Abstract
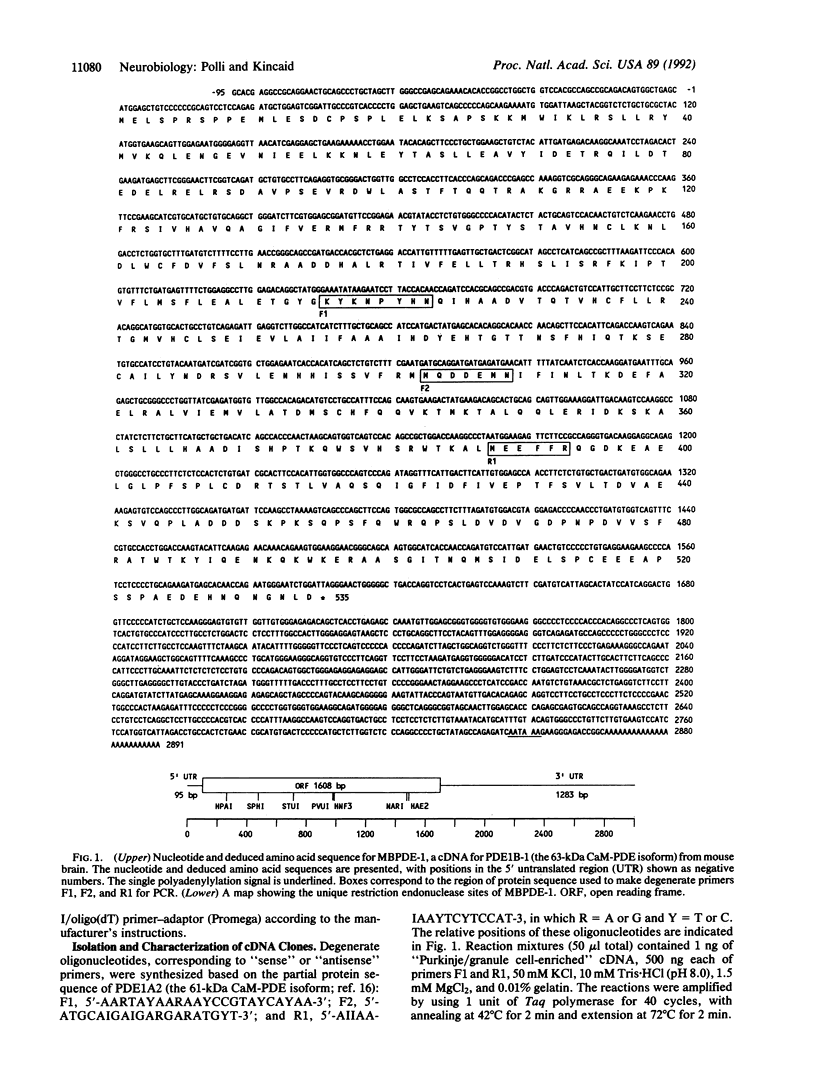
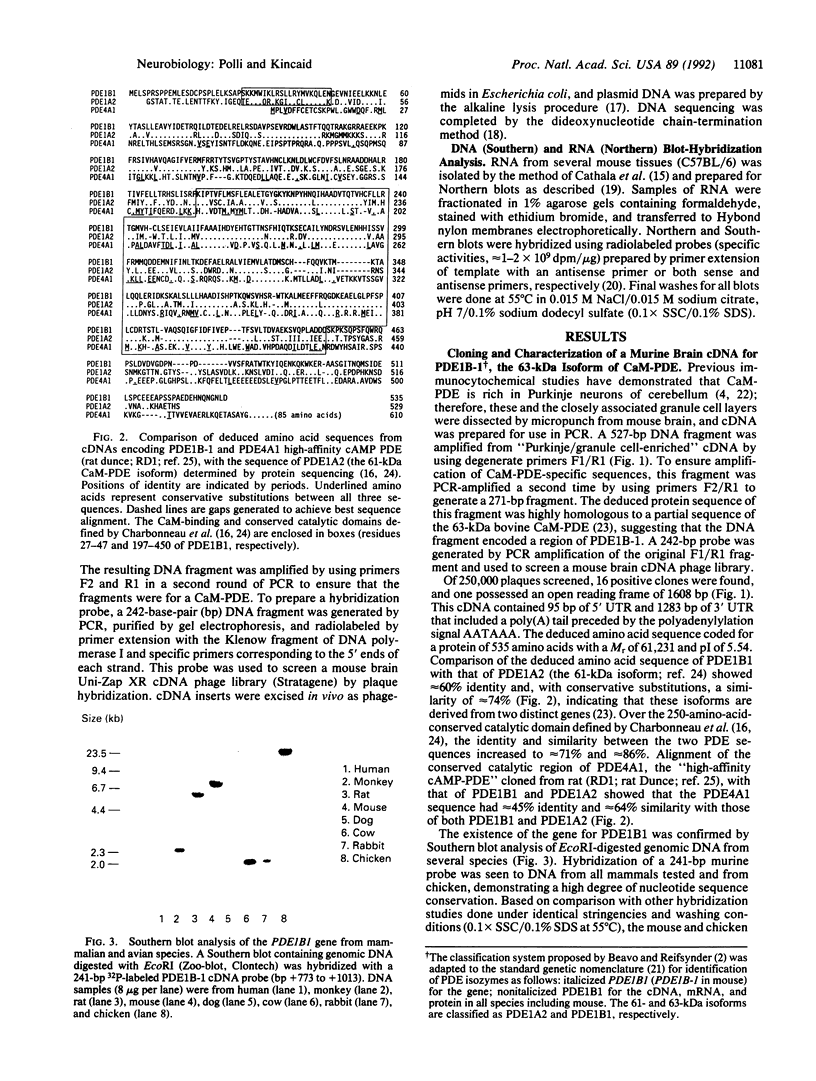
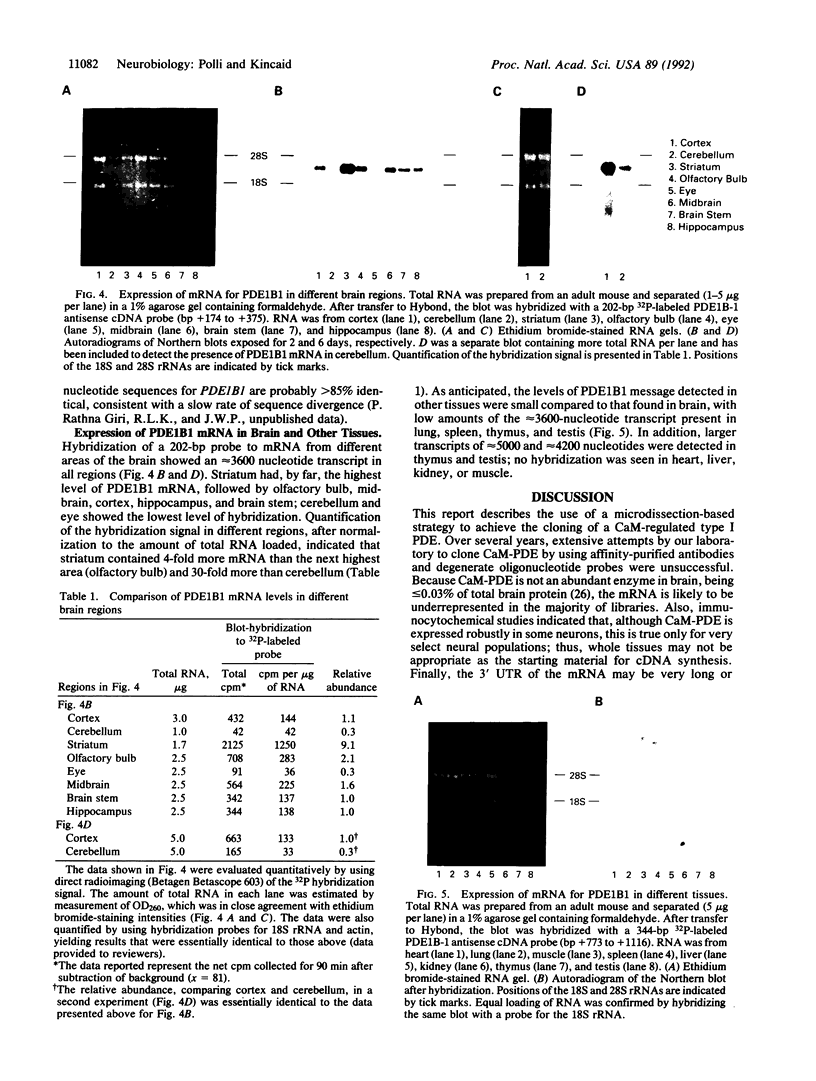
A murine cDNA for the 63-kDa calmodulin-dependent phosphodiesterase (CaM-PDE), PDE1B-1, was isolated by using polymerase chain reaction with degenerate primers followed by the cloning of a full-length cDNA from a whole-brain phage library. The nucleotide sequence of 2986 base pairs contains an open reading frame encoding a protein of 535 amino acids (M(r) = 61,231) with a predicted isoelectric point of 5.54. The deduced protein sequence shows approximately 60% identity with that of the 61-kDa isoform (PDE1A2), consistent with the proposal that these proteins arise from two separate genes [Novack, J. P., Charbonneau, H., Bentley, J. K., Walsh, K. A. & Beavo, J. A. (1991) Biochemistry 30, 7940-7947]. Southern blot analysis suggests high nucleotide-sequence conservation of the PDE1B1 gene among mammalian and avian species. A single approximately 3600-nucleotide mRNA transcript was seen in all brain regions, with striatum containing 4- to 30-fold higher levels than other areas. In nonneural tissues, low amounts of PDE1B1 mRNA were detected in lung, spleen, thymus, and testis; hybridization to several larger mRNA species was also seen in thymus and testis. By using nucleic acid probes for PDE1B1, the mechanisms that control its highly selective gene expression can now be studied at the molecular level.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban C. D., Billingsley M. L., Kincaid R. L. Evidence for transsynaptic regulation of calmodulin-dependent cyclic nucleotide phosphodiesterase in cerebellar Purkinje cells. J Neurosci. 1989 Jul;9(7):2374–2381. doi: 10.1523/JNEUROSCI.09-07-02374.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Beavo J. A., Reifsnyder D. H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol Sci. 1990 Apr;11(4):150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- Billingsley M. L., Polli J. W., Balaban C. D., Kincaid R. L. Developmental expression of calmodulin-dependent cyclic nucleotide phosphodiesterase in rat brain. Brain Res Dev Brain Res. 1990 May 1;53(2):253–263. doi: 10.1016/0165-3806(90)90015-q. [DOI] [PubMed] [Google Scholar]
- Borisy F. F., Ronnett G. V., Cunningham A. M., Juilfs D., Beavo J., Snyder S. H. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992 Mar;12(3):915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Beier N., Walsh K. A., Beavo J. A. Identification of a conserved domain among cyclic nucleotide phosphodiesterases from diverse species. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9308–9312. doi: 10.1073/pnas.83.24.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Kumar S., Novack J. P., Blumenthal D. K., Griffin P. R., Shabanowitz J., Hunt D. F., Beavo J. A., Walsh K. A. Evidence for domain organization within the 61-kDa calmodulin-dependent cyclic nucleotide phosphodiesterase from bovine brain. Biochemistry. 1991 Aug 13;30(32):7931–7940. doi: 10.1021/bi00246a009. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Wolgemuth D. J., Hsu M. T., Darnell J. E., Jr Transcription and accurate polyadenylation in vitro of RNA from the major late adenovirus 2 transcription unit. Cell. 1982 Mar;28(3):575–584. doi: 10.1016/0092-8674(82)90212-4. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Takayasu H., Eberwine M., Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P. M., Moraski S., Jr, Hachisu R. Identification and characterization of a Ca2+-calmodulin-sensitive cyclic nucleotide phosphodiesterase in a human lymphoblastoid cell line. Biochem J. 1987 Apr 15;243(2):533–539. doi: 10.1042/bj2430533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R. S., Beavo J. A. Purification of two calcium/calmodulin-dependent forms of cyclic nucleotide phosphodiesterase by using conformation-specific monoclonal antibody chromatography. Proc Natl Acad Sci U S A. 1982 May;79(9):2788–2792. doi: 10.1073/pnas.79.9.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Sharma R. K., Soderling T. R. Regulation of Ca2+/calmodulin-dependent cyclic nucleotide phosphodiesterase by the autophosphorylated form of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1989 Jun 25;264(18):10884–10887. [PubMed] [Google Scholar]
- Hurwitz R. L., Hirsch K. M., Clark D. J., Holcombe V. N., Hurwitz M. Y. Induction of a calcium/calmodulin-dependent phosphodiesterase during phytohemagglutinin-stimulated lymphocyte mitogenesis. J Biol Chem. 1990 May 25;265(15):8901–8907. [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Differential localization of calmodulin-dependent enzymes in rat brain: evidence for selective expression of cyclic nucleotide phosphodiesterase in specific neurons. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1118–1122. doi: 10.1073/pnas.84.4.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid R. L., Balaban C. D., Billingsley M. L. Regulated expression of calmodulin-dependent cyclic nucleotide phosphodiesterase in the central nervous system. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):473–486. [PubMed] [Google Scholar]
- Kincaid R. L., Giri P. R., Higuchi S., Tamura J., Dixon S. C., Marietta C. A., Amorese D. A., Martin B. M. Cloning and characterization of molecular isoforms of the catalytic subunit of calcineurin using nonisotopic methods. J Biol Chem. 1990 Jul 5;265(19):11312–11319. [PubMed] [Google Scholar]
- Lipkin V. M., Khramtsov N. V., Vasilevskaya I. A., Atabekova N. V., Muradov K. G., Gubanov V. V., Li T., Johnston J. P., Volpp K. J., Applebury M. L. Beta-subunit of bovine rod photoreceptor cGMP phosphodiesterase. Comparison with the phosphodiesterase family. J Biol Chem. 1990 Aug 5;265(22):12955–12959. [PubMed] [Google Scholar]
- Ludvig N., Burmeister V., Jobe P. C., Kincaid R. L. Electron microscopic immunocytochemical evidence that the calmodulin-dependent cyclic nucleotide phosphodiesterase is localized predominantly at postsynaptic sites in the rat brain. Neuroscience. 1991;44(2):491–500. doi: 10.1016/0306-4522(91)90072-v. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Novack J. P., Charbonneau H., Bentley J. K., Walsh K. A., Beavo J. A. Sequence comparison of the 63-, 61-, and 59-kDa calmodulin-dependent cyclic nucleotide phosphodiesterases. Biochemistry. 1991 Aug 13;30(32):7940–7947. doi: 10.1021/bi00246a010. [DOI] [PubMed] [Google Scholar]
- Palkovits M. Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res. 1973 Sep 14;59:449–450. doi: 10.1016/0006-8993(73)90290-4. [DOI] [PubMed] [Google Scholar]
- Polli J. W., Kincaid R. L., Torris J., Billingsley M. L. Expression of calmodulin-dependent enzymes in developing rat striatum is not affected by perturbation of dopaminergic systems. Synapse. 1991 Oct;9(2):136–143. doi: 10.1002/syn.890090208. [DOI] [PubMed] [Google Scholar]
- Rossi P., Giorgi M., Geremia R., Kincaid R. L. Testis-specific calmodulin-dependent phosphodiesterase. A distinct high affinity cAMP isoenzyme immunologically related to brain calmodulin-dependent cGMP phosphodiesterase. J Biol Chem. 1988 Oct 25;263(30):15521–15527. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K., Adachi A. M., Adachi K., Wang J. H. Demonstration of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isozymes by monoclonal antibodies. J Biol Chem. 1984 Jul 25;259(14):9248–9254. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Calmodulin and Ca2+-dependent phosphorylation and dephosphorylation of 63-kDa subunit-containing bovine brain calmodulin-stimulated cyclic nucleotide phosphodiesterase isozyme. J Biol Chem. 1986 Jan 25;261(3):1322–1328. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Purification and characterization of bovine lung calmodulin-dependent cyclic nucleotide phosphodiesterase. An enzyme containing calmodulin as a subunit. J Biol Chem. 1986 Oct 25;261(30):14160–14166. [PubMed] [Google Scholar]
- Swinnen J. V., Joseph D. R., Conti M. Molecular cloning of rat homologues of the Drosophila melanogaster dunce cAMP phosphodiesterase: evidence for a family of genes. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5325–5329. doi: 10.1073/pnas.86.14.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J. Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol Ther. 1991;51(1):13–33. doi: 10.1016/0163-7258(91)90039-o. [DOI] [PubMed] [Google Scholar]





