Highlight
Analysis of rice plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants reveals that starch synthesis in pollen grains requires the production of glucose-1-P and ADP-glucose in the plastids.
Key words: ADP-glucose pyrophosphorylase, male sterility, Oryza sativa, phosphoglucomutase, plastid, pollen, starch.
Abstract
To elucidate the starch synthesis pathway and the role of this reserve in rice pollen, we characterized mutations in the plastidic phosphoglucomutase, OspPGM, and the plastidic large subunit of ADP-glucose (ADP-Glc) pyrophosphorylase, OsAGPL4. Both genes were up-regulated in maturing pollen, a stage when starch begins to accumulate. Progeny analysis of self-pollinated heterozygous lines carrying the OspPGM mutant alleles, osppgm-1 and osppgm-2, or the OsAGPL4 mutant allele, osagpl4-1, as well as reciprocal crosses between the wild type (WT) and heterozygotes revealed that loss of OspPGM or OsAGPL4 caused male sterility, with the former condition rescued by the introduction of the WT OspPGM gene. While iodine staining and transmission electron microscopy analyses of pollen grains from homozygous osppgm-1 lines produced by anther culture confirmed the starch null phenotype, pollen from homozygous osagpl4 mutant lines, osagpl4-2 and osagpl4-3, generated by the CRISPR/Cas system, accumulated small amounts of starch which were sufficient to produce viable seed. Such osagpl4 mutant pollen, however, was unable to compete against WT pollen successfully, validating the important role of this reserve in fertilization. Our results demonstrate that starch is mainly polymerized from ADP-Glc synthesized from plastidic hexose phosphates in rice pollen and that starch is an essential requirement for successful fertilization in rice.
Introduction
Starch is one of the main storage reserves in various tissues and organs of higher plant species. The starch synthesis pathways in photosynthetic source leaves and in heterotrophic sink seed endosperms have been defined based on the analysis of a large number of mutants identified in many plant species. Such studies have revealed that the key regulatory step of the starch synthesis pathway is mediated by ADP-glucose (ADP-Glc) pyrophosphorylase (AGP) (Müller-Röber et al., 1990; Okita, 1992; Tetlow et al., 2004; Lee et al., 2007). AGP catalyses the formation of ADP-Glc and inorganic pyrophosphate (PPi) from glucose-1-phosphate (Glc-1-P) and ATP. The resulting ADP-Glc molecule serves as the glucosyl donor for starch synthesis.
AGP functions as a heterotetrameric enzyme and is composed of two large subunits (LSs) and two small subunits (SSs) with slightly different molecular masses (Okita et al., 1990; Smith-White and Preiss, 1992; Villand et al., 1993; Seferoglu et al., 2014). Higher plants contain multiple AGP LS and SS isoforms that are expressed in different tissues and organs and/or have different subcellular locations (Beckles et al., 2001a, b ; James et al., 2003; Vigeolas et al., 2004; Lee et al., 2007; Huang et al., 2014; Okamura et al., 2014). In photosynthetic tissue, AGP is located in the chloroplast where it is responsible for starch synthesis, as mutations in the major leaf isoform exhibit reduced plastidic AGP activity and impaired starch synthesis (Tsai and Nelson, 1966; Dickinson and Preiss, 1969; Lin et al., 1988a, b ; Smith et al., 1989; Wang et al., 1997, 1998; Lee et al., 2007; Cook et al., 2012; Huang et al., 2014; Okamura et al., 2014). By contrast, in cereal endosperms, although there is an AGP detected in the specialized starch-containing amyloplasts, the major AGP activity is located in the cytosol (Greene and Hannah, 1998; Sikka et al., 2001; James et al., 2003; Johnson et al., 2003; Lee et al., 2007; Tuncel et al., 2014).
During photosynthesis in leaves, triose-phosphates from the Calvin–Benson cycle are exported to the cytosol for the production of sucrose. A portion of the triose-phosphates are retained in the chloroplast where they are metabolized into transitory starch by co-ordinated sequential enzyme reactions involving the plastidic phosphoglucomutase (pPGM), that interconverts glucose-6-phosphate (Glc-6-P) and Glc-1-P, and plastidic AGP. Mutants with reduced pPGM activity have decreased levels of leaf starch (Caspar et al., 1985; Hanson and McHale, 1988; Harrison et al., 1998, 2000; Tauberger et al., 2000; Fernie et al., 2001). These results clearly demonstrate that Glc-1-P and, in turn, ADP-Glc is synthesized in the leaf chloroplasts. Similarly, studies in the sink organs, including seeds, of non-cereal plants also provide evidence that AGP activity is present exclusively in the plastids (Smith et al., 1995; Beckles et al., 2001b ).
By contrast, a number of studies have revealed that the cytosolic AGP activity is a prerequisite for normal starch synthesis in the seed endosperm of cereal plants such as rice, barley, and maize (Greene and Hannah, 1998; Sikka et al., 2001; James et al., 2003; Johnson et al., 2003; Lee et al., 2007; Tuncel et al., 2014). For instance, the low-starch endosperm mutant of barley, Risø16, lacks the cytosolic AGP SS isoform (Johnson et al., 2003). The low-starch maize mutant bt2 possesses a mutation in a cytosolic AGP SS (Giroux and Hannah, 1994; Hannah et al., 2001). Similarly, mutants of the rice osagps2 and osagpl2 lack the cytosolic AGP SS and LS, respectively, and exhibit impaired starch synthesis during seed development (Lee et al., 2007). In addition to null mutations, missense mutations in the OsAGPL2 gene resulted in impaired AGP enzyme activity and, in turn, severely shrivelled, starch-deficient rice seeds (Tuncel et al., 2014). These results clearly demonstrate that the cytosolic form of AGP is essential for normal starch synthesis in the developing seed endosperm of cereal plants.
In many plants including most of the world’s major cereal crops, rice, maize, wheat, and barley, starch is the main storage reserve in mature pollen grains and used for supplying energy and a carbon skeleton to support pollen germination and pollen tube growth for proper fertilization. Thus, insufficient starch synthesis in pollen grains is believed to be responsible for male sterility (Dickinson, 1968; Wen and Chase, 1999; Datta et al., 2002; Wu et al., 2016). As such, manipulation of the starch content in pollen grains has provided a novel strategy of inducing male sterility (Wu et al., 2016). Male sterile lines are used in hybrid seed production which is widely employed for the production of high yield (Virmani and Kumar, 2009; Jeon et al., 2011; Wu et al., 2016). Despite its importance, little is known about the starch synthesis pathway in cereal pollen grains, an important heterotrophic organ essential for plant reproduction.
In the present study, we show that mutations in the rice plastidic PGM, OspPGM, and the plastidic AGP LS, OsAGPL4, result in starch deficiency in pollen grains and induce male sterility. These results clearly demonstrate that the main pathway of starch synthesis in this sink organ requires the synthesis of Glc-1-P and ADP-Glc in the plastid.
Materials and methods
Plant materials
Greenhouse-grown japonica rice [cultivar (cv.) Dongjin] WT and mutants were mainly used in the experiments. Rice plants were grown in a greenhouse at 30 °C during the day and at 20 °C at night in a light/dark cycle of 14/10h and approximately 80% humidity. Anthers from WT rice plants for RT-PCR were harvested at different development stages.
RT-PCR analysis
Total RNA was extracted using the Trizol reagent and reverse transcription was performed with the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). First strand cDNAs were used in RT-PCR reactions with gene-specific primers and control primers for the rice housekeeping gene Ubiquitin5 (OsUBQ5; LOC_Os01g22490). The primers were designed in the region encompassing at least one intron for each gene to exclude genomic DNA contamination. The gene-specific primers used for RT-PCR are in Supplementary Table S1 at JXB online.
Subcellular localization of the OspPGM-GFP fusion protein
The OspPGM full-length cDNA without stop codon was fused to the Green Fluorescent Protein (GFP) gene in-frame. The OspPGM full-length cDNA was amplified by PCR using the forward primer containing the SalI restriction site (5′-CACCGTCGACATGGCCTCGCACGCGCTCCGCCTC-3′) and the reverse primer containing the SmaI restriction site (5′-TCCCCCGGGATGTTATGACAGTAGGCTTATCTC-3′). The amplified fragment was digested with the respective enzymes and cloned between the CaMV35S promoter and GFP of pJJ461 which was derived from pC1300intC (Ouwerkerk et al., 2001). The resulting GFP fusion construct was then transformed into maize protoplasts. GFP signals were detected by excitation with the 488nm line of the argon laser and a capturing emission at 522nm by laser-scanning confocal microscopy (LSM 510 META, Carl Zeiss, Jena, Germany).
Isolation of the osppgm and osagpl4 T-DNA mutants
The osppgm-1, osppgm-2, and osagpl4-1 mutant alleles were identified from the rice T-DNA insertion sequence database (Jeon et al., 2000; Jeong et al., 2006; http://www.postech.ac.kr/life/pfg/risd/index.html). Genomic DNA was isolated from young leaves of rice plants using a simple miniprep method (Chen and Ronald, 1999). Pollen grains were collected with fine pipette tips under a microscope and ground using a micropestle in a PCR tube for use as the genomic DNA template. Genotypes for the T-DNA insertion were determined by genomic DNA PCR analysis using PF1/PR1 and L1/PR1 primer sets for osppgm-1, PF2/PR2 and PF2/G1 sets for osppgm-2, and LF1/LR1 and LR1/G1 sets for osagpl4-1. The sequences of primers used for genotyping are listed in Supplementary Table S1.
Reciprocal crosses
For the reciprocal crosses, OspPGM/osppgm-1, OspPGM/osppgm-2, and OsAGPL4/osagl4-1 were crossed with a japonica cv. Ilpum WT rice plants. Genotypes of the crossed lines were determined by PCR using the same primer sets used for T-DNA insertions.
Anther culture of osppgm mutants
To generate homozygous mutant plants of OspPGM, anther culture was performed as described by Eom et al. (2016), with slight modifications, using anthers isolated from the heterozygous plants of OspPGM/osppgm-1 and OspPGM/osppgm-2. After surface sterilization of the panicles with 70% (v/v) ethanol, anthers removed from the spikelet were cultured on the N6 callus induction medium containing 2.0mg l–1 NAA, 0.2mg l–1 kinetin, and 5% (w/v) gelrite at 25 °C for 1 month. The induced callus was moved to the N6 regeneration medium containing 0.2mg l–1 IAA, 2.0mg l–1 kinetin, 2g l–1 casein hydrolysate, 2mg l–1 ABA, 40g l–1 maltose, and 5% (w/v) gelrite for regeneration.
Creation of OsAGPL4 mutants using the CRISPR/Cas system
To find an effective protospacer adjacent motif (PAM) and avoid any off-target, we screened possible target sequences using the CRISPRdirect program (Naito et al., 2015; http://crispr.dbcls.jp/). Designed guide RNA (5′-GCAGTTCCTGTGGCTATTTG-3′) was cloned into an entry vector, pOs-sgRNA and then cloned into a destination vector, pH-Ubi-cas9-7, using the GatewayTM system (Miao et al., 2013). The resulting vector was transformed into the japonica rice cv. Dongjin by Agrobacterium mediation (Jeon et al., 2000). The target PAM site sequence of transgenic plants was determined.
Analysis of PGM activity by a native polyacrylamide gel electrophoresis
The in-gel PGM activity assay of the homozygous osppm-1 mutant plants was performed as described by Egli et al. (2010) with slight modifications. Leaf samples were homogenized in ice-cold extraction buffer containing 100mM TRIS–HCl (pH 7.0), 10mM MgCl2,100mM KCl, 42mM β-mercaptoethanol, and 15% (v/v) glycerol. Proteins were resolved in non-denaturing polyacrylamide gel containing 8% (w/v) acrylamide [30% (w/v) acrylamide/0.8% (w/v) bis-acrylamide] and 375mM TRIS–HCl (pH 8.8). The gels were washed in the washing solution containing 50mM TRIS–HCl, pH 7.0, and 5mM MgCl2 for 1min and were then incubated in the staining solution containing 50mM TRIS–HCl, pH 7.0, 5mM MgCl2, 5.3mM Glc-1-P, 0.25mM NADP, 0.25mM NAD, 0.1mM phenazine methosulphate, 0.25mM nitroblue tetrazolium, and 40 units Glc-6-P dehydrogenase at 37 °C.
Determination of sucrose and starch
Approximately 100mg of rice leaves and 3mg of mature anthers containing pollen grains, respectively, were harvested at the end of the day from 12-week old plants and mature flowers. The soluble sugar sucrose and insoluble starch were measured using NAD(P)H-coupled enzymatic tests in the ethanol-soluble and -insoluble fractions (Lee et al., 2008). The measured metabolite contents were normalized to the leaf fresh weights.
Pollen staining and light microscopy
For staining pollen nuclei, pollen grains were incubated in phosphate-buffered saline containing 4ng ml–1 Hoechst 33342 (Sigma, St Louis, MO, USA) for 1h at 65 °C. The pollen starch was stained with 10% (v/v) Lugol solution (Sigma). The nuclear- and starch-stained pollen grains were monitored under UV light and white light, respectively, with an Olympus BX61 microscope.
Transmission electron microscopy
Pollen grains at the mature stage of development were fixed in sodium phosphate buffer (pH 7.2) containing 3% (v/v) glutaraldehyde, and then post-fixed using 2% (v/v) osmium tetroxide. After dehydration, the specimens were put into Spurr’s low-viscosity embedding mixture. Ultrathin sections (40–60nm in thickness) were stained with 2.5% (w/v) uranyl acetate and 2.5% (w/v) lead citrate aqueous solutionsbefore being examined with a transmission electron microscope, Tecnai G2 Spirit (FEI Co., Hillsboro, OR, USA).
Measurement of AGP activity
Anthers containing pollen grains (approximately 3mg) were resuspended in 100 μl of lysis buffer containing 50mM TRIS–HCl (pH 7.6), 150mM NaCl, 5% (v/v) glycerol, 5mM EDTA, 5mM DTT, 1×proteinase inhibitors, and 1mM PMSF. The samples were collected at the bottom of a tube by centrifugation at 1 000 g for 5min at 4 °C. They were then ground on ice using a pestle in a 1.5ml tube and sonicated twice for 5s using a Sonic Dismembrator (Model 100, Fisher Scientific, Pittsburgh, PA, USA). Soluble proteins were obtained by centrifugation at 15 000 g for 10min at 4 °C and used for the enzyme assay. AGP activities were measured in the starch synthesis (ADP-Glc formation) direction as described in Hwang et al. (2007) with slight modifications. Briefly, the reactions were performed at 30 °C for 40min in 0.1ml of 100mM HEPES–NaOH (pH 8.0), 5mM DTT, 10mM MgCl2, 2mM ATP, 5mM 3-phosphoglycerate, 0.15 units/reaction inorganic pyrophosphatase (Sigma), 0.4mg ml–1 BSA, 2mM [14C] Glc-1-P (517 dpm nmol–1). [14C] ADP-Glc formation was measured using the Tri-Carb 2100TR Liquid Scintillation Counter (PerkinElmer, Boston, MA, USA).
Results
Identification of a rice plastidic phosphoglucomutase gene highly expressed at the starch synthesis stage during pollen development
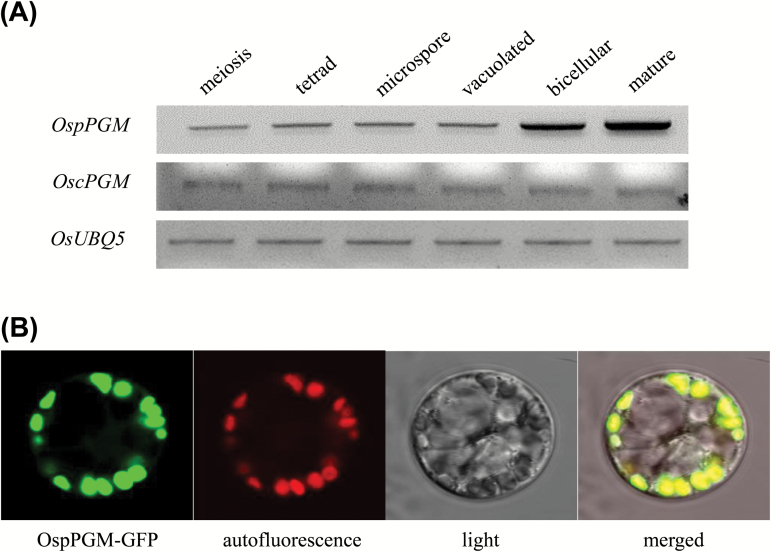
To resolve the starch synthesis pathway in rice pollen grains, it was necessary to see whether a rice PGM gene is highly expressed in the bicellular and mature stages of anthers when starch begins to accumulate (Raghavan, 1988; Yamagata et al., 2010). Analysis of the rice genomic sequence identified two PGM genes, LOC_Os03g50480 and LOC_Os10g11140 that are predicted to encode a cytosolic isoform, OscPGM, and a plastidic isoform, OspPGM, respectively (see below). Their expression was examined by semi-quantitative RT-PCR at various stages of the developing anthers. OspPGM expression had increased at the bicellular pollen stage and reached a peak at the mature pollen stage (Fig. 1A). By contrast, the OscPGM gene was expressed weakly throughout all the stages of pollen development. This result indicates that OspPGM expression coincides with the onset of starch accumulation during pollen development, supporting a direct role of this enzyme in starch synthesis.
Fig. 1.
Expression profile of two rice phosphoglucomutase genes (A) and subcellular localization of OspPGM-GFP protein (B). (A) RT-PCR analysis of OspPGM (LOC_Os03g50480) and OscPGM (LOC_Os10g11140) at different stages of pollen development. OsUBQ5 was used as the PCR control. (B) Chloroplast localization of OspPGM–GFP fusion protein in maize protoplasts. A full-length OspPGM cDNA was fused in-frame to GFP and expressed under the control of the CaMV35S promoter. The fluorescent GFP signal, chloroplast autofluorescence, light microscope view, and a merged image are shown from left to right.
Subcellular localization prediction using the ChloroP program (http://www.cbs.dtu.dk/services/ChloroP/; Emanuelsson et al., 2000) identified a putative N-terminal chloroplast transit peptide of the first 44 amino acids of OspPGM. To verify its subcellular localization, a full-length OspPGM cDNA was fused in-frame to the GFP gene under the control of the CaMV35S promoter of a plant expression vector. The resulting construct was expressed in maize protoplasts. Analysis of the microscope images clearly showed that GFP signals and chlorophyll autofluorescence completely overlapped (Fig. 1B). This result confirms the plastidic localization of OspPGM.
Isolation and characterization of OspPGM mutants
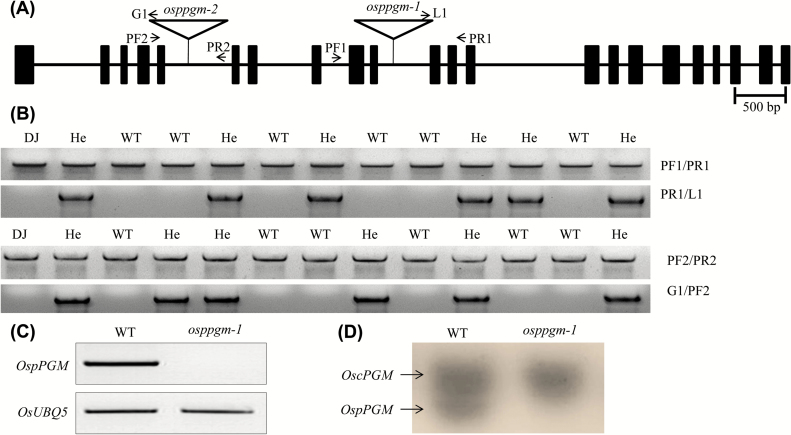
To understand its in vivo role, we isolated two OspPGM mutant alleles, osppgm-1 and osppgm-2, from our T-DNA mutant population (Jeon et al., 2000; An et al., 2005a, b; Jeong et al., 2006). The isolated osppgm-1 and osppm-2 mutant alleles harbour their T-DNA insertion in the tenth and fifth introns of the OspPGM gene, respectively (Fig. 2A). To isolate homozygous lines for these two mutant alleles from their segregating progeny, genomic DNA PCR analysis was performed using gene- and T-DNA specific primers (Supplementary Table S1). No homozygous T-DNA insertion mutant line was found as all appeared to be WT or heterozygote (Fig. 2B). Segregation ratios of progeny of self-pollinated heterozygous plants OspPGM/osppgm-1 and OspPGM/osppgm-2 were found to be nearly 1:1:0 for WT, heterozygous, and homozygous genotype (37:39:0 plants from OspPGM/osppgm-1 and 46:49:0 plants from OspPGM/osppgm-2) (Fig. 2B; Table 1). This raises the possibility that osppgm-1 and osppgm-2 mutant pollen is sterile due to the loss of Glc-1-P synthesis in the plastid.
Fig. 2.
Molecular characterization of OspPGM mutants. (A) Schematic depiction of positions of the inserted T-DNAs in the osppgm-1 and osppgm-2 mutant alleles. The 22 exons of OspPGM are indicated by the filled boxes. Primers for genotyping are marked with arrows. In the osppgm-1 and osppgm-2 mutants, T-DNAs are inserted into the tenth and the fifth introns of OspPGM, respectively. (B) Genomic DNA PCR analysis of progeny plants of OspPGM/osppgm-1 and OspPGM/osppgm-2. PF1/PR1 and L1/PR1 primers, and PF2/PR2 and G1/PF2 primers, respectively, were used for the genotyping of OspPGM/osppgm-1 (top) and OspPGM/osppgm-2 (bottom). DJ, mutant background genotype; WT, wild type; He, heterozygote. (C) RT-PCR analysis of the osppgm-1 homozygote generated by anther culture. (D) In-gel activity assay of PGM following native polyacrylamide gel electrophoresis of leaf protein extracts. Two distinct activities are visible and OspPGM activity is completely missing in the osppgm-1 homozygote as the lower faster moving band is assigned as OspPGM than the upper slower moving OscPGM.
Table 1.
Distorted segregation in the progeny of self-pollinated OspPGM/osppgm-1, OspPGM/osppgm-2, and OsAGPL4/osagpl4-1 plants
Genotypes of progeny plants were determined by PCR using gene- and T-DNA-specific primers.
| Parent plant | Observed/expected genotype of progeny in % (Observed/analysed plants in number) |
||
|---|---|---|---|
| OspPGM/osppgm-1 |
OspPGM/OspPGM
48.7/25 (37/76) |
OspPGM/osppgm-1
51.3/50 (39/76) |
osppgm-1/osppgm-1
0/25 (0/76) |
| OspPGM/osppgm-2 |
OspPGM/OspPGM
48.4/25 (46/95) |
OspPGM/osppgm-2
51.6/50 (49/95) |
osppgm-2/osppgm-2
0/25 (0/95) |
| OsAGPL4/osagpl4-1 |
OsAGPL4/OsAGPL4
48.5/25 (50/103) |
OsAGPL4/osagpl4-1
51.5/50 (53/103) |
osagpl4-1/osagpl4-1
0/25 (0/103) |
In order to determine whether the gametophytic defect is caused by a male or a female organ, we performed reciprocal crosses between the WT and each of the heterozygotes OspPGM/osppgm-1 or OspPGM/osppgm-2, respectively. When OspPGM/osppgm-1 or OspPGM/osppgm-2 was used as the pollen donor, none of the F1 plants yielded the heterozygous OspPGM/osppgm-1 or OspPGM/osppgm-2 genotype. However, when the heterozygous lines were used as the female part, the genotypes of F1 plants were segregated nearly to 1:1 for WT and heterozygote for both mutant alleles (Table 2), clearly indicating that impaired pPGM activity in rice pollen caused male sterility.
Table 2.
Results of reciprocal crosses between each of OspPGM/osppgm-1, OspPGM/osppgm-2 or OsAGPL4/osagl4-1 and wild-type plants
Genotypes of F1 plants were determined by PCR using gene- and T-DNA-specific primers.
| Genetic cross | Observed/expected genotype of progeny in % (Observed/analysed plants in number) |
||
|---|---|---|---|
| Paternal parent | Maternal parent | ||
| OspPGM/osppgm-1 | OspPGM/OspPGM |
OspPGM/OspPGM
100/50 (93/93) |
OspPGM/osppgm-1
0/50 (0/93) |
| OspPGM/OspPGM | OspPGM/osppgm-1 |
OspPGM/OspPGM
51.5/50 (52/101) |
OspPGM/osppgm-1
48.5/50 (49/101) |
| OspPGM/osppgm-2 | OspPGM/OspPGM |
OspPGM/OspPGM
100/50 (97/97) |
OspPGM/osppgm-2
0/50 (0/97) |
| OspPGM/OspPGM | OspPGM/osppgm-2 |
OspPGM/OspPGM
48.1/50 (52/108) |
OspPGM/osppgm-2
51.9/50/(56/108) |
| OsAGPL4/osagl4-1 | OsAGPL4/OsAGPL4 |
OsAGPL4/OsAGPL4
100/50 (87/87) |
OsAGPL4/osagl4-1
0/50 (0/87) |
| OsAGPL4/OsAGPL4 | OsAGPL4/osagl4-1 |
OsAGPL4/OsAGPL4
48.4/50 (45/93) |
OsAGPL4/osagl4-1
51.4/50 (48/93) |
Genetic complementation of the OspPGM mutant
To determine if the osppgm mutation is responsible for the male sterile phenotype, we introduced the WT OspPGM cDNA under the control of the constitutive maize (Zea mays) Ubiquitin1 (ZmUbi1) promoter into hygromycin-resistant scutellum-derived calli from the OspPGM/osppgm-1 heterozygous seeds by Agrobacterium-mediated transfer. We obtained three Ubi:OspPGM transgenic lines by the Phosphomannose Isomerase/mannose selection system (Eom et al., 2011). Genomic DNA PCR analysis indicated that these lines were all heterozygous for the osppgm-1 allele and harboured the WT OspPGM cDNA transgene. We then analysed progeny plants of the transgenic lines and found a number of OspPGM cDNA-carrying osppgm-1/osppgm-1 homozygous plants among the progeny of the independent transgenic lines (see Supplementary Fig. S1A at JXB online). Expression of the OspPGM transgene was confirmed in the subsequently selected complemented plants (i.e. osppgm-1/osppgm-1//Ubi:OspPGM/Ubi:OspPGM; hereafter referred to as Comp) (Supplementary Fig. S1B). This result demonstrates that the defect in fertility of osppgm-1 mutant pollen was rescued by overexpression of the WT OspPGM in the complemented lines. The line (Comp #4) with the highest expression of the introduced OspPGM transgene was chosen for further analysis (Supplementary Fig. S1B).
Production and phenotype of homozygous osppgm mutant
To examine in detail the role of OspPGM in developing pollen, as well as in other organs including the photosynthetic leaf, we generated homozygous osppgm mutants by anther culture. In total, 29 and 17 independent plants were produced from the anthers of OspPGM/osppgm-1 and OspPGM/osppgm-2, respectively. We successfully generated 14 normal green osppgm-1/osppgm-1 homozygotes and 15 WT at a segregation ratio close to 1:1. By contrast, all regenerated WT and osppgm-2/osppgm-2 homozygotes were albino, a phenotype most likely due to a background mutation. Thus, we only analysed the osppgm-1/osppgm-1 homozygous plants.
RT-PCR analysis confirmed that the OspPGM endogenous mRNA expression was completely abolished in osppgm-1/osppgm-1 plants (Fig. 2C). In order to determine the loss of its enzymatic activity, crude extracts of the leaf tissues were separated by a native polyacrylamide gel electrophoresis followed by staining for PGM activity (Egli et al., 2010). In the WT extract, two distinct bands were observed but only the upper band was visible in the osppgm-1/osppgm-1 mutant. Because the rice genome contains two PGMs, cytosolic and plastidic isoforms, the lower faster-moving band is attributable to the plastidic form OspPGM as it is missing in the osppgm-1/osppgm-1 mutant, while the upper slower-moving band is the cytosolic OscPGM (Fig. 2D). Based on these results, we concluded that pPGM activity was abolished in the osppgm-1/osppgm-1 plants.
To understand the male sterile phenotype of osppgm mutants, we then examined the starch-staining capacity of pollen grains from osppgm-1/osppgm-1 homozygous plants as well as those from OspPGM/osppgm-1, OspPGM/osppgm-2, and Comp #4. We found that all the pollen grains of osppgm-1/osppgm-1 were not stained by iodine solution, indicating that they contain little or no starch. By contrast, all pollen grains of Comp #4 and about 50% of the pollen from OspPGM/osppgm-1 and OspPGM/osppgm-2 stained dark with iodine (Fig. 3A–E). In addition, transmission electron microscopy analysis verified that the osppgm-1/osppgm-1 pollen did not contain any starch granules, while the WT had large ones (Fig. 3F, G). Based on nuclear staining with Hoechst 33342, all WT and mutant pollen contained one vegetative nucleus and two generative nuclei (Fig. 3H, I) suggesting that osppgm mutant pollen developed normally but did not accumulate any starch. This result clearly supports the view that osppgm pollen grains develop normally but cannot fertilize due to limited accumulation of reserve starch.
Fig. 3.
Phenotype of mature pollen grains of osppgm-1/osppgm-1, OspPGM/osppgm-1, OspPGM/osppgm-2, and Comp #4 complemented plant. (A–E) Iodine-stained pollen. (F, G) Transmission electron microscopy images of pollen. (H, I) Nuclear stained pollen by Hoechst 33342. (A, F, H) Wild type; (B) OspPGM/osppgm-1 (C) OspPGM/osppgm-2 (D, G, I) osppgm-1 homozygote, and (E) complemented line # 4. White arrowhead, generative nucleus; red arrowhead, vegetative nucleus. WT, wild type; S, starch.
The plastidic PGM is known to be a key enzyme for starch synthesis during the day in the mesophyll cells of a photosynthetic leaf. Thus, we determined the starch content in the leaves of the osppgm-1/osppgm-1 homozygote, WT, and Comp #4 plants. The second leaves collected from the top of the plants immediately prior to heading were analysed at the end of the day. Starch did not accumulate in the leaf of osppgm-1/osppgm-1 (Supplementary Fig. S2A), while WT and Comp #4 accumulated starch normally. This starch-deficient phenotype of osppgm-1/osppgm-1 is identical to that observed in the chloroplast PGM mutant lines from other plant species (Caspar et al., 1985; Hanson and McHale, 1988; Harrison et al., 1998, 2000; Tauberger et al., 2000; Fernie et al., 2001).
As newly fixed carbon is normally partitioned between sucrose and starch, we determined the sucrose levels in these rice lines. Sucrose levels were found to be higher in the osppgm-1/osppgm-1 than in the control lines (Supplementary Fig. S2B), while no significant difference in the levels of sucrose and starch between WT and Comp #4 was evident. Our observations are consistent with the results of other reported pPGM mutants where sucrose levels are elevated when starch synthesis is suppressed (Caspar et al., 1985; Hanson and McHale, 1988; Fernie et al., 2001).
Although carbon partitioning is clearly affected, we observed no detectable differences in the size, morphology or development of osppgm-1/osppgm-1 plants compared with the controls (Supplementary Fig. S2C) under our growth conditions, with the exception that all osppgm-1/osppgm-1 plants generated by anther culture remained sterile over the many years that they were maintained. No visible phenotypic differences in vegetative growth were observed for the starchless rice mutants, osagps2 and apl1 (Lee et al., 2007; Rösti et al., 2007). The absence of any effect on growth and development by leaf starch deficiency in rice may simply be due to the fact that this plant has limited starch synthesis capacity and that its growth relies more on the synthesis of sucrose (Lee et al., 2008, 2014).
Identification of an ADP-glucose pyrophosphorylase gene highly expressed in the starch synthesis stage of pollen
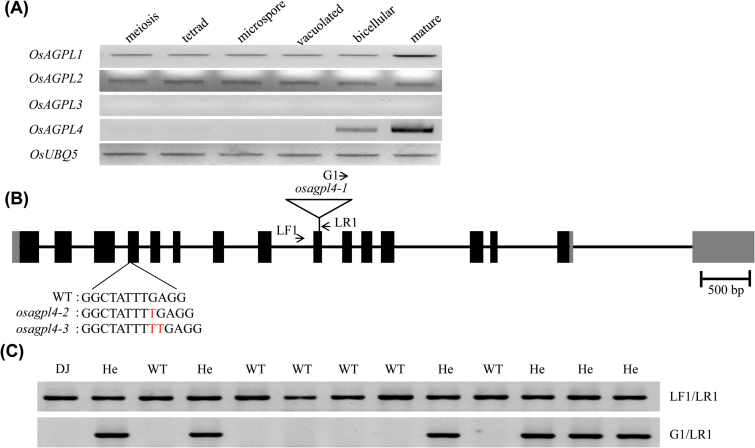
The aforementioned results indicate that the plastidic PGM-deficient rice mutants exhibit impaired starch synthesis in pollen grains that renders them male sterile. This suggests that the hexose phosphate pool within the pollen plastids is used for ADP-Glc synthesis, unlike a large cytosolic ADP-Glc pool used for starch synthesis in cereal endosperm including rice (Sullivan et al., 1995; Sikka et al., 2001; Lee et al., 2007; Cakir et al., 2016). This prompted us to identify a plastidic AGP isoform that functions mainly in starch synthesis of rice pollen. For this, we analysed the expression of the four rice AGP LS isoform genes at various stages of the developing anthers. Such analysis revealed that OsAGPL4 (LOC_Os07g13980) began to be expressed at the bicellular stage and reached its highest level at the mature stage when the pollen grains accumulate starch (Fig. 4A). Consistently, expression analysis of publically available datasets from the Rice Massively Parallel Signature Sequencing (rice MPSS; https://mpss.danforthcenter.org/rice/mpss_index.php) and the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) supported the pollen preferential expression of OsAGPL4 (Supplementary Fig. S3). The other AGP LS isoforms were constitutively expressed at much lower levels, with the exception of OsAGPL3 whose transcript was not detectable at all of the stages examined. We had previously shown that the OsAGPL4 is located in the plastids (Lee et al., 2007).
Fig. 4.
Expression of rice OsAGP large subunit genes during pollen development (A) and molecular characterization of osagpl4 mutant (B, C). (A) RT-PCR analysis of OsAGPL genes. Transcript levels of different stage tissues are comparable for each gene in the PCR reactions. OsUBQ5 was used as the PCR control. (B) Schematic depiction of positions of the T-DNA insertion in the osagpl4-1. The 16 exons of OsAGPL4 are shown by filled boxes. Primers for genotyping are marked with arrows. The other two alleles, osagpl4-2 and osagpl4-3, generated by the CRISPR/Cas system have one and two nucleotide insertions, respectively. (C) Genomic DNA PCR analysis of progeny of the self-pollinated OsAGPL4/osagpl4-1. LF1/LR1 and G1/LR1 primer sets were used for genotyping of the wild type OsAGPL4 copy and T-DNA insertion allele, respectively. DJ, mutant background genotype; WT, wild type; He, heterozygote.
Isolation and characterization of the OsAGPL4 mutant
We had previously demonstrated that the rice OsAGPL2 mutant line exhibits a shrunken seed endosperm phenotype (Lee et al., 2007). The OsAGPS2 mutant, lacking both alternative-spliced transcripts encoding the plastidic (OsAGPS2a) and cytosolic (OsAGPS2b) forms, produced starchless leaves and a shrunken endosperm phenotype (Lee et al., 2007). An OsAGPL3 mutant, apl1, also showed impaired starch synthesis in leaves (Rösti et al., 2007) while an OsAGPL1 mutant, apl3, lacked starch in the culm (Cook et al., 2012). As none of these mutants shows any distortion in progeny segregation or male sterile phenotype, we isolated a mutant of OsAGPL4, highly expressed in the starch synthesis stage of pollen, osagpl4-1, from the rice T-DNA mutant library (Jeon et al., 2000; An et al., 2005a, b ; Jeong et al., 2006).
The osagpl4-1 mutant allele harboured a T-DNA insertion in the ninth exon (Fig. 4B). PCR analysis of the genomic DNA of progeny plants of the OsAGPL4 heterozygous mutant using gene- and T-DNA specific primers (Supplementary Table S1) revealed no osagpl4-1/osagpl4-1 homozygous individuals. Instead, progeny segregated 1:1 (50:53 plants) for WT and heterozygote (Fig. 4C; Table 1). This raised the possibility that osagpl4-1 mutant pollen is sterile possibly due to a defect in starch synthesis. We performed reciprocal crosses between the WT and OsAGPL4/osagpl4-1. When the female parent was the heterozygous line, F1 genotypes were segregated nearly 1:1 for WT and heterozygote (Table 2). By contrast, the other cross did not transmit the osagpl4-1 allele to their F1 plants. This result strongly indicates that the loss of OsAGPL4 in rice pollen caused male sterility.
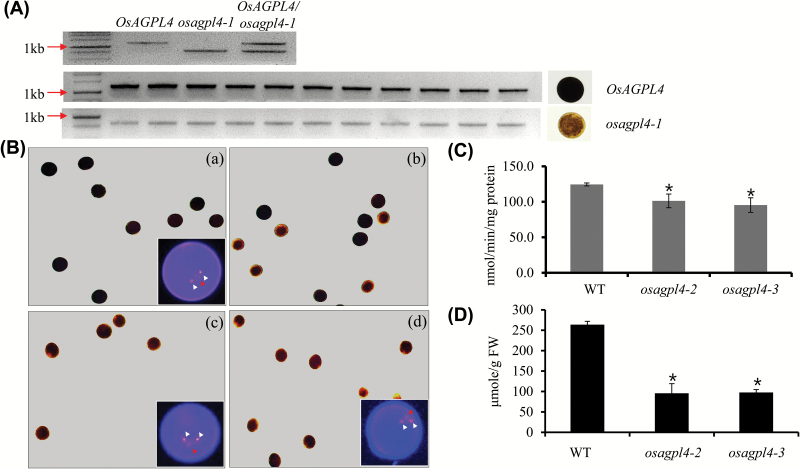
We next examined starch levels by iodine staining of the pollen grains of the OsAGPL4/osagpl4-1 heterozygous plant, and found that about a half of the pollen was strongly stained and the other weakly stained, while all of that from the WT stained dark (Fig. 5B). To examine if the level of starch staining is correlated with the OsAGPL4 genotype of each pollen, PCR analysis was performed with genomic DNAs isolated from individual pollen grains. The result showed that all dark-stained pollen produced the WT copy of OsAGPL4, while the weakly stained pollen amplified the osagpl4-1 allele (Fig. 5A). This indicates that impaired function of OsAGPL4 reduces starch synthesis.
Fig. 5.
Molecular, phenotypic, and biochemical analysis of osagpl4 mutant pollen. (A) Genomic DNA PCR analysis of individual pollen grains of OsAGPL4/osagpl4-1. (Top) The LF1/LR1 primer set was used for the wild-type OsAGPL4 gene, and the G1/LR1 set for osagpl4-1. (Middle and bottom) LF1, LR1, and G1 primers were mixed in the PCR reactions from genomic DNAs of individual pollen of OsAGPL4/osagpl4-1. All pollen grains darkly stained by iodine solution contain the wild-type OsAGPL4 (middle) and all weakly stained pollen grains carry osagpl4-1 (bottom). (B) Iodine stained pollen. (a) Wild type, (b) OsAGPL4/osagpl4-1, (c) osagpl4-2/osagpl4-2, (d) osagpl4-3/osagpl4-3. Pollen grains stained with Hoechst 33342 are shown in insets for the wild type and two homozygous mutants, osagpl4-2/osagpl4-2 and osagpl4-3/osagpl4-3, generated by the CRISPR/Cas system. White arrowhead, generative nucleus; red arrowhead, vegetative nucleus. (C) AGP activity of mature anthers of the wild type and homozygous osagpl4-2 and osagpl4-3 lines. (D) Starch contents of the mature anthers of the wild type and homozygous osagpl4-2 and osagpl4-3 lines. WT, wild type. Each data point represents the mean ±SD from at least three different plants. *P <0.01.
Production and characterization of osagpl4 homozygous mutants
To understand further the role of OsAGPL4 in the starch synthesis of rice pollen, we adopted the CRISPR/Cas system, as an alternative for anther culture, to produce homozygous mutant lines. A region of the fourth exon was selected as the target of guide RNA. Of 43 independent transgenic lines, we found two lines, osagpl4-2 and osagpl4-3, with their homozygous mutant alleles at the target site. The osagpl4-2 and osagpl4-3 alleles had one and two nucleotide insertions, respectively (Fig. 4B). Pollen of the homozygous osagpl4-2 and osagpl4-3 lines was examined by starch staining. The results showed that all pollen grains from both lines stained weakly (Fig. 5B). In nuclear staining with Hoechst 33342, all pollen grains carrying the WT OsAGPL4, osagpl4-2 or osagpl4-3 allele contained one vegetative nucleus and two generative nuclei (Fig. 5B, insets), suggesting that the osagpl4 mutant pollen developed normally.
The AGP activities of two homozygous osagpl4 lines were measured according to the previously described method using 14C-labelled Glc-1-P (Hwang et al., 2007). We collected the anthers containing mature pollen grains from the WT, osagpl4-2, and osagpl4-3 lines. The levels of AGP activity in osagpl4-2 and osagpl4-3 were found to be significantly reduced by 18.6% and 23.4%, respectively, in the mature anthers, compared with that of the WT (Fig. 5C). Our quantitative measurement clearly confirmed that the levels of starch in osagpl4-2 and osagpl4-3 were significantly reduced by about 65% in the mature anthers, compared with that of the WT (Fig. 5D). This result demonstrates that OsAGPL4 is a major AGP LS isoform in rice pollen. The other AGP isoforms, which were also expressed during pollen development (Fig. 4A), are probably responsible for the remaining AGP activity in the mutants. The AGP activity from the anther walls could also contribute to the AGP activity in the mutants.
Interestingly, the homozygous alleles of osagpl4-2 and osagpl4-3 bore a number of seeds, resulting in about 10–20% fertility in our growth conditions. Genomic DNA PCR analysis of these progeny plants confirmed their homozygous mutant genotypes. This result suggests that osagpl4 mutant pollen with a reduced starch content retained some fertilization ability but cannot compete with the WT pollen when both are present in the heterozygous plants.
Discussion
Many major food crop plants, including rice, store starch in their pollen to provide the building blocks and energy reserves for pollen germination and subsequent elongation of the pollen tube. By contrast, Arabidopsis thaliana, a dicot model plant, utilizes lipid bodies as their storage reserve in pollen grains. Thus, the presence of starch in pollen grains is critical for normal pollen fertilization and it is commonly used as a viable pollen indicator, but our current understanding on how carbon is routed into starch in this vital plant organ has not been defined until now. In cereals, it is now well established that ADP-Glc, the sugar nucleotide utilized by starch synthase, is synthesized by two different pathways. In source leaves, the synthesis of ADP-Glc is restricted to the chloroplast and used for the production of transitory starch while, in the developing sink organ, seed endosperm, the synthesis of ADP-Glc involves cytosolic events. In our present study, based on our analyses of mutants of the rice plastidic PGM, OspPGM, and the plastidic AGP LS isoform, OsAGPL4, we demonstrate that starch synthesis in the sink organ pollen depends upon the pool of plastidic synthesized ADP-Glc.
Loss-of-function of OspPGM results in a defect in starch synthesis and causes male sterility
In rice, pollen grains start accumulating starch at the bicellular stage and the starch levels gradually increase until maturity (Raghavan, 1988; Yamagata et al., 2010). Many pollen mutants that lack starch are non-viable in the fertilization process (Min et al., 2013; Zhang et al., 2013; Khan et al., 2015; Wu et al., 2016). Because PGM is an important enzyme in starch synthesis, we showed that rice contains both cytosolic and plastidic isoforms for PGM and that the plastidic isoform gene, OspPGM, is highly expressed in the mature pollen stages (Fig. 1A). Native gel activity analysis of the homozygous pPGM mutant, osppgm-1, supports the view that the rice genome encodes a unique pPGM (Fig. 2D). Here, we found that the osppgm mutant pollen grains lack starch (Fig. 3) and were male sterile (Tables 1, 2). Direct evidence that male sterility is caused by starch deficiency in pollen is provided by the result that fertile pollen grains were produced when the osppgm mutation was complemented by the WT OspPGM gene (Supplementary Fig. S1). The male-sterile phenotype has not been reported from the pPGM mutants of other plants, including Arabidopsis that utilizes lipid bodies as a major reserve in pollen grains (Caspar et al., 1985; Hanson and McHale, 1988; Harrison et al., 1998, 2000; Tauberger et al., 2000; Fernie et al., 2001).
It has been well established that the accumulation and metabolism of leaf starch is important for the growth of dicot plant species, including Arabidopsis, as readily evident by mutations in pPGM, AGP, β-amylase, α-glucan water dikinase (GWD1), and the maltose transporter (Caspar et al., 1985, 1991; Lin et al., 1988a, b ; Niittylä et al., 2004; Fulton et al., 2008). Moreover, the elevation of leaf starch was demonstrated to enhance photosynthetic capacity and plant growth in rice as well as in Arabidopsis (Gibson et al., 2003, 2011). Unlike Arabidopsis, however, rice leaves normally accumulate relatively little starch. Our mutant analyses of the rice cytosolic fructose 1,6 bisphosphatase and the plastidic triose phosphate/phosphate translocator demonstrated that rice has a limited capacity for starch synthesis in the source leaf (Lee et al., 2008, 2014). Hence, the impairment of the synthesis of transitory leaf starch mediated by mutations in apl1, an OsAGPL3 mutant, and osagps2, an OsAGPS2 mutant, would not be expected to disrupt normal plant growth (Lee et al., 2007; Rösti et al., 2007). Similarly, the rice leaf starch excess1 (lse1), a GWD1 mutant, grew normally unlike the mutants of the Arabidopsis homologous gene (Hirose et al., 2013). In the present study, the osppgm-1 homozygous mutant did not synthesize starch in the source leaf during the day and grew normally (Supplementary Fig. S2). This result strongly supports our previous results that rice plant growth relies more on the synthesis of sucrose under normal condition.
OsAGPL4 encodes a major large subunit of AGP for starch synthesis in rice pollen
OspPGM is the enzyme isoform that converts Glc-6-P to Glc-1-P in the plastids of rice. The finding that osppgm mutants abolish starch synthesis in rice pollen grains supports the view that ADP-Glc, the substrate for starch synthesis, is mainly synthesized from Glc-1-P in the plastids of pollen rather than in the cytosol. To verify this hypothesis, we identified the plastidic OsAGPL4 as the dominant AGP LS which is preferentially expressed at the bicellular and mature stages of pollen development (Fig. 4A). Its localization was previously determined as plastidic (Lee et al., 2007). Our observation of OsAGPL4 alleles, osagpl4-1, osaspl4-2, and osagpl4-3, showed that OsAGPL4 deficiency resulted in reduced starch content as indicated by starch staining and quantitative measurement (Fig. 5B, D) and mediated a male sterile phenotype (Tables 1, 2). AGP activity in anthers containing osagpl4 mutant pollen was significantly reduced (Fig. 5C), although substantial amounts of enzyme were probably contributed by OsAGPL1, another plastidic AGP LS isoform (Lee et al., 2007), which may compensate starch synthesis in osagpl4 mutant pollen. Previous mutant analysis on the rice AGP genes from our and other groups did not show any male sterile phenotype (Lee et al., 2007; Rösti et al., 2007; Cook et al., 2012; Okamura et al., 2014), supporting that OsAGPL4 is critical for starch synthesis in rice pollen.
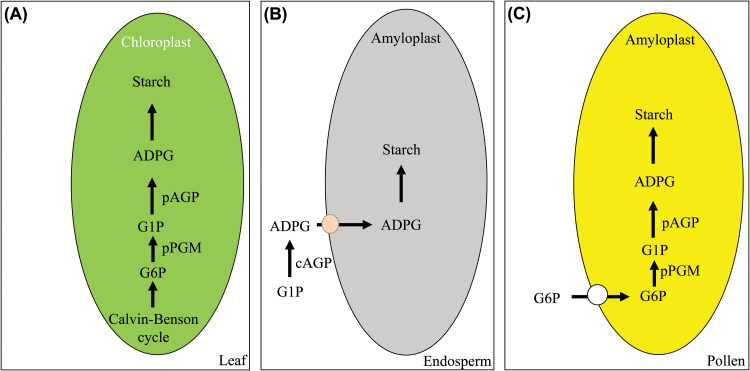
A model for the starch synthesis pathway in rice pollen
Previous and current results suggest that, in rice leaves, the photoassimilates produced from the Calvin–Benson cycle is stored as transitory starch by the co-ordinated function of both plastidic OspPGM and OsAGP isoforms, mainly OsAGPS2a and OsAGPL3 (Fig. 6A). Each of their mutations, osppgm, osagps2, and apl1, caused starch deficiency in rice leaves (Supplementary Fig. S2) (Lee et al., 2007; Rösti et al., 2007). In rice seed endosperm, the cytosolic AGP isoforms, the OsAGPS2b/OsAGPL2 complex, comprise the dominant enzyme activity in starch synthesis (Fig. 6B) (Lee et al., 2007). A lesion of one of the two subunits, OsAGPL2 and OsAGPS2b, produced a shrunken seed endosperm due to a remarkable reduction in starch synthesis. Thus, starch synthesis in the rice leaf and seed endosperm, mainly depends on the plastidic and cytosolic ADP-Glc synthesis pathway, respectively.
Fig. 6.
Proposed pathways of starch synthesis in the plastid of leaf (A), endosperm (B), and pollen grains (C) in rice. (A) In rice leaves, photoassimilates produced from the Calvin–Benson cycle is stored as transitory starch by the co-ordinated action of the plastidic OspPGM and OsAGP isoforms, mainly OsAGPS2a and OsAGPL3. Starch synthesis in rice leaves depends on the synthesis of ADP-Glc in the chloroplast. (B) In rice seed endosperm, the cytosolic AGP isoforms, OsAGPS2b/OsAGPL2 complex, constitute the dominant enzyme activity in starch synthesis. Starch synthesis in the seed endosperm mainly depends on the cytosolic ADP-Glc synthesis. Developing endosperms are exposed to low oxygen stress as the seeds mature. Thus, the cytosolic AGP activity enforces the UDP-glucose pyrophosphorylase metabolic flux using PPi as an alternative to the ATP-dependent enzyme/pathway. (C) In rice pollen, Glc-6-P is converted to Glc-1-P via pPGM and Glc-1-P is then converted to ADP-Glc in the amyloplasts. This suggests that starch synthesis in rice pollen grains mainly depends on the plastidic ADP-Glc synthesis, similar to that seen in rice leaves. This model suggests the potential roles of the plastidic ATP/ADP translocator and Glc-6-P/phosphate translocator in rice pollen grains. pAGP, plastidic ADP-Glc pyrophosphorylase; cAGP, cytosolic ADP-Glc pyrophosphorylase; pPGM, plastidic phosphoglucomutase; ADPG, ADP-Glc; G1P, Glc-1-P; G6P, Glc-6-P. (Online in colour.)
The developing endosperms are subjected to low oxygen stress as the seeds mature (Rolletschek et al., 2002, 2011). Hence, energy is produced mainly by glycolysis instead of by respiration. Cereal endosperm compensates for the reduction in energy production by conserving nucleoside triphosphates and PPi (Tuncel et al., 2014; Cakir et al., 2016). Sucrose imported from source leaves is broken down by sucrose synthase, which forms UDP-glucose (UDP-Glc) and fructose. UDP-Glc, together with PPi, are then acted upon by UDP-Glc pyrophosphorylase (UGPase) to form Glc-1-P and UTP. The newly synthesized Glc-1-P, together with ATP, is used to synthesize ADP-Glc by AGP. In this series of reactions, the high-energy PPi is consumed in the UGPase reaction but is re-synthesized in the AGP step. Although ATP is consumed UTP is synthesized, thereby conserving nucleoside triphosphates.
Our present finding proposes a model for the starch synthesis pathway in the sink organ pollen that has been unknown until now (Fig. 6C). Lesions in the plastidic isoforms, OsAGPL4 and OspPGM, impair starch synthesis that, in turn, causes sterile pollen (Figs 2–5). This result indicates that Glc-6-P is converted to Glc-1-P via pPGM and that Glc-1-P is then converted to ADP-Glc in the amyloplasts of rice pollen. This suggests that starch synthesis in rice pollen mainly depends on the plastidic ADP-Glc synthesis, as in photosynthetic leaves. In the photosynthetic leaves, ATP for starch synthesis is supplied by photophosphorylation during photosynthesis. In the heterotrophic organ pollen, ATP is supplied through respiration in the mitochondria, suggesting a potential involvement of a plastidic ATP/ADP translocator protein (AATP). In addition, it is likely that Glc-6-P is preferentially imported into the amyloplasts of rice pollen grains by a Glc-6-P/phosphate translocator (GPT). This is consistent with the results from dicot plants, including Arabidopsis, that plastids of heterotrophic tissues preferentially import Glc-6-P (Niewiadomski et al., 2005; Weber et al., 2005; Facchinelli and Weber, 2011). The Arabidopsis GPT1 mutants caused severe defects especially during pollen development due to less lipid body formation (Niewiadomski et al., 2005). Putative plastidic AATPs and GPTs have previously been identified in rice (Toyota et al., 2006). Expression analysis with publicly available microarray datasets indicated that two AATP genes (LOC_Os01g45910 and LOC_Os02g11740) and two GPT genes (LOC_Os08g08840 and LOC_Os07g33910) are expressed in developing rice pollen (Supplementary Fig. S3). Therefore, it would be valuable in future to determine a role of these plastidic translocators that function in rice pollen.
The OsAGPL4 mutant may be used for the production of hybrid rice
Hybrid breeding exploits the increased vigour or heterosis of F1 hybrid plants. Cytoplasmic male sterility, photoperiod-sensitive genic male sterility, and thermo-sensitive genic male sterility systems are all used for hybrid rice breeding (Virmani and Kumar, 2009; Jeon et al., 2011). An alternative strategy for engineering male sterility has recently been developed by simply abolishing starch accumulation in maize pollen resulting from the expression of α-amylase (Wu et al., 2016). In the present study, the homozygous osagl4-2 and osagpl4-3 lines created by CRISPR/Cas yielded a considerable number of homozygous mutant seeds. We showed that the heterozygous OsAGPL4/osagpl4-1 did not produce any osagpl4-1/osagpl4-1 homozygous progeny plants (Table 1), and that the reciprocal cross experiment confirmed that the osagpl4-1 allele could not be transmitted to the next generation through pollen of the heterozygote OsAGPL4/osagpl4-1 (Table 2). The production of homozygous progeny of osagpl4-2 and osagpl4-3 is not surprising, considering that the mutant pollen accumulated a low level of starch. This result most likely suggests that osagpl4 mutant pollen retains their fertilization ability, although they are unable to compete against WT pollen. This raises an alternative way for engineering male sterility by creating homozygous mutants of a pollen-specific gene that codes for a major carbon metabolic gene of starch synthesis with functionally redundant isoforms. Nevertheless, this possibility needs to be investigated in the future.
In conclusion, our present study identified the OspPGM and OsAGP isoforms that are essential for starch synthesis in rice pollen grains. We also established a model of starch synthesis among different organs during rice growth and development. The new information provided in this study may also facilitate the fine control of the starch synthesis pathway in the future. This has the potential to enhance fertility in order to increase yield productivity under environmental stress conditions and to control the male sterility trait in hybrid rice production.
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of PCR primers used in this study.
Figure S1. Genomic DNA PCR (A) and RT-PCR (B) analysis of genetically complemented lines of osppgm-1 with wild type OspPGM copy under the control of the maize Ubi1 promoter.
Figure S2. Metabolite and phenotypic analysis of osppgm-1/osppgm-1 mutant rice plant generated by anther culture.
Figure S3. Digital expression profile of OsAGPL4, the putative plastidic ATP/ADP translocator, and Glc-6-P/phosphate translocator genes.
Acknowledgements
We thank Professor Li-Jia Qu (Peking University, China) for providing the CRISPR/Cas vectors pOs-sgRNA and pH-Ubi-cas9-7. This work was supported by a grant from the Next Generation BioGreen 21 Program of the Rural Development Administration of Korea (PJ011798012016 to J-SJ). S-KH and TWO are supported by the US Department of Agriculture National Institute of Food and Agriculture (WNP00590) and the Agricultural Research Center, College of Agricultural, Human, and Natural Resource Sciences, Washington State University. The authors declare no conflict of interest.
References
- An G, Jeong D-H, Jung K-H, Lee S. 2005. a Reverse genetic approaches for functional genomics of rice. Plant Molecular Biology 59, 111–123. [DOI] [PubMed] [Google Scholar]
- An G, Lee S, Kim S-H, Kim S-R. 2005. b Molecular genetics using T-DNA in rice. Plant and Cell Physiology 46, 14–22. [DOI] [PubMed] [Google Scholar]
- Beckles DM, Craig J, Smith AM. 2001. a ADP-glucose pyrophosphorylase is located in the plastid in developing tomato fruit. Plant Physiology 126, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckles DM, Smith AM, ap Rees T. 2001. b A cytosolic ADP-glucose pyrophosphorylase is a feature of graminaceous endosperms, but not of other starch-storing organs. Plant Physiology 125, 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Shiraishi S, Tuncel A, et al. 2016. Analysis of the rice ADP-glucose transporter (OsBT1) indicates the presence of regulatory processes in the amyloplast stroma that control ADP-glucose flux into starch. Plant Physiology 170, 1271–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Huber S, Somerville C. 1985. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiology 79, 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar T, Lin TP, Kakefuda G, Benbow L, Preiss J, Somerville C. 1991. Mutants of Arabidopsis with altered regulation of starch degradation. Plant Physiology 95, 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DH, Ronald PC. 1999. A rapid DNA minipreparation method suitable for AFLP and other PCR applications. Plant Molecular Biology Reports 17, 53–57. [Google Scholar]
- Cook FR, Fahy B, Trafford K. 2012. A rice mutant lacking a large subunit of ADP-glucose pyrophosphorylase has drastically reduced starch content in the culm but normal plant morphology and yield. Functional Plant Biology 39, 1068–1078. [DOI] [PubMed] [Google Scholar]
- Datta R, Chamusco KC, Chourey PS. 2002. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiology 130, 1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DB. 1968. Rapid starch synthesis associated with increased respiration in germinating lily pollen. Plant Physiology 43, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DB, Preiss J. 1969. Presence of ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize endosperm. Plant Physiology 44, 1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli B, Kölling K, Köhler C, Zeeman SC, Streb S. 2010. Loss of cytosolic phosphoglucomutase compromises gametophyte development in Arabidopsis. Plant Physiology 154, 1659–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology 300, 1005–1016. [DOI] [PubMed] [Google Scholar]
- Eom J-S, Cho J-I, Reinders A, et al. 2011. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiology 157, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J-S, Nguyen CD, Lee D-W, Lee S-K, Jeon JS. 2016. Genetic complementation analysis of rice sucrose transporter genes in Arabidopsis SUC2 mutant atsuc2 . Journal of Plant Biology 59, 231–237. [Google Scholar]
- Facchinelli F, Weber APM. 2011. The metabolite transporters of the plastid envelope: an update. Frontiers in Plant Science 2, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Roessner U, Trethewey RN, Willmitzer L. 2001. The contribution of plastidial phosphoglucomutase to the control of starch synthesis within the potato tuber. Planta 213, 418–426. [DOI] [PubMed] [Google Scholar]
- Fulton DC, Stettler M, Mettler T, et al. 2008. β-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active beta-amylases in Arabidopsis chloroplasts. The Plant Cell 20, 1040–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Hwang S-K, Edwards GE, Okita TW, Kato C, Matsui H, Ito H. 2003. Metabolic engineering of starch for enhanced plant productivity and yields. Journal of Applied Glycoscience 50, 201–206. [Google Scholar]
- Gibson K, Park J-S, Nagai Y, et al. 2011. Exploiting leaf starch synthesis as a transient sink to elevate photosynthesis, plant productivity and yields. Plant Science 181, 275–281. [DOI] [PubMed] [Google Scholar]
- Giroux MJ, Hannah LC. 1994. ADP-glucose pyrophosphorylase in shrunken-2 and brittle-2 mutants of maize. Molecular & General Genetics 243, 400–408. [DOI] [PubMed] [Google Scholar]
- Greene T, Hannah L. 1998. Maize endosperm ADP-glucose pyrophosphorylase SHRUNKEN2 and BRITTLE2 subunit interactions. The Plant Cell 10, 1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah LC, Shaw JR, Giroux MJ, Reyss A, Prioul JL, Bae JM, Lee JY. 2001. Maize genes encoding the small subunit of ADP-glucose pyrophosphorylase. Plant Physiology 127, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KR, McHale NA. 1988. A starchless mutant of Nicotiana sylvestris containing a modified plastid phosphoglucomutase. Plant Physiology 88, 838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison CJ, Mould RM, Leech MJ, et al. 2000. The rug3 locus of pea encodes plastidial phosphoglucomutase. Plant Physiology 122, 1187–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Hedley C, Wang T. 1998. Evidence that the rug3 locus of pea (Pisum sativum L.) encodes plastidial phosphoglucomutase confirms that the imported substrate for starch synthesis in pea amyloplasts is glucose-6-phosphate. The Plant Journal 13, 753–762. [Google Scholar]
- Hirose T, Aoki N, Harada Y, Okamura M, Hashida Y, Ohsugi R, Akio M, Hirochika H, Terao T. 2013. Disruption of a rice gene for α-glucan water dikinase, OsGWD1, leads to hyperaccumulation of starch in leaves but exhibits limited effects on growth. Frontiers in Plant Science 4, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Hennen-Bierwagen TA, Myers AM. 2014. Functions of multiple genes encoding ADP-glucose pyrophosphorylase subunits in maize endosperm, embryo, and leaf. Plant Physiology 164, 596–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SK, Hamada S, Okita TW. 2007. Catalytic implications of the higher plant ADP-glucose pyrophosphorylase large subunit. Phytochemistry 68, 464–477. [DOI] [PubMed] [Google Scholar]
- James MG, Denyer K, Myers AM. 2003. Starch synthesis in the cereal endosperm. Current Opinion in Plant Biology 6, 215–222. [DOI] [PubMed] [Google Scholar]
- Jeon JS, Lee S, Jung KH., et al. 2000. T-DNA insertional mutagenesis for functional genomics in rice. The Plant Journal 22, 561–570. [DOI] [PubMed] [Google Scholar]
- Jeon J-S, Jung K-H, Kim H-B, Suh J-P, Khush GS. 2011. Genetic and molecular insights into the enhancement of rice yield potential. Journal of Plant Biology 54, 1–9. [Google Scholar]
- Jeong D-H, An S, Park S, et al. 2006. Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. The Plant Journal 45, 123–132. [DOI] [PubMed] [Google Scholar]
- Johnson PE, Patron NJ, Bottrill AR, Dinges JR, Fahy BF, Parker ML, Waite DN, Denyer K. 2003. A low-starch barley mutant, risø 16, lacking the cytosolic small subunit of ADP-glucose pyrophosphorylase, reveals the importance of the cytosolic isoform and the identity of the plastidial small subunit. Plant Physiology 131, 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MMR, Hasnunnahar M, Iwayoshi M, Ogura-Tsujita Y, Isshiki S. 2015. Pollen degeneration in three functional male-sterile lines of eggplant with the wild Solanum cytoplasms. Horticulture, Environment, and Biotechnology 56, 350–357. [Google Scholar]
- Lee S-K, Eom J-S, Voll LM, Prasch CM, Park Y-I, Hahn T-R, Ha S-H, An G, Jeon J-S. 2014. Analysis of a triose phosphate/phosphate translocator-deficient mutant reveals a limited capacity for starch synthesis in rice leaves. Molecular Plant 7, 1705–1708. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Hwang S-K, Han M, et al. 2007. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Molecular Biology 65, 531–546. [DOI] [PubMed] [Google Scholar]
- Lee S-K, Jeon J-S, Börnke F, et al. 2008. Loss of cytosolic fructose-1,6-bisphosphatase limits photosynthetic sucrose synthesis and causes severe growth retardations in rice (Oryza sativa). Plant, Cell & Environment 31, 1851–1863. [DOI] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville C, Preiss J. 1988. a Isolation and characterization of a starchless mutant of Arabidopsis thaliana (L.) Heynh lacking ADPglucose pyrophosphorylase activity. Plant Physiology 86, 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TP, Caspar T, Somerville CR, Preiss J. 1988. b A starch deficient mutant of Arabidopsis thaliana with low ADPglucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiology 88, 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Guo D, Zhang J, Huang Q, Qin G, Zhang X, Wan J, Gu H, Qu L-J. 2013. Targeted mutagenesis in rice using the CRISPR-Cas system. Cell Research 23, 1233–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Zhu L, Tu L, Deng F, Yuan D, Zhang X. 2013. Cotton GhCKI disrupts normal male reproduction by delaying tapetum programmed cell death via inactivating starch synthase. The Plant Journal 75, 823–835. [DOI] [PubMed] [Google Scholar]
- Müller-Röber BT, Kossmann J, Hannah LC, Willmitzer L, Sonnewald U. 1990. One of two different ADP-glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Molecular & General Genetics 224, 136–146. [DOI] [PubMed] [Google Scholar]
- Naito Y, Hino K, Bono H, Ui-Tei K. 2015. CRISPRdirect: software for designing CRISPR/Cas guide RNA with reduced off-target sites. Bioinformatics 31, 1120–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niittylä T, Messerli G, Trevisan M, Chen J, Smith AM, Zeeman SC. 2004. A previously unknown maltose transporter essential for starch degradation in leaves. Science 303, 87–89. [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Knappe S, Geimer S, et al. 2005. The Arabidopsis plastidic glucose 6-phosphate/phosphate translocator GPT1 is essential for pollen maturation and embryo sac development. The Plant Cell 17, 760–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M, Hirose T, Hashida Y, Yamagishi T, Ohsugi R, Aoki N. 2014. Starch reduction in rice stems due to a lack of OsAGPL1 or OsAPL3 decreases grain yield under low irradiance during ripening and modifies plant architecture. Functional Plant Biology 40, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Okita TW. 1992. Is there an alternative pathway for starch synthesis? Plant Physiology 100, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita TW, Nakata PA, Anderson JM, Sowokinos J, Morell M, Preiss J. 1990. The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiology 93, 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwerkerk PB, de Kam RJ, Hoge JH, Meijer AH. 2001. Glucocorticoid-inducible gene expression in rice. Planta 213, 370–378. [DOI] [PubMed] [Google Scholar]
- Raghavan V. 1988. Anther and pollen development in rice (Oryza sativa). American Journal of Botany 75, 183–196. [Google Scholar]
- Rolletschek H, Borisjuk L, Koschorreck M, Wobus U, Weber H. 2002. Legume embryos develop in a hypoxic environment. Journal of Experimental Botany 53, 1099–1107. [DOI] [PubMed] [Google Scholar]
- Rolletschek H, Melkus G, Grafahrend-Belau E, Fuchs J, Heinzel N, Schreiber F, Jakob PM, Borisjuk L. 2011. Combined noninvasive imaging and modeling approaches reveal metabolic compartmentation in the barley endosperm. The Plant Cell 23, 3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösti S, Fahy B, Denyer K. 2007. A mutant of rice lacking the leaf large subunit of ADP-glucose pyrophosphorylase has drastically reduced leaf starch content but grows normally. Functional Plant Biology 34, 480–489. [DOI] [PubMed] [Google Scholar]
- Seferoglu AB, Koper K, Can FB, Cevahir G, Kavakli IH. 2014. Enhanced heterotetrameric assembly of potato ADP-glucose pyrophosphorylase using reverse genetics. Plant and Cell Physiology 55, 1473–1483. [DOI] [PubMed] [Google Scholar]
- Sikka VK, Choi SB, Kavakli IH, Sakulsingharoj C, Gupta S, Ito H, Okita TW. 2001. Subcellular compartmentation and allosteric regulation of the rice endosperm ADPglucose pyrophosphorylase. Plant Science 161, 461–468. [Google Scholar]
- Smith A, Bettey M, Bedford ID. 1989. Evidence that the rb locus alters the starch content of developing pea embryos through an effect on ADP glucose pyrophosphorylase. Plant Physiology 89, 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Denyer K, Martin CR. 1995. What controls the amount and structure of starch in storage organs? Plant Physiology 107, 673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-White BJ, Preiss J. 1992. Comparison of proteins of ADP-glucose pyrophosphorylase from diverse sources. Journal of Molecular Evolution 34, 449–464. [DOI] [PubMed] [Google Scholar]
- Sullivan T, Kaneko Y. 1995. The maize brittle1 gene encodes amyloplast membrane polypeptides. Planta 196, 477–484. [DOI] [PubMed] [Google Scholar]
- Tauberger E, Fernie a R, Emmermann M, Renz A, Kossmann J, Willmitzer L, Trethewey RN. 2000. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. The Plant Journal 23, 43–53. [DOI] [PubMed] [Google Scholar]
- Tetlow IJ, Morell MK, Emes MJ. 2004. Recent developments in understanding the regulation of starch metabolism in higher plants. Journal of Experimental Botany 55, 2131–2145. [DOI] [PubMed] [Google Scholar]
- Toyota K, Tamura M, Ohdan T, Nakamura Y. 2006. Expression profiling of starch metabolism-related plastidic translocator genes in rice. Planta 223, 248–257. [DOI] [PubMed] [Google Scholar]
- Tsai CY, Nelson OE. 1966. Starch-deficient maize mutant lacking adenosine dephosphate glucose pyrophosphorylase activity. Science 151, 341–343. [DOI] [PubMed] [Google Scholar]
- Tuncel A, Kawaguchi J, Ihara Y, et al. 2014. The rice endosperm ADP-glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme. Plant and Cell Physiology 55, 1169–1183. [DOI] [PubMed] [Google Scholar]
- Vigeolas H, Mohlmann T, Martini N, Neuhaus HE, Geigenberger P. 2004. Embryo-specific reduction of ADP-Glc pyrophosphorylase leads to an inhibition of starch synthesis and a delay in oil accumulation in developing seeds of oilseed rape. Plant Physiology 136, 2676–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villand P, Olsen OA, Kleczkowski LA. 1993. Molecular characterization of multiple cDNA clones for ADP-glucose pyrophosphorylase from Arabidopsis thaliana . Plant Molecular Biology 23, 1279–1284. [DOI] [PubMed] [Google Scholar]
- Virmani SS, Kumar I. 2009. Hybrid rice technology. In: Datta SK. ed.Rice improvement in the genomics era. Boca Raton, Florida: CRC Press, 105–137. [Google Scholar]
- Wang SM, Chu B, Lue WL, Yu TS, Eimert K, Chen J. 1997. adg2-1 represents a missense mutation in the ADPG pyrophosphorylase large subunit gene of Arabidopsis thaliana . The Plant Journal 11, 1121–1126. [DOI] [PubMed] [Google Scholar]
- Wang SM, Lue WL, Yu TS, Long JH, Wang CN, Eimert K, Chen J. 1998. Characterization of ADG1, an Arabidopsis locus encoding for ADPG pyrophosphorylase small subunit, demonstrates that the presence of the small subunit is required for large subunit stability. The Plant Journal 13, 63–70. [DOI] [PubMed] [Google Scholar]
- Weber APM, Schwacke R, Flügge UI. 2005. Solute transporters of the plastid envelope membrane. Annual Review of Plant Biology 56, 133–164. [DOI] [PubMed] [Google Scholar]
- Wen LY, Chase CD. 1999. Mitochondrial gene expression in developing male gametophytes of male-fertile and S male-sterile maize. Sexual Plant Reproduction 11, 323–330. [Google Scholar]
- Wu Y, Fox TW, Trimnell MR, Wang L, Xu R, Cigan AM, Huffman GA, Garnaat CW, Hershey H, Albertsen MC. 2016. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnology Journal 14, 1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y, Yamamoto E, Aya K, et al. 2010. Mitochondrial gene in the nuclear genome induces reproductive barrier in rice. Proceedings of the National Academy of Sciences, USA 107, 1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu C, He Y, Zong J, Yang X, Si H, Sun Z, Hu J, Liang W, Zhang D. 2013. Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proceedings of the National Academy of Sciences, USA 110, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.