Abstract
The walls of the cerebral ventricles in the developing embryo harbor the primary neural stem cells from which most neurons and glia derive. In many vertebrates, neurogenesis continues postnatally and into adulthood in this region. Adult neurogenesis at the ventricle has been most extensively studied in organisms with small brains, such as reptiles, birds, and rodents. In reptiles and birds, these progenitor cells give rise to young neurons that migrate into many regions of the forebrain. Neurogenesis in adult rodents is also relatively widespread along the lateral ventricles but migration is largely restricted to the rostral migratory stream into the olfactory bulb. Recent work indicates that the wall of the lateral ventricle is highly regionalized with progenitor cells giving rise to different types of neurons depending on their location. In species with larger brains, young neurons born in these spatially specified domains become dramatically separated from potential final destinations. Here we hypothesize that the increase in size and topographical complexity (e.g. intervening white matter tracts) in larger brains may severely limit the long-term contribution of new neurons born close to, or in, the ventricular wall. We compare the process of adult neuronal birth, migration, and integration across different species with different brain sizes, and discuss how early regional specification of progenitor cells may interact with brain size and affect where and when new neurons are added.
GRAPHICAL ABSTRACT
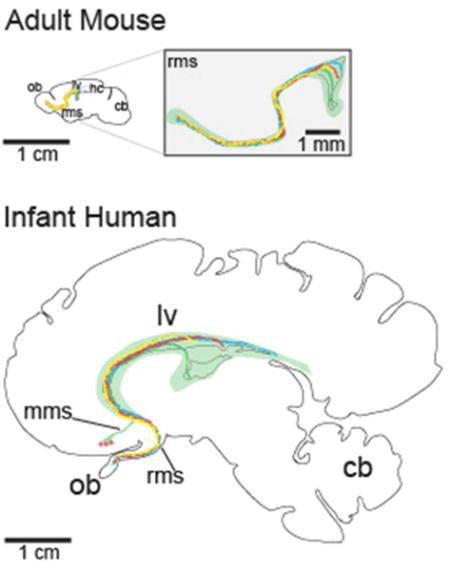
We discuss current work on adult neurogenesis across several species and propose that the tremendous increase in brain size between these species has significantly influenced the manner in which newly born neurons are able to contribute to adult brain circuits. This graphic illustrates the dramatic increase in distances that young neurons must migrate within the rostral migratory stream (RMS) in the human brain compared to the mouse brain; such changes may limit the circuits able to receive new neurons and the duration of neurogenesis through life.
Historical Perspective
In his book Degeneration and Regeneration of the Nervous System, Ramon y Cajal wrote that: “In adult centres the nerve paths are something fixed, ended, immutable. Everything may die, nothing may be regenerated” (Ramon y Cajal, 1928). Following Cajal’s identification of nerve cells as independent units of brain circuits, it became deeply established in the neurosciences, as well as in the general public, that no new neurons are added to the brain once fetal development is complete. This dogma persisted for most of the 20th century until [3H]-thymidine became available for cellular birth dating. The incorporation [3H]-thymidine into dividing cells was used in conjunction with Nissl staining to identify newly born cells with neuronal morphology. Work using this approach suggested that new neurons are born in multiple brain regions in adulthood. The regions where labeled cells were found included the cortex of the rat, the granule cell layer of the hippocampal dentate gyrus in the rat and the cat (Altman, 1963), and the rat olfactory bulb (OB) (Altman, 1967; Bayer and Altman, 1975). Subsequent ultrastructural studies supported the idea that the adult rat dentate gyrus and OB contain young neurons (Kaplan and Hinds, 1977). The interpretation of these results was questioned for several reasons: (i) the possibility that [3H]-thymidine labelling was not in new neurons but in closely-adjoining proliferative glial cells, (ii) whether the radioactive-labeling (relatively few grains per cell) was sufficient to reflect true cell division, and (iii) that the putative labeled cells could have incorporated [3H]-thymidine due to DNA repair (Rakic, 1985, 2002a). While controversial, this initial work was the first evidence for adult incorporation of new neurons in the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus and the OB, sites which have been confirmed in subsequent studies (reviewed in Fuentealba et al., 2012; Yu et al., 2014).
Renewed interest in adult neurogenesis came from independent studies in songbirds, a model organism to study vocal learning (Thorpe, 1954; Nottebohm, 2004). Seasonal changes in the size of a key region of the song control pathway, the high vocal center (HVC), were found to correlate with changing levels of testosterone (Nottebohm, 1981). Short survivals after [3H]-thymidine exposure revealed the presence of dividing cells within the ventricular zone (VZ) on the walls of the lateral ventricles (Fig. 1). With longer [3H]-thymidine post-labeling intervals, labeled neurons were found in the HVC (Goldman and Nottebohm, 1983). Further work using electrophysiology confirmed the neuronal identity of the labeled cells (Paton and Nottebohm, 1984). New neurons were found to synaptically integrate within the HVC (Paton and Nottebohm, 1984; Burd and Nottebohm, 1985) and send projections to the distant nucleus robustus archistriatalis (RA) (Alvarez-Buylla and Kirn, 1997). The amount of adult neurogenesis in birds correlates with seasonal and hormonal patterns, and with complex experiences, suggesting a possible functional role in plasticity and/or learning (Barnea and Nottebohm, 1994; Nottebohm et al., 1994; Alvarez-Buylla and Kirn, 1997; Nottebohm, 2004). This work clearly demonstrated that the newly added neurons can become part of functional circuits in the adult brain, and are involved in an ongoing process of neuronal replacement (Nottebohm, 2004). Early investigators assumed that the progenitor cells for adult neurogenesis would be simple and undifferentiated, possibly cells retained from fetal development (Altman, 1969), but work in songbirds demonstrated that this is not the case. Radial glia (RG) cells in the adult songbird VZ (Fig. 2) were found to incorporate [3H]-thymidine and their division correlated with the production of young neurons, providing the first evidence that these cells are the adult neural stem cells (NSCs) (Alvarez-Buylla et al., 1990a). These and other studies using birds provided several levels of evidence for adult neurogenesis including the identification of proliferating neuronal progenitors, electrophysiological and ultrastructural identification of the new neurons, and a possible functional importance for their integration into established adult circuits.
Figure 1.
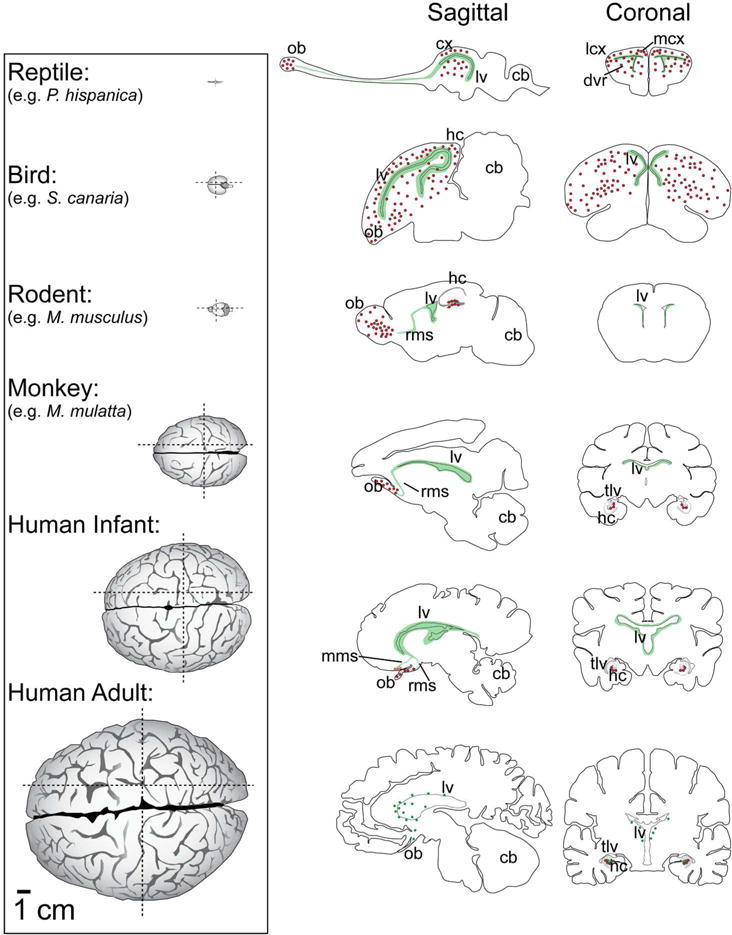
Neurogenic zones and migration destinations across several species and their relationship to brain size. (Left column) Dorsal view of the brains of several species all scaled according to the 1 cm scale bar at the bottom left. (Middle column) Sagittal view of each brain at the parasagittal plane of the RMS and olfactory bulb in each species. Green regions indicate germinal zones. Red dots indicate destinations of the migratory young neurons. Bottom row is scaled according to the lower left 1 cm scale bar, other sections are not to scale. (Right column) Coronal view of each brain. (Reptile): The lizard P. hispanica is shown as an example of adult neurogenesis in the reptile. (Bird): The canary is shown, highlighting the extensive ventricular germinal zone and regions that continue to receive adult born neurons. (Rodent): The adult mouse has a pronounced rostral migratory stream from the ventricles as well as abundant hippocampal neurogenesis. (Monkey): The macaque is one of the original non-human primates where adult neurogenesis was studied. Progenitor cells are abundant along the SVZ, cells proliferate and migrate along the RMS to the OB, and the SGZ in the hippocampus contains proliferating progenitor cells that produce adult-born granule cells. (Human infant:) During early postnatal life there are still many dividing cells and clusters of DCX+ cells along the LV walls. The rostral migratory and medial migratory streams are present after birth until approximately 18 months of age, however the medial stream is directed out of the plane of the section towards the reader, so its destination is represented by red dots with dashed lines. (Human adult:) Potential neurogenic sites in the adult human brain are shown, however the extent of these processes are still controversial. Progenitor cell proliferation has been detected in the SVZ, but most samples do not contain evidence of an RMS. cb, cerebellum; cx, cortex; dvr, dorsal ventricular ridge; hc, hippocampus; lcx, lateral cortex; lv, lateral ventricle; mcx, medial cortex; mms, medial migratory stream; ob, olfactory bulb; rms, rostral migratory stream; tlv, temporal lobe of the lateral ventricle. References: Reptile: (Font et al., 2001; Luzzati et al., 2009; Gonzalez-Granero, S.; Lezameta, M; Garcia-Verdugo, 2011) Bird: (Alvarez-Buylla et al., 1988; Vellema et al., 2011) Mouse: (Lein et al., 2007) Monkey: (Mikula et al., 2007) Human infant: (Sanai et al., 2004, 2011; Quiñones-Hinojosa et al., 2006; Bayer and Altman, 2007; Wang et al., 2011) Human Adult: ((Mikula et al., 2007; Woolsey and Gado, 2007).
Figure 2.
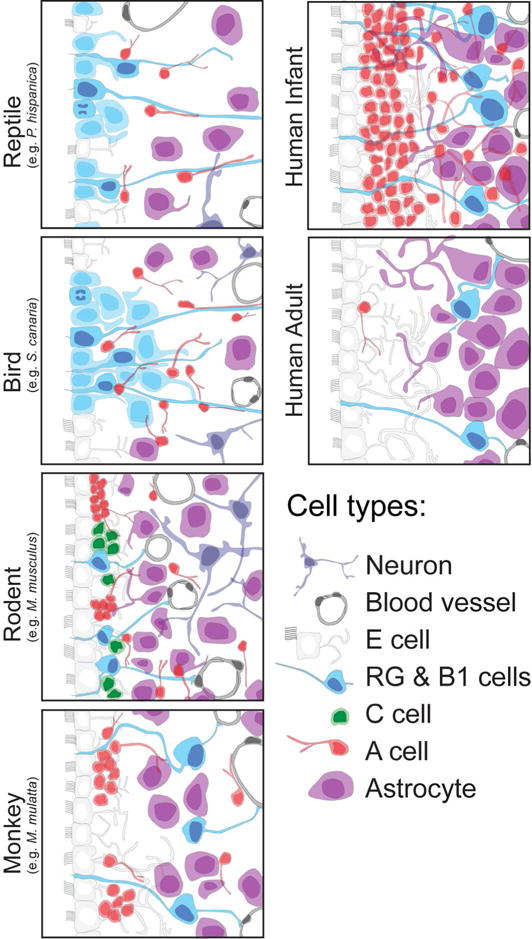
Schematic of the different neuronal precursor cells within the SVZ across several species. The adult SVZ consists of many of the same cell types across different species. Multicilliated E cells (white) line the walls of the lateral ventricles and are separated from a layer of glial cells (purple) and proliferating neuronal progenitors (blue) by migrating type A neuroblasts (red). In the adult human, the SVZ contains a hypocellular gap layer between the ependymal cells (white), astrocytes (purple) and rare progenitor cells (blue).
The challenge of adding new neurons
The addition of new neurons into an adult network requires the coordination of cell division, migration, maturation, and integration into existing circuits. To begin, progenitor cells must be maintained for extended periods of time past embryogenesis into adulthood to generate new neurons. This continued proliferation in the adult brain must be regulated, as the presence of dividing NSCs into adulthood has been proposed as a possible source for brain tumors (Jacques et al., 2010; Alcantara Llaguno et al., 2011; Cuddapah et al., 2014). Next, young neuroblasts need to migrate varying distances based on their birthplace using cues to guide them to their final destination (Rousselot et al., 1995; Lim et al., 1997; Wu et al., 1999; Hack et al., 2002; Bolteus and Bordey, 2004). The distances that separate sites of origin from destinations in the juvenile and adult brain result in migratory routes that (i) are orders of magnitude longer than in the embryo, when the brain is relatively small, (Figs. 3, 4), and (ii) contain more complexities, such as extensive vascularization, increasing regions of white matter, and a very dense network of mature dendrites, axons and synapses. Once the young neurons complete this journey, they must begin the process of integration into fully functional networks, without deleterious effects on the working of these circuits (Nottebohm, 2004; Song et al., 2005; Lazarini and Lledo, 2011). We suggest that amidst these challenges, one of the most important restrictions to widespread adult neurogenesis is brain size. Given the early regional specification of NSCs in development, the increased migration requirements on young neurons and the changes in the architecture of neurogenic zones in a larger brain may impose strict limitations for the delivery of neurons to many brain regions. These structural constraints may also lead to variability in adult neurogenesis between species.
Figure 3.
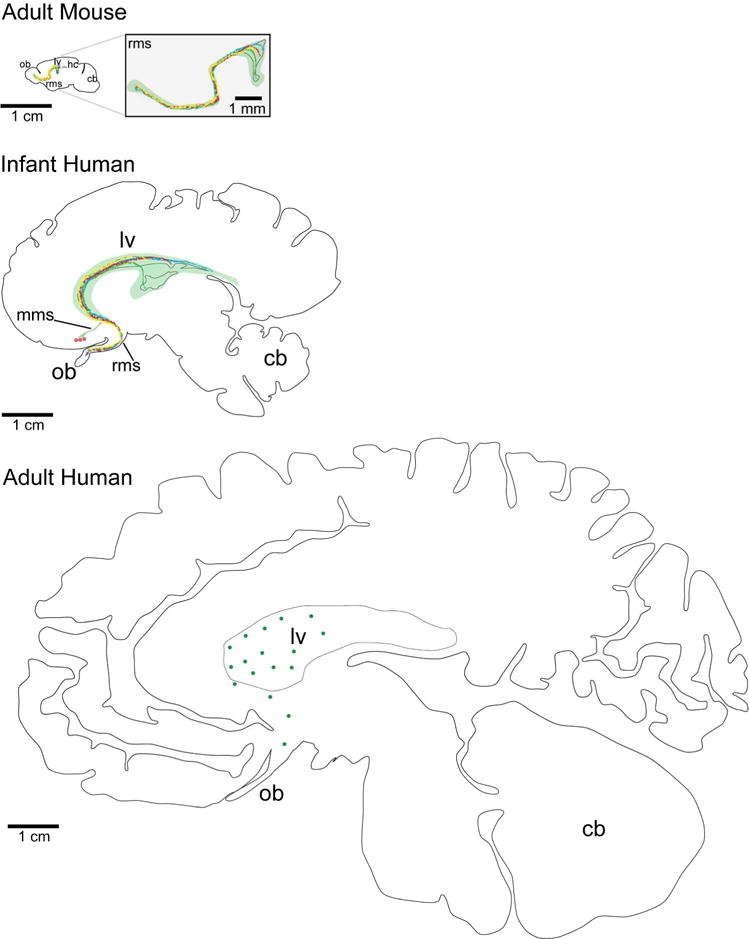
Sagittal representation of the spatial separation of neurogenic niches through development in the mouse and human brain. Sagittal sections at the parasagittal plane of the RMS and olfactory bulb in the mouse (top), human infant (middle), and adult human (bottom). With increased size, the progenitor domains (the migratory routes are represented by different colored lines) of the adult human V-SVZ are likely to have expanded and be further separated to cover equivalent areas. Additionally, potential brain targets are further away from the adult V-SVZ in the human than in the mouse resulting in longer and more variable neuronal migratory routes. All sections are scaled to the same 1 cm scale bar, except for the magnified view of the mouse RMS. cb, cerebellum; hc, hippocampus; lv, lateral ventricle; mms, medial migratory stream; ob, olfactory bulb; rms, rostral migratory stream.
Figure 4.
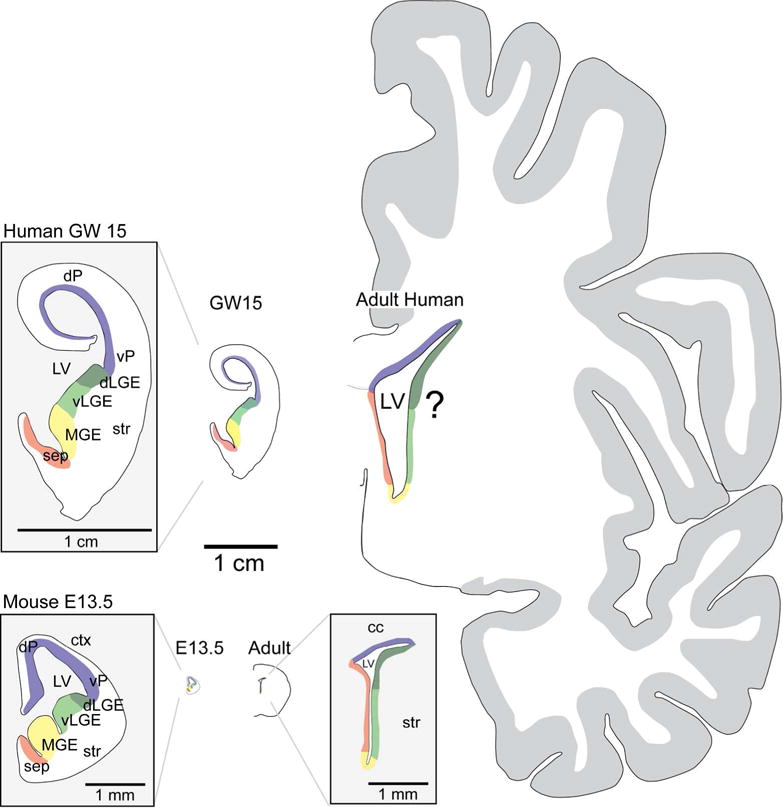
Coronal representation of the neurogenic niches through development in the mouse and human brain. Coronal sections of the human (top row) and mouse (bottom row) brain at embryonic (GW 15, E 13.5) and adult ages showing divisions of the ventricular wall into neurogenic microdomains (represented by different colors). These domains become more clearly established as development progresses and are detailed in the embryonic mouse and human (magnified views of GW 15 human and E 13.5 mouse). Adult NSCs retain this positional information in the mouse V-SVZ, however whether similar preservation occurs in the adult human is unknown. All unmagnified views are scaled relative to the 1 cm scale bar in the center. cc, corpus callosum; ctx, cortex; dLGE, dorsal lateral ganglionic eminence; dP, dorsal pallium; GW, gestational weeks; LV, lateral ventricle; MGE, medial ganglionic eminence; sep, septum; str, striatum; vLGE, ventral lateral ganglionic eminence; vP, ventral pallium.
Adult neurogenesis in species with different brain sizes
Adult neurogenesis in the V-SVZ has been described in greatest detail in species with small brains like rodents, birds, and reptiles, making it difficult to evaluate the influence of brain size on this process. Species with larger brains (especially humans) are more technically challenging to study, which greatly limits our ability to accurately ascertain the presence of adult neurogenesis. In this section we will evaluate the evidence for adult neurogenesis and its unique features in several species with brains of different sizes (Figs 1, 2). We will consider humans and primates alongside other mammals where more experimental data are available.
Reptiles
In the adult reptile brain, many new neurons are born and migrate to brain regions throughout the telencephalon; however, most reptiles where this process has been identified have very small brains (Gonzalez-Granero, et al., 2011). In the adult lizard VZ, proliferating RG cells persist, suggesting that these cells function as progenitors (Yanes et al., 1990; Font et al., 2001). The generation of neurons from dividing RG has not been directly demonstrated by lineage tracing, but young migratory neurons can be found nearby, and the vast majority of [3H]-thymidine labeled cells in the VZ have characteristics of RG (Garcia-Verdugo, et al., 2002). Most young neurons migrate either a short distance along RG into regions near the VZ like the medial, dorsal, and lateral cortices, and the striatum, but some make the relatively long journey to the OB (Fig. 1) (Pérez-Cañellas and García-Verdugo, 1996; Font et al., 2001). Adult-born cells in lizards apparently differentiate into various types of mature neurons including cortical projection neurons (Peñafiel et al., 1996; Lopez-Garcia et al., 2002). The medial cortex is the reptile homologue to the mammalian dentate gyrus (Rodríguez et al., 2002; Kaslin et al., 2008; Kempermann, 2012). This region, which likely has a role in spatial navigation, receives many new neurons in adulthood from the VZ and may take as long as 2 weeks to migrate (Lopez-Garcia et al., 1988; Day et al., 2001; Rodríguez et al., 2002). Interestingly, VZ-derived neurons can also replace damaged neurons in the lizard medial cortex following chemical (Font et al., 1991, 1997; Ramirez-Castillejo 2002) or physical injury (Romero-Alemán et al., 2004). Newly born neurons in the lizard brain contribute greatly to the pallium; in the turtle brain, however, neuroblasts target the OB and striatum in greater proportion (Pérez-Cañellas et al., 1997). It remains unclear to what extent adult neurogenesis in reptiles is part of a process of continuous brain growth, neuronal replacement or both. The above studies suggest that in reptiles, cells born in the walls of the lateral ventricles can reach many telencephalic structures. It will be interesting to determine to what extent, and in what brain regions, neurogenesis continues in reptiles with larger brains (e.g. in large species of Crocodilia) where target regions are further from the VZ.
Birds
As described by the early studies in songbirds, new neurons are born in the VZ in the walls of the lateral ventricles where proliferating RG persist. From this germinal zone, young neurons migrate widely into many structures of the telencephalon (Fig. 1). Neuronal dispersal has been best studied in canaries (Alvarez-Buylla and Nottebohm, 1988), where many of the cells are destined for the nearby avian striatum (previously known as the lobus parolfactorius). Pallial regions, including the HVC, also receive many new neurons. Interestingly, proliferation is most abundant in hot spots on the ventral VZ (Alvarez-Buylla, et al., 1988), facing the avian striatum and the border between hyperpallium and mesopallium (previously termed hyperstriatum and hyperstriatum ventralis, respectively). Adult neurogenesis has been found in several bird species, but as with reptiles, most of the brains are relatively small and there is variability between species. Canaries and other songbirds have large brains in relation to their body mass, but their absolute brain size is still small. In most birds, neurons need to migrate no more than a centimeter, at most, from their site of birth in the VZ to their final destination anywhere in the telencephalon. The amount of time it takes for this process to occur in canaries is approximately 2–3 weeks after the cells are born, based on [3H]-thymidine labeling (Alvarez-Buylla and Nottebohm, 1988). Although adult neurogenesis has been found in the forebrain of small parrots like budgerigars (Nottebohm, 1987), larger parrots have not been studied. Other birds where adult neurogenesis has been found include adult doves (Ling et al., 1997), chickadees (Barnea and Nottebohm, 1994), and chickens and quails (Nottebohm, 1985; Nottebohm and Alvarez-Buylla, 1993). During embryonic development, the zebra finch forebrain has a second layer of proliferating cells separated from the VZ, in what appears to be an avian version of a developmental sub-ventricular zone (SVZ) (Charvet and Streidter, 2009). Interestingly, this layer of proliferating cells is diminished, or possibly absent, in the adult (DeWulf and Bottjer, 2002). As we will discuss next, an expanded ventricular-subventricular zone (V-SVZ) for adult neurogenesis is a prominent feature in the developing mammalian brain and may have emerged as a place to generate the larger numbers of new neurons required to build larger brains. We next turn to mammals where, although adult neurogenesis is less widespread, we have an opportunity to compare adult neurogenesis at the V-SVZ between species with much greater brain size variability.
Mammals
Adult mammals also have neurogenesis along the walls of the lateral ventricle, similar to lizards and birds; however, the migratory routes of these young neurons are mostly restricted to a rostral migratory stream (RMS) that is directed to the OB. These restrictions in mammals may not only be a consequence of which adult circuits continue to receive new neurons, but may be influenced by the more complex brain structure. Mammals also have a second primary site of adult neurogenesis away from the wall of the lateral ventricle in the dentate gyrus of the hippocampus. Unlike birds and reptiles, where new neurons in the medial cortex (the DG homolog) are born in the VZ, in mammals the progenitor cells for this region are away from the ventricle, in the SGZ. As we will discuss below, the presence of progenitors in the SGZ places them closer to the circuits that receive the new neurons. These new neurons migrate a much shorter distance than those born in the mammalian V-SVZ, and are therefore perhaps less affected by changes in brain size.
Mice
We will begin our survey of the unique features of mammalian V-SVZ adult neurogenesis in the relatively small brains of rodents, where recent advances have progressed most rapidly due to the availability of genetic tools. In the adult mouse, the primary precursors, also known as B1 cells, have many characteristics of astrocytes (Doetsch et al., 1999; Kriegstein and Alvarez-Buylla, 2009) and maintain epithelial properties of their predecessors which are RG (Merkle et al., 2004; Mercier et al., 2002; Mirzadeh et al., 2008). RG in the developing mammalian brain function as primary progenitors for neurons (Miyata et al., 2001; Noctor et al., 2001; Campbell and Götz, 2002; Weissman et al., 2003; Anthony et al., 2004). In adult mammals, most RG cells transform into parenchymal astrocytes perinatally. B1 cells retain an apical contact with the ventricular lumen like RG cells, but do not have contact with the pial surface, as many RG cells do (Fig. 2). Instead, most B1 cells have their cell body in the SVZ with end-feet on blood vessels, similar to that of many astrocytes. Therefore, the adult mammalian periventricular germinal zone includes ventricular and subventricular domains and this has lead to the more recent denomination V-SVZ (Fuentealba et al., 2012). The apical-ventricular contact of B1 cells is surrounded by ependymal cells (E cells). E cells are multi-ciliated, epithelial cells that play a key role in CSF homeostasis and circulation, but also contribute to the regulation of B1 cell proliferation (Lim and Alvarez-Buylla, 1999; Gajera et al., 2010; Khatri et al., 2014). It has been suggested that B1 cells can divide to either self-renew or generate C cells (the transit amplifying, or intermediate progenitor cell), but the actual mode of B1 cell division in vivo remains unknown. C cells, in turn, give rise to A cells, the young migratory neurons, some of which continue to divide as they migrate away from the V-SVZ. (Lois and Alvarez-Buylla, 1994; Menezes and Luskin, 1994; García-Verdugo et al., 1998; Doetsch et al., 1999). A cells express doublecortin (DCX), a cytoskeletal-associated protein required for proper neuronal migration, as well as a polysialylated version of the neuronal adhesion molecule NCAM (PSA-NCAM). These neuroblasts move along each other using chain migration through the V-SVZ and then anteriorly along a structure called the rostral migratory stream (RMS) (Altman, 1969; Lois and Alvarez-Buylla, 1994). Neuroblasts in the RMS travel to the OB, where they mature primarily into granule cells (GCs) and some peri-glomerular (PGCs) interneurons. This journey ranges in distance from 3 to 8 mm depending on their site of birth (Doetsch and Alvarez-Buylla, 1996) and takes cells between 3–7 days (Lois and Alvarez-Buylla, 1994, Petreanu and Alvarez-Buylla 2002).
Other mammals
Despite broad similarities in sites of neuronal birth and migration in mammals, the architecture of the V-SVZ can vary considerably between species. Unlike in rodents, in many mammals including rabbits (Ponti et al., 2006), sheep (Brus et al., 2013b), and cows (Rodriguez-Perez et al., 2003), the adult V-SVZ contains regions with a hypo-cellular gap layer that is largely devoid of cell bodies separating the ependymal cells from astrocytes. This hypo-cellular gap is a prominent feature of the adult human V-SVZ (Sanai et al., 2004) as we will discuss in further detail below; however, in most other mammals the V-SVZ also contains many chains of young neurons migrating to the OB. Whereas in rodents and humans the olfactory ventricle closes during development, the RMS of many adult mammals (e.g. rabbit, cat, sheep, and cow) contains an open olfactory ventricle with a prominent V-SVZ along it. In the young postnatal rabbit (Ponti et al., 2006), chains of migrating neurons extending into the neighboring parenchyma are present in a dorsolateral expansion of the V-SVZ (called the abventricular SVZ). While the rabbit V-SVZ has a GFAP+ glial meshwork, this is less prominent within the abventricular SVZ: in this region, neuroblasts are tightly associated with blood vessels and axonal processes. These parenchymal chains appear directed to cortex, though no specific target has been established, and suggest the possibility of additional migratory routes during the early postnatal period. The architecture of the RMS also varies considerably, even within species. In domesticated cats, when brain size is held constant, the RMS length and width are also consistent, whereas in different dog breeds where brain size is more variable, the length and width of the RMS are also more varied (Malik et al., 2012). In this study, the RMS was measured to be between 10–15 mm in cats and 20–30 mm in dogs, however migration rates and lineage tracing were not evaluated.
The migration and maturation rates of young neurons appear greatly protracted in species with larger brains. In the adult sheep, migration from the SVZ to the OB may take as long as one month (Brus, et al., 2013a, 2013b). This is compared to 3–7 days in mice that it takes for young neurons to make the journey from the V-SVZ to the OB (Rousselot, et al., 1995; Petreanu and Alvarez-Buylla 2002). Compared to mice, where the RMS is approximately 4 mm long, in the sheep it is ~20 mm long. If the cells migrate at a similar speed between the two species, it would take about 5 times longer for cells to make the journey from the proximal V-SVZ to the OB in the sheep compared to mice. Migrations are likely even longer in larger species taking into consideration that many cells originate from caudal the walls of the lateral ventricles in the caudal telencephalon. These studies suggest that despite the similarities of many key features of adult neurogenesis, the requirement for longer and more tortuous migration routes in larger brains may lead to dramatic differences in the kinetics of this process. The maturation of new neurons in the sheep brain also appears longer compared to mice. Mature BrdU+/NeuN+ cells are scarce in the adult sheep OB 3 months after BrdU injections and their total number keeps increasing up to 8 months post-BrdU (Brus et al., 2013b). This is compared to 2–3 weeks that it takes for new neurons to mature in mice (Petreanu and Alvarez-Buylla 2002). Therefore, both migration and maturation of new neurons destined for the OB take much longer in the larger brain of sheep compared to mice.
Humans and non-human primates
In primates the cortex has dramatically expanded (Fig. 1), in many cases accompanied by cortical tissue folding (gyrification) and higher cognitive function (Ghosh and Jessberger, 2013). Even in species with brains that have undergone this growth there is variability in brain size that is associated with differences in the anatomy of neurogenic sites and the perdurance of these proliferative regions. Interest in studying adult human neurogenesis was stimulated by an early study using rare BrdU-treated adult brain samples (Eriksson 1998), where a few cells were identified in the V-SVZ that had incorporated BrdU. We next review the limited evidence for human V-SVZ neurogenesis, and then compare this with evidence from smaller non-human primate brains.
In the adult human brain, proliferation in the V-SVZ is scarce. Nonetheless, small numbers of cells cultured from intraoperative samples of the adult human V-SVZ can proliferate and generate immature neurons in vitro (Sanai et al., 2004). This study also found rare TUJ1, PSA-NCAM+ cells in the V-SVZ. More recent studies showed that DCX+ neuroblasts in the V-SVZ and the RMS of the adult human are extremely rare and only a small portion express proliferative markers (Wang et al., 2011). The numbers of DCX+ cells found in adults in this study were much lower compared to those observed at the fetal ages tested, in agreement with work from other groups (Bédard and Parent, 2004; Sanai et al., 2004). These studies also found only a few DCX+ neuroblasts in the olfactory tract and none in the OB. The inability to regularly birthdate human specimens has stimulated the development of new strategies, such as the use of carbon 14 (14C) (Spalding et al., 2005). This approach takes advantage of the increased atmospheric levels of 14C during the height of atomic bomb testing between the 1940s and the mid-1960s. Cells that were dividing during these years would be expected to have elevated levels of 14C in their DNA. Work using 14C birthdating also concluded that there is little to no postnatal neurogenesis in the adult human OB (Bergmann et al., 2012).
A study of the postnatal development of the human V-SVZ revealed many young neuroblasts immediately adjacent to the ependyma; these cells are mostly gone by two years of age (Sanai et al., 2011). This same layer adjacent to the ependyma in older humans is largely devoid of cell bodies and is referred to as the “hypocellular gap”. The decrease in migrating neurons in this region coincides with a reduction in proliferation. Similar to humans, adult macaques and marmosets also have a hypocellular gap layer next to the wall of the lateral ventricle (Sanai et al., 2004; Quiñones-Hinojosa et al., 2006; Gil-Perotin et al., 2009; Sawamoto et al., 2011) (Fig. 2). The emergence of this hypocellular gap in the V-SVZ could correlate with the disappearance of cells migrating to the OB in adulthood. However, this relationship has not been investigated, and we know that in humans there are other potential targets, in addition to the OB, for periventricular migrating young neurons present in infants.
In the infant human, a medial migratory stream (MMS) was identified containing many neuroblasts migrating in the medial prefrontal cortex. This collection of young neurons, not found in the mouse brain, or yet in other primates, appears to be destined for the ventral medial prefrontal cortex, an area important for social decision-making and risk processing (van den Bos and Güroglu, 2009; Schonberg et al., 2012). Novel migratory pathways may be more prevalent in brains with a protracted development or during the early postnatal period (as described above in rabbits).
The extent of continued neurogenesis in the adult non-human primate V-SVZ also remains in question. There is evidence for the adult production of new neurons in the V-SVZ of some non-human primates, and the V-SVZ in these species has unique features. In the adult marmoset brain the RMS does not appear as a continuous path with chains of migrating neuroblasts, particularly within the olfactory tract (Sawamoto et al., 2011), though it was present in neonatal samples. In rhesus monkeys, a cell-dense region is present at the ventral anterior V-SVZ, termed the vfSVZ (Pencea et al., 2001; Rakic, 2002b), which is equivalent to the anterior V-SVZ in the mouse brain but is more expanded ventrally. An elaboration of the V-SVZ was observed in adult squirrel monkeys where it gives rise to two ventral extensions, a rostral and caudal expansion populated by PSA-NCAM+ Tuj1+ cells. They converge to form a unified RMS that is anteriorly directed to the OB (Bedard et al., 2002a). It would be interesting to test whether this caudal ventral extension serves as a shortcut to the longer dorsal route along the lateral ventricle.
Primate brains, including humans, provide a dramatic example of how brain size could influence the presence of neurogenesis in the adult. Migratory paths of young neurons to the OB greatly increase from infancy into adulthood (Fig 3). The minimum distance from the ventral anterior V-SVZ to the olfactory tract (OT) in the human brain at birth is about 12–15 mm, but cells must travel nearly the same distance again once they enter the tract to reach destinations in the main olfactory bulb. So the putative RMS in the human newborn is about 30 mm. Furthermore, cells could be born more caudally in the LV, which would easily double or triple the length of their total migration (Fig. 3). In the adult human brain all of these sizes are further increased, with the distance from the ventral anterior V-SVZ to the entrance of the OT measuring closer to 25–30 mm (Mikula et al., 2007). Once entering the OB, cells must migrate nearly the same distance again to reach most distal portions of the OB. The RMS in adult humans would therefore be 50–60 mm long if this migratory path was retained in the adult. In line with our hypothesis, these long distances imposed by bigger brains might create unique challenges to young neurons and ultimately limit their presence with age. As indicated above, rare, individual cells expressing markers of young migrating neurons and a clear leading process have been detected along this path (Nader et al. 2004; Wang et al., 2011). This raises the question of whether the rare lone-ranger neurons still make this very long journey in the adult human brain.
Fundamental questions remain regarding the duration and locations where adult neurogenesis persists in the human brain. While most studies support the presence of V-SVZ neurogenesis in the infant primate brain, there is little evidence for the presence of a significant number of new neurons in the V-SVZ of adolescent and adult human brains. However, significant growth of the brain occurs after infancy, exemplified in humans (Figs. 1, 3), and this could limit the contribution of neurons born in the V-SVZ to targets that become too distant. These conclusions must be tempered by the current limitations of our tools. Sample variability, particularly in adult ages, may be partially responsible for generating data that are difficult to compare directly. Advances in approaches and techniques will hopefully provide novel ways to study adult neurogenesis more consistently in humans.
Widening the search for adult neurogenesis
Different sites in mammals
Immunostaining for progenitor cell markers, and the expression of DCX and PSA-NCAM in young migrating neuroblasts (Brown et al., 2003; Couillard-Despres et al., 2005) have suggested that there may be additional brain regions where adult neurogenesis occurs. Adult-born BrdU+ cells expressing markers of young neurons (such as DCX and TUJ1) have been reported in the adult rat neocortex and striatum (Dayer et al., 2005) and the adult rabbit striatum (Luzzati et al., 2006). BrdU+ cells have also been observed in the hypothalamus of adult mice, sheep, and voles (Fowler et al., 2002; Kokoeva et al., 2005, 2007; Migaud et al., 2010) and the amygdala of adult voles (Fowler et al., 2002, 2005). In the adult rodent neurogenic niche, the process of neuronal birth, migration, and maturation has been documented using not just marker expression and BrdU incorporation, but also genetic lineage tracing, retroviral labeling, ultrastructural characterization, and co-labeling with other cell-type markers. As these tools are not always available in all species, the expression of markers of young neurons is also used to explore new species in addition to new brain regions (Chawana et al., 2013; Patzke et al., 2013, 2014). However, it is important to keep in mind that each of these tools on their own has significant limitations. Some of the original criticisms of adult neurogenesis still apply: thymidine analogue-labeling can also be found in non-dividing cells and the timing and dosing directly affect the extent of labeling and the health of labeled cells. Furthermore, thymidine analogues can be incorporated into cells during DNA repair or DNA replication that does not accompany cell division (Rakic, 2002a, 2002b; Gould, 2007). In addition, the dosing and detection of BrdU are frequently variable between studies. The neuroblast marker, DCX, has been used as a proxy for evidence of active neurogenesis; however, both DCX and PSA-NCAM expression can persist in mature neurons (Knoth et al., 2010; Klempin et al., 2011; Kohler et al., 2011) and this expression is associated with features of plasticity and remodeling (Bonfanti, 2006; Bloch et al., 2011;Nacher et al., 2002, 2004, Vellema, et al., 2014). Work on novel adult neurogenic sites must be supported by fate mapping and lineage tracing studies to identify progenitors and mature, integrated adult-born neurons, as has been performed for the forebrain in songbirds and the V-SVZ-OB, and SGZ-DG in rodents.
Different sites in monkey
As in other species, alternative sites for adult neurogenesis have been suggested in primates, including the cortex (Gould et al., 1999; Cai et al., 2009; Fung et al., 2011), striatum (Bédard et al., 2002b), and the amygdala (Bernier et al., 2002; Zhang et al., 2009). Rare BrdU+ NeuN+ cells were found in the striatum of adult squirrel monkeys three weeks post-injection (Bédard et al., 2002b); however, a different study used expression profiling to demonstrate that most if not all striatal interneurons in macaque monkeys are made during gestation and found no evidence for BrdU-labeling in neurons in this region (Wang et al., 2014). Conflicting observations have also been made in the neocortex. In early adult macaque studies, BrdU labeling of NeuN+ cells was found in the prefrontal cortex, the inferior temporal lobe, and the striatum (Gould et al., 1999). These results contradicted studies in macaques that showed no [3H]-thymidine labeling (Rakic, 1985) or little to no BrdU incorporation (Kornack and Rakic, 2001; Koketsu et al., 2003) in neurons of the adult cortex, despite evidence of proliferation in the dentate gyrus. Koketsu, et al. did find rare BrdU+/NeuN+ cells (<0.01% of NeuN+ cells) and BrdU+/DCX+ cells in the frontal cortex, but the morphology and level of staining in these cells was different from those observed in the OB of juvenile monkeys. Thus the incorporation of new neurons in the primate cortex still remains uncertain; if present, the available evidence suggests that this is a rare phenomenon.
Different sites in humans
14C labeling has been used to ask whether other human brain regions have adult-born neurons. A recent study found evidence for adult neurogenesis in the striatum (Ernst et al., 2014). Retrospective birth-dating was performed using tissue from patients who had received the thymidine analogue, IdU, for radiosensitization. Idu+/NeuN+ cells were found in the striatum of patients exposed to higher doses of this thymidine analog, contradicting other work suggesting that striatal interneurons are generated embryonically (Wang et al., 2014). In another use of 14C birth-dating, glial and vascular cells, but not neurons, were shown to have significant turnover in brain tissue of patients who had ischemic stroke (Huttner et al., 2014). This study’s finding of no neurogenesis in response to cortical injury in humans did not agree with other human studies (Jin et al., 2006; Martí-Fàbregas et al., 2010) or the finding of post-injury neurogenesis in rodents (Parent et al., 2002; Parent, 2003; Kaneko and Sawamoto, 2009; Magnusson et al., 2014). Although the use of 14C birthdating is an innovative way to use existing brain bank material, additional validation of this approach as a method to evaluate the age of individual cells is necessary. Other processes, such as DNA damage or methylation could affect the extent of 14C incorporation; DNA methylation levels, for example, are variable even within a sorted population of neuronal nuclei (Iwamoto et al., 2011). Despite how little is currently known about the accuracy of this approach, it represents a novel way to evaluate cellular age in postmitotic cells.
The study of human brain tissue is singularly challenging. The protocols used to prepare human samples can have high variability, and fixation methods are not standardized between brain banks or institutions. Issues also arise when considering the genetic and environmental individuality, lifestyle characteristics, and the clinical history (eg. epilepsy, tumor) of the tissue. Some of these experimental uncertainties could explain the difficulty in reaching a consensus over whether and to what extent adult human neurogenesis persists.
We next consider the growing data on the early regionalization of NSCs in germinal regions, and how this particular feature of development might interact with increasing brain size to limit adult neurogenesis.
Embryonic regional specification: possible limits on adult neurogenesis
The work we have reviewed suggests that adult V-SVZ neurogenesis may be less prevalent in larger brains. One possible explanation is that brain growth anatomically limits the ability of young neurons to move from different progenitor regions to their final destination. Neuronal diversity arises from differences in progenitor cell specification that is highly linked to the location within the developing and adult V-SVZ. Neural circuits, which require multiple neuronal types, recruit them from different pools of progenitors within these highly regionalized germinal niches. Building the brain, therefore, requires the eventual relocation of young neurons from their sites of production to their final destination.
Progenitor heterogeneity is established very early during the development of the neural tube (Puelles et al., 2000). Both spatial (dorsal-ventral and rostro-caudal) gradients of morphogens (secreted signaling molecules) and temporal patterning determine the types of neurons produced in specific locations. These signals are integrated for a given position and transduced into differential expression of transcription factors that will direct the production of specific neuronal subtypes. The combinatorial expression of Hox genes divides the caudal neural tube along the A-P axis into the hindbrain segments called rhombomeres, and segments of the spinal cord (Keynes and Lumsden, 1990; Gavalas et al., 1998). In the spinal cord, progenitors have differential exposure to Shh based on their dorsal-ventral position, resulting in a variety of spinal cord neurons (Jessell, 2000). Establishment of these gradients of morphogens must happen when the CNS is very small. As the CNS grows, positional segmentation becomes fixed, however progenitor areas continue to expand as they pattern the development of the neuroaxis into the major CNS subdivisions. In the forebrain, this gene expression topography dictates not only the large pallial/subpallial division between the progenitor zones that will produce projection neurons and interneurons, respectively, but also more specific boundaries of structures that will emerge in the adult brain. These mechanisms of regional specification appear to be highly conserved in vertebrates (Macdonald et al., 1994; Bachy et al., 2001; Marshall and Goldman, 2002; Marín and Rubenstein, 2003; Puelles and Rubenstein, 2003; Kessaris et al., 2006).
In mice, the spatial organization established in the embryo appears to be preserved in the adult V-SVZ (Fig. 4). Regional specification during early development leads to a graded expression of transcription factors in B1 cells (Kohwi et al., 2005; Young et al., 2007; Sequerra, 2014). These cues segregate the V-SVZ into regions that produce different subtypes of OB interneurons depending on whether the progenitor cells are located on the septal, lateral, or dorsal wall of the lateral ventricle (Merkle et al., 2007). For example, superficial GCs and GABAergic tyrosine hydroxylase (TH)+ PGCs come from progenitors in the dorsal and cortical V-SVZ, while deep GC and GABAergic calbindin (CB)+ PGCs arise from the ventral V-SVZ. These regions can be further subdivided. In the anterior ventral V-SVZ alone, there are microdomains of B1 cells that produce four previously undescribed OB neuron subtypes (Merkle et al., 2014). Recent work indicates that regional B1 cell specification occurs during very early stages of forebrain development (Fuentealba et al., 2015). Therefore, progenitor cell parcellation, essential for creating diversity in the neuronal progeny, is established early in development and continues into the adult neurogenic niche. Although it has not been studied as extensively, regional restrictions likely also exist in the avian brain (Scott and Lois, 2007). Whether such spatial organization exists in the dentate SGZ of the rodent brain is under investigation (Li, et al.2013; Sugiyama et al., 2013; Bekiari et al., 2014).
How much of the spatial patterning of adult NSCs observed in rodents is conserved in larger and more complex primate brains? During embryonic development, many of the same transcriptional domains are present in primate brains as in mouse brains; however the exact territories of these domains and the subtypes of cells they produce are expanded (Reinchisi et al., 2012; Hansen et al., 2013; Ma et al., 2013) (Fig. 4). This creates a topographical challenge in linking germinal zones to the destinations where neurons might be needed as the brain grows during normal development (Fig. 3).
Shortening the migratory requirements through displaced progenitors
One method for dealing with the increase in size during development is to have adult germinal zones closer to the sites where the new neurons are required. A striking example of displaced progenitor cells is a greatly expanded fetal progenitor zone in primates called the outer V-SVZ. This region contains RG-like progenitor cells unanchored in the VZ, called outer RG cells (Hansen et al., 2010). The outer RG cells generate a large population of projection neurons and in humans this region may have grown in size to facilitate cortical expansion (Lui et al., 2011). To produce this additional layer of progenitors during development, human RG at the ventricle divide horizontally via a mechanism called mitotic somatic translocation (MST) (LaMonica et al., 2013; Ostrem et al., 2014). This is a process where the soma of the progenitor quickly jumps in the direction of the developing cortical plate, away from the ventricle, before dividing. This mode of cell division moves the progenitors closer to the target areas of cortex decreasing the migratory distances for young neurons.
Another example of progenitors that reside near where the neurons are required is the SGZ of the hippocampus. SGZ progenitors are developmentally derived from the ventricular wall, and eventually move closer to their target regions. The primary progenitors in the SGZ have astroglial characteristics and are known as radial astrocytes (Seri et al., 2001, 2004; Kriegstein and Alvarez-Buylla, 2009) based on their prominent radial process that traverses the granule cell layer, or Type I cells (Gage, et al., 1998; Kronenberg, et al., 2003). In the SGZ, radial astrocytes give rise to intermediate progenitors known as D cells, or type II progenitors (Seri et al., 2001, 2004; Filippov et al., 2003; Hodge et al., 2008). The type II progenitors in turn give rise to type III cells, young neurons that express DCX, prior to their final maturation in the granule cell layer of the DG. These processes occur within the SGZ, requiring only a short migration of the newly produced neurons from their site of birth into the granule cell layer of the DG.
For many neuronal cell types, radial displacement of progenitor cells alone is not sufficient to reduce the distance from sites of birth to final destination. Some neuronal precursors, such as those that generate cortical interneurons, are not radially linked to target areas; their progeny require long tangential migrations. In rodents, young migrating interneurons use fast migration speeds and long tangential migratory routes to go from the subpallium, the ventral source of most interneurons in the mouse brain, to their dorsal cortical destinations (Anderson et al., 1999, 2001; Wichterle et al., 2001; Marín et al., 2010). How large can the brain grow while maintaining a productive tangential migration mechanism to feed areas like the cortex with sufficient interneurons? The primate and human subpallium contains the ventral progenitor areas for these cells, but its organization is more complex with an outer layer of progenitor cells not observed in the mouse (Hansen et al., 2013). Some groups have even proposed that larger non-human primate and human brains may generate more interneurons by the addition of a neurogenic zone in the dorsal ventricular wall (Zecevic et al., 2005; Jakovcevski et al., 2011; Radonjić et al., 2014); this idea is still under debate (Molnár and Butt, 2013). There is also recent data that in both rabbit and human brains, the birth of new neurons persists late into gestation (Malik et al., 2013; Arshad et al., 2015); such protracted neurogenesis could provide an extended source of neurons for larger brains.
The regionalization of early progenitor zones and their preservation in the adult niche is not completely understood, particularly in humans. Regional specification happens early in development and appears to be conserved among vertebrates, underscoring the importance of preserved genetic mechanisms in establishing the V-SVZ germinal micro domains. Progenitor zones and their potential target areas become greatly separated in bigger brains (see Fig. 3). The resulting distances and possible increase in complexity (e.g. white matter expansion) may severely limit or even impede neuronal navigation in larger brains. This hypothesis could explain the growing evidence that migration from the walls of the lateral ventricles to the OB is rare, or non-existent, in the adult human brain.
A functional role for new neurons in small brains
What is the function of adult-born neurons, and can larger brains do without them? Thus far we have focused on how increasing brain size might limit the presence of adult neurogenesis. However, there might be examples where the functional benefit of newborn neurons for certain regions may outweigh the challenges of maintaining neurogenesis and neuronal migration in a larger brain. Unfortunately, the purpose of adult neurogenesis remains unknown, and studies focusing on possible functions are generally performed in animals with small brains like birds and rodents. (For reviews see: Nottebohm, 2004; Zhao et al., 2008; Lazarini and Lledo, 2011; Aimone et al., 2014). The findings from these studies suggest that adult-born neurons contribute to neural circuit plasticity.
In songbirds, correlations between neuronal replacement and environmental complexity, or with the changing environment at different times of the year, suggest that adult neurogenesis may be linked to circuit plasticity possibly associated with learning and memory (Nottebohm, 1981, 2004). Consistently, higher levels of adult neurogenesis are observed in open-ended learners like canaries compared to zebra finches, which are closed-ended learners (Alvarez-Buylla et al., 1990b). Targeted deletion of HVC projection neurons, that are normally replaced in adult male zebra finches, results in a severe deterioration of song (Scharff et al., 2000). Interestingly, with time these cells were replaced via adult neurogenesis, and the birds’ original song was restored to varying degrees. These data suggest that the newly formed neurons participate in song production and are integral part of the circuits required for this behavior, but likely are not the only substrate for memory. Song learning in birds clearly provides a powerful system to further investigate the function of new neurons, but future experiments may require better tools to directly manipulate the different components of neuronal addition (birth, migration and integration), cell replacement (cell death) or the functioning of newly incorporated nerve cells. Methods for the genetic modification of songbirds have been developed (Agate et al., 2009; Scott et al., 2010) and these techniques could allow for more direct testing of the function of the new neurons in the avian brain.
Studies in the rodent olfactory system, which can take advantage of current genetic tools, suggest that adult neurogenesis may also be related to plasticity, but the precise function of new neurons in the OB remains unclear. Granule cells are the most commonly recruited neuronal type in the adult OB. Their survival appears to be closely tied to their level of activity. While proliferation, migration, and integration were not affected by decreased activity (i.e. loss of olfaction), olfactory deprivation has been shown to increase cell death (Petreanu and Alvarez-Buylla, 2002) and decrease the total number of newborn cells in the OB (Alonso et al., 2006; Bastien-Dionne et al., 2010). Conversely, an odor-enriched environment is associated with increased numbers of newly born cells (Bovetti et al., 2009). Genetic modification of the electrical properties of adult-generated neurons, via viral-induced expression of K+ or Na+ channels, showed that their intrinsic activity level, but not their overall firing patterns, affects their survival (Lin et al, 2010). This mechanism of linking neuronal activity with survival could allow for precise modification of the OB circuit in relation to sensory experience, but how the new neurons enhance olfactory function is not known. Other studies have attempted to correlate V-SVZ proliferation to olfactory behavioral changes. Use of irradiation to reduce proliferation at the V-SVZ did not alter olfactory detection or short-term olfactory memory, but decreased long-term olfactory memory Lazarini et al., 2009). Other groups have also linked changes in proliferation at the V-SVZ to changes in olfactory memory (Rochefort et al., 2002; Sultan et al., 2010) as well as other odor-dependent tasks, such as olfactory fear-conditioning (Valley et al., 2009).
Direct ablation of newly born OB cells, via cre-induced diptheria toxin (DTA) expression, does not appear to have dramatic effects on spontaneous or innate odor discrimination (Imayoshi et al., 2008). However, in aged mice where V-SVZ neurogenesis is lower, fine odor discrimination (but not discrete odor discrimination) was impaired relative to young mice, and this impairment was partially reproduced by decreasing EGFR signaling in young mice (Enwere et al., 2004). Thus, depending on the specific odor task, the effect of adult neurogenesis on olfactory function can vary. Mathematical modeling of the OB circuit also suggests that neurogenesis could permit maximal discrimination of odors in an unsupervised manner and maintain this task in changing environments (Cecchi, et al., 2001). Such a function for OB neurons is reminiscent of pattern separation, a term borrowed from computational neuroscience modeling.
Pattern separation is also one of the main roles postulated for continued neuronal production in the adult SGZ. This process describes the task of making similar neural patterns of activity more distinct, which could be key for learning and memory. A role for adult-born neurons in the SGZ has been found in contextual fear-conditioned learning, recall in a radial arm maze task (Clelland et al., 2009; Creer et al., 2010; Sahay et al., 2011), memory consolidation (Kitamura and Inokuchi, 2014) and forgetting (Akers et al., 2014). Thus, in two different circuits, there appears to be a similar role and possible adaptive benefit from the continual addition of young neurons. The modulation of neurogenesis in the SGZ is also influenced by extrinsic factors such as an enriched environment (Kempermann et al., 1997), exercise (van Pragg et al., 1999), and stress (Duman et al., 2001; Veenema et al., 2007). Aging can also decrease SGZ proliferation (Kuhn et al., 1996) as was observed in the V-SVZ (Enwere et al., 2004). Tools to more precisely alter neurogenesis or neuronal recruitment will be required to ascertain the function(s) of adult born neurons in these circuits and how this dynamic process is regulated.
While the specific role of adult neuronal addition and replacement remains unclear, available evidence suggests that it is a mechanism of plasticity. The potential constraints associated with larger brains (as discussed above) and the requirement for longer neuronal migrations, need to be balanced with the potential functional benefits of having new neurons in particular brain circuits. Birds, for example, may have adapted neuronal replacement as a method to increase synaptic space for learning while still maintaining a small brain to facilitate flight (Fernando Nottebohm, personal communication). Humans, on the other hand, do not rely on olfaction as much as other species, such as dogs and mice, which might explain the lack of a prominent RMS in the adult human brain. If plasticity is the main function of new neurons, larger brains may have less of a need for the continued production of new neurons into adulthood because they already have enough neurons as a substrate for new learning and memory.
Conclusion
Cajal’s proclamation that in the adult “ ….Everything may die, nothing may be regenerated ” was followed by the careful statement: “It is for the scientists of the future to change, if possible, this harsh decree” (Ramon y Cajal, 1928). A century has passed, and technological advances and new experimental data have shown that adult neurogenesis does in fact occur in reptiles, birds and mammals. However, the location and magnitude varies in different species and there is little evidence that this process contributes to normal regeneration or repair in mammals. Comparative studies thus far suggest that the continued production of young neurons in the V-SVZ and migration into the OB is reduced in species with larger brains. Early regional specification of NSCs during fetal development establishes the sites where specific neuronal types are generated, and these proliferative regions become increasingly separated as the brain grows. In species with larger brains, such as humans, the dramatic brain growth between the late fetal period and infancy may subsequently result in a severe limitation for adult neurogenesis to continue from increasingly distant V-SVZ sources. The extensive expansion of the human forebrain could also impose migratory distances and obstacles that are unsurpassable for young migrating neurons. However, other factors, such as a functional benefit from adding new neurons to specific circuits, could work in conjunction with or in opposition to brain size. There may be examples of large brains that have devised developmental mechanisms to bypass limitations of neuronal migration. Additional comparative studies between diverse species (Patzke et al., 2013) could help fill these gaps in the field.
Even if it is rare, the process of adult neurogenesis has taught us much about how new neurons can be produced, migrate, and integrate into existing circuits in an adult brain. These processes could underlie new approaches to brain repair. It will be important to understand which brain circuits can incorporate new neurons and how to enhance delivery of new neurons to these regions. These are key challenges for future work in adult neurogenesis and neuronal replacement.
Acknowledgments
We thank Sara Gil-Perontin, Kirsten Obernier, and Trevor Sorrells for their comments on the manuscript.
This work is funded by the US National Institutes of Health (NS28478 and HD032116). Arturo Alvarez-Buylla is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at UCSF. Mercedes Paredes is supported by a California Regenerative Medicine Clinical Fellowship. Shawn Sorrells is supported by a NRSA NIH Fellowship (F32MH103003).
Footnotes
CONFLICT OF INTEREST
The authors do not have any financial or personal relationships with people or organizations that could have influenced their work and the opinions expressed in this review.
AUTHOR CONTRIBUTIONS
The authors take responsibility for the accuracy of how the data presented and discussed. MFP, SFS, and AAB wrote the original article and JV, MFP, AAB contributed to edits and graphics, prepared by SFS. MFP and SFS contributed equally to this manuscript.
LITERATURE CITED
- Agate RJ, Scott BB, Haripal B, Lois C, Nottebohm F. Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proc Natl Acad Sci U S A. 2009;106:17963–17967. doi: 10.1073/pnas.0909139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of Adult Neurogenesis: From Genes to Cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers KG, Martinez-canabal A, Restivo L, Yiu AP, Cristofaro A, De Hsiang HL, Wheeler AL, Guskjolen A, Niibori Y, Shoji H, Ohira K, Richards BA, Miyakawa T, Josselyn SA, Frankland PW. Hippocampal neurogenesis Regulates Forgetting During Adulthood and Infancy. Science. 2014:598–602. doi: 10.1126/science.1248903. [DOI] [PubMed] [Google Scholar]
- Alcantara Llaguno SR, Chen Y, McKay RM, Parada LF. Stem cells in brain tumor development. 1st. Elsevier Inc; Dallas, TX: 2011. [DOI] [PubMed] [Google Scholar]
- Alonso M, Viollet C, Gabellec M-M, Meas-Yedid V, Olivo-Marin J-C, Lledo P-M. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Postnatal neurogenesis in the guinea-pig. Nature. 1967;214:1098–1101. doi: 10.1038/2141098a0. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic investigation of cell proliferation in the brains of rats and cats. Anat Rec. 1963;145:573–591. doi: 10.1002/ar.1091450409. [DOI] [PubMed] [Google Scholar]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. J Comp Neurol. 1969;136:269–293. doi: 10.1002/cne.901360303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–4. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Mapping of radial glia and of a new cell type in adult canary brain. J Neurosci. 1988;8:2707–2712. doi: 10.1523/JNEUROSCI.08-08-02707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990a;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990b;249:1444–1446. doi: 10.1126/science.1698312. (80) [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Anderson S, Mione M, Yun K, Rubenstein JLR. Differential origins of neocortical projection and local circuit neurons: Role of Dlx genes in neocortical interneuron ogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. doi: 10.1016/s0896-6273(04)00140-0. [DOI] [PubMed] [Google Scholar]
- Arshad a, Vose LR, Vinukonda G, Hu F, Yoshikawa K, Csiszar a, Brumberg JC, Ballabh P. Extended Production of Cortical Interneurons into the Third Trimester of Human Gestation. Cereb Cortex. 2015:1–15. doi: 10.1093/cercor/bhv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachy I, Vernier P, Retaux S. The LIM-homeodomain gene family in the developing Xenopus brain: conservation and divergences with the mouse related to the evolution of the forebrain. J Neurosci. 2001;21:7620–7629. doi: 10.1523/JNEUROSCI.21-19-07620.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea A, Nottebohm F. Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A. 1994;91:11217–11221. doi: 10.1073/pnas.91.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien-Dionne PO, David LS, Parent A, Saghatelyan A. Role of sensory activity on chemospecific populations of interneurons in the adult olfactory bulb. J Comp Neurol. 2010;518:1847–1861. doi: 10.1002/cne.22307. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. The Human Brain During the Early First Trimester. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- Bedard A, Levesque M, Bernier PJ, Parent A. The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur J Neurosci. 2002a;16:1917–1924. doi: 10.1046/j.1460-9568.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- Bédard A, Cossette M, Lévesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002b;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- Bédard A, Parent A. Evidence of newly generated neurons in the human olfactory bulb. Brain Res Dev Brain Res. 2004;151:159–68. doi: 10.1016/j.devbrainres.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Bekiari C, Giannakopoulou A, Siskos N, Grivas I, Tsingotjidou A, Michaloudi H, Papadopoulos GC. Neurogenesis in the septal and Temporal Part of the Adult Rat Dentate Gyrus. Hippocampus. 2014;13:1–13. doi: 10.1002/hipo.22388. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Liebl J, Bernard S, Alkass K, Yeung MSY, Steier P, Kutschera W, Johnson L, Landén M, Druid H, Spalding KL, Frisén J. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch J, Kaeser M, Sadeghi Y, Rouiller EM, Redmond DE, Brunet JF. Doublecortin-positive cells in the adult primate cerebral cortex and possible role in brain plasticity and development. J Comp Neurol. 2011;519:775–789. doi: 10.1002/cne.22547. [DOI] [PubMed] [Google Scholar]
- Bolteus AJ, Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J Neurosci. 2004;24:7623–31. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–164. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Van den Bos W, Güroglu B. The role of the ventral medial prefrontal cortex in social decision making. J Neurosci. 2009;29:7631–7632. doi: 10.1523/JNEUROSCI.1821-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brus M, Keller M, Lévy F. Temporal features of adult neurogenesis: differences and similarities across mammalian species. Front Neurosci. 2013a;7:135. doi: 10.3389/fnins.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Gheusi G, Keller M, Lledo PM, Lévy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J Comp Neurol. 2013b;521:169–188. doi: 10.1002/cne.23169. [DOI] [PubMed] [Google Scholar]
- Burd GD, Nottebohm F. Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J Comp Neurol. 1985;240:143–52. doi: 10.1002/cne.902400204. [DOI] [PubMed] [Google Scholar]
- Cai Y, Xiong K, Chu Y, Luo D-W, Luo X-G, Yuan X-Y, Struble RG, Clough RW, Spencer DD, Williamson A, Kordower JH, Patrylo PR, Yan X-X. Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp Neurol. 2009;216:342–356. doi: 10.1016/j.expneurol.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K, Götz M. Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci. 2002;25:235–238. doi: 10.1016/s0166-2236(02)02156-2. [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO. Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci. 2001;11:175–182. doi: 10.1023/a:1012849801892. [DOI] [PubMed] [Google Scholar]
- Charvet CJ, Striedter GF. Developmental Origins of Mosaic Brain Evolution: Morphometric Analysis of the Developing Zebra Finch. 2009;213:203–213. doi: 10.1002/cne.22005. [DOI] [PubMed] [Google Scholar]
- Chawana R, Patzke N, Kaswera C, Gilissen E, Ihunwo AO, Manger PR. Adult neurogenesis in eight Megachiropteran species. Neuroscience. 2013;244:159–172. doi: 10.1016/j.neuroscience.2013.04.020. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn H-G, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddapah VA, Robel S, Watkins S, Sontheimer H. A neurocentric perspective on glioma invasion. Nat Rev Neurosci. 2014;15:455–65. doi: 10.1038/nrn3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Crews D, Wilczynski W. Effects of medial and dorsal cortex lesions on spatial memory in lizards. Behav Brain Res. 2001;118:27–42. doi: 10.1016/s0166-4328(00)00308-9. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf V, Bottjer SW. Age and Sex Differences in Mitotic Activity within the Zebra Finch Telencephalon. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Alvarez-Buylla A. Network of tangential pathways for neuronal migration in adult mammalian brain. Proc Natl Acad Sci U S A. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Enwere E, Shingo T, Gregg C, Fujikawa H, Ohta S, Weiss S. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24:8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisén J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, Wang LP, Yamaguchi M, Kettenmann H, Kempermann G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Pérez-Cañellas M, Alćantara S, García-Verdugo JM. 3-Acetylpyridine-induced degeneration and regeneration in the adult lizard brain: a qualitative and quantitative analysis. Brain Res. 1997;754:245–259. doi: 10.1016/s0006-8993(97)00085-1. [DOI] [PubMed] [Google Scholar]
- Font E, Desfilis E, Pérez-Cañellas MM, García-Verdugo JM. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav Evol. 2001;58:276–295. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Font E, García-Verdugo JM, Alcántara S, López-García C. Neuron regeneration reverses 3-acetylpyridine induced cell loss in the cerebral cortex of adult lizards. Brain Res. 1991;551:230–235. doi: 10.1016/0006-8993(91)90937-q. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Johnson F, Wang Z. Estrogen regulation of cell proliferation and distribution of estrogen receptor alpha in the brains of adult female prairie and meadow voles. J Comp Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Obernier K, Alvarez-Buylla A. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012 doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba LC, Rompani SB, Parraguez JI, Obernier K, Romero R, Cepko CL, Alvarez-Buylla A. Embryonic origin of postnatal neural stem cells. Cell. 2015;161:1644–1655. doi: 10.1016/j.cell.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung SJ, Joshi D, Allen KM, Sivagnanasundaram S, Rothmond DA, Saunders R, Noble PL, Webster MJ, Weickert CS. Developmental patterns of doublecortin expression and white matter neuron density in the postnatal primate prefrontal cortex and schizophrenia. PLoS One. 2011;6:e25194. doi: 10.1371/journal.pone.0025194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor Cells in the Adult Dentate Gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gajera CR, Emich H, Lioubinski O, Christ A, Beckervordersandforth-Bonk R, Yoshikawa K, Bachmann S, Christensen EI, Götz M, Kempermann G, Peterson AS, Willnow TE, Hammes A. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123:1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- García-Verdugo JM, Doetsch F, Wichterle H, Lim DA, Alvarez-Buylla A. Architecture and cell types of the adult subventricular zone: n search of the stem cells. J Neurobiol. 1998;36:234–248. doi: 10.1002/(sici)1097-4695(199808)36:2<234::aid-neu10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Garcìa-Verdugo JM, Ferron S, Flames N, Collado L, Desfilis E, Font E. The proliferative ventricular zone in adult vertebrates: A comparative study using reptiles, birds, and mammals. 2002;57:765–775. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Gavalas A, Studer M, Lumsden A, Rijli FM, Krumlauf R, Chambon P. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development. 1998;125:1123–1136. doi: 10.1242/dev.125.6.1123. [DOI] [PubMed] [Google Scholar]
- Ghosh L, Jessberger S. Supersize me-new insights into cortical expansion and gyration of the mammalian brain. EMBO J. 2013;32:1793–1795. doi: 10.1038/emboj.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Perotin S, Duran-Moreno M, Belzunegui S, Luquin MR, Garcia-Verdugo JM. Ultrastructure of the subventricular zone in Macaca fascicularis and evidence of a mouse-like migratory stream. J Comp Neurol. 2009;514:533–554. doi: 10.1002/cne.22026. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Granero S, Lezameta M, Garcia-Verdugo JM. Neurogenesis in the Adult Brain I: Adult Neurogenesis in Reptiles. Brain. 2011:257–270. [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nature Reviews Neuroscience. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Hack I, Bancila M, Loulier K, Carroll P, Cremer H. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002;5:939–945. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nature Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Parker PRL, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Kowalczyk TD, Wolf SA, Encinas JM, Rippey C, Enikolopov G, Kempermann G, Hevner RF. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J Neurosci. 2008;28:3707–3717. doi: 10.1523/JNEUROSCI.4280-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner HB, Bergmann O, Salehpour M, Rácz A, Tatarishvili J, Lindgren E, Csonka T, Csiba L, Hortobágyi T, Méhes G, Englund E, Solnestam BW, Zdunek S, Scharenberg C, Ström L, Ståhl P, Sigurgeirsson B, Dahl A, Schwab S, Possnert G, Bernard S, Kokaia Z, Lindvall O, Lundeberg J, Frisén J. The age and genomic integrity of neurons after cortical stroke in humans. Nat Neurosci. 2014;17:801–803. doi: 10.1038/nn.3706. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Ueda J, Oldham MC, Ukai W, Hashimoto E, Saito T, Geschwind DH, Kato T. Neurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome Res. 2011;21:688–696. doi: 10.1101/gr.112755.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, O’ Malley C, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29:222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci U S A. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Sawamoto K. Adult neurogenesis and its alteration under pathological conditions. Neurosci Res. 2009;63:155–164. doi: 10.1016/j.neures.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, Hinds JW. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977;197:1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2008;363:101–122. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G. New neurons for “survival of the fittest. Nature Reviews Neuroscience. 2012;13:727–736. doi: 10.1038/nrn3319. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes R, Lumsden A. Segmentation and the origin of regional diversity in the vertebrate central nervous system. Neuron. 1990;4:1–9. doi: 10.1016/0896-6273(90)90438-l. [DOI] [PubMed] [Google Scholar]
- Khatri P, Obernier K, Simeonova IK, Hellwig A, Hölzl-Wenig G, Mandl C, Scholl C, Wölfl S, Winkler J, Gaspar JA, Sachinidis A, Ciccolini F. Proliferation and cilia dynamics in neural stem cells prospectively isolated from the SEZ. Sci Rep. 2014;4:3803. doi: 10.1038/srep03803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Inokuchi K. Role of adult neurogenesis in hippocampal-cortical memory consolidation. Mol Brain. 2014;7:13. doi: 10.1186/1756-6606-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Kronenberg G, Cheung G, Kettenmann H, Kempermann G. Properties of doublecortin-(DCX)-expressing cells in the piriform cortex compared to the neurogenic dentate gyrus of adult mice. PLoS One. 2011;6:e25760. doi: 10.1371/journal.pone.0025760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler SJ, Williams NI, Stanton GB, Cameron JL, Greenough WT. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc Natl Acad Sci U S A. 2011;108:10326–31. doi: 10.1073/pnas.1017099108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M, Osumi N, Rubenstein JLR, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu D, Mikami A, Miyamoto Y, Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J Comp Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- Kornack DR, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. (Journal??) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Delbru M. Subpopulations of Proliferating Cells of the Adult Hippocampus Respond Differently to Physiologic Neurogenic Stimuli. 2003;463:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonica BE, Lui JH, Hansen DV, Kriegstein AR. Mitotic spindle orientation predicts outer radial glial cell generation in human neocortex. Nat Commun. 2013;4:1665. doi: 10.1038/ncomms2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Mouthon Ma, Gheusi G, de Chaumont F, Olivo-Marin JC, Lamarque S, Abrous DN, Boussin FD, Lledo PM. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4:1–11. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen T-M, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong H-W, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf K-R, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Li G, Fang L, Fernandez G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–672. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Fishell GJ, Alvarez-Buylla A. Postnatal mouse subventricular zone neuronal precursors can migrate and differentiate within multiple levels of the developing neuraxis. Proc Natl Acad Sci U S A. 1997;94:14832–14836. doi: 10.1073/pnas.94.26.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-W, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron. 2010;65:32–9. doi: 10.1016/j.neuron.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zuo M, Alvarez-Buylla A. Neurogenesis in juvenile and adult Ring Doves. J Comp Neurol. 1997;312:300–312. [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Molowny A, Garcia-Verdugo JM, Ferrer I. Delayed postnatal neurogenesis in the cerebral cortex of lizards. Brain Res. 1988;471:167–174. doi: 10.1016/0165-3806(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia C, Molowny A, Nacher J, Ponsoda X, Sancho-Bielsa F, Alonso-Llosa G. The lizard cerebral cortex as a model to study neuronal regeneration. An Acad Bras Cienc. 2002;74:85–104. doi: 10.1590/s0001-37652002000100006. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, Bonfanti L, Fasolo A, Peretto P. DCX and PSA-NCAM Expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb Cortex. 2009;19:1028–1041. doi: 10.1093/cercor/bhn145. [DOI] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. Neurogenesis in the caudate nucleus of the adult rabbit. J Neurosci. 2006;26:609–621. doi: 10.1523/JNEUROSCI.4371-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013:1–12. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Xu Q, Barth KA, Mikkola I, Holder N, Fjose A, Krauss S, Wilson SW. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron. 1994;13:1039–1053. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EMK, Lindvall O, Kokaia Z, Frisen J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BBR, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33:411–23. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik SZ, Lewis M, Isaacs A, Haskins M, van Winkle T, Vite CH, Watson DJ. Identification of the rostral migratory stream in the canine and feline brain. PLoS One. 2012;7:1–9. doi: 10.1371/journal.pone.0036016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O, Rubenstein JLR. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marín O, Valiente M, Ge X, Tsai L-H. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CAG, Goldman JE. Subpallial dlx2-expressing cells give rise to astrocytes and oligodendrocytes in the cerebral cortex and white matter. J Neurosci. 2002;22:9821–9830. doi: 10.1523/JNEUROSCI.22-22-09821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martí-Fàbregas J, Romaguera-Ros M, Gómez-Pinedo U, Martínez-Ramírez S, Jiménez-Xarrié E, Marín R, Martí-Vilalta JL, García-Verdugo JM. Proliferation in the human ipsilateral subventricular zone after ischemic stroke. Neurology. 2010;74:357–365. doi: 10.1212/WNL.0b013e3181cbccec. [DOI] [PubMed] [Google Scholar]
- Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol. 2002;451:170–188. doi: 10.1002/cne.10342. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17:207–214. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migaud M, Batailler M, Segura S, Duittoz A, Franceschini I, Pillon D. Emerging new sites for adult neurogenesis in the mammalian brain: a comparative study between the hypothalamus and the classical neurogenic zones. Eur J Neurosci. 2010;32:2042–2052. doi: 10.1111/j.1460-9568.2010.07521.x. [DOI] [PubMed] [Google Scholar]
- Mikula S, Trotts I, Stone J, Jones EG. Internet-Enabled High-Resolution Brain Mapping and Virtual Microscopy. NeuroImage. 2007;35(1):9–15. doi: 10.1016/j.neuroimage.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh Z, Merkle FT, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–78. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Butt SJB. Best-laid schemes for interneuron origin of mice and men. Nat Neurosci. 2013;16:1512–4. doi: 10.1038/nn.3557. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2002;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Pham K, Gil-Fernandez V, Mcewen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Alvarez-Buylla A. Neurogenesis and neuronal re- placement in adult birds. In: Cuello AC, editor. Restorative neurology, vol. 6: Neuronal cell death and repair. Amsterdam: Elsevier; 1993. pp. 227–236. [Google Scholar]
- Nottebohm F, O’Loughlin B, Gould K, Yohay K, Alvarez-Buylla A. The life span of new neurons in a song control nucleus of the adult canary brain depends on time of year when these cells are born. Proc Natl Acad Sci U S A. 1994;91:7849–7853. doi: 10.1073/pnas.91.17.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adulthood. Ann NY Acad Sci. 1985;457:143–161. doi: 10.1111/j.1749-6632.1985.tb20803.x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Plasticity in the adult avian central nervous system: possible relation between hormones, learning, and brain repair. Handb Physiol Nerv Syst. 1987:85–108. [Google Scholar]
- Nottebohm F. The road we travelled: Discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Ostrem BEL, Lui JH, Gertz CC, Kriegstein AR. Control of outer Radial Glial Stem Cell Mitosis in the Human Brain. Cell Rep. 2014;8:656–664. doi: 10.1016/j.celrep.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parent JM. Injury-Induced neurogenesis in the Adult Mammalian Brain. Neurosci. 2003;9:261–272. doi: 10.1177/1073858403252680. [DOI] [PubMed] [Google Scholar]
- Paton Ja, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Patzke N, LeRoy A, Ngubane NW, Bennett NC, Medger K, Gravett N, Kaswera-Kyamakya C, Gilissen E, Chawana R, Manger PR. The distribution of doublecortin-immunopositive cells in the Brains of Four Afrotherian Mammals: the Hottentot Golden Mole (Amblysomus hottentotus), the Rock Hyrax (Procavia capensis), the Eastern Rock Sengi (Elephantulus myurus) and the Four-Toed Sengi. Brain Behav Evol. 2014;84:227–41. doi: 10.1159/000367934. [DOI] [PubMed] [Google Scholar]
- Patzke N, Spocter Ma, Karlsson KA, Bertelsen MF, Haagensen M, Chawana R, Streicher S, Kaswera C, Gilissen E, Alagaili AN, Mohammed OB, Reep RL, Bennett NC, Siegel JM, Ihunwo AO, Manger PR. In contrast to many other mammals, cetaceans have relatively small hippocampi that appear to lack adult neurogenesis. Brain Struct Funct. 2013:1–23. doi: 10.1007/s00429-013-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñafiel A, Gutiérrez A, Martín R, Mar Pérez-Cañellas M, de la Calle A. A tangential neuronal migration in the olfactory bulbs of adult lizards. Neuroreport. 1996;7:1257–1260. doi: 10.1097/00001756-199605170-00007. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp Neurol. 2001;172:1–16. doi: 10.1006/exnr.2001.7768. [DOI] [PubMed] [Google Scholar]
- Pérez-Cañellas MM, García-Verdugo JM. Adult neurogenesis in the telencephalon of a lizard: a [3H]thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Dev Brain Res. 1996;93:49–61. doi: 10.1016/0165-3806(96)00014-4. [DOI] [PubMed] [Google Scholar]
- Pérez-Cañellas MM, Font E, García-Verdugo JM. Postnatal neurogenesis in the telencephalon of turtles: evidence for nonradial migration of new neurons from distant proliferative ventricular zones to the olfactory bulbs. Dev Brain Res. 1997;101:125–137. doi: 10.1016/s0165-3806(97)00058-8. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Aimar P, Bonfanti L. Cellular composition and cytoarchitecture of the rabbit subventricular zone and its extensions in the forebrain. 2006;507:491–507. doi: 10.1002/cne.21043. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JLR. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L, Rubenstein JLR. Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci. 2003;26:469–476. doi: 10.1016/S0166-2236(03)00234-0. [DOI] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Radonjić NV, Ayoub AE, Memi F, Yu X, Maroof A, Jakovcevski I, Anderson SA, Rakic P, Zecevic N. Diversity of cortical Interneurons in Primates: The Role of the Dorsal Proliferative Niche. Cell Rep. 2014:1–13. doi: 10.1016/j.celrep.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Limits of neurogenesis in primates. Science. 1985;227:1054–1056. doi: 10.1126/science.3975601. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002a;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurogenesis in adult primates. Prog Brain Res. 2002b;138:3–14. doi: 10.1016/S0079-6123(02)38067-1. [DOI] [PubMed] [Google Scholar]
- Ramirez Castillejo C, Nacher J, Molowny A, Ponsoda X, Lopez-Garcia C. PSA-NCAM immunocytochemistry in the cerebral cortex and other telencephalic areas of the lizard Podarcis hispanica: Differential expression during medial cortex neuronal regeneration. J Comp Neurol. 2002;453:145–156. doi: 10.1002/cne.10390. [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S. In: Degeneration and regeneration of the nervous system. May R, translator. New York: Hafner; 1928. [Google Scholar]
- Reinchisi G, Ijichi K, Glidden N, Jakovcevski I, Zecevic N. COUP-TFII expressing interneurons in human fetal forebrain. Cereb Cortex. 2012;22:2820–30. doi: 10.1093/cercor/bhr359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent J-D, Lledo P-M. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F, López JC, Vargas JP, Broglio C, Gómez Y, Salas C. Spatial memory and hippocampal pallium through vertebrate evolution: insights from reptiles and teleost fish. Brain Res Bull. 2002;57:499–503. doi: 10.1016/s0361-9230(01)00682-7. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pérez LM, Pérez-Martín M, Jiménez aJ, Fernández-Llebrez P. Immunocytochemical characterisation of the wall of the bovine lateral ventricle. Cell Tissue Res. 2003;314:325–335. doi: 10.1007/s00441-003-0794-1. [DOI] [PubMed] [Google Scholar]
- Romero-Alemán MM, Monzón-Mayor M, Yanes C, Lang D. Radial glial cells, proliferating periventricular cells, and microglia might contribute to successful structural repair in the cerebral cortex of the lizard Gallotia galloti. Exp Neurol. 2004;188:74–85. doi: 10.1016/j.expneurol.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Rousselot P, Lois C, Alvarez-Buylla A. Embryonic (PSA) N-CAM reveals chains of migrating neuroblasts between the lateral ventricle and the olfactory bulb of adult mice. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H-H, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo J-M, Rowitch DH, Alvarez-Buylla A. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT, McDermott MW, Parsa AT, Manuel García-Verdugo J, Berger MS, Alvarez-Buylla A. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 2004;427:740–744. doi: 10.1038/nature02301. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Hirota Y, Alfaro-Cervello C, Soriano-Navarro M, He X, Hayakawa-Yano Y, Yamada M, Hikishima K, Tabata H, Iwanami A, Nakajima K, Toyama Y, Itoh T, Alvarez-Buylla A, Garcia-Verdugo JM, Okano H. Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J Comp Neurol. 2011;519:690–713. doi: 10.1002/cne.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Kirn JR, Grossman M, Macklis JD, Nottebohm F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron. 2000;25:481–92. doi: 10.1016/s0896-6273(00)80910-1. [DOI] [PubMed] [Google Scholar]
- Schonberg T, Fox CR, Mumford JA, Congdon E, Trepel C, Poldrack RA. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an FMRI investigation of the balloon analog risk task. Front Neurosci. 2012;6:1–11. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott BB, Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol. 2007;502:202–14. doi: 10.1002/cne.21296. [DOI] [PubMed] [Google Scholar]
- Scott BB, Velho TA, Sim S, Lois C. Applications of avian transgenesis. ILAR J. 2010;51:353–361. doi: 10.1093/ilar.51.4.353. [DOI] [PubMed] [Google Scholar]
- Sequerra EB. Subventricular zone progenitors in time and space: generating neuronal diversity. 2014;8:1–6. doi: 10.3389/fncel.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisén J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Osumi N, Katsuyama Y. The germinal matrices in the developing dentate gyrus are composed of neuronal progenitors at distinct differentiation stages. Dev Dyn. 2013;242:1442–1453. doi: 10.1002/dvdy.24035. [DOI] [PubMed] [Google Scholar]
- Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 2010;24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- Thorpe WH. The Process of Song-Learning in the Chaffinch as Studied by Means of the Sound Spectrograph. Nature. 1954;173:465–469. [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, de Kloet ER, de Wilde MC, Roelofs AJ, Kawata M, Buwalda B, Neumann ID, Koolhaas JM, Lucassen PJ. Differential effects of stress on adult hippocampal cell proliferation in low and high aggressive mice. J Neuroendocrinol. 2007;19:489–98. doi: 10.1111/j.1365-2826.2007.01555.x. [DOI] [PubMed] [Google Scholar]
- Vellema M, Verschueren J, Van Meir V, Van der Linden A. A customizable 3-dimensional digital atlas of the canary brain in multiple modalities. Neuroimage. 2011;57:352–361. doi: 10.1016/j.neuroimage.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Vellema M, Hertel M, Urbanus SL, Van Der Linden A, Gahr M. Evaluating the predictive Value of Doublecortin as a Marker for Adult Neurogenesis in Canaries (Serinus canaria) 2014;1315:1299–1315. doi: 10.1002/cne.23476. [DOI] [PubMed] [Google Scholar]
- Wang C, Liu F, Liu Y-Y, Zhao C-H, You Y, Wang L, Zhang J, Wei B, Ma T, Zhang Q, Zhang Y, Chen R, Song H, Yang Z. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011;21:1534–1550. doi: 10.1038/cr.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, You Y, Qi D, Zhou X, Wang L, Wei S, Zhang Z, Huang W, Liu Z, Liu F, Ma L, Yang Z. Human and monkey striatal interneurons are derived from the medial ganglionic eminence but not from the adult subventricular zone. J Neurosci. 2014;34:10906–10923. doi: 10.1523/JNEUROSCI.1758-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman T, Noctor SC, Clinton BK, Honig LS, Kriegstein AR. Neurogenic radial glial cells in reptile, rodent and human: from mitosis to migration. Cereb Cortex. 2003;13:550–559. doi: 10.1093/cercor/13.6.550. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Woolsey T, Gado JH. The brain Atlas: A Visual Guide to the Human Central Nervous System. John Wiley & Sons; 2007. [Google Scholar]
- Wu W, Wong K, Chen J, Jiang Z, Dupuis S, Wu JY, Rao Y. Directional guidance of neuronal migration in the olfactory system by the protein Slit. Nature. 1999;400:331–336. doi: 10.1038/22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes C, Monzon-Mayor M, Ghandour MS, de Barry J, Gombos G. Radial glia and astrocytes in developing and adult telencephalon of the lizard Gallotia galloti as revealed by immunohistochemistry with anti-GFAP and anti-vimentin antibodies. J Comp Neurol. 1990;295:559–68. doi: 10.1002/cne.902950405. [DOI] [PubMed] [Google Scholar]
- Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–8296. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Marchetto MC, Gage FH. How to make a hippocampal dentate gyrus granule neuron. Development. 2014;141:2366–75. doi: 10.1242/dev.096776. [DOI] [PubMed] [Google Scholar]
- Zecevic N, Chen Y, Filipovic R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J Comp Neurol. 2005;491:109–122. doi: 10.1002/cne.20714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cai Y, Chu Y, Chen E, Feng J, Luo X, Xiong K, Struble RG, Clough RW, Patrylo PR, Kordower JH, Yan X. Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat. 2009;3:17. doi: 10.3389/neuro.05.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional Implications of Adult Neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]


