Abstract
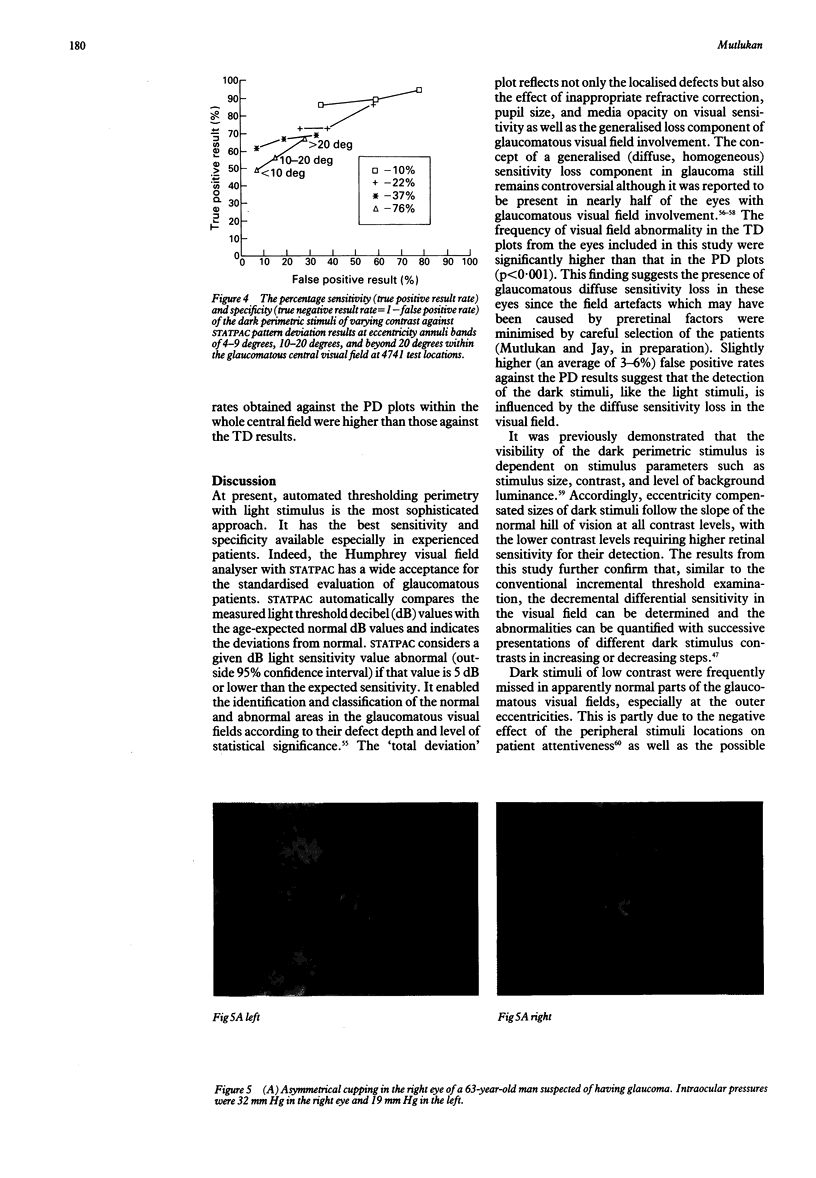
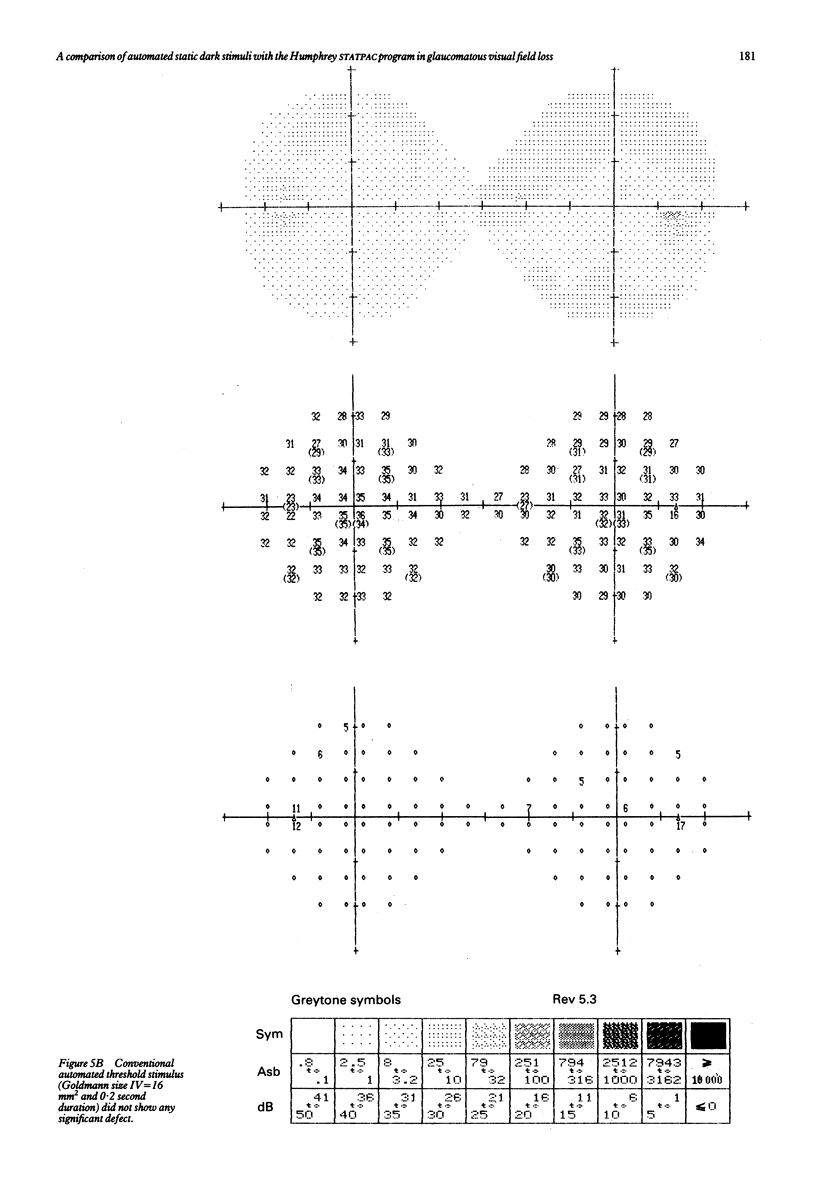
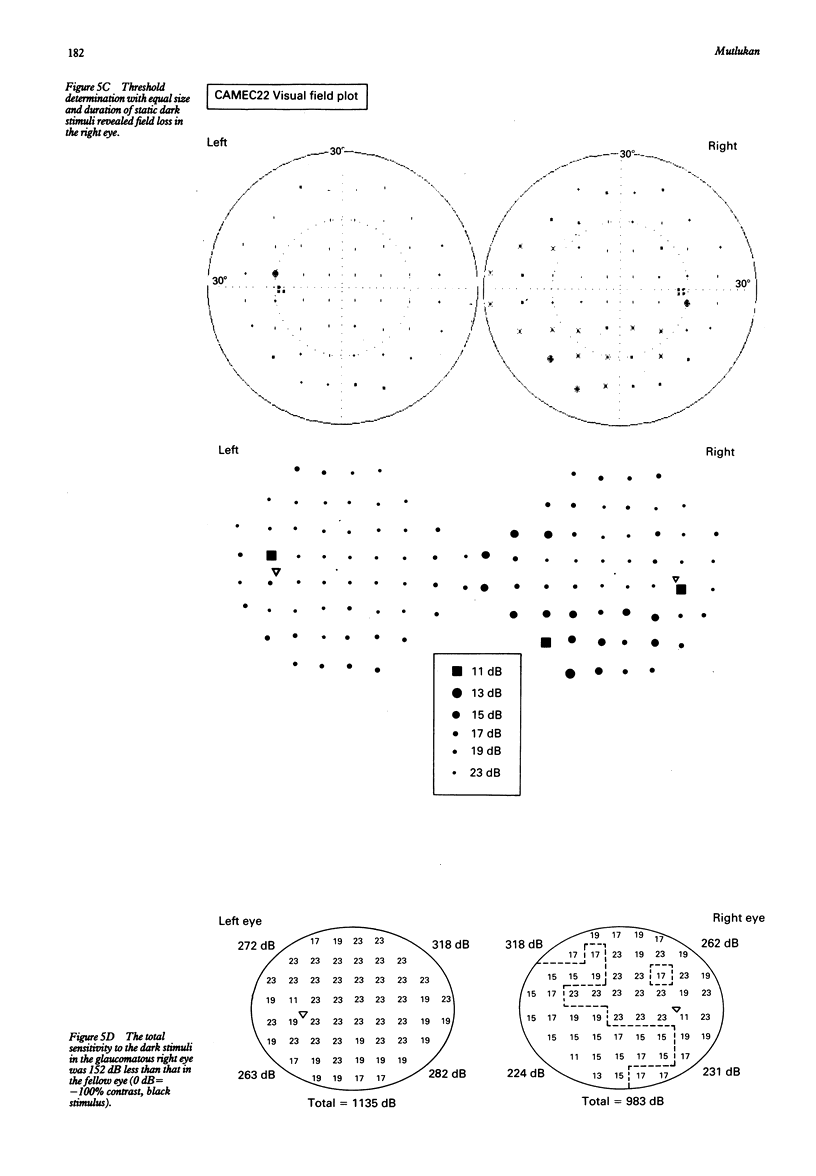
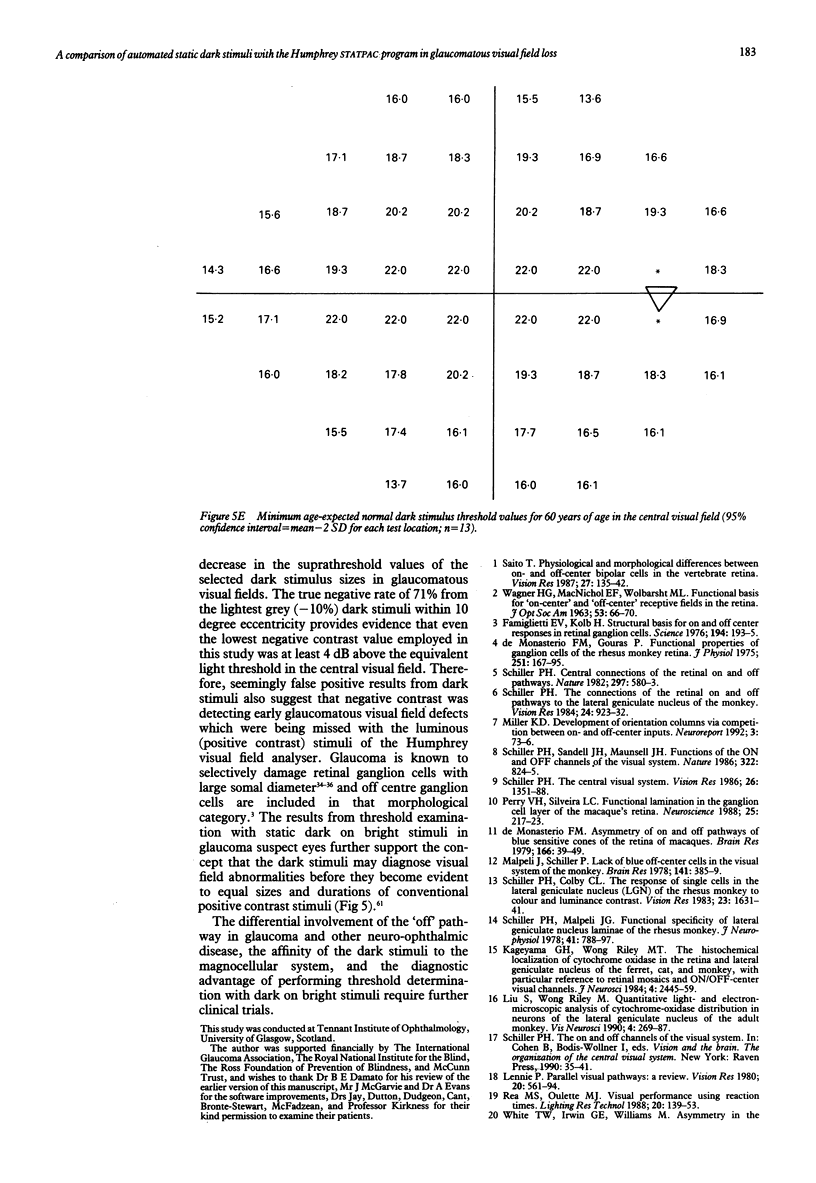
Visual field examination is conventionally performed with bright stimuli on a dark background. Dark stimuli on a bright background, however, may provide different information as light increases and decreases are subject to parallel processing in the visual pathway. Twenty five eyes with primary open angle glaucoma and visual field loss were examined with the Humphrey visual field analyser thresholding program 30-2 and the computer assisted moving eye campimeter (CAMEC) using static dark stimuli at four different Weber contrast levels of -10 (n = 9), -22 (n = 25), -37 (n = 14), and -76% (n = 25) on a cathode ray tube with a background luminance of 10 cd/m2. The cumulative results obtained with STATPAC 'pattern deviation' empirical probability maps and the results from each contrast of the dark stimulus at identical test locations were compared at eccentricity annuli bands of 4-9, 10-20, and 21-28 degrees. Dark stimuli of lower contrast provided higher abnormal point detection rates. Furthermore, visual field defects to the low contrast dark stimuli were more extensive than those to the luminous stimuli. In conclusion, dark stimuli allowed the delineation between glaucomatous field defects and the normal regions in the central visual field.
Full text
PDF
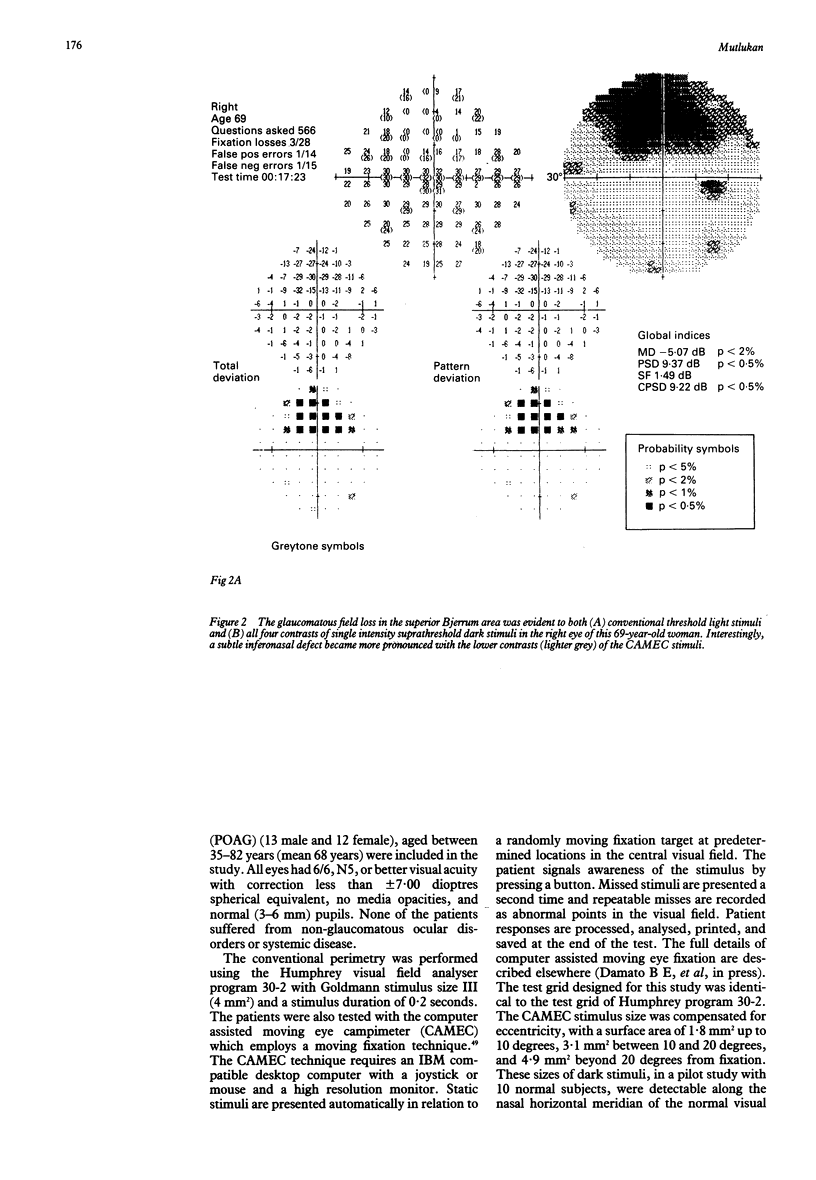
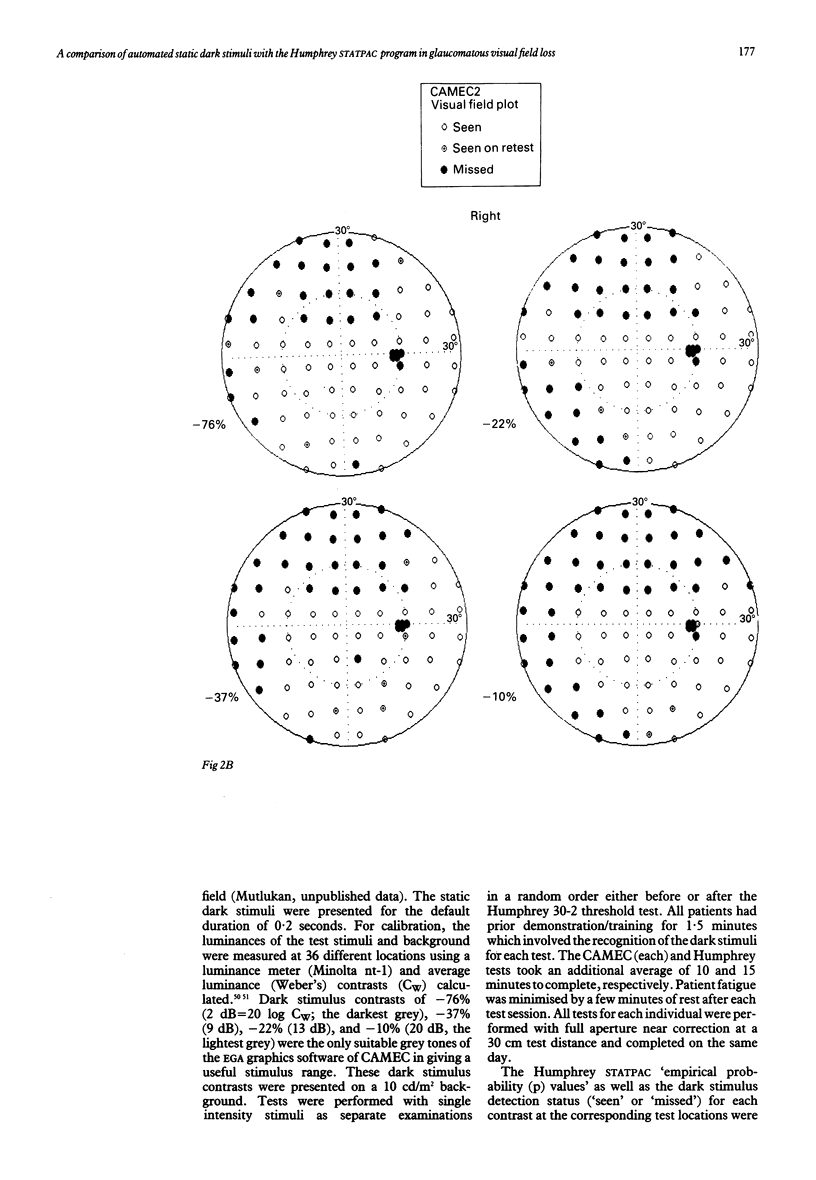
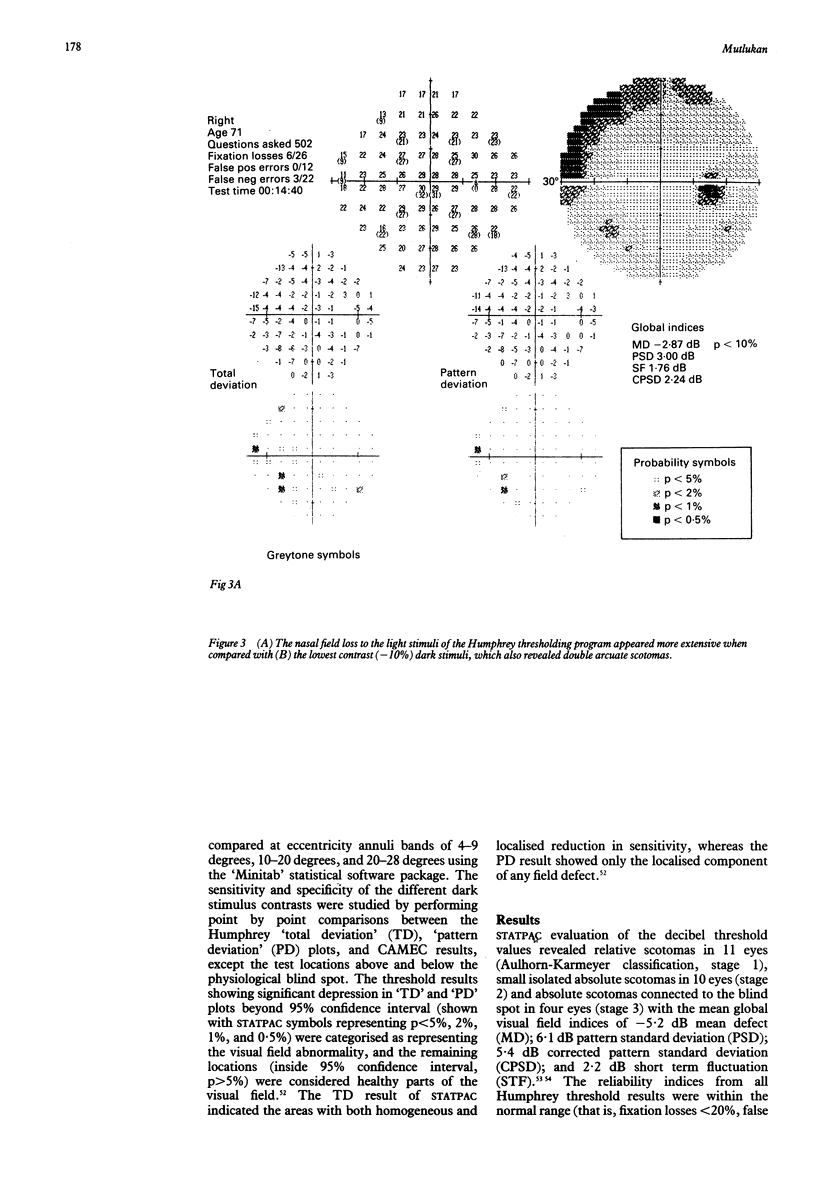
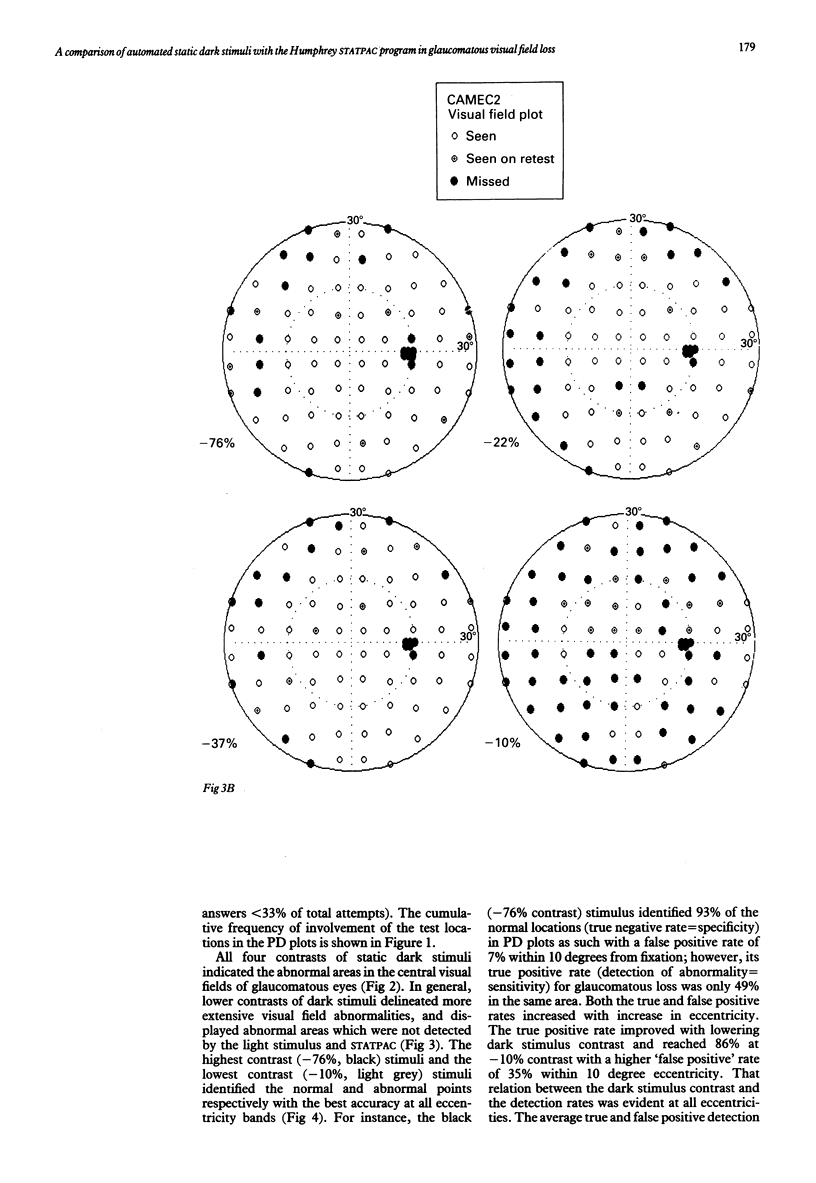

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asai T., Katsumori N., Mizokami K. [Retinal ganglion cell damage in human glaucoma. 2. Studies on damage pattern]. Nippon Ganka Gakkai Zasshi. 1987 Dec;91(12):1204–1213. [PubMed] [Google Scholar]
- Bassi C. J., Lehmkuhle S. Clinical implications of parallel visual pathways. J Am Optom Assoc. 1990 Feb;61(2):98–110. [PubMed] [Google Scholar]
- Brannan J. R., Bodis-Wollner I. Evidence for two systems mediating perceived contrast. Vis Neurosci. 1991 Jun;6(6):587–592. doi: 10.1017/s0952523800002571. [DOI] [PubMed] [Google Scholar]
- Brunsmann J., Brunsmann F., Krastel H. Prototyp eines programmgesteuerten Video-Kampimeters. Fortschr Ophthalmol. 1985;82(6):578–580. [PubMed] [Google Scholar]
- Damato B. E. Oculokinetic perimetry: a simple visual field test for use in the community. Br J Ophthalmol. 1985 Dec;69(12):927–931. doi: 10.1136/bjo.69.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drance S. M. Diffuse visual field loss in open-angle glaucoma. Ophthalmology. 1991 Oct;98(10):1533–1538. doi: 10.1016/s0161-6420(91)32092-x. [DOI] [PubMed] [Google Scholar]
- Elenius V. Rod saturation perimetry. Testing of the cone function with achromatic objects. Arch Ophthalmol. 1985 Apr;103(4):519–523. doi: 10.1001/archopht.1985.01050040061018. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976 Oct 8;194(4261):193–195. doi: 10.1126/science.959847. [DOI] [PubMed] [Google Scholar]
- Flammer J., Drance S. M., Augustiny L., Funkhouser A. Quantification of glaucomatous visual field defects with automated perimetry. Invest Ophthalmol Vis Sci. 1985 Feb;26(2):176–181. [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Color coding in primate retina. Vision Res. 1981;21(11):1591–1598. doi: 10.1016/0042-6989(81)90039-0. [DOI] [PubMed] [Google Scholar]
- Heijl A., Lindgren A., Lindgren G. Test-retest variability in glaucomatous visual fields. Am J Ophthalmol. 1989 Aug 15;108(2):130–135. doi: 10.1016/0002-9394(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Heijl A., Lindgren G., Olsson J., Asman P. Visual field interpretation with empiric probability maps. Arch Ophthalmol. 1989 Feb;107(2):204–208. doi: 10.1001/archopht.1989.01070010210024. [DOI] [PubMed] [Google Scholar]
- Highman V. N. Examination of the central visual field at a reading distance. Br J Ophthalmol. 1968 May;52(5):408–414. doi: 10.1136/bjo.52.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama G. H., Wong-Riley M. T. The histochemical localization of cytochrome oxidase in the retina and lateral geniculate nucleus of the ferret, cat, and monkey, with particular reference to retinal mosaics and ON/OFF-center visual channels. J Neurosci. 1984 Oct;4(10):2445–2459. doi: 10.1523/JNEUROSCI.04-10-02445.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krastel H., Meyer-Josten C., Brunsmann J., Brunsmann F. Hochauflösende Kontrolle des zentralen Gesichtsfeldes mit dem Video-Kampimeter. Fortschr Ophthalmol. 1988;85(6):750–755. [PubMed] [Google Scholar]
- Langerhorst C. T., van den Berg T. J., Greve E. L. Is there general reduction of sensitivity in glaucoma? Int Ophthalmol. 1989 Jan;13(1-2):31–35. doi: 10.1007/BF02028634. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Pokorny J., Smith V. C., Martin P. R., Valberg A. Luminance and chromatic modulation sensitivity of macaque ganglion cells and human observers. J Opt Soc Am A. 1990 Dec;7(12):2223–2236. doi: 10.1364/josaa.7.002223. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Liu S., Wong-Riley M. Quantitative light- and electron-microscopic analysis of cytochrome-oxidase distribution in neurons of the lateral geniculate nucleus of the adult monkey. Vis Neurosci. 1990 Mar;4(3):269–287. doi: 10.1017/s0952523800003400. [DOI] [PubMed] [Google Scholar]
- Malpeli J. G., Schiller P. H. Lack of blue OFF-center cells in the visual system of the monkey. Brain Res. 1978 Feb 10;141(2):385–389. doi: 10.1016/0006-8993(78)90211-1. [DOI] [PubMed] [Google Scholar]
- Merigan W. H. Chromatic and achromatic vision of macaques: role of the P pathway. J Neurosci. 1989 Mar;9(3):776–783. doi: 10.1523/JNEUROSCI.09-03-00776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D. Development of orientation columns via competition between ON- and OFF-center inputs. Neuroreport. 1992 Jan;3(1):73–76. doi: 10.1097/00001756-199201000-00019. [DOI] [PubMed] [Google Scholar]
- Mutlukan E., Bradnam M., Keating D., Damato B. E. Visual evoked cortical potentials from transient dark and bright stimuli. Selective 'on' and 'off-pathway' testing? Doc Ophthalmol. 1992;80(2):171–181. doi: 10.1007/BF00161243. [DOI] [PubMed] [Google Scholar]
- Mutlukan E., Damato B. E. The dark perimetric stimulus. Br J Ophthalmol. 1992 May;76(5):264–267. doi: 10.1136/bjo.76.5.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlukan E. Glaucomatous optic neuropathy causes sensitivity loss to light offsets in the visual field. Neuroreport. 1993 Sep 3;4(10):1159–1162. [PubMed] [Google Scholar]
- Perry V. H., Silveira L. C. Functional lamination in the ganglion cell layer of the macaque's retina. Neuroscience. 1988 Apr;25(1):217–223. doi: 10.1016/0306-4522(88)90020-6. [DOI] [PubMed] [Google Scholar]
- Purpura K., Kaplan E., Shapley R. M. Background light and the contrast gain of primate P and M retinal ganglion cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4534–4537. doi: 10.1073/pnas.85.12.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H. A., Sanchez R. M., Dunkelberger G. R., L'Hernault N. L., Baginski T. A. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):913–920. [PubMed] [Google Scholar]
- Regan D., Milner B. A., Heron J. R. Delayed visual perception and delayed visual evoked potentials in the spinal form of multiple sclerosis and in retrobulbar neuritis. Brain. 1976 Mar;99(1):43–66. doi: 10.1093/brain/99.1.43. [DOI] [PubMed] [Google Scholar]
- Saito T. Physiological and morphological differences between On- and Off-center bipolar cells in the vertebrate retina. Vision Res. 1987;27(2):135–142. doi: 10.1016/0042-6989(87)90176-3. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Colby C. L. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vision Res. 1983;23(12):1631–1641. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Malpeli J. G. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978 May;41(3):788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Sandell J. H., Maunsell J. H. Functions of the ON and OFF channels of the visual system. 1986 Aug 28-Sep 3Nature. 322(6082):824–825. doi: 10.1038/322824a0. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. The central visual system. Vision Res. 1986;26(9):1351–1386. doi: 10.1016/0042-6989(86)90162-8. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. The connections of the retinal on and off pathways to the lateral geniculate nucleus of the monkey. Vision Res. 1984;24(9):923–932. doi: 10.1016/0042-6989(84)90067-1. [DOI] [PubMed] [Google Scholar]
- Shechter S., Hochstein S. On and off pathway contributions to apparent motion perception. Vision Res. 1990;30(8):1189–1204. doi: 10.1016/0042-6989(90)90174-j. [DOI] [PubMed] [Google Scholar]
- Wehrhahn C., Rapf D. ON- and OFF-pathways form separate neural substrates for motion perception: psychophysical evidence. J Neurosci. 1992 Jun;12(6):2247–2250. doi: 10.1523/JNEUROSCI.12-06-02247.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westheimer G. The oscilloscopic view: retinal illuminance and contrast of point and line targets. Vision Res. 1985;25(8):1097–1103. doi: 10.1016/0042-6989(85)90098-7. [DOI] [PubMed] [Google Scholar]
- White T. W., Irvin G. E., Williams M. C. Asymmetry in the brightness and darkness Broca-Sulzer effects. Vision Res. 1980;20(8):723–726. doi: 10.1016/0042-6989(80)90098-x. [DOI] [PubMed] [Google Scholar]
- Zemon V., Gordon J., Welch J. Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Vis Neurosci. 1988;1(1):145–150. doi: 10.1017/s0952523800001085. [DOI] [PubMed] [Google Scholar]
- de Monasterio F. M. Asymmetry of on- and off-pathways of blue-sensitive cones of the retina of macaques. Brain Res. 1979 Apr 20;166(1):39–48. doi: 10.1016/0006-8993(79)90647-4. [DOI] [PubMed] [Google Scholar]



