Abstract
Induction of cathelicidin-mediated antimicrobial pathway against intracellular M. tuberculosis by 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), the active form of vitamin D, has been documented in vitro. However, in in vivo studies related to inflammatory disorders, 1,25(OH)2D3 has been demonstrated to induce an anti-inflammatory response. We therefore examined whether in the murine model of tuberculosis, the anti-inflammatory effects of 1,25(OH)2D3 would affect the outcome of M. tuberculosis infection. We show here that administration of 1,25(OH)2D3 to M. tuberculosis infected mice led to a change in lung granuloma architecture, characterized by a marked decrease in B cell lymphocytic aggregates. Consistent with the altered granulomas, 1,25(OH)2D3-treated mice also exhibited significantly higher bacterial burden in the lungs compared to the control group. These findings highlight the need to further investigate the effect of vitamin D on host immunity to M. tuberculosis in the context of the granulomatous response.
Vitamin D exerts regulatory control over a multitude of biological functions, including immune regulation1,2. The synthesis of the physiological form of vitamin D, vitamin D3 (cholecalciferol) is initiated with the photolysis of 7-dehydrocholestrol in the skin. Upon UVB radiation exposure, 7-dehydrocholestrol is converted to pre-vitamin D3 which is subsequently isomerized to vitamin D3. Conversion of vitamin D3 to 25-hydroxyvitamin D3 (25(OH)D3) takes place in the liver, and subsequently to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in the kidney. Extra-renal conversion of 25(OH)D3 to 1,25(OH)2D3 occurs in other tissue types including immune cells such as dendritic cells, macrophages and T cells3,4,5. 1,25(OH)2D3, the bioactive form of vitamin D mediates its effect in various cell types by binding to its nuclear receptor known as vitamin D receptor (VDR) which functions as a heterodimer with the retinoid X receptor and together with co-regulatory complexes, affects the transcription of target genes5,6,7,8.
Previous studies have noted that 1,25(OH)2D3 restricts intracellular Mycobacterium tuberculosis (Mtb) replication via the induction of anti-microbial peptides9. The convergence of Interleukin (IL)-1β and VDR signaling pathways in antimicrobial response has also been reported10,11. The vitamin D-mediated anti-microbial pathway can be activated by CD40L and IFNγ12. 1,25(OH)2D3 also inhibits intracellular growth of Mtb by interfering with the accumulation of infection-induced lipid droplets13. In contrast to its antimicrobial host protective functions, 1,25(OH)2D3 also down-modulates proinflammatory adaptive immune responses14,15,16,17. 1,25(OH)2D3 inhibits differentiation of dendritic cells resulting in the suppression of the proinflammatory cytokine IL-12 and an increase in the anti-inflammatory cytokine IL-1018,19. Similarly, a number of in vitro studies suggest that 1,25(OH)2D3 induces differentiation of T regulatory cells20,21,22. In murine models of autoimmune diseases, 1,25(OH)2D3 has been reported to suppress the generation of Th1 and Th17 effector cells23,24,25,26,27; the same T cell subsets that are involved in protection against tuberculosis28,29. With the identification of many immunomodulatory properties of 1,25(OH)2D3, interest in vitamin D supplementation as a therapeutic approach to treat chronic inflammatory diseases is gaining momentum. Although a definite link between vitamin D supplementation and amelioration of disease severity is yet to be established, several studies show an improvement in clinical outcome. Treatment of patients with remitting multiple sclerosis (MS) with a 6 month supplementation of high dose dietary vitamin D3 resulted in beneficial immunomodulatory effects30. In another randomized placebo controlled trial, high dose vitamin D supplementation led to decreased inflammatory cytokine levels and moderate clinical improvement in patients with systemic lupus erythematosus (SLE)31. A randomized, double-blind, placebo-controlled trial in adults with chronic obstructive pulmonary disease (COPD) indicated that vitamin D3 supplementation reduced the risk of severe exacerbations32. Because 1,25(OH)2D3 can initiate potent anti-inflammatory response in the host and suppress Th1 and Th17 responses26,27,33, it becomes imperative to ascertain whether 1,25(OH)2D3 treatment will have any repercussions on the induction of host protective response against Mtb infection.
Cathelicidin and β defensin genes, that have been shown to play a key role in anti-mycobacterial mechanisms in humans34, are not regulated by VDR in rodents due to the lack of vitamin D response elements (VDRE)34. However, a large number of VDR responsive lymphoid and myeloid cell functions have been studied in murine models of infection and autoimmune disease. Administration of 1,25(OH)2D3 was shown to protect against experimental autoimmune encephalomyelitis (EAE) as well as against experimental inflammatory bowel disease (IBD)25. Vitamin D was also reported to suppress proinflammatory immune response in experimental cerebral malaria in mice35. 1,25(OH)2D3 treatment resulted in increased susceptibility to C. rodentium infection27 and its infusion in M. paratuberculosis-infected mice led to exacerbation of the disease resulting in increased bacterial burden36. Overall these studies suggest that the murine model can be used to investigate the impact of vitamin D on host resistance against Mtb, which has remained undetermined.
In this study, we therefore, investigated whether administration of 1,25(OH)2D3, the active form of vitamin D, would affect host immunity to Mtb in a murine model of tuberculosis. We report here that Mtb-infected mice treated with 1,25(OH)2D3 exhibited altered pulmonary granuloma formation and reduced ability to contain bacterial burden in the lung as compared to the control group of mice. These findings are significant since they provide a framework to further explore the potential role of vitamin D in disrupting the inflammatory networks involved in granuloma formation and control of Mtb growth.
Results
Administration of 1,25(OH)2D3 during Mtb infection alters cellular recruitment to the lungs
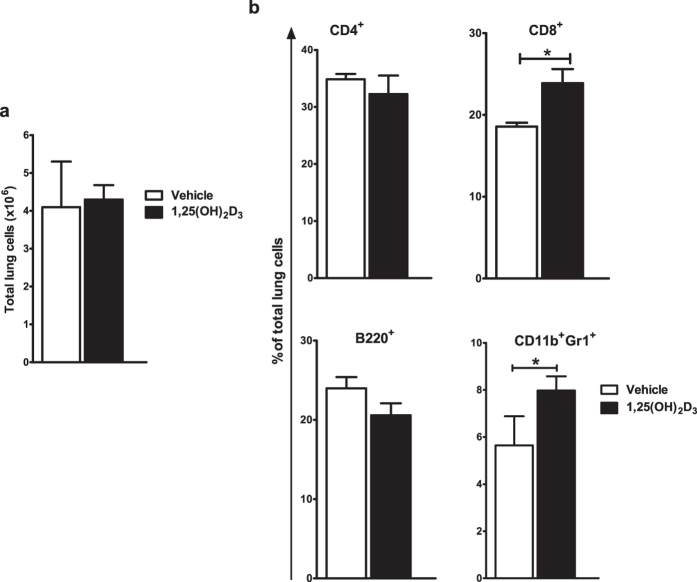
In a low dose aerosol exposure model, Mtb infection leads to an increased recruitment of various immune cell types to lungs that reaches a peak at four weeks post infection28,37. Therefore, in order to investigate the impact of vitamin D on lung cellularity during this acute phase of Mtb-infection, 1,25(OH)2D3-treated and control mice were sacrificed at four weeks post infection and flow cytometric analysis of lung single cell suspensions was carried out. The total number of cells recruited to the lungs of Mtb infected mice was observed to be the same in both the groups (Fig. 1a). Characterization of these cell populations was carried out via FACS (Fig. S1). We observed similar recruitment of CD4+ T cells in the lungs of both the groups of mice, however, percentage of CD8+ T cells was observed to be increased in the lungs of 1,25(OH)2D3-treated mice (Fig. 1b). We also observed a lower percentage of B220+ B cells, albeit not statistically significant and a significant increase in the percentage of CD11b+Gr1+ neutrophils in the lungs of 1,25(OH)2D3-treated mice compared to the control group (Fig. 1b).
Figure 1. 1,25(OH)2D3 treatment leads to altered cellular recruitment to the lungs in Mtb infected mice.
Lung single cell suspensions were prepared from 1,25(OH)2D3-treated and control groups of mice at four weeks post Mtb infection. Total lung cells were counted via Trypan blue dye exclusion method (a). Percentage of CD4+, CD8+, B220+ and CD11b+Gr1+ cells in the lungs were quantitated by flow cytometry (b). Five mice were used in each group and data are presented as mean ± SD.
1,25(OH)2D3 administration alters the organization of pulmonary granulomas
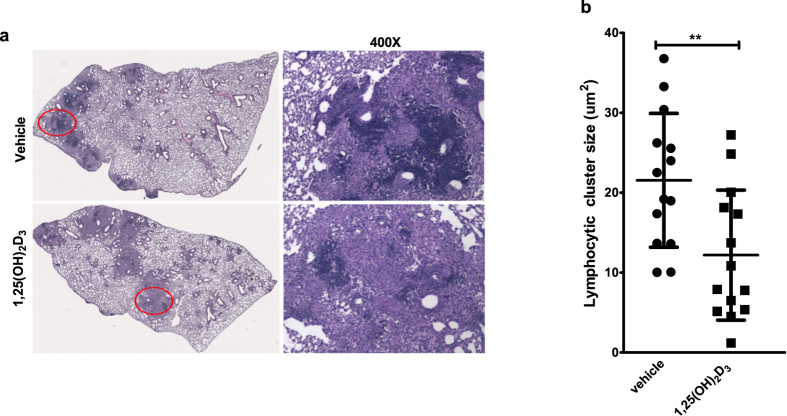
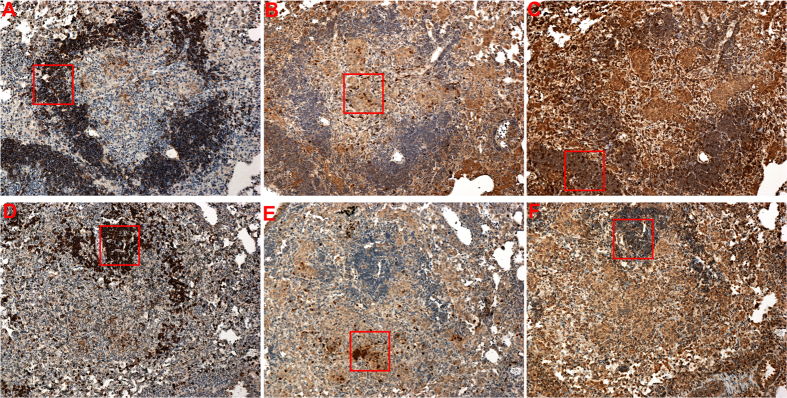
In the murine model of tuberculosis, the acute phase of infection is followed by establishment of a granuloma that consists of small foci of lymphocytic aggregates interspersed with macrophages and other cell types38,39,40. The granulomatous response leads to containment of inflammation and controls bacterial growth41. Since at four weeks post infection lung single cell suspension derived from the two groups of mice exhibited unique cellular pattern, we therefore compared the subsequent development of inflammatory lesions in the lungs of 1,25(OH)2D3-treated mice to that of control mice at six weeks following Mtb infection. A similar level of inflammation was observed in both groups of mice (Fig. 2a). However, lymphocytic aggregates in the inflammatory zones were significantly smaller in size in the 1,25(OH)2D3-treated lungs compared to the control group (Fig. 2b). Immunohistochemical evaluation of lung sections showed that these lymphocytic clusters are rich in CD20 positive B cells (3A) and are associated with Ly6G (3B) positive neutrophils. The granulomas of 1,25(OH)2D3-treated mice exhibited an overall decrease in CD20 (3D) cell staining but showed enhanced Ly6G+ cells (Fig. 3E).
Figure 2. Lymphocytic cluster size is reduced in the pulmonary granulomas of 1,25(OH)2D3-treated mice.
Formalin-fixed, paraffin-embedded lung tissue sections at six weeks post infection were stained with H&E (a). Nikon Microphoto FXA equipped with Objective Imagining surveyor and a motorized stage and NIS Elements Advanced Research software were used to calculate the lymphocytic cluster size in the granulomas of these mice. Each point in the graph represents surface area of one granuloma. Granulomas sampled from the left lung of five mice in each group are plotted (b) and are presented as mean ± SD. Lungs from all the animals in each group were used for analysis and representative image from each group is shown in Fig. 2a.
Figure 3. B cell rich lymphocytic cluster are diminished in the granulomas of 1,25(OH)2D3-treated mice.
Immunohistochemistry was performed on lung tissue sections at six weeks post infection to detect CD20+ (A, D), Ly6G+ (B, E) and CD3+(C, F) cells in the lymphocytic clusters. Tissue sections were stained individually with antibodies against indicated surface markers. Image (100X) was captured using Nikon Eclipse E 800 microscope. The boxed area in each section is presented at higher magnification (200X) in Fig. S2. Lungs from all the animals in each group were used for analysis and representative images from each group are shown here.
Mice treated with 1,25(OH)2D3 exhibit reduced ability to contain bacterial burden during the chronic phase of Mtb infection
We next compared the bacterial burden in the two groups of mice at four and six week time intervals following Mtb infection. In the control group, as expected, there was an increase in bacterial burden at four weeks, and at six weeks as mice entered the chronic phase of infection, the bacterial burden decreased significantly (Fig. 4). In the 1,25(OH)2D3-treated mice, a similar increase in bacterial burden was also seen at four weeks, but, unlike in the control group, these mice did not exhibit a decrease in bacterial burden at six weeks and there was a significant difference in the bacterial burden in the two groups of mice at this stage (Fig. 4).
Figure 4. 1,25(OH)2D3-treated mice fail to control the bacterial burden.
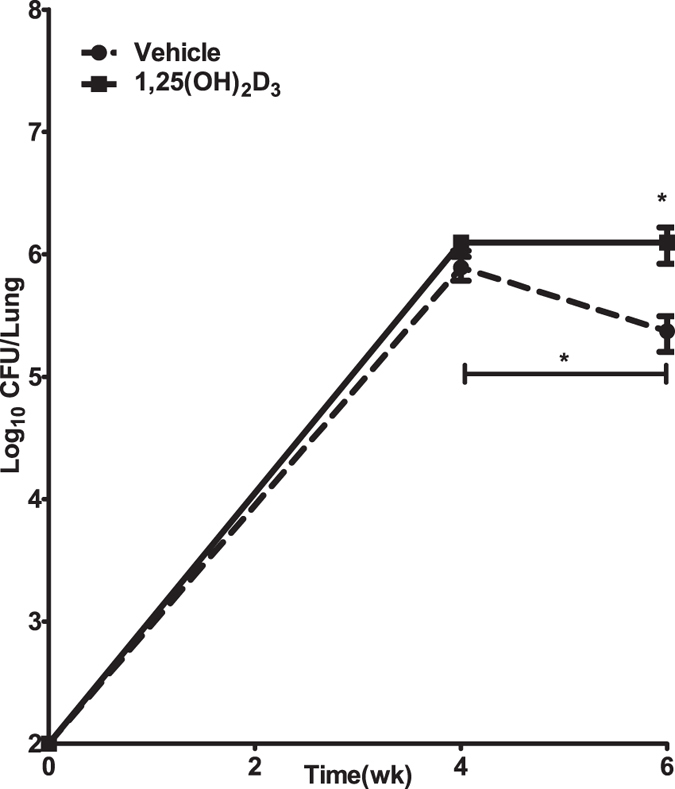
C57BL/6 mice were treated with 1,25(OH)2D3 or vehicle and infected via aerosol with ~100 CFU of Mtb Erdman strain. Mice were sacrificed at four and six weeks following infection and viable bacterial burden was determined by plating the lung homogenates on 7H11 agar pates. Each time point includes five mice per group. Experiment was performed twice and data from one experiment is presented as mean CFU ± SD.
1,25(OH)2D3-treated mice exhibit an overall increase in inflammatory gene expression
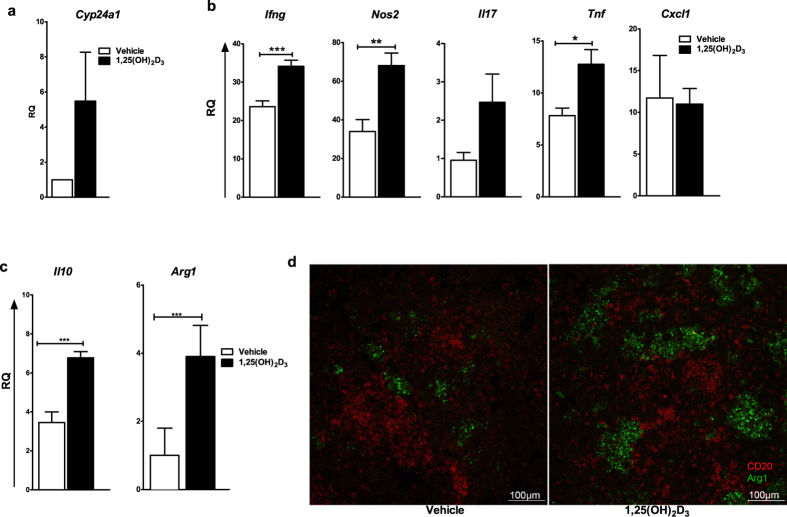
We next evaluated the gene expression profile of key inflammation-related genes including Cyp24a, at four weeks post infection by real-time PCR. We observed that Cyp24a expression was induced in 1,25(OH)2D3 treated group of animals (Fig. 5a). Increased expression of Ifng, Nos2, Il17, and Tnf was observed in 1,25(OH)2D3-treated-mice compared to control mice (Fig. 5b). The 1,25(OH)2D3-treated-mice also exhibited significantly elevated gene expression for Il10 and Arg1 (Fig. 5c), indicating that the overall increase in both pro- and anti-inflammatory genes is likely a reflection of the increased bacterial burden in the lungs of these mice. Immunofluorescence staining confirmed the increased expression of arginase -1 in the lungs of 1,25(OH)2D3-treated mice (Fig. 5d).
Figure 5. Enhanced expression of cytokines in the lungs of 1,25(OH)2D3-treated mice and in situ detection of arginase-1.
Total RNA was isolated from the lungs of of Mtb infected mice at four weeks post infection. Gene expression was assessed by real-time RT-PCR and fold induction in gene expression compared to uninfected lungs was determined (a, b, c). Immunofluorescence was used to detect arginase-1 expression in situ (d). Lung sections from mice infected with Mtb at four weeks post infection were stained for CD20 (red) and arginase-1 (green). Lungs from all the animals in each group were used for analysis. Representative sections from each group are shown. For RT PCR, five mice were used in each group and data are presented as mean ± SD.
Discussion
Host resistance to Mtb infection is critically dependent on the complex interplay between innate and adaptive immune responses to the pathogen42. Although proinflammatory responses are key to the host’s ability to contain the infection, anti-inflammatory immune response pathways are critical for the prevention of excessive inflammation-induced damage to the host42. Vitamin D deficiency and VDR polymorphism are associated with increased susceptibility or progression to tuberculosis disease43,44,45. It has also been suggested that vitamin D as an adjunctive therapy during tuberculosis treatment may accelerate clinical recovery and inflammation resolution46,47. Although a number of studies conducted in vitro strongly suggest that 1,25(OH)2D3 directly and indirectly activates host antimicrobial pathways9,11,48, there is a lack of in vivo studies that focus on immunomodulatory role of vitamin D in the context of Mtb infection. The key innate and adaptive immune response pathways generated in response to Mtb infection bear similarities in humans and mice. The availability of mice strains that exhibit disease pathology similar to humans is increasingly making this model an attractive tool in preclinical testing of drugs and vaccines49,50,51. Therefore, in this study we sought to employ the murine model of tuberculosis to investigate the impact of 1,25(OH)2D3 on the outcome of Mtb infection. Our data indicating altered cellular recruitment to the lungs and change in granuloma architecture of 1,25(OH)2D3-treated mice, suggest an important role for vitamin D in regulating Mtb infection induced immune response. Particularly, the reduction in the size of B cell rich lymphocytic clusters in the 1,25(OH)2D3 -treated lungs suggest that vitamin D may affect the immune mechanisms involved in B cell follicular response in the tuberculosis granuloma with consequent impact on the host’s ability to control Mtb growth.
Several studies have indicated that B cell functions are modulated by 1,25(OH)2D36,52,53,54,55,56. It has been reported that although B cells do not express VDR constitutively52,57, VDR expression is induced by B cell activating signals52,55. Whether 1,25(OH)2D3 directly regulates B cells has been a matter of debate. It has been suggested that 1,25(OH)2D3 mediated inhibition of B cell function may be indirect, through the modulation of T cell or monocyte functions53,58. B cell rich lymphocytic aggregates, bearing features of secondary lymphoid follicles, are characteristic of both human and murine tuberculosis granulomas38,39,59,60. B cell follicles in tuberculosis granuloma have been suggested to provide a site for continuing cellular proliferation in response to Mtb antigens and have been implicated in the regulation of the granulomatous response61. In a study by Chen et al.6, 1,25(OH)2D3 was shown to inhibit the proliferation of activated B cells and induce their apoptosis in vitro. However, we were not able to detect enhanced apoptosis in the lymphocytic aggregates in the 1,25(OH)2D3-treated mice compared to the control group. Mtb infection in B cell deficient mice38 resulted in an increased influx of neutrophils to the lungs, higher expression of IL-1762 and increased bacterial burden. It is thus plausible that modulation of B cell responses by 1,25(OH)2D3, either directly or indirectly, may be a contributing factor to the observed increase in the number of neutrophils in the lungs of 1,25(OH)2D3-treated mice. Because of the altered granuloma environment, 1,25(OH)2D3-treated mice are subsequently unable to restrict bacterial burden in the lungs as efficiently as control animals as they enter the chronic phase of infection. Although we observed increased expression of several pro-inflammatory cytokines, expression of IL-10 and arginase-1 was also increased in 1,25(OH)2D3-treated mice. Macrophage-derived IL-10 induces arginase-1 in alternatively activated macrophages63, and furthermore, arginase-1 controls Mtb growth and T cell mediated immunopathology64. Therefore, future studies should evaluate how these cytokines modulate the inflammatory response in acute infection and also the immunopathology that is induced in chronic infection in 1,25(OH)2D3-treated mice.
In a recent study, Reeme et al.65 reported that high dietary vitamin D suppressed proinflammatory cytokine response, accompanied by mitigated pulmonary immunopathology in late stage Mtb infection in C3HeB/FeJ mice. It is of interest that similar to this study65 we also observed increased proinflammatory cytokine response and neutrophil influx in 1,25(OH)2D3-treated mice. Although, in the Reeme study, the authors did not characterize B cells in the lymphocytic clusters, they observed a reduction in lymphocytic cluster size in the groups of mice that were fed high vitamin D diet. The reduction in B cell rich lymphocytic cluster size in response to 1,25(OH)2D3 treatment suggests that vitamin D may modulate B cell mediated immune response to Mtb infection. Our future experiments will dissect the mechanisms involved in vitamin D mediated modulation of B cell function in Mtb infection. Another difference between our finding in the C57BL/6 mice and the C3HeB/FeJ data is that the latter study did not find increased bacterial numbers in the lung of mice fed high dietary vitamin D. The increased bacterial burden in response to 1,25(OH)2D3 seen in our study may be the result of mouse genotype specific differences. It is also possible that the strain of bacteria may influence the outcome of vitamin D or 1,25(OH)2D3 treatment since our study used Mtb Erdman while the C3HeB/FeJ mice were infected with Mtb H37Rv. It is therefore critical that investigations are conducted on mice with varying genetic backgrounds such as the Diversity Outbred mice66 and with diverse clinical Mtb strains to delineate the effects of vitamin D on host immunity to Mtb. Another limitation of our study is that mice do not express human cathelcidin, which has been shown to be involved in antimicrobial activity against Mtb in vitro9. Further in vivo studies are needed in LL37 transgenic mice (expressing the human cathelicidin gene) to fully evaluate the interplay between vitamin D and the host immune response during Mtb infection and to dissect the underlying mechanisms.
Methods
Mice
C57BL/6 female mice (6–8 weeks old) were purchased from the National Cancer Institute (Frederick, MD, USA). Mtb-infected mice were housed in the animal BSL3 facility and guidelines from R-NJMS-Institutional Animal Care and Use Committee were followed in handling the animals. All experimental protocols in this study were approved by R-NJMS-Institutional Animal Care and Use Committee.
Determination of pulmonary bacterial burden
1,25(OH)2D3 was obtained from Cayman Chemical Company, (Ann Arbor, MI, USA). Mice were injected with 20 ng 1,25(OH)2D3 or vehicle (90% 1,2-propanediol in ethanol) via subcutaneous route three times a week. The dose of 1,25(OH)2D3 was chosen on the basis of previous in vivo studies with mice67. The treatment was initiated a day before the infection and continued until the completion of the study. Mice were infected with a low dose of Mtb Erdman strain (Trudeau Institute, Saranac, NY) in a whole body inhalation exposure system (Glas-Col, LLC, Terre Haute, IN). The number of bacteria deposited in the lungs were determined by plating the whole lung homogenates on 7H11 plates 24 hrs following aerosolization. At each time interval studied, infected animals were sacrificed by cervical dislocation and the right superior lobe of the lung was homogenized in PBS containing 0.05% Tween 80. Serial dilutions of the homogenates were plated onto 7H11 agar. The plates were incubated at 37 °C and colonies counted after 21 days. The rest of the lung was reserved for single cell preparation, RNA extraction and histological studies.
Lung single cell preparation
Lungs were perfused with 5 ml sterile PBS, cut into small pieces and incubated with 2 mg/ml collagenase D (Roche) for 30 min. The digestion was stopped by adding 5 mM EDTA. The digested tissue was transferred to a 40-μm nylon cell strainer and disrupted using a syringe plunger to obtain single cell suspensions. RBCs were lysed with ACK lysing buffer and viable cell number was determined by trypan blue dye exclusion method.
Immunohistochemistry
Mtb-infected mice were sacrificed at indicated time intervals and lungs were perfused with PBS. Excised lungs were fixed in 4% paraformaldehyde for a week and subsequently stored in 70% ethanol until they were embedded in paraffin. Five micrometer sections were cut and stained using the standard H&E protocol. For immunohistochemistry, four to six micrometer sections were cut and mounted onto Superfrost/Plus microscope slides (Fisherbrand). Tissue sections were de-paraffinized with xylene and rehydrated with ethanol gradations and water. CD3, Ly6G, CD20 and arginase-1 epitopes were retrieved using heat induced epitope retrieval method as described previously68. The tissue sections were dipped in 10 mM citrate retrieval buffer (pH 6.0) and heated in a microwave pressure cooker (Nordic ware). Tissue sections were then blocked with Background Buster (Innovex Biosciences) for 30 minutes. Primary antibodies against CD20 (M-20), and arginase-1 (H-52), were obtained from Santa Cruz Biotechnology. CD3 (Rabbit polyclonal) was obtained from Abcam and Ly6G (1A8) was obtained from Biolegend. Sections were stained with these antibodies at 4 °C overnight. The sections were washed (PBS containing 0.5% tween 20) and reacted with biotinylated (1:100, Vector Laboratories) or fluorescent-labeled secondary antibody (1:1000 Life Technologies) for 45 minutes at room temperature. For fluorescent detection of CD20 and arginase-1, Alexa 568 and Alexa 488 conjugated donkey – anti rabbit (Life Technologies) secondary antibodies were used. Relevant isotype controls were used for each primary antibody. The streptavidin horseradish peroxidase substrate (BioGenex) was used for immunodetection using DAB as a chromogen (BioGenex). The sections were counterstained with hematoxylin, and subsequently dehydrated in 95% and 100% ethanol followed by xylene. Finally, they were mounted on coverslips for microscopic visualization. Sections stained with fluorescent-labeled antibodies were directly mounted with ProLong antifade mounting media (Molecular Probes). Nikon Microphot-FXA, equipped with Objective Imaging Surveyor and a motorized microscope stage was used to capture tiled images. Granuloma area was measured using NIS Elements Advanced Research software. Fluorescent images were captured using NikonA1R laser scanning confocal microscope equipped with 20X planApo - numerical aperture 0.75.
Flow cytometry
The following anti-mouse mAbs used for the study: anti-CD4 (RM4–5), anti-CD8 (Ly-2), anti-B220 (RA3-6B2), anti-CD11b (M1/70) and anti-Ly-6G & Ly-6C (RB6-8C5) were purchased from BD Biosciences. All antibodies were directly conjugated to fluorochromes and isotype controls were included for each antibody type. Single cell suspensions from the lungs were made as described above and cell density was determined. Approximately one million cells were washed and re-suspended in FACS buffer containing appropriate concentrations of fluorochrome-conjugated antibodies. After thirty minutes incubation at 4°C, the cells were washed again in FACS buffer and fixed with 4% paraformaldehyde for 30 min. The cells were acquired on BD LSR II flow cytometer and data were analyzed using FlowJo (Tree Star).
Real-Time RT PCR
Lung tissues were homogenized in TRIzol® (Ambion™) and stored at −80 °C till further processing. RNA was extracted from TRIzol® and further purified using RNeasy kit (Qiagen) and reverse transcribed using High Capacity RNA to cDNA kit (Applied Biosystems™). cDNA was amplified using Taqman® reagents (Applied Biosystems™) on the ABI PRISM 7900 HT Sequence Detection System and fold induction in gene expression relative to uninfected tissue (RQ) was calculated by 2−ΔΔCT method69 by the Applied Biosystems software. Briefly, relative gene expression (fold induction) is calculated as 2−ΔΔCt, where ΔCt = Ct (gene of interest) – Ct (normalizer = β-actin) and the ΔΔCt = ΔCt (sample) – ΔCt (calibrator). The calibrator in our study is lung tissue obtained from uninfected mice. Due to the undetectable Cyp24a1 amplification in uninfected tissues, a Ct value of 40 was assigned to calculate relative fold induction. The following primer/probe sets from Applied Biosystems™ were used; Nos2 (Mm00440502_m1), Tnf (Mm00443260_g1), Ifng (Mm01168134_m1), Il17a (Mm00439618_m1), Il10 (Mm00439614_m1), Arg1 (Mm00475988_m1), Cyp24a1 (Mm00487244_m), Cxcl1 (Mm04207460_m1), and β actin (Mm00607939_s1).
Statistical analysis
For statistical analysis, GraphPad Prism Software (version 5) was used. The unpaired Student t test was used to determine statistical significance between the two groups. Values of *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001 were considered significant.
Additional Information
How to cite this article: Bhatt, K. et al. 1,25 (OH)2D3 treatment alters the granulomatous response in M. tuberculosis infected mice. Sci. Rep. 6, 34469; doi: 10.1038/srep34469 (2016).
Supplementary Material
Acknowledgments
This study was supported by a grant from NJ Health Foundation Program and NIH grant AI-121621 awarded to P.S. & S.C. We thank Luke Fritzky (Confocal Imaging Facility, Rutgers - NJMS) for excellent assistance with confocal microscopy and Vaishali Veldurthy for helpful discussions.
Footnotes
Author Contributions The study was designed and planned by P.S., S.C., K.B. and resources were secured by P.S., S.C. K.B., W.R. and N.S. carried out the experiments and contributed to data acquisition. Data analysis was carried out by K.B. and P.S. and S.C. K.B. wrote the original draft. P.S. and S.C. contributed to the review and editing of the manuscript. All authors read and approved the final manuscript.
References
- Hewison M. Vitamin D and immune function: an overview. Proceedings of the Nutrition Society 71, 50–61, doi: 10.1017/S0029665111001650 (2012). [DOI] [PubMed] [Google Scholar]
- Lips P. Vitamin D physiology. Progress in Biophysics and Molecular Biology 92, 4–8, doi: 10.1016/j.pbiomolbio.2006.02.016 (2006). [DOI] [PubMed] [Google Scholar]
- Lagishetty V., Liu N. Q. & Hewison M. Vitamin D metabolism and innate immunity. Molecular and cellular endocrinology 347, 97–105, doi: 10.1016/j.mce.2011.04.015 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadranel J. et al. 1,25(OH)2D2 production by T lymphocytes and alveolar macrophages recovered by lavage from normocalcemic patients with tuberculosis. The Journal of clinical investigation 85, 1588–1593, doi: 10.1172/jci114609 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H. et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nature immunology 8, 285–293, doi: http://www.nature.com/ni/journal/v8/n3/suppinfo/ni1433_S1.html (2007). [DOI] [PubMed] [Google Scholar]
- Chen S. et al. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. The Journal of Immunology 179, 1634–1647, doi: 10.4049/jimmunol.179.3.1634 (2007). [DOI] [PubMed] [Google Scholar]
- Christakos S., Dhawan P., Verstuyf A., Verlinden L. & Carmeliet G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiological reviews 96, 365–408, doi: 10.1152/physrev.00014.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J. W. & Meyer M. B. Fundamentals of vitamin D hormone-regulated gene expression. The Journal of steroid biochemistry and molecular biology 144 Pt A, 5–11, doi: 10.1016/j.jsbmb.2013.11.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. T. et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311, 1770–1773 (2006). [DOI] [PubMed] [Google Scholar]
- Liu P. T. et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One 4, e5810, doi: 10.1371/journal.pone.0005810 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verway M. et al. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog 9, e1003407, doi: 10.1371/journal.ppat.1003407 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug-Micu G. M. et al. CD40 ligand and interferon-gamma induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology 139, 121–128, doi: 10.1111/imm.12062 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamon H. et al. Cutting Edge: Vitamin D Regulates Lipid Metabolism in Mycobacterium tuberculosis Infection. J Immunol, doi: 10.4049/jimmunol.1400736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun R. F., Liu P. T., Modlin R. L., Adams J. S. & Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Frontiers in physiology 5, 151, doi: 10.3389/fphys.2014.00151 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M. T. Mechanisms underlying the effect of vitamin D on the immune system. The Proceedings of the Nutrition Society 69, 286–289, doi: 10.1017/s0029665110001722 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R. & Christakos S. Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients 7, 8251–8260, doi: 10.3390/nu7105392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeke F., Takiishi T., Korf H., Gysemans C. & Mathieu C. Vitamin D: modulator of the immune system. Current opinion in pharmacology 10, 482–496, doi: 10.1016/j.coph.2010.04.001 (2010). [DOI] [PubMed] [Google Scholar]
- Penna G. & Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. Journal of immunology 164, 2405–2411 (2000). [DOI] [PubMed] [Google Scholar]
- Griffin M. D. et al. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America 98, 6800–6805, doi: 10.1073/pnas.121172198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger W. W., Laban S., Kleijwegt F. S., van der Slik A. R. & Roep B. O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. European journal of immunology 39, 3147–3159, doi: 10.1002/eji.200839103 (2009). [DOI] [PubMed] [Google Scholar]
- Van Belle T. L. et al. 1,25-Dihydroxyvitamin D3 and its analog TX527 promote a stable regulatory T cell phenotype in T cells from type 1 diabetes patients. PloS one 9, e109194, doi: 10.1371/journal.pone.0109194 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery L. E. et al. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. Journal of immunology 183, 5458–5467, doi: 10.4049/jimmunol.0803217 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier J. A., Nashold F. E., Olson J. K. & Hayes C. E. The Ifng gene is essential for Vdr gene expression and vitamin D(3)-mediated reduction of the pathogenic T cell burden in the central nervous system in experimental autoimmune encephalomyelitis, a multiple sclerosis model. Journal of immunology (Baltimore, Md.: 1950) 189, 3188–3197, doi: 10.4049/jimmunol.1102925 (2012). [DOI] [PubMed] [Google Scholar]
- Daniel C. et al. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. The Journal of pharmacology and experimental therapeutics 319, 622–631, doi: 10.1124/jpet.106.107599 (2006). [DOI] [PubMed] [Google Scholar]
- Cantorna M. T. Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch Biochem Biophys 523, 103–106, doi: 10.1016/j.abb.2011.11.001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S. et al. 1,25-dihydroxyvitamin D(3) ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Molecular and cellular biology 31, 3653–3669, doi: 10.1128/mcb.05020-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryz N. R. et al. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal Th17 responses. American journal of physiology. Gastrointestinal and liver physiology 303, G1299–1311, doi: 10.1152/ajpgi.00320.2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V., Nolt D. & Flynn J. L. Long-term control of Mycobacterium tuberculosis infection is mediated by dynamic immune responses. Journal of immunology 175, 1107–1117 (2005). [DOI] [PubMed] [Google Scholar]
- Cooper A. M. & Khader S. A. The role of cytokines in the initiation, expansion, and control of cellular immunity to tuberculosis. Immunological reviews 226, 191–204, doi: 10.1111/j.1600-065X.2008.00702.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotirchos E. S. et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology 86, 382–390, doi: 10.1212/wnl.0000000000002316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Raya A., Abou-Raya S. & Helmii M. The effect of vitamin D supplementation on inflammatory and hemostatic markers and disease activity in patients with systemic lupus erythematosus: a randomized placebo-controlled trial. The Journal of rheumatology 40, 265–272, doi: 10.3899/jrheum.111594 (2013). [DOI] [PubMed] [Google Scholar]
- Martineau A. R. et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. The Lancet. Respiratory medicine 3, 120–130, doi: 10.1016/s2213-2600(14)70255-3 (2015). [DOI] [PubMed] [Google Scholar]
- Reich K. M., Fedorak R. N., Madsen K. & Kroeker K. I. Vitamin D improves inflammatory bowel disease outcomes: basic science and clinical review. World journal of gastroenterology 20, 4934–4947, doi: 10.3748/wjg.v20.i17.4934 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A. F., Borregaard N. & Koeffler H. P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 19, 1067–1077, doi: 10.1096/fj.04-3284com (2005). [DOI] [PubMed] [Google Scholar]
- He X. et al. Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. Journal of immunology (Baltimore, Md.: 1950) 193, 1314–1323, doi: 10.4049/jimmunol.1400089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabel J. R., Goff J. P., Whipple D. L., Ackermann M. R. & Reinhardt T. A. Low calcium diet and 1,25-dihydroxyvitamin D(3) infusion modulate immune responses during Mycobacterium paratuberculosis infection in beige mice. Veterinary immunology and immunopathology 50, 127–143 (1996). [DOI] [PubMed] [Google Scholar]
- Bhatt K. et al. B7 Costimulation Is Critical for Host Control of Chronic Mycobacterium tuberculosis Infection. The Journal of Immunology 182, 3793–3800, doi: 10.4049/jimmunol.0802996 (2009). [DOI] [PubMed] [Google Scholar]
- Maglione P. J., Xu J. & Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. Journal of immunology 178, 7222–7234 (2007). [DOI] [PubMed] [Google Scholar]
- Maglione P. J. & Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. European journal of immunology 39, 676–686, doi: 10.1002/eji.200839148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader S. A. et al. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. Journal of immunology 187, 5402–5407, doi: 10.4049/jimmunol.1101377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S. & Schaible U. E. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Frontiers in immunology 3, 411, doi: 10.3389/fimmu.2012.00411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M., Robinson R. T. & Cooper A. M. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 16, 57–63, doi: 10.1038/ni.3048 (2015). [DOI] [PubMed] [Google Scholar]
- Wilkinson R. J. et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west London: a case-control study. The Lancet 355, 618–621, doi: 10.1016/S0140-6736(99)02301-6 (2000). [DOI] [PubMed] [Google Scholar]
- Martineau A. R. et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. Proceedings of the National Academy of Sciences of the United States of America 108, 19013–19017, doi: 10.1073/pnas.1111825108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield T. et al. The Seasonality of Tuberculosis, Sunlight, Vitamin D, and Household Crowding. Journal of Infectious Diseases, doi: 10.1093/infdis/jiu121 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens A. K. et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proceedings of the National Academy of Sciences 109, 15449–15454, doi: 10.1073/pnas.1200072109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A. R. et al. High-dose vitamin D(3) during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377, 242–250, doi: 10.1016/s0140-6736(10)61889-2 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. S. et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. Journal of immunology 182, 4289–4295, doi: 10.4049/jimmunol.0803736 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. The mouse as a useful model of tuberculosis. Tuberculosis (Edinburgh, Scotland) 83, 112–115 (2003). [DOI] [PubMed] [Google Scholar]
- Flynn J. L. Lessons from experimental Mycobacterium tuberculosis infections. Microbes and Infection 8, 1179–1188, doi: 10.1016/j.micinf.2005.10.033 (2006). [DOI] [PubMed] [Google Scholar]
- Kramnik I. & Beamer G. Mouse models of human TB pathology: roles in the analysis of necrosis and the development of host-directed therapies. Seminars in immunopathology 38, 221–237, doi: 10.1007/s00281-015-0538-9 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. W. et al. Requirements for induction of vitamin D-mediated gene regulation in normal human B lymphocytes. Journal of immunology 157, 2900–2908 (1996). [PubMed] [Google Scholar]
- Muller K., Heilmann C., Poulsen L. K., Barington T. & Bendtzen K. The role of monocytes and T cells in 1,25-dihydroxyvitamin D3 mediated inhibition of B cell function in vitro. Immunopharmacology 21, 121–128 (1991). [DOI] [PubMed] [Google Scholar]
- Heine G. et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. European journal of immunology 38, 2210–2218, doi: 10.1002/eji.200838216 (2008). [DOI] [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J. & Manolagas S. C. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. The Journal of Immunology 136, 2734–2740 (1986). [PubMed] [Google Scholar]
- Shirakawa A.-K. et al. 1,25-Dihydroxyvitamin D3 Induces CCR10 Expression in Terminally Differentiating Human B Cells. The Journal of Immunology 180, 2786–2795, doi: 10.4049/jimmunol.180.5.2786 (2008). [DOI] [PubMed] [Google Scholar]
- Veldman C. M., Cantorna M. T. & DeLuca H. F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374, 334–338, doi: 10.1006/abbi.1999.1605 (2000). [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Adams J. S., Sakai R. & Jordan S. C. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. The Journal of clinical investigation 74, 657–661, doi: 10.1172/JCI111465 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrichs T. et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol 204, 217–228 (2004). [DOI] [PubMed] [Google Scholar]
- Tsai M. C. et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cellular microbiology 8, 218–232, doi: 10.1111/j.1462-5822.2005.00612.x (2006). [DOI] [PubMed] [Google Scholar]
- Ulrichs T. et al. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. The Journal of Pathology 204, 217–228, doi: 10.1002/path.1628 (2004). [DOI] [PubMed] [Google Scholar]
- Kozakiewicz L. et al. B Cells Regulate Neutrophilia during Mycobacterium tuberculosis Infection and BCG Vaccination by Modulating the Interleukin-17 Response. PLoS Pathog 9, e1003472, doi: 10.1371/journal.ppat.1003472 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T. et al. Autocrine IL-10 induces hallmarks of alternative activation in macrophages and suppresses antituberculosis effector mechanisms without compromising T cell immunity. Journal of immunology (Baltimore, Md.: 1950) 183, 1301–1312, doi: 10.4049/jimmunol.0803567 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque-Correa M. A. et al. Macrophage arginase-1 controls bacterial growth and pathology in hypoxic tuberculosis granulomas. Proceedings of the National Academy of Sciences of the United States of America 111, E4024–4032, doi: 10.1073/pnas.1408839111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeme A. E. & Robinson R. T. Dietary Vitamin D3 Suppresses Pulmonary Immunopathology Associated with Late-Stage Tuberculosis in C3HeB/FeJ Mice. The Journal of Immunology 196, 1293–1304, doi: 10.4049/jimmunol.1500931 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G. A., Gatti D. M., Munger S. C. & Svenson K. L. The Diversity Outbred mouse population. Mammalian genome: official journal of the International Mammalian Genome Society 23, 713–718, doi: 10.1007/s00335-012-9414-2 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca H. F. & Cantorna M. T. Vitamin D: its role and uses in immunology. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 15, 2579–2585, doi: 10.1096/fj.01-0433rev (2001). [DOI] [PubMed] [Google Scholar]
- Bhatt K., Kim A., Kim A., Mathur S. & Salgame P. Equivalent functions for B7.1 and B7.2 costimulation in mediating host resistance to Mycobacterium tuberculosis. Cellular Immunology 285, 69–75, doi: 10.1016/j.cellimm.2013.09.004 (2013). [DOI] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25, 402–408, doi: 10.1006/meth.2001.1262 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.