Abstract
Caudal visceral mesoderm (CVM) cells migrate from posterior to anterior of the Drosophila embryo as two bilateral streams of cells to support the specification of longitudinal muscles along the midgut. To accomplish this long-distance migration, CVM cells receive input from their environment, but little is known about how this collective cell migration is regulated. In a screen we found that wunen mutants exhibit CVM cell migration defects. Wunens are lipid phosphate phosphatases known to regulate the directional migration of primordial germ cells (PGCs). PGC and CVM cell types interact while PGCs are en route to the somatic gonadal mesoderm, and previous studies have shown that CVM impacts PGC migration. In turn, we found here that CVM cells exhibit an affinity for PGCs, localizing to the position of PGCs whether mislocalized or trapped in the endoderm. In the absence of PGCs, CVM cells exhibit subtle changes, including more cohesive movement of the migrating collective, and an increased number of longitudinal muscles is found at anterior sections of the larval midgut. These data demonstrate that PGC and CVM cell migrations are interdependent and suggest that distinct migrating cell types can coordinately influence each other to promote effective cell migration during development.
KEY WORDS: Caudal visceral mesoderm, Longitudinal visceral muscle founder, Cell migration, Primordial germ cells, Wunen, Lipid phosphate phosphatase, Tre1, Drosophila melanogaster
Summary: Distinct migrating cell types – muscle founder cells and primordial germ cells – coordinately influence each other to promote effective cell migration during development.
INTRODUCTION
Cell migration is crucial during embryonic development. It results in cell rearrangement within a developing embryo, effectively controlling cell-cell and cell-environment interactions to drive cell differentiation and organogenesis. Migrating cells can move directionally based on the recognition of region-specific cues that generally promote attraction or repulsion (Kurosaka and Kashina, 2008; Pocha and Montell, 2014). Most studies have focused on the cues emanating from non-motile cell types, which act essentially as targets to influence cell migration paths (e.g. Duchek et al., 2001; McDonald et al., 2006). However, in a few cases, it has also been found that distinct cell types can jointly influence each other's migration and/or morphogenesis (Bunt et al., 2010; Theveneau et al., 2013). Thus, directed cell migration may be regulated by a series of events that include fixed and/or moving cues.
Drosophila caudal visceral mesoderm (CVM) cell migration is an excellent system in which to study how collective cell migration is regulated to support proper organogenesis (Bae et al., 2012; Rørth, 2009). It has the potential to provide novel insight into both the mechanisms of guidance and the influence of homotypic and heterotypic cell-cell interactions. In Drosophila, CVM cells undergo the longest-distance migration seen during embryogenesis, during which they must interact with several tissues (Bae et al., 2012). They originate from a cluster of ∼50 cells located at the posterior-most end of the embryo (the caudal mesoderm) and migrate as two distinct groups on either side of the embryonic body towards the anterior over the course of 6 h (Kadam et al., 2012). At the end of this anteriorly directed movement, CVM cells fuse with fusion-competent myoblasts originating from the trunk visceral mesoderm (TVM) to form the longitudinal muscles that ensheath the gut (Lee et al., 2006). This collective behavior differs from other commonly studied cell migration models in Drosophila (i.e. border cells, salivary gland, germ cells, macrophage, salivary gland; reviewed by Pocha and Montell, 2014) and instead appears more similar to those studied in vertebrates, including the neural crest and lateral line, which move as cell streams (Friedl and Gilmour, 2009; Rorth, 2011; Theveneau and Mayor, 2012; Weijer, 2009).
Little is known about the specific cues that CVM cells utilize to complete their long-distance journey through the embryo. Previous studies have suggested that FGF signaling might serve multiple roles in guiding CVM cell migration, including as a chemoattractant to direct movement, survival factor and modulator of cell adhesion properties, as well as serving to promote cell proliferation (Kadam et al., 2012; Mandal et al., 2004; Reim et al., 2012). However, even in the absence of FGF signaling, CVM cells remain competent to initiate their movement towards the anterior, albeit misdirected and slow, suggesting that additional factors operate as guidance cues to CVM cells (Kadam et al., 2012; Reim et al., 2012).
The migration of another cell population, the primordial germ cells (PGCs), coincides spatiotemporally with that of CVM cells (Broihier et al., 1998; Reim et al., 2012). Here, we investigated whether CVM cells and PGCs share guidance cues and provide evidence that, instead, these distinct cell migrations are interdependent.
RESULTS
Spatiotemporal analysis of CVM cells and their association with PGCs
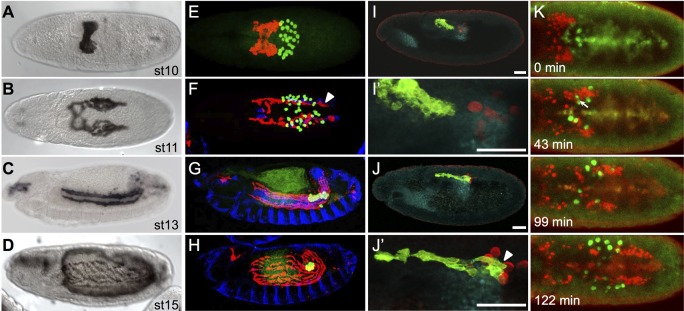
CVM cells can be tracked during different stages of development using reporter genes that track nuclei (HC3; Kadam et al., 2012) or cell outlines (GV2) (see the supplementary Materials and Methods). CVM cells originate from the most posterior region of the mesoderm in the early embryo and, through germband elongation, are carried to a dorsal position in the embryo (Fig. 1A). Subsequently, the cluster of cells separates bilaterally into two symmetric groups of ∼30 cells, which then move in a synchronous manner towards the anterior of the embryo (Fig. 1B) (reviewed by Bae et al., 2012). The active migration of these cells together with germband retraction supports the movement of CVM cells to anterior regions of the embryo, so that these muscle founder cells may extend along the length of the developing midgut (Fig. 1C) and are properly positioned to ultimately ensheath it (Fig. 1D).
Fig. 1.
CVM cell and PGC migrations overlap spatiotemporally. (A-D) Wild-type Drosophila embryos of the indicated stages expressing the CVM-specific reporter GV2 (HLH54F>Gap-Venus) detected with an anti-GFP antibody and DAB colorimetric staining. (E-J′) Colocalization of CVM cells relative to PGCs, TVM and/or pmg in wild-type embryos containing the GV2 reporter. anti-GFP, anti-Vasa, anti-FasIII and anti-Hb9 antibodies were used to detect CVM cells (red, E-H; green, I-J′), PGCs (green, E-H; red, I-J′), TVM (blue, E-H) or midgut primordium (cyan, I-J′), respectively. (A,B,E,F) Dorsal views; (C,D,G-J′) lateral views. In this and subsequent figures, all embryos are oriented with anterior to the left and dorsal side up unless otherwise noted. E-H are stage matched to A-D. Arrowheads (F,J′) indicate CVM cells elongated in the anterior-posterior orientation. Scale bars in I′ and J′ indicate relative magnification to images I and J, respectively. (K) Movie stills from live imaging of wild-type embryos containing PGC (vasa-GFP) and CVM cell (HLH54F>H2A-mCherry, ‘HC3’) reporters, visualized over the course of 3 h from a dorsal view (see Movie 1). Movie initiates at stage 10 (time 0 min) and continues for ∼3 h until germband elongation occurs at stage 12. Arrow (43 min) indicates the position of a germ cell deforming to move between CVM cells.
Through co-staining with antibodies against the GV2 GFP reporter to identify CVM cells and against Vasa, a germ cell-specific protein, to identify PGCs, our results confirm that CVM cells and PGCs are in close association during these developmental stages (e.g. Fig. 1E-J) (Broihier et al., 1998; Ismat et al., 2010). When CVM cells first initiate their anteriorly directed migration, they move onto the posterior midgut primordium (pmg) (Fig. 1E,I,I′) (Ismat et al., 2010). Simultaneously, PGCs exit from their position inside the pmg, moving upwards (ventrally, at this stage) (Fig. 1J,J′). Shortly afterwards, as both CVM cells and PGCs migrate anteriorly, the paths of the two cell types intersect and, concomitantly, cells intermingle generally within two bilateral groups (Fig. 1F). Eventually, PGCs take a different course as they move towards the somatic gonadal precursors (SGPs), while CVM cells continue their migration course towards the anterior, all the time retaining close association with the TVM (Fig. 1G). The PGCs remain in the developing gonad, whereas the CVM cells subsequently fuse with fusion-competent myoblasts and spread to cover the midgut (Fig. 1H).
We followed the migration of these cells over the 3-h period during which they co-migrate using live in vivo imaging (Movie 1, Fig. 1K). The live imaging shows that PGCs intermingle with CVM cells. At times, PGCs appear to squeeze through tight spaces between CVM cells (Fig. 1K, 43 min; Movie 1), and this observed deformation of PGCs strongly suggests that the two cell types contact each other directly. Ultimately, CVM cells overtake the PGCs midway through stage 11 (Fig. 1K, 99 min; Movie 1). CVM cells continue to move towards the anterior of the embryo (moving downwards, dorsally at this stage, out of the field of view), while the PGCs slow down as they approach the SGP (Fig. 1K, 122 min; Movie 1).
CVM migration is defective in wunen mutants
The close association of CVM cells with PGCs prompted us to investigate whether guidance cues for PGC migration impact CVM cell migration. In particular, two Drosophila genes, wunen (wun) and wunen 2 (wun2), were of interest owing to their previously documented role as repellents and survival factors that support the directional migration of PGCs (reviewed by Montell, 2006; Zhang et al., 1996, 1997).
Wunens are lipid phosphate phosphatases that have various functions, including the dephosphorylation of extracellular phospholipids (Pyne et al., 2004). The prevailing view is that they modify cues that guide the migration of PGCs, but they are also important for supporting PGC survival (Ile and Renault, 2013; Renault et al., 2004). wun and wun2 are required to orient germ cell migration out of the pmg and towards the SGP, and their expression at the midline within the central nervous system promotes bilateral sorting of the PGCs (Renault et al., 2010; Sano et al., 2005). Wunens also function to limit PGC homotypic cell-cell attraction. This particular repellant mechanism requires the expression of maternally derived, catalytically active Wun2 within PGCs themselves (Hanyu-Nakamura et al., 2004; Renault et al., 2004; Starz-Gaiano et al., 2001).
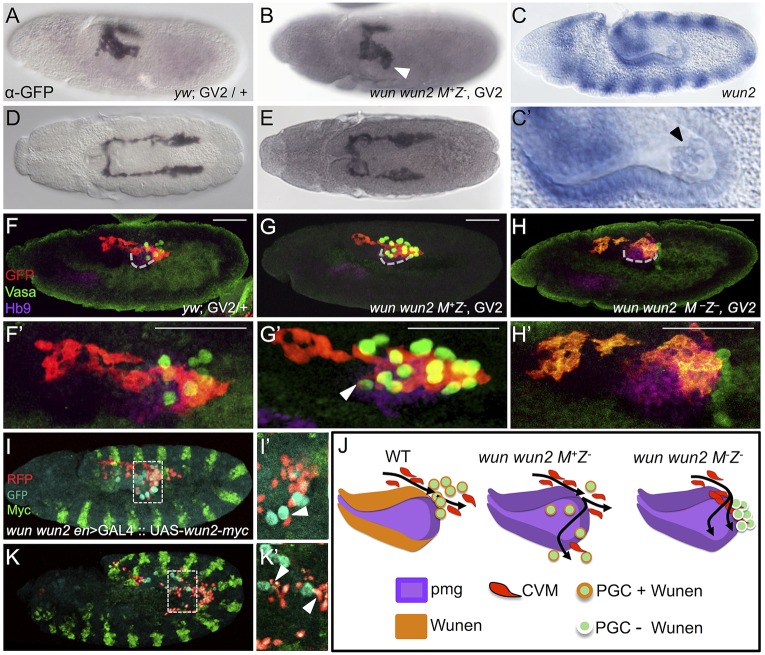
wun and wun2 are expressed in identical patterns in the ectoderm and within the posterior domain of the pmg epithelium (Fig. 2C,C′) (Renault et al., 2002; Starz-Gaiano et al., 2001) during the stage that CVM cells begin their migration over the pmg (Fig. 2A) and move anteriorly along the TVM (Fig. 2D). As expression of Wunens supports bilateral sorting of PGCs, we hypothesized that Wunens might also similarly influence the bilateral sorting of CVM cells.
Fig. 2.
CVM cell migration is more strongly affected by the loss of maternal than zygotic wunen genes. (A,B,D,E) Wild-type (A,D) and wun wun2 zygotic (B,E) embryos expressing the CVM reporter GV2 (heterozygous) detected with anti-GFP antibody and DAB staining. (A,B) Early stage 11 embryos oriented dorsolaterally showing migration of the CVM along the lateral side of the pmg in wun wun2 zygotic mutants (arrowhead in B) compared with wild type (A). (D,E) Late stage 11 embryos, dorsal view, showing CVM cell migration in wun wun2 zygotic mutants (E) compared with wild type (D). (C,C′) In situ hybridization using a riboprobe to wun2 within a stage 10 embryo, lateral view. C′ is a magnified view of PGCs within the pmg; the arrowhead indicates wun2 expression within the germ cells. (F-H′) Lateral views of early stage 11 embryos containing the GV2 reporter of wild-type (F,F′), wun wun2 M+Z− (G,G′) or wun wun2 M−Z−(H,H′) backgrounds in which antibodies to GFP (red), Vasa (green) and Hb9 (purple) were used to assay colocalization of CVM cells, PGCs and the pmg, respectively. Dashed gray line (F,G,H) marks the ventral side of pmg. Arrowhead (G′) indicates CVM cells that have migrated almost to the ventral side of the pmg, colocalized with a mislocalized PGC. Scale bars in F'-H' show relative magnification to images in F-H. (I,I′,K,K′) Lateral view of stage 11 (I,I′) and stage 12 (K,K′) wun wun2 M+Z− en>GAL4::UAS-wun2-myc; HC3 embryos with fluorescent antibody detection of CVM (anti-RFP, red), PGC (anti-Vasa, cyan) and ectopic Wun2 (anti-Myc, green). I′ and K′ show a single z-slice from I and K, respectively. Arrowheads indicate CVM cells that are mismigrating downwards (dorsally, at this stage; I') or laterally (K′) near mismigrated PGCs. (J) Model showing the aberrant movement of CVM cells and PGCs in the absence of zygotic wun wun2 (G) and both maternal and zygotic wun wun2 (H) compared with wild type (F).
As the wun and wun2 genes are linked, we used a transheterozygous combination of two previously characterized mutant chromosomes that disrupt both wun and wun2 function on each chromosome [wunCE/wunGL (Zhang et al., 1996); see the Materials and Methods] to assay the wun wun2 double-mutant somatic loss-of-function phenotypes (hereafter referred to as zygotic wun wun2 mutants). In the absence of zygotic wun wun2, the first sign of germ cell migration defects occurs soon after the PGCs exit the pmg (Starz-Gaiano et al., 2001; Zhang et al., 1996). At this early stage, one or several PGCs often aberrantly localize to the outside of the pmg, with a failure to migrate onto the lateral mesoderm. Those PGCs that do migrate away from the pmg do so aberrantly, moving off track from the SGP such that they are found even in the adjacent ectoderm (Starz-Gaiano et al., 2001; Zhang et al., 1996). As identified previously, loss of somatic Wunen from the pmg and ectoderm results in this mismigration of PGCs (Zhang et al., 1996). Similarly, in this mutant background, CVM cells also exhibit variable mismigration phenotypes. CVM cells are often positioned off to the side of the pmg in zygotic wun wun2 mutants (Fig. 2B illustrates a case of the most severe phenotype identified); a position that appears generally lower (i.e. more dorsal at this stage) and/or spread out compared with wild type (Fig. 2A). However, the frequency of CVM cell midline crossing was no higher in zygotic wun wun2 mutants than in wild type, as most clusters appear to migrate with bilateral symmetry (Fig. 2E).
Using an anti-Hb9 (ExEx – FlyBase) antibody to visualize the pmg, in the wun wun2 zygotic mutants CVM cells often mislocalize with PCGs that have mismigrated upon exit from the pmg (Fig. 2G,G′, compare with Fig. 2F,F′). CVM cells misroute, moving along the lateral sides of the pmg in wun wun2 mutants, as compared with their movement along the top of the pmg in wild type. Eventually, in these zygotic wun wun2 mutants, the majority of CVM cells are able to migrate along their typical path (Fig. S1E, compare with Fig. S1D). It does not appear that Wunens act as repellents to guide the bilateral migration of CVM cells, as is the case for PGCs. No significant differences in the frequency of asynchronous clusters or the mismigration of cells to the midline were associated with zygotic wun wun2 mutants. However, live analysis of wun wun2 mutants with the HC3 reporter in this zygotic mutant background revealed that CVM migration proceeds toward the anterior, but with some erratic movement (Movie 2). This is especially apparent at the rear of each cluster, where some cells appear to localize to the midline for prolonged periods, and on the lateral sides of the clusters where cells are off track and not proceeding at the same speed as the other CVM cells. This contrasts with the behavior of CVM cells in wild-type embryos, where CVM cells may approach the midline but do not remain there for any prolonged period of time (Movies 1 and 3).
At stages 12 and 13, an even more marked effect can be seen in zygotic wun wun2 mutants, as many CVM cells become detached from the TVM (Fig. S1H,H′, compare with Fig. S1G,G′). In wild-type embryos two distinct lines of CVM cells can be seen as they round the posterior of the embryo (Fig. S1A), whereas in the zygotic wun wun2 mutants the migration is disorganized as cells cluster around the mislocalized PGCs (Fig. S1B). Using a FasIII antibody as a TVM marker, it becomes clear that although the CVM cells make it to the most anterior position of their migration path, a large percentage of them do not make direct contact with the TVM (compare Fig. S1H′ with Fig. S1G′).
CVM cells are not excluded from tissues expressing Wun2
In zygotic wun wun2 mutants, the paths of wayward PGCs can be influenced additionally by ectopically expressing wun2 in the ectoderm within stripes, using engrailed (en)>GAL4 to drive expression via UAS-wun2-myc (Fig. 2I,I′,K,K′) (Mukherjee et al., 2013). As previously shown (Mukherjee et al., 2013), at stage 11 wayward PGCs move to ‘Wunen-free’ interstripe domains and are also restricted from mesodermal domains that are adjacent to ectodermal cells expressing ectopic Wun2 (Fig. 2I,I′, see also Fig. S2G-G″). By stage 12, as the PGCs cluster between the Wun2-expressing stripes, groups of CVM cells can be seen to be off course, specifically in the vicinity of these PGC clusters within interstripe domains (Fig. 2K,K′). Unlike the PGCs, however, the CVM cells are not generally affected by the ectodermal expression of wun2 and continue on their normal migratory course, except when near a ‘mismigrated’ PGC (Fig. S2G-G″). CVM migration, for the most part, appears similar to that seen in a wun wun2 zygotic mutant without ectopic expression of wun2 (compare Fig. S2D-F with Fig. S2A-C). Wunens are therefore unlikely to directly provide a guidance cue for the CVM cells, as they do for the PGCs.
Germ cell-derived Wun is required for proper CVM migration
Instead, we hypothesized that these wun wun2 mutant phenotypes affecting CVM cell migration could stem indirectly from the PGCs as a consequence of their mismigration in these mutant backgrounds (Fig. 2J). Zygotically expressed Wunen impacts the direction of PGC migration, whereas maternally expressed Wunen impacts PGC survival as well as homotypic interactions (Hanyu-Nakamura et al., 2004; Starz-Gaiano et al., 2001; Zhang et al., 1997). Therefore, we investigated the wun wun2 maternal phenotype to investigate whether germ cell-expressed Wunens support an additional role in CVM cell migration.
Embryos were obtained from females containing wun wun2 germline clones to eliminate maternal expression of both genes [i.e. maternal and zygotic mutants (M−Z−), see the Materials and Methods] and effects on CVM cell migration were examined. Previous studies had demonstrated that M−Z−wun wun2 mutants display more severe defects in PGC migration (Renault et al., 2004; Starz-Gaiano et al., 2001; Zhang et al., 1996, 1997). PGCs fail to cross the pmg epithelium until later in stage 11 and remain at their point of emergence from the pmg (Fig. 2H,H′), and subsequently the cells die (Renault et al., 2010). CVM cell migration defects are also exacerbated in M−Z− wun wun2 mutants compared with the zygotic mutants, as many CVM cells fail to migrate anteriorly past the gut (Fig. 2H,H′). At early stages, the majority of CVM cells appear unable to move past the germ cells, which are amassed at the leading edge of the pmg. Eventually though, CVM cells are able to proceed along their course (Fig. S1C,F), suggesting that maternally provided Wunens exert a transient effect on the early migration of CVM cells (Fig. 2J; see Discussion).
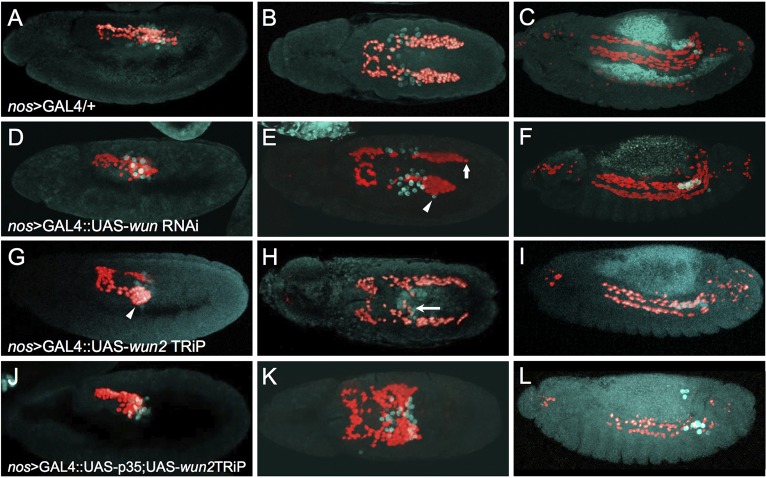
To assess directly if the pool of Wunen required for CVM migration includes maternal Wunen derived from the germ cells, we disabled Wunen function in the PGCs using tissue-specific RNAi-mediated knockdown of wun or wun2. The nos>GAL4.VP16 driver (Van Doren et al., 1998) was used to support knockdown of wun or wun2 from PGCs via RNAi constructs. Knockdown of each gene was investigated for the purposes of comparison, although previous results suggest that wun2 function within PGCs is most important. First, wun2 transcripts are detectable within germ cells (Fig. 2C,C′) whereas wun transcripts are not significantly enriched in germ cells (Renault et al., 2004; Starz-Gaiano et al., 2001). Second, loss of maternal wun alone has no effect on germ cell survival, whereas loss of maternal wun2 alone is sufficient to cause many germ cells to die (Renault et al., 2004; Starz-Gaiano et al., 2001). Perhaps not surprisingly, knockdown of wun from PGCs by expression of an RNAi UAS-hairpin construct via the nos>GAL4.VP16 driver had little effect (Fig. 3D-F, compare with Fig. 3A-C). The most severe effects involved the mismigration of a few PGCs (Fig. 3E, arrowhead) and the asynchrony of CVM clusters (Fig. 3E, arrow), which are phenotypes also exhibited by wild type. By contrast, knockdown of wun2 from PGCs resulted in strong CVM migration defects that included aggregation of CVM cells on the pmg at early stages (Fig. 3G, arrowhead) and mismigration of the CVM to the midline in proximity to mismigrated PGCs (Fig. 3H, arrow). All images shown utilized the wun2 TRiP line, but consistent results were seen when using an independent wun2 RNAi line (see Materials and Methods). Knockdown of wun2 in the CVM cells or pmg did not affect migration (Fig. S3A-G), demonstrating a PGC-specific role. Collectively, these data demonstrate that germ cell-derived wun2 expression influences CVM migration.
Fig. 3.
Germ cell-derived Wun is required for proper CVM cell migration. Immunofluorescence on embryos expressing HC3 to detect CVM (anti-RFP; red) and PGCs (anti-Vasa; cyan) in early stage 11 (A,D,G,J, lateral view), late stage 11 (B,E,H,K, dorsal view) and stage 13 (C,F,I,L, lateral view). All embryos express nos>GAL4.VP16 (nos>GAL4), which activates expression of UAS constructs specifically in the PGCs at these stages of development. (A-C) The nos>GAL4.VP16 driver alone shows wild-type CVM cell and PGC migration. (D-F) UAS-wun6446 RNAi driven by nos>GAL4.VP16 shows a weak phenotype, if any, as asymmetric migration (arrow) and a few lost PGCs (arrowhead) were observed at late stage 11. (G-I) Knocking down wun2 expression in PGCs by expression of UAS-wun232423TRiP causes the CVM cells to aggregate on the lateral side of the pmg in early stage 11 (arrowhead, G) and to mismigrate towards PGCs at late stage 11 (H, arrow), as well as causing a loss of CVM cells and PGCs at stage 13 (I). (J-L) Coexpressing the anti-apoptotic factor p35 along with the wun232423TRiP causes severe mismigration phenotypes. PGCs exit the pmg (J) but subsequently stall (K), resulting in sequestration of CVM cells at this position (K). More PGCs remain alive upon coexpression of p35 (compare L and I; see Fig. S4) but cause the opposite effect on CVM cells, as fewer cells are present at stage 13 (L). Presumably, more CVM cells die because they fail to reach the appropriate position along the TVM or otherwise mismigrate and die.
Since loss of Wunen activity from the germ cells also results in the death of PGCs at stage 10 when they exit the pmg (Hanyu-Nakamura et al., 2004), we controlled for the possibilities that (1) CVM cells are non-specifically attracted to PGCs because they are dying or that (2) the death of PGCs diminishes the attractant effect on CVM cells. Programmed cell death of the PGCs associated with wun2 knockdown was prevented by coexpression of the baculovirus caspase inhibitor p35 (Hay et al., 1994; Miller, 1997) along with a wun2 hairpin construct supporting RNAi within the germ cells. Previous studies have suggested that PGC death is caspase independent, but a clear effect on PGC number/viability was observed upon expression of p35 together with wun2 knockdown in this experimental setup, arguing that cell death is at least partially caspase dependent under these conditions (Fig. S4). Upon coexpression of p35 and wun2 RNAi, PGCs were similarly misdirected, moving only a short distance and remaining clumped at the midline, but PGCs were detected in normal numbers, even at later stages, suggesting that PGC death had been prevented through the coexpression of p35 (Fig. 3L compared with Fig. 3I; Fig. S4). CVM cells associated with the germ cells upon PGC exit from the pmg (Fig. 3J), as in the previous experiment with wun2 RNAi alone (Fig. 3G), but, surprisingly, remained associated with them for longer, appearing almost ‘stuck’ and aggregated to the PGCs. In this case, the majority of CVM cells were located at the midline well after the two CVM clusters should have completely separated (Fig. 3K, compare with Fig. 3B,H). Expression of p35 within the PGCs alone, in the absence of wun2 RNAi, did not affect CVM cell migration (Fig. S3H,I), nor did expression of lacZ, as a control, rescue PGC numbers (see Fig. S4). These results are consist with the idea that by preventing cell death upon wun2 knockdown, more PGCs are present, causing increased attraction of CVM cells to the PGCs, and also suggest that Wunens function to release the CVM cells from the PGCs since the ability of PGCs to derail CVM cells was increased in potency upon loss of Wun2 (see Discussion).
CVM cells are rerouted to germ cells immobilized in the pmg
To further explore the possibility that PGCs act as an attractant for CVM cells, we examined the migration of CVM cells when PGC migration had been perturbed by a Wunen-independent mechanism. In Trapped in endoderm 1 (Tre1) mutants, PGCs become immobilized in the lumen of the pmg, as they are unable to cross the epithelium (Kunwar et al., 2003). Tre1 encodes a G protein-coupled receptor that is expressed in PGCs, as well as in other somatic tissues, and is required cell-autonomously within PGCs for their migration out of the endoderm/pmg (Kunwar et al., 2003).
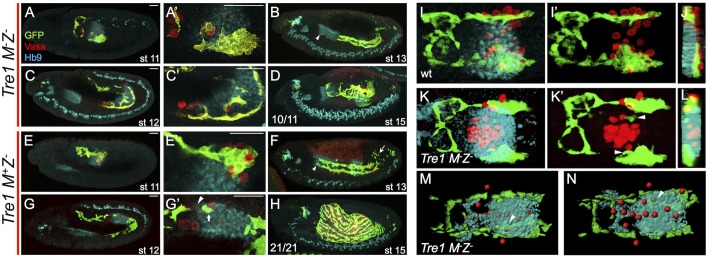
Tre1 M−Z− mutants exhibit strong phenotypes affecting both PGC and CVM cell migrations. As has been shown previously (Kunwar et al., 2003), except for a few escapers, the PGCs in M−Z− mutants are unable to exit the pmg. They exhibit a loss of polarity as well as an inability to cross the endoderm epithelium (Kunwar et al., 2003). In Tre1 M−Z− mutants, the CVM migrates to the lateral side of the pmg (Fig. 4A,A′,K-L), instead of migrating along the dorsal side as is most often observed in wild type (Fig. 4I-J). Strikingly, some CVM cells enter the pmg at the stage when they are normally moving over it and make contact with the PGCs that fail to emerge at stage 11 (Fig. 4K-L, arrowheads in K′) as well as later in the migration (Fig. 4M,N). The ability of CVM cells to penetrate the pmg epithelium suggests that they access a diffusible attractive cue emanating from the PGCs and demonstrates that CVM cells can move invasively through epithelium to find their targets. As a result of this attraction of CVM cells to PGCs positioned in the pmg, the front of the migrating CVM collective remains associated with the pmg and the PGCs contained within it (Fig. 4C,C′). CVM cells fail to migrate to the anterior end of the pmg (Fig. 4B, arrowhead) and fail to properly ensheath the gut at stage 15 (Fig. 4D). Furthermore, these phenotypes were similar for M−Z− and M−Z+ mutants (Fig. S5), suggesting that the key determinate of these PGC and CVM cell migration defects is maternally derived.
Fig. 4.
Immobilization of germ cells within the pmg redirects CVM cell migration. Lateral view (A-H) or dorsal view (I-N) of embryos fluorescently stained for GFP (GV2, CVM), Vasa (PGCs) and Hb9 (midgut primordium) in Tre1 mutants that are either M−Z− (A-D,K-N) or M+Z− (E-H) or wt (GV2/+) (I,J). Scale bars in A′,C′,E′,G′ show relative magnification to images in A,C,E and G, respectively. (A,E) Stage 11 embryos show colocalization of PGCs and CVM internal (A,A′) or just external (E,E′) to the Hb9 (pmg-associated endoderm) staining. (C,G) In stage 12 Tre1 M−Z− mutants (C,C″) most if not all of the PGCs are still internal to the pmg and the CVM migration does not continue past the end of the midgut, whereas in the M+Z− Tre1 mutants (G,G′) there are a few PGCs trapped in the pmg (arrowhead) and CVM cells are associated with them (arrow), although the majority of the CVM cells migrate normally. (B,F) At stage 13, the CVM in the Tre1 M−Z−mutants (B) fails to migrate to the anterior end of the pmg (marked by arrowheads in B and F) and all but one PGC remain internal to the pmg, whereas in the M+Z− mutants the PGCs have populated the gonadal mesoderm and the CVM appears somewhat normal except for cell death in the posterior (arrow). (D,H) The CVM in stage 15 Tre1 M−Z− mutants (D) fails to cover the gut and most if not all of the PGCs are lost. Ten out of the eleven embryos examined at this stage showed this severe phenotype, whereas 21 out of 21 M+Z− Tre1 mutant embryos examined look grossly normal at stage 15 (H). (I-L) Stage 11 embryos showing GV2/+ (I,I′) or Tre1 M−Z− (K,K′). (J,L) yz slice through the center of the pmg, with dorsal to the right, shows that the CVM in GV2/+ embryos migrates over the dorsal side of the pmg (I,J), whereas in the Tre1 M−Z− mutants the CVM cells invade the center of the pmg where the PGCs are localized (K,L, arrowheads in K′). (M,N) 3D projection model of stage 12 Tre1 M−Z− embryo created in Imaris using the surface detector (for CVM and pmg) or the spot detector (for PGCs) to clearly delineate the three cell groups. Arrowhead indicates CVM cells that are internal to the pmg when viewed in a single embryo both from the dorsal (M) and ventral (N) side of the projection.
Nevertheless, to directly investigate a role for Tre1 in somatic tissues that would indicate PGC-independent effects, we assayed Tre1 zygotic mutants (i.e. M+Z−). Subtle defects were observed, predominantly at early stages of the migration. PGCs appear to have a slight delay in exiting the pmg, and this is also likely to impact CVM cells, which can be found associated with them at stage 11 (Fig. 4E,E′) and stage 12 (Fig. 4G,G′). Despite some CVM cell death in the posterior of the embryo (Fig. 4F, arrow), the CVM cells complete their migration to the anterior end of the gut (Fig. 4F, arrowhead) and ultimately are able to properly ensheath the midgut (Fig. 4H). These zygotic phenotypes are less severe than those exhibited by the maternal Tre1 mutants, supporting the view that the maternal source of Tre1 is the most influential.
CVM cell migration is affected in the absence of germ cells
In the previous sections we showed evidence that PGCs act as a guidance cue for CVM cells. We tested this hypothesis in another way by examining CVM cell migration in genetic backgrounds lacking germ cells. CVM migration phenotypes were examined in embryos from germ cell-less (gcl) maternal mutants that either lack germ cells completely or have a greatly reduced number of germ cells. Importantly, this mutant background is known not to have pleiotropic effects, such as defective abdominal patterning (Robertson et al., 1999). We used the gclΔ mutation in trans with the chromosomal deficiency Df(2R)Exel7098 (referred to as gclΔ/Df) to reduce the chance of background effects from the gclΔ chromosome.
Embryos from gclΔ/Df maternal mutants showed a modest but significant difference in the number of embryos with CVM cells that cross the midline, as compared with those from wild-type females (Fig. S6A). The synchrony of the forward movement of the clusters, which is another previously characterized CVM phenotype (Kadam et al., 2012), did not differ from wild type (Fig. S6B), nor did the length of the CVM clusters (Fig. S6C).
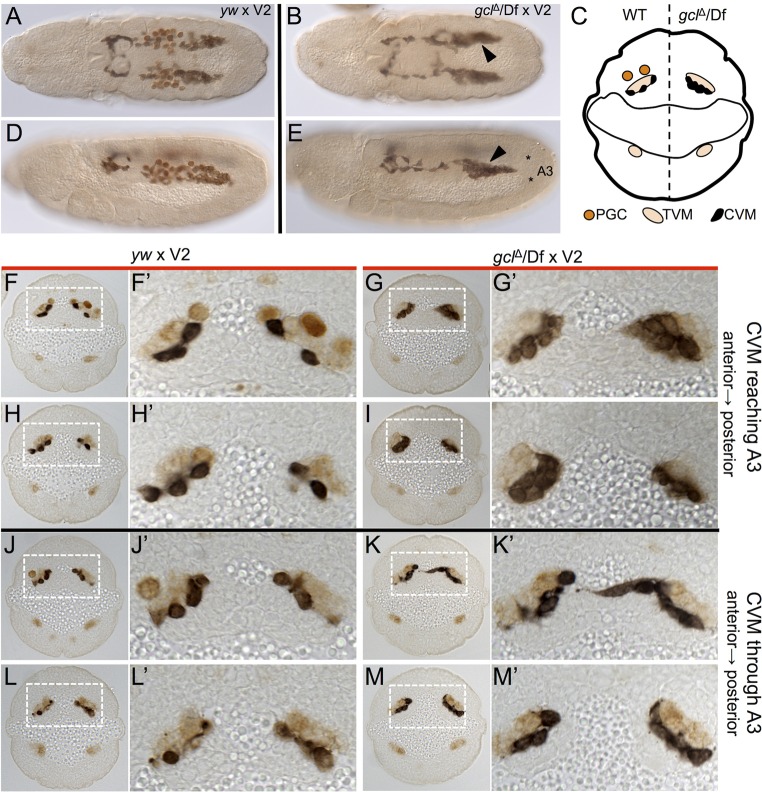
We observed a unique phenotype in embryos from gclΔ/Df maternal mutants, in which the clusters of CVM cells appeared more compact (Fig. 5, compare A with B, and D with E). This phenotype is present in embryos with no germ cells (Fig. 5B,E) and in those with a reduced number of germ cells (Fig. S6E). To explore this phenotype further, we examined CVM in transverse sections of embryos immunostained for CVM, TVM, and germ cells (Fig. 5C,F-M′). In embryos from gclΔ/Df maternal mutants without germ cells, CVM cells properly localize to the TVM but appear clumped and multilayered without the spaces between cells that are visible in embryos from wild-type females (Fig. 5, compare F,F′ with G,G′, and H,H′ with I,I′). This effect was present at multiple stages of CVM migration (Fig. 5, compare J-L′ with K-M′). We observed equivalent phenotypes in embryos from other maternal mutant backgrounds that also yield progeny without germ cells (Fig. S6D-K).
Fig. 5.
CVM cells are more tightly organized in the absence of germ cells. (A,B,D,E) Immunostaining for CVM (anti-GFP, black) and germ cells (anti-Vasa, brown) in embryos from wild type (yw) females (A,D) and from gclΔ/Df maternal mutants (B,E) crossed to GV2 males. (A,B) Dorsal views; (D,E) lateral views. Arrowheads (B,E) indicate regions of tighter CVM association. (C) Schematic depicting the spatial relationship between CVM, TVM and germ cells in a transverse section of a stage 11 embryo, near the back of the CVM cluster that overlaps the germ cells on the anterior-posterior axis. An embryo from a wild-type female is depicted on the left half of the section, and an embryo from a germ cell-less embryo from a gclΔ/Df maternal mutant on the right half of the section. (F-M′) Transverse sections of embryos immunostained for CVM (black, anti-GFP), PGCs (anti-Vasa, strong brown) and TVM (anti-FasIII, pale brown) of the indicated genotypes. F′-M′ show a magnified view of the boxed regions. CVM is more widely spaced in embryos from yw females (F,H,J,L) as compared with the CVM that is more tightly clumped and forms multilayers in embryos from gclΔ/Df females (G,I,K,M). Staging of embryos was matched by determining the progress of CVM cells through the abdominal segments. Asterisks (E) mark the approximate boundaries of the third abdominal segment (A3) for reference of CVM progression in F-M.
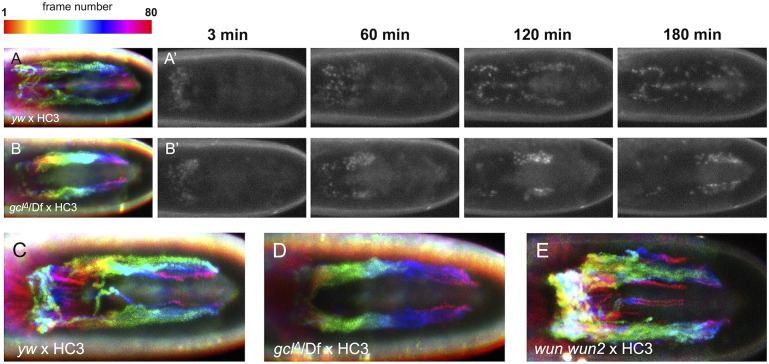
As in the fixed tissue analysis, live imaging of embryos from gclΔ/Df maternal mutants showed a consistently tighter cluster of CVM cells, as evidenced by their more ordered forward movement, compared with wild type, as migration proceeded (Fig. 6A,A′,C, compared with B,B′,D and Movie 3 compared with Movie 4). This suggested that the PGCs act to disperse CVM cells, possibly acting as a ‘drag’ to support the streaming migration profile as opposed to migrating as a tighter cohesive collective. A similar analysis of movies from wun wun2 zygotic mutants (Fig. 6E; Movie 2, embryo 2) also showed that a small subset of CVM cells stay permanently off track in this mutant background, as compared with wild type in which cells may venture off course, approaching the midline, but eventually rejoin the migrating cluster.
Fig. 6.
Cohesive forward movement of migrating CVM cell clusters is affected in embryos from gcl and wun wun2 maternal mutants. Temporal color-coded projections of CVM cell migration in wild-type (A,C), gclΔ/Df (B,D) and wun wun2 (E) embryos expressing the HC3 reporter. Each projection is compiled from 80 movie stills of time points taken every 3 min over a 4-h span (A′,B′), with each still assigned a unique color code corresponding to a specific time point. (A,A′,C) CVM migration in wild-type embryos proceeds in a cohesive yet dynamic fashion. Although some cells occasionally deviate towards the midline, these eventually rejoin and resume forward movement as part of a collective cluster. A′ and C are embryos 2 and 1, respectively, in Movie 3. By contrast, embryos from gcl maternal mutants migrate in a relatively undeviating and compact fashion (B,B′,D). B′ and D are embryos 2 and 3, respectively, in Movie 4. In wun wun2 mutants, some CVM cells wander towards the midline but fail to rejoin either of the two migrating clusters (E).
To examine if these CVM cell migration phenotypes in embryos obtained from gcl maternal mutants that lack germ cells translate into lasting effects on the development of the gut musculature, the longitudinal muscles associated with larval midguts were examined using a phalloidin stain to visualize musculature (Fig. 7A,B). Significantly more longitudinal muscles were present at the proventriculus, anterior section of the midgut, in the gcl mutant background than in wild type (Fig. 7C, P<0.05).
Fig. 7.
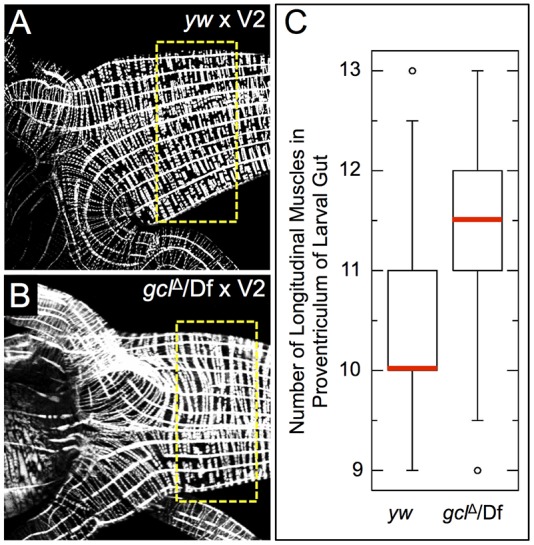
Extra longitudinal muscles are present in the midguts of larvae derived from gcl maternal mutant mothers. (A,B) Phalloidin staining of the proventriculus of third instar larval midguts to mark longitudinal and circular muscles. Longitudinal muscle runs left to right in panels. Dashed box indicates regions analyzed in C. (C) Quantification of the number of longitudinal muscles per half-side in the anterior midgut of the larvae. Red lines on the plot indicate the median value for each data set, which are significantly different (P=0.0048, Mann–Whitney–Wilcoxon test). yw females, n=25; gclΔ/Df maternal mutants, n=29.
DISCUSSION
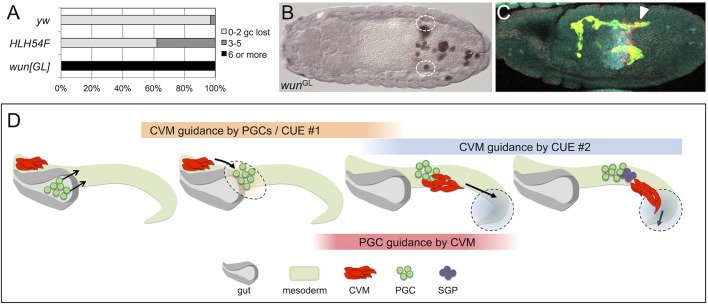
Our data provide evidence that the migration of CVM cells is guided in part by PGCs. Immobilization of the germ cells within the pmg results in the aberrant accumulation of CVM cells at this position and causing, at minimum, stalling of CVM cell migration, or stronger phenotypes that include the mismigration of CVM cells into the pmg epithelium when PGCs are trapped there (Fig. 4). The mismigration of PGCs has more of a negative impact on CVM cell migration than the absence of PGCs: the CVM cell migration phenotypes exhibited by mutants that lack germ cells are more subtle and, at most, the CVM cells become clumped and multilayered compared with wild type (Figs 5 and 6). However, to our knowledge, prior to this study Drosophila PGCs had not been shown to affect the migration of other somatic cell types. In addition, our data complement previous studies that show a dependence of PGC migration on the CVM and Wunens (Fig. 8A,B) (Broihier et al., 1998; Starz-Gaiano et al., 2001). It was previously proposed that the CVM helps to ensure faithful movement of the PGCs onto the lateral mesoderm upon exit of the PGCs from the pmg, with direct contact occurring between the two cell populations (Broihier et al., 1998). This is supported by the appearance of caudally located, mismigrated PGCs and, concomitantly, a reduction in the number of PGCs that reach the gonads in mutants that lack CVM (i.e. HLH54F, Fig. 8A) (Ismat et al., 2010). Our data now show that CVM cells are influenced by PGCs and, together with prior studies that have demonstrated the inverse relationship, support the view that the migrations of these two cell types are interdependent.
Fig. 8.
CVM cell and PGC migrations rely on multiple cues, including guidance from each other. (A) Quantification of the penetrance and severity of germ cell (gc) loss in wild-type embryos (yw, n=65), zygotic wunGL mutants (i.e. zygotic wun wun2, n=30) and HLH54F mutants (n=72). (B) Anti-Vasa staining of a wunGL homozygous embryo scored in A, showing many lost germ cells outside of the presumptive gonads (dashed ovals). (C) A M+Z− Tre1 mutant embryo showing CVM cell migration (arrowhead) relative to germ cells. (D) Model depicting how the migration of CVM cells could be guided by multiple cues. The overall successful migration of CVM cells in the absence of germ cells suggests that, besides the PGCs (Cue#1), another cue (Cue#2) is present in the vicinity of the pmg that acts in concert with the PGCs to initiate movement of the CVM cells towards the anterior and onto the TVM.
PGCs act as a cue for CVM cells
The ability of germ cells to act as a cue for CVM cells is highlighted by the general colocalization of CVM cells with mismigrated PGCs, whether due to loss of wunen, ectopic expression of wunen, or loss of Tre1. For example, in a Tre1 M+Z− embryo where the PGCs have migrated out of only one side of the endoderm, the CVM cells are associated with them, whereas on the other side, where PGCs remain temporarily stalled at the endoderm, the CVM cells are likewise stalled, seemingly awaiting direction (Fig. 8C).
Wunens appear to modulate the attraction of the CVM cells to the PGCs, but do not directly act as an attractant or repellent to the CVM. Ectopic Wun2 expression failed to impact CVM cell migration directly, but instead indirectly impacted cells by causing the PGCs to move off course. The failure of ectopic Wun2 expression to impact CVM cell migration directly, as cells predominantly migrate towards the anterior, suggests that the CVM guidance cues do not depend on zygotic Wunens. However, we did uncover a role for maternal Wun2 in supporting PGC-mediated attraction of CVM cells. The CVM cell guidance cue produced by PGCs appears to be influenced by Wunens. When PGCs are prevented from undergoing cell death in the nos>GAL4.VP16 wun2 knockdown through the expression of p35 (Fig. 3J,K), the prolonged association of CVM cells with these altered PGCs suggests that Wunens normally function to weaken the association of PGCs and CVM cells. It is possible that Wunens influence the levels of CVM attractant produced by the PGCs or the ability of the CVM to respond to that attractant. This invoked PGC-derived CVM attractant remains unknown, as does the specific attractant acted upon by Wunens that influences PGCs (Kunwar et al., 2006). Our data suggest that Wun2 is able to ‘tune’ the attraction of CVM cells to PGCs to ensure that the association between these two cells types is normally transient.
Furthermore, our results provide evidence that this PGC-derived guidance cue, ‘Cue#1’, is likely to be diffusible and, surprisingly, that CVM cells will invade epithelial tissue to reach its source (Fig. 8D). CVM cells become localized near PGCs immobilized inside the endoderm of Tre1 mutants, suggesting that CVM cells can access this cue through the pmg epithelium. The remodeling of the pmg to a permissive mesenchymal state, which normally allows passage of PGCs, does not require Tre1 or PGCs (Callaini et al., 1995; Jaglarz and Howard, 1995; Seifert and Lehmann, 2012), suggesting that the localization of the CVM cells to the pmg is not due to a physical change to the pmg epithelium in Tre1 mutants.
In summary, our results support the view that PGCs are likely to emit an early-acting CVM attractant (i.e. Cue#1), which is diffusible and influenced by PGC-derived maternal Wun2, but that additional CVM attractants (‘Cue#2’) also exist that act later and are PGC (and Wunen) independent (see Fig. 8C). Multiple cues are likely to be necessary to keep the CVM cells that are undergoing this long-distance migration on track.
Interdependence of germ cell and CVM cell migrations
To our knowledge, the PGC and CVM cells are the first pair of co-migrating cell populations shown to be interdependent in Drosophila. This interdependence most likely functions to enhance the fidelity of each migration process, as opposed to acting as a necessary factor for either. In HLH54F mutants, which lack CVM, PGCs do reach the gonadal mesoderm, although with decreased efficiency (Ismat et al., 2010). In mutants lacking mesoderm, exit of PGCs from the pmg remains oriented (Jaglarz and Howard, 1994), demonstrating that CVM cells are unlikely to be required until PGCs have exited the pmg. This suggests that the CVM cells are unlikely to play a role in guiding the PGCs out of the pmg and are more likely to affect PGC migration once CVM cells and PGCs meet spatiotemporally. It is possible that these heterotypic cell-cell interactions serve to regulate the timing/synchrony of developmental events. For instance, if the PGCs have not exited the pmg, then CVM cells may also be ‘held back’ to support the co-migration.
Our results also show that embryos lacking germ cells may exhibit subtle changes in the longitudinal muscles formed by the CVM, as revealed by increased muscle counts at the anterior end of the midgut (Fig. 7), and the fitness of these muscles might be altered in the absence of germ cells. As germ cells migrate in proximity to muscle tissues in many animals (Weidinger et al., 1999), it is possible that the interdependent migration of muscles and germ cells observed here in Drosophila embryos is a conserved relationship. The implications are that animals that are sterile due to a lack of germ cells could have defective muscles, and possibly impaired gut function, which could influence the physiological state of the animal yet remain undetected.
Interdependent cell migrations are emerging as a common phenomenon (reviewed by Pocha and Montell, 2014), and might be underappreciated because reciprocal interactions have not been widely investigated. For example, it has been shown in Xenopus that neural crest and placode cells undergo a ‘chase and run’ mode of migration, in which the neural crest cells chase placode cells through chemotaxis and placode cells run when they are contacted by neural crest cells (Theveneau et al., 2013). In Drosophila embryos, hemocytes and renal tubules undergo migrations that are temporally distinct, yet have been shown to guide each other's migration/morphogenesis (Bunt et al., 2010). Interdependent cell migrations might be commonplace during development and, despite the limited temporal role of such interactions, these interactions are likely to contribute to the efficient and robust movement of cells that is required to support proper development.
MATERIALS AND METHODS
Fly strains, genetics and generation of transgenic lines
All crosses and strains were maintained at 23-25°C, unless noted otherwise. For details of the fly stocks used and the generation of the transgenic lines employed see the supplementary Materials and Methods.
Fixation, in situ hybridization and antibody staining
Embryos were fixed and stained using standard protocols (Frasch, 1995; Jiang et al., 1991; Kosman et al., 2004). For additional information regarding staining procedures see the supplementary Materials and Methods.
Live imaging and image processing
The CVM cells were visualized by introduction of HC3 reporter into each mutant stock. Live imaging was conducted as described previously (Kadam et al., 2012) with some modifications, as detailed in the supplementary Materials and Methods.
Acknowledgements
We thank Manfred Frasch, Paul Lasko, Ruth Lehmann, Andrew Renault, Jim Skeath, the TRiP Stock Center and the Developmental Studies Hybridoma Bank for providing fly stocks and antibodies used in this study.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
V.S., L.D., Y.-K.B. and A.S. designed the experiments; V.S., L.D., Y.-K.B., F.M., N.T. and J.S. conducted the experiments; V.S., L.D., F.M. and A.S. analyzed the data and wrote the paper.
Funding
This work was funded by the National Institutes of Health [R21HD073860 and R01GM104838 to A.S.]; the American Heart Association [11POST7600181 to Y.-K.B.]; and the American Cancer Society [PF-15-202-01-DDC to V.S.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.134346.supplemental
References
- Arkov A. L., Wang J.-Y., Ramos A. and Lehmann R. (2006). The role of Tudor domains in germline development and polar granule architecture. Development 133, 4053-4062. 10.1242/dev.02572 [DOI] [PubMed] [Google Scholar]
- Bae Y.-K., Trisnadi N., Kadam S. and Stathopoulos A. (2012). The role of FGF signaling in guiding coordinate movement of cell groups: guidance cue and cell adhesion regulator? Cell Adhes. Migr. 6, 397-403. 10.4161/cam.21103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F. and Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312-3317. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broihier H. T., Skeath J. B. (2002). Drosophila homeodomain protein dHb9 directs neuronal fate via crossrepressive and cell-nonautonomous mechanisms. Neuron 35, 39-50. 10.1016/S0896-6273(02)00743-2 [DOI] [PubMed] [Google Scholar]
- Broihier H. T., Moore L. A., Van Doren M., Newman S. and Lehmann R. (1998). zfh-1 is required for germ cell migration and gonadal mesoderm development in Drosophila. Development 125, 655-666. [DOI] [PubMed] [Google Scholar]
- Bunt S., Hooley C., Hu N., Scahill C., Weavers H. and Skaer H. (2010). Hemocyte-secreted type IV collagen enhances BMP signaling to guide renal tubule morphogenesis in Drosophila. Dev. Cell 19, 296-306. 10.1016/j.devcel.2010.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaini G., Riparbelli M. G. and Dallai R. (1995). Pole cell migration through the gut wall of the Drosophila embryo: analysis of cell interactions. Dev. Biol. 170, 365-375. 10.1006/dbio.1995.1222 [DOI] [PubMed] [Google Scholar]
- Dahanukar A., Foster K., van der Goes van Naters W. M. and Carlson J. R. (2001). A Gr receptor is required for response to the sugar trehalose in taste neurons of Drosophila. Nat. Neurosci. 4, 1182-1186. 10.1038/nn765 [DOI] [PubMed] [Google Scholar]
- Duchek P., Somogyi K., Jékely G., Beccari S. and Rørth P. (2001). Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107, 17-26. 10.1016/S0092-8674(01)00502-5 [DOI] [PubMed] [Google Scholar]
- Ephrussi A., Dickinson L. K. and Lehmann R. (1991). Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66, 37-50. 10.1016/0092-8674(91)90137-N [DOI] [PubMed] [Google Scholar]
- Frasch M. (1995). Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 374, 464-467. 10.1038/374464a0 [DOI] [PubMed] [Google Scholar]
- Friedl P. and Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445-457. 10.1038/nrm2720 [DOI] [PubMed] [Google Scholar]
- Georgias C., Wasser M. and Hinz U. (1997). A basic-helix-loop-helix protein expressed in precursors of Drosophila longitudinal visceral muscles. Mech. Dev. 69, 115-124. 10.1016/S0925-4773(97)00169-X [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K., Kobayashi S. and Nakamura A. (2004). Germ cell-autonomous Wunen2 is required for germline development in Drosophila embryos. Development 131, 4545-4553. 10.1242/dev.01321 [DOI] [PubMed] [Google Scholar]
- Hay B., Jan L. Y. and Jan Y. N. (1990). Localization of vasa, a component of Drosophila polar granules, in maternal-effect mutants that alter embryonic anteroposterior polarity. Development 109, 425-433. [DOI] [PubMed] [Google Scholar]
- Hay B. A., Wolff T. and Rubin G. M. (1994). Expression of baculovirus P35 prevents cell death in Drosophila. Development 120, 2121-2129. [DOI] [PubMed] [Google Scholar]
- Ile K. E. and Renault A. D. (2013). Compartmentalizing the embryo: lipids and septate junction mediated barrier function. Fly 7, 18-22. 10.4161/fly.22938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismat A., Schaub C., Reim I., Kirchner K., Schultheis D. and Frasch M. (2010). HLH54F is required for the specification and migration of longitudinal gut muscle founders from the caudal mesoderm of Drosophila. Development 137, 3107-3117. 10.1242/dev.046573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglarz M. K. and Howard K. R. (1994). Primordial germ cell migration in Drosophila melanogaster is controlled by somatic tissue. Development 120, 83-89. [DOI] [PubMed] [Google Scholar]
- Jaglarz M. K. and Howard K. R. (1995). The active migration of Drosophila primordial germ cells. Development 121, 3495-3503. [DOI] [PubMed] [Google Scholar]
- Jiang J., Kosman D., Ip Y. T. and Levine M. (1991). The dorsal morphogen gradient regulates the mesoderm determinant twist in early Drosophila embryos. Genes Dev. 5, 1881-1891. 10.1101/gad.5.10.1881 [DOI] [PubMed] [Google Scholar]
- Kadam S., Ghosh S. and Stathopoulos A. (2012). Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development 139, 699-708. 10.1242/dev.068791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J., Smith J. L. and Macdonald P. M. (1991). oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 66, 23-35. 10.1016/0092-8674(91)90136-M [DOI] [PubMed] [Google Scholar]
- Kosman D., Mizutani C. M., Lemons D., Cox W. G., McGinnis W. and Bier E. (2004). Multiplex detection of RNA expression in Drosophila embryos. Science 305, 846 10.1126/science.1099247 [DOI] [PubMed] [Google Scholar]
- Kunwar P. S., Starz-Gaiano M., Bainton R. J., Heberlein U. and Lehmann R. (2003). Tre1, a G protein-coupled receptor, directs transepithelial migration of Drosophila germ cells. PLoS Biol. 1, e80 10.1371/journal.pbio.0000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar P. S., Siekhaus D. E. and Lehmann R. (2006). In vivo migration: a germ cell perspective. Annu. Rev. Cell Dev. Biol. 22, 237-265. 10.1146/annurev.cellbio.22.010305.103337 [DOI] [PubMed] [Google Scholar]
- Kurosaka S. and Kashina A. (2008). Cell biology of embryonic migration. Birth Defects Res. C Embryo Today 84, 102-122. 10.1002/bdrc.20125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-H., Zaffran S. and Frasch M. (2006). Development of the larval visceral musculature. In Muscle Development in Drosophila, pp. 62-78. New York: Springer. [Google Scholar]
- Mandal L., Dumstrei K. and Hartenstein V. (2004). Role of FGFR signaling in the morphogenesis of the Drosophila visceral musculature. Dev. Dyn. 231, 342-348. 10.1002/dvdy.20088 [DOI] [PubMed] [Google Scholar]
- McCabe J. B. and Berthiaume L. G. (1999). Functional roles for fatty acylated amino-terminal domains in subcellular localization. Mol. Biol. Cell 10, 3771-3786. 10.1091/mbc.10.11.3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Pinheiro E. M., Kadlec L., Schupbach T. and Montell D. J. (2006). Multiple EGFR ligands participate in guiding migrating border cells. Dev. Biol. 296, 94-103. 10.1016/j.ydbio.2006.04.438 [DOI] [PubMed] [Google Scholar]
- Miller L. K. (1997). Baculovirus interaction with host apoptotic pathways. J. Cell. Physiol. 173, 178-182. [DOI] [PubMed] [Google Scholar]
- Montell D. J. (2006). The social lives of migrating cells in Drosophila. Curr. Opin. Genet. Dev. 16, 374-383. 10.1016/j.gde.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Neher R. A. and Renault A. D. (2013). Quantifying the range of a lipid phosphate signal in vivo. J. Cell Sci. 126, 5453-5464. 10.1242/jcs.136176 [DOI] [PubMed] [Google Scholar]
- Nakamura A., Amikura R., Hanyu K. and Kobayashi S. (2001). Me31B silences translation of oocyte-localizing RNAs through the formation of cytoplasmic RNP complex during Drosophila oogenesis. Development 128, 3233-3242. [DOI] [PubMed] [Google Scholar]
- Pocha S. M. and Montell D. J. (2014). Cellular and molecular mechanisms of single and collective cell migrations in Drosophila: themes and variations. Annu. Rev. Genet. 48, 295-318. 10.1146/annurev-genet-120213-092218 [DOI] [PubMed] [Google Scholar]
- Pyne S., Kong K.-C. and Darroch P. I. (2004). Lysophosphatidic acid and sphingosine 1-phosphate biology: the role of lipid phosphate phosphatases. Semin. Cell Dev. Biol. 15, 491-501. 10.1016/j.semcdb.2004.05.007 [DOI] [PubMed] [Google Scholar]
- Reim I., Hollfelder D., Ismat A. and Frasch M. (2012). The FGF8-related signals Pyramus and Thisbe promote pathfinding, substrate adhesion, and survival of migrating longitudinal gut muscle founder cells. Dev. Biol. 368, 28-43. 10.1016/j.ydbio.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault A. D., Starz-Gaiano M. and Lehmann R. (2002). Metabolism of sphingosine 1-phosphate and lysophosphatidic acid: a genome wide analysis of gene expression in Drosophila. Mech. Dev. 119 Suppl. 1, S293-S301. 10.1016/S0925-4773(03)00131-X [DOI] [PubMed] [Google Scholar]
- Renault A. D., Sigal Y. J., Morris A. J. and Lehmann R. (2004). Soma-germ line competition for lipid phosphate uptake regulates germ cell migration and survival. Science 305, 1963-1966. 10.1126/science.1102421 [DOI] [PubMed] [Google Scholar]
- Renault A. D., Kunwar P. S. and Lehmann R. (2010). Lipid phosphate phosphatase activity regulates dispersal and bilateral sorting of embryonic germ cells in Drosophila. Development 137, 1815-1823. 10.1242/dev.046110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S. E., Dockendorff T. C., Leatherman J. L., Faulkner D. L. and Jongens T. A. (1999). germ cell-less is required only during the establishment of the germ cell lineage of Drosophila and has activities which are dependent and independent of its localization to the nuclear envelope. Dev. Biol. 215, 288-297. 10.1006/dbio.1999.9453 [DOI] [PubMed] [Google Scholar]
- Rørth P. (2009). Collective cell migration. Annu. Rev. Cell Dev. Biol. 25, 407-429. 10.1146/annurev.cellbio.042308.113231 [DOI] [PubMed] [Google Scholar]
- Rørth P. (2011). Whence directionality: guidance mechanisms in solitary and collective cell migration. Dev. Cell 20, 9-18. 10.1016/j.devcel.2010.12.014 [DOI] [PubMed] [Google Scholar]
- Sano H., Renault A. D. and Lehmann R. (2005). Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J. Cell Biol. 171, 675-683. 10.1083/jcb.200506038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert J. R. K. and Lehmann R. (2012). Drosophila primordial germ cell migration requires epithelial remodeling of the endoderm. Development 139, 2101-2106. 10.1242/dev.078949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starz-Gaiano M., Cho N. K., Forbes A. and Lehmann R. (2001). Spatially restricted activity of a Drosophila lipid phosphatase guides migrating germ cells. Development 128, 983-991. [DOI] [PubMed] [Google Scholar]
- Tearle R. G. and Nusslein-Volhard C. (1987). Tubingen mutants and stock list. Drosophila Information Service 66, 209-269. [Google Scholar]
- Theveneau E. and Mayor R. (2012). Neural crest migration: interplay between chemorepellents, chemoattractants, contact inhibition, epithelial-mesenchymal transition, and collective cell migration. Wiley Interdiscip. Rev. Dev. Biol. 1, 435-445. 10.1002/wdev.28 [DOI] [PubMed] [Google Scholar]
- Theveneau E., Steventon B., Scarpa E., Garcia S., Trepat X., Streit A. and Mayor R. (2013). Chase-and-run between adjacent cell populations promotes directional collective migration. Nat. Cell Biol. 15, 763-772. 10.1038/ncb2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno K., Ohta M., Morita H., Mikuni Y., Nakajima S., Yamamoto K. and Isono K. (2001). Trehalose sensitivity in Drosophila correlates with mutations in and expression of the gustatory receptor gene Gr5a. Curr. Biol. 11, 1451-1455. 10.1016/S0960-9822(01)00450-X [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A. L. and Lehmann R. (1998). Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 8, 243-246. 10.1016/S0960-9822(98)70091-0 [DOI] [PubMed] [Google Scholar]
- Wang C. I. and Lehmann R. (1991). Nanos is the localized posterior determinant in Drosophila. Cell 66, 637-647. 10.1016/0092-8674(91)90110-K [DOI] [PubMed] [Google Scholar]
- Weidinger G., Wolke U., Koprunner M., Klinger M. and Raz E. (1999). Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. Development 126, 5295-5307. [DOI] [PubMed] [Google Scholar]
- Weijer C. J. (2009). Collective cell migration in development. J. Cell Sci. 122, 3215-3223. 10.1242/jcs.036517 [DOI] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Cheng Y. and Howard K. (1996). Identification and genetic analysis of wunen, a gene guiding Drosophila melanogaster germ cell migration. Genetics 143, 1231-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Zhang J., Purcell K. J., Cheng Y. and Howard K. (1997). The Drosophila protein Wunen repels migrating germ cells. Nature 385, 64-67. 10.1038/385064a0 [DOI] [PubMed] [Google Scholar]