Abstract
In this study, we sought to investigate how concomitant hyperglycemia influences the impact of combination antiretroviral therapy on blood-brain barrier (BBB) endothelial function. Immortalized human brain microvascular endothelial cell line (hCMEC/D3) was exposed to azidothymidine (AZT; a nucleoside reverse transcriptase inhibitor) and/or indinavir (IND; protease inhibitor) in normal glycemic (5.5mM) or hyperglycemic (HG; 25mM) media containing D-glucose for 24-72h. Cellular reactive oxygen species (ROS) and mitochondria-specific superoxide levels were assayed in addition to membrane potential to determine the extent of mitochondrial dysfunction. Nrf2 expression was analyzed by immunofluorescence. Our results indicated a significant increase in BBB endothelial toxicity (decreased ATP) by HG and AZT+IND with progression of time (24-72h). Concurrent HG and antiviral drug combination synergistically elevated BBB endothelial ROS induced by either condition alone. Further, HG and AZT+IND mutually interact to elicit a pronounced increase in mitochondrial superoxide levels post 24h (vs. either condition alone or controls). In addition, HG and AZT+IND complemented each other to induce potential loss of mitochondrial membrane potential. While HG or AZT+IND alone for 24h increased Nrf2 nuclear distribution, co-exposure conditions induced a potential loss of Nrf2 expression/nuclear translocation in BBB endothelium. In summary, our data strongly suggest that antiretroviral drug combination potentially interacts with concomitant HG and triggers exacerbated mitochondrial dysfunction and BBB endothelial toxicity, possibly through dysregulation of Nrf2 signaling. Thus, this study warrants the critical need for safety evaluation and monitoring of neurovascular complications of HAART regimens in HIV-infected diabetic patient cohort.
Keywords: BBB, Diabetes, HAART, Mitochondria, Nrf2, Oxidative stress, Zidovudine
1. Introduction
While the initiation of highly active antiretroviral drug therapy (HAART) has dramatically improved the treatment and survival rates in HIV infected patients, emerging evidence indicates a trajectory of HAART-associated adverse events predisposing this population to increased risk of diabetes and cardiovascular disorders (Chastain et al., 2015; Dagogo-Jack, 2008; Kalra et al., 2011). Particularly, metabolic complications and onset of diabetes are strongly evident in patients treated with HAART regimen containing protease inhibitors (PI) such as indinavir (Lee et al., 2004) and/or azidothymidine, a nucleoside reverse transcriptase inhibitor (NRTI) (De et al., 2008; Karamchand et al., 2016). In addition, long-term HAART worsens the prognosis of HIV-associated neurocognitive disorders with increased CNS infestation of immune cells and ensuing neuronal damage (Langford et al., 2002).
Overarching evidence has provided extensive mechanistic basis for HAART-associated vascular complications at various sites (Blas-Garcia et al., 2011). For instance, azidothymidine (AZT) and indinavir (IND) significantly impaired mitochondrial function and cellular bioenergetics leading to profound endothelial dysfunction in periphery (Barbaro and Iacobellis, 2009; Hebert et al., 2004). Increased endothelial oxidative stress through escalated generation of reactive oxygen/nitrogen species (ROS/RNS) was shown as potential molecular mechanism critical to antiretroviral drug-induced mitochondrial toxicity and vascular pathogenesis (Baliga et al., 2004; Barbaro and Iacobellis, 2009; Jiang et al., 2007). For example, HAART induced decline in mitochondrial membrane potential correlated with increased endothelial ROS generation (Jiang et al., 2007).
Recent studies also highlighted the deleterious impact of these antiretroviral drugs on the functional integrity of blood-brain barrier (BBB), a dynamic neuroprotective barrier primarily constituted by brain microvascular endothelium in cellular (Fiala et al., 2004; Manda et al., 2011) and animal models (Dasuri et al., 2016). In fact, AZT and IND combination at high doses significantly decreased the stringency of endothelial barrier with increased paracellular permeability to dextrans (Manda et al., 2011). BBB dysfunction is widely recognized as a potential mechanism in the etiology of various CNS disorders including HIV encephalitis and its ramifications (Sajja et al., 2016). Given the increasing evidence of BBB disruption in clinical and experimental diabetes, downstream to endothelial oxidative injury and mitochondrial dysfunction (Allen and Bayraktutan, 2009; Prasad et al., 2014), we hypothesized that HAART regimen containing PI and NRTI would elicit exacerbated BBB endothelial toxicity in diabetic subjects. Thus, our objective was to assess the impact of concomitant hyperglycemia and antiretroviral drug combination (AZT and IND) on BBB endothelial redox homeostasis and mitochondrial function.
2. Materials and methods
2.1.Cell culture
Immortalized human cerebrovascular endothelial cell line (hCMEC/D3) was used in this study as an in vitro model of human BBB (Manda et al., 2011; Toth et al., 2014). Cell monolayers (p28-31) were cultured in buffered endothelial basal media containing 5% FBS, antibiotics and all supplements provided in EGM™ 2MV BulletKit (Lonza), as described previously (Sajja et al., 2015). Media was replaced every 2 days and cells were maintained in treatment media (basal media with 1% human serum and antibiotics) overnight prior to treatment exposure.
2.2.Drug Treatment
Confluent cell monolayers were exposed to AZT (5 or 10μM), IND (6 or 12μM) or AZT (5μM) and IND (6μM) combination in normal (NG) or hyperglycemic (HG) media containing 5.5mM or 25mM D-glucose, respectively, for 24-72h. These drug concentrations are relevant to observed steady state therapeutic plasma levels in patients receiving HAART (Jiang et al., 2007; Wang et al., 2009) and AZT+IND combination for the indicated time intervals was based on previous reports (Hebert et al., 2004; Wang et al., 2009).
2.3.Cell viability
The effects of treatment on cell viability (metabolically active cells) were determined by CellTiter-Glo® 2.0 assay (Promega) as per the manufacturer’s instructions. Briefly, following 24-72h treatment, cells were added the reagent to determine the ATP levels by luminescence using Synergy 2 Multimode microplate reader (BioTek).
2.4.Total cellular ROS measurement by Flow Cytometry
Following various treatment conditions for 24h, cells cultured in 6-well plates were washed and incubated with a cell permeable and florescent probe, dichlorodihydrofluorescein diacetate (H2-DCFDA; 10μM) in cell medium for another 30 mins at 37 °C. Cells were washed and collected in cold PBS and analyzed by Flow Cytometer (Accuri C6) as mentioned earlier (Fofaria et al., 2014).
2.5.MitoSOX Red Assay
To measure mitochondria-specific superoxide generation, hCMEC/D3 monolayers were exposed to various treatment conditions for 24h. Following a wash, cells were incubated with 5μM of MitoSOX Red reagent (ThermoFisher Scientific) for 15min and subsequently washed with ice-cold PBS and florescence was determined by BioTek Synergy2 Multimode microplate reader. The fluorescence was normalized per unit μg of protein as quantified by BCA method (Sajja et al., 2015).
2.6.Mitochondrial membrane potential (ΔΨm) measurement
To assess the mitochondrial function, cells were incubated with lipophilic cationic dye, JC-1 (2μM), for 15min at 37 °C following treatment, according to the manufacturer’s instructions (ThermoFisher Scientific). Red (aggregates) to green (monomer) florescence ratio was measured using BioTek Synergy2 Multimode microplate reader.
2.7.Immunofluorescence
Following treatment, cells were fixed with 10% formalin and permeabilized with 0.2% triton x-100 in PBS (Sajja et al., 2015). After blocking with 10% goat serum, cells were incubated with rabbit anti-Nrf2 (Santa Cruz) overnight at 4°C followed by 1h incubation with Alexa Fluor 555-secondary antibody. Cells were mounted with DAPI in Prolonged Gold Anti-fade reagent (Invitrogen). Images were captured with EVOS digital inverted fluorescence microscope at 40x magnitude and analysed by Image J software (https://imagej.nih.gov/ij/download.html).
2.8.Statistical analysis
Data were expressed as mean ± SEM and analyzed by one-way ANOVA followed by Dunnet’s post hoc test using GraphPad Prism 6.07. For statistical significance, p value was set to less than 0.05.
3. Results
3.1.Concomitant exposure to hyperglycemia and AZT+IND potentiates BBB endothelial toxicity
As shown in Fig. 1, 24h or 72h exposure to AZT (5μM) or/and IND (6μM) in NG did not affect the cell viability (population of metabolically active cells) as indicated by ATP levels (compared to control). Similarly, HG alone did not significantly influence the cell viability at 24 or 72h when compared to respective controls (Fig. 1). However, there was a marked decline (by 25-30%) in viable cell population following 72h exposure to drug combination or HG when compared to their effects at 24h (Figs. 1A and 1B). Importantly, AZT+IND combination in presence of HG significantly decreased the endothelial viability with progression of time at 72h (vs. control or either condition alone; Fig. 1B). Based on these cell viability studies, we chose the 24h duration of exposure in further experiments.
Figure 1.
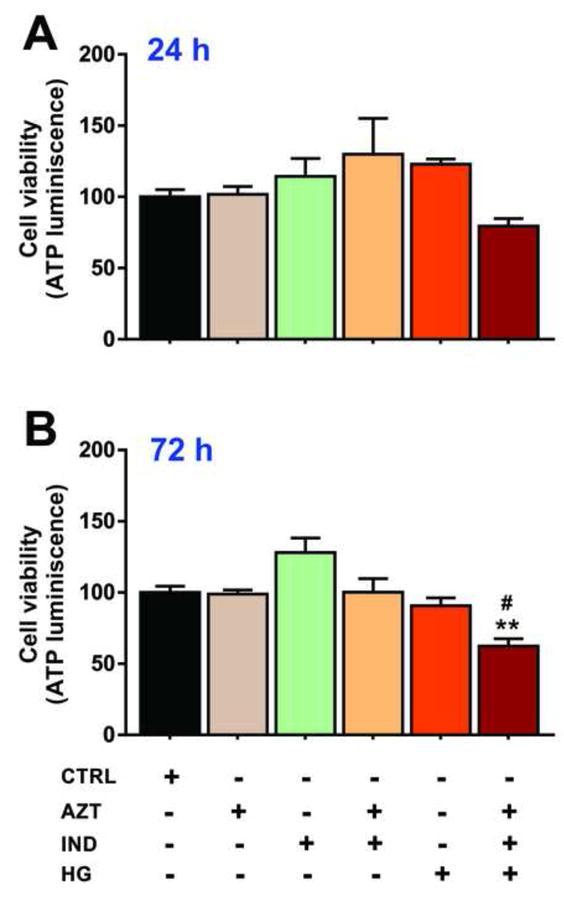
Concomitant exposure to HG and antiretroviral drug combination synergistically increases BBB endothelial toxicity. hCMEC/D3 cells were exposed to AZT (5μM) or/and IND (6μM) in NG or HG media for 24-72h and cell viability was determined by ATP luminescence (see Methods). Data were expressed as mean ± SEM (% control). **p<0.01 vs. control and #p<0.05 vs. AZT+IND or HG. The corresponding treatment conditions were shown below for each bar graph. N=5-6 biological replicates.
3.2.HG and antiretroviral drug combination exacerbate BBB endothelial oxidative stress response
Total ROS levels were measured by Flow cytometry to assess the extent of oxidative stress (and damage) in hCMEC/D3 cells by various treatment conditions. One-way ANOVA revealed a significant main effect of the treatment on endothelial ROS generation (Fig. 2). AZT (5μM) and IND (6μM) combination significantly elevated BBB endothelial ROS levels represented by mean florescence intensity of oxidized DCF (p<0.05 vs. control; Fig 2). Similarly, HG alone induced significant oxidative stress load in hCMEC/D3 cells post 24h exposure (p<0.05 vs. control). Further analysis indicated that concomitant exposure to HG and antiretroviral drug combination additively up-regulated ROS generation (oxidative stress) in BBB endothelial cells (p<0.05 vs. AZT+IND or HG alone; p<0.01 vs control). Thus, our data show synergistic and mutual interactions between HG and antiretroviral drug combination leading to a significant increase in endothelial oxidative stress and possible BBB dysfunction.
Figure 2.
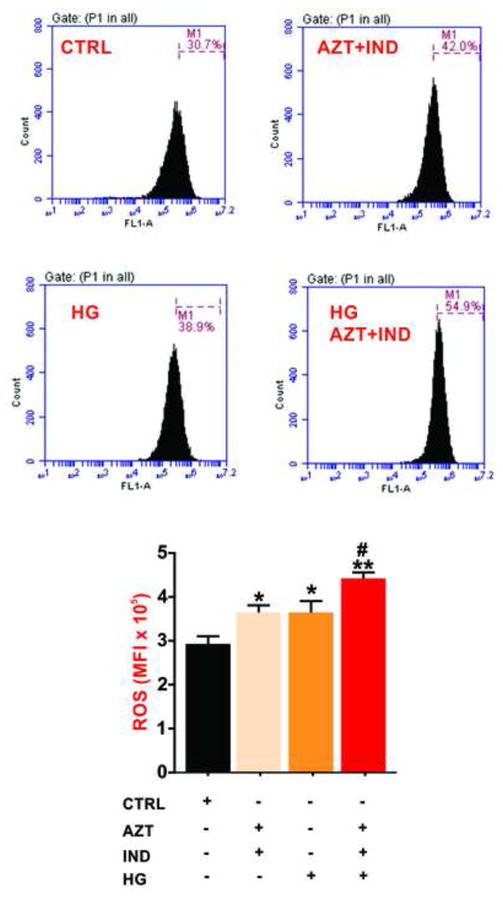
Combined exposure to HG and antiretroviral drugs combination exacerbates BBB endothelial oxidative stress. Total cellular ROS levels were determined by flow cytometry in hCMEC/D3 cells post 24h exposure to NG or HG media containing AZT or/and IND. The bar graph represents the data (expressed as mean ± SEM of the mean florescence intensity, MFI) shown in the histograms. *p<0.05 and **p<0.01 vs. control; #p<0.05 vs. HG or AZT+IND. N=3 biological replicates.
3.3.HG and AZT+IND aggravate BBB endothelial mitochondrial dysfunction
We next determined the mutual effects of HG and AZT+IND combination on mitochondria- specific superoxide (ROS) generation in hCMEC/D3 cell monolayers using the MitoSOX Red assay. As shown in Fig. 3A, one-way ANOVA revealed a significant effect of treatment on mitochondrial ROS production. Further post hoc analysis suggested that concomitant exposure to HG and AZT+IND elicited a significant surge in mitochondrial superoxide production in BBB endothelial cells (p<0.05 vs. control or either treatment condition) implicating a substantial increase in oxidative stress and mitochondrial damage. This was further evident by a potential loss of ΔΨm (or alternatively an increase in mitochondrial depolarization), a strong measure of mitochondrial function (bioenergetics) in cells exposed simultaneously to HG and antiretroviral drug combination (p<0.01 vs. control and p<0.05 vs. HG or AZT+IND). However, individual exposures to AZT, IND or HG alone did not significantly affect the ΔΨm (compared to control). Thus, our data implicate that antiretroviral drugs in presence of existing hyperglycemia would have detrimental effects on the mitochondrial function leading to BBB endothelial toxicity.
Figure 3.
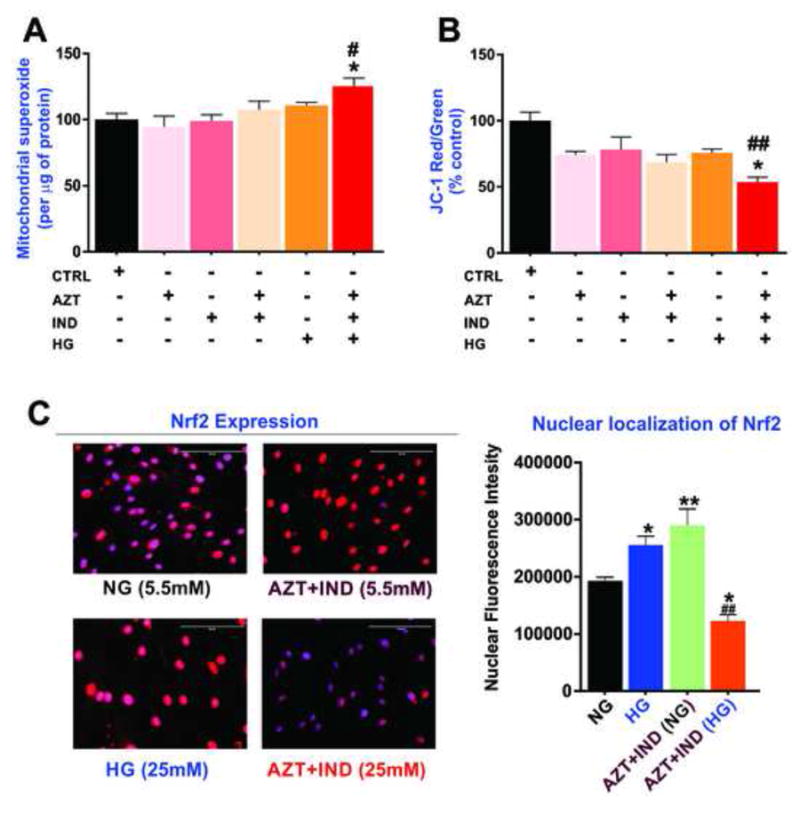
Effects of concomitant exposure to HG and antiretroviral drug combination on mitochondrial function and Nrf2 expression in BBB endothelial cells. 24h exposure to HG and AZT+IND combination significantly increases mitochondrial superoxide (ROS) content as measured by MitoSOX Red (A) and impair the mitochondrial membrane potential assayed by the ratio of JC-dye red aggregates to green monomers (% control) in hCMEC/D3 cells (B). The mitochondrial superoxide content was further normalized to the protein content. (C) Immunofluorescence analysis of Nrf2 expression and nuclear localization with images captured at 40x (scale = 100μm). Data were expressed as mean ± SEM. *p<0.05 vs HG or AZT+IND alone; #p<0.05 and ##p<0.01 vs. control. N=5-6 biological replicates. For immunofluorescence experiments, N=3 biological replicates.
3.4.HG and AZT+IND synergistically downregulate BBB endothelial Nrf2 expression
NF-E2 related factor (Nrf2) is a ubiquitously expressed redox-sensitive transcription factor responsible for activating the antioxidant response element genes to protect the cell against oxidative stress and inflammation (Alfieri et al., 2011; Holmstrom et al., 2013). As shown in Fig. 3C, immunofluorescent analysis revealed that 24h treatment with HG or AZT+IND significantly increases Nrf2 expression and nuclear translocation in BBB endothelium. Interestingly, we observed a pronounced downregulation of Nrf2 expression and nuclear localization following concomitant exposure to HG and AZT+IND. These data suggest that the antiretroviral drug combination under HG conditions potentially impairs the BBB endothelial redox hemostasis by suppressing Nrf2-mediated antioxidant responses, thus explaining the mechanistic basis for increased endothelial oxidative stress shown in Fig. 2.
4. Discussion
Although, existing clinical studies indicate an increased onset and prevalence of diabetes in HIV infected patients receiving HAART chronically, the impact of antiviral drug combination on vascular endothelium in diabetic subjects is largely unknown. In addition, the detrimental effects of AZT and IND combination on BBB endothelial function are relatively less studied despite the significant evidence showing an escalation of oxidative/inflammatory stress by these drugs leading to vascular endothelial dysfunction in periphery (Blas-Garcia et al., 2011). Thus, our results provide unprecedented evidence that combination of AZT and IND potentially interacts with HG to induce an aggravated BBB endothelial oxidative stress and mitochondrial toxicity, potential mechanisms of endothelial dysfunction and loss of BBB integrity (Freeman and Keller, 2012; Sajja et al., 2016).
Previously, it was reported that AZT alone or in combination with IND at higher concentrations significantly increased tight junction (TJ) disruption at endothelial cell-cell contacts in congruent with decreased trans-endothelial electrical resistance (Fiala et al., 2004). In a subsequent study, Manda and colleagues demonstrated that AZT and IND combination significantly elevated BBB permeability to dextrans (and reduction in TEER) across hCMEC/D3 monolayers, possibly though increased ROS and loss of mitochondrial membrane potential (Manda et al., 2011). Further, recent findings from our group indicate a significant disruption of ZO-1 bands at endothelial cell-cell contents and marked increase in BBB permeability following HG exposure (Sajja et al., 2014). In this line, our data implicate that HG complements AZT and IND combination (or vice versa) resulting in an exacerbated BBB disruption. Also it should be noted that indinavir was shown to promote umbilical vein microvascular endothelial cell proliferation (HUVEC) through the release of endothelin-1(Hebert et al., 2004). Therefore, increased ATP by IND alone post 72h exposure we observed in our study (Fig. 1) can be explained by similar mechanism where indinavir promotes angiogenesis and therefore cell proliferation in BBB microvascular endothelial cells. However, this hypothesis needs to be verified in further studies
It should also be noted that the onset of significant endothelial oxidative load and mitochondrial dysfunction as reported by Manda et al was observed at much higher concentrations of AZT+IND combination (50-200μM) (Manda et al., 2011), that may not be clinically feasible (Jiang et al., 2007; Wang et al., 2009). However, lower therapeutically relevant concentrations of AZT and IND combination alone independent of HG failed to elicit BBB endothelial toxicity and alter cell metabolism (Manda et al., 2011), thus supporting our data shown in Figs. 1-3. It is also reasonable to assume that HG may sensitize the BBB endothelium to antiretroviral drug induced toxicity through profound mitochondrial dysfunction and redox imbalance.
Oxidative stress and mitochondrial dysfunction are shown to have crucial role in HAART induced endothelial dysfunction and vascular complications (Blas-Garcia et al., 2011; Jiang et al., 2007; Mondal et al., 2004; Wang et al., 2009). Interestingly, IND alone or in HAART potentiated endothelial inflammatory stress manifested by increased expression of endothelial adhesion molecules and monocyte recruitment (Mondal et al., 2004). In corroboration with these findings, we observed that AZT and IND combination (or HG alone) for 24h significantly increased total cellular oxidative stress in hCMEC/D3 cells (Fig. 2). However, either condition did not affect mitochondrial ROS production (Fig. 3). It is plausible that the lack of effects of AZT or/and IND including HG could be due to transient nature (instability) of mitochondrial ROS and inclusion of shorter time frames (4-8h) could possibly elucidate drug-induced changes in mitochondrial ROS (Jiang et al., 2007). Nevertheless, our data suggest that HG exposure concomitant with AZT+IND exacerbates mitochondrial superoxide levels and membrane depolarization with potential alterations in cell metabolism.
We previously demonstrated that Nrf2, a master regulator of constitutive and inducible expression of cellular anti-oxidant gene networks, is critical for BBB integrity and function (Sajja et al., 2015). In this context, we studied the impact of antiretroviral drug combination in presence or absence of HG on Nrf2 expression and its nuclear distribution in BBB endothelial cells. While treatment with either condition increased the nuclear localization of Nrf2 that implicates an ensuing activation of antioxidant responses, AZT+IND co-exposure with HG potentially suppressed Nrf2 expression and nuclear translocation in BBB endothelium (see Fig. 3C). Importantly, the synergistic deficit in BBB Nrf2 signaling strongly correlated with increased cellular oxidative stress (ROS generation; Fig. 2) and mitochondrial dysfunction (Fig. 3A & B) induced by antiretroviral drug combination with accompanying HG. Alternatively, augmentation of Nrf2 nuclear localization by AZT+IND or HG could be explained as a plausible mechanism leading to an increased ATP generation by these conditions post 24h (see Fig. 1). Given the pivotal role of Nrf2 in mitochondrial function and cellular bioenergetics (Dinkova-Kostova and Abramov, 2015), our results suggest that impaired endothelial Nrf2 signaling could be a potential mechanism that underlies the exacerbated BBB toxicity by AZT+IND in presence of HG.
5. Conclusions
In summary, concomitant HG and antiretroviral drug combination (AZT+IND) synergistically or additively interact to induce an exacerbated BBB endothelial dysfunction that involves significant down-regulation of Nrf2 expression/nuclear translocation leading to an increased oxidative stress and mitochondrial toxicity. Thus, our data suggest that use of HAART regimen containing PI and NRTIs in HIV-infected diabetic subjects could pose a significant clinical challenge due to increased progression and pathogenesis of neurovascular and cognitive disorders that ensue BBB disruption.
Acknowledgments
The hCMEC/D3 cell line was provided by Dr. P.O. Couraud (INSERM, France). The authors also thank Taylor R. Liles for his technical assistance.
Funding
These studies were supported by Alternatives Research and Development Foundation and in part by NIH/NIDA R01-DA029121-01A1 to Dr. Luca Cucullo.
Footnotes
Author Contributions
Conceived and designed the experiments: RKS, LC; Performed the experiments: SP, RKS, MAK; Data analysis and interpretation: RKS, SP, LC; Contributed reagents/materials/analysis tools: LC; Manuscript preparation: SP, RKS, LC. Manuscript revision: MAK, LC. All authors approved the final version of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes Metab. 2009;11:480–490. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- Baliga RS, Liu C, Hoyt DG, Chaves AA, Bauer JA. Vascular endothelial toxicity induced by HIV protease inhibitor: evidence of oxidant-related dysfunction and apoptosis. Cardiovasc Toxicol. 2004;4:199–206. doi: 10.1385/ct:4:2:199. [DOI] [PubMed] [Google Scholar]
- Barbaro G, Iacobellis G. Metabolic syndrome associated with HIV and highly active antiretroviral therapy. Curr Diab Rep. 2009;9:37–42. doi: 10.1007/s11892-009-0008-7. [DOI] [PubMed] [Google Scholar]
- Blas-Garcia A, Apostolova N, Esplugues JV. Oxidative stress and mitochondrial impairment after treatment with anti-HIV drugs: clinical implications. Curr Pharm Des. 2011;17:4076–4086. doi: 10.2174/138161211798764951. [DOI] [PubMed] [Google Scholar]
- Chastain DB, Henderson H, Stover KR. Epidemiology and management of antiretroviral-associated cardiovascular disease. Open AIDS J. 2015;9:23–37. doi: 10.2174/1874613601509010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagogo-Jack S. HIV therapy and diabetes risk. Diabetes Care. 2008;31:1267–1268. doi: 10.2337/dc08-0459. [DOI] [PubMed] [Google Scholar]
- Dasuri K, Pepping JK, Fernandez-Kim SO, Gupta S, Keller JN, Scherer PE, Bruce-Keller AJ. Elevated adiponectin prevents HIV Protease inhibitor toxicity and preserves cerebrovascular homeostasis in mice. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.02.009. [DOI] [PubMed] [Google Scholar]
- De WS, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, El-Sadr W, Monforte A, Fontas E, Law MG, Friis-Moller N, Phillips A. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31:1224–1229. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala M, Murphy T, MacDougall J, Yang W, Luque A, Iruela-Arispe L, Cashman J, Buga G, Byrns RE, Barbaro G, Arthos J. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol. 2004;4:327–337. doi: 10.1385/ct:4:4:327. [DOI] [PubMed] [Google Scholar]
- Fofaria NM, Kim SH, Srivastava SK. Piperine causes G1 phase cell cycle arrest and apoptosis in melanoma cells through checkpoint kinase-1 activation. PLoS One. 2014;9:e94298. doi: 10.1371/journal.pone.0094298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Keller JN. Oxidative stress and cerebral endothelial cells: regulation of the blood-brain-barrier and antioxidant based interventions. Biochim Biophys Acta. 2012;1822:822–829. doi: 10.1016/j.bbadis.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR. Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc Toxicol. 2004;4:117–131. doi: 10.1385/ct:4:2:117. [DOI] [PubMed] [Google Scholar]
- Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production but not apoptosis. Toxicol Appl Pharmacol. 2007;224:60–71. doi: 10.1016/j.taap.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3:2. doi: 10.1186/1758-5996-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamchand S, Leisegang R, Schomaker M, Maartens G, Walters L, Hislop M, Dave JA, Levitt NS, Cohen K. Risk Factors for Incident Diabetes in a Cohort Taking First-Line Nonnucleoside Reverse Transcriptase Inhibitor-Based Antiretroviral Therapy. Medicine (Baltimore) 2016;95:e2844. doi: 10.1097/MD.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, Mallory ME, Hansen LA, Archibald S, Jernigan T, Masliah E. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS. 2002;16:1019–1029. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GA, Rao MN, Grunfeld C. The Effects of HIV Protease Inhibitors on Carbohydrate and Lipid Metabolism. Curr Infect Dis Rep. 2004;6:471–482. doi: 10.1007/s11908-004-0067-5. [DOI] [PubMed] [Google Scholar]
- Manda KR, Banerjee A, Banks WA, Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med. 2011;50:801–810. doi: 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Pradhan L, Ali M, Agrawal KC. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovasc Toxicol. 2004;4:287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja RK, Green KN, Cucullo L. Altered Nrf2 signaling mediates hypoglycemia-induced blood-brain barrier endothelial dysfunction in vitro. PLoS One. 2015;10:e0122358. doi: 10.1371/journal.pone.0122358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja RK, Prasad S, Cucullo L. Impact of altered glycaemia on blood-brain barrier endothelium: an in vitro study using the hCMEC/D3 cell line. Fluids Barriers CNS. 2014;11:8. doi: 10.1186/2045-8118-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja RK, Rahman S, Cucullo L. Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. J Cereb Blood Flow Metab. 2016;36:539–554. doi: 10.1177/0271678X15616978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth AE, Walter FR, Bocsik A, Santha P, Veszelka S, Nagy L, Puskas LG, Couraud PO, Takata F, Dohgu S, Kataoka Y, Deli MA. Edaravone protects against methylglyoxal-induced barrier damage in human brain endothelial cells. PLoS One. 2014;9:e100152. doi: 10.1371/journal.pone.0100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chai H, Lin PH, Yao Q, Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. Am J Pathol. 2009;174:771–781. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]


