Abstract
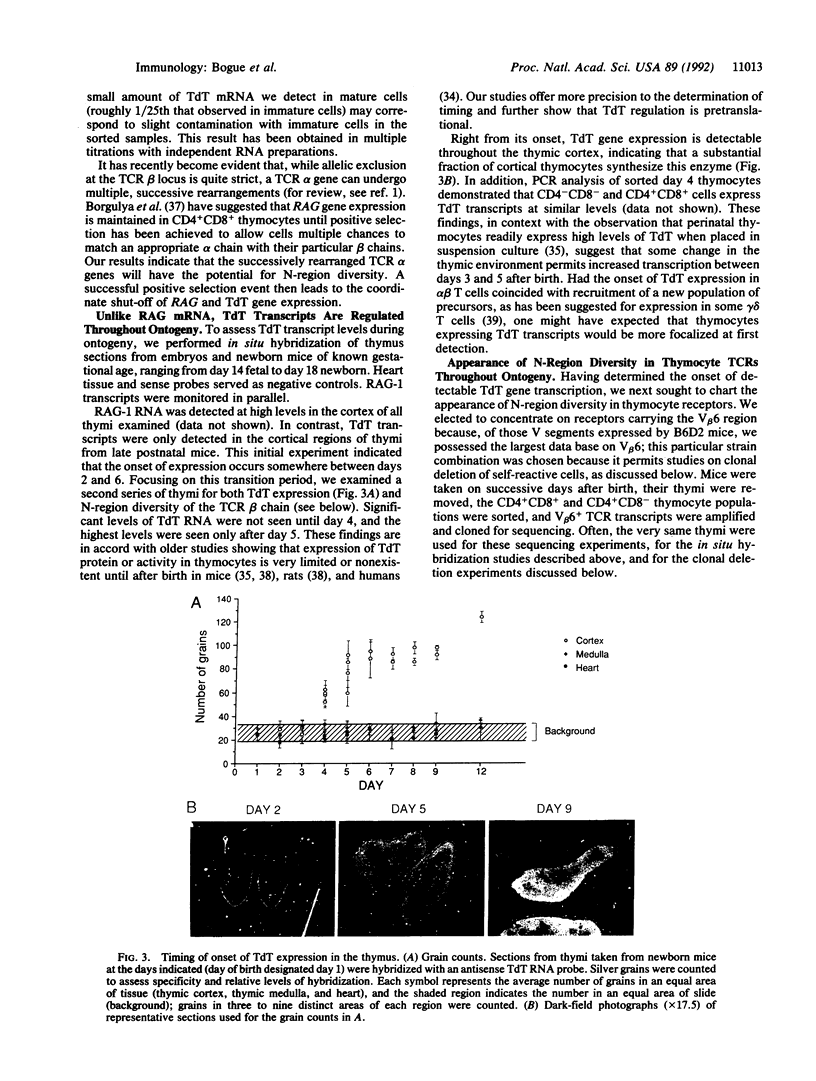
The random addition of "N nucleotides" by terminal deoxynucleotidyltransferase (TdT) is an important component of the diversity of T-cell receptor genes. We have investigated the expression of TdT during thymocyte differentiation and thymus ontogeny. TdT gene transcripts are confined to immature thymocytes of the cortex, being down-regulated concomitantly with recombination-activating gene transcripts after positive selection of mature medullary T cells. According to in situ hybridization, TdT RNA is absent from the neonatal thymus, but it appears 3 to 5 days after birth, just before the appearance of significant N-region diversity in T-cell receptor junctional sequences but clearly after the thymus attains competence at clonal deletion.
Full text
PDF

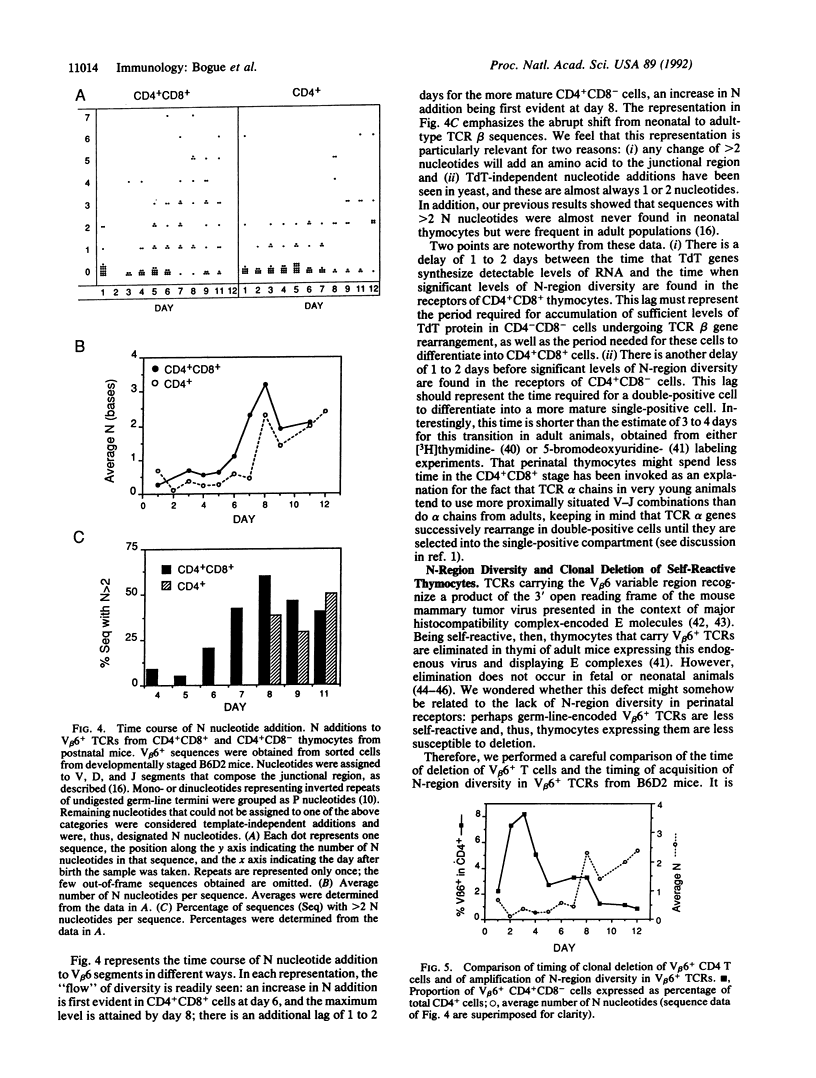

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar L. K., Belmont J. W. V gamma 3 T cell receptor rearrangement and expression in the adult thymus. J Immunol. 1991 Feb 15;146(4):1348–1352. [PubMed] [Google Scholar]
- Bangs L. A., Sanz I. E., Teale J. M. Comparison of D, JH, and junctional diversity in the fetal, adult, and aged B cell repertoires. J Immunol. 1991 Mar 15;146(6):1996–2004. [PubMed] [Google Scholar]
- Benoist C., Mathis D. Generation of the alpha beta T-cell repertoire. Curr Opin Immunol. 1992 Apr;4(2):156–161. doi: 10.1016/0952-7915(92)90005-y. [DOI] [PubMed] [Google Scholar]
- Bogue M., Candéias S., Benoist C., Mathis D. A special repertoire of alpha:beta T cells in neonatal mice. EMBO J. 1991 Dec;10(12):3647–3654. doi: 10.1002/j.1460-2075.1991.tb04931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992 May 1;69(3):529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Candéias S., Katz J., Benoist C., Mathis D., Haskins K. Islet-specific T-cell clones from nonobese diabetic mice express heterogeneous T-cell receptors. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6167–6170. doi: 10.1073/pnas.88.14.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candéias S., Waltzinger C., Benoist C., Mathis D. The V beta 17+ T cell repertoire: skewed J beta usage after thymic selection; dissimilar CDR3s in CD4+ versus CD8+ cells. J Exp Med. 1991 Nov 1;174(5):989–1000. doi: 10.1084/jem.174.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson L., Holmberg D. Genetic basis of the neonatal antibody repertoire: germline V-gene expression and limited N-region diversity. Int Immunol. 1990;2(7):639–643. doi: 10.1093/intimm/2.7.639. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Overmo C., Holmberg D. Selection against N-region diversity in immunoglobulin heavy chain variable regions during the development of pre-immune B cell repertoires. Int Immunol. 1992 May;4(5):549–553. doi: 10.1093/intimm/4.5.549. [DOI] [PubMed] [Google Scholar]
- Cayre Y., de Sostoa A., Silverstone A. E. Isolation of a subset of thymocytes inducible for terminal transferase biosynthesis. J Immunol. 1981 Feb;126(2):553–556. [PubMed] [Google Scholar]
- Ceredig R. Intrathymic proliferation of perinatal mouse alpha beta and gamma delta T cell receptor-expressing mature T cells. Int Immunol. 1990;2(9):859–867. doi: 10.1093/intimm/2.9.859. [DOI] [PubMed] [Google Scholar]
- Chun J. J., Schatz D. G., Oettinger M. A., Jaenisch R., Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991 Jan 11;64(1):189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Cosgrove D., Gray D., Dierich A., Kaufman J., Lemeur M., Benoist C., Mathis D. Mice lacking MHC class II molecules. Cell. 1991 Sep 6;66(5):1051–1066. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Bandeira A., Pereira P., Portnoi D., Holmberg D., Martinez C., Freitas A. A. Selection of lymphocyte repertoires: the limits of clonal versus network organization. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):159–170. doi: 10.1101/sqb.1989.054.01.020. [DOI] [PubMed] [Google Scholar]
- Deibel M. R., Jr, Riley L. K., Coleman M. S., Cibull M. L., Fuller S. A., Todd E. Expression of terminal deoxynucleotidyl transferase in human thymus during ontogeny and development. J Immunol. 1983 Jul;131(1):195–200. [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Rock E. P., Patten P. A., Davis M. M., Chien Y. H. The adult T-cell receptor delta-chain is diverse and distinct from that of fetal thymocytes. Nature. 1988 Feb 18;331(6157):627–631. doi: 10.1038/331627a0. [DOI] [PubMed] [Google Scholar]
- Feeney A. J. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med. 1991 Jul 1;174(1):115–124. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- George J. F., Jr, Schroeder H. W., Jr Developmental regulation of D beta reading frame and junctional diversity in T cell receptor-beta transcripts from human thymus. J Immunol. 1992 Feb 15;148(4):1230–1239. [PubMed] [Google Scholar]
- Gregoire K. E., Goldschneider I., Barton R. W., Bollum F. J. Ontogeny of terminal deoxynucleotidyl transferase-positive cells in lymphohemopoietic tissues of rat and mouse. J Immunol. 1979 Sep;123(3):1347–1352. [PubMed] [Google Scholar]
- Gu H., Förster I., Rajewsky K. Sequence homologies, N sequence insertion and JH gene utilization in VHDJH joining: implications for the joining mechanism and the ontogenetic timing of Ly1 B cell and B-CLL progenitor generation. EMBO J. 1990 Jul;9(7):2133–2140. doi: 10.1002/j.1460-2075.1990.tb07382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesmann M., Scott B., Kisielow P., von Boehmer H. Kinetics and efficacy of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 1991 Aug 9;66(3):533–540. doi: 10.1016/0092-8674(81)90016-7. [DOI] [PubMed] [Google Scholar]
- Ikuta K., Weissman I. L. The junctional modifications of a T cell receptor gamma chain are determined at the level of thymic precursors. J Exp Med. 1991 Nov 1;174(5):1279–1282. doi: 10.1084/jem.174.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasato T., Yamagishi H. Novel excision products of T cell receptor gamma gene rearrangements and developmental stage specificity implied by the frequency of nucleotide insertions at signal joints. Eur J Immunol. 1992 Jan;22(1):101–106. doi: 10.1002/eji.1830220116. [DOI] [PubMed] [Google Scholar]
- Jones L. A., Chin L. T., Merriam G. R., Nelson L. M., Kruisbeck A. M. Failure of clonal deletion in neonatally thymectomized mice: tolerance is preserved through clonal anergy. J Exp Med. 1990 Nov 1;172(5):1277–1285. doi: 10.1084/jem.172.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallenbach S., Doyen N., Fanton d'Andon M., Rougeon F. Three lymphoid-specific factors account for all junctional diversity characteristic of somatic assembly of T-cell receptor and immunoglobulin genes. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2799–2803. doi: 10.1073/pnas.89.7.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Vakil M., Solvason N. The role of idiotypic interactions and B-cell subsets in development of the B-cell repertoire. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):203–207. doi: 10.1101/sqb.1989.054.01.025. [DOI] [PubMed] [Google Scholar]
- Koiwai O., Yokota T., Kageyama T., Hirose T., Yoshida S., Arai K. Isolation and characterization of bovine and mouse terminal deoxynucleotidyltransferase cDNAs expressible in mammalian cells. Nucleic Acids Res. 1986 Jul 25;14(14):5777–5792. doi: 10.1093/nar/14.14.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyes S., Pao W., Hayday A. Influence of site of expression on the fetal gamma delta T-cell receptor repertoire. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7830–7833. doi: 10.1073/pnas.88.17.7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaille J. J., DeCloux A., Bonneville M., Takagaki Y., Tonegawa S. Junctional sequences of T cell receptor gamma delta genes: implications for gamma delta T cell lineages and for a novel intermediate of V-(D)-J joining. Cell. 1989 Dec 1;59(5):859–870. doi: 10.1016/0092-8674(89)90609-0. [DOI] [PubMed] [Google Scholar]
- Lafaille J. J., Haas W., Coutinho A., Tonegawa S. Positive selection of gamma delta T cells. Immunol Today. 1990 Mar;11(3):75–78. doi: 10.1016/0167-5699(90)90030-d. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Schatz D. G., Rosa M., Baltimore D. Increased frequency of N-region insertion in a murine pre-B-cell line infected with a terminal deoxynucleotidyl transferase retroviral expression vector. Mol Cell Biol. 1987 Sep;7(9):3237–3243. doi: 10.1128/mcb.7.9.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo K., Landau N. R., Smale S. T. LyF-1, a transcriptional regulator that interacts with a novel class of promoters for lymphocyte-specific genes. Mol Cell Biol. 1991 Oct;11(10):5229–5243. doi: 10.1128/mcb.11.10.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Stella G., Postema C. E., Thompson C. B. Chicken T-cell receptor beta-chain diversity: an evolutionarily conserved D beta-encoded glycine turn within the hypervariable CDR3 domain. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7699–7703. doi: 10.1073/pnas.88.17.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek K. Analysis of junctional diversity during B lymphocyte development. Science. 1990 Nov 9;250(4982):820–823. doi: 10.1126/science.2237433. [DOI] [PubMed] [Google Scholar]
- Payne J., Huber B. T., Cannon N. A., Schneider R., Schilham M. W., Acha-Orbea H., MacDonald H. R., Hengartner H. Two monoclonal rat antibodies with specificity for the beta-chain variable region V beta 6 of the murine T-cell receptor. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7695–7698. doi: 10.1073/pnas.85.20.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. J., Lesley J., Trotter J., Schulte R., Hyman R., Sefton B. M. Changes in the relative abundance of type I and type II lck mRNA transcripts suggest differential promoter usage during T-cell development. Mol Cell Biol. 1990 Aug;10(8):4266–4270. doi: 10.1128/mcb.10.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983 Apr;130(4):1627–1633. [PubMed] [Google Scholar]
- Schneider R., Lees R. K., Pedrazzini T., Zinkernagel R. M., Hengartner H., MacDonald H. R. Postnatal disappearance of self-reactive (V beta 6+) cells from the thymus of Mlsa mice. Implications for T cell development and autoimmunity. J Exp Med. 1989 Jun 1;169(6):2149–2158. doi: 10.1084/jem.169.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwager J., Bürckert N., Courtet M., Du Pasquier L. The ontogeny of diversification at the immunoglobulin heavy chain locus in Xenopus. EMBO J. 1991 Sep;10(9):2461–2470. doi: 10.1002/j.1460-2075.1991.tb07785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Vremec D., Egerton M. The kinetics of T cell antigen receptor expression by subgroups of CD4+8+ thymocytes: delineation of CD4+8+3(2+) thymocytes as post-selection intermediates leading to mature T cells. J Exp Med. 1991 Feb 1;173(2):323–332. doi: 10.1084/jem.173.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli K., Benoist C., Mathis D. Why is clonal deletion of neonatal thymocytes defective? Eur J Immunol. 1992 Oct;22(10):2487–2493. doi: 10.1002/eji.1830221004. [DOI] [PubMed] [Google Scholar]
- Turka L. A., Schatz D. G., Oettinger M. A., Chun J. J., Gorka C., Lee K., McCormack W. T., Thompson C. B. Thymocyte expression of RAG-1 and RAG-2: termination by T cell receptor cross-linking. Science. 1991 Aug 16;253(5021):778–781. doi: 10.1126/science.1831564. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]