Abstract
Background. Pediatric Kawasaki disease (KD) and human immunodeficiency virus (HIV)+ adult Kawasaki-like syndrome (KLS) are dramatic vasculitides with similar physical findings. Both syndromes include unusual arterial histopathology with immunoglobulin (Ig)A+ plasma cells, and both impressively respond to pooled Ig therapy. Their distinctive presentations, histopathology, and therapeutic response suggest a common etiology. Because blood is in immediate contact with inflamed arteries, we investigated whether KD and KLS share an inflammatory signature in serum.
Methods. A custom multiplex enzyme-linked immunosorbent assay (ELISA) defined the serum cytokine milieu in 2 adults with KLS during acute and convalescent phases, with asymptomatic HIV+ subjects not taking antiretroviral therapy serving as controls. We then prospectively collected serum and plasma samples from children hospitalized with KD, unrelated febrile illnesses, and noninfectious conditions, analyzing them with a custom multiplex ELISA based on the KLS data.
Results. Patients with KLS and KD subjects shared an inflammatory signature including acute-phase reactants reflecting tumor necrosis factor (TNF)-α biologic activity (soluble TNF receptor I/II) and endothelial/smooth muscle chemokines Ccl1 (Th2), Ccl2 (vascular inflammation), and Cxcl11 (plasma cell recruitment). Ccl1 was specifically elevated in KD versus febrile controls, suggesting a unique relationship between Ccl1 and KD/KLS pathogenesis.
Conclusions. This study defines a KD/KLS inflammatory signature mirroring a dysfunctional response likely to a common etiologic agent. The KD/KLS inflammatory signature based on elevated acute-phase reactants and specific endothelial/smooth muscle chemokines was able to identify KD subjects versus febrile controls, and it may serve as a practicable diagnostic test for KD.
Keywords: Kawasaki disease, Kawasaki-like syndrome, KD, KLS
Kawasaki disease (KD) is diagnosed based on a constellation of findings including fever of at least 5 days plus at least 4 of the following 5: nonexudative conjunctivitis, rash, changes of the oropharynx, erythema and/or painful swelling of hands and feet, and sentinel lymph node. Kawasaki disease has a predilection for coronary arteries, causing aneurysms with residual risk for cardiovascular morbidity or mortality [1]. Intravenous immunoglobulin (IVIG) reduces aneurysm formation from 20% to 4% [2, 3]. Coronary arteries in fatal KD show a mixed inflammatory pattern that includes immunoglobulin (Ig)A+ plasma cells [4, 5]. Immunoglobulin A+ plasma cell infiltration occurs in vascular and nonvascular tissues, which suggests an infectious agent invading via respiratory tract or gut mucosa [6]. Periodic outbreaks suggest a ubiquitous infectious etiology [7, 8] with an atypical presentation in susceptible individuals. Diagnosing KD is problematic because children with atypical presentations may not receive IVIG but can develop aneurysms and sudden death [9].
In the 1980s, an adult syndrome resembling KD, termed Kawasaki-like syndrome (KLS), came to medical attention in human immunodeficiency virus (HIV)+ adults with advanced disease [10]. Patients with KLS are treated with IVIG like KD patients, and they have similar responses (see reviews in Johnson et al [11] and Stankovic et al [12]). In a setting of limited evaluations, no aneurysms have been documented in KLS; however, aneurysms occur in HIV-seronegative adults with KD [13, 14]. In 2003, a KLS conjunctival biopsy showed IgA+ plasma cells infiltrating arterial walls, linking KLS to KD by histopathology. The same study showed elevated soluble tumor necrosis factor receptor (sTNFR)II levels, suggesting a role for TNF-α in pathophysiology [15].
Understanding pathophysiology may improve KD/KLS diagnosis and treatment. Inflammatory responses can be categorized by cytokine patterns of activated CD4 T cells. In broad terms, responses to intracellular pathogens (eg, viruses) feature interferon gamma ([IFN-γ] labeled Th1) to (1) parasites and allergens interleukin (IL)-4 and IL-13 (Th2) and to (2) some bacterial infections and autoimmune diseases IL-17 (Th17) (reviewed in [16]). Chemokines recruit specific cell types to inflamed tissues, they are cell lineage specific, and they are differentially regulated in Th1/Th2 cytokine milieus (reviewed in [17]). We used this immunobiology as the framework to design (1) a multiplex ELISA investigation of cytokine milieu in 2 KLS cases and (2) a subsequent KD investigation that allowed us to compare the KD and KLS inflammatory signatures.
Case 1
Patient 1 is 23-year-old male with fevers, rash, strawberry tongue, painful swelling of hands and feet, and nonexudative conjunctivitis, preceded by diarrhea and abdominal pain (Figure 1). Admission evaluation revealed HIV/acquired immune deficiency syndrome (CD4 = 3; viral load 180 000 copies/mL). On hospital day 9, the patient had persistent fevers (103.9°F), and developed severe hypotension (maximum fluid resuscitation and pressors) in spite of empiric antimicrobials plus hydrocortisone; the workup for infectious etiologies was entirely negative (Supplementary Table 1). In the intensive care unit, he was diagnosed with KLS and treated with IVIG without aspirin due to thrombocytopenia, with a rapid response including fever resolution and pressor discontinuation within 24 hours. Without deterioration, fevers relapsed 4 days after IVIG no. 1, which led to a second infusion (IVIG no. 2). The relapsed fever only resolved with subsequent initiation of aspirin therapy enabled by improved platelet counts. With permission from the patient, serum was collected before IVIG no. 1 and 13 weeks postdischarge.
Figure 1.
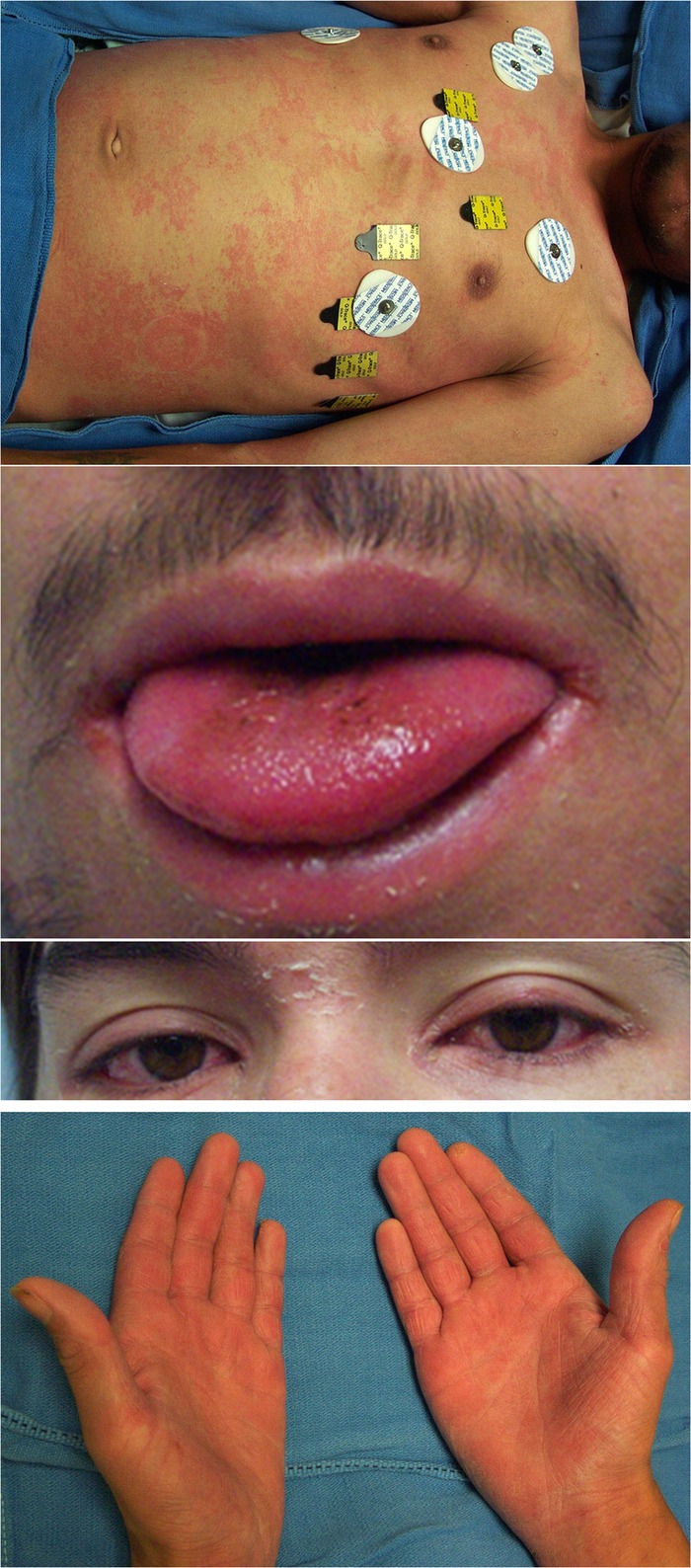
Physical findings in patient no. 1. Sequential panels showing rash, changes of the oropharynx, nonexudative conjunctivitis, and erythema with mild swelling of hands.
Case 2
Patient 2 is a 31-year-old HIV+ male (CD4 = 19, viral load 139 000 copies/mL) with idiopathic eosinophilia who presented with 10 days of fever (102.7°F), myalgia, mild abdominal pain and diarrhea, painful swollen hands and feet. Findings included nonexudative conjunctivitis, cracked lips, mild thrush, cervical lymphadenopathy, impressive painful swelling of hands and feet, and rash. On hospital day 5, the patient remained febrile (103°F) but testing was negative (Supplementary Table 1). He was treated with IVIG and aspirin, defervesced during the infusion, and felt remarkably better. He was discharged the following day on previously prescribed antiretroviral medication plus aspirin. He returned to clinic at 2 and 5 weeks, and he appeared well on both visits. With permission serum was collected before IVIG and 5 weeks postdischarge.
Both patients had desquamation involving the hands at approximately 2 weeks, as typically seen in KD. Neither patient had a recurrence in the following 5 years; detailed clinical summaries are included in the Supplementary Data.
METHODS
Kawasaki-Like Syndrome Investigation
A case-control study was performed with approval from the Indiana University Institutional Review Board (IRB). A custom multiplex ELISA with analytes representing cytokine polarization, tissue/cell-type specific inflammation, and/or KD/KLS association was designed (Supplementary Table 2). Analyte levels in acute and convalescent KLS serum were compared with levels in asymptomatic HIV+ male serum (CD4 > 350 not taking combination antiretroviral therapy) leftover from an unrelated study [18]. Serum from a healthy female was included to preliminarily identify analytes abnormally low in HIV+ individuals. A commercial laboratory routinely servicing large clinical trials ([19, 20]) (Aushon Biosystems, Woburn, MA) performed multiplex ELISA on blinded samples. Samples 1 and 2 comprised acute/convalescent sera from patient 1; samples 3 and 4 comprised acute/convalescent sera from patient 2; samples 5–7 comprised HIV controls; and sample 8 comprised a seronegative female (dataset in Supplementary Table 3). An in-house ELISA was performed to quantify IFN-γ (human IFN-γ ELISA kit; Pierce-Endogen, Rockford, IL).
Kawasaki Disease Study
Based on our KLS investigation, we hypothesized that IL-6, IL-13, and sTNFRI/II along with chemokines Ccl1, Ccl2, and Cxcl11 would be elevated in KD and that elevated sTNFRI/II plus Ccl1, Ccl2, and Cxcl11 would selectively identify KD cases. A custom multiplex ELISA was designed for the KD study including IL-1β (anticipated negative acute-phase reactant), IL-4 (Th2), IL-13 (Th2), Ccl1 (Th2 chemokine), Ccl2 (vascular inflammation), Ccl11 (Th2 chemokine), Ccl4 (anticipated negative Th1 chemokine), Cxcl9 (neutrophil recruitment), and Cxcl11 (plasma cell recruitment). In the context of a small study, paired serum and plasma samples (pseudo-duplicates) were obtained to address outliers and determine optimal sample type. Blood sampling was done before IVIG for KD subjects.
The prospective KD study was performed between September 2014 and July 2015. Children >6 months and <8 years were enrolled into 3 groups: (1) KD, (2) febrile illness (febrile controls [FC]), and (3) noninfectious illness (healthy controls [HC]). Inclusion criteria included formal KD diagnosis (KD), fever >38.2°C during evaluation, a non-KD working diagnosis without rash (FC), and admission for noninfectious condition (HC). Exclusion criteria included known HIV+ status or genetic disorder (all) and absence of a working diagnosis or presence of rash (FC). Serum and plasma datasets are in Supplementary Tables 5 and 6. The IRBs at Indiana University Riley Children's Hospital and Children's Hospitals and Clinics of Minnesota approved this study.
Statistical Analysis
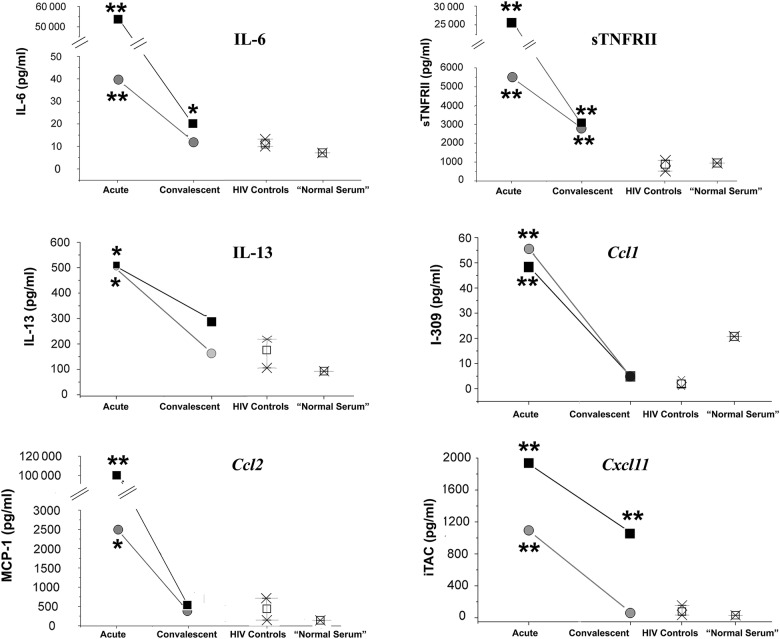
For KLS, data were analyzed as individual comparisons of patient 1 and 2 acute and convalescent values to the combined analyte data from 3 HIV+ control subjects with a Student's t test. “Normal serum” was not included in analyses. For within-study comparisons, P values of <.05 were considered significant. Power analyses to guide follow-up studies were performed, and these results are included in the legend of Figure 3.
Figure 3.
Analytes elevated in Kawasaki-like syndrome (KLS) patients in the acute phase that, during the convalescent phase, return to or toward levels seen in asymptomatic human immunodeficiency virus (HIV)+ control subjects. Patient 1 (severe KLS), black squares; patient 2 (typical KLS), gray circles. For chemokines, the common nomenclature is used on the y-axis; the graph body is labeled with the systematic name. Power analyses of these pilot data suggest a confirmatory sample size as small as 3 controls and 3 cases for interleukin (IL)-6, tumor necrosis factor receptor (TNFRII), Ccl1, Ccl2, and Cxcl11; 6 controls and 3 cases for IL-13. *P value <.05; **P value <.01. Abbreviations: MCP, monocyte chemoattractant protein; sTNFRII, soluble TNFRII.
Based on our KLS data and the literature [15, 21], we postulated that KD would be inextricably linked to a marked elevation in sTNFRI/II or IL-6 and that elevations in Ccl1, Ccl2, and w Cxcl11 would identify KD versus FC. Elevations in sTNFRIl/II were confirmed during preliminary examination of data. One-way analysis of variance with Tukey post hoc tests and receiver operator curve (ROC) analyses were performed on sTNFRI/II and IL-6 to determine optimal cutoffs. An sTNFRII cutoff of >1900 pg/mL eliminated all HC and 3 FC, step 1 of a 2-step algorithm. We then performed t tests with Welch correction and ROC analyses on subjects with sTNFRII levels >1900 pg/mL to determine whether remaining analytes were uniquely elevated in KD versus FC. For pathogenesis-specific chemokines Ccl1 (P value = .036), Ccl2 (P value = .024), and Cxcl11 (P value = .13) identified in our KLS study as possible “KD predictors”, a ROC analysis was performed to find optimal cutoffs; Ccl1 (>3.55 pg/mL), Ccl2 (>715 pg/mL), and Cxcl11 (>39.4 pg/mL). Educated by samples 12 (KD) and 26 (FC) with similar measurements (Table 1), we found that at least 2 KD predictors were needed to appropriately identify these subjects; a comprehensive statistical analysis description is in Supplementary Data.
Table 1.
Testing the Kawasaki Disease Algorithm
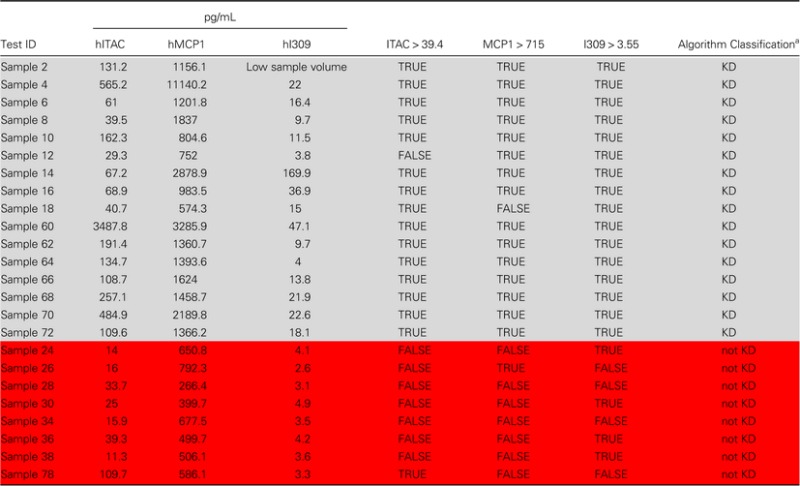 |
Samples highlighted in light gray are KD subjects; samples highlighted in red are FC subjects with sTNFRII values >1900 pg/mL.
Abbreviations: I309, inflammatory cytokine 309; ITAC, interferon-inducible T-cell chemokine; MCP, monocyte chemoattractant protein; KD, Kawasaki Disease.
a>2 true equals a diagnosis of KD.
RESULTS
Kawasaki-Like Syndrome Study
Kawasaki-like syndrome results can be grouped into 3 categories. In the first category, analytes not elevated in KLS were compared with HIV+ controls. This category includes IL-17 and Ccl5 (Supplementary Figure 1) and IL-1β (below limit of detection).
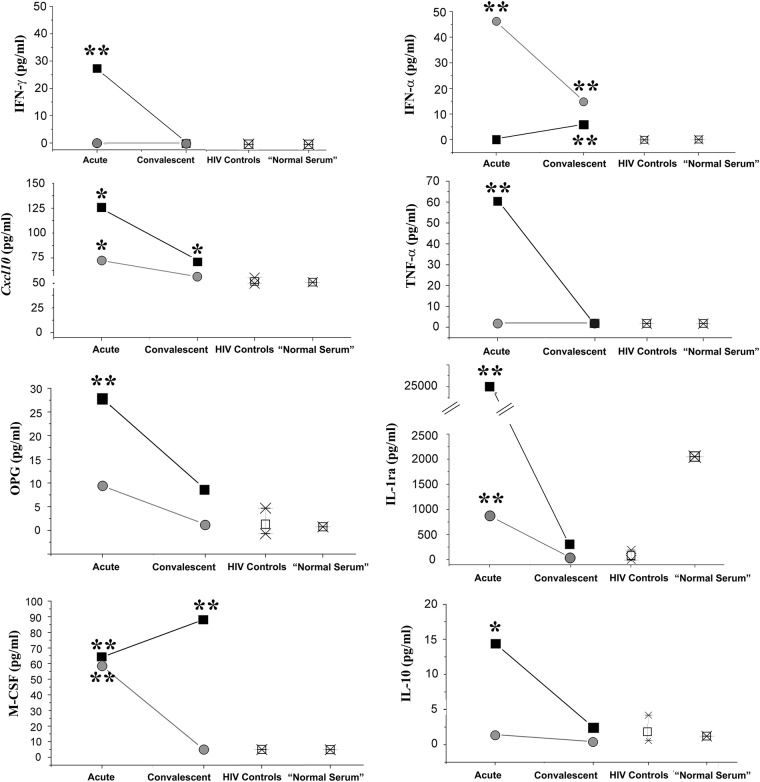
The second category includes analytes related to KLS severity. Of these, IFN-γ, Cxcl10, osteoprotegerin, TNF-α, IL-1ra, and IL-10 were elevated only in patient 1 with shock (Figure 2). Although the results were statistically significant, Cxcl10 is included in this category because the level in patient 2 was only 30% higher than HIV+ controls; IL-1ra was included here because the “elevated” level in patient 2 was lower than the level found in normal serum. Macrophage colony-stimulating factor was elevated in typical KLS and persistently elevated in severe KLS. Interferon-α (Figure 2, top right panel) was elevated in typical KLS and absent in KLS shock (KLSS). Although conclusions cannot be drawn based on 2 patients, an inadequate IFN-α response may be a marker for severe disease.
Figure 2.
Analytes reflecting severity of Kawasaki-like syndrome (KLS). Patient 1 (severe KLS shock), black squares; patient 2 (typical KLS), gray circles. The control human immunodeficiency virus (HIV) subjects' mean (open square) and range of analyte values are indicated in the third column. The level of analyte in a single HIV-negative “normal serum” is shown as a square in the final column. For interleukin (IL)-1ra, note (1) the break in the scale and (2) that the level of IL-1ra in HIV-negative normal serum is higher than in HIV+ control subjects. Interferon (IFN)-γ and tumor necrosis factor (TNF)-α for the HIV+ controls and normal serum were below the limit of detection and were plotted as “0”. *P value <.05; **P value <.01. OPG, osteoprotegrin.
The third (interesting) category includes analytes elevated in acute KLS that, during the convalescent phase, decreased to or toward levels seen in HIV+ controls. The category includes IL-6, sTNFRII, IL-13, Ccl1, Ccl2, and Cxcl11 (Figure 3). As previously reported in KD [22, 23], IL-6 and Ccl2 were elevated in KLS. As previously reported in KLS [15], sTNFRII was markedly elevated in both KLS patients. Particularly interesting were elevations in IL-13 (Th2), and chemokines Ccl1 (Th2), and Cxcl11, a plausible link to pathognomonic IgA+ plasma cell infiltration of arterial walls in KD/KLS. Supplementary Table 4 summarizes the KLS results versus published data for KD and non-KD illnesses.
Kawasaki Disease Study Results
Sixteen KD, 13 FC, and 10 HC subjects were enrolled. Two FC were excluded before analysis: subject-2101 was 9 years old (too old), and subject-2102 had a prominent rash of unknown etiology (a predefined exclusion criterion). Sixteen KD (1 with aneurysms, 1 incomplete, 1 IVIG-resistant), 11 FC, and 10 HC were included in the final analyses; demographics and clinical findings are in Supplementary Tables 7–9.
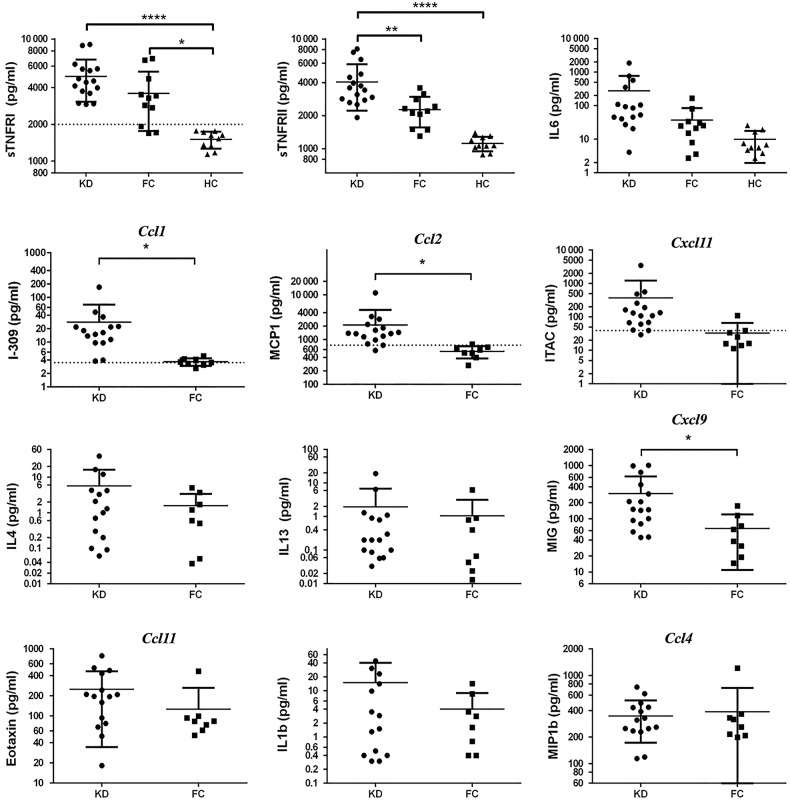
Multiplex ELISA testing (Figure 4) showed that KD universally included marked elevations in acute-phase reactants IL-6 or TNF-α surrogates TNFRI/II but usually in both. There were significant differences or trends in KD versus FC in KLS-implicated pathogenesis-chemokines Ccl1, Ccl2, and Cxcl11. Cxcl11 did not reach significance (P =.13) due to a high level (3488 pg/mL) in KD subject-1101. Subject-1101 was arguably the sickest subject with an echocardiography left anterior descending z-score of +2.69, marked perivascular brightness around the coronaries, and the highest C-reactive protein (CRP) in the study at 34.4 mg/dL (see Supplementary Table 8). The markedly elevated Cxcl11 may have reflected the severity of the vasculitis. The high Cxcl11 level for subject-1101 did not appear to be erroneous because it was similarly elevated in paired-plasma (pseudo-duplicate as per study design). The KD Cxcl11 mean without subject-1101 was 163 pg/mL. With subject-1101 absent, the Cxcl11 P value based on 15 KD subjects was .008. The acute-phase reactant IL-1β and the Th1 chemokine Ccl4 were not specifically elevated in KD nor was the Th2 chemokine Ccl11. Elevated IL-13 documented in KLS was not seen in KD nor was previously reported elevation in Th2 cytokine IL-4. Cxcl9 was elevated in KD, but ROC analysis showed that Cxcl9 did not separate KD from FC. Before the KD study, we hypothesized that elevated sTNFRI/II combined with elevated pathogenesis-chemokines Ccl1, Ccl2, and Cxcl11 would be a diagnostic test for KD. Based on that hypothesis, we tested a 2-step algorithm. Using a TNFRII level of >1900 pg/mL as the cutoff for considering a KD diagnosis, secondary ROC analysis showed that elevations in 2 or more pathogenesis-chemokines (Ccl1, Ccl2, Cxcl11) accurately identified all KD versus FC (Figure 5; Table 1).
Figure 4.
Results of Kawasaki disease (KD) study. Top row shows acute-phase reactants: soluble tumor necrosis factor receptors (sTNFR) and interleukin (IL)-6. Row 2 illustrates pathogenesis-specific chemokines. Rows 3 and 4 show analytes related to polarization of the immune response. For chemokines only, common nomenclature is used on the y-axis and the graph body is labeled with the systematic name. Note that data are plotted on log scale. *P value <.05; **P value < .01; ****P value < .0001. Abbreviations: ITAC, interferon-inducible T-cell chemokine; MCP, monocyte chemoattractant protein; MIG, monocyte interferon-γ-inducible protein; MIP, macrophage inflammatory protein.
Figure 5.
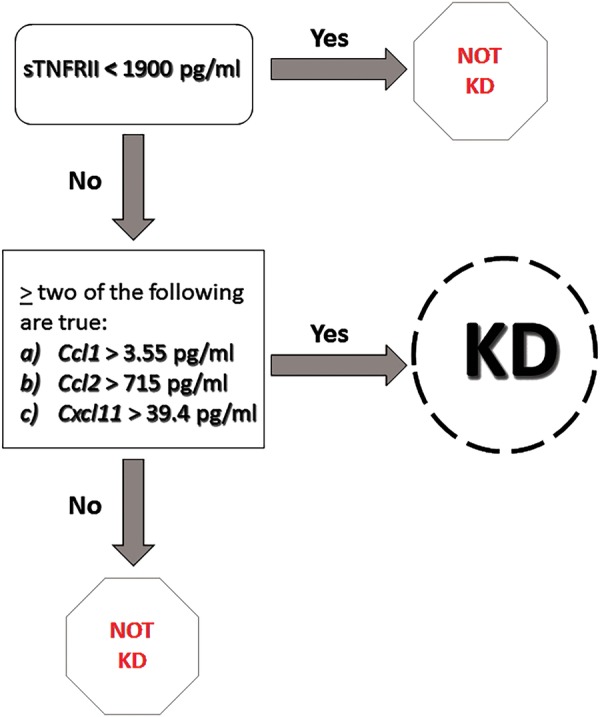
Two-step Kawasaki disease (KD) diagnostic algorithm. Abbreviation: sTNFR, soluble tumor necrosis factor receptor.
DISCUSSION
Our investigation shows that KD and KLS share an inflammatory signature including a robust acute-phase response (sTNFRI/II) and elevations in pathogenesis-specific chemokines Ccl1, Ccl2, and likely Cxcl11. We posit that KD and KLS are the same disease based on inflammatory signatures, combined with unusual arterial histopathology (IgA+ plasma cells), similar physical findings, and rapid clinical responses to IVIG. The KD/KLS inflammatory signature is compatible with an infectious agent, perhaps the ribonucleic acid (RNA) virus-like particles visualized in pathology specimens using reverse-engineered KD IgA antibodies [24, 25].
This is the first reported case of KLSS. It manifested similar to KD shock syndrome ([26–28]). The mechanism appears to be a cytokine storm with high TNF-α and IL-6 and an ineffective counter-inflammatory response (IL-10/IL-1ra). This case demonstrates that KLS is potentially fatal and that IVIG should be administered beyond the 10-day febrile cutoff used in pediatrics.
Our investigation of 2 KLS patients at clinical extremes suggests that IFN-α is important for host protection in KD/KLS. The typical KLS patient (Case 2) had elevated IFN-α (46 ρg/mL) with undetectable IFN-γ; the KLSS patient had elevated IFN-γ (27 ρg/mL) with undetectable IFN-α, a starkly inverted pattern. For comparison, IFN-γ serum levels during viral illnesses run 8–30 ρg/mL [29–31]; IFN-α levels in children with influenza had a mean of 602 ρg/mL [32]. Kawasaki disease investigators have documented low to undetectable serum IFN-α levels [33–35]; however, the KD coronary artery transcriptome shows a prominent type I IFN molecular fingerprint; ie, evidence for local IFN activity during KD arteritis [36]. If KD susceptibility is determined by suboptimal innate IFN-α responses, then low IFN-α levels do not argue against its role in protection or against an RNA virus-like etiologic agent.
Our KD results are consistent with published data. We found Cxcl9 elevated in KD, consistent with a recent serum study [37] and the coronary artery transcriptome [36]. Soluble TNFRI/II (TNF-α activity) within the inflammatory signature has been individually reported by others [15, 21] and seems to play a central role in pathogenesis. The mechanism-specific chemokines Ccl2, Cxcl11, and Ccl1 investigated in this study are the unique aspect of the KD/KLS inflammatory signature.
Ccl2 (endothelial/smooth muscle cells) is a biomarker for myocardial infarction risk [38] and was previously shown to be elevated in KD [39, 40]. In this study, we identify Cxcl11 as a likely new KD-associated chemokine (unadjusted P value of .13; adjusted P value of .008). Its receptor Cxcr3 is present and functional on human IgA+ plasma cells [41], consistent with recruitment into arterial walls. Elevated Cxcl11 is associated with human aortic aneurysms, and Cxcr3 is required for aneurysm formation in mice [42]. Engagement of Cxcr3 by Cxcl9, Cxcl10, and Cxcl11 results in its removal from the cell surface. Children with KD have reduced Cxcr3 on circulating T cells attributed to Cxcl10 [43]; Cxcl11 is a more potent Cxcr3 internalization trigger than Cxcl10 [44–46]. Cxcr3 inhibitors, potentially KD therapeutics, have entered clinical trials for other purposes [47, 48].
Ccl1 was uniquely elevated in KD in this 39-subject study. Ccl1 is produced by human endothelial cells exposed to atherogenic apolipoprotein A. The Ccl1 receptor Ccr8 is found on Th2 cells [49]; however, Ccr8 is also expressed by human vascular smooth muscle cells. In a mouse model, femoral artery luminal trauma induced Ccl1 production by smooth muscle cells, suggesting that Ccl1/Ccr8 is involved in vascular injury repair [50]. Ccl1 seems to be intimately and uniquely tied to KD/KLS-triggered vascular injury.
The Th1/Th2/Th17 polarization paradigm has been used to interpret KD pathogenesis. Interferon-γ, the prototype Th1 cytokine, is not typically elevated in KD [35, 51]; children with elevated IFN-γ are at higher risk of aneurysms [34]. In this report, the adult HIV+ patient with detectable IFN-γ went into shock. In contrast, IL-4, the prototypic Th2 cytokine, has previously been reported elevated in children with KD [35], and the Th2 chemokine Ccl17 recently shown elevated in KD [37]. Idiopathic eosinophilia (Th2 phenomenon) is seen in KD [52–54] and in non-HIV adult KD [14]. KLS patient 2 with typical KLS had idiopathic eosinophilia, a recognized condition in advanced HIV disease [55, 56]. We investigated the Th2 chemokine Ccl11 (regulated by IL-4), which was not elevated. The Th1 chemokines elevated in KD (Cxcl9, Cxcl10, Cxc11, Ccl2) would recruit Th1 cells as available, but those chemokine levels are regulated by IFNs (including IFN-α) and/or TNF-α unrelated to cytokine polarization. We investigated Th1 chemokine Ccl4 produced by activated cells of several types, which was not elevated. We found that IL-13, a quintessential Th2 cytokine, was elevated in both KLS patients. The IL-13 levels in KD children were low, and differences between KD and FC were not significant, perhaps reflecting the difference between reactivation/recall in KLS versus a primary response in KD. We and others [57–59] have shown that CD8 T cells produce IL-13. CD8 T cells predominate in acutely inflamed KD coronary arteries [60]. It is unclear whether the CD4-based Th1/Th2/Th17 paradigm is a good fit for KD/KLS pathogenesis.
Our results suggest a KD/KLS diagnostic test based on elevated sTNFRI/II combined with elevations in pathogenesis-specific chemokines Ccl1, Ccl2, and Cxcl11. A previously published KD diagnostic algorithm based on 12 conventional laboratory parameters (CRP, total white blood cell count, etc) had a sensitivity of 74.3% and a false-positive KD diagnosis rate of 37.2% for FC [61]. An improved version of this algorithm was published during the revision process for this manuscript [62]. Kawasaki disease pathophysiology has been investigated using advanced transcriptomics, proteomics, and multiplex ELISA. Urine proteomics identified KD biomarkers including meprin A [63], and peripheral blood mononuclear cells (PBMCs) transcriptomics identified KD transcripts, including 130 differing between KD and adenovirus-infected children [64–66]. The analytes in our inflammatory signature are not among the 130 transcripts. It is not clear whether urine protein or circulating PBMC mRNA optimally reflect vascular wall inflammation. Ko et al [43] recently used commercial multiplex ELISA arrays to investigate KD versus FC and demonstrated that Cxcl10 was differentially elevated in KD. Our custom 12-plex KD ELISA contained 3 analytes investigated in that study with the same results: there was no difference in IL-1β and insignificant trends toward elevated IL-4 and IL-6 in KD versus FC.
CONCLUSIONS
Kawasaki disease and KLS are likely the same disease, based on a shared inflammatory signature. Specific signature components, elevations in sTNFRII, and at least 2 of 3 pathogenesis-associated chemokines (Ccl1, Ccl2, and Cxcl11), were able to identify KD subjects versus FC and healthy children as diagnosed by experienced clinicians at 2 major pediatric tertiary referral hospitals. This was not a definitive study of the KD inflammatory signature's utility in general practice. Children with febrile illness and rash were specifically excluded because incomplete KD is a difficult diagnosis, with existing diagnostic criteria based solely on expert opinion [67]. Inclusion of febrile illnesses with rash as controls (eg, viral syndrome) in this initial investigation would have required study-dictated diagnostic viral testing and echocardiography to ensure that cases of incomplete KD did not get assigned to the febrile control group. Follow-up studies will include controls with defined febrile rashes (eg, adenoviral infections), and an investigation to determine whether KD diagnoses can be made as early as day 3 of fever in the setting of at least 1 of the following: nonexudative conjunctivitis, rash, painful swelling, or erythema of hands or feet. Results of this study also suggest that investigations of type 1 IFNs, Cxcl11/Cxcr3, and Ccl1/Ccr8 will provide new insights into KD and KLS pathogenesis.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank the following individuals: Drs. Mitch Goldman and Carol Langley who identified patients 1 and 2 as possible Kawasaki-like syndrome cases; Samir Gupta for the asymptomatic human immunodeficiency virus control sera; Bryan Schneider for providing the digital photographs included in this report; and Morton Davidson for insightful criticism of the manuscript.
Financial support. Resources for the study were from R. M. J.'s Indiana University academic discretionary account (internal academic speaking honoraria, grant-related bonuses) and Yale University start-up funds.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Gordon JB, Krahn AM, Burns JC. When children with Kawasaki disease grow up: myocardial and vascular complications in adulthood. J Am Coll Cardiol 2009; 54:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Burns JC et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med 1986; 315:341–7. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Takahashi M, Beiser AS et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med 1991; 324:1633–9. [DOI] [PubMed] [Google Scholar]
- 4.Rowley AH, Eckerley CA, Jack HM et al. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol 1997; 159:5946–55. [PubMed] [Google Scholar]
- 5.Rowley AH, Shulman ST, Spike BT et al. Oligoclonal IgA response in the vascular wall in acute Kawasaki disease. J Immunol 2001; 166:1334–43. [DOI] [PubMed] [Google Scholar]
- 6.Rowley AH, Shulman ST, Mask CA et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis 2000; 182:1183–91. [DOI] [PubMed] [Google Scholar]
- 7.Yanagawa H, Nakamura Y, Yashiro M et al. A nationwide incidence survey of Kawasaki disease in 1985–1986 in Japan. J Infect Dis 1988; 158:1296–301. [DOI] [PubMed] [Google Scholar]
- 8.Yanagawa H, Nakamura Y, Yashiro M et al. Incidence of Kawasaki disease in Japan: the nationwide surveys of 1999–2002. Pediatr Int 2006; 48:356–61. [DOI] [PubMed] [Google Scholar]
- 9.Rowley AH. Incomplete (atypical) Kawasaki disease. Pediatr Infect Dis J 2002; 21:563–5. [DOI] [PubMed] [Google Scholar]
- 10.Viraben R, Dupre A. Kawasaki disease associated with HIV infection. Lancet 1987; 1:1430–1. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RM, Little JR, Storch GA. Kawasaki-like syndromes associated with human immunodeficiency virus infection. Clin Infect Dis 2001; 32:1628–34. [DOI] [PubMed] [Google Scholar]
- 12.Stankovic K, Miailhes P, Bessis D et al. Kawasaki-like syndromes in HIV-infected adults. J Infect 2007; 55:488–94. [DOI] [PubMed] [Google Scholar]
- 13.Gomard-Mennesson E, Landron C, Dauphin C et al. Kawasaki disease in adults: report of 10 cases. Medicine (Baltimore) 2010; 89:149–58. [DOI] [PubMed] [Google Scholar]
- 14.Fraison JB, Seve P, Dauphin C et al. Kawasaki disease in adults: observations in France and literature review. Autoimmun Rev 2016; 15:242–9. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard JN, Powell HC, Freeman WR et al. Recurrent Kawasaki disease-like syndrome in a patient with acquired immunodeficiency syndrome. Clin Infect Dis 2003; 36:105–11. [DOI] [PubMed] [Google Scholar]
- 16.Leung S, Liu X, Fang L et al. The cytokine milieu in the interplay of pathogenic Th1/Th17 cells and regulatory T cells in autoimmune disease. Cell Mol Immunol 2010; 7:182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol 2002; 283:R7–28. [DOI] [PubMed] [Google Scholar]
- 18.Gupta SK, Johnson RM, Saha C et al. Improvement in HIV-related endothelial dysfunction using the anti-inflammatory agent salsalate: a pilot study. AIDS 2008; 22:653–5. [DOI] [PubMed] [Google Scholar]
- 19.Nixon AB, Pang H, Starr MD et al. Prognostic and predictive blood-based biomarkers in patients with advanced pancreatic cancer: results from CALGB80303 (Alliance). Clin Cancer Res 2013; 19:6957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valle JW, Wasan H, Lopes A et al. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol 2015; 16:967–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa S, Matsubara T, Umezawa Y et al. Serum levels of p60 soluble tumor necrosis factor receptor during acute Kawasaki disease. J Pediatr 1994; 124:721–5. [DOI] [PubMed] [Google Scholar]
- 22.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr 1992; 121:924–6. [DOI] [PubMed] [Google Scholar]
- 23.Simonini G, Masi L, Giani T et al. Osteoprotegerin serum levels in Kawasaki disease: an additional potential marker in predicting children with coronary artery involvement. J Rheumatol 2005; 32:2233–8. [PubMed] [Google Scholar]
- 24.Rowley AH, Baker SC, Shulman ST et al. Cytoplasmic inclusion bodies are detected by synthetic antibody in ciliated bronchial epithelium during acute Kawasaki disease. J Infect Dis 2005; 192:1757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowley AH, Baker SC, Shulman ST et al. RNA-containing cytoplasmic inclusion bodies in ciliated bronchial epithelium months to years after acute Kawasaki disease. PLoS One 2008; 3:e1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Natterer J, Perez MH, Di Bernardo S. Capillary leak leading to shock in Kawasaki disease without myocardial dysfunction. Cardiol Young 2012; 22:349–52. [DOI] [PubMed] [Google Scholar]
- 27.Thabet F, Bafaqih H, Al-Mohaimeed S et al. Shock: an unusual presentation of Kawasaki disease. Eur J Pediatr 2011; 170:941–3. [DOI] [PubMed] [Google Scholar]
- 28.Flynn E, Kowalski R, Burgner D. Kawasaki disease shock syndrome with retrograde diastolic aortic flow. J Pediatr 2016; 336–336.e1. [DOI] [PubMed] [Google Scholar]
- 29.Kaiser L, Fritz RS, Straus SE et al. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol 2001; 64:262–8. [DOI] [PubMed] [Google Scholar]
- 30.Oda K, Yamamoto Y. Serum interferon-gamma, interleukin-4, and interleukin-6 in infants with adenovirus and respiratory syncytial virus infection. Pediatr Int 2008; 50:92–4. [DOI] [PubMed] [Google Scholar]
- 31.Chakravarti A, Kumaria R. Circulating levels of tumour necrosis factor-alpha & interferon-gamma in patients with dengue & dengue haemorrhagic fever during an outbreak. Indian J Med Res 2006; 123:25–30. [PubMed] [Google Scholar]
- 32.Masuyama T, Matsuo M, Ichimaru T et al. Possible contribution of interferon-alpha to febrile seizures in influenza. Pediatr Neurol 2002; 27:289–92. [DOI] [PubMed] [Google Scholar]
- 33.Ogle JW, Waner JL, Joffe LS et al. Absence of interferon in sera of patients with Kawasaki syndrome. Pediatr Infect Dis J 1991; 10:25–9. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol 1990; 56:29–36. [DOI] [PubMed] [Google Scholar]
- 35.Hirao J, Hibi S, Andoh T, Ichimura T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int Arch Allergy Immunol 1997; 112:152–6. [DOI] [PubMed] [Google Scholar]
- 36.Rowley AH, Wylie KM, Kim KY et al. The transcriptional profile of coronary arteritis in Kawasaki disease. BMC Genomics 2015; 16:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng S, Yadav SK, Gao F, Yi Q. Plasma levels of monokine induced by interferon-gamma/chemokine (C-X-X motif) ligand 9, thymus and activation-regulated chemokine/chemokine (C-C motif) ligand 17 in children with Kawasaki disease. BMC Pediatr 2015; 15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Quesada C, Frangogiannis NG. Monocyte chemoattractant protein-1/CCL2 as a biomarker in acute coronary syndromes. Curr Atheroscler Rep 2009; 11:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung HS, Kim HY, Kim HS et al. Production of chemokines in Kawasaki disease, Henoch-Schonlein purpura and acute febrile illness. J Korean Med Sci 2004; 19:800–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shikishima Y, Saeki T, Matsuura N. Chemokines in Kawasaki disease: measurement of CCL2, CCL22 and CXCL10. Asian Pac J Allergy Immunol 2003; 21:139–43. [PubMed] [Google Scholar]
- 41.Johansson C, Ahlstedt I, Furubacka S et al. Differential expression of chemokine receptors on human IgA+ and IgG+ B cells. Clin Exp Immunol 2005; 141:279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo A, Saad A, Ali R et al. Circulating interferon-gamma-inducible Cys-X-Cys chemokine receptor 3 ligands are elevated in humans with aortic aneurysms and Cys-X-Cys chemokine receptor 3 is necessary for aneurysm formation in mice. J Thorac Cardiovasc Surg 2012; 143:704–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko TM, Kuo HC, Chang JS et al. CXCL10/IP-10 is a biomarker and mediator for Kawasaki disease. Circ Res 2015; 116:876–83. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal M, He C, Siddiqui J et al. CCL11 (eotaxin-1): a new diagnostic serum marker for prostate cancer. Prostate 2013; 73:573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colvin RA, Campanella GS, Manice LA, Luster AD. CXCR3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mol Cell Biol 2006; 26:5838–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem 2004; 279:30219–27. [DOI] [PubMed] [Google Scholar]
- 47.Jenh CH, Cox MA, Cui L et al. A selective and potent CXCR3 antagonist SCH 546738 attenuates the development of autoimmune diseases and delays graft rejection. BMC Immunol 2012; 13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wijtmans M, Verzijl D, Leurs R et al. Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem 2008; 3:861–72. [DOI] [PubMed] [Google Scholar]
- 49.Soler D, Chapman TR, Poisson LR et al. CCR8 expression identifies CD4 memory T cells enriched for FOXP3+ regulatory and Th2 effector lymphocytes. J Immunol 2006; 177:6940–51. [DOI] [PubMed] [Google Scholar]
- 50.Haque NS, Fallon JT, Pan JJ et al. Chemokine receptor-8 (CCR8) mediates human vascular smooth muscle cell chemotaxis and metalloproteinase-2 secretion. Blood 2004; 103:1296–304. [DOI] [PubMed] [Google Scholar]
- 51.Matsubara T, Katayama K, Matsuoka T et al. Decreased interferon-gamma (IFN-gamma)-producing T cells in patients with acute Kawasaki disease. Clin Exp Immunol 1999; 116:554–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Byrne ML, Cohen MS. Marked eosinophilia in a patient with history of severe atypical Kawasaki disease. Congenit Heart Dis 2013; 8:E130–3. [DOI] [PubMed] [Google Scholar]
- 53.Kuo HC, Yang KD, Liang CD et al. The relationship of eosinophilia to intravenous immunoglobulin treatment failure in Kawasaki disease. Pediatr Allergy Immunol 2007; 18:354–9. [DOI] [PubMed] [Google Scholar]
- 54.Tremoulet AH, Jain S, Chandrasekar D et al. Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J 2011; 30:1022–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen AJ, Steigbigel RT. Eosinophilia in patients infected with human immunodeficiency virus. J Infect Dis 1996; 174:615–8. [DOI] [PubMed] [Google Scholar]
- 56.Tietz A, Sponagel L, Erb P et al. Eosinophilia in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis 1997; 16:675–7. [DOI] [PubMed] [Google Scholar]
- 57.Fuschiotti P, Larregina AT, Ho J et al. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 2013; 65:236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dakhama A, Collins ML, Ohnishi H et al. IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy 2013; 68:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johnson RM, Kerr MS, Slaven JE. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 2014; 142:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown TJ, Crawford SE, Cornwall ML et al. CD8T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis 2001; 184:940–3. [DOI] [PubMed] [Google Scholar]
- 61.Ling XB, Kanegaye JT, Ji J et al. Point-of-care differentiation of Kawasaki disease from other febrile illnesses. J Pediatr 2013; 162:183–8e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hao S, Jin B, Tan Z et al. A classification tool for differentiation of Kawasaki disease from other febrile illnesses. J Pediatr 2016; June 22 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kentsis A, Shulman A, Ahmed S et al. Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease. EMBO Mol Med 2013; 5:210–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Popper SJ, Shimizu C, Shike H et al. Gene-expression patterns reveal underlying biological processes in Kawasaki disease. Genome Biol 2007; 8:R261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Popper SJ, Watson VE, Shimizu C et al. Gene transcript abundance profiles distinguish Kawasaki disease from adenovirus infection. J Infect Dis 2009; 200:657–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ling XB, Lau K, Kanegaye JT et al. A diagnostic algorithm combining clinical and molecular data distinguishes Kawasaki disease from other febrile illnesses. BMC Med 2011; 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newburger JW, Takahashi M, Gerber MA et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 2004; 110:2747–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.