Abstract
Background
Response to disease modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) is often heterogeneous. We aimed to identify types of disease activity trajectories following the initiation of a new biologic DMARD (bDMARD).
Methods
Pooled analysis of nine national registries of patients with diagnosis of RA, who initiated Abatacept and had at least two measures of disease activity (DAS28). We used growth mixture models to identify groups of patients with similar courses of treatment response, and examined these patients' characteristics and effectiveness outcomes.
Findings
We identified three types of treatment response trajectories: ‘gradual responders’ (GR; 3576 patients, 91·7%) had a baseline mean DAS28 of 4·1 and progressive improvement over time; ‘rapid responders’ (RR; 219 patients, 5·6%) had higher baseline DAS28 and rapid improvement in disease activity; ‘inadequate responders’ (IR; 103 patients, 2·6%) had high DAS28 at baseline (5·1) and progressive worsening in disease activity. They were similar in baseline characteristics. Drug discontinuation for ineffectiveness was shorter among inadequate responders (p = 0.03), and EULAR good or moderate responses at 1 year was much higher among ‘rapid responders’ (p < 0.001).
Interpretation
Clinical information and baseline clinical characteristics do not allow a reliable prediction of which trajectory the patients will follow after bDMARD initiation.
Keywords: Abatacept, Rheumatoid arthritis, Disease activity, DAS28, Longitudinal data, Drug retention, Response rate
Highlights
-
•
This study examined disease activity trajectories in a multinational cohort of 3898 rheumatoid arthritis patients.
-
•
Growth mixture models identified three groups: gradual, rapid, and inadequate responders (GR: 91·7%, RR: 5·6%, IR: 2·6%).
-
•
At baseline, groups were similar in demographic and clinical characteristics, and moderately different in function and disease activity.
-
•
The groups had large difference in drug retention and in good or moderate response rate.
Using nine national registries, this study of 3898 established RA patients initiating a new bDMARD identified distinct types of responders: gradual, rapid and inadequate responders. Neither socio-demographic nor clinical characteristics at baseline allowed the prediction of the type of response trajectory after treatment initiation, but effectiveness outcomes strongly differed, suggesting that these empirically derived subgroups have clinical relevance.
As a major aim of precision medicine is to make anti-rheumatic therapy more personalized, the detection of responder types following initiation of a specific bDMARD underscores the need to find reliable predictors of trajectories to identify patients needing a distinct treatment strategy.
1. Introduction
The effect of disease modifying antirheumatic drugs (DMARDs) in rheumatoid arthritis (RA) on disease activity is generally presented using population means (Combe et al., 2015, Gabay et al., 2015, Littlejohn et al., 2015). The use of biologic DMARDs (bDMARD) has revolutionized the therapy of severe RA (Sanmarti et al., 2015). However, the response to treatment is heterogeneous, both to cDMARDs (Aga et al., 2015), and to the various bDMARD agents (Kiely, 2015). As a major aim in the new era of precision medicine is to make anti-rheumatic therapy more personalized, identifying and predicting distinct treatment responses trajectories to DMARDs has major implications for clinical practice. Studies (range n: 568–2752) focused on identifying types of patients with similar evolutions in disease activity (Siemons et al., 2014), physical activity (Demmelmaier et al., 2016), functional limitation (Norton et al., 2013), or psychological distress (Norton et al., 2011) and found subsets of patients with less favorable trajectories. The identification of predictors of response type trajectories could enable an early identification of patients needing a distinct treatment strategy.
In RA, disease activity measures are the main clinical outcome used by practitioners to appraise the evolution of RA (Finckh et al., 2007), to modify and adapt treatment, and to determine if patients have reached a state of low disease activity (Inoue et al., 2007) or remission (Mohammed et al., 2015). The Disease Activity Score based on 28 joints (DAS28) is a well-established instrument to assess disease activity (Prevoo et al., 1995).
A study of early RA patients (Siemons et al., 2014) (n = 568) found three types of trajectories during the first year after treatment initiation: the most frequent type (82·6% of patients) was a good responder group, the second type (14·1%) comprised patients with a slower response to treatment, and the third one was composed of a very small group (3·3%) of patients who showed no improvement after 1 year. However, the trajectories of disease activity in patients initiating a specific bDMARD or in patients with established disease have not been studied.
The aim of this study was to identify different types of trajectories in RA disease activity following the initiation of a new bDMARD and to examine the determinants of each responder type in a large multi-national observational cohort.
2. Materials and Methods
2.1. Study Design
This is a pooled analysis of data from nine national registries of RA patients: ARTIS (Sweden), ATTRA (Czech Republic), DANBIO (Denmark), GISEA (Italy), NOR-DMARD (Norway), ORA (France), REUMA.PT (Portugal), RHUMADATA (Canada), and SCQM (Switzerland), collected from 2006 to 2015. Each of the registries was approved by the local Ethics Committee and national guidelines for collection of informed consent form before enrolment in the study in accordance with the Declaration of Helsinki were followed. A more detailed description of the registries is available elsewhere (Curtis et al., 2010, Finckh et al., 2015). Inclusion criteria for this analysis were a diagnosis of RA, initiation of ABA treatment and at least two assessments of DAS28. The primary outcome to model response trajectories was the disease activity score based on 28 joints (DAS28) (Prevoo et al., 1995). Most registries used DAS28-ESR; when not available, we used the DAS28-CRP instead. All patients had either complete data for the DAS28-ESR or complete data for the DAS28-CRP. Almost all registries had some patients with missing DAS28-ESR data. The Italian registry, GISEA, was the only registry with only DAS28-CRP data.
In addition to the DAS28, we extracted demographic variables, BMI, smoking status, comorbidities, seropositivity according to rheumatoid factor or anti-citrullinated protein antibodies (ACPAs), disease duration, and disability as measured by the health assessment questionnaire (HAQ). We also extracted information about treatment, including number of past biologics treatment, conventional DMARD (cDMARD) and glucocorticosteroid therapy. We further computed ABA drug retention, which integrates both drug effectiveness and tolerance, ABA drug retention until stopping for ineffectiveness, reason for ABA discontinuation, EULAR good or moderate response rate at 6 months, one year, and two years (van Riel, 2014) corrected for drug discontinuation (Lundex) (Kristensen et al., 2006). ABA drug retention was defined as the time between drug initiation and last administration, plus one dispensation interval. Patients lost to follow-up were censored at the last registered visit.
2.2. Statistical Analysis
We used growth mixture models (GMM) to identify groups of patients with similar courses of treatment response, modeling time since beginning of treatment as polynomials with a linear, quadratic and cubic terms and derived empirically based trajectory subgroups. GMM are used to model unobserved types of evolution of disease activity over time. GMM estimate groups of patients in which the trajectories of DAS28 are similar within each trajectory type and different from the trajectories in the other types (Reineke and Seddig, 2011). To determine the optimal number of types (also called latent classes in growth mixture models terminology) of disease activity trajectories, we used two information criteria (Akaike's information criteria, and Bayesian information criteria), with lower value indicating a better fit of the model to the data.
We then examined the association of these groups with demographic-, disease-, and treatment-related covariates, as well as treatment effectiveness. We analyzed patients and disease characteristics at treatment initiation using standard descriptive statistics and Fisher exact test for categorical variables or Wilcoxon rank sum test for continuous variables. We ran a sensitivity analysis using probability-weighted regression with weights based on posterior probability of classification in each trajectory group. Data are presented as means (SD) or medians (interquartile ranges) depending on their distribution. ABA drug retention was analyzed by the Kaplan Meier method. Given the inherent differences between registries (Finckh et al., 2015), we tested for effect modification by registry using an interaction term between types of trajectories and national registry using a Cox proportional hazard model. Analyses were performed using R v3.2.4 (R foundation, Vienna, Austria) and the lcmm package (Proust-Lima et al., 2015).
3. Results
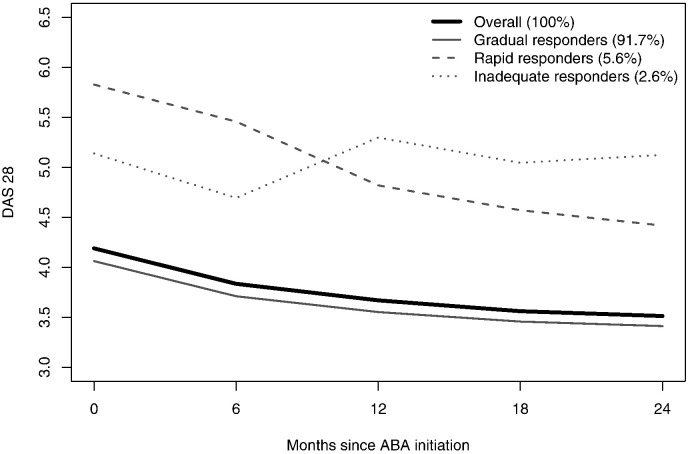
A total of 3898 patients initiated ABA with a mean number of 3.94 DAS28 assessments. Follow-up time ranged from 1 month to 11.7 years. Trajectory analysis of the entire sample identified three types of disease activity trajectories with low misclassification (for goodness of fit indices, see Appendix 1). The largest group (3576 patients, 91·7%) can be labeled as the ‘gradual responders’ (GR) type, with a mean DAS28 at baseline of 4·1 and a progressive improvement over time. Fig. 1 presents the observed means for patients based on assigned types of trajectories. Estimated mean trajectories were quite similar (data not shown). The second group (219 patients, 5·6%) can be described as the ‘rapid responders’ (RR) type, with higher DAS28 values at baseline, and a rapid improvement in disease activity. The third group (103 patients, 2·6%) can be identified as ‘inadequate responders’ (IR) type, with higher DAS28 values at baseline, a short improvement during the first 6 months, followed by a return to initial disease activity level (for exact estimates of the DAS28 trajectories for these three types of patients, see Appendix 2).
Fig. 1.
Class-specific DAS28 trajectories based on observed means. Time horizon was cut at 2 years to focus on the period with most available data.
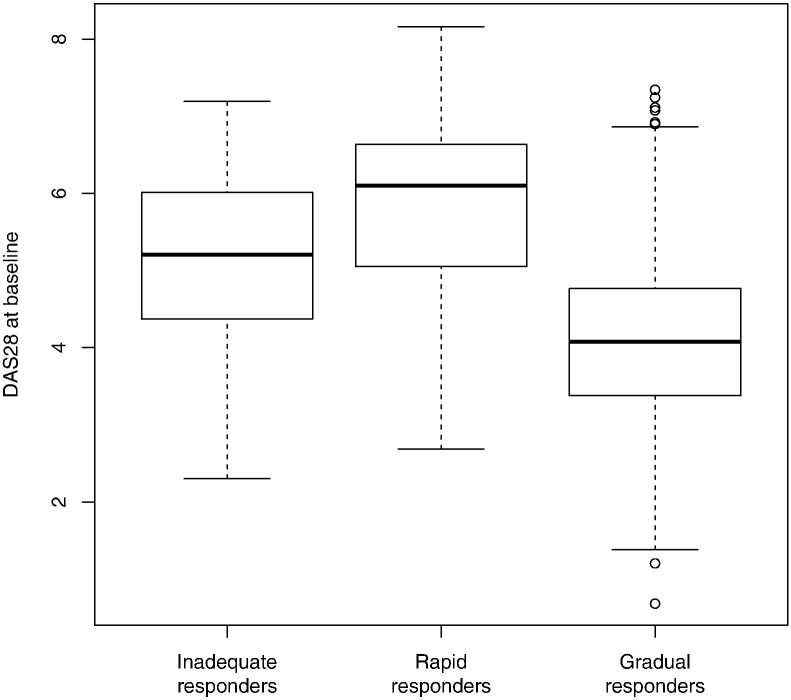
The three types were similar in age, sex, BMI distributions, disease duration, and comorbidities (Table 1). ‘Gradual responders’ group had less disability at baseline (mean HAQ score: GR, 1·1; RR, 1·7; IR, 1·3, p < 0·001), and less previous treatment failures with cDMARDs and bDMARDs. Groups differed in mean DAS28 at baseline (p < 0·001), with ‘gradual responders’ generally presenting lower disease activity at baseline. However, these differences were not the main determinant of group membership since the variability of DAS28 at baseline was large, and there was a large overlap of DAS28 values between groups (Fig. 2). Groups also differed in the components of the DAS28 score (i.e., tender joints, swollen joints, ESR or CRP, and patient global assessment). The sensitivity analysis using probability-weighted regression accounting for uncertainty in classification of patients into three groups found similar results. In particular, significant and non-significant results remained the same.
Table 1.
Characteristics and outcomes of the patients in each of the three trajectories' type.
| ‘Gradual Responders’ | ‘Rapid responders’ | ‘inadequate responders’ | p | |
|---|---|---|---|---|
| Number of patients, n (%) | 3576 (91.7%) | 219 (5.6%) | 103 (2.6%) | |
| Time follow-up, years, mean (SD) | 2.0 (1.7) | 2.2 (2.2) | 2.1 (1.6) | |
| Number of assessments, mean (SD) | 3.9 (3.9) | 5.0 (4.9) | 4.5 (2.4) | |
| Age, years, mean (SD) | 57.6 (13.0) | 56.6 (12.7) | 59.2 (12.6) | 0.22 |
| BMI, mean (SD) | 25.8 (5.2) | 26.1 (5.7) | 28.1 (5.1) | 0.10 |
| Female, n (%) | 2866 (80.1%) | 187 (85.4%) | 85 (82.5%) | 0.14 |
| Ever smoker, n (%) | 582 (17.6%) | 36 (17.2%) | 14 (14.3%) | 0.70 |
| RF, n (%) | 1521 (68.2%) | 87 (64.4%) | 47 (74.6%) | 0.36 |
| ACPA, n (%) | 1165 (57.8%) | 67 (51.5%) | 41 (68.3%) | 0.09 |
| Disease duration, years, mean (SD) | 12.5 (9.9) | 11.9 (9.1) | 12.7 (9.0) | 0.63 |
| HAQ, mean (SD) | 1.1 (0.7) | 1.7 (0.6) | 1.3 (0.8) | < 0.001 |
| Tender joints at baseline, mean (SD) | 4.6 (5.3) | 10.1 (7.1) | 5.2 (6.6) | < 0.001 |
| Swollen joints at baseline, mean (SD) | 3.1 (3.7) | 7.3 (6.2) | 4.4 (4.4) | < 0.001 |
| ESR at baseline, mean (SD) | 23.3 (18.6) | 36.2 (28.1) | 32.1 (22.0) | < 0.001 |
| CRP at baseline, mean (SD) | 9.5 (16.1) | 20.3 (32.7) | 11.7 (16.0) | < 0.001 |
| Patient global assessment, mean (SD) | 47.5 (25.4) | 61.5 (21.2) | 45.0 (28.5) | < 0.001 |
| DAS 28 at baseline, mean (SD) | 4.1 (1.0) | 5.8 (1.1) | 5.1 (1.1) | < 0.001 |
| N. past biologics, median [IQR] | 1 [1; 2] | 2 [1; 3] | 2 [1; 3.5] | < 0.001 |
| N past cDMARD, median [IQR] | 2 [1; 4] | 2.5 [1; 4] | 3 [1; 4] | 0.03 |
| Glucocorticoids at baseline, n (%) | 1773 (66.4%) | 120 (67.4%) | 66 (77.6%) | 0.09 |
| Comorbidities, n (%) | ||||
| Metabolic disease | 47 (1.3%) | 4 (1.8%) | 0 (0.0%) | 0.49 |
| CV disease | 80 (2.2%) | 6 (2.7%) | 2 (1.9%) | 0.86 |
| Infectious disease | 35 (1.0%) | 1 (0.5%) | 0 (0.0%) | 0.69 |
| Cancer | 8 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1.00 |
| ABA drug retentiona, years, median[95% CI] | ||||
| Overall | 2.3 [2.1; 2.5] | 1.6 [1.3; 3.0] | 1.5 [1.4; 2.0] | 0.11 |
| Until ineffectiveness | 4.7 [4.3; 5.1] | 5.3 [2.6; −] | 2.0 [1.7; −] | 0.03 |
| ABA discontinuation, n (%) | 1981 (55.4%) | 135 (61.6%) | 78 (75.7%) | < 0.001 |
| Reasons for discontinuationb, n (%) | 0.17 | |||
| For adverse events | 333 (16.8%) | 12 (8.9%) | 9 (11.5%) | |
| For remission | 19 (1.0%) | 1 (0.7%) | 0 (0.0%) | |
| For other reasons | 483 (24.4%) | 31 (23.0%) | 20 (25.6%) | |
| For ineffectiveness | 1146 (57.8%) | 91 (67.4%) | 49 (62.8%) | |
| EULAR moderate or good response | ||||
| At 6 months, n (%) | 645 (24.3) | 31 (21.2) | 31 (32.9) | 0.11 |
| At 6 months, % Lundex | 21.8 | 16.3 | 32.9 | |
| At 1 year, n (%) | 600 (30.8) | 70 (66.0) | 6 (8.2) | < 0.001 |
| At 1 year, % Lundex | 22.1 | 39.2 | 6.4 | |
| At 2 years, n(%) | 647 (54.9) | 68 (94.4) | 3 (10.0) | < 0.001 |
| At 2 years, % Lundex | 29.8 | 46.1 | 3.9 | |
| Low disease activity (DAS28 < 3.2) | ||||
| At 6 months, N (%) | 1120 (33.4) | 15 (7.3) | 16 (16.5) | < 0.001 |
| At 1 year, N (%) | 1256 (36.5) | 36 (17.1) | 1 (1.0) | < 0.001 |
| At 2 years, N (%) | 1433 (41.3) | 54 (25.7) | 1 (1.0) | < 0.001 |
Estimated using Kaplan-Meier estimation.
Other reasons include discontinuation due to pregnancy, surgery, and missing information on the specific reason for drug discontinuation.
Fig. 2.
Boxplots of DAS28 values at baseline for the three types of trajectories.
ABA overall drug retention time was similar across all groups (p = 0·11). However, as could be expected, ABA drug retention until discontinuation for ineffectiveness was much shorter among ‘inadequate responders’ (median time in years: GR, 4·7, RR: 5·3, IR: 2·0, p = 0·03). The proportion of patients with EULAR good or moderate response rate (Lundex corrected) at 1 year was higher among ‘rapid responders’ (GR: 22·1%, RR: 39·2%, IR: 6·4%).
4. Discussion
Safety and efficacy of ABA in early and established RA has been demonstrated in several studies, using population means or – one could say – a single trajectory (Westhovens et al., 2009, Westhovens et al., 2014, Kremer et al., 2014, Schiff et al., 2011, Schiff et al., 2014). The present study focused on trajectory analyses of disease activity following the initiation of ABA, using growth mixture modeling to identify subgroups with similar response patterns. This study, which is a collaboration of nine national registries, is the first to analyze trajectories of disease activity in patients with established RA. Analysis of the entire sample identified three types of disease activity trajectories: a larger group of ‘gradual responders’ (91·7%), who improved gradually over time; a group of ‘rapid responders’ (5·6%), who started with a high DAS28 at baseline and improved quickly; and a smaller group of ‘inadequate responders’ (2·6%), who had a stable and relatively high disease activity over the first two years. Overall, socio-demographic and clinical characteristics at baseline were not strongly associated with future trajectory of disease activity after ABA treatment initiation. The importance of identifying these trajectories is reflected in the close association between clinical effectiveness and type of disease activity trajectory: The ‘inadequate responders’ discontinued ABA due to ineffectiveness much earlier compared to gradual and rapid responders. Furthermore, EULAR moderate or good response at 1 year was reached by almost none of the “inadequate responders”, compared to more than a third of the ‘rapid responders’.
Similarly to studies that examined disease activity trajectories in early RA (Barnabe et al., 2015, Siemons et al., 2014), we identified a large group of gradual responders and a small group of rapid responders. However, the present analysis also detected one group that displayed no improvement of their disease activity over time. The differences in findings could be due to study population or to the smaller sample size of previous studies. Whereas other studies focused on early RA patients on their first DMARD treatment, our analysis included more treatment resistant patients, often initiating a second or third line treatment, who often had long disease duration. In this difficult to treat patient group, it is not surprising to find inadequate responders, a subgroup probably composed of both primary non-responders and patients with secondary failures to this biologic agent. It is also possible that the smaller sample size and limited follow-up of other studies did not allow the detection of small trajectory subgroups.
In general, the patients in the three trajectory types could not be separated by baseline characteristics, except for higher disease activity and functional disability at baseline among rapid responders. This finding is in line with previous studies of patients with established RA showing that high DAS28 (Narvaez et al., 2016) and high HAQ score at baseline are associated with good response to bDMARD at 3 months (Kristensen et al., 2008). In contrast, studies of patients with early RA (i.e., with less chronicity) described a group of rapid responders with a lower DAS 28 at baseline, and found that patients' trajectory types differed in socio-demographic characteristics (e.g., sex, race, education) (Barnabe et al., 2015). The discrepancies in findings may be explained by differences in study population.
Much research is currently directed at identifying biomarkers to predict response and move towards personalized medicine; however no biomarkers have currently reached a level of discrimination to allow their use in clinical practice. Seropositivity for rheumatoid factor or anti-CCP antibodies has been consistently associated with a better effectiveness of ABA (Gottenberg et al., 2016), but were not associated with a specific disease activity trajectory in this analysis. Clinical effectiveness outcomes strongly differed between trajectories' types, in line with previous studies of disease activity or disability trajectories over time, in which type of trajectories was associated with mortality (Norton et al., 2013), remission (Siemons et al., 2014), or radiographic progression (Barnabe et al., 2015).
A limitation of this study is the observational nature of the data with the potential bias generated from attrition. In addition, unmeasured baseline characteristics, such as socioeconomic factors, may be associated with disease trajectories (Finckh et al., 2015). Another limitation is that DAS28 is a composite score, and the trajectories found in this study may not correspond to trajectories of the underlying scores. The strengths of this study include the large number of patients treated in a real-life setting, resulting from an international collaboration that allowed a pooled analysis of nine RA registries.
In conclusion, after ABA treatment initiation, different types of responders to treatment were identified: gradual, rapid and inadequate response groups, with differing drug discontinuation and response rates. However, clinical information such as seropositivity or disease duration, and baseline characteristics, do not allow to predict reliably the trajectory a patient will follow after ABA initiation. Other predictors of responder types should be explored to support clinical decision making.
Funding
The study is investigator initiated and supported by an unrestricted research grant from Bristol Myers-Squibb. Funders had no role in study design, data collection, data analysis, interpretation and writing.
Author Contribution
DSC did the data analysis, all authors contributed to data interpretation, provided comments on the manuscript writing, and approved the final manuscript.
Appendix 1. Goodness-of-fit statistics for growth mixture modeling
| Model | AIC | BIC | Percentage of total sample |
|||
|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 3 | Type 4 | |||
| M1. 1 class, random intercept | 46,626.4 | 46,664.1 | 100 | |||
| M2. 1 class, random slope | 46,424.0 | 46,467.8 | 100 | |||
| M3. 2 classes, random slope | 46,269.5 | 46,344.7 | 80.2 | 19.8 | ||
| M4. 3 classes, random slope | 46,163.0 | 46,269.6 | 91.7 | 5.6 | 2.6 | |
| M5. 4 classes, random slope | 52,549.8 | 52,713.0 | 82.8 | 11.1 | 4.5 | 1.6 |
For M4, Type 1 (Gradual responders), Type 2 (rapid responders), Type 3 (inadequate responders).
Posterior classification was 0.77% in Type 1, 0.81% in Type 2, and 0.94% in Type 3.
Appendix 2. Estimates of best-fitting growth mixture model
| Coefficient | p | 95% CI | |
|---|---|---|---|
| Gradual responders | |||
| Intercept | 4.06 | < 0.001 | 3.92; 4.20 |
| Time | − 0.69 | < 0.001 | − 0.88; − 0.50 |
| Time2 | 0.28 | < 0.001 | 0.19; 0.37 |
| Time3 | − 0.03 | < 0.001 | − 0.05; − 0.02 |
| Rapid responders | |||
| Intercept | 5.83 | < 0.001 | 5.47; 6.34 |
| Time | − 2.63 | < 0.001 | − 3.32; − 1.94 |
| Time2 | 0.81 | < 0.001 | 0.47; 1.15 |
| Time3 | − 0.08 | < 0.001 | − 0.12; − 0.3 |
| Inadequate responders | |||
| Intercept | 5.14 | < 0.001 | 4,53; 5.75 |
| Time | 3.68 | < 0.001 | 2.46; 4.90 |
| Time2 | − 1.51 | < 0.001 | − 2.09; − 0.93 |
| Time3 | 0.17 | < 0.001 | 0.09; 0.25 |
References
- Aga A.B., Lie E., Uhlig T., Olsen I.C., Wierod A., Kalstad S., Rodevand E., Mikkelsen K., Kvien T.K., Haavardsholm E.A. Time trends in disease activity, response and remission rates in rheumatoid arthritis during the past decade: results from the NOR-DMARD study 2000–2010. Ann. Rheum. Dis. 2015;74:381–388. doi: 10.1136/annrheumdis-2013-204020. [DOI] [PubMed] [Google Scholar]
- Barnabe C., Sun Y., Boire G., Hitchon C.A., Haraoui B., Thorne J.C., Tin D., van der Heijde D., Curtis J.R., Jamal S., Pope J.E., Keystone E.C., Bartlett S., Bykerk V.P. Heterogeneous disease trajectories explain variable radiographic, function and quality of life outcomes in the Canadian Early Arthritis Cohort (CATCH) PLoS One. 2015;10 doi: 10.1371/journal.pone.0135327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe B., Logeart I., Belkacemi M.C., Dadoun S., Schaeverbeke T., Daures J.P., Dougados M. Comparison of the long-term outcome for patients with rheumatoid arthritis with persistent moderate disease activity or disease remission during the first year after diagnosis: data from the ESPOIR cohort. Ann. Rheum. Dis. 2015;74:724–729. doi: 10.1136/annrheumdis-2013-204178. [DOI] [PubMed] [Google Scholar]
- Curtis J.R., Jain A., Askling J., Bridges S.L., Jr., Carmona L., Dixon W., Finckh A., Hyrich K., Greenberg J.D., Kremer J., Listing J., Michaud K., Mikuls T., Shadick N., Solomon D.H., Weinblatt M.E., Wolfe F., Zink A. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin. Arthritis Rheum. 2010;40:2–14.e1. doi: 10.1016/j.semarthrit.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmelmaier I., Dufour A.B., Nordgren B., Opava C.H. Trajectories of physical activity over two years in persons with rheumatoid arthritis. Arthritis Care Res. 2016 doi: 10.1002/acr.22799. [DOI] [PubMed] [Google Scholar]
- Finckh A., Ciurea A., Brulhart L., Kyburz D., Moller B., Dehler S., Revaz S., Dudler J., Gabay C. B cell depletion may be more effective than switching to an alternative anti-tumor necrosis factor agent in rheumatoid arthritis patients with inadequate response to anti-tumor necrosis factor agents. Arthritis Rheum. 2007;56:1417–1423. doi: 10.1002/art.22520. [DOI] [PubMed] [Google Scholar]
- Finckh A., Neto D., Iannone F., Loza E., Lie E., van Riel P., Hetland M.L., Pavelka K., Gottenberg J.E., Canhao H., Mariette X., Turesson C. The impact of patient heterogeneity and socioeconomic factors on abatacept retention in rheumatoid arthritis across nine European countries. RMD Open. 2015;1 doi: 10.1136/rmdopen-2014-000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C., Riek M., Scherer A., Finckh A. Effectiveness of biologic DMARDs in monotherapy versus in combination with synthetic DMARDs in rheumatoid arthritis: data from the Swiss clinical quality management registry. Rheumatology (Oxford) 2015;54:1664–1672. doi: 10.1093/rheumatology/kev019. [DOI] [PubMed] [Google Scholar]
- Gottenberg J.E., Courvoisier D.S., Hernandez M.V., Iannone F., Lie E., Canhao H., Pavelka K., Hetland M.L., Turesson C., Mariette X., Finckh A. Rheumatoid factor and anti-citrullinated protein antibody positivity are associated with a better effectiveness of abatacept: results from the Pan-European registry analysis. Arthritis Rheum. 2016 doi: 10.1002/art.39595. [DOI] [PubMed] [Google Scholar]
- Inoue E., Yamanaka H., Hara M., Tomatsu T., Kamatani N. Comparison of Disease Activity Score (DAS)28 - erythrocyte sedimentation rate and DAS28 - C-reactive protein threshold values. Ann. Rheum. Dis. 2007;66:407–409. doi: 10.1136/ard.2006.054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely P.D. Biologic efficacy optimization-a step towards personalized medicine. Rheumatology (Oxford) 2015 doi: 10.1093/rheumatology/kev356. [DOI] [PubMed] [Google Scholar]
- Kremer J.M., Peterfy C., Russell A.S., Emery P., Abud-Mendoza C., Sibilia J., Becker J.C., Westhovens R., Genant H.K. Longterm safety, efficacy, and inhibition of structural damage progression over 5 years of treatment with abatacept in patients with rheumatoid arthritis in the abatacept in inadequate responders to methotrexate trial. J. Rheumatol. 2014;41:1077–1087. doi: 10.3899/jrheum.130263. [DOI] [PubMed] [Google Scholar]
- Kristensen L.E., Saxne T., Geborek P. The LUNDEX, a new index of drug efficacy in clinical practice: results of a five-year observational study of treatment with infliximab and etanercept among rheumatoid arthritis patients in southern Sweden. Arthritis Rheum. 2006;54:600–606. doi: 10.1002/art.21570. [DOI] [PubMed] [Google Scholar]
- Kristensen L.E., Kapetanovic M.C., Gulfe A., Soderlin M., Saxne T., Geborek P. Predictors of response to anti-TNF therapy according to ACR and EULAR criteria in patients with established RA: results from the south Swedish Arthritis Treatment Group Register. Rheumatology (Oxford) 2008;47:495–499. doi: 10.1093/rheumatology/ken002. [DOI] [PubMed] [Google Scholar]
- Littlejohn G., Roberts L., Bird P., De Jager J., Griffiths H., Nicholls D., Young J., Zochling J., Tymms K.E. Patients with rheumatoid arthritis in the Australian OPAL cohort show significant improvement in disease activity over 5 years: a multicenter observational study. J. Rheumatol. 2015;42:1603–1609. doi: 10.3899/jrheum.141575. [DOI] [PubMed] [Google Scholar]
- Mohammed R.H., Farahat F., Kewan H.H., Bukhari M.A. Predictors of European League Against Rheumatism (EULAR) good response, DAS-28 remission and sustained responses to TNF-inhibitors in rheumatoid arthritis: a prospective study in refractory disease. Springerplus. 2015;4:207. doi: 10.1186/s40064-015-0979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez J., Magallares B., Diaz Torne C., Hernandez M.V., Reina D., Corominas H., Sanmarti R.J.M.L.L., Rodriguez De La Serna A., Nolla J.M. Predictive factors for induction of remission in patients with active rheumatoid arthritis treated with tocilizumab in clinical practice. Semin. Arthritis Rheum. 2016;45:386–390. doi: 10.1016/j.semarthrit.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Norton S., Sacker A., Young A., Done J. Distinct psychological distress trajectories in rheumatoid arthritis: findings from an inception cohort. J. Psychosom. Res. 2011;71:290–295. doi: 10.1016/j.jpsychores.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Norton S., Sacker A., Dixey J., Done J., Williams P., Young A. Trajectories of functional limitation in early rheumatoid arthritis and their association with mortality. Rheumatology (Oxford) 2013;52:2016–2024. doi: 10.1093/rheumatology/ket253. [DOI] [PubMed] [Google Scholar]
- Prevoo M.L., Van't Hof M.A., Kuper H.H., van Leeuwen M.A., van de Putte L.B., van Riel P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- Proust-Lima C., Philipps V., Liquet B. 2015. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package Lcmm. [Google Scholar]
- Reineke J., Seddig D. Growth mixture models in longitudinal research. Adv. Stat. Anal. 2011;95:415–434. [Google Scholar]
- Sanmarti R., Garcia-Rodriguez S., Alvaro-Gracia J.M., Andreu J.L., Balsa A., Caliz R., Fernandez-Nebro A., Ferraz-Amaro I., Gomez-Reino J.J., Gonzalez-Alvaro I., Martin-Mola E., Martinez-Taboada V.M., Ortiz A.M., Tornero J., Marsal S., Moreno-Muelas J.V. 2014 update of the consensus statement of the Spanish Society of Rheumatology on the use of biological therapies in rheumatoid arthritis. Reumatol. Clin. 2015;11:279–294. doi: 10.1016/j.reuma.2015.05.001. [DOI] [PubMed] [Google Scholar]
- Schiff M., Keiserman M., Codding C., Songcharoen S., Berman A., Nayiager S., Saldate C., Aranda R., Becker J.C., Nys M., Le Bars M., Reed D.M., Poncet C., Dougados M. Clinical response and tolerability to abatacept in patients with rheumatoid arthritis previously treated with infliximab or abatacept: open-label extension of the ATTEST study. Ann. Rheum. Dis. 2011;70:2003–2007. doi: 10.1136/annrheumdis-2011-200316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M., Weinblatt M.E., Valente R., van der Heijde D., Citera G., Elegbe A., Maldonado M., Fleischmann R. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann. Rheum. Dis. 2014;73:86–94. doi: 10.1136/annrheumdis-2013-203843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemons L., Ten Klooster P.M., Vonkeman H.E., Glas C.A., van de Laar M. Distinct trajectories of disease activity over the first year in early rheumatoid arthritis patients following a treat-to-target strategy. Arthritis Care Res. 2014;66:625–630. doi: 10.1002/acr.22175. [DOI] [PubMed] [Google Scholar]
- van Riel P.L. The development of the disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) Clin. Exp. Rheumatol. 2014;32:S-65-74. [PubMed] [Google Scholar]
- Westhovens R., Robles M., Ximenes A.C., Nayiager S., Wollenhaupt J., Durez P., Gomez-Reino J., Grassi W., Haraoui B., Shergy W., Park S.H., Genant H., Peterfy C., Becker J.C., Covucci A., Helfrick R., Bathon J. Clinical efficacy and safety of abatacept in methotrexate-naive patients with early rheumatoid arthritis and poor prognostic factors. Ann. Rheum. Dis. 2009;68:1870–1877. doi: 10.1136/ard.2008.101121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhovens R., Kremer J.M., Emery P., Russell A.S., Alten R., Barre E., Dougados M. Long-term safety and efficacy of abatacept in patients with rheumatoid arthritis and an inadequate response to methotrexate: a 7-year extended study. Clin. Exp. Rheumatol. 2014;32:553–562. [PubMed] [Google Scholar]