Abstract
Status epilepticus (SE) is a life-threatening emergency that can cause neurodegeneration with debilitating neurological disorders. However, the mechanism by which convulsive SE results in neurodegeneration is not fully understood. It has been shown that epileptic seizures produce markedly increased levels of nitric oxide (NO) in the brain, and that NO induces Ca2 + release from the endoplasmic reticulum via the type 1 ryanodine receptor (RyR1), which occurs through S-nitrosylation of the intracellular Ca2 + release channel. Here, we show that through genetic silencing of NO-induced activation of the RyR1 intracellular Ca2 + release channel, neurons were rescued from seizure-dependent cell death. Furthermore, dantrolene, an inhibitor of RyR1, was protective against neurodegeneration caused by SE. These results demonstrate that NO-induced Ca2 + release via RyR is involved in SE-induced neurodegeneration, and provide a rationale for the use of RyR1 inhibitors for the prevention of brain damage following SE.
Keywords: Ryanodine receptor, Nitric oxide, Calcium, Seizures, Neurodegeneration
Highlights
-
•
NO-induced activation of RyR1 is critical for seizure-induced neuronal cell death.
-
•
Dantrolene is protective against neurodegeneration caused by epileptic seizures.
-
•
RyR1 is a therapeutic candidate to ameliorate the brain damage following seizures.
Status epilepticus (SE) is a life-threatening condition that can cause neurodegeneration. Mikami et al. report that NO-induced activation of type 1 ryanodine receptors (RyR1) is involved in SE-induced neuron death. Furthermore, dantrolene, an inhibitor of RyR1, has been found to be protective against neurodegeneration caused by SE.
1. Introduction
Epilepsy is caused by a wide variety of insults to the brain as well as by congenital abnormalities in ionic channels (Berkovic et al., 2006, Jentsch et al., 2004, Meisler and Kearney, 2005). Status epilepticus (SE) is a neurological emergency and results in brain damage that increases the risk of recurrent seizures and debilitating neuronal abnormalities including death (Chang and Lowenstein, 2003, Goldberg and Coulter, 2013). Its high morbidity and mortality makes SE one of the most significant neurological disorders in terms of high social costs (Betjemann and Lowenstein, 2015). Therefore, understanding of the pathophysiology of SE-induced brain damage is required for the treatment of the neurological emergency. During epileptic seizures, NO formation has been observed to increase as a result of Ca2 +-dependent activation of NO synthase (NOS) in neurons (Mülsch et al., 1994). This increase in NO levels has been implicated in seizure-induced neuronal cell loss based on the finding that neurodegeneration is attenuated in neuronal NOS (nNOS)-deficient mice (Parathath et al., 2007). Thus NO is strongly implicated as a neurodegenerative factor in epileptic states. However, the molecular mechanisms by which NO exerts its role in SE-induced neurodegeneration requires clarification. Thus, rational treatment for the prevention of brain damage associated with SE has yet to be explored.
In addition to the cyclic guanosine monophosphate-dependent protein kinase pathway, NO is known to regulate the function of target proteins through S-nitrosylation of cysteine residues (Hess et al., 2005, Jaffrey et al., 2001). One such target is the type 1 ryanodine receptor (RyR1), which is the Ca2 +-induced Ca2 + release (CICR) channel in the endoplasmic reticulum (ER). NO induces the opening of the RyR1 channel through S-nitrosylation (Aghdasi et al., 1997, Eu et al., 2000, Sun et al., 2001). The activity of RyR1 due to this activation mechanism has been implicated in Ca2 + leakage from skeletal muscle Ca2 + stores that has been attributed to certain pathological conditions (Bellinger et al., 2009, Durham et al., 2008). A single cysteine residue at 3635 (C3635) in rabbit RyR1 is responsible for sensitizing the skeletal muscle Ca2 + release channel to NO (Sun et al., 2001). An alanine substitution for C3635 (C3635A) of RyR1 expressed in human embryonic kidney (HEK) 293 cells has been shown to reduce S-nitrosylation levels and abolish the regulation of the skeletal muscle Ca2 +-release channel by physiological concentrations of NO (Sun et al., 2001). In the brain, NO induces Ca2 + release from the ER through S-nitrosylation of RyR1, which results in an increased concentration of intracellular Ca2 + in neurons (Kakizawa et al., 2012). Ca2 + release via RyR1 has also been implicated in NO-induced neuronal cell death, as shown by studies in which cell death is significantly milder in cultured neurons taken from RyR1-deficient mice than in controls (Kakizawa et al., 2012). These studies raise the possibility that NO-induced Ca2 + release (NICR) is involved in certain pathological states in the brain; however, the pathophysiological role of NICR remains to be established. Furthermore, pathophysiological significance of NICR in vivo has not been examined.
In this study, we examined whether NICR via S-nitrosylated RyR1 is involved in SE-induced neurodegeneration. In order to study the role of NICR in vivo, we generated a knock-in mouse line, in which the essential cysteine residue at 3636 of mouse RyR1 (corresponding to cysteine 3635 in humans and rabbits) was replaced by alanine (Ryr1C3636A) to prevent its S-nitrosylation. We show that NICR was indeed silenced in neurons from Ryr1C3636A knock-in mice, which allowed us to examine the role of NICR in a kainic acid (KA)-model of temporal lobe epilepsy. Here we provide evidence that NICR exacerbates neurodegeneration in the hippocampus following KA-induced seizures, suggesting that RyR1 is a promising therapeutic target candidate to ameliorate the neurodegenerative effect of SE.
2. Materials and Methods
2.1. Chemicals
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), D(+)-glucose, ethanol, 2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (HEPES), magnesium chloride, N-ethylmaleimide (NEM), Nonidet P 40 (NP-40), potassium permanganate, sodium chloride, sodium dodecylsulfate (SDS) and tris(hydroxymethyl)aminomethane were purchased from Nacalai Tesque (Kyoto, Japan). Calcium chloride dehydrates, dantrolene sodium salt, l-glutamic acid, β-mercaptoethanol, neocuproine and TritonX-100 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid, acetone, caffeine, dithiothreitol (DTT) and potassium chloride were purchased from Wako Pure Chemicals (Osaka, Japan). Calcein-AM, 4′-6-diamidino-2-phenylindole (DAPI) solution, ethylenediaminetetraacetic acid (EDTA), Hoechst33342, 1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene (NOC7), 3-morpholinosydnonimine (SIN-1), were purchased from Dojindo (Kumamoto, Japan). Kainic acid (KA), (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801) and N-methyl-d-aspartate (NMDA) were purchased from Tocris Bioscience (Bristol, UK).
2.2. Animals
All animal-related procedures were in accordance with the guidelines of the University of Tokyo. Mice were housed in a transparent plastic cage, fed with food and water ad libitum, and kept under controlled lighting conditions (12 h-light/12 h-dark) in specific pathogen-free conditions in the University of Tokyo animal facility. The maximum number of adult mice in a cage was 7. The Ryr1+/C3636A knock-in mouse was generated at UNITECH (Kashiwa, Japan). Briefly, the targeting vector contained genomic DNA encompassing exon 74 of the mouse Ryr1 gene with a nucleotide change encoding for the mutation at codon 3636 (TGT → GCT, C3636A). The targeting vector was transfected into embryonic stem (ES) cells, and targeted ES clones were screened and confirmed by Southern analysis. The correctly targeted ES cells were injected into blastocysts to generate chimeric mice. Mice carrying the targeted allele were crossed with Tg-Cre transgenic mice to remove the floxed neomycin cassette and produce heterozygous Ryr1+/C3636A mice. Crossing of heterozygous Ryr1+/C3636A mice yielded homozygous Ryr1C3636A/C3636A mice, designated as Ryr1C3636A mice.
The primer sequences for genotyping were as follows: forward, 5′-GCTTAAGGACTGGACATAGAGCTAA-3′; and reverse, 5′-CTGAATATGTGGATATGGGTATAGG-3′. PCR was conducted using Thermococcus kodakaraensis (KOD-FX) DNA polymerase (Toyobo, Osaka, Japan) with the following amplification cycle: 94 °C for 1 min followed by 40 cycles of 94 °C for 10 s and 68 °C for 1 min. The 471 and/or 361 bp bands were detected by 2.5% (w/v) agarose gel electrophoresis stained with ethidium bromide.
2.3. Preparation of Cerebral Neuronal Culture
Neurons were prepared from the cerebral cortices of mice fetuses (postnatal day 0) based on a modification of a previously described procedure (Kakizawa et al., 2012, Kanemaru et al., 2007). Briefly, minced cerebral cortices were treated with 1.0% (w/v) trypsin and 0.1% (w/v) Deoxyribonulease I (Sigma-Aldrich) in Ca2 +/Mg2 +-free phosphate-buffered saline (PBS) (Takara, Shiga, Japan) for 5 min at room temperature (RT). Cells were washed with Neurobasal-A medium supplemented with 5% (v/v) fetal bovine serum (FBS), penicillin (100 units mL− 1), streptomycin (100 units mL− 1), B-27 supplement, and 2 mM l-glutamine (Gibco, ThermoFisher Scientific, Grand Island, NY, USA) and dissociated by triturating with a fire-polished Pasteur pipette in Ca2 +/Mg2 +-free PBS containing 0.05% (w/v) Deoxyribonulease I and 0.03% (w/v) trypsin inhibitor (Sigma-Aldrich). Dispersed cells were plated at 1.0 × 105 cells cm− 2 on glass slide coated with poly-l-lysine and laminin (Sigma-Aldrich). Cells were then cultured at 37 °C under a humidified atmosphere containing 5% CO2. The medium was changed every 2 d by replacing half of the old medium with fresh FBS-free medium. Cells cultured for 7–10 d were used for experiments.
2.4. Preparation of Skeletal Muscle Primary Culture
Primary cultured myoblasts from newborn mice were prepared based on a modification of a previously described procedure (Rando and Blau, 1994). Briefly, the forelimbs and hindlimbs were removed and bones dissected away. The muscle was cut into small fragments and enzymatically dissociated with collagenase (from Clostridium histolyticum, 2.5 mg mL− 1, Wako Pure Chemicals) at 37 °C for 15 min. The fragments were passed through 40-μm cell strainer and the suspension subjected to low-speed centrifugation. The pellet was resuspended in Dulbecco's modified Eagle medium (DMEM) supplemented with 20% (v/v) FBS, penicillin (100 units mL− 1), streptomycin (100 units mL− 1), 2 mM l-glutamine and 10 ng mL− 1 recombinant human fibroblast growth factor (FGF)-basic (Gibco). Myoblasts were differentiated into myotubes with DMEM containing 2% horse serum.
2.5. Intracellular Ca2 + Imaging
Ca2 + imaging was carried out based on a modification of the previously described procedure (Kakizawa et al., 2012). Briefly, neurons and myocytes were loaded with 5 μM Fura-2 acetoxymethyl ester (Fura-2AM) (Molecular Probes, ThermoFisher Scientific, Eugene, OR, USA) for 30 min in HEPES-buffered saline (150 mM NaCl, 4 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 5.6 mM glucose, pH adjusted to 7.4 with NaOH). Fluorescence images were acquired at 510 nm using an inverted microscope (IX81, Olympus, Tokyo, Japan) with a UApo/340 40 × (numerical aperture (NA) 1.35; Olympus) and a cooled CCD camera (EM-CCD C9100, Hamamatsu Photonics, Shizuoka, Japan) at a rate of one frame every 3 s. Excitation wavelengths were 340 and 380 nm. Ca2 + imaging experiments were conducted at RT. Image analyses were carried out using ImageJ64 (National Institutes of Health, Bethesda, MD, USA). Regions of interests (ROIs) corresponding to individual cells were selected and the mean fluorescence intensity (F) of each ROI minus the background intensity was calculated for each frame to obtain F340/F380 as an indicator of [Ca2 +]i. In Fig. 1c and S3e, F340/F380 was converted to [Ca2 +]i using the calibration equation (Grynkiewicz et al., 1985) with the Kd value of 224 nM. Myocytes were stimulated with an electrical pulse (10 V, 100 ms) using SEN-3201 stimulator (Nihonkohden, Tokyo, Japan).
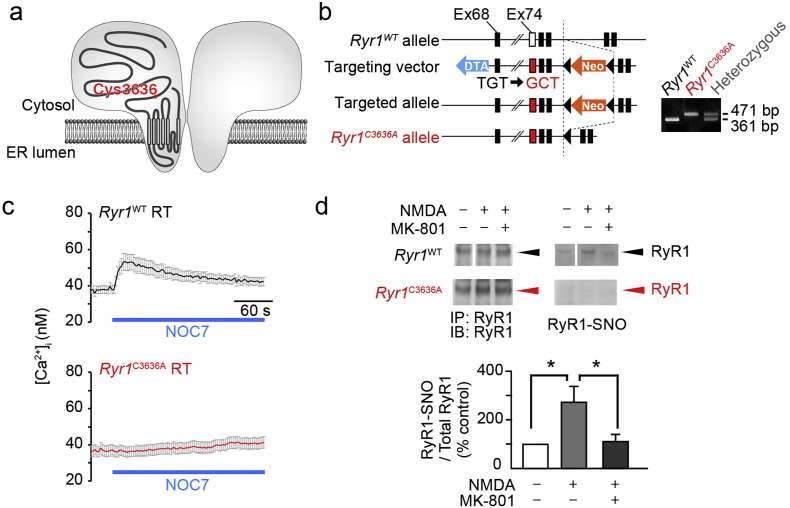
Fig. 1.
Generation of Ryr1C3636A mice and characterization of RyR1C3636A channels in brain (a) Schema of the RyR1 channel. (b) Creation of Ryr1C3636A mutant allele. (c) NOC7 (500 μM)-induced intracellular Ca2 + increase in Ryr1WT and Ryr1C3636A neurons; n = 45–67 neurons. (d) S-nitrosylation of RyR1 in the hippocampal slices. Bottom panel shows the levels of S-nitrosylated RyR1 (SNO-RyR1) in Ryr1WT slices (n = 4). Error bars indicate s.e.m. Statistical significance was determined by analysis of variance (ANOVA) followed by a Tukey-Kramer post-hoc test. * p < 0.05. SNO-RyR1 was not detected in Ryr1C3636A slices (n = 4). See also Fig. S1, S2 and S3.
2.6. NO Donor and Caffeine Application
To prepare a stock solution of NOC7 (100 mM), NOC7 was dissolved in 0.3 N NaOH. NO generation was induced by adding an appropriate amount of NOC7 stock solution to saline containing the same amount of 0.3 N HCl. After a 2-min incubation, the saline-containing NOC7 was applied to the primary culture.
Caffeine was dissolved in saline and applied to the primary culture by pipet. To ensure steady-state ER Ca2 + loading, cells were subjected to short application (10 s) of high potassium saline (114 mM NaCl, 40 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 5 mM HEPES, 5.6 mM glucose, pH adjusted to 7.4 with NaOH) before caffeine was applied.
2.7. Analysis of Neuronal Cell Death
Neurons in primary culture (maintained at 37 °C) were exposed to 500 μM NOC7 or vehicle. After 5 h, cultures were stained with JC-1 (J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide) Mitochondrial Membrane Potential Assay Kit (Cayman Chemical, Ann Arbor, MI USA) for 10 min at RT. Fluorescence images were obtained at 515–550 nm (excitation at 480 nm) and 600 nm (excitation at 540 nm) using an inverted microscope equipped with PlanApo 60 × (NA, 1.42; Olympus) objective and CCD camera. Cell viability was expressed as the intensity of red fluorescence divided by that of green fluorescence in the cell body of neurons.
To examine the morphology of neurons, cultured neurons were fixed with 4% (w/v) paraformaldehyde in PBS and permeabilized with 0.3% (v/v) Triton X-100 in PBS. After rinsing in PBS, cells were incubated with 10% (v/v) bovine serum albumin (Nakarai Tesque, Tokyo, Japan) in PBS at RT for 1 h. For immunofluorescence staining, cells were incubated overnight at 4 °C with antibody against mouse anti-neuronal class III β-tubulin clone TUJ1 (1:1000; Covance, Berkeley, CA; MMS-435P). The immunoreaction was visualized with secondary subclass-specific AlexaFluor 488-conjugated antiserum (Invitrogen, ThermoFisher Scientific, Eugene, OR, USA) at a dilution of 1:1000. Images were acquired with an Olympus IX81 inverted microscope equipped with a 20 × objective (NA = 0.45) and a CCD camera.
2.8. Western Blotting
Anesthetized mice were decapitated and the cortical gray matter, hippocampus or skeletal muscle were dissected in ice-cold potassium phosphate buffer containing 0.32 M sucrose, 1 mM DTT, 1 mM EDTA, the protease inhibitor cocktail Complete (Roche Diagnostics, Mannheim, Germany) and calpain inhibitor I (Roche Diagnostics) using a Potter type glass homogenizer with a Teflon pestle and centrifuged for 10 min at 4 °C and 1000 × g, with a centrifuge to remove nuclei and intact cells. The supernatant was centrifuged for 10 min at 4 °C and 1300 × g to obtain post-nuclear supernatant. The supernatant were centrifuged for 10 min at 4 °C and 17,000 × g, with a high-speed refrigerated micro centrifuge to obtain cytosol and microsomal fraction. Protein quantification was performed on the supernatant using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). SDS-loading buffer, containing β-mercaptoethanol was added to the samples before heat incubation at 95 °C for 5 min. Five micrograms of protein samples were separated by SDS–PAGE on a 5–20% (w/v) gradient polyacrylamide gel (Wako Pure Chemicals) and electroblotted onto a polyvinylidene fluoride (PVDF) membrane (Bio-Rad) at 200 mA for 4 h in electrophoretic transfer cell (Bio-Rad). Membranes were blocked in TBSN (0.1% (v/v) NP-40 in tris-buffered saline) containing 5% (w/v) skim milk for 1 h at RT. The blots were incubated with either rabbit anti-RyR1 polyclonal antibody (Kakizawa et al., 2007) (1:1000), mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal antibody (1:3000, Merck Millipore, Darmstadt, Germany; MAB374) or mouse anti-nNOS monoclonal antibody (1:250; Invitrogen, ThermoFisher Scientific; 61-7000) in the blocking solution overnight at 4 °C. The membrane was washed in TBSN 3 times for 10 min and incubated with a horseradish peroxidase-conjugated anti-rabbit IgG (1:10,000; Medical & Biological Laboratories, Aichi, Japan) or anti-mouse IgG (1:20,000; Medical & Biological Laboratories) in TBSN for 1 h at RT. The horseradish peroxidase was visualized using Western Lightning Plus-ECL (PerkinElmer, Waltham, MA, USA) and chemiluminescence was detected using an Amersham Hyperfilm ECL film (GE Healthcare Life Sciences, Piscataway, NJ, USA).
2.9. DAR-4M Imaging
For NO imaging, NO indicator Diaminorhodamine-4M (DAR-4M) (Kojima et al., 2001) was used. Primary cultured cells were loaded with 5 μM DAR-4M AM (Sekisui Medical, Tokyo, Japan) in saline at RT for 5 min. Then time-lapse fluorescence images were captured every 3 s using the IX81 microscope.
2.10. Organotypic Cultures of Hippocampal Slices
Hippocampal organotypic slice culture was carried out based on a modification of the previously described procedure (Koyama et al., 2007, Koyama et al., 2012). Hippocampal slice cultures were prepared from P6–9 mice. Hippocampal slices were incubated at 37 °C in a humidified incubator with 5% CO2 and 95% air and slices for 7–10 d in vitro were used for experiments.
2.11. Biotin Switch Assay
For detect the S-nitrosylation of RyR1, biotin switch assay was carried out based on a modification of the previously described procedure (Kakizawa et al., 2012). Hippocampal slice cultures were rinsed with HEN buffer (25 mM HEPES, pH 7.7, 0.1 mM EDTA, and 0.01 mM neocuproine). Slices were homogenized in HEN buffer containing 0.5% (w/v) CHAPS, 0.1% (w/v) SDS, 20 mM NEM, protease inhibitor cocktails Complete and calpain inhibitor I by a Potter type glass homogenizer with a Teflon pestle and lysed by rocking for 30 min at 4 °C to block sulfhydryl groups. Lysates were centrifuged at 10,000 × g for 10 min at 4 °C. The supernatant was recovered, supplemented with SDS at a final concentration of 1% (w/v), and further incubated for 30 min at RT to achieve complete blockade of sulfhydryl groups. Excess NEM was removed by protein precipitation with acetone, and the pellet resuspended and incubated in HEN buffer containing 1% (w/v) SDS, 10 mM ascorbic acid and S-Nitrosylation Labeling reagents in the kit as per manufacturer's instructions (Cayman Chemical) for reduction of S-nitrosothiols and labeling with biotin. Extra label was removed by a second acetone precipitation. Proteins were resuspended in lysis buffer containing 25 mM Tris-HCl, pH 7.5, 100 mM NaCl, 2 μM EDTA, 0.05% (v/v) TritonX-100, protease inhibitor cocktails Complete and calpain inhibitor I. For coimmunoprecipitations, 2 μL of anti-RyR1 polyclonal antibody (Merck Millipore; AB9078) was added to lysate and allowed to bind to the antibody for 1 h shaking at 4 °C before addition of 40 μL preequilibrated Protein G sepharose 4 FastFlow (GE Healthcare Life Science). After shaking at 4 °C for another 1 h, beads were washed 5 times in lysis buffer and bound protein was eluted at 95 °C for 5 min in 50 μL of SDS-loading buffer. Protein was separated by SDS-PAGE and electroblotted onto a PVDF membrane. Membranes were blocked in TBSN containing 3% bovine serum albumin (NakaraiTesque) for 1 h at RT. Protein was detected by immunoblotting using a polyclonal antibody against RyR1 (Kakizawa et al., 2007). The blot was then stripped and re-probed with streptavidin-HRP (horse radish peroxidase; Sigma-Aldrich) by chemiluminescence to identify S-nitrosylation of RyR1. S-nitrosylated RyR1 was normalized by the intensity of the RyR1 band.
2.12. KA-induced Seizures Model Mice
Homozygous Ryr1C3636A male mice and age-matched wild-type male littermates were used. Intraperitoneal injection of KA (40 mg kg− 1) in sterilized PBS was used to induce an SE at postnatal 8–12 weeks. Dantrolene (10 mg kg− 1 body weight) in sterilized water was intraperitoneally injected 30 min after KA injection, when required. KA injection was carried out between 1 p.m. and 5 p.m. Animals were observed in the home cage and classified according to the seizure scale: (0) no response; (1) staring, rigid posture with straight and rigid tail; (2) head nodding, rearing and repetitive movement; (3) jumping, wobbling and/or falling; (4) non-intermittent seizure activity persisting for 30 min; (5) death. Only animals reaching at least stage 3–4 were considered for this study (38/72).
For light microscopic analysis, mice were anesthetized and perfused with 4% (w/v) paraformaldehyde in PBS 24 h after KA administration. Brains were removed and post-fixed with 4% paraformaldehyde in PBS overnight at 4 °C. Brains were cryoprotected by sequential immersion in 15 and then 30% (w/v) sucrose/PBS, and then embedded in Tissue Freezing Medium (Leica Microsystems). Sections were cut to 25-μm thickness using a cryostat (CM1900, Leica Instruments, Nussloch, Germany) at − 20 °C. Tissue slices were mounted on PlatinumPro-coated slides (Matsunami, Osaka, Japan) and dried.
Slides were incubated in 0.1% (w/v) cresyl violet acetate (MP Biomedicals, Solon, OH, USA) and 0.1% (v/v) acetic acid for 5 min, followed by dehydration in ascending ratios of ethanol and xylene, and cover-slipped with Malinol mounting medium (Muto Pure Chemicals, Tokyo, Japan).
2.13. Fluoro-Jade C Staining
Hippocampal cryo-sections were immersed in a solution of 5% (w/v) NaOH in 80% (v/v) ethanol for 5 min, then sequentially transferred to 70% (v/v) ethanol for 2 min, distilled water for 2 min, 0.06% (w/v) potassium permanganate for 10 min, and distilled water for 2 min at RT. Slices were then incubated for 20 min with 0.0002% (w/v) Fluoro-Jade C (Merck Millipore) (Schmued et al., 2005) and 2 μM DAPI in a 0.1% (v/v) acetic acid, and rinsed by three washes with distilled water. Sections were dried at 50 °C, dehydrated in xylene, and mounted with DPX Mountant (Sigma-Aldrich). Images of the hippocampal region were captured with a Leica TCS SP8 laser-scanning microscope equipped with a 10 × objective. To quantify Fluoro-Jade C-positive cells, four mice per condition were used. For each hippocampus, six sections were analyzed. Neuronal degeneration was expressed as the intensity of CA3 fluorescence divided by that of CA1 fluorescence in the pyramidal cell layer.
2.14. [3H]Ryanodine Binding Assay
Microsomes were prepared from forelimb and hindlimb muscles of adult mice. [3H]Ryanodine binding assay was carried out based on a previously described procedure with some modifications (Murayama et al., 2015). Briefly, microsomes were incubated with 5 nM [3H]ryanodine in a buffer containing 0.17 M NaCl, 20 mM 2-Hydroxy-3-morpholinopropanesulfonic acid (MOPSO), pH 7.0, 2 mM DTT, 1 mM β,γ-methylene adenosine triphosphate (AMPPCP) and various concentrations of free Ca2 +. The protein-bound [3H]ryanodine was separated by filtering through polyethyleneimine-treated glass filters (Whatman GF/B; GE Helthcare) using Micro 96 Cell Harvester (Molecular Device, Sunnyvale, CA, USA). Nonspecific binding was determined in the presence of 20 μM unlabeled ryanodine. The [3H]ryanodine binding data (B) were normalized to the maximal binding sites for [3H]ryanodine (Bmax) that was separately determined by Scatchard plot analysis using varied concentrations of [3H]ryanodine (3–40 nM).
2.15. Vector Construction and Lentivirus Production
The coding sequence of TagRFP (Evrogen JSC, Moscow, Russia) (Merzlyak et al., 2007) with the mitochondrial targeting sequence was expressed by the mouse excitatory neuron-specific Camk2a promoter (pLenti-Camk2a-mito-TagRFP). Human embryonic kidney 293T (HEK293T) cells were cultured in DMEM supplemented with 5% (v/v) FBS, penicillin (100 units mL− 1), streptomycin (100 units mL− 1) and 8 mM l-glutamine. When HEK293T cells were grown to 90% confluence, culture medium was replaced with DMEM without antibiotics. Lentiviruses were produced by reverse transfection of HEK293T cells using 15 μg lentiviral vectors, 4 μg plasmids expressing the vesicular stomatitis virus glycoprotein (VSV-G), 8 μg packing plasmids Δ8.9 and Lipofectamine 2000 (Thermo Fisher Scientific) in 10 cm collagen type I-coated dishes (Asahi glass, Tokyo, Japan). Cells were incubated at 37 °C under 5% CO2. After 15 h, the medium was replaced with fresh media and cells were incubated for 36 h at 32 °C. The lentivirus-containing medium was collected and cleared by centrifugation at 1500 rpm for 5 min (Centrifuge 5702, Eppendorf, Hamburg, Germany). For concentration of lentivirus, centrifugation was performed at 10,000 rpm overnight at 4 °C using microcentrifuge (Tomy, MRX-150). Supernatant was completely removed and virus pellets resuspended in 50 μL PBS and stored at − 80 °C until use.
2.16. Analysis of Mitochondrial Circularity in Neurons
Primary cultured neurons at 4 d in vitro were infected with lentiviral mito-TagRFP. Four days after lentiviral infection, neurons were examined for morphological change of mitochondria. For the assessment of mitochondrial fragmentation, mitochondrial circularity (4π [area]/[perimeter]2) was analyzed using ImageJ64 software (National Institute of Health, Bethesda, MD, U.S.A.).
2.17. Statistics
All statistical analyses of the data were performed using Microsoft Excel 2004 for Mac (Microsoft, Redmond, WA, USA) with the add-in software Statcel2 (OMS, Saitama, Japan). Differences between two groups were analyzed with Student's t-test. Differences between three or more groups were analyzed by ANOVA. Post hoc multiple comparisons were made using the Tukey-Kramer test.
3. Results
3.1. Generation and Characterization of NICR-deficient Mice
To examine the potential pathophysiological significance of NICR in vivo, we generated a mouse line Ryr1C3636A in which the cysteine residue (Cys3636) critical for NICR (Kakizawa et al., 2012, Sun et al., 2001) is replaced by alanine residue (Fig. 1a and b). The Ryr1C3636A mice exhibited no significant change in the body weight, gross anatomy of the brain or the protein levels of RyR1 or nNOS in the hippocampus and cerebral cortex (Fig. S1a–S1d). Moreover, there was no significant difference in the protein levels of RyR1 or nNOS, the ryanodine binding of sarcoplasmic reticulum fractions, which reflects CICR activity of RyRs (Meissner, 1994, Ogawa, 1994), or in depolarization-induced Ca2 + release via RyR1 in skeletal myocytes (Fig. S2a–S2e). When we compared the effect of caffeine, which is an agonist of CICR, on CICR in Ryr1C3636A and Ryr1WT neurons, we observed no significant difference (Fig. S3a and S3b). However, when we compared the effect of NOC7, an NO donor, on [Ca2 +]i in Ryr1C3636A and Ryr1WT neurons, we observed a marked increase in [Ca2 +]i only in Ryr1WT neurons (Fig. 1c). Moreover, when we applied 50 μM N-methyl-d-aspartic acid (NMDA) to hippocampal slice cultures for 2 h to induce seizure-like activity, we found significant increases in S-nitrosylation of RyR1 in Ryr1WT mice but not in Ryr1C3636A mice (Fig. 1d), although NMDA-induced NO production was similarly observed in both Ryr1WT and Ryr1C3636A neurons (Fig. S3c and S3d). Collectively, these results indicate that in Ryr1C3636A mice, Ca2 + release induced by NO is silenced, but other Ca2 + release modes via RyR1 are not.
3.2. Kainic Acid-induced Neuronal Cell Death in the Hippocampus was Reduced in the Ryr1C3636A Mice
Prompted by our observation that NICR is genetically silenced in Ryr1C3636A mice, we next studied the pathophysiological significance of NICR in the KA-induced temporal lobe epilepsy model (Nadler et al., 1978). KA induced severe seizure phenotype in both Ryr1WT and Ryr1C3636A mice (Fig. 2a). In Ryr1WT mice, 24 h after KA administration, the CA3 region showed darkly stained shrunken pyramidal cells, indicative of neurodegeneration. In contrast, in KA-treated Ryr1C3636A mice, the CA3 region was packed with large pyramidal cells similar to those found in sham-treated mice (Fig. 2b). These observations indicate that genetic inhibition of NICR ameliorates seizure-induced neurodegeneration, suggesting that pharmacological inhibition of NICR may also have a neuroprotective effect. Dantrolene is a well-known inhibitor of RyR1 at body temperature (Ohta and Endo, 1986). Indeed, we found that NICR was blocked by dantrolene in Ryr1WT neurons at 37 °C (Fig. S3e). To examine whether dantrolene is protective against neurodegeneration caused by epileptic seizures in vivo, we administered dantrolene to Ryr1WT and Ryr1C3636A mice after KA-induced seizures. Although dantrolene had no significant effect on the maximum level of seizures (Fig. 2a), it circumvented morphological changes in the CA3 region in Ryr1WT mice (Fig. 2b). In contrast, dantrolene administration had no apparent effect in Ryr1C3636A mice.
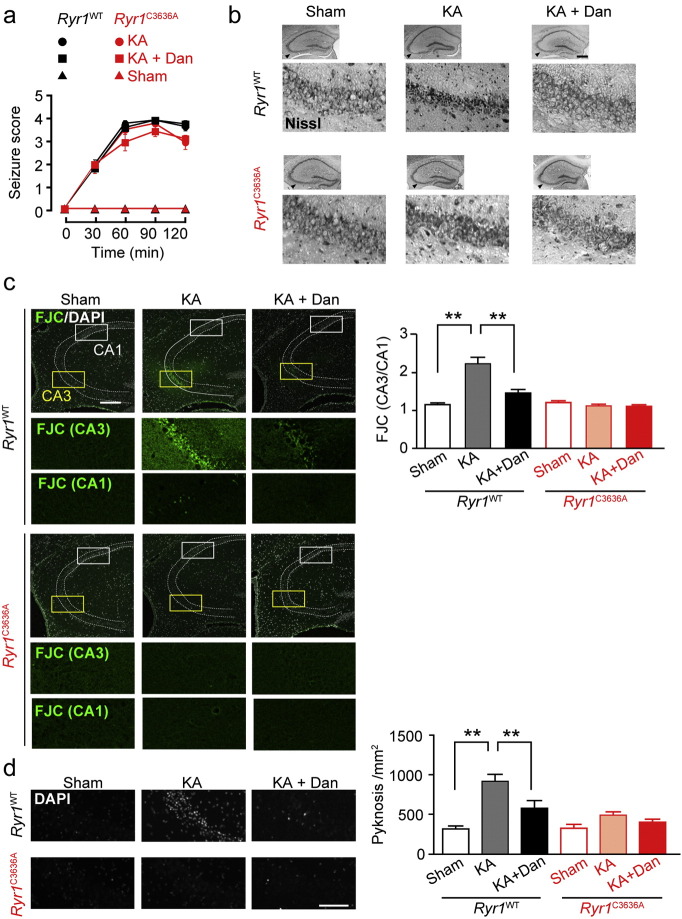
Fig. 2.
Kainic acid-induced neurodegeneration in CA3 region was reduced in Ryr1C3636A mice (a) KA (40 mg kg− 1, i.p.) triggered limbic seizures. n = 5–7. Dan, dantrolene. (b) Nissl-staining of hippocampus 24 h after KA injection. Scale bar: 500 μm. (c) Fluoro-Jade C staining of hippocampus. Dotted lines show the position of stratum pyramidale. Yellow (CA3) and white (CA1) boxed areas in upper panels are shown enlarged in bottom panels. n = 28–40 slices from 5 to 7 mice. Scale bar: 200 μm. Error bars indicate s.e.m. Data are analyzed for significance using ANOVA followed by a Tukey-Kramer post-hoc test. ** p < 0.01. (d) DAPI staining of hippocampus. n = 30–43 slices from 5 to 7 mice. Scale bar: 100 μm. Error bars indicate s.e.m. Data are analyzed for significance using ANOVA followed by a Tukey-Kramer post-hoc test. ** p < 0.01.
We next examined the extent of seizure-induced neuronal damage using the neurodegeneration marker Fluoro-Jade C (FJC) (Schmued et al., 2005). FJC positve neurons were clearly visualized in CA3 region of KA-treated Ryr1WT mice but scarcely observed in KA-treated Ryr1C3636A mice, which showed similar staining as sham-treated animals (Fig. 2c). Moreover, 4′,6-Diamidino-2-phenylindole (DAPI) staining revealed cells with condensed, pyknotic nuclei, representative of apoptotic cells, in the CA3 region of KA-treated Ryr1WT mice but not in Ryr1C3636A mice (Fig. 2d). Thus, the hippocampus of Ryr1C3636A mice deficient in NICR was protected from seizure-induced neurodegeneration. Furthermore, in vivo dantrolene administration reduced the amount of neurodegeneration analyzed using FJC staining in Ryr1WT mice (Fig. 2c and d). In contrast, dantrolene treatment had no effect on Ryr1C3636A mice. These results indicate that the NICR inhibitors such as dantrolene may have therapeutic potential for treating neurodegeneration after epileptic seizures.
3.3. NO-induced Neuronal Cell Death was Milder in the Ryr1C3636A Neurons In Vitro
These in vivo results led us to study the role of NICR in NO-induced neuronal cell death. Five hours after the application of NOC7, Ryr1WT cultured neurons showed a distinctive pattern of morphological changes characterized by short and curly neurites, whereas such shortening of neurites was absent in Ryr1C3636A neurons (Fig. 3a). In Ryr1WT neurons, dantrolene exerted a protective effect against NOC7-induced morphological changes. In contrast, no significant effect of dantrolene was detected in Ryr1C3636A neurons (Fig. 3a).
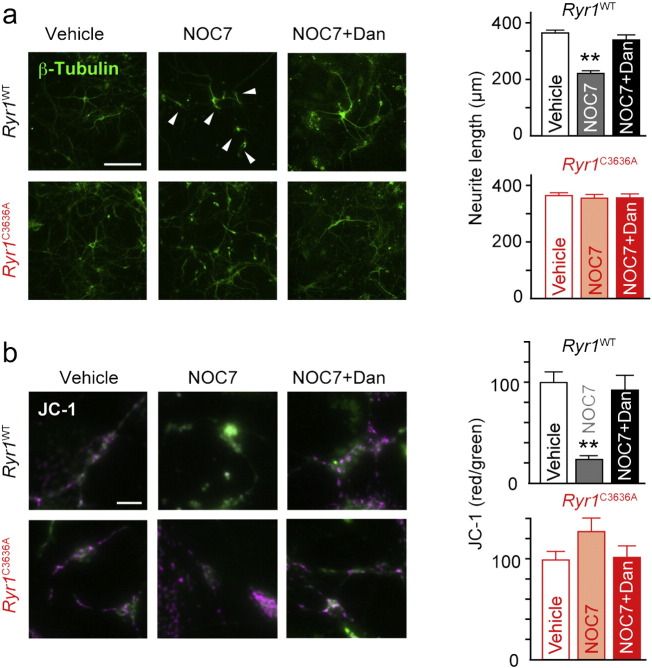
Fig. 3.
Involvement of NICR in NO-induced neuronal cell death (a) Effect of NO on neuron morphology (stained with anti-β-III tubulin antibody). Arrowheads indicate short and curly neurites. Scale bar: 100 μm. Graphs show the length of the longest neurite of each neuron; n = 52–116; error bars indicate s.e.m. Concentrations of NOC7 and dantrolene (Dan) were 500 μM and 10 μM, respectively. Data were analyzed for significance using ANOVA followed by a Tukey-Kramer post-hoc test. ** p < 0.01. (b) Cell viability examined by JC-1 assay. Scale bar: 10 μm. n = 49–65. Error bars indicate s.e.m. Data were analyzed for significance using ANOVA followed by a Tukey-Kramer post-hoc test. ** p < 0.01. See also Fig. S4.
We next examined the mitochondrial membrane potential (ΔΨm), which is dissipated in apoptotic cells, by using the JC-1 assay (Kroemer and Reed, 2000). In Ryr1WT neurons exposed to NOC7, the red/green ratio of JC-1 decreased, indicative of reduced ΔΨm, whereas in Ryr1C3636A neurons, the red/green ratio was sustained after NOC7 application (Fig. 3b). Thus, NO induced mitochondrial dysfunction in Ryr1WT but not in Ryr1C3636A neurons. Again, dantrolene reversed the NO-induced reduction of ΔΨm in Ryr1WT neurons, but had no significant effect in Ryr1C3636A neurons (Fig. 3b).
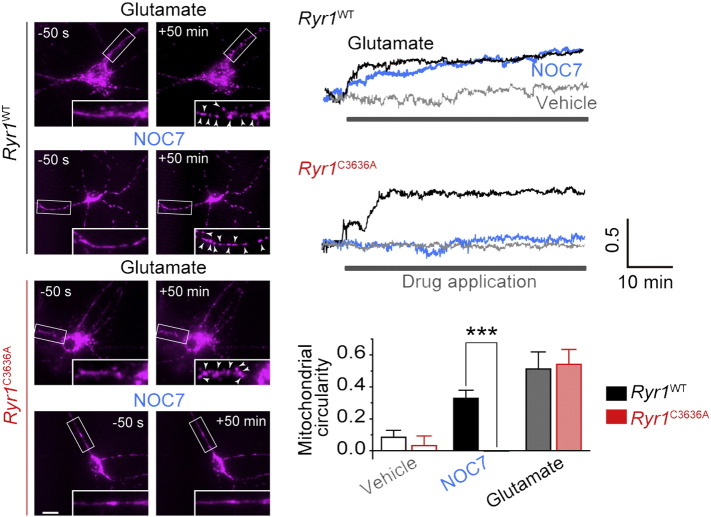
Mitochondrial fragmentation occurs early in apoptosis and plays a key role in cell death progression (Knott et al., 2008). We, therefore, carried out time-lapse imaging of the mitochondrial morphology in cultured neurons, in which mitochondria-targeted TagRFP was expressed. Upon application of 100 μM glutamate, which is known as a strong inducer of neuronal apoptosis (Choi, 1988), a significant increase in mitochondrial fragmentation was observed (Fig. 4 top left). Similarly, after NOC7 application, we observed a significant increase in mitochondrial fragmentation in Ryr1WT neurons (Fig. 4 middle left). However, NOC7-induced mitochondrial fragmentation was scarcely observed in Ryr1C3636A neurons (Fig. 4 bottom left). We quantified the mitochondrial fragmentation analyzing the circularity of the mitochondrial morphology (see Materials and methods). Although glutamate induced mitochondrial fragmentation in both Ryr1WT and Ryr1C3636A neurons to the same extent, NOC7 induced mitochondrial fragmentation only in Ryr1WT neurons, and NOC7-induced mitochondrial fragmentation was absent in Ryr1C3636A neurons (Fig. 4 right panels). We also examined the effect of dantrolene on NOC7-induced mitochondrial fragmentation, but did not find a significant effect of the drug (data not shown). These results suggest that neuronal cell death induced by NICR is accompanied by mitochondrial dysfunction, but the causal relationship requires further clarification.
Fig. 4.
Involvement of NICR in NO-induced mitochondrial fragmentation. Left panels show mitochondrial morphology before and 50 min after drug application. Glutamate (Glu; 100 μM) was used in positive control experiments. NOC7 concentration was 500 μM. Right upper panels show the representative time courses of fragmentation assessed by circularity of mitochondria. Right bottom panel show the average circularity of mitochondria between 55 and 60 min after drug application. Scale bar: 5 μm. n = 10–16. Data are analyzed for significance using t-test. *** p < 0.001.
NO may also exert its cytotoxic effect through the formation of peroxynitrite (Szabó et al., 2007). However, the peroxynitrite donor 3-morpholino-sydnonimine (SIN-1) induced neuronal cell death in both Ryr1WT and Ryr1C3636A neurons to the same extent (Fig. S4a and S4b). Thus, Cys3636 in RyR1 is unlikely to be the key residue affected by peroxynitrite.
4. Discussion
These results indicate the involvement of NICR in neurodegeneration following SE. Moreover, this data set is consistent with the finding that epileptic seizure-induced neurotoxicity is reduced in nNOS-deficient mice (Parathath et al., 2007). Although the targets of NO have been elusive, the present results highlight the importance of the S-nitrosylation of RyR1 at Cys3636 (corresponding to Cys3635 in rabbits and humans). This cysteine residue has been shown to undergo various redox modifications such as oxidation by H2O2 and S-glutathionylation in addition to S-nitrosylation (Aracena-Parks et al., 2006). However, H2O2 has been shown to activate both rabbit RyR1WT and RyR1C3635A (Aracena-Parks et al., 2006). Furthermore, while it was reported that S-nitrosoglutathione (GSNO) preferentially S-glutathionylated Cys3635 (Aracena-Parks et al., 2006), another study found that GSNO activated RyR1C3635A similarly to RyR1WT (Sun et al., 2003). Thus, neither oxidation nor S-glutathionylation of Cys3636 is likely to be involved in the activation of RyR1.
The RyR1 antagonist dantrolene is an approved drug for the treatment of malignant hyperthermia, which is a pharmacogenetic disorder in patients characterized by an increased susceptibility of muscular RyR1 to general anesthetics (Hirshey Dirksen et al., 2011). Several studies have raised the possibility of dantrolene as a treatment for neurodegeneration (Mody and MacDonald, 1995, Muehlschlegel and Sims, 2009). However, the precise mechanism for its therapeutic effect has remained elusive. The present results shed light on this important question, and pave the way for exploring RyR1 antagonists as therapeutics for brain damage associated with epileptic seizures.
NO is implicated in various other pathological conditions in the brain, including excitotoxicity during ischemic brain injury (Nakamura et al., 2013, Pacher et al., 2007). For instance, in the middle cerebral artery occlusion model, the brain injury is milder in mice treated with an nNOS-specific inhibitor and in nNOS-deficient mice (Huang et al., 1994). Thus, it seems possible that NICR may also play a role in ischemic brain injury (Kakizawa et al., 2012), and that RyR1 antagonist may have a potential therapeutic value in other neurodegenerative diseases associated with excitotoxicity.
Author Contributions
MI and SK conceived the research; YM, KK, YO and MI designed the research; YM, KK, TN and TM carried out the experiments; KS, HS, RK, AI, TY, YI, TS, NS and SK provided experimental tools; YM, KK, YO, TN, JS, TM and MI analyzed the data; YM and MI wrote the manuscript; and MI supervised the study.
Abbreviations
- AM
acetoxymethyl ester
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CICR
Ca2 +-induced Ca2 + release
- Dan
dantrolene
- DAPI
4′,6-Diamidino-2-phenylindole
- DAR-4M
Diaminorhodamine-4M
- DMEM
Dulbecco's modified Eagle medium
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic reticulum
- ES
embryonic stem
- FBS
fetal bovine serum
- FGF
fibroblast growth factor
- FJC
Fluoro-Jade C
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSNO
S-nitrosoglutathione
- HEK
human embryonic kidney
- HEPES
2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HRP
horseradish peroxidase
- KA
kainic acid
- JC-1
J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolcarbocyanine iodide
- MK-801
(5S,10R)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine maleate
- NA
numerical aperture
- NICR
nitric oxide-induced Ca2 + release
- NMDA
N-methyl-d-aspartic acid
- NEM
N-ethylmaleimide
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOC7
1-hydroxy-2-oxo-3-(N-methyl-3-aminopropyl)-3-methyl-1-triazene
- NOS
nitric oxide synthase
- NP-40
Nonidet P 40
- PBS
phosphate-buffered saline
- PVDF
polyvinylidene fluoride
- ROI
regions of interest
- RT
room temperature
- RyR1
type 1 ryanodine receptor
- SDS
sodium dodecyl sulfate
- SE
status epilepticus
- SIN-1
3-morpholino-sydnonimine
- TBS
tris-buffered saline
- ΔΨm
mitochondrial membrane potential
Acknowledgement
We thank Dr. Masahiko Watanabe for providing rabbit anti-RyR1 antibody and Mr. Yasuhiro Oda for technical assistance. This work was supported by JSPS KAKENHI Grant Number JP21229004 and JP25221304 to MI, JP13J00025 and JP15K08227 to YM, JP15H05648 to KK and JP23659037 and JP15K06774 to SK, and grants from Takeda Science Foundation and Mochida Memorial Foundation to SK.
The authors have declared that no conflict of interest exists.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.08.020.
Contributor Information
Sho Kakizawa, Email: kakizawa.sho.4u@kyoto-u.ac.jp.
Masamitsu Iino, Email: iino@m.u-tokyo.ac.jp.
Appendix A. Supplementary Data
Supplementary figures.
References
- Aghdasi B., Reid M.B., Hamilton S.L. Nitric oxide protects the skeletal muscle Ca2 + release channel from oxidation induced activation. J. Biol. Chem. 1997;272:25462–25467. doi: 10.1074/jbc.272.41.25462. [DOI] [PubMed] [Google Scholar]
- Aracena-Parks P., Goonasekera S.A., Gilman C.P., Dirksen R.T., Hidalgo C., Hamilton S.L. Identification of cysteines involved in S-nitrosylation, S-glutathionylation, and oxidation to disulfides in ryanodine receptor type 1. J. Biol. Chem. 2006;281:40354–40368. doi: 10.1074/jbc.M600876200. [DOI] [PubMed] [Google Scholar]
- Bellinger A.M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A.R. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 2009;15:325–330. doi: 10.1038/nm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic S.F., Mulley J.C., Scheffer I.E., Petrou S. Human epilepsies: interaction of genetic and acquired factors. Trends Neurosci. 2006;29:391–397. doi: 10.1016/j.tins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Betjemann J.P., Lowenstein D.H. Status epilepticus in adults. Lancet Neurol. 2015;14:615–624. doi: 10.1016/S1474-4422(15)00042-3. [DOI] [PubMed] [Google Scholar]
- Chang B.S., Lowenstein D.H. Epilepsy. N. Engl. J. Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Choi D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Durham W.J., Aracena-Parks P., Long C., Rossi A.E., Goonasekera S.A., Boncompagni S., Galvan D.L., Gilman C.P., Baker M.R., Shirokova N. RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell. 2008;133:53–65. doi: 10.1016/j.cell.2008.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eu J.P., Sun J., Xu L., Stamler J.S., Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- Goldberg E.M., Coulter D.A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nat. Rev. Neurosci. 2013;14:337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2 + indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hess D.T., Matsumoto A., Kim S.O., Marshall H.E., Stamler J.S. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell. Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Hirshey Dirksen S.J., Larach M.G., Rosenberg H., Brandom B.W., Parness J., Lang R.S., Gangadharan M., Pezalski T. Future directions in malignant hyperthermia research and patient care. Anesth. Analg. 2011;113:1108–1119. doi: 10.1213/ANE.0b013e318222af2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Huang P.L., Panahian N., Dalkara T., Fishman M.C., Moskowitz M.A. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Jaffrey S.R., Erdjument-Bromage H., Ferris C.D., Tempst P., Snyder S.H. Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat. Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- Jentsch T.J., Hubner C.A., Fuhrmann J.C. Ion channels: function unravelled by dysfunction. Nat. Cell Biol. 2004;6:1039–1047. doi: 10.1038/ncb1104-1039. [DOI] [PubMed] [Google Scholar]
- Kakizawa S., Kishimoto Y., Hashimoto K., Miyazaki T., Furutani K., Shimizu H., Fukaya M., Nishi M., Sakagami H., Ikeda A., Kondo H., Watanabe M., Iino M., Takeshima H. Junctophilin-mediated channel crosstalk essential for cerebellar synaptic plasticity. EMBO J. 2007;26:1924–1933. doi: 10.1038/sj.emboj.7601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S., Yamazawa T., Chen Y., Ito A., Murayama T., Oyamada H., Kurebayashi N., Sato O., Watanabe M., Mori N., Oguchi K., Sakurai T., Takeshima H., Saito N., Iino M. Nitric oxide-induced calcium release via ryanodine receptors regulates neuronal function. EMBO J. 2012;31:417–428. doi: 10.1038/emboj.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K., Okubo Y., Hirose K., Iino M. Regulation of neurite growth by spontaneous Ca2 + oscillations in astrocytes. J. Neurosci. 2007;27:8957–8966. doi: 10.1523/JNEUROSCI.2276-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Hirotani M., Nakatsubo N., Kikuchi K., Urano Y., Higuchi T., Hirata Y., Nagano T. Bioimaging of nitric oxide with fluorescent indicators based on the rhodamine chromophore. Anal. Chem. 2001;73:1967–1973. doi: 10.1021/ac001136i. [DOI] [PubMed] [Google Scholar]
- Koyama R., Muramatsu R., Sasaki T., Kimura R., Ueyama C., Tamura M., Tamura N., Ichikawa J., Takahashi N., Usami A. A low-cost method for brain slice cultures. J. Pharmacol. Sci. 2007;104:191–194. doi: 10.1254/jphs.sc0070119. [DOI] [PubMed] [Google Scholar]
- Koyama R., Tao K., Sasaki T., Ichikawa J., Miyamoto D., Muramatsu R., Matsuki N., Ikegaya Y. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat. Med. 2012;18:1271–1278. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Meisler M.H., Kearney J.A. Sodium channel mutations in epilepsy and other neurological disorders. J. Clin. Invest. 2005;115:2010–2017. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2 + release channels and their regulation by endogenous effectors. Annu. Rev. Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Merzlyak E.M., Goedhart J., Shcherbo D., Bulina M.E., Shcheglov A.S., Fradkov A.F., Gaintzeva A., Lukyanov K.A., Lukyanov S., Gadella T.W. Bright monomeric red fluorescent protein with an extended fluorescence lifetime. Nat. Methods. 2007;4:555–557. doi: 10.1038/nmeth1062. [DOI] [PubMed] [Google Scholar]
- Mody I., MacDonald J.F. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2 + release. Trends Pharmacol. Sci. 1995;16:356–359. doi: 10.1016/s0165-6147(00)89070-7. [DOI] [PubMed] [Google Scholar]
- Muehlschlegel S., Sims J.R. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit. Care. 2009;10:103–115. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Busse R., Mordvintcev P.I., Vanin A.F., Nielsen E.O., Scheel-Krüger J., Olesen S.P. Nitric oxide promotes seizure activity in kainate-treated rats. Neuroreport. 1994;5:2325–2328. doi: 10.1097/00001756-199411000-00029. [DOI] [PubMed] [Google Scholar]
- Murayama T., Kurebayashi N., Yamazawa T., Oyamada H., Suzuki J., Kanemaru K., Oguchi K., Iino M., Sakurai T. Divergent activity profiles of type 1 ryanodine receptor channels carrying malignant hyperthermia and central core disease mutations in the amino-terminal region. PLoS One. 2015;10 doi: 10.1371/journal.pone.0130606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler J.V., Perry B.W., Cotman C.W. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature. 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Tu S., Akhtar M.W., Sunico C.R., Okamoto S., Lipton S.A. Aberrant protein S-nitrosylation in neurodegenerative diseases. Neuron. 2013;78:596–614. doi: 10.1016/j.neuron.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Crit. Rev. Biochem. Mol. Biol. 1994;29:229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Ohta T., Endo M. Inhibition of calcium-induced calcium release by dantrolene at mammalian body-temperature. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1986;62:329–332. [Google Scholar]
- Pacher P., Beckman J.S., Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parathath S.R., Gravanis I., Tsirka S.E. Nitric oxide synthase isoforms undertake unique roles during excitotoxicity. Stroke. 2007;38:1938–1945. doi: 10.1161/STROKEAHA.106.478826. [DOI] [PubMed] [Google Scholar]
- Rando T.A., Blau H.M. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued L.C., Stowers C.C., Scallet A.C., Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Sun J., Xin C., Eu J.P., Stamler J.S., Meissner G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11158–11162. doi: 10.1073/pnas.201289098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Xu L., Eu J.P., Stamler J.S., Meissner G. Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine receptor by different mechanisms. An allosteric function for O2 in S-nitrosylation of the channel. J. Biol. Chem. 2003;278:8184–8189. doi: 10.1074/jbc.M211940200. [DOI] [PubMed] [Google Scholar]
- Szabó C., Ischiropoulos H., Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.