Abstract
Chronic inflammation contributes to the development of various forms of cancer. The polyamine catabolic enzyme spermine oxidase (SMOX) is induced in chronic inflammatory conditions, including Helicobacter pylori-associated gastritis, where its production of hydrogen peroxide contributes to DNA damage and subsequent tumorigenesis. MicroRNA expression levels are also altered in inflammatory conditions; specifically, the tumor suppressor miR-124 becomes silenced by DNA methylation. We sought to determine if this repression of miR-124 is associated with elevated SMOX activity and concluded that miR-124 is indeed a negative regulator of SMOX. In gastric adenocarcinoma cells harboring highly methylated and silenced mir-124 gene loci, 5-azacytidine treatment allowed miR-124 re-expression and decreased SMOX expression. Overexpression of an exogenous miR-124-3p mimic repressed SMOX mRNA and protein expression as well as H2O2 production by >50% within 24 hours. Reporter assays indicated that direct interaction of miR-124 with the 3′-untranslated region of SMOX mRNA contributes to this negative regulation. Importantly, overexpression of miR-124 prior to infection with H. pylori prevented the induction of SMOX believed to contribute to inflammation-associated tumorigenesis. Compelling human in vivo data from H. pylori-positive gastritis tissues indicated that the mir-124 gene loci are more heavily methylated in a Colombian population characterized by elevated SMOX expression and a high risk for gastric cancer. Furthermore, the degree of mir-124 methylation significantly correlated with SMOX expression throughout the population. These results indicate a protective role for miR-124 through the inhibition of SMOX-mediated DNA damage in the etiology of H. pylori-associated gastric cancer.
Keywords: spermine oxidase, Helicobacter pylori, polyamines, miR-124, epigenetic, gastritis
Introduction
Spermine oxidase (SMOX), an inducible enzyme in the polyamine catabolic pathway, is responsible for the direct back-conversion of spermine to spermidine.1 This FAD-dependent reaction also generates significant amounts of the ROS precursor hydrogen peroxide (H2O2) as a byproduct. SMOX activity occurs in the cytoplasm as well as the nucleus,2, 3 where its production of H2O2 in close proximity to DNA results in oxidative DNA damage that elevates the potential for neoplastic transformation. To further exacerbate this potential, spermine, the substrate of SMOX, functions as a free-radical scavenger4 and is diminished during SMOX-mediated ROS generation. SMOX is induced in response to various stimuli, including bacterial infection,5–7 pro-inflammatory cytokines,8 the natural polyamines, and certain polyamine analogues.1 Importantly, significant increases in SMOX protein expression have been detected in tissues from patients harboring conditions characterized by chronic inflammation, including Helicobacter pylori-associated gastritis,9 prostatic intraepithelial neoplasia (PIN),10 and ulcerative colitis.11 Each of these conditions increases the risk of tumorigenesis, and the inhibition or loss of SMOX activity in animal models representing these conditions decreases tumor occurrence.7, 12, 13 Elevated SMOX thereby serves as a molecular link between infection and/or inflammatory stimuli and the development of chronic inflammation-associated tumorigenesis.
Much research has focused on H. pylori-mediated induction of SMOX in gastric epithelial cells as a mechanism linking chronic gastritis and gastric carcinogenesis. A Gram-negative, microaerophilic bacterium, H. pylori inhabits the gastric mucosae of greater than half of the world’s population, and chronic infection with H. pylori is causally linked to gastritis and peptic ulcer disease.14 Furthermore, H. pylori infection is considered the predominant risk factor for the development of gastric cancer, with approximately 90% of newly diagnosed noncardia gastric cancer cases attributable to chronic H. pylori infection.15 With a 5-year survival rate of less than 15%, gastric cancer is the third leading cause of cancer-related deaths worldwide.16–18
The prevalence of H. pylori infection is greatest in developing countries, and throughout the Department of Nariño, Colombia, approximately 80% of children are H. pylori-positive by the age of 5.19 However, the risk of eventually developing gastric cancer differs greatly between residents of two geographically isolated regions: those inhabiting the rural Andes mountain villages have an approximately 25-fold greater risk of developing gastric cancer than those residing in the low-risk region along the Pacific coast.20 Importantly, gastric tissues biopsied from gastritis patients in the high-risk region demonstrate elevated SMOX activity that results in increased oxidative DNA damage, relative to similarly staged patients from the low-risk region.13 These results, combined with related studies in a Mongolian gerbil model,13 implicate SMOX as the mediator of increased H. pylori-associated gastric cancer risk in the Andean population and suggest the use of aberrant SMOX induction as an indicator of gastric cancer risk and a rational target for chemoprevention.
The dysregulation of specific microRNAs (miRNAs) with tumor suppressive or oncogenic roles is prevalent in cancer. miRNAs are short, ~22-nt-long, non-coding RNA molecules that negatively regulate target mRNA transcripts, typically through base-pairing with a region in the 3′-UTR. As tumor suppressor genes, mature miRNAs bind to the mRNA transcripts of genes with potentially oncogenic functions, down-regulating their expression via message destabilization or translational inhibition. Tumor suppressive miRNAs are frequently inactivated in cancer through multiple mechanisms, including epigenetic changes such as aberrant promoter-region DNA hypermethylation. Of interest, human miR-124 is encoded by 3 loci: mir-124-1 (8p23.1), mir-124-2 (8q12.3), and mir-124-3 (20q13.33). Each of these is associated with a canonical CpG island that becomes densely hypermethylated, resulting in tumor-specific epigenetic repression that has been observed in many cancer types, including gastric, colon, and prostate.21–24
A chronic inflammatory microenvironment contributes to epigenetic silencing through DNA hypermethylation, which accumulates in non-cancerous gastric mucosae prior to the development of malignancy.25 Several studies have implicated correlations between miR-124 epigenetic inactivation and a predisposition to tumorigenesis. In particular, mir-124 hypermethylation has been reported in several premalignant conditions that are associated with chronic inflammation and/or infection, and mir-124 hypermethylation is frequently observed in gastric biopsies of individuals with H. pylori infection.21 However, the relationship of mir-124 methylation to the mechanism of gastric carcinogenesis has not been determined. Similarly, mir-124 DNA hypermethylation was detected in colonic tissues of both pediatric and adult patients with active ulcerative colitis,22, 26 a colorectal cancer predisposition, as well as in premalignant cervical lesions.27
The above-mentioned infection/inflammation-associated conditions under which mir-124 becomes silenced through DNA hypermethylation mimic those in which SMOX becomes activated. Therefore, we hypothesized that miR-124 is a negative regulator of SMOX that prevents the DNA-damaging and tumorigenic effects of SMOX induction. We directly tested this hypothesis and herein present both in vitro and in vivo human data implicating the epigenetic inactivation of mir-124 during H. pylori-associated gastritis as a key player in the induction of SMOX-mediated oxidative DNA damage and potential for gastric carcinogenesis.
Results
Exogenous expression of miR-124 decreases SMOX expression
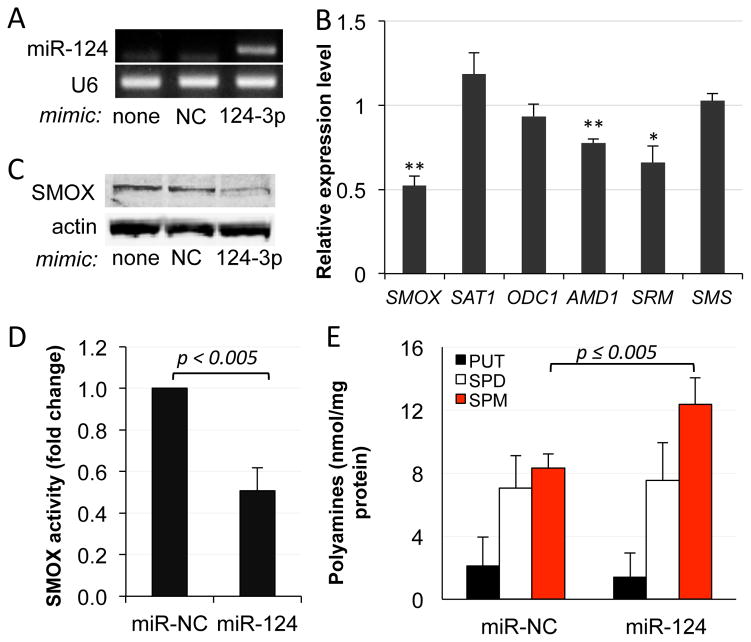
AGS human gastric adenocarcinoma cells express nearly undetectable levels of mature miR-124,21 and we have previously demonstrated that AGS cells induce SMOX expression in response to H. pylori infection.6, 13 To determine if increasing miR-124 expression in these cells would alter the expression of SMOX, transient transfections were conducted using a miRNA mimic corresponding to hsa-miR-124-3p: after 24 h, miR-124 was highly expressed while SMOX mRNA expression was significantly down-regulated (>50%), compared to cells transfected with a negative control miRNA mimic (miR-NC) (Fig. 1A and B). SMOX protein levels were also diminished upon miR-124 expression, as indicated by Western blot analyses (Fig. 1C). Both the decreased generation of H2O2 specifically from the oxidation of spermine (Fig. 1D) and the increased level of spermine (SPM)(Fig. 1E), the substrate of SMOX and a free-radical scavenger,4, 28 verified the reduction in enzymatically active SMOX following expression of miR-124. Intracellular concentrations of the other natural polyamines were not significantly affected, as the inhibition of SMOX does not elicit a complete blockade of polyamine metabolism. Of the other polyamine metabolic enzymes, S-adenosylmethionine decarboxylase (AMD1) and spermidine synthase (SRM) mRNA expression levels were also decreased following miR-124 transfection (Fig. 1B). As neither of these transcripts is predicted to bind miR-124, it is likely that this down-regulation of biosynthesis is due to feedback from the observed increase in intracellular spermine concentration.29
Figure 1. Exogenous expression of miR-124 decreases SMOX expression in gastric adenocarcinoma cells.
AGS cells were transfected with the miR-124-3p or negative control mimic (miR-NC) for 24 h and analyzed for the following: A. RT-PCR of miR-124 expression with U6 snRNA amplification as a normalization control; B. qRT-PCR of key polyamine pathway enzymes, normalized to GAPDH. Data are presented as the relative expression level in cells containing miR-124 versus miR-NC. (SAT1: spermidine/spermine N1-acetyltransferase; ODC1: ornithine decarboxylase; AMD1: S-adenosylmethionine decarboxylase; SRM: spermidine synthase; SMS: spermine synthase); C. Western blot using antibodies to SMOX and β-actin; D. SMOX activity assays measured as pmol H2O2 produced/mg protein/minute; E. intracellular polyamine pool concentrations (SPM: spermine; SPD: spermidine; PUT: putrescine). Histograms in B, D, and E represent the means of 4 independent experiments with error bars indicating SEM. Student’s t-test was used to calculate two-tailed p-values (**p < 0.01; *p < 0.05).
miR-124 directly targets the 3′-UTR of SMOX
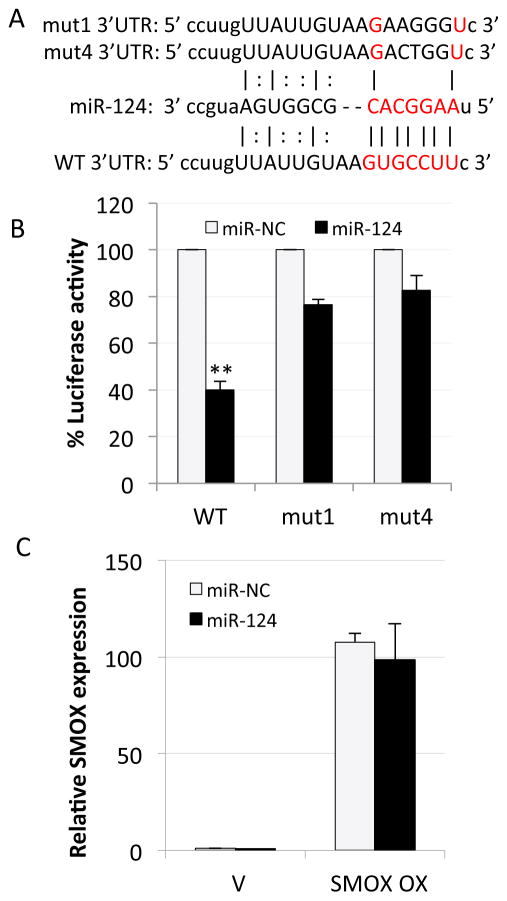
To determine if SMOX is a direct target of miR-124, the 3′-UTR of the human SMOX gene was examined for predicted miRNA recognition sites. Bioinformatic analysis revealed a miR-124 target site starting at position 262 of the human SMOX 3′-UTR that consists of an exact match to positions 2–8 of the mature miRNA (the seed + position 8) (Fig. 2A). This region of the human SMOX gene, which is broadly conserved among vertebrates, was isolated and inserted downstream of the firefly luciferase gene to generate a reporter construct. Transfection of the resulting plasmid into AGS cells induced robust luciferase activity that was attenuated approximately 60% upon cotransfection with the miR-124 mimic (Fig. 2B). miR-124 was unable to significantly decrease luciferase activity when cotransfected with mutated versions of the same region. Furthermore, AGS cells stably overexpressing the SMOX coding region but lacking the predicted miR-124 binding site continued to express elevated SMOX mRNA following miR-124 mimic transfection (Fig. 2C). These results indicate that miR-124 regulates SMOX, at least in part, through direct targeting of its 3′-UTR.
Figure 2. miR-124 directly targets the SMOX 3′-UTR.
A. Complementarity of the 3′-UTR of wild-type (WT) or mutant (mut) human SMOX mRNA (starting at nucleotide +262) with the hsa-miR-124 seed sequence (red text). B. Luciferase assay indicating miR-124 mimic expression attenuates reporter plasmid activity through direct interaction with the WT SMOX 3′-UTR. Luciferase activity was measured in RLUs and normalized to β-galactosidase activity (n = 2 measured in triplicate; error bars = SEM; **p < 0.05). C. AGS cells transfected with the SMOX coding region lacking the 3′-UTR (SMOX OX) or empty vector (V). qRT-PCR data depicts SMOX expression levels after transfection with miR-124 or miR-NC mimics, relative to GAPDH. Columns represent the means (n = 2) with SEM.
miR-124 inhibits H. pylori-mediated induction of SMOX
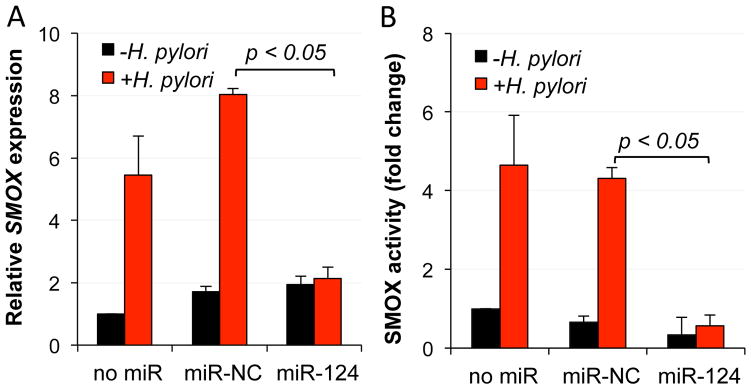
Infection of gastric epithelial cells with H. pylori induces SMOX expression that results in increased DNA damage in association with gastric tumorigenesis.6, 9, 12, 13, 30 To determine if the expression of miR-124 could influence H. pylori-mediated SMOX induction, AGS gastric epithelial cells were transfected with miR-124 or the negative control mimic 48 h prior to infection with H. pylori. Quantitative PCR results demonstrated significant (6–8-fold) increases in SMOX mRNA in wild-type AGS cells or those containing the negative control miRNA mimic following co-incubation with H. pylori, while the cells transfected with the miR-124 mimic maintained SMOX mRNA levels that were not significantly greater than basal levels (Fig. 3A). These results indicate that miR-124 is capable of negatively regulating the increased SMOX transcript levels that occur with H. pylori infection, thereby potentially preventing the SMOX-mediated production of DNA-damaging H2O2.
Figure 3. Overexpression of miR-124 inhibits H. pylori-mediated induction of SMOX.
AGS cells expressing the miR-NC or miR-124 mimics were exposed to H. pylori for 6 h. A. qRT-PCR demonstrates that miR-124 expression represses the increase in SMOX mRNA typically observed in response to H. pylori infection. B. Spermine oxidase activity assay demonstrating a lack of H. pylori-mediated spermine oxidation (measured as pmol H2O2 produced/mg protein/minute and presented as fold-change) in the presence of miR-124. Columns represent the means (n ≥3; error bars = SEM).
To confirm this protective role for miR-124, SMOX activity and the resultant generation of H2O2 were analyzed following the exposure of miR-124-expressing AGS cells to H. pylori (Fig. 3B). As with SMOX mRNA, significantly increased SMOX activity occurred following 6 h of co-incubation with H. pylori in either wild-type or negative control AGS cells. Importantly, the AGS cells overexpressing miR-124 generated diminished levels of spermine oxidation-specific H2O2, regardless of the presence of H. pylori. These data verify that miR-124 is a negative regulator of SMOX and as such, serves to protect cells from the DNA-damaging effects known to result from increased spermine oxidation in association with H. pylori infection.
Re-expression of endogenous miR-124 correlates with decreased SMOX
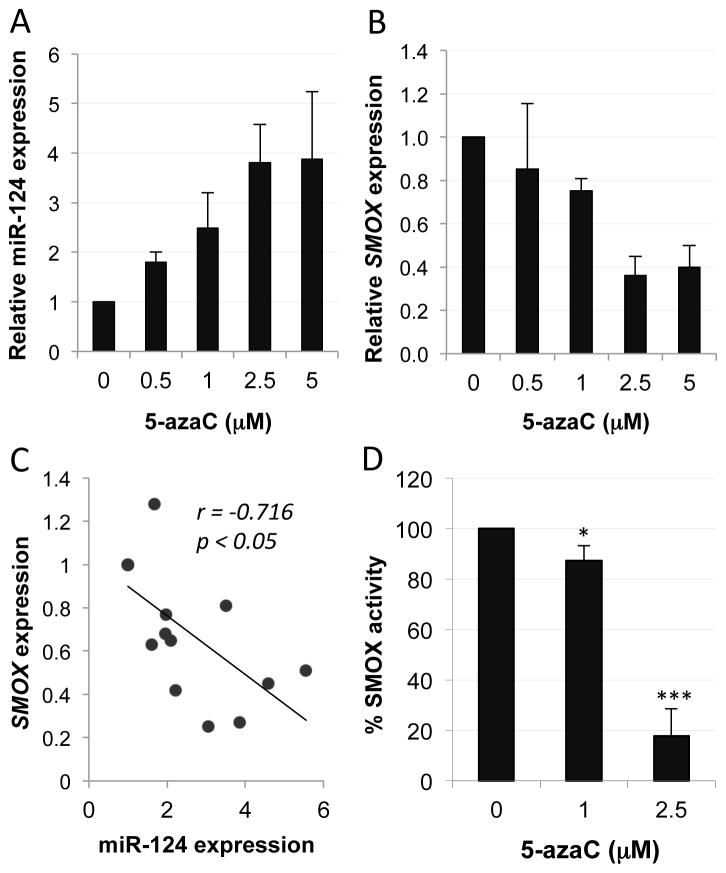
Previous studies have demonstrated that simultaneous CpG-island DNA hypermethylation of the promoter regions of the 3 mir-124 genes is responsible for its lack of expression in AGS cells.21 We therefore treated AGS cells with the DNA methyltransferase (DNMT) inhibitor 5-azacytidine (5-azaC) to induce re-expression of endogenous miR-124 and observe its effects on SMOX expression. Treatment with 5-azaC resulted in a dose-dependent increase in miR-124 expression (Fig. 4A) that correlated with decreased expression of the SMOX transcript (Fig. 4B). This negative correlation was confirmed by regression analysis of the individual expression levels at each 5-azaC concentration, with r and p-values calculated using Spearman’s rank correlation (Fig. 4C). Consequently, the production of SMOX-specific hydrogen peroxide was decreased with increasing concentrations of 5-azaC (Fig. 4D), suggesting that re-expression of endogenous miR-124 was capable of down-regulating SMOX. Among the other genes in the polyamine pathway, only ODC1 expression was significantly altered by 5-azaC (SI Fig. 1).
Figure 4. Re-expression of endogenous miR-124 correlates with repressed SMOX expression.
AGS cells were treated with 5-azacytidine (5-azaC) every 48 h (3 treatments total). RNA expression levels were quantified using qRT-PCR with primers specific to miR-124 (A) or SMOX (B). miR-124 transcript levels are relative to U6, and SMOX levels are relative to GAPDH. Columns indicate the means (n = 3) with error bars indicating SEM. In C, expression levels from the individual experiments in A and B were used for regression analysis. Spearman’s rank correlation was used to calculate r and p values. SMOX activity (D) following 5-azaC treatment was measured as pmol H2O2/mg protein/minute (n = 3 independent experiments measured in triplicate; error bars = SEM; *p < 0.01; ***p < 0.001).
mir-124 DNA methylation in gastric biopsies from at-risk Colombian gastritis populations
To investigate the clinical implications of the miR-124 – SMOX interaction, methylation levels of the 3 hsa-mir-124 genes were quantitatively analyzed using Pyrosequencing of gastric mucosae DNA obtained from 90 patients with dyspeptic symptoms who resided in the high- or low-risk regions of Nariño, Colombia. We previously demonstrated that these same high-risk patients maintain significantly elevated levels of DNA-damaging SMOX activity that correlates with their increased risk of developing gastric cancer, relative to the low-risk patients.13 The majority of patients (90%) was H. pylori-positive and included 43 persons from the low-risk coastal region and 47 persons from the high-risk Andean region. The overall DNA methylation levels of mir-124 were significantly elevated in the high-risk population compared to those of the low-risk group at each of the 3 gene loci (Fig. 5). Notably, the methylation level at each genomic locus in an individual patient was positively correlated with those at the other 2 loci in the same patient (mir-124-1 vs. mir-124-2: r = 0.679, p < 0.0001; mir-124-1 vs. mir-124-3: r = 0.618, p < 0.0001; mir-124-2 vs. mir-124-3: r = 0.742, p < 0.0001), strengthening the potential for epigenetic silencing of mir-124 in the patient as well as in the Andean population as a whole. These observations are consistent with mir-124 epigenetic down-regulation as a mechanism that allows for the chronically elevated SMOX activity and oxidative DNA damage that contributes to the high tumorigenic risk of the Andean community.13
Figure 5. Methylation levels of mir-124 genes are elevated in gastritis patient biopsies from the high-risk region versus the low-risk region.
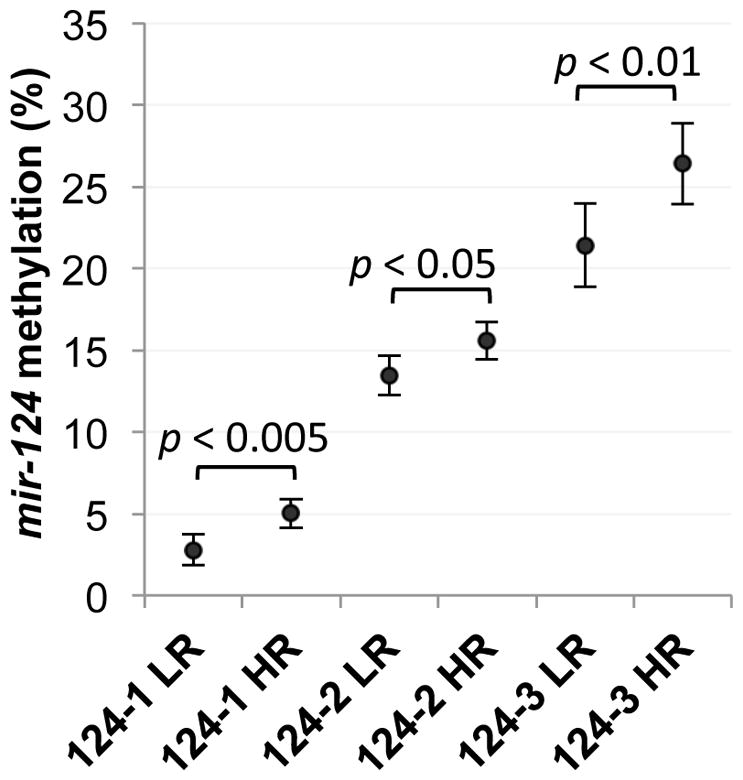
The CpG methylation percentage at each mir-124 gene locus was determined using Pyrosequencing of bisulfite-modified DNA from patients residing in the low-risk (LR) coastal region (n = 43) or the high-risk (HR) (n = 47) mountain region (assays were performed using coded case numbers). The mean methylation percentages of each patient population are presented as dots, with error bars indicating 95% confidence interval. Statistically significant differences between the 2 populations (p < 0.05) are as indicated.
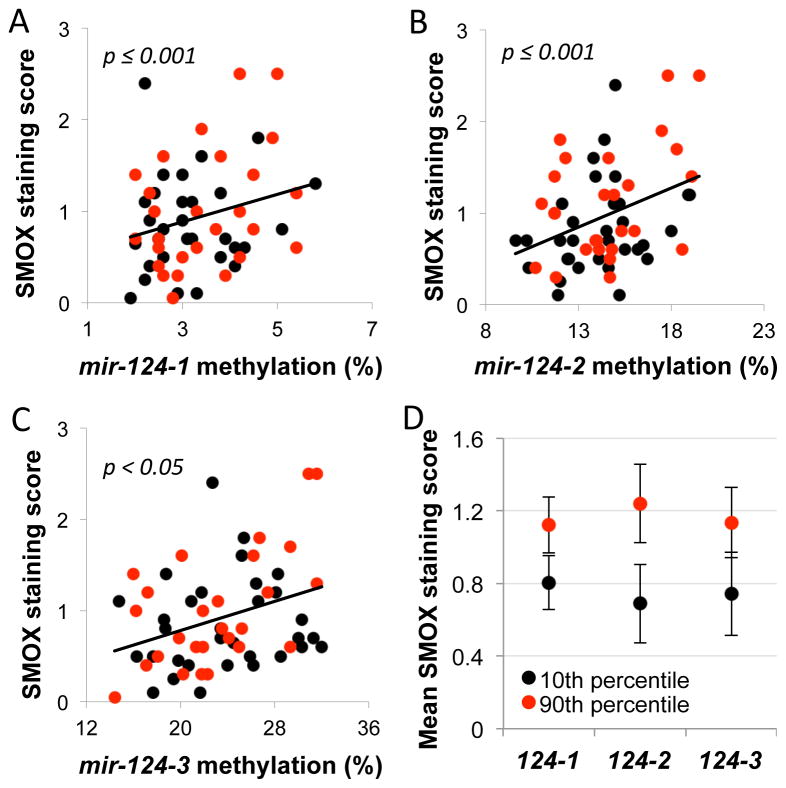
It is important to note that although the coastal Colombian population has been historically referred to as “low-risk” relative to the Andean population, inhabitants of the coastal region remain at significant risk for developing gastric cancer. Considering this risk and the fact that patients in both regions have advanced lesions, we applied multivariate analyses using the entire Colombian cohort to estimate the overall relationship between methylation levels of the mir-124 gene loci and expression of SMOX, which was previously determined by immunohistochemistry.13 After adjusting for confounding variables, including H. pylori infection status and cagA status, age, histopathology score, and risk region, generalized linear models indicated that SMOX expression was significantly and positively associated with the degree of DNA methylation at each of the 3 mir-124 gene loci (Fig. 6A–D). These compelling human in vivo data imply that increased methylation of mir-124 is positively associated with increased expression of SMOX in Colombian patients with H. pylori-associated gastritis, regardless of region of residence. Therefore, methylation of mir-124 potentially serves as a critical factor in determining general susceptibility to H. pylori-associated oxidative damage in the etiology of gastric cancer.
Figure 6. Methylation levels of mir-124 genes correlate with SMOX expression in gastritis patients at various stages of disease.
In A, B, and C, generalized linear models were used to analyze the relationships between mir-124 promoter methylation and SMOX expression in the human gastric biopsies from both high- and low-risk regions combined. Regression plots present SMOX immunohistochemistry staining scores versus the percent methylation of mir-124-1, -2, or -3 (black dots: low-risk region patients; red dots: high-risk region patients; patients within the top and bottom 10th percentiles of mir-124 methylation were excluded from the plots but included in the multivariate analyses and calculations of p values). In D, the mean SMOX staining scores at the 10th and 90th percentiles of mir-124 methylation (at each locus) are presented with error bars indicating 95% confidence intervals.
Discussion
Chronic inflammation associated with H. pylori infection is perceived to play a causative role in epigenetic gene silencing through DNA hypermethylation, which increases as an individual progresses through the cascade from non-atrophic gastritis to cancer.31–33 As shown here with mir-124, methylation levels of other tumor suppressor genes involved in gastric carcinogenesis are also increased in the high-risk Colombian gastritis patients,34, 35 suggesting a fundamental difference affecting the methylation process. Genetic differences, including ancestral variation in both the human and bacterial populations, also contribute to cancer risk.36 Our group recently isolated and characterized multiple H. pylori strains from individuals inhabiting the high- or low-risk regions; interestingly, compared to those obtained from low-risk individuals, the strains from high-risk patients were capable of inducing significantly higher levels of SMOX activity and DNA damage with reduced apoptosis levels that were associated with activation of EGFR.9, 13 Therefore, in high-risk-region patients already predisposed to SMOX expression due to hypermethylated mir-124 genes, there is also an increased chance of becoming colonized by an H. pylori strain with heightened ability to induce SMOX without triggering apoptosis.
The current study did not investigate the effects of miR-124 expression on cell proliferation and tumorigenic potential, as these have been previously reported in AGS cells among others. As would be expected for a miRNA with tumor suppressive function, overexpressing miR-124 decreased proliferation rates and anchorage-independent growth in all tumor cell types examined, and mouse tumor xenografts originating from miR-124-overexpressing gastric carcinoma cells, including the AGS cell line used in the current study, resulted in significantly slower tumor growth.37 As miR-124 has multiple potentially oncogenic targets, including regulators of proliferation such as CDK6,24 it should be noted that in cells with silenced miR-124, growth is significantly upregulated concomitant with SMOX-mediated DNA damage and decreased apoptosis, again exacerbating the potential for malignancy.
Through identifying SMOX as a novel target of miR-124, the results of the current study suggest the aberrant epigenetic silencing of miR-124 as a potential risk indicator for H. pylori-associated gastric carcinogenesis. As exemplified in Fig. 3 and schematically illustrated in Fig. 7, when expressed in gastric epithelial cells, miR-124 targets and prevents the induction of SMOX-generated ROS that occurs in the presence of inflammatory stimuli, such as H. pylori-associated gastritis. As a result, gastritis patients expressing higher levels of miR-124 maintain lower levels of SMOX and appear less likely to progress through the higher-grade, pre-malignant lesions that lead to gastric cancer. In contrast, patients at high risk for progressing to gastric cancer demonstrate significantly higher levels of SMOX and oxidative DNA damage that is associated with increased levels of mir-124 CpG island hypermethylation.
Figure 7. The proposed mechanism of miR-124-mediated protection from H. pylori-induced tumorigenesis.
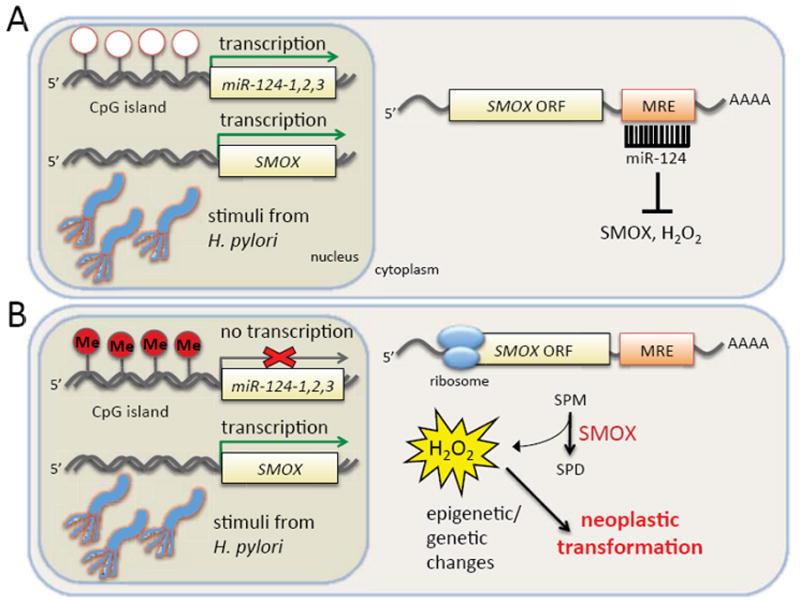
(A) In H. pylori-positive gastritis patients with low levels of DNA methylation at the mir-124 gene promoters, sufficient transcription occurs to enable post-transcriptional silencing of SMOX mRNA by mature miR-124. The result is a low level of SMOX protein induction in spite of transcriptional activation by H. pylori-associated inflammatory stimuli. (B) In H. pylori-positive patients with increased CpG island methylation (Me) in the mir-124 promoters, the abundance of mature miR-124 is reduced, and SMOX protein production is uncontrolled. The result is reduced concentrations of free-radical scavenging spermine combined with persistent ROS production, thereby increasing the likelihood of oxidative DNA damage known to contribute to the genetic and epigenetic changes associated with cancer. MRE: microRNA recognition element.
Our studies therefore suggest the potential use of methylated mir-124 as a valuable indicator of gastric cancer risk in H. pylori-positive individuals. As greater than half of the world’s population is infected with H. pylori, global antibiotic eradication of the bacteria is not feasible.38 In addition to cost prohibitions, antibiotic resistance is a factor, and eradication only reduces the risk of gastric cancer if conducted prior to the occurrence of pre-malignant lesions.39 As a biomarker, methylation of mir-124 could provide an indicator for those patients most likely to progress to the pre-malignant lesions and cancer, who could then receive more intensive monitoring with appropriate eradication and/or chemopreventive strategies. In fact, without regard to its downstream effects, methylation of mir-124-3 has shown utility as an accurate indicator of metachronous gastric cancers, in a multicenter cohort study.40
Due to the limitations of eradication, strategies for the chemoprevention of H. pylori-associated gastric carcinogenesis are needed to significantly reduce the risk associated with this pathogen. Pre-clinical data using a Mongolian gerbil model of H. pylori-associated gastric cancer has provided promising results with α-difluoromethylornithine (DFMO), an inhibitor of polyamine biosynthesis, or MDL72 527, an inhibitor of spermine oxidase.13 Both agents inhibited SMOX-mediated DNA damage and reduced the gastric dysplasia and carcinoma associated with H. pylori infection. A clinical trial using DFMO, which reduces the abundance of spermine available for oxidation in the pre-clinical gerbil model,13 is being initiated in Colombia. In this regard, the detection of mir-124 hypermethylation could provide a feasible method for identifying the patients most likely to benefit from a therapy targeting the polyamine pathway. Additionally, although not specifically targeting SMOX, the fact that the mode of mir-124 silencing is epigenetic indicates its reversibility and suggests the use of DNMT inhibitors, such as 5-azacytidine, which have been extensively studied in the clinic and are currently being revisited for their lasting demethylating effects using transient dosing schedules at less cytotoxic concentrations.41 Finally, the utility of miRNAs themselves as potential anticancer drugs is an area of active investigation currently limited by a lack of efficient delivery systems.42
Hypermethylation of mir-124 has been observed in many other cancer types as well as in several pre-malignant conditions associated with chronic inflammation and/or infection. In particular, mir-124 methylation increases in ulcerative colitis and has been identified as a potential risk marker for colitis-associated cancer,22 a condition that, like gastritis, varies greatly among individuals. As we have previously reported that elevated SMOX activity in an ETBF-induced model of mouse colitis is a mechanism linking inflammatory stimuli with tumorigenesis,7 we can speculate that miR-124 expression influences SMOX activity in this system as well. Moreover, the lack of miR-124 expression in multiple systems further implicates a fundamental role for SMOX induction in the etiology of chronic inflammation/infection-associated carcinogenesis that warrants further investigation.
It should also be noted that infection/inflammation-associated, ROS-induced DNA damage has been directly implicated in the epigenetic silencing of tumor suppressor genes.43 Consequently, the intriguing possibility exists that mir-124 methylation and SMOX expression/activity may constitute a feed-forward mechanism where increased SMOX expression resulting from mir-124 methylation leads to more ROS production, increased mir-124 methylation, and thus more SMOX expression, as the lesions progress towards malignancy.
In conclusion, the current study links the aberrant DNA methylation of mir-124 with the activation of spermine oxidation and consequential generation of oxidative DNA damage in the etiology of H. pylori-associated gastric cancer. Methylation levels of the mir-124 genes are elevated in patients at heightened risk of progression to H. pylori-associated gastric adenocarcinoma, while low methylation levels in the low-risk population are associated with miR-124-mediated protection from SMOX induction. Therefore, the methylation or expression level of miR-124 may provide a useful marker for identifying those H. pylori-positive individuals at increased risk for developing gastric cancer.
Materials and Methods
Cell lines, culture conditions, and chemicals
The AGS human gastric cancer cell line, obtained from and authenticated by ATCC (CRL-1739, Manassas, VA, USA), was maintained in F12K medium containing 10% fetal bovine serum at 37°C, 5% CO2. Mycoplasma testing was performed using the MycoAlert detection kit (Lonza, Walkersville, MD, USA). All custom primers were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Restriction and DNA modification enzymes were purchased from New England Biolabs (Billerica, MA, USA). Treatments with the DNA methyltransferase inhibitor 5-azaC (Sigma, St. Louis, MO, USA) were conducted at the doses indicated and included a change of medium with freshly diluted inhibitor every 48 hours for a total of 3 treatments, followed by a 3-day rest.
RNA extraction, gene expression, and miRNA expression studies
RNAiMAX (Life Technologies, Grand Island, NY, USA) was used to transfect AGS cells (2.5 × 105 cells/well in 6-well plates) with mirVana miRNA mimics (Life Technologies) corresponding to hsa-miR-124-3p or a negative control. After 24 h, total RNA was extracted using TRIzol reagent (Life Technologies) and quantified by spectrophotometry; cDNA was synthesized using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD, USA). SYBR green-mediated, real-time PCR was performed using the primer pairs and annealing temperatures in SI Table 1 with SYBR green SuperMix for iQ (Quanta Biosciences). The optimum annealing temperature for each primer pair was determined using temperature gradients followed by melt curve analyses and visualization on 2% agarose gels with GelStar staining (Lonza) and KODAK Digital Science Image Analysis Software (Rochester, NY, USA). Amplification was performed on a BioRad MyiQ2 real-time PCR detection system with data collection by the iQ5 optical system software (Hercules, CA, USA). In each qPCR experiment, samples were analyzed in triplicate, normalized to the GAPDH reference gene, and the fold-change in expression was determined relative to cDNA from untreated cells using the 2−δδCt algorithm.
For miRNA expression analysis, 1 μg of TRIzol-extracted RNA was converted to cDNA using the miScript II PCR System (SABiosciences, Frederick, MD, USA). SYBR-green-mediated qPCR was performed using a miR-124-specific sense primer24 with a universal antisense primer (SABiosciences), according to the manufacturer’s recommendations. U6 snRNA was amplified as the normalization control. Amplification products were electrophoresed on 2% agarose gels, stained with GelStar, and visualized and photographed using KODAK Digital Science Image Analysis software.
Analyses of spermine oxidase protein and activity and intracellular polyamine pools
AGS cells were transfected with the hsa-miR-124-3p or negative control miRNA mimic for 24 or 48 h, collected, and quick-frozen for analysis. Spermine oxidase activity was measured using a luminol-based assay measuring the production of hydrogen peroxide, as previously described.44 Polyamine concentrations were determined as previously described.45 Both assays were normalized relative to milligrams of total cellular protein determined using the method of Bradford.46
For Western blots, total protein (50 μg per lane) was separated on pre-cast 4–12% Bis-Tris NuPAGE gels with 1 × MOPS running buffer (Invitrogen) and transferred onto Immun-Blot PVDF membranes (BioRad). Blots were blocked for 1 hour at room temperature in Odyssey blocking buffer (LI-COR, Lincoln, NE, USA), followed by overnight incubation at 4°C with antibodies specific to SMOX (1:1000 dilution), as previously described,2 and β-actin (#sc-8432, Santa Cruz Biotechnology, Dallas, TX, USA). Incubation with species-specific, fluorophore-conjugated secondary antibodies allowed the visualization and quantification of immunoreactive proteins using the Odyssey infrared detection system and software (LI-COR).
Analysis of the 3′-UTR of spermine oxidase
Putative hsa-miR-124 binding sites in the 3′-UTR of the human SMOX transcript were predicted using bioinformatic tools available at www.microrna.org47 and TargetScan (version 6.2).48 To experimentally verify that miR-124 could directly influence SMOX mRNA expression, an 86-bp region of the human SMOX 3′-UTR (+227 to +315) including the putative recognition element for miR-124 was PCR-amplified and inserted downstream of the luciferase gene in the pMIR-REPORT Luciferase expression vector (Life Technologies). Site-directed mutagenesis of the miR-124 seed recognition sequence in the SMOX 3′-UTR was accomplished using the QuikChange II system (Agilent Technologies, Santa Clara, CA, USA), and resulting plasmids were verified by sequencing. Cotransfection of the pMIR-Luc-SMOX plasmid (wild type or mutant), the mirVana hsa-miR-124-3p mimic or negative control mimic, and the pMIR-REPORT-βGal reporter control plasmid into AGS cells was performed using Lipofectamine 2000 (Life Technologies). Cell lysates were collected 48 h later in reporter lysis buffer, and luciferase and β-galactosidase activities were measured using Luciferase and β-Galactosidase Enzyme Assay Systems, respectively (Promega, Madison, WI, USA). Luciferase activity was measured as relative light units and normalized to β-gal activity measurements determined by absorbance at OD420. Additionally, a stable AGS cell line was created by transfection with a plasmid containing the SMOX coding region and the first 158 nucleotides of the 3′UTR (lacking the miR-124 target site). Following transfection with the miR-124 or -NC mimic, qRT-PCR for SMOX was conducted as described above.
H. pylori culture and infection of AGS cells
H. pylori strain 60190 (ATCC) was maintained on trypticase soy agar plates containing 5% sheep blood at 37°C, 5% CO2. In preparation for the infection of AGS cells, the bacteria were transferred to 75-cm2 tissue culture flasks containing Brucella broth supplemented with 10% fetal bovine serum and grown for 48 hours in an upright position with occasional agitation at 37°C, 5% CO2. Bacteria were then collected by centrifugation, washed with PBS, and cell number was estimated based on OD600.
AGS gastric epithelial cells transfected with the miR-NC or miR-124-3p mimic were divided and replated in antibiotic-free F12K medium supplemented with 10% fetal bovine serum for 24 h prior to the addition of H. pylori at an MOI of 100 for 6 h. Uninfected cells from the same transfected population were used as negative controls. Cells were harvested and lysates prepared for RNA extraction or SMOX activity and protein analysis.
Human subjects
The human gastric biopsies used in the current study were obtained previously following the provision of informed consent and according to Institutional Review Board protocols approved by Vanderbilt University and Universidad del Valle Ethics Committees.13, 34, 35 Briefly, samples were obtained by upper endoscopy from 90 patients with dyspeptic symptoms who resided in either the high-risk Andean Mountain community of Tuquerres (47 patients) or the low-risk Pacific Coast city of Tumaco (43 patients), both in the Department of Nariño, Colombia. H. pylori infection status, cagA genotype, and SMOX expression values were scored by a pathologist (MBP) blinded to the patient groups, as previously described.13, 35, 49
Quantitative DNA methylation analyses
DNA was isolated from the frozen human gastric antral biopsies and used for bisulfite modification, PCR amplification, and Pyrosequencing as previously described.35 Multiple primer pairs were designed specifically for each of the 3 gene loci of hsa-mir-124 as follows: mir-124-1 forward (mir124a-1PF) 5′-GGAGTTTTTTAGAAGTAGGTTTGATGTT-3′ and reverse (mir124a-1PR) 5′-biotin-CTCCCCTCCCTAAACCCTCCAAC-3′50; mir-124-2 forward (mir124a-2G) 5′-AGGAGGGAATTATTGTTTTTTAGATAGTTG-3′ and reverse (mir124a-2H) 5′-biotin-AAAAAAAACTCCTACTTTTCCATTACAAC-3′; and mir-124-3 forward (mir124a-3F) 5′-AAAGAGAAGAGTTTTTATTTTTGAGTAT-3′ and reverse (mir124a-3R) 5′-biotin-TCCTCCTCAACTACCTTCCCCTA-3′50. The production of a single product for each primer pair was verified by electrophoresis in a 2% agarose gel. Methylated HeLa cell DNA (New England Biolabs) and normal human blood DNA were included in each experiment as positive and negative controls, respectively. Sequencing primers for each amplification product were as follows: mir-124-1 (mir124a-1PS) 5′-TAGAAGTAGGTTTGATGT-3′; mir-124-2 (mir124a-2GS) 5′-AATTTTTTTAGGAGATT-3′; and mir-124-3 (mir124a-3FS) 5′-GAGATTAGTTTTTTTAAT-3′. Methylation was quantitated across the following regions of hg19: mir-124-1, chr8 (9764472-9764488); mir-124-2, chr8 (65291958-65291986); and mir-124-3, chr20 (61809423-61809438).
Statistical analyses
Data from in vitro experiments were analyzed using Student’s t-test and Spearman’s rank correlations, where appropriate. For patient data, correlation coefficients and their corresponding p-values were used to characterize bivariate relationships for variables normally distributed (Pearson) or not (Spearman). Generalized linear models with a Gaussian link were used to estimate the association between SMOX expression and mir-124 methylation after adjusting for confounding variables that included age, H. pylori status, cagA status, and histopathology score. Multivariate analyses were conducted using STATA 14 (College Station, TX, USA).
Supplementary Material
Acknowledgments
The authors thank Alberto Delgado for his technical expertise and performance of SMOX immunohistochemistry studies. This study was supported by National Institutes of Health Grants R01CA051085 and R01CA098454 (to RAC), R01DK053620 and R01CA190612 (to KTW), P01CA028842 (to PC and KTW), P01CA116087 (to KTW), K01AT007324 (to RC), the Vanderbilt Digestive Disease Research Center grant (P30DK058404), UL1RR024975 (Vanderbilt CTSA, Pilot Project to KTW) and Merit Review Grant 1I01BX001453 from the Office of Medical Research, Department of Veterans Affairs (to KTW).
Footnotes
Author contributions: T.M.S., K.T.W., and R.A.C. designed the research; T.M.S., J.C.S., M.B.P., R.C., and B.G.S. performed the research; L.E.B. and P.C. provided unique human samples; T.M.S, M.B.P., R.M.M., K.T.W, and R.A.C. analyzed the data; and T.M.S., K.T.W., and R.A.C. wrote the paper. All authors revised and approved the final manuscript.
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
References
- 1.Wang Y, Devereux W, Woster PM, Stewart TM, Hacker A, Casero RA., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 2.Murray-Stewart T, Wang Y, Goodwin A, Hacker A, Meeker A, Casero RA., Jr Nuclear localization of human spermine oxidase isoforms - possible implications in drug response and disease etiology. Febs J. 2008;275:2795–2806. doi: 10.1111/j.1742-4658.2008.06419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cervelli M, Bellini A, Bianchi M, Marcocci L, Nocera S, Polticelli F, et al. Mouse spermine oxidase gene splice variants. Nuclear subcellular localization of a novel active isoform. Eur J Biochem. 2004;271:760–770. doi: 10.1111/j.1432-1033.2004.03979.x. [DOI] [PubMed] [Google Scholar]
- 4.Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA., Jr The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi R, Cheng Y, Asim M, Bussiere FI, Xu H, Gobert AP, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–40173. doi: 10.1074/jbc.M401370200. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–8525. doi: 10.1158/0008-5472.CAN-04-3511. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, et al. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:15354–15359. doi: 10.1073/pnas.1010203108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babbar N, Casero RA., Jr Tumor necrosis factor-alpha increases reactive oxygen species by inducing spermine oxidase in human lung epithelial cells: a potential mechanism for inflammation-induced carcinogenesis. Cancer Res. 2006;66:11125–11130. doi: 10.1158/0008-5472.CAN-06-3174. [DOI] [PubMed] [Google Scholar]
- 9.Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, et al. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology. 2014;146:1739–1751. e1714. doi: 10.1053/j.gastro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodwin AC, Jadallah S, Toubaji A, Lecksell K, Hicks JL, Kowalski J, et al. Increased spermine oxidase expression in human prostate cancer and prostatic intraepithelial neoplasia tissues. Prostate. 2008;68:766–772. doi: 10.1002/pros.20735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong SK, Chaturvedi R, Piazuelo MB, Coburn LA, Williams CS, Delgado AG, et al. Increased expression and cellular localization of spermine oxidase in ulcerative colitis and relationship to disease activity. Inflamm Bowel Dis. 2010;16:1557–1566. doi: 10.1002/ibd.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaturvedi R, Asim M, Romero-Gallo J, Barry DP, Hoge S, de Sablet T, et al. Spermine oxidase mediates the gastric cancer risk associated with Helicobacter pylori CagA. Gastroenterology. 2011;141:1696–1708. e1691–1692. doi: 10.1053/j.gastro.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene. 2015;34:3429–3440. doi: 10.1038/onc.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin CS. Helicobacter pylori gastritis, peptic ulcer, and gastric cancer: clinical and molecular aspects. Clin Infect Dis. 1997;25:1017–1019. doi: 10.1086/516077. [DOI] [PubMed] [Google Scholar]
- 15.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 16.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Sipponen P, Naumann M, Moayyedi P, Megraud F, Xiao SD, et al. Helicobacter pylori eradication has the potential to prevent gastric cancer: a state-of-the-art critique. Am J Gastroenterol. 2005;100:2100–2115. doi: 10.1111/j.1572-0241.2005.41688.x. [DOI] [PubMed] [Google Scholar]
- 18.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 19.Camargo MC, Yepez MC, Ceron C, Guerrero N, Bravo LE, Correa P, et al. Age at acquisition of Helicobacter pylori infection: Comparison of two areas with contrasting risk of gastric cancer. Helicobacter. 2004;9:262–270. doi: 10.1111/j.1083-4389.2004.00221.x. [DOI] [PubMed] [Google Scholar]
- 20.Correa P, Cuello C, Duque E, Burbano LC, Garcia FT, Bolanos O, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027–1035. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 21.Ando T, Yoshida T, Enomoto S, Asada K, Tatematsu M, Ichinose M, et al. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: Its possible involvement in the formation of epigenetic field defect. Int J Cancer. 2009;124:2367–2374. doi: 10.1002/ijc.24219. [DOI] [PubMed] [Google Scholar]
- 22.Ueda Y, Ando T, Nanjo S, Ushijima T, Sugiyama T. DNA methylation of microRNA-124a is a potential risk marker of colitis-associated cancer in patients with ulcerative colitis. Dig Dis Sci. 2014;59:2444–2451. doi: 10.1007/s10620-014-3193-4. [DOI] [PubMed] [Google Scholar]
- 23.Shi XB, Xue L, Ma AH, Tepper CG, Gandour-Edwards R, Kung HJ, et al. Tumor suppressive miR-124 targets androgen receptor and inhibits proliferation of prostate cancer cells. Oncogene. 2013;32:4130–4138. doi: 10.1038/onc.2012.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lujambio A, Ropero S, Ballestar E, Fraga MF, Cerrato C, Setién F, et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007;67:1424–1429. doi: 10.1158/0008-5472.CAN-06-4218. [DOI] [PubMed] [Google Scholar]
- 25.Ushijima T, Hattori N. Molecular Pathways: Involvement of Helicobacter pylori–triggered inflammation in the formation of an epigenetic field defect, and its usefulness as cancer risk and exposure markers. Clin Cancer Res. 2012;18:923–929. doi: 10.1158/1078-0432.CCR-11-2011. [DOI] [PubMed] [Google Scholar]
- 26.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, et al. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–852. e842. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilting SM, van Boerdonk RA, Henken FE, Meijer CJ, Diosdado B, Meijer GA, et al. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer. 2010;9:167–181. doi: 10.1186/1476-4598-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rider JE, Hacker A, Mackintosh CA, Pegg AE, Woster PM, Casero RA., Jr Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007;33:231–240. doi: 10.1007/s00726-007-0513-4. [DOI] [PubMed] [Google Scholar]
- 29.Pegg AE, Xiong H, Feith DJ, Shantz LM. S-adenosylmethionine decarboxylase: structure, function and regulation by polyamines. Biochem Soc Trans. 1998;26:580–586. doi: 10.1042/bst0260580. [DOI] [PubMed] [Google Scholar]
- 30.Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013;4:475–481. doi: 10.4161/gmic.25583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maekita T, Nakazawa K, Mihara M, Nakajima T, Yanaoka K, Iguchi M, et al. High levels of aberrant DNA methylation in Helicobacter pylori-infected gastric mucosae and its possible association with gastric cancer risk. Clin Cancer Res. 2006;12:989–995. doi: 10.1158/1078-0432.CCR-05-2096. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima T, Maekita T, Oda I, Gotoda T, Yamamoto S, Umemura S, et al. Higher methylation levels in gastric mucosae significantly correlate with higher risk of gastric cancers. Cancer Epi Biomarkers Prev. 2006;15:2317–2321. doi: 10.1158/1055-9965.EPI-06-0436. [DOI] [PubMed] [Google Scholar]
- 33.Niwa T, Tsukamoto T, Toyoda T, Mori A, Tanaka H, Maekita T, et al. Inflammatory processes triggered by Helicobacter pylori infection cause aberrant DNA methylation in gastric epithelial cells. Cancer Res. 2010;70:1430–1440. doi: 10.1158/0008-5472.CAN-09-2755. [DOI] [PubMed] [Google Scholar]
- 34.Schneider BG, Piazuelo MB, Sicinschi LA, Mera R, Peng DF, Roa JC, et al. Virulence of infecting Helicobacter pylori strains and intensity of mononuclear cell infiltration are associated with levels of DNA hypermethylation in gastric mucosae. Epigenetics. 2013;8:1153–1161. doi: 10.4161/epi.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider BG, Peng DF, Camargo MC, Piazuelo MB, Sicinschi LA, Mera R, et al. Promoter DNA hypermethylation in gastric biopsies from subjects at high and low risk for gastric cancer. Int J Cancer. 2010;127:2588–2597. doi: 10.1002/ijc.25274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kodaman N, Pazos A, Schneider BG, Piazuelo MB, Mera R, Sobota RS, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc Natl Acad Sci U S A. 2014;111:1455–1460. doi: 10.1073/pnas.1318093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie Y, et al. MicroRNA-124 inhibits proliferation and induces apoptosis by directly repressing EZH2 in gastric cancer. Mol Cell Biochem. 2014;392:153–159. doi: 10.1007/s11010-014-2028-0. [DOI] [PubMed] [Google Scholar]
- 38.Dalal RS, Moss SF. At the Bedside: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:213–224. doi: 10.1189/jlb.4BT0214-100R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ford AC, Forman D, Hunt RH, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:3174–3187. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asada K, Nakajima T, Shimazu T, Yamamichi N, Maekita T, Yokoi C, et al. Demonstration of the usefulness of epigenetic cancer risk prediction by a multicentre prospective cohort study. Gut. 2015;64:388–396. doi: 10.1136/gutjnl-2014-307094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai H-C, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, et al. Transient low doses of DNA demethylating agents exert durable anti-tumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Jiang Y, Peng H, Chen Y, Zhu P, Huang Y. Recent progress in microRNA delivery for cancer therapy by non-viral synthetic vectors. Adv Drug Deliv Rev. 2015;81:142–160. doi: 10.1016/j.addr.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 43.O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–619. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 45.Kabra PM, Lee HK, Lubich WP, Marton LJ. Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 46.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 47.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36:D149–153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 49.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–1195. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimizu T, Suzuki H, Nojima M, Kitamura H, Yamamoto E, Maruyama R, et al. Methylation of a panel of microRNA genes is a novel biomarker for detection of bladder cancer. Eur Urol. 2013;63:1091–1100. doi: 10.1016/j.eururo.2012.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.