Abstract
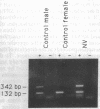
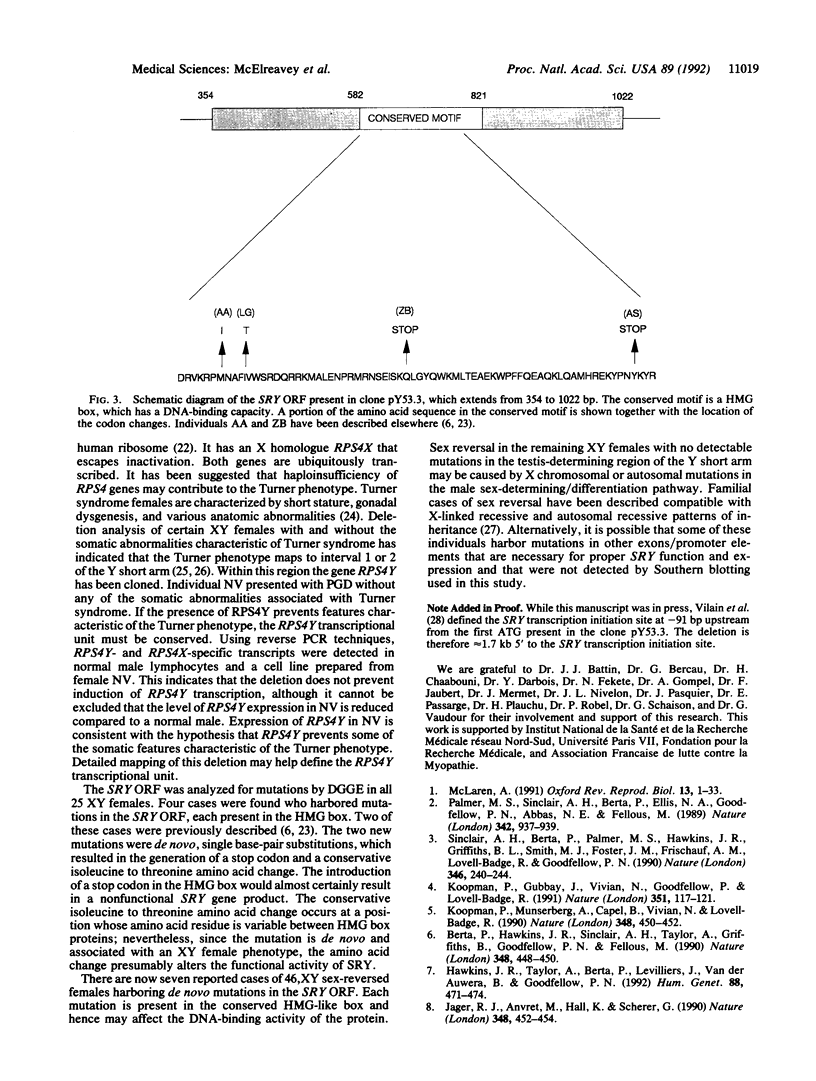
The human testis-determining factor resides within a 35-kilobase (kb) region of the Y chromosome immediately adjacent to the pseudoautosomal boundary. A candidate gene for human sex determination (SRY) was isolated in this region. Here, we describe a study of 25 cases of XY females with pure gonadal dysgenesis for mutations on the Y chromosome short arm, including SRY. Southern blotting revealed a sex-reversed female harboring a deletion extending from approximately 8 kb from the pseudoautosomal boundary of the Y chromosome to at least 33 kb and no more than 60 kb upstream, toward the centromere. The deletion begins no more than 1.8 kb upstream from the first ATG of the SRY open reading frame present in the clone pY53.3. To our knowledge, no mutation has been described previously outside the SRY "HMG box" on the short arm of the Y chromosome, which is associated with sex reversal. Since the 5' extent of the SRY transcriptional unit has not been defined, the deletion may remove upstream exons of SRY and/or transcriptional regulatory motifs, either situation resulting in lack of testicular development. It cannot be formally excluded that the mutation removes a second locus, independent of SRY, that is critical for sex determination. Denaturant gradient gel electrophoresis analysis of the SRY open reading frame in the remaining 24 cases revealed de novo single base-pair transitions in the SRY conserved domain in 4 cases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Ferguson-Smith M. A., Tolmie J., Kwok K., Mitchell M., Jamieson D., Cooke A., Florentin L. Variable transfer of Y-specific sequences in XX males. Nucleic Acids Res. 1986 Jul 11;14(13):5375–5387. doi: 10.1093/nar/14.13.5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attree O., Vidaud D., Vidaud M., Amselem S., Lavergne J. M., Goossens M. Mutations in the catalytic domain of human coagulation factor IX: rapid characterization by direct genomic sequencing of DNA fragments displaying an altered melting behavior. Genomics. 1989 Apr;4(3):266–272. doi: 10.1016/0888-7543(89)90330-3. [DOI] [PubMed] [Google Scholar]
- Berta P., Hawkins J. R., Sinclair A. H., Taylor A., Griffiths B. L., Goodfellow P. N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990 Nov 29;348(6300):448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Disteche C. M., Casanova M., Saal H., Friedman C., Sybert V., Graham J., Thuline H., Page D. C., Fellous M. Small deletions of the short arm of the Y chromosome in 46,XY females. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7841–7844. doi: 10.1073/pnas.83.20.7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E. M., Beer-Romero P., Brown L. G., Ridley A., McNeil J. A., Lawrence J. B., Willard H. F., Bieber F. R., Page D. C. Homologous ribosomal protein genes on the human X and Y chromosomes: escape from X inactivation and possible implications for Turner syndrome. Cell. 1990 Dec 21;63(6):1205–1218. doi: 10.1016/0092-8674(90)90416-c. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Hawkins J. R., Taylor A., Berta P., Levilliers J., Van der Auwera B., Goodfellow P. N. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet. 1992 Feb;88(4):471–474. doi: 10.1007/BF00215684. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Individual-specific 'fingerprints' of human DNA. Nature. 1985 Jul 4;316(6023):76–79. doi: 10.1038/316076a0. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991 May 9;351(6322):117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Koopman P., Münsterberg A., Capel B., Vivian N., Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990 Nov 29;348(6300):450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Magenis R. E., Tochen M. L., Holahan K. P., Carey T., Allen L., Brown M. G. Turner syndrome resulting from partial deletion of Y chromosome short arm: localization of male determinants. J Pediatr. 1984 Dec;105(6):916–919. doi: 10.1016/s0022-3476(84)80077-3. [DOI] [PubMed] [Google Scholar]
- McElreavey K. D., Vilain E., Boucekkine C., Vidaud M., Jaubert F., Richaud F., Fellous M. XY sex reversal associated with a nonsense mutation in SRY. Genomics. 1992 Jul;13(3):838–840. doi: 10.1016/0888-7543(92)90164-n. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Noonan K. E., Roninson I. B. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page D. C., Mosher R., Simpson E. M., Fisher E. M., Mardon G., Pollack J., McGillivray B., de la Chapelle A., Brown L. G. The sex-determining region of the human Y chromosome encodes a finger protein. Cell. 1987 Dec 24;51(6):1091–1104. doi: 10.1016/0092-8674(87)90595-2. [DOI] [PubMed] [Google Scholar]
- Palmer M. S., Sinclair A. H., Berta P., Ellis N. A., Goodfellow P. N., Abbas N. E., Fellous M. Genetic evidence that ZFY is not the testis-determining factor. Nature. 1989 Dec 21;342(6252):937–939. doi: 10.1038/342937a0. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Goodfellow P. N., Smith K. D. Report of the committee on the genetic constitution of the Y chromosome. Cytogenet Cell Genet. 1989;51(1-4):438–449. doi: 10.1159/000132802. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A. The Y-chromosomal and autosomal testis-determining genes. Development. 1987;101 (Suppl):33–38. [PubMed] [Google Scholar]