cell 22: 1898–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel S, Pedrioli PG, Bucher T, Brost R, Costanzo M, Schmidt A, Aebersold R, Boone C, Hofmann K, Peter M (2009) Ubiquitin-related modifier Urm1 acts as a sulphur carrier in thiolation of eukaryotic transfer RNA. Nature 458: 228–232 [DOI] [PubMed] [Google Scholar]
- Leihne V, Kirpekar F, Vågbø CB, van den Born E, Krokan HE, Grini PE, Meza TJ, Falnes PO (2011) Roles of Trm9- and ALKBH8-like proteins in the formation of modified wobble uridines in Arabidopsis tRNA. Nucleic Acids Res 39: 7688–7701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner J, Retzer K, Malenica N, Bartkeviciute R, Lucyshyn D, Jäger G, Korbei B, Byström A, Luschnig C (2015) Meta-regulation of Arabidopsis auxin responses depends on tRNA maturation. Cell Reports 11: 516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mapolelo DT, Dingra NN, Naik SG, Lees NS, Hoffman BM, Riggs-Gelasco PJ, Huynh BH, Johnson MK, Outten CE (2009) The yeast iron regulatory proteins Grx3/4 and Fra2 form heterodimeric complexes containing a [2Fe-2S] cluster with cysteinyl and histidyl ligation. Biochemistry 48: 9569–9581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Mapolelo DT, Randeniya S, Johnson MK, Outten CE (2012) Human glutaredoxin 3 forms [2Fe-2S]-bridged complexes with human BolA2. Biochemistry 51: 1687–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc 1: 387–396 [DOI] [PubMed] [Google Scholar]
- Lu J, Huang B, Esberg A, Johansson MJ, Byström AS (2005) The Kluyveromyces lactis gamma-toxin targets tRNA anticodons. RNA 11: 1648–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D, Bernard DG, Balk J, Hai H, Cui X (2012) The DUF59 family gene AE7 acts in the cytosolic iron-sulfur cluster assembly pathway to maintain nuclear genome integrity in Arabidopsis. Plant Cell 24: 4135–4148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford JI, Behringer FJ, Callos JD, Feldmann KA (1992) Normal and abnormal development in the Arabidopsis vegetative shoot apex. Plant Cell 4: 631–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlgarten C, Jablonowski D, Wrackmeyer U, Tschitschmann S, Sondermann D, Jäger G, Gong Z, Byström AS, Schaffrath R, Breunig KD (2010) Elongator function in tRNA wobble uridine modification is conserved between yeast and plants. Mol Microbiol 76: 1082–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR (1986) Genealogy of principal strains of the yeast genetic stock center. Genetics 113: 35–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlenhoff U, Molik S, Godoy JR, Uzarska MA, Richter N, Seubert A, Zhang Y, Stubbe J, Pierrel F, Herrero E, et al. (2010) Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron-sulfur cluster. Cell Metab 12: 373–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa A, Sakamoto S, Takahashi M, Morikawa H, Sakamoto A (2007) The RNAi-mediated silencing of xanthine dehydrogenase impairs growth and fertility and accelerates leaf senescence in transgenic Arabidopsis plants. Plant Cell Physiol 48: 1484–1495 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Nakai M, Lill R, Suzuki T, Hayashi H (2007) Thio modification of yeast cytosolic tRNA is an iron-sulfur protein-dependent pathway. Mol Cell Biol 27: 2841–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandakumar J, Schwer B, Schaffrath R, Shuman S (2008) RNA repair: an antidote to cytotoxic eukaryal RNA damage. Mol Cell 31: 278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedialkova DD, Leidel SA (2015) Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161: 1606–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H, De Groeve S, Fleury D, Neyt P, Bruno L, Bitonti MB, Vandenbussche F, Van der Straeten D, Yamaguchi T, Tsukaya H, et al. (2010) Plant Elongator regulates auxin-related genes during RNA polymerase II transcription elongation. Proc Natl Acad Sci USA 107: 1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen H, Fleury D, Bruno L, Robles P, De Veylder L, Traas J, Micol JL, Van Montagu M, Inzé D, Van Lijsebettens M (2005) The elongata mutants identify a functional Elongator complex in plants with a role in cell proliferation during organ growth. Proc Natl Acad Sci USA 102: 7754–7759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraskevopoulou C, Fairhurst SA, Lowe DJ, Brick P, Onesti S (2006) The Elongator subunit Elp3 contains a Fe4S4 cluster and binds S-adenosylmethionine. Mol Microbiol 59: 795–806 [DOI] [PubMed] [Google Scholar]
- Philipp M, John F, Ringli C (2014) The cytosolic thiouridylase CTU2 of Arabidopsis thaliana is essential for posttranscriptional thiolation of tRNAs and influences root development. BMC Plant Biol 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciocchi A, Saguez C, Boussac A, Cassier-Chauvat C, Chauvat F (2007) CGFS-type monothiol glutaredoxins from the cyanobacterium Synechocystis PCC6803 and other evolutionary distant model organisms possess a glutathione-ligated [2Fe-2S] cluster. Biochemistry 46: 15018–15026 [DOI] [PubMed] [Google Scholar]
- Pujol-Carrion N, Belli G, Herrero E, Nogues A, de la Torre-Ruiz MA (2006) Glutaredoxins Grx3 and Grx4 regulate nuclear localisation of Aft1 and the oxidative stress response in Saccharomyces cerevisiae. J Cell Sci 119: 4554–4564 [DOI] [PubMed] [Google Scholar]
- Pyke KA, Marrison JL, Leech RM (1991) Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heynh. J Exp Bot 42: 1407–1416 [Google Scholar]
- Riegler H, Geserick C, Zrenner R (2011) Arabidopsis thaliana nucleosidase mutants provide new insights into nucleoside degradation. New Phytol 191: 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Smyth GK (2008) Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9: 321–332 [DOI] [PubMed] [Google Scholar]
- Rolland T, Taşan M, Charloteaux B, Pevzner SJ, Zhong Q, Sahni N, Yi S, Lemmens I, Fontanillo C, Mosca R, et al. (2014) A proteome-scale map of the human interactome network. Cell 159: 1212–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roret T, Tsan P, Couturier J, Zhang B, Johnson MK, Rouhier N, Didierjean C (2014) Structural and spectroscopic insights into BolA-glutaredoxin complexes. J Biol Chem 289: 24588–24598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, Berriz GF, Gibbons FD, Dreze M, Ayivi-Guedehoussou N, et al. (2005) Towards a proteome-scale map of the human protein-protein interaction network. Nature 437: 1173–1178 [DOI] [PubMed] [Google Scholar]
- Saito Y, Shibayama H, Tanaka H, Tanimura A, Matsumura I, Kanakura Y (2011) PICOT is a molecule which binds to anamorsin. Biochem Biophys Res Commun 408: 329–333 [DOI] [PubMed] [Google Scholar]
- Scheidt V, Jüdes A, Bar CB, Klassen R, Schaffrath R (2014) Loss of wobble uridine modification in tRNA anticodons interferes with TOR pathway signaling. Microb Cell 1: 416–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieker CD, Van der Veen AG, Damon JR, Spooner E, Ploegh HL (2008) A functional proteomics approach links the ubiquitin-related modifier Urm1 to a tRNA modification pathway. Proc Natl Acad Sci USA 105: 18255–18260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvadurai K, Wang P, Seimetz J, Huang RH (2014) Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism. Nat Chem Biol 10: 810–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KJ, Olson MV (1984) Effects of altered 5′-flanking sequences on the in vivo expression of a Saccharomyces cerevisiae tRNATyr gene. Mol Cell Biol 4: 657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftel A, Stehling O, Lill R (2010) Iron-sulfur proteins in health and disease. Trends Endocrinol Metab 21: 302–314 [DOI] [PubMed] [Google Scholar]
- Sherman F. (2002) Getting started with yeast. Methods Enzymol 350: 3–41 [DOI] [PubMed] [Google Scholar]
- Stehling O, Mascarenhas J, Vashisht AA, Sheftel AD, Niggemeyer B, Rösser R, Pierik AJ, Wohlschlegel JA, Lill R (2013) Human CIA2A-FAM96A and CIA2B-FAM96B integrate iron homeostasis and maturation of different subsets of cytosolic-nuclear iron-sulfur proteins. Cell Metab 18: 187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehling O, Vashisht AA, Mascarenhas J, Jonsson ZO, Sharma T, Netz DJ, Pierik AJ, Wohlschlegel JA, Lill R (2012) MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337: 195–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarassov K, Messier V, Landry CR, Radinovic S, Serna Molina MM, Shames I, Malitskaya Y, Vogel J, Bussey H, Michnick SW (2008) An in vivo map of the yeast protein interactome. Science 320: 1465–1470 [DOI] [PubMed] [Google Scholar]
- Van Leene J, Eeckhout D, Cannoot B, De Winne N, Persiau G, Van De Slijke E, Vercruysse L, Dedecker M, Verkest A, Vandepoele K, et al. (2015) An improved toolbox to unravel the plant cellular machinery by tandem affinity purification of Arabidopsis protein complexes. Nat Protoc 10: 169–187 [DOI] [PubMed] [Google Scholar]
- Van Leene J, Eeckhout D, Persiau G, Van De Slijke E, Geerinck J, Van Isterdael G, Witters E, De Jaeger G (2011) Isolation of transcription factor complexes from Arabidopsis cell suspension cultures by tandem affinity purification. Methods Mol Biol 754: 195–218 [DOI] [PubMed] [Google Scholar]
- van Wietmarschen N, Moradian A, Morin GB, Lansdorp PM, Uringa EJ (2012) The mammalian proteins MMS19, MIP18, and ANT2 are involved in cytoplasmic iron-sulfur cluster protein assembly. J Biol Chem 287: 43351–43358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Ding Y, Yao J, Zhang Y, Sun Y, Colee J, Mou Z (2015) Arabidopsis Elongator subunit 2 positively contributes to resistance to the necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola. Plant J 83: 1019–1033 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Nakagawa A, Izumi S, Shimada H, Sakamoto A (2010) RNA interference-mediated suppression of xanthine dehydrogenase reveals the role of purine metabolism in drought tolerance in Arabidopsis. FEBS Lett 584: 1181–1186 [DOI] [PubMed] [Google Scholar]
- Witte CP, Rosso MG, Romeis T (2005) Identification of three urease accessory proteins that are required for urease activation in Arabidopsis. Plant Physiol 139: 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Lin J, Liu JZ, Wang X, Lim W, Oh M, Park J, Rajashekar CB, Whitham SA, Cheng NH, et al. (2012) Ectopic expression of Arabidopsis glutaredoxin AtGRXS17 enhances thermotolerance in tomato. Plant Biotechnol J 10: 945–955 [DOI] [PubMed] [Google Scholar]
- Wu TD, Nacu S (2010) Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 26: 873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Li B, Zhang Z, Wang Q, Qiao T, Li K (2015) Human glutaredoxin 3 can bind and effectively transfer [4Fe-4S] cluster to apo-iron regulatory protein 1. Biochem Biophys Res Commun 465: 620–624 [DOI] [PubMed] [Google Scholar]
- Xu D, Huang W, Li Y, Wang H, Huang H, Cui X (2012) Elongator complex is critical for cell cycle progression and leaf patterning in Arabidopsis. Plant J 69: 792–808 [DOI] [PubMed] [Google Scholar]
- Zarepour M, Kaspari K, Stagge S, Rethmeier R, Mendel RR, Bittner F (2010) Xanthine dehydrogenase AtXDH1 from Arabidopsis thaliana is a potent producer of superoxide anions via its NADH oxidase activity. Plant Mol Biol 72: 301–310 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Wu X, An X, Stubbe J, Huang M (2011) Investigation of in vivo diferric tyrosyl radical formation in Saccharomyces cerevisiae Rnr2 protein: requirement of Rnr4 and contribution of Grx3/4 AND Dre2 proteins. J Biol Chem 286: 41499–41509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinshteyn B, Gilbert WV (2013) Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet 9: e1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.