Abstract
Measles virus (MV) infects dendritic cells (DCs) resulting in immunosuppression. Human DCs express two MV receptors: CD46 and human signaling lymphocyte activation molecule (hSLAM); thus, the role played by either alone is unclear. Because wild-type (wt) MV uses hSLAM receptor preferentially, we dissected the molecular basis of MV–DC interaction and resultant immunosuppression through the hSLAM receptor by creating transgenic (tg) mice expressing hSLAM on DCs. After infection with wt MV, murine splenic DCs expressing hSLAM receptor had less B7-1, B7-2, CD40, MHC class I, and MHC class II molecules on their surfaces and displayed an increased rate of apoptosis when compared to uninfected DCs. Further, MV-infected DCs failed to stimulate allogeneic T cells and inhibited mitogen-dependent T-cell proliferation. Individual expression of human SLAM, interferon α/β receptor, tumor necrosis factor-α, and lymphotoxin-α or β from T cells was not required for MV-infected DCs to inhibit the proliferation of T cells.
Keywords: Measles virus, Dendritic cells, Transgenic mice, Immunosuppression, SLAM, Lymphocyte proliferation
Introduction
Measles virus (MV) is a contagious human pathogen that continues to infect 30–40 million humans annually leading to approximately 1 million deaths despite the availability of an effective attenuated vaccine (The World Health Report, 2002). MV infects cells of the immune system and induces a transient but profound suppression of immune responses, which is the major cause of related fatalities (Griffin, 1995; McChesney and Oldstone, 1989). How MV impairs immunity has been extensively studied and likely involves multiple mechanisms. T lymphocytes are permissive to MV infection (Hyypia et al., 1985; Joseph et al., 1975; Sullivan et al., 1975) leading subsequently to an inhibition of their proliferative responses to mitogens, alloantigens, and recalled antigens (McChesney et al., 1988; Naniche et al., 1999; Sun et al., 1998). When MV infects antigen-presenting cells (APC) such as dendritic cells (DCs), the outcome is believed to be modulation of cross-talk between T cells and APC, thereby aborting the induction of adaptive immune responses (Schneider-Schaulies et al., 2003; Servet-Delprat et al., 2003). Human DCs obtained from cultures of blood CD34+ cells, monocytes, and Langerhans cells are susceptible to MV infection in vitro (Dubois et al., 2001; Fugier-Vivier et al., 1997; Grosjean et al., 1997; Schnorr et al., 1997; Servet-Delprat et al., 2000a, 2000b). Such infection enhances apoptosis of DCs and inhibits CD40 ligand-dependent terminal differentiation of DCs. Additionally, cocultivation of MV-infected DCs with T cells increases Fas-mediated DC apoptosis as well as virus production. MV-infected DCs also upregulate their expression of TRAIL (Vidalain et al., 2000) and inhibit proliferation of naive and activated T cells.
Human DCs express the two known receptors for MV: CD46 and human signaling lymphocyte activation molecule (hSLAM) (Dorig et al., 1993; Erlenhoefer et al., 2001; Hsu et al., 2001; Manchester et al., 1994; Naniche et al., 1993; Tatsuo et al., 2000). Human complement regulatory protein (CD46) is constitutively expressed on all nucleated cells (McQuaid and Cosby, 2002), and transgenic (tg) mice expressing the human CD46 receptor have been generated and widely used to investigate the interaction of MV with neurons and cells of the immune system (Horvat et al., 1996; Lawrence et al., 1999; Oldstone et al., 1999; Patterson et al., 2002; Rall et al., 1997; Slifka et al., 2003; Yannoutsos et al., 1996). However, wild-type (wt) MV or MV passaged on B95-8 cells does not use the CD46 receptor for entry into cells as efficiently as MV vaccine strains do. That is, the interaction of wt MV with CD46 is weaker than its interaction with hSLAM (Erlenhofer et al., 2002; Manchester et al., 2000; Ono et al., 2001a; Schneider et al., 2002). SLAM is a prototypic receptor belonging to a SLAM subfamily within the CD2 immunoglobulin superfamily and facilitates modulation of lymphocyte proliferation and cytokine production (Cocks et al., 1995; Veillette and Latour, 2003). SLAM family receptors mediate intracellular protein tyrosine phosphorylation signals dependent on the binding affinity to SLAM-associated protein (SAP) or EAT-2 (Chan et al., 2003; Engel et al., 2003). The expression of SLAM is restricted to immune-system cells such as immature thymocytes, memory T cells, activated T cells, B cells, monocytes, and DCs (Cocks et al., 1995; McQuaid and Cosby, 2002; Minagawa et al., 2001; Murabayashi et al., 2002; Punnonen et al., 1997; Sidorenko and Clark, 1993). MV binds to the V domain of hSLAM but does not bind to the mouse counterpart indicating that murine SLAM does not serve as a cellular receptor for MV (Ohno et al., 2003; Ono et al., 2001b).
Despite numerous studies performed in vitro, the mechanism of immunosuppression caused by MV infection of human DCs remains obscure primarily because DCs simultaneously express the two MV receptors CD46 and SLAM, whose activities are, so far, inseparable. To address this issue and focus directly on MV interaction with SLAM on DCs, we created tg mice whose DCs expressed hSLAM by using the CD11c promoter. We report here that CD11c+ DCs from tg mice expressed hSLAM protein and were permissive to MV, whereas DCs from non-tg mice had neither property. With this model, we showed that DCs infected with MV via hSLAM were deficient in stimulating allogeneic T cells in a mixed lymphocyte reaction (MLR) and causing naive T cells to proliferate in the presence of mitogens. These events were associated with MV-induced downregulation of MHC class I and II and B7-1, B7-2, and CD40 co-activation molecules on DCs, as well as enhanced apoptosis of DCs.
Results
Generation of tg mice expressing hSLAM on DCs
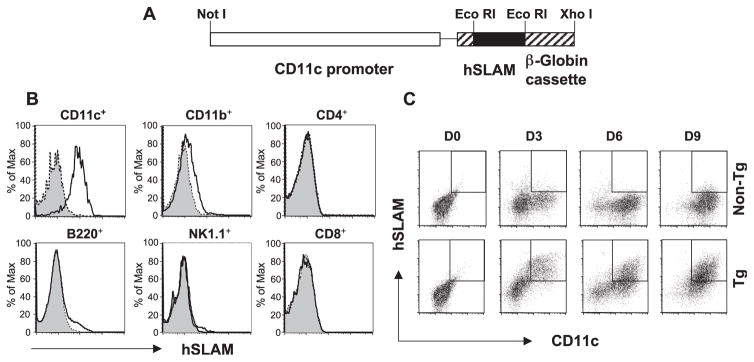
To express hSLAM on murine DCs, we created tg mice with hSLAM transcriptionally regulated by the CD11c promoter (Fig. 1A). Single cell suspensions from spleens of CD11c-hSLAM tg and non-tg mice were analyzed by flow cytometry for hSLAM protein expression on diverse immune cells. As shown in Fig. 1B, hSLAM protein was expressed on the surface of splenic CD11c+ DCs, but not on T cells (CD4+ and CD8+), B cells (B220+), or NK cells (NK1.1+). A minimal level of hSLAM expression was detected on CD11c−CD11b+ macrophages. Because bone marrow BM cells contain DC progenitors and have been widely used to generate CD11c+ DCs in the presence of the cytokine GM-CSF, we generated BM-derived CD11c+ DCs from hSLAM tg mice and investigated for their expression of hSLAM protein. Over time, as shown in Fig. 1C, BM cells in response to GM-CSF yielded equivalent numbers of CD11c+ DCs in hSLAM tg as compared to non-tg mice. However, in BM-derived CD11c+ DCs from tg mice, amounts of hSLAM protein increased over time (both CD11c+ and hSLAM+ cells: d3: 23%, d6: 34%, d9: 52%) whereas CD11c+ cells from non-tg mice did not (Fig. 1C). These data indicate that hSLAM protein was expressed on the surfaces of CD11c+ DCs obtained from splenocytes and precursor BM stem cells.
Fig. 1.
Tg construct of CD11c-hSLAM fusion gene and analysis of hSLAM protein expression. Panel (A) represents the fusion gene construct used to generate CD11c-hSLAM tg mice. Briefly, hSLAM cDNA (black box) was introduced into an EcoRI-treated pCD11c vector, between the CD11c promoter (open box) and β-Globin cassette (striped box). (B) hSLAM expression on several cell types from tg mice. Splenocytes were stained with antibody to mouse CD11c, CD11b, CD4, CD8, B220, or NK1.1 molecules combined with an antibody to hSLAM. Histograms represent the expression of hSLAM on specific cells from tg mice (open histogram) or non-tg mice (filled histogram). Panel (C) illustrates the generation of CD11c+ hSLAM+ cells from BM cultures in the presence of GM-CSF. At day 0, 3, 6, and 9 after culturing BM cells from non-tg (upper panels) or tg mice (lower panels), CD11c+ hSLAM+ cells are shown as an open box in each graph.
MV infects and alters DCs from CD11c-hSLAM tg mice
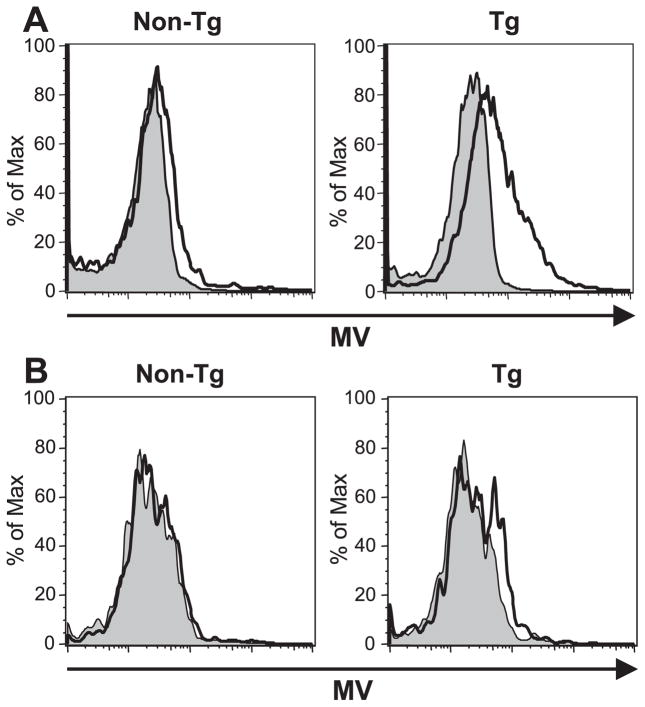
To determine whether hSLAM expression on mouse CD11c+ DCs rendered them susceptible to MV infection, DCs were enriched from splenocytes of tg or non-tg mice and then infected in vitro with wt JW strain of MVat 0.3 MOI. At 2 dpi, the expression of viral proteins on CD11c+ DCs was assessed by flow cytometry (Hahm et al., 2003). About 35% of CD11c+ DCs from CD11c-hSLAM tg mice were infected with MV and expressed viral proteins on their surfaces. In contrast, fewer than 6% of CD11c+ DCs from non-tg mice scored positive for MV proteins after incubation with MV (Fig. 2A). The low level of expression of MV proteins on DCs from non-hSLAM tg mice incubated with MV likely resulted from either nonreceptor-specific binding of MV to mouse DCs or from the intrinsic phagocytic ability of DCs, because nonreplicating, UV-inactivated MV induced an equivalent level of viral glycoprotein expression on similar numbers (5%) of DCs (unpublished data). No significant difference in MV infectivity was observed for other cell types of CD4+ and CD8+ T cells, B cells, and CD11b+ cells between tg and non-tg mice inoculated with MV. In addition, infectious viruses were produced from in vitro infected DCs of tg mice as detected by cocultivation assay when MV-infected DCs cocultured with B95-8 cells and cytopathic effects and syncytia formation observed on B95-8 cells (unpublished data). When tg and non-tg mice were infected intravenously with MV in vivo, 2–5% of splenic DCs from tg mice expressed viral proteins, although <1% of DCs from non-tg mice were positive for MV proteins (Fig. 2B). In vivo, MV infectivity was consistently observed in several independent experiments. However, because 2–5% of CD11c+ DCs were infected in vivo compared to >35% of CD11c+ DCs in vitro, we focused our next series of experiments on the in vitro infection model.
Fig. 2.
MV infection of CD11c+ DCs from CD11c-hSLAM tg mice in vitro and in vivo. (A) In vitro MV infection of CD11c+ DCs from CD11c-hSLAM tg mice. DCs enriched from splenocytes of non-tg or tg mice were either mock-infected (filled histogram) or infected with MV-JWB (open histogram) at 0.3 MOI. Two dpi, the expression of MV glycoproteins on CD11c+ DCs, was analyzed by flow cytometry. (B) In vivo infection of CD11c-hSLAM tg mice. Adult animals of both tg mice and non-tg mice were mock-infected (filled histogram) or injected intravenously with MV-JWB (open histogram) at 2 × 106 TCID50. Four dpi, splenic CD11c+ DCs were analyzed for expression of MV proteins. Data shown represent three independent experiments.
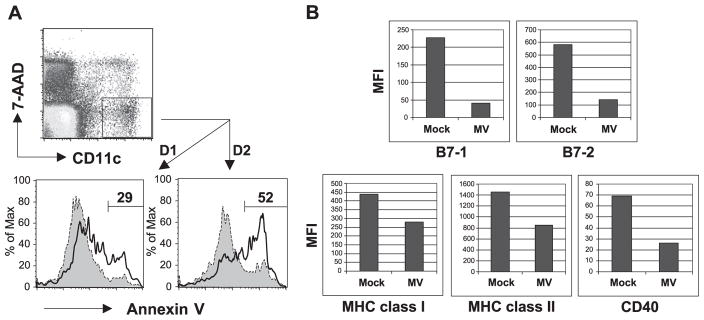
Human DCs undergo apoptosis during MV infection (Fugier-Vivier et al., 1997; Servet-Delprat et al., 2000a). However, because human DCs express both CD46 and also SLAM receptor, it is not known if both or only one of these receptors is responsible. Therefore, MV-infected splenocytes from tg mice were stained for CD11c, Annexin V as an early marker of apoptosis, and 7-AAD to exclude dead cells, and analyzed hereafter by flow cytometry. As shown in Fig. 3A, 29% of splenic CD11c+ DCs from tg mice were undergoing apoptosis within 1 day after MV infection, whereas only 9% of mock-infected control cells were Annexin V-positive. By 2 dpi, 52% of MV-incubated splenic DCs scored positive for apoptosis but only 18% of mock-infected cells were positive. BM-derived CD11c+ DCs infected with MV also showed enhanced rate of apoptosis (10–15% above the mock-infected BM-DCs) (unpublished data). Thus, hSLAM expression alone on mouse DCs rendered them permissive to MV infection and resulted in programmed cell death.
Fig. 3.
Alteration of DCs from CD11c-hSLAM tg mice by MV infection. (A) MV infection induces apoptosis of splenic DCs. Splenocytes were mock-infected (filled histogram) or MV-infected (open histogram) and stained with Annexin V and 7-AAD at 1 and 2 dpi. Living DCs (CD11c+ 7-AAD− cells) were analyzed for Annexin V positivity. Apoptotic cells are represented in each graph. (B) MV infection downregulated expression of DC markers on splenic DCs. Splenocytes from hSLAM tg mice were mock-infected or MV-infected. At 2 dpi, CD11c+ DCs were stained with anti-CD40, anti-B7-1, anti-B7-2, anti-MHC class I, or anti-MHC class II antibodies. The geometric mean fluorescence intensity (MFI) for each marker is depicted on graphs. Data shown represent three independent experiments.
We next examined the expression of several important DC surface proteins on splenic CD11c+ DCs after MV infection. Costimulatory molecules B7-1, B7-2, and CD40 and MHC class I and II proteins on the surfaces of CD11c+ DCs were all downregulated following MV infection (Fig. 3B). When non-tg DCs were treated with MV, the expression of costimulatory molecules and MHC molecules on their surfaces hardly changed compared to their expression on mock-infected non-tg DCs (unpublished data). Further, MV infection of tg DCs as detected by MV protein expression closely correlated with the reduction in surface expression of these molecules (unpublished data). Because these proteins are known to play a decisive role in DC-induced generation of adaptive T-cell responses, we next evaluated the ability of MV-infected DCs to activate and expand naive T cells.
MV-infected DCs inhibit syngeneic and allogeneic T-cell proliferation
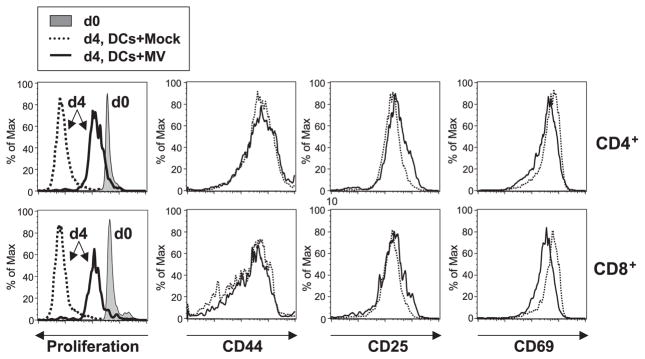
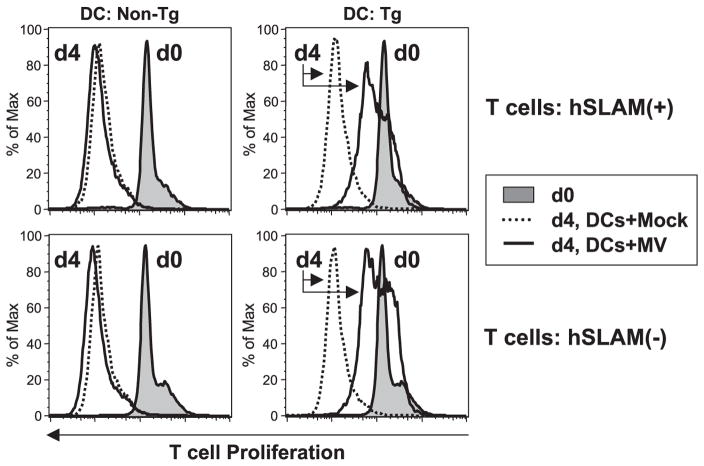
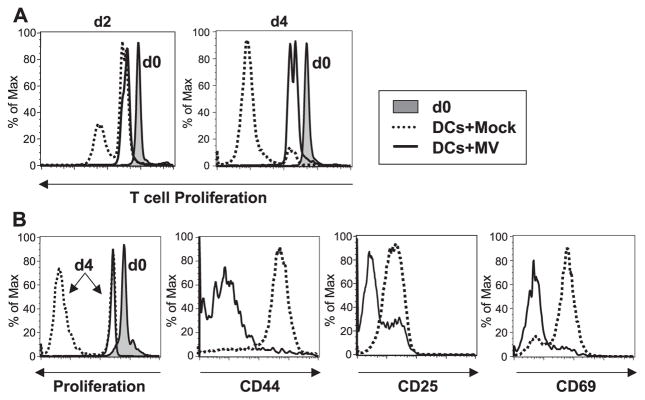
DCs expressing hSLAM were either mock-infected or MV-infected and, at 3 dpi, were UV-irradiated to block trans-infection of T cells. Then, CFSE-labeled syngeneic T lymphocytes from mice engineered to express hSLAM only on those cells through use of an Lck promoter (Hahm et al., 2003) were added to the culture along with PMA and ionomycin. In response to these mitogens, CD4+ and CD8+ T lymphocytes proliferated normally when incubated with either mock-infected DCs from CD11c-hSLAM tg mice (Fig. 4) or DCs from non-tg mice inoculated with MV (Fig. 6). In contrast, MV infected, hSLAM+ CD11c+ DCs from tg mice greatly inhibited T-cell proliferation (Fig. 4). Although T cells exhibited significantly reduced proliferation in response to mitogens in the presence of MV-infected CD11c+ DCs, the phenotypic expression of CD44, CD25, and CD69 was not blocked.
Fig. 4.
Inhibition of mitogen-dependent T-cell proliferation by MV-infected DCs from CD11c-hSLAM tg mice. CD11c+ DCs derived from tg BM cells cultured for 10 days with GM-CSF were mock-infected (dotted line) or infected with MV-JWB (solid line). Three dpi, they were UV-irradiated and then mixed with CFSE-labeled lymphocytes in the presence of PMA and ionomycin. The CD4+ and CD8+ T cells were analyzed for proliferation and the expression of CD44, CD25, and CD69 by flow cytometry. Left histograms show the baseline CFSE-labeled cells at day 0 (d0, filled histogram) and proliferation at day 4 (d4, solid line: T cells with MV-infected DCs, or dotted line: T cells with mock-infected DCs).
Fig. 6.
hSLAM expression on T cells is not required for the inhibition of T-cell proliferation by MV-infected DCs. BM-derived DCs from CD11c-hSLAM tg mice or non-tg mice were mock-infected or MV-infected. They were cocultured with CFSE-stained T cells from Lck-hSLAM tg mice or non-tg mice under mitogenic stimulus. Filled histogram is basal level of CFSE at day 0 of coculture. Dotted lines and solid lines represent the proliferation of CD4+ T cells at day 4 with a culture of mock-infected DCs and MV-infected DCs, respectively.
Now we addressed the question whether MV-infected hSLAM+ CD11c+ DCs could generate an MLR. As expected, in coculture of mock-infected hSLAM+ CD11c+ DCs from FVB/N mice (H-2q) and naive T cells from C57BL/6 (H-2b), T cells rapidly proliferated at 2 and 4 days. In contrast, MV-infected hSLAM+ CD11c+ DCs lost their ability to stimulate allogeneic T cells (Fig. 5A). Analysis of CD8+ T cells showed that they were not or were poorly activated, as judged by their low expression levels of CD44, CD25, and CD69 molecules (Fig. 5B), and by their inability to proliferate.
Fig. 5.
Impairment of allostimulatory capacity of DCs expressing hSLAM by MV infection. Panel (A) shows the inhibition of allogeneic T-cell proliferation by MV infection of DCs in a MLR. BM-derived DCs from CD11c-hSLAM tg mice of FVB/N (H-2q) strain were mock-infected or MV-infected. Three dpi, they were cocultured with CFSE-labeled T lymphocytes from C57BL/6 (H-2b). Histograms show the baseline CFSE-labeled at day 0 (d0; filled histogram) and proliferation at days 2 or 4. Dotted lines represent the proliferation of CD8+ T cells primed by mock-infected DCs, and solid lines show their impaired proliferation when in contact with MV-infected DCs. (B) Inhibition of T-cell activation by MV-infected DCs in an allogeneic MLR. At day 4 after coculture of T cells with mock-infected DCs (dotted line) or with MV-infected DCs (solid line), the level of CFSE fluorescence and CD44, CD25, and CD69 expression on the CD8+ T cells was assessed by flow cytometry.
No role for hSLAM, IFN-α/β-R, TNF-α, LT-α, and LT-β from T cells in the MV-induced CD11c+ DC-mediated inhibition of T-cell proliferation
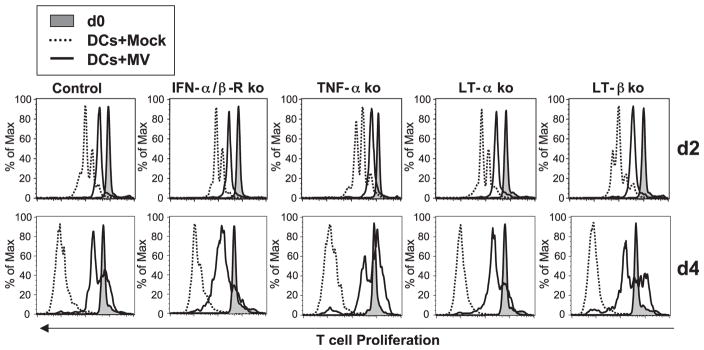
Lastly, we evaluated whether hSLAM, IFN-α/β-R, TNF-α, LT-α, and LT-β from T cells played a role in DC-mediated inhibition of T-cell proliferation subsequent to MV infection because these molecules are thought involved in lymphocyte proliferation or survival. First, to test the possibility that MV glycoproteins interacted with hSLAM on T cells thereby sending negative signal to T cells that inhibited T-cell proliferation, we mixed various combinations of either hSLAM+ CD11c+ DCs or hSLAM−CD11c+ DCs with or without MV and added either hSLAM+ or hSLAM− T cells. As anticipated, mock-infected or MV-incubated hSLAM− DCs did not inhibit the proliferation of hSLAM(+ or −) T cells because DCs were not infected (Fig. 6). In contrast, hSLAM+ DCs infected with MV significantly impaired proliferation of T lymphocytes either devoid of or bearing hSLAM (Fig. 6). Therefore, the expression of hSLAM on T cells was not necessary for the inhibition of T-cell proliferation induced by MV-infected DCs, including those from genetically different knock-out (ko) mice that did not express hSLAM on their T cells. The last series of experiments showed that T cells genetically deficient for IFN-α/β-R, TNF-α, LT-α, or LT-β did not proliferate in response to mitogens in the presence of MV-infected CD11c+ DCs (Fig. 7), indicating that none of these T-cell molecules played an individual role in MV-induced CD11c+ DC-mediated inhibition of T-cell proliferation.
Fig. 7.
IFN-α/β-R, TNF-α, LT-α, or LT-β from T cells are not essential components for the inhibition of T-cell proliferation by MV-infected DCs. BM-derived DCs from CD11c-hSLAM tg mice were mock-infected or MV-infected as in Fig. 6. CFSE-stained T cells from ko mice deficient in IFN-α/β-R, TNF-α, LT-α, or LT-β were cocultured with mock-infected DCs (dotted line) or MV-infected DCs (solid line) with mitogenic stimulus. The proliferation of CD4+ T cells was traced and shown at days 0 (filled histogram), 2, and 4.
Discussion
We report here that murine DCs expressing hSLAM were obtained by generating tg mice expressing hSLAM, an MV receptor, and that these DCs were now permissive to wt MV infection. CD11c+ DCs isolated from the spleen and BM of these tg mice expressed hSLAM and, when infected with MV, lost the ability to function normally. Such impaired MV-infected DCs did not support T-cell proliferation.
Transgenic mice expressed the hSLAM protein under control of a CD11c promoter, and the presence of this protein on DCs conferred susceptibility to MV infection. Human DCs express SLAM and CD46 and both molecules act as receptors for MV. MV infection was previously shown to induce maturation of human immature DCs derived from blood CD34+ cells and monocytes (Schnorr et al., 1997; Servet-Delprat et al., 2000b). It is likely that phenotypic maturation of DCs helps immature DCs to transport MV from its primary MV infection area of infection to a secondary site, a lymphoid organ, where T lymphocytes reside. However, MV-infected mouse mature DCs in spleen were highly susceptible to apoptosis and downregulated B7-1, B7-2, CD40, MHC class I, and MHC class II molecules (Fig. 3), implying that MV-infected mature DCs do not send danger signals to T cells. Rather, MV might exploit the DC network for travel to secondary lymphoid organs by infecting immature DCs that are induced to mature then migrate to these organs. In such organs, subsequently DCs loaded with MV proteins inhibit stimulation of naive T cells. Therefore, MV can likely amplify itself in lymphocytes without being counteracted by newly acquired anti-MV immune responses (Hyypia et al., 1985; Joseph et al., 1975; McChesney et al., 1988; Sullivan et al., 1975).
Failure to respond to recalled antigenic, mitogenic, and allogeneic stimulation of T cells is a characteristic of MV infection. Similarly, we observed here inhibition of T-cell proliferation using MV-infected mouse CD11c+ DCs bearing hSLAM receptor (Figs. 4 and 5). Without direct viral infection of T cells, mitogen-dependent T-cell proliferation was significantly inhibited by MV-infected DCs, which might have occurred by contact of viral proteins from MV-infected DCs with T cells (Dubois et al., 2001; Klagge et al., 2000; Laine et al., 2003; Marie et al., 2001) or by yet undiscovered soluble factors secreted by the cocultures (Sun et al., 1998). Interestingly, the expression of T-cell activation markers (CD25, CD69, and CD44) was not impaired by MV-infected DCs when mitogenic stimulus was given for T-cell activation to directly trigger protein kinase C signaling pathway (Fig. 4). However, when the stimulus was allogeneic, these molecules were poorly expressed on T cells (Fig. 5), indicating MHC class II-dependent allostimulatory capacity of DCs was severely impaired by MV infection. In both cases, MV-infected DCs strongly inhibited T-cell proliferation regardless of the surface expression level of T-cell activation markers. On studying the defect in proliferation, we found that individual expression of hSLAM, type I IFN receptor, TNF-α, LT-α, or LT-β from T cells was not essential for MV-induced CD11c+ DC-mediated inhibition of T-cell proliferation (Figs. 6 and 7).
Although hSLAM expression on T cells was not required for MV-infected DC-mediated inhibition of T-cell proliferation, MV bound directly to hSLAM; therefore, this protein may alter normal immune system function. SLAM has been identified as the cellular target protein for several viruses (Sidorenko and Clark, 2003). For example, Morvilliviruses such as rinderpest virus, canine distemper virus, as well as MV use SLAM as their receptor (Tatsuo et al., 2001), while Molluscum contagiosum virus encodes proteins that are homologous to SLAM (Bugert et al., 2000). Moreover, individuals infected with HIV display altered expression of SLAM on their T cells (Meroni et al., 1999). These observations, in concert, imply that SLAM may play an important role in regulating immune responses to pathogens and is thus chosen by many viruses to subordinate the hosts protective immune arsenal. Because MV downregulates hSLAM on lymphocytes (Hahm et al., 2003; Tanaka et al., 2002) and SLAM associates with SAP and Fyn tyrosine kinase (Chan et al., 2003; Latour et al., 2003), the effect of MV infection on SLAM-mediated signaling and immune modulation promises to be an area of interest and importance. For this analysis, the tg mice expressing hSLAM receptor separately but specifically on T cells and on DCs should be useful tools. Both human and mouse SLAM possess 100% identity of three tyrosine-based motifs within their cytoplasmic region (Wang et al., 2001). This mediates SLAM-SAP binding. Therefore, MV binding to human SLAM may likely affect intracellular SLAM-SAP signaling pathway in mouse cells.
In summary, our findings show that MV infection of DCs restricted through the SLAM receptor disrupts DC function by a multifacet strategy. First, the virus infects CD11c+ DCs and inhibits their terminal maturation by disordering MHC and costimulatory molecules necessary for antigen presentation. Second, MV infection renders DCs susceptible to apoptosis, which restricts quantitatively the number of DCs capable of generating adaptive immune responses. The events surrounding MV infection are likely to shed knowledge not only on how MV induces immunosuppression but may be mirrored, in part, by a variety of other viruses such as HIV (Knight and Patterson, 1997), lymphocytic choriomeningitis virus (Sevilla et al., 2003), cytomegalovirus (Raftery et al., 2001), herpes simplex virus (Kruse et al., 2000), dengue virus (Wu et al., 2000), and other infectious agents who have also developed strategies to avert or usurp the function of DCs for their benefit.
Materials and methods
Construction of expression plasmid
The hSLAM cDNA was PCR-amplified by using pCAG-SLAM, which was provided by Yusuke Yanagi (Kyushu University), as a template in combination with the following primers: 5′-CGGAATTCACCATGGATCCCAAGGGGCTCCT-3′ and 5′-CGGAATTCCCTTCAGAAAGTCCCTTTGTTGG-3′. To construct pCD11c-hSLAM, amplified hSLAM cDNA was digested with EcoRI and then cloned into an EcoRI-treated pCD11c vector, which was obtained from Dan R. Littman (New York University Medical Center). The pCD11c vector contains CD11c promoter and a β-globin expression cassette that has a transcription terminator for efficient gene expression in tg mice (Brocker et al., 1997). The entire hSLAM gene and the junction sites between the vector and the inserted gene were sequenced to ensure proper construction.
Generation of tg mice
The 7.7 kb transgene of CD11c promoter-hSLAM fragment was isolated by digestion of pCD11c-hSLAM with XhoI and NotI and was microinjected into either FVB/N or FVB/N × C57BL/6 fertilized oocytes. The oocytes were then implanted into pseudopregnant CD1 female mice at the TSRI transgenic facility. Tail biopsies were performed on pups, and DNA was isolated for detection of hSLAM cDNA. Five FVB/N and one FVB/N × C57BL/6 tg mouse founders were identified using a PCR assay as described (Hahm et al., 2003).
Mice
Were maintained in the closed breeding colony of TSRI. The origin, genotyping, and use of Lck-hSLAM tg mice (Hahm et al., 2003), IFN-α/β-receptor (R) ko mice (Muller et al., 1994), tumor necrosis factor (TNF)-α ko mice (Korner et al., 1997), lymphotoxin (LT)-α ko mice (Fu et al., 1997), and LT-β ko mice (Koni et al., 1997; Oldstone et al., 2002) has been reported.
Culture of DCs
BM cells were isolated and cultured as described with minor modifications (Lutz et al., 1999). BM cells obtained by flushing femurs and tibias with a PBS solution were resuspended in 0.83% of ammonium chloride for 3 min to lyse red blood cells. Then 3–4 million cells were cultured on a 100-mm tissue culture plate with RPMI complete medium containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10 mM HEPES, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 0.05 mM β-mercaptoethanol, and 200 U/ml rmGM-CSF (Peprotech). Every 2–3 days of culture, half of the medium was removed and replaced by fresh rmGM-CSF-supplemented culture medium. After 10 days of culture, nonadherent cells were pooled by gentle pipetting and used as a source of BM-derived DCs. From spleens of tg or non-tg littermates, DCs were obtained after digestion with collagenase D (1 mg/ml, type II, Sigma) at 37 °C for 15 min. To disrupt T-cell–DC complexes, 0.01 M of EDTA was added and incubation continued for 5 min. Single cells were then obtained by mechanical disruption as described (Hahm et al., 2003). When necessary, splenic DCs were enriched (50–70%) with appropriate columns (Stem cell Technologies Inc.) and cultured in complete medium containing rmGM-CSF.
Analysis of MV infection
Wt MV, JW strain, required several passages on B95-8 cells (fewer than 10 times) to sufficiently enhance viral titers for experimental study. This virus is referred to as MV-JWB (Hahm et al., 2003; Manchester et al., 2000). B95-8 cells were maintained in RPMI 1640 medium containing 7% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. For MV-binding to DCs in vitro, BM-derived DCs or splenic DCs from CD11c-hSLAM tg or non-tg mice were incubated with either MV-free media as a negative control (mock infection) or MV-JWB at 0.1–0.5 multiplicity of infection (MOI) on ice for 2 h. After washing with PBS, cells were cultured in complete media containing rmGM-CSF. For MV infection in vivo, 8- to 12-week-old tg or non-tg mice were injected intravenously with MV-JWB at a dose of 2 × 106 TCID50 in a volume of 250 μl. Mice were sacrificed 3–4 days after inoculation and splenocytes harvested as above. For a negative control, MV-free cell supernatants were prepared identically to JW virus stock and used for mock in vivo infection analysis.
Flow cytometric analysis
Incubation of cells with various fluorochrome-conjugated antibodies was carried out for 20 min on ice in PBS containing 1% FBS and 0.1% sodium azide. After washing three times, cells were fixed in 1% formaldehyde in PBS. Antibodies used in this study were specific for murine CD4, CD8, B220, CD11b, CD11c, NK1.1, CD44, CD25, CD69, H-2Kq MHC class I, I-A/I-E MHC class II, CD40, B7-1, and B7-2 antibodies (BD PharMingen). Staining for hSLAM was performed with an anti-hSLAM antibody (Advanced ImmunoChemical Inc.) conjugated to Cy5 using a monoclonal antibody labeling kit (Amersham Biosciences). For the detection of surface MV proteins, cells were incubated with a human polyclonal serum to MV (Hahm et al., 2003) for 20 min, washed 3 times, and then stained with FITC or PE-conjugated donkey anti-human IgG antibody (Jackson Immunoresearch Laboratories). Fluorescent cells were acquired with a FACSCalibur flow cytometer (Becton Dickinson) then analyzed with FlowJo (Treestar) software.
Proliferation assay
To examine the effect of MV-infected DCs on T-cell proliferation, BM-derived CD11c+ DCs from tg or non-tg mice of the FVB/N strain (H-2q) were either mock-infected or MV-infected and then UV-irradiated at 3 days post-infection (dpi) to block trans-infection of T cells. MV-infected DCs on 24-well plates were UV-irradiated by 30 min of exposure with a 254-nm UV lamp. This treatment inactivated infectious virus as no infected T cells were observed by FACS analysis in the coculture experiment. Lymphocytes obtained from various Lck-hSLAM tg, non-tg, or ko mice were labeled with carboxyfluorescein succidimyl ester (CFSE; Molecular Probes, Eugene, OR) as described (Hahm et al., 2003), mixed with DCs, treated with 20 ng/ml PMA, and 1 μg/ml ionomycin, and cultured in complete medium containing 50 U/ml of IL-2. For MLR, splenic T cells of C57BL/6 strain (H-2b) were used in the coculture with mock-infected DCs or MV-infected DCs from CD11c-hSLAM tg mice. The level of CFSE fluorescence from T cells was traced by flow cytometric analysis.
Apoptotic analysis
Splenocytes obtained from CD11c-hSLAM tg mice were either mock-infected or infected with MV and cultured with complete media containing GM-CSF. At day 1 and 2 post-infection, cells were stained for surface antigen (CD11c-allophycocyanin conjugated), washed, and subsequently incubated for 15 min with Annexin V-FITC and 7-amino-actinomycin D (7-AAD) according to the manufacturer’s recommendations (Pharmingen). After staining, cells were immediately acquired and analyzed.
Acknowledgments
This is publication No. 16196-NP from Department of Neuropharmacology, The Scripps Research Institute. This work was supported by a NIH grant AI36222 to M.B.A.O. N.A. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research and by a Shiley Scholar from the Scripps Clinic. We thank Sam H. Shin for technical assistance, Yusuke Yanagi (Kyushu University, Japan) and Dan R. Littman (New York University Medical Center) for graciously supplying the hSLAM gene and pCD11c vector, respectively.
References
- Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugert JJ, Melquiot NV, Darai G. Mapping of mRNA transcripts in the genome of molluscum contagiosum virus: transcriptional analysis of the viral slam gene family. Virus Genes. 2000;21:189–192. doi: 10.1023/a:1008187430053. [DOI] [PubMed] [Google Scholar]
- Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- Cocks BG, Chang CCJ, Carballido JM, Yssel H, de Vries JE, Aversa G. A novel receptor involved in T-cell activation. Nature. 1995;376:260–263. doi: 10.1038/376260a0. [DOI] [PubMed] [Google Scholar]
- Dorig R, Marcel A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- Dubois B, Lamy PJ, Chemin K, Lachaux A, Kaiserlian D. Measles virus exploits dendritic cells to suppress CD4+ T-cell proliferation via expression of surface viral glycoproteins independently of T-cell trans-infection. Cell Immunol. 2001;214:173–183. doi: 10.1006/cimm.2001.1898. [DOI] [PubMed] [Google Scholar]
- Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev, Immunol. 2003;3:813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- Erlenhoefer C, Wurzer WJ, Löffler S, Schneider-Schaulies S, ter Meulen V, Schneider-Schaulies J. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J Virol. 2001;75:4499–4505. doi: 10.1128/JVI.75.10.4499-4505.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlenhofer C, Duprex WP, Rima BK, ter Meulen V, Schneider-Schaulies J. Analysis of receptor (CD46, CD150) usage by measles virus. J Gen Virol. 2002;83:1431–1436. doi: 10.1099/0022-1317-83-6-1431. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Matsumoto M, Molina H, Chaplin DD. Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc Natl Acad Sci USA. 1997;94:5739–5743. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Immune responses during measles virus infection. In: Billeter M, ter Meulen V, editors. Measles Virus. Springer; Berlin: 1995. pp. 117–134. [Google Scholar]
- Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm B, Arbour N, Naniche D, Homann D, Manchester M, Oldstone MBA. Measles virus infects and suppresses proliferation of T lymphocytes from transgenic mice bearing human signaling lymphocytic activation molecule. J Virol. 2003;77:3505–3515. doi: 10.1128/JVI.77.6.3505-3515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat B, Rivailler P, Varior-Krishnan G, Cardoso A, Gerlier D, Rabourdin-Combe C. Transgenic mice expressing human measles virus (MV) receptor provide cells exhibiting different permissivities to MV infection. J Virol. 1996;70:6673–6681. doi: 10.1128/jvi.70.10.6673-6681.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu EC, Iorio C, Sarangi F, Khine AA, Richardson CD. CDw150 (SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology. 2001;279:9–21. doi: 10.1006/viro.2000.0711. [DOI] [PubMed] [Google Scholar]
- Hyypia T, Korkiamaki P, Vainionpaa R. Replication of measles virus in human lymphocytes. J Exp Med. 1985;161:1261–1271. doi: 10.1084/jem.161.6.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph BS, Lampert PW, Oldstone MBA. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975;16:1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klagge IM, ter Meulen V, Schneider-Schaulies S. Measles virus-induced promotion of dendritic cell maturation by soluble mediators does not overcome the immunosuppressive activity of viral glycoproteins on the cell surface. Eur J Immunol. 2000;30:2741–2750. doi: 10.1002/1521-4141(200010)30:10<2741::AID-IMMU2741>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Knight SC, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Korner H, Cook M, Riminton DS, Lemckert FA, Hoek RM, Ledermann B, Kontgen F, Fazekas de St Groth B, Sedgwick JD. Distinct roles for lymphotoxin-α and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur J Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine D, Trescol-Biemont MC, Longhi S, Libeau G, Marie JC, Vidalain PO, Azocar O, Diallo A, Canard B, Rabourdin-Combe C, Valentin H. Measles virus (MV) nucleoprotein binds to a novel cell surface receptor distinct from FcgammaRII via Its C-terminal domain: role in MV-induced immunosuppression. J Virol. 2003;77:11332–11346. doi: 10.1128/JVI.77.21.11332-11346.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- Lawrence DMP, Vaughn MM, Belman AR, Cole JS, Rall GF. Immune response-mediated protection of adult but not neonatal mice from neuron-restricted measles virus infection and central nervous system disease. J Virol. 1999;73:1795–1801. doi: 10.1128/jvi.73.3.1795-1801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Manchester M, Liszewski MK, Atkinson JP, Oldstone MBA. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–2165. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchester M, Eto DS, Valsamakis A, Liton PB, Fernandez-Munoz R, Rota PA, Bellini WJ, Forthal DN, Oldstone MBA. Clinical isolates of measles virus use CD46 as a cellular receptor. J Virol. 2000;74:3967–3974. doi: 10.1128/jvi.74.9.3967-3974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie JC, Kehren J, Trescol-Biemont MC, Evlashev A, Valentin H, Walzer T, Tedone R, Loveland B, Nicolas JF, Rabourdin-Combe C, Horvat B. Mechanism of measles virus-induced suppression of inflammatory immune responses. Immunity. 2001;14:69–79. doi: 10.1016/s1074-7613(01)00090-5. [DOI] [PubMed] [Google Scholar]
- McChesney MB, Oldstone MBA. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- McChesney MB, Altman A, Oldstone MBA. Suppression of T lymphocyte function by measles virus is due to cell cycle arrest in G1. J Immunol. 1988;140:1269–1273. [PubMed] [Google Scholar]
- McQuaid S, Cosby SL. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab Invest. 2002;82:403–409. doi: 10.1038/labinvest.3780434. [DOI] [PubMed] [Google Scholar]
- Meroni L, Fusi ML, Varchetta S, Biasin M, Rusconi S, Villa ML, De Vries JE, Aversa G, Galli M, Clerici M. Altered signaling lymphocytic activation molecule (SLAM) expression in HIV infection and redirection of HIV-specific responses via SLAM triggering. Clin Immunol. 1999;92:276–284. doi: 10.1006/clim.1999.4747. [DOI] [PubMed] [Google Scholar]
- Minagawa H, Tanaka K, Ono N, Tatsuo H, Yanagi Y. Induction of the measles virus receptor SLAM (CD150) on monocytes. J Gen Virol. 2001;82:2913–2917. doi: 10.1099/0022-1317-82-12-2913. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Murabayashi N, Kurita-Taniguchi M, Ayata M, Matsumoto M, Ogura H, Seya T. Susceptibility of human dendritic cells (DCs) to measles virus (MV) depends on their activation stages in conjunction with the level of CDw150: role of Toll stimulators in DC maturation and MV amplification. Microbes Infect. 2002;4:785–794. doi: 10.1016/s1286-4579(02)01598-8. [DOI] [PubMed] [Google Scholar]
- Naniche D, Varior-Krishnan G, Cervino F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–6032. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naniche D, Reed SI, Oldstone MBA. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J Virol. 1999;73:1894–1901. doi: 10.1128/jvi.73.3.1894-1901.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S, Seki F, Ono N, Yanagi Y. Histidine at position 61 and its adjacent amino acid residues are critical for the ability of SLAM (CD150) to act as a cellular receptor for measles virus. J Gen Virol. 2003;84:2381–2388. doi: 10.1099/vir.0.19248-0. [DOI] [PubMed] [Google Scholar]
- Oldstone MBA, Lewicki H, Thomas D, Tishon A, Dales S, Patterson J, Manchester M, Omán D, Caniche D, Holz A. Measles virus infection in a transgenic model: virus-induced central nervous system disease and immunosuppression. Cell. 1999;98:629–640. doi: 10.1016/s0092-8674(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Oldstone MBA, Race R, Thomas D, Lewicki H, Homann D, Smelt S, Holz A, Koni P, Lo D, Chesebro B, Flavell R. Lymphotoxin-alpha- and lymphotoxin-beta-deficient mice differ in susceptibility to scrapie: evidence against dendritic cell involvement in neuroinvasion. J Virol. 2002;76:4357–4363. doi: 10.1128/JVI.76.9.4357-4363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Hidaka Y, Aoki T, Minagawa H, Yanagi Y. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J Virol. 2001a;75:4399–4401. doi: 10.1128/JVI.75.9.4399-4401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono N, Tatsuo H, Tanaka K, Minagawa H, Yanagi Y. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J Virol. 2001b;75:1594–1600. doi: 10.1128/JVI.75.4.1594-1600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CE, Lawrence DM, Echols LA, Rall GF. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J Virol. 2002;76:4497–4506. doi: 10.1128/JVI.76.9.4497-4506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punnonen J, Cocks BG, Carballido JM, Bennett B, Peterson D, Aversa G, de Vries JE. Soluble and membrane-bound forms of signaling lymphocytic activation molecule (SLAM) induce proliferation and Ig synthesis by activated human B lymphocytes. J Exp Med. 1997;185:993–1004. doi: 10.1084/jem.185.6.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery MJ, Schwab M, Eibert SM, Samstag Y, Walczak H, Schonrich G. Targeting the function of mature dendritic cells by human cytomegalovirus: a multilayered viral defense strategy. Immunity. 2001;15:997–1009. doi: 10.1016/s1074-7613(01)00239-4. [DOI] [PubMed] [Google Scholar]
- Rall GF, Manchester M, Daniels LR, Callahan E, Belman A, Oldstone MBA. A transgenic mouse model for measles virus infection of the brain. Proc Natl Acad Sci USA. 1997;94:4659–4663. doi: 10.1073/pnas.94.9.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U, Messling V, von Devaux P, Cattaneo R. Efficiency of measles virus entry and dissemination through different receptors. J Virol. 2002;76:7460–7467. doi: 10.1128/JVI.76.15.7460-7467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Schaulies S, Klagge IM, ter Meulen V. Dendritic cells and measles virus infection. Curr Top Microbiol Immunol. 2003;276:77–101. doi: 10.1007/978-3-662-06508-2_4. [DOI] [PubMed] [Google Scholar]
- Schnorr JJ, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Azocar O, Le Deist F, Fischer A, Rabourdin-Combe C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000a;74:4387–4393. doi: 10.1128/jvi.74.9.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Bausinger H, Manie S, Le Deist F, Azocar O, Hanau D, Fischer A, Rabourdin-Combe C. Measles virus induces abnormal differentiation of CD40L-activated human dendritic cells. J Immunol. 2000b;164:1753–1760. doi: 10.4049/jimmunol.164.4.1753. [DOI] [PubMed] [Google Scholar]
- Servet-Delprat C, Vidalain PO, Valentin H, Rabourdin-Combe C. Measles virus and dendritic cell functions: how specific response cohabits with immunosuppression. Curr Top Microbiol Immunol. 2003;276:103–123. doi: 10.1007/978-3-662-06508-2_5. [DOI] [PubMed] [Google Scholar]
- Sevilla N, Kunz S, McGavern D, Oldstone MBA. Infection of dendritic cells by lymphocytic choriomeningitis virus. Curr Top Microbiol Immunol. 2003;276:125–144. doi: 10.1007/978-3-662-06508-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J Immunol. 1993;151:4614–4624. [PubMed] [Google Scholar]
- Sidorenko SP, Clark EA. The dual-function CD150 receptor subfamily: the viral attraction. Nat Immunol. 2003;4:19–24. doi: 10.1038/ni0103-19. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Homann D, Tishon A, Pagarigan R, Oldstone MBA. Measles virus infection results in suppression of both innate and adaptive immune responses to secondary bacterial infection. J Clin Invest. 2003;111:805–810. doi: 10.1172/JCI13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JL, Barry DW, Lucas SJ, Albrecht P. Measles infection of human mononuclear cells: I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975;142:773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Burns JB, Howell JM, Fujinami RS. Suppression of antigen-specific T cell proliferation by measles virus infection: role of a soluble factor in suppression. Virology. 1998;246:24–33. doi: 10.1006/viro.1998.9186. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Minagawa H, Xie MF, Yanagi Y. The measles virus hemagglutinin downregulates the cellular receptor SLAM (CD150) Arch Virol. 2002;147:195–203. doi: 10.1007/s705-002-8312-0. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–897. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- Tatsuo H, Ono N, Yanagi Y. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J Virol. 2001;75:5842–5850. doi: 10.1128/JVI.75.13.5842-5850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The World Health Report. Reducing Risks, Promoting Healthy Life. World Health Organization; Geneva: 2002. pp. 186–197. [DOI] [PubMed] [Google Scholar]
- Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15:277–285. doi: 10.1016/s0952-7915(03)00041-4. [DOI] [PubMed] [Google Scholar]
- Vidalain PO, Azocar O, Lamouille B, Astier A, Rabourdin-Combe C, Servet-Delprat C. Measles virus induces functional TRAIL production by human dendritic cells. J Virol. 2000;74:556–559. doi: 10.1128/jvi.74.1.556-559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Morra M, Wu C, Gullo C, Howie D, Coyle T, Engel T, Terhorst C. CD150 is a member of a family of genes that encode glycoproteins on the surface of hematopoietic cells. Immunogenetics. 2001;53:382–394. doi: 10.1007/s002510100337. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- Yannoutsos N, Ijzermans JN, Harkes C, Bonthuis F, Zhou CY, White D, Marquet RL, Grosveld F. A membrane cofactor protein transgenic mouse model for the study of discordant xenograft rejection. Genes Cells. 1996;1:409–419. doi: 10.1046/j.1365-2443.1996.d01-244.x. [DOI] [PubMed] [Google Scholar]