Abstract
Human cytomegalovirus (CMV)-induced adaptive natural killer (NK) cells display distinct phenotypic and functional characteristics, including properties of immune memory. We hypothesized that these cells may be more resistant to suppression mediated by immune regulatory cell subsets, making them attractive for use in cancer therapy. Here we report that relative to conventional NK cells, adaptive NK cells express lower levels of the inhibitory receptor TIGIT which results in resistance to immune suppression mediated by myeloid-derived suppressor cells (MDSC), as derived from cytokine induction in normal blood or patients with myelodysplastic syndrome (MDS). In contrast, conventional NK cells were potently suppressed by MDSC, an effect abrogated completely by TIGIT blockade. Mechanistically, TIGIT signaling in NK cells after MDSC co-culture led to a decrease in the phosphorylation of ZAP70/Syk and ERK1/2. These effects were reversed by blocking TIGIT on NK cells or by inhibiting production of reactive oxygen species (ROS) by MDSC, the latter of which upregulated the TIGIT ligand CD155 on MDSC. Accordingly, the blunted cytotoxicity of NK cells co-cultured with MDSC against tumor cells could be reversed by blocking TIGIT or ROS production. Overall, our results show how adaptive NK cells arising in response to CMV infection can escape MDSC-mediated suppression, and defined TIGIT antagonists as a novel type of checkpoint inhibitor to enhance NK cell-mediated responses against cancer and infection.
Keywords: Adaptive NK cell, TIGIT, myeloid-derived suppressor cells, cancer
Introduction
Natural killer cells are lymphocytes of the innate immune system (1,2). Although they share similar mechanisms of killing with cytotoxic T cells (3,4), NK cells recognize targets through families of activating and inhibitory receptors. The balance between these receptors determines the function of NK cells (5). A down-regulation of MHC class I on damaged cells, or a mismatch between inhibitory subgroups of killer immunoglobulin-like receptors (KIRs) and their respective human leukocyte antigen (HLA) ligands on cells will render targets susceptible to NK cell killing (6,7). Therefore, tuning down the expression of inhibitory receptors on NK cells would increase their response to tumor cells. Like T cells, NK cell anti-tumor activity is limited by highly suppressive tumor microenvironment, which leads to dampened immunological function and poor prognosis (8–11). Emerging studies indicate that inhibitory receptors such as cytotoxic T lymphocyte-associated 4 (CTLA-4), programmed cell death 1 (PD-1) and T cell Ig and ITIM domain (TIGIT) on T and NK cells can suppress anti-tumor responses (12–16). While the physiologic role of inhibitory receptors is to maintain immune homeostasis, the goal in cancer immunotherapy is to unleash this control.
TIGIT is an inhibitory receptor that binds with high affinity to CD155 and with lower affinity to CD112. CD155 and CD112 are expressed in epithelial cells and antigen-activated T cells at steady state. However these ligands are defined as “stress-induced” and their expression is increased upon viral infection and malignant transformation (17). Engagement of TIGIT with CD155 competes with the interaction between the activating receptor DNAM-1 and CD155, resulting in decreased NK cell cytolytic activity and IFNγ production (16,18). Recently, TIGIT was found to be highly expressed on tumor-infiltrating lymphocytes (TILs), and co-blockade of TIGIT and PD-1 synergistically augmented CD8+ T cell activity against autologous tumor cells (19). TIGIT is also expressed on polyclonal NK cells (20), but little is known with respect to how TIGIT regulates human NK cell function in the tumor microenvironment.
We have recently identified heterogeneous subsets of highly specialized human NK cells that arise in response to cytomegalovirus (CMV) infection. We refer to these cells as “adaptive” and they are defined by epigenetic silencing of one or more of the proximal signaling molecules SYK, EAT-2, and FcεRγ along with silencing of the transcription factor PLZF. Interestingly, adaptive NK cells exhibit a whole-genome methylation signature that is remarkably similar to effector CD8+ T cells (21). Adaptive NK cells express the activating receptor NKG2C and maturation marker CD57 and these cells are virtually absent in CMV seronegative individuals. These cells produce markedly more inflammatory cytokines following CD16 ligation and are long-lived. Our group has recently shown that adaptive NK cell expansion after CMV reactivation in hematopoietic cell transplant recipients is associated with lower relapse rates (22).
In the present study, we examined the interaction between adaptive NK cells and myeloid-derived suppressor cells (MDSCs). MDSCs are a heterogeneous population of myeloid progenitor cells and immature myeloid cells. In humans, MDSCs commonly express CD11b, CD33, low or no HLA-DR and are either CD14+ (monocytic MDSCs [mMDSCs]) or CD15+CD66b+ (granulocytic MDSCs [gMDSCs]) (23). These cells are induced by tumors and contribute to inhibition of both innate and adaptive anti-tumor immunity by producing TGF-β, IL-10, reactive oxygen species (ROS), and arginase (24). We found that, compared with conventional NK cells, adaptive NK cells expressed lower TIGIT and consequently resisted functional suppression by cancer patient-derived MDSCs. These data suggest that strategies to expand adaptive NK cells or approaches to block the CD155-TIGIT interaction should be considered for enhancement of anti-tumor NK cell immune responses.
Material and Methods
Patients and healthy donors
Peripheral blood mononuclear cells (PBMC) were obtained fresh from CMV-seropositive healthy subjects or cryopreserved from myelodysplastic syndrome patients (MDS, n=15) obtained from the National Marrow Donor Program (NMDP)/Center for International Blood and Marrow Transplant Research Repository. All samples were de-identified and use was approved by the University of Minnesota and NMDP institutional review board in accordance with the Declaration of Helsinki.
Cell isolation
PBMC were seeded at 2×106/ml in RPMI medium containing 10% heat inactivated FBS, IL-6 (10 ng/ml, Sigma-Aldrich) and GM-CSF (10 ng/ml, R&D systems) for a week and refreshed on day 3 of culture to generate MDSCs (25). Next, HLA-DR+ cells were isolated with anti-human HLA-DR microbeads (Miltenyi Biotech), and MDSCs were thereafter purified from the HLA-DR− fraction using anti-CD33 microbeads (Miltenyi Biotech). Monocytic MDSC were used in all shown experiments (≥85%CD14+HLA-DR−). NK and T cells were isolated from overnight rested PBMC by negative depletion (Stemcell Technologies) or CD3 microbeads (Miltenyi Biotech). Control monocytes were isolated from overnight rested PBMC using anti-CD33 microbeads.
Proliferation assays
Monocytes or MDSCs were seeded in 96 well U-bottom plates in duplicate at 1:1–1:16 ratios with CellTrace violet dye (5 uM, Invitrogen)-labeled autologous T or NK cells (1×105) in RPMI medium (Gibco) supplemented with 10% FBS (referred below as medium). T cells were stimulated with anti-CD3/CD28 activation beads (40 beads/well) and IL-15 (1 ng/ml) or IL-15 (10 ng/ml) alone for NK cells and cultured for 3–5 days. Cells were acquired by LSRII flow cytometer (BD Biosciences) and data analyzed by FlowJo (Tree Star).
Flow cytometry analysis
Purified NK cells were cultured with monocytes or MDSCs for 5 days, in contact or separated by transwell inserts (0.4um pores) (Corning), and in the presence of IL-15 (10 ng/ml) prior to staining. In some experiments NK cells were cultured overnight in medium alone or in the presence of IL-15 (10 ng/ml), and with the addition of IL-18 (100 ng/ml) and IL-12 (10 ng/ml), or agonistic anti-CD16 (3G8, 1 ug/ml). Cryopreserved PBMC were rested overnight in medium to recover from freezing and then cultured for 6 hours in the presence of IL-15 (10 ng/ml) and anti-CD16 (1 ug/ml) prior to staining. Cells were stained with fluorochrome-conjugated antibodies detailed in Supplementary Table 1. Detection of CD107a, Ki67, IFN-γ, and TNFα production was performed following fixation and permeabilization (eBioscience) according to the manufacturer’s instructions. In some experiments, MDSCs were cultured overnight and stained for CD155 (PVR) in the presence of reagents targeting MDSC suppressive pathways (26) including 10 μg/mL TGFβ neutralizing antibodies (R&D systems), 200 IU/mL of the ROS scavenger catalase (Sigma-Aldrich) or superoxide dismutase (Sigma-Aldrich), 500 μmol/L arginase inhibitor N(ω)-hydroxy-nor-l-arginine (nor-NOHA; Calbiochem) or iNOS inhibitor NG-monomethyl-l-arginine (L-NMMA; Sigma-Aldrich). All cells were acquired by LSRII and analyzed by FlowJo. Adaptive and conventional NK cells were gated and identified according to the gating strategy in Supplementary Figure S1.
Confocal microscopy
MDSCs, monocytes, and NK cells were prelabeled with CellTracker Blue for 20 min (20 μM, Invitrogen) and co-cultured overnight in the presence of IL-15 (10 ng/ml). Cells were loaded onto poly-lysine pretreated chamber slides. Following overnight culture, cells were stimulated with agonistic anti-CD16 for 6 hours, fixed in 4% paraformaldehyde for 30 min and then blocked with 3% BSA at RT. Following blocking, cells were incubated with primary anti-TIGIT and anti-PVR (CD155) overnight at 4°C and then 1 hour with the fluorescence-labeled secondary antibodies before confocal microscopy (objective 20X).
Phosflow
NK cells from healthy blood donors were co-cultured with MDSCs or monocytes at a 2:1 ratio in the presence of IL-15 (10 ng/ml) and in the presence or absence of catalase (200 IU/ml) or blocking antibodies against TIGIT (10 ug/ml) for 5 days. Cells were then washed, rested for 4 hours, and stimulated with anti-CD16 agonist antibody for 10 and 30 min. before analysis of Zap-70 and ERK1/2 phosphorylation respectively. Cells were fixed and permeabilized with BD fixation buffer and permeabilization buffer III and stained for pZap-70 (pY319)/Syk (pY352) and pERK1/2 (pT202/pY204) according to the manufacturer’s instructions (BD biosciences).
Chromium release assays
Anti-TIGIT antibodies were functionally tested by cross-linking with chromium (51Cr)-labeled FcRII+ murine cell line p815 (authenticated from ATCC, used within three months of the first passage). NK cell function was analyzed after 4 hours against p815 in the presence of, IgG, anti-TIGIT (10 ug/ml) or an agonistic anti-CD158b (anti-KIR) (10 ug/ml, Biolegend) control. Following 5 days of co-culture with monocytes or MDSCs in the presence or absence of blocking antibodies against TIGIT (10 ug/ml) or ROS scavenger catalase (200 IU/ml), polyclonal-NK cell cytotoxicity was analyzed by 51Cr-release assays (4 hours) against K562 (authenticated from ATCC, used within three months of the first passage) cells at a 5:1-2.5:1 effector:target ratios.
Statistical analysis
All data were first analyzed in the software mentioned above and summarized by Prism Version 6 software (GraphPad). All data were first tested for normal distribution. Thereafter, differences among groups were analyzed by a Student’s t test, One or Two-Way ANOVA, or nonparametric Mann–Whitney U tests (as indicated in the figure legends). Representative histograms or images were chosen based on the average values.
Results
MDSCs suppress T and NK cell proliferation and NK cell function
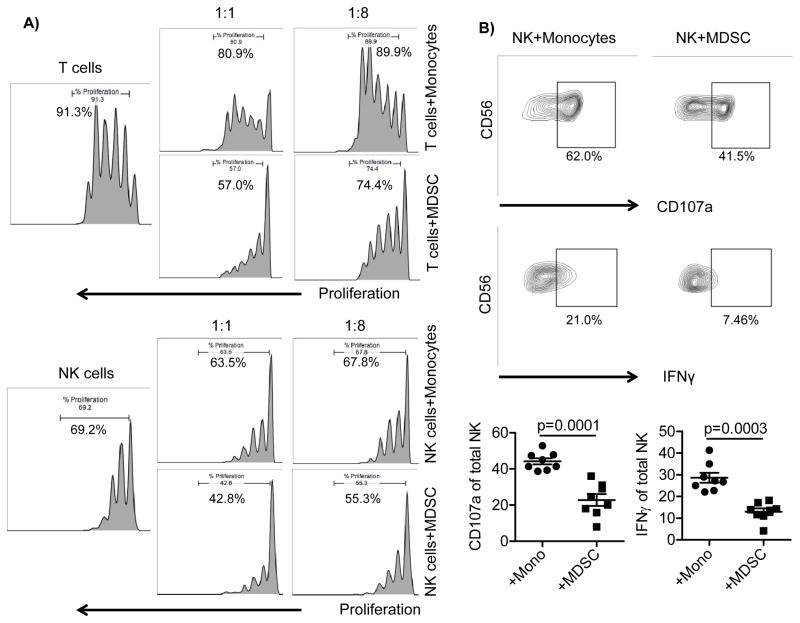
To investigate the interaction between MDSCs and NK cell subsets, MDSCs were generated from healthy donors cultured with IL-6 and GM-CSF for one week (25), (Supplementary Figure S2A). Fresh monocytes were used as a myeloid cell control for these experiments. Purified T and NK cells were co-cultured with monocytes or MDSCs at different ratios and evaluated for proliferation following 3–4 days of culture. While monocytes had a little effect on proliferation, MDSCs induced a 2.7±1.6-fold T cell suppression (p=0.005) of proliferation with a similar effect on NK cells (1.5±0.27-fold suppression, p=0.006) (Figure 1A). Similarly, CD16 engagement stimulated NK cell degranulation and IFN-γ production that was significantly suppressed by MDSC (2.4±1.4 fold suppression, p=0.0001 and 2.6±1.5 fold suppression, p=0.003, respectively) relative to NK cells cultured with monocytes (Figure 1B).
Figure 1. MDSCs suppress T and NK cell proliferation and NK cell functions.
A) Purified T and NK cells from healthy blood donors were labeled by CellTrace Violet and co-cultured with MDSCs or monocytes at different ratios in the presence of CD3/CD28 beads (40 beads/1×105 cells) and IL-15 (1 ng/ml) for T cells or IL-15 (10 ng/ml) alone for NK cells. Proliferation was assessed on day 3 or 4, and representative data is shown of six independent experiments. B) Purified NK cells were co-cultured with monocytes or MDSCs at a 2:1 ratio in the presence of IL-15 (10 ng/ml) for 5 days. Cells were stimulated with agonistic CD16 (1 μg/ml) for 6 hours prior to staining and evaluated for degranulation (CD107a) and IFN-γ production. Cumulative (n=8) data are shown as mean ± SEM. The Student’s t test was used for statistical analysis.
Adaptive NK cells resist MDSC suppression
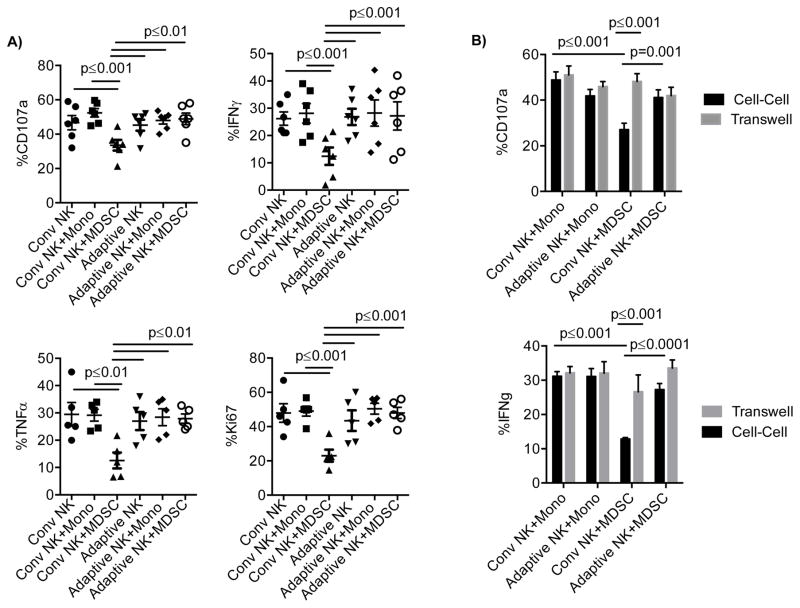
We next examined whether adaptive NK cells could resist MDSC suppression compared with conventional NK cells. We defined adaptive NK cells by flow cytometry as NK cells from CMV-seropositive donors that were CD57+NKG2C+FcεR1γ− (21) and conventional NK cells as CD57+NKG2C−. Since adaptive NK cells are less responsive to cytokines (21), they were instead stimulated with an anti-CD16 agonistic antibody. Purified polyclonal NK cells were co-cultured with monocytes or MDSCs for 5 days and examined for degranulation, proliferation and cytokine production following CD16 stimulation. Conventional and adaptive NK cells express equal amounts of CD16 (Supplementary Figure S2B). Similar NK cell function was observed when cultured alone or with the addition of monocytes in the presence of IL-15 (Figure 2A). Compared to monocyte controls, MDSCs mediated significant suppression of CD107a (52.4±2.4% vs. 33.5±3.1%, p≤0.001), IFN-γ (31.3±3.8% vs. 13.8±3.0%, p≤0.001), TNF (29.1±2.1% vs. 13.6±3.3%, p≤0.01) and proliferation (49.0±2.9% vs. 23.0±3.5%, p≤0.001) (measured by Ki67) within the population of conventional NK cells. However, adaptive NK cells were resistant to the same MDSC population (Figure 2A). Moreover, conventional NK cell degranulation and IFNγ production were completely restored when MDSC were separated from NK cells by a transwell (Figure 2B). Thus, CMV infection induces a population of adaptive NK cells that are resistant to contact dependent MDSC suppression.
Figure 2. Adaptive NK cells resist MDSC suppression.
A) Purified NK cells from healthy blood donors were cultured alone or in contact with MDSCs or monocytes at a 2:1 ratio in the presence of IL-15 (10 ng/ml) for 5 days or B) in transwells allowing exchange of soluble factors only. Cells were stimulated with anti-CD16 six hours prior to staining, and degranulation, IFN-γ and TNF-production, as well as proliferation (Ki67) were assessed by flow cytometry. Pooled data of 5–7 independent experiments are shown as the mean ± SEM and statistical analysis were done using the Two-Way ANOVA.
Adaptive NK cell resistance to MDSC suppression correlates with lower TIGIT expression
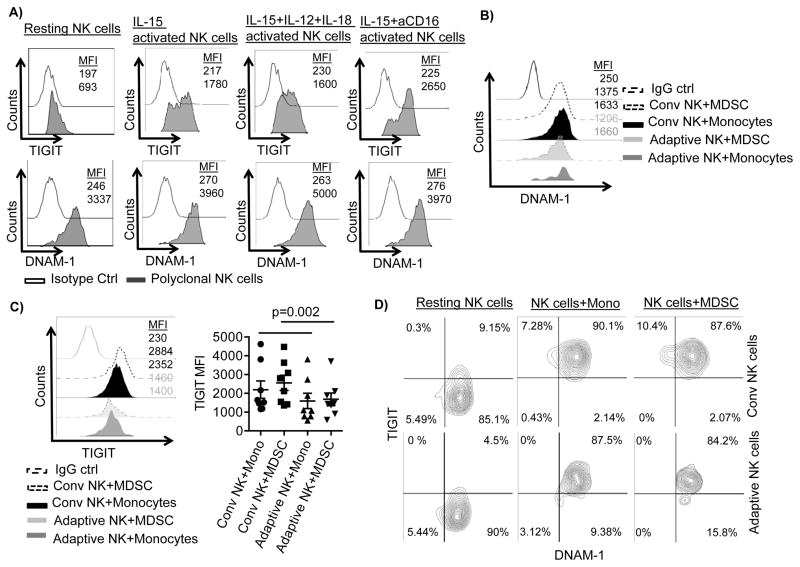
NK cells were cultured overnight in the absence or the presence of IL-15 (10 ng/ml) alone or with additional stimulation of IL-12 (10 ng/ml) and IL-18 (100 ng/ml) or anti-CD16 (1 μg/ml) prior to staining. TIGIT expression was low without stimulation and was upregulated with IL-15 stimulation alone. Additional stimulation by anti-CD16 further increased TIGIT expression. However, DNAM-1 could not be further increased due to high baseline expression level (Figure 3A). Interestingly, the staining pattern for TIGIT on these polyclonal activated NK cells showed a bimodal expression (Figure 3A). To explore this phenomenon further, we examined the expression of TIGIT and the two counterparts DNAM-1 and CD96, and other receptors on adaptive and conventional NK cells when cultured with monocytes or MDSC. There were no expression differences between adaptive and conventional NK cells for DNAM-1, CD96, NKp44, NKp46, PD-1, Tim3, or NKG2A (Figure 3B, Supplementary Figure S2C). In contrast, TIGIT expression was significantly less on adaptive vs. conventional NK cells whether co-cultured with monocytes (p=0.008) or MDSCs (p=0.002) (Figure 3C). Although, conventional and adaptive NK cells co-expressed TIGIT and DNAM-1 at similar levels before and after co-culture with monocytes or MDSC (NK alone: 18%±10% vs. 14%±11.5%, NK+monocytes: 86%±8% vs. 83%±9%, NK+MDSC: 84%±9% vs. 82%±6%, Figure 3D), adaptive NK cell expression of TIGIT remained low.
Figure 3. Conventional NK cells express higher TIGIT compared to adaptive NK cells.
A) Purified NK cells from healthy blood donors were cultured before staining in the absence or presence of IL-15 (10 ng/ml) alone or with the additional stimulation of IL-12 (10 ng/ml) and IL-18 (100 ng/ml) for 18 hours or with stimulation with anti-CD16 (1 μg/ml) for 6 hours. One of 4 independent experiments is shown. NK cells were cultured with MDSCs or monocytes at a 2:1 ratio in presence of IL-15 (10 ng/ml) for 5 days. Cells were stimulated with anti-CD16 six hours prior to analysis. Representative histograms for B) DNAM-1 and C) TIGIT expression and aggregate data for TIGIT expression (n=8) are shown as mean fluorescence intensity (MFI) ± SEM. Two-Way ANOVA was used for statistical analysis. D) NK cells before and after co-culture with monocytes or MDSC were analyzed for co-expression of DNAM-1 and TIGIT. Representative data is shown of 3 independent experiments and 7 replicates.
TIGIT-dependent suppression of conventional NK cells by MDSCs
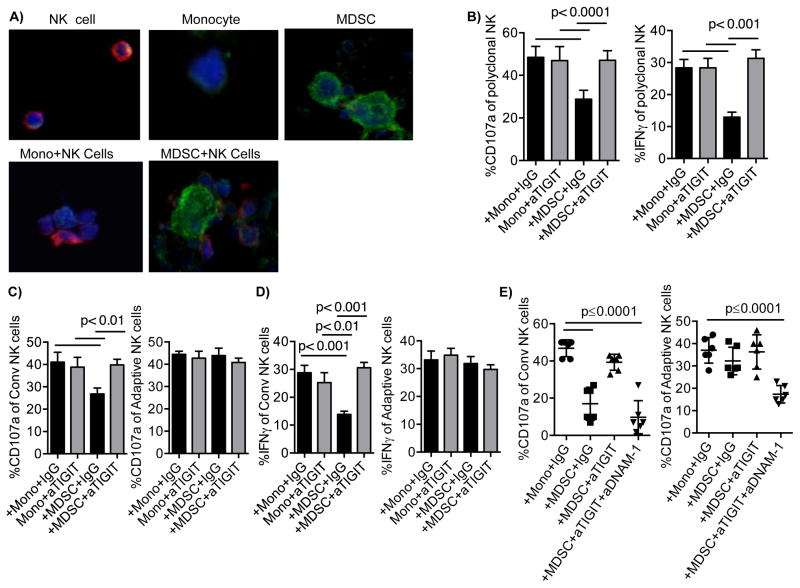
Monocytes, MDSCs, and NK cells were labeled with CellTracker Blue, co-cultured in chamber slides overnight. Cells were stimulated with anti-CD16 prior stained with anti-CD155 (green) and anti-TIGIT (red) and distinguished by size. As expected, TIGIT on NK cells co-localized with CD155 on MDSCs as a result of high expression of CD155 on MDSC compared to a minimal expression on monocytes (Figure 4A). To assess whether TIGIT plays a role in MDSC-dependent regulation of NK cells, IFN-γ production was evaluated in conventional NK cells co-cultured with MDSCs based on differential high versus low TIGIT expression after 6 hours of CD16 stimulation. Cells with low TIGIT expression produce significantly more IFN-γ relative to NK cells with high TIGIT expression (36.2% vs. 19.9%, p=0.0005, Supplementary Figure S2D). Next, we examined whether engagement of TIGIT is responsible for driving the MDSC-suppression of NK cells. We tested the function of the anti-TIGIT antibody as previously described in a P815 assay with normal NK cells (27). While the presence of anti-CD158b control inhibited NK cell cytotoxicity, NK cell function was not affected in the presence of anti-TIGIT (Supplementary Figure S3A) indicating the lack of agonistic function. NK cells were then co-cultured with monocytes or MDSCs for 5 days in the presence or absence of blocking antibodies against TIGIT. MDSC-induced suppression of polyclonal NK cell function was completely abrogated by blocking TIGIT (Figure 4B). As TIGIT blockade had little effect on adaptive NK cells, this release of suppression was entirely based on the large conventional NK cell population (Figure 4C and 4D). Simultaneous blockade of TIGIT and DNAM-1 in conventional NK cells co-cultured with MDSC reversed the effect of TIGIT-blockade and inhibited the degranulation and IFN-γ of adaptive NK cells (Figure 4E, Supplementary Figure S3B), indicating a TIGIT-dependent inhibition of DNAM-1 signaling.
Figure 4. TIGIT-dependent suppression of conventional NK cells by MDSC.
A) Monocytes, MDSCs, and NK cells were labeled with CellTracker Blue, co-cultured on slides overnight then stimulated with anti-CD16 prior stained with anti-CD155 (green) and anti-TIGIT (red) followed by confocal microscopy. Individual cell types are shown at the upper panel or at the lower panel when co-cultured. Representative data of 2 independent experiments and 6 donors is shown. NK cells were cultured with monocytes or MDSCs in the presence of IL-15 and IgG control (10 ug/ml) or blocking antibodies against TIGIT (10 ug/ml) for 5 days. Degranulation (n=9) and IFN-γ production (n=8) were evaluated in B) polyclonal NK cells, C) conventional (n=8) and D) adaptive NK cells (n=9). E) Alternatively, cells were co-blocked by anti-TIGIT and anti-DNAM-1(10 ug/ml) (n=6). Pooled data are shown as mean ± SEM of n number of replicates, and the Two-Way and One-way (E) ANOVA were used for statistical analysis.
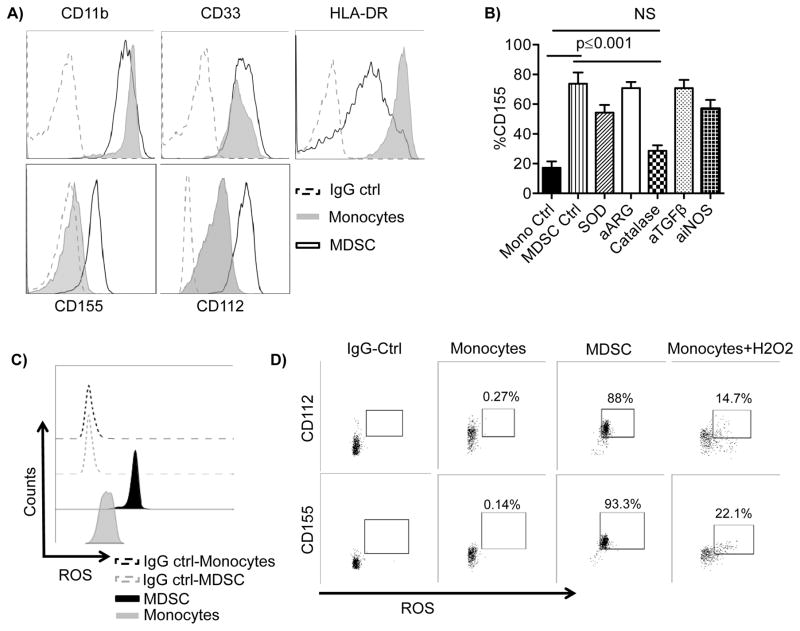
ROS-induces CD155 expression on the surface of MDSCs
We further examined the expression of the TIGIT ligands CD155 and CD112 in monocytes and MDSCs. MDSCs expressed high levels of CD155 compared with almost no expression in monocytes (MFI: 675±124 vs. 107±23, p=0.015). Moreover, CD112 expression was significantly higher in MDSCs compared to monocytes (MFI: 1714±331 vs. 865±196, p=0.015) (Figure 5A). To further investigate the mechanisms of MDSC-induced conventional NK cell suppression, we individually blocked pathways utilized by MDSCs including superoxide, arginase, ROS, TGF-β, and iNOS overnight at the end of MDSC generation. Inhibition of ROS production with catalase resulted mainly in the significant decrease in the expression of CD155 on MDSCs down to the level seen in control monocytes (55%±23 decrease, p=0.03) (Figure 5B).
Figure 5. Reactive Oxygen Species (ROS) induce CD155 expression on MDSCs.
A) MDSCs and monocytes were stained for the antigens shown. One representative example from 10 independent experiments is shown. B) Induced MDSCs were stained for CD155 and analyzed by flow cytometry following overnight treatment with superoxide dismutase (SOD, 200 IU/mL), arginase inhibitor (a-ARG, arginase inhibitor N(ω)-hydroxy-nor-l-arginine, 500 μM), ROS scavenger (Catalase, 200 IU/mL), blocking antibodies against TGF-β (10 μg/ml), iNOS inhibitor (aiNOS, NG-monomethyl-l-arginine, 500 μM), or left untreated compared to control monocytes. Pooled (n=4) data from n independent experiments are shown as mean ± SEM, and statistical analysis were done using the Two-way ANOVA. C) Unstimulated monocytes and MDSCs were stained for total ROS and analyzed by flow cytometry. D) Unstimulated or H2O2 (250 μM) monocytes for 1 hour and unstimulated MDSCs were stained for total ROS, CD112, and CD155 and analyzed by flow cytometry. Cells double positive for ROS and CD112 or CD155 are shown. One representative donor of 6 is shown. One representative isotype control is shown for all groups for simplicity as individual controls were similar between conditions.
Several studies have shown that increased ROS production in MDSCs correlates with suppression of T cell function. We examined the ROS production levels in MDSCs compared to freshly isolated monocytes. Monocytes produced almost no ROS and were predominantly CD155 negative. In contrast, MDSCs produced high basal levels of ROS and were uniformly CD155 positive (Figures 5C). Furthermore, inducing ROS production in monocytes by H2O2 treatment induced the expression of CD155 in ROS+ monocytes (Figure 5D).
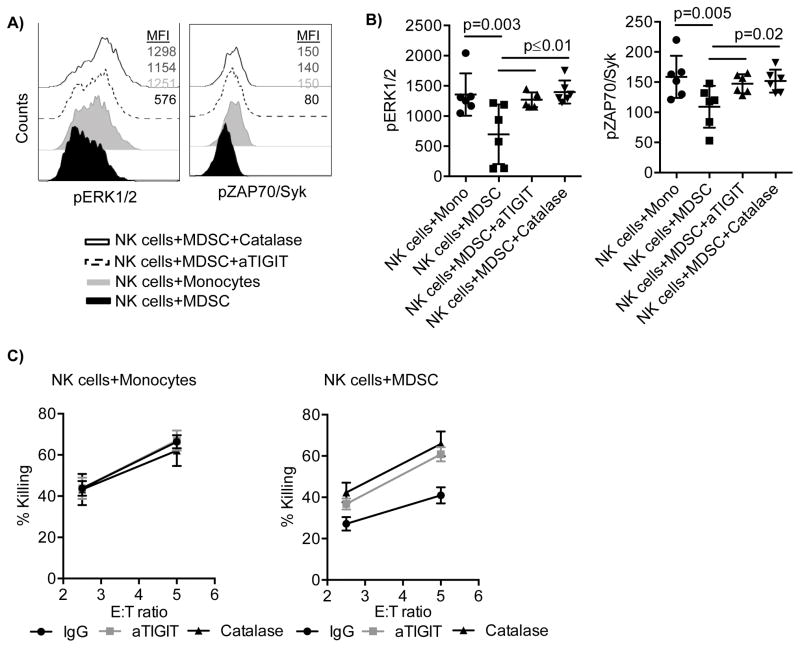
TIGIT engagement inhibits pZAP70/Syk and pERK1/2 and results in inhibition of NK cell cytotoxicity
Given the strong suppressive effect of TIGIT engagement on conventional NK cell function and proliferation, we next analyzed the CD16 induced signaling interaction with TIGIT in NK cells co-cultured with MDSC. Compared to control monocytes, NK cells co-cultured with MDSCs exhibited decreased phosphorylation of ERK1/2 (p=0.003) and ZAP70/Syk (p=0.005). Furthermore, blocking TIGIT or inhibiting ROS restored the phosphorylation of ERK1/2 and ZAP70/Syk (p≤0.02, Figure 6A, B). We next investigated whether blocking TIGIT or ROS could recover the cytotoxicity of NK cells cultured with MDSCs against tumor cells. Neither anti-TIGIT nor catalase had any effect on NK cells cultured alone (data not shown). NK cells cultured with either monocytes or MDSCs were pre-treated with TIGIT blocking antibodies or catalase, washed, and then incubated with 51Cr-labeled K562 cells. NK cell cytotoxicity was significantly decreased after co-culture with MDSCs while monocytes had no effect (Figure 6C). Both TIGIT blockade and ROS inhibition completely reversed the suppressive effect mediated by MDSCs (Figure 6C). Moreover, blocking TIGIT combined with catalase treatment in NK cell and MDSC co-cultures had no additive effect on either pZAP70/Syk and pERK1/2 or NK cell cytotoxicity (data not shown).
Figure 6. TIGIT engagement inhibits pZAP70/Syk and pERK1/2 and results in inhibition of NK cell cytotoxicity.
A) Purified NK cells were co-cultured with MDSCs or monocytes at a 2:1 ratio in the presence of IL-15 (10 ng/ml), IgG control (10 μg/ml) and in the presence or absence of blocking antibodies against TIGIT (10 μg/ml), or catalase (200 IU/mL) for 5 days. Cells were stimulated for 10 and 30 min with anti-CD16, and stained for pZAP/Syk or pERK1/2 respectively. Representative A) or cumulative B) data are shown from 3 independent experiments and 6 donors as mean ± SEM. Statistical analysis were done using the Two-Way ANOVA. C) NK cells from monocyte and MDSC co-cultures in the presence or absence of anti-TIGIT or catalase were washed and incubated with 51Cr-labeled K562 for 4 hours to assess NK cell cytotoxicity. Representative data from 3 independent experiments is shown as mean ± SEM.
TIGIT-dependent suppression of conventional NK cells by myelodysplastic syndrome (MDS) patient MDSCs
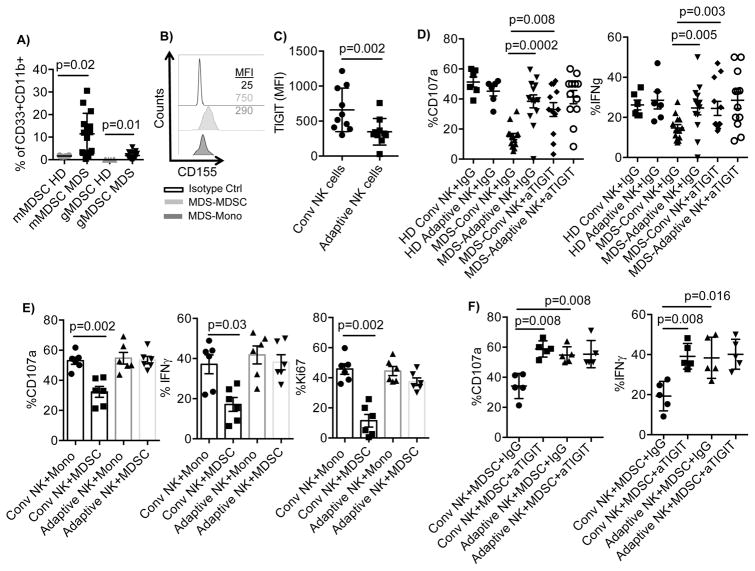
Having identified the contact-mediated suppressive mechanism of cytokine-generated MDSCs, we next investigated whether this mechanism was operant in a physiologic setting in vivo. PBMC from CMV-seropositive MDS patients and healthy donors (HD) were analyzed for the frequency of adaptive NK cells and MDSCs. Although there was a high frequency of adaptive NK cells in the blood of CMV+ MDS patients (n= 10, 17%±15% vs 7%±5%), the total NK cell frequency was significantly lower compared to HD (n=8, 1.3%±1.2 vs. 8%±7%). Monocytic MDSCs (mMDSCs) were defined as CD45+Lin−CD11b+CD33+HLA-DR−/lowCD14+ and granulocytic MDSCs (gMDSCs) as CD45+Lin−CD11b+CD33+CD15+. Compared to healthy blood donors, we found a significant increase in the frequency of both mMDSCs (p=0.02) and gMDSCs (p=0.01) in the blood of MDS patients (Figure 7A). In addition, MDS-MDSCs have increased CD155 expression compared to MDS-monocytes (Figure 7B). PBMC from MDS patients were then evaluated for the expression of TIGIT on conventional and adaptive NK cells. In MDS patients, TIGIT expression was significantly lower on adaptive compared to conventional NK cells (MFI: 347±189 vs. 660±311, p=0.002; Figure 7C). MDS patient CMV-induced adaptive NK cells exhibited significantly greater function after activation with IL-15 and CD16 stimulation relative to MDS-conventional NK cells. Notably, adaptive NK cells displayed similar degranulation and IFNγ production as in healthy donor NK cells (Figure 7D). Blocking TIGIT signaling in conventional NK cells from MDS patients rescued their functional hyporesponsiveness, but there was little added effect on adaptive NK cells that have inherently low levels of TIGIT expression (Figure 7D).
Figure 7. TIGIT-dependent suppression of conventional NK cells by MDS MDSCs.
A) PBMC (n=15) from MDS patients and healthy donors (n=6) were rested overnight, stained and the MDSC frequency were determined by flow cytometry. Monocytic MDSCs (mMDSCs) were defined as CD45+Lin−CD11b+CD33+HLA-DR−/lowCD14+ and granulocytic MDSCs (gMDSCs) as CD45+Lin−CD11b+CD33+CD15+. B) MDS-PBMC were stained for CD155 and gated for mMDSC and monocytes. Representative histograms are shown of 15. C) PBMCs (n=10) from MDS patients were rested overnight and evaluated for TIGIT expression by flow cytometry. D) PBMCs from healthy donors (HD, n=6) or MDS patients (n=13) were stimulated with IL-15 (10 ng/ml) in the presence of IgG control or anti-TIGIT, and anti-CD16 (1 ug/ml) for 6 hours and assessed for NK cell degranulation and IFN-γ production. E) Purified NK cells (n=6) from healthy blood donors were co-cultured with autologous monocytes or allogeneic MDSCs enriched from the blood of MDS patients at a 2:1 ratio in the presence of IL-15 (10 ng/ml) for 5 days. Following 6 hours stimulation with anti-CD16, degranulation and IFN-γ production was evaluated in conventional and adaptive NK cells by flow cytometry. F) Purified NK cells (n=5) from healthy blood donors were co-cultured with allogeneic MDSCs enriched from the blood of MDS patients at a 2:1 ratio in the presence of IL-15 (10 ng/ml), IgG control (10 ug/ml) and in the presence or absence of anti-TIGIT (10 ug/ml) for 5 days. 6 hours prior staining, cells were stimulated with anti-CD16 and degranulation and IFN-γ production was evaluated in conventional and adaptive NK cells by flow cytometry. Representative data are shown as mean ± SD, and statistical analysis were done on pooled data using the Student’s t test for (A), (C), (D), and Mann-Whitney test for (E) and (F).
We next evaluated the suppressive capacity of MDSCs circulating in the blood of MDS patients on allogeneic NK cells from healthy volunteers. Following 5 days of co-culture in the presence of IL-15 and CD16 stimulation, we observed a marked reduction in allogeneic conventional NK cell function compared to that of adaptive NK cells in the same sample (Figure 7E and Supplementary Figure S3C). TIGIT blockade completely reversed the suppressive function of primary MDSCs from MDS patients on conventional NK cells, while no effect was seen on the CMV-induced adaptive NK cells (Figure 7F). Thus, our data definitively demonstrate that the MDSC suppressive mechanism observed with cytokine-generated MDSCs from normal donors are identical to those of primary MDSCs from MDS patients.
Discussion
There has been an explosion of new data in the immunotherapy literature describing the potential therapeutic benefits of NK and T cell immunotherapy in patients with active cancer (28–31). This excitement is fueled by unexpected strong clinical results with checkpoint blockade against T cell PD-1/PD-L1 and/or CTLA-4 pathways (32,33). While it is well known that NK cell responses to targets are determined by a counterbalance of signals via activating and inhibitory receptors, similar checkpoint blockade mechanisms are less understood. In this study, we identified TIGIT/CD155 as a key axis underlying MDSC-induced suppression of conventional NK cells. Importantly, CMV-induced adaptive NK cells expressed low levels of TIGIT and were not susceptible to MDSC suppression. The function of MDSCs induced from normal blood by cytokine was equivalent to that of MDSCs naturally induced in MDS cancer patients, which highlights the physiologic relevance of these findings and their potential translational importance. There are at least two ways to overcome TIGIT-induced immunosuppression of NK cells. The first is TIGIT blockade, which restores CD16 signaling in conventional NK cells to normal levels. The second is the expansion of MDSC-resistant adaptive NK cells after CMV-exposure.
Immunosuppressive cell types, including MDSCs, accumulate in the tumor microenvironment and exert suppression and diminished tumor clearance (34). In agreement with other studies (26,35,36), we show that cytokine-induced MDSCs suppress polyclonal NK cell proliferation, degranulation and IFN-γ production. However, by segregating adaptive and conventional NK cells, we unexpectedly found that adaptive NK cells are resistant to MDSC suppression. We next investigated this mechanism. We found that TIGIT expression was significantly lower in adaptive NK cells compared with conventional NK cells. In a recent study, Wang et al have shown that different expression levels of TIGIT in NK cells from healthy individuals was associated with functional heterogeneity, and high TIGIT expression inversely correlated with IFN-γ production in response to IL-12 stimulation (20). Our results are in agreement with this study. In addition, we found that TIGIT blockade interferes with DNAM-1 dependent signaling in conventional NK cells. Although CD96 detection decreased following co-culture with MDSC, CD96 blockade had no effect (data not shown) indicating a dominant role of TIGIT to suppress conventional NK cells by CD155 expressing MDSC.
CD112 and CD155 are regulated by cellular stress and bind TIGIT with low and high affinity respectively. Both receptors are highly expressed on transformed cells (37–39). Here we show that conversion of monocytes into MDSCs is associated with increased CD112 and induction of CD155 expression. CD155 but not CD112 expression was dependent on reactive oxygen species (ROS) production, and MDSCs produced high levels of ROS. Corzo et al have shown a substantial up-regulation of ROS by MDSCs in different tumor models and in patients with head and neck cancer. These increases in ROS were explained by greater activity of NADPH oxidase (NOX2) (40). In addition, an earlier study has revealed that the CD155 gene promoter contains a binding site for Nrf-1, a transcription factor regulated by oxidative stress (41). Hence, the CD155 expression may indirectly be controlled by oxidative stress that is generated by an increase in ROS production.
TIGIT engagement on NK cells following co-culture with MDSCs resulted in substantially less phosphorylation of ZAP70/Syk and ERK1/2 compared with NK cells co-cultured with CD155− monocytes. Importantly, blocking TIGIT or inhibiting ROS production reversed this defect in proximal signaling and restored K562 function. We also found that circulating MDSCs from patients with MDS were highly suppressive of conventional NK cells, an effect reversed by TIGIT blockade. This shows that the phenotypic MDSCs induced by MDS are functionally suppressive, a finding that may contribute to the clinical progression of this disease (42).
Our data suggest that it will be of value to consider blockade of TIGIT to enhance the anti-tumor role of NK cells in cancer immunotherapy. Alternatively, clonal expansion of TIGIT-low adaptive NK cells in cancer patients could improve anti-tumor immunotherapy with minimal suppression of the tumor microenvironment. Such expansion is seen after CMV reactivation in immunosuppressed patients (43,44), and could be promoted by infusion of adaptive NK cells or by CMV vaccines. Treatment with CMV envelope glycoprotein B and genetic immunization with dendritic cell CMV vaccines have been shown to be safe and feasible (45–47). In summary, our novel data provide a new perspective on the suppression of different NK cell subsets by MDSCs and highlight the importance of TIGIT in directing this interaction.
Supplementary Material
Acknowledgments
Financial support: This work was supported by a fellowship to D. Sarhan from Karolinska Institutet, Sweden and the following NIH grants: P01 CA111412 (J.S. Miller, S. Cooley, M.R. Verneris), P01 CA65493 (J.S. Miller, S. Cooley, M.R. Verneris, B.R. Blazar), R35 CA197292 (J.S. Miller, S. Cooley, M.R. Verneris), R01 HL122216 (J.S. Miller), R01 AI34495 (B.R. Blazar) and R01 CA72669 (B.R. Blazar). The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA076518 from the National Cancer Institute, the NHLBI and the NIAID.
We would like to thank Andy Price and Carolyn Meyer at the Panoskaltsis-Mortari lab and Guillermo Marques at the imaging center of UMN for helping with the confocal microscopy imaging.
Footnotes
Conflict of interest statement: Dr. JS Miller serves on the Scientific Advisory Board of Celgene, Fate Therapeutics and Oxis Biotech. Dr. BR Blazar declares a financial conflict with Tmunity and Kadmon Corp. Dr. Miller’s and Blazar’s relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict of interest polices. None of these relationships had any role in this research, which was funded by the NIH. The other authors have no conflict of interest to declare.
References
- 1.Kiessling R, Klein E, Pross H, Wigzell H. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. European journal of immunology. 1975;5(2):117–21. doi: 10.1002/eji.1830050209. [DOI] [PubMed] [Google Scholar]
- 2.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity International journal of cancer. Journal international du cancer. 1975;16(2):216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 3.Narni-Mancinelli E, Vivier E, Kerdiles YM. The ‘T-cell-ness’ of NK cells: unexpected similarities between NK cells and T cells. International immunology. 2011;23(7):427–31. doi: 10.1093/intimm/dxr035. [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nature reviews Immunology. 2004;4(3):190–8. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 5.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annual review of immunology. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AK, Wickstrom SL, Tallerico R, Salam S, Lakshmikanth T, Brauner H, et al. Retuning of mouse NK cells after interference with MHC class I sensing adjusts self tolerance but preserves anti-cancer response. Cancer immunology research. 2015 doi: 10.1158/2326-6066.CIR-15-0001. [DOI] [PubMed] [Google Scholar]
- 8.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer research. 2015;75(11):2200–10. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donatelli SS, Zhou JM, Gilvary DL, Eksioglu EA, Chen X, Cress WD, et al. TGF-beta-inducible microRNA-183 silences tumor-associated natural killer cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(11):4203–8. doi: 10.1073/pnas.1319269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(49):20847–52. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Xu Y, Chen L, Xu B, Wu C, Jiang J. B7-H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. International journal of clinical and experimental pathology. 2015;8(8):9428–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Kohlhapp FJ, Broucek JR, Hughes T, Huelsmann EJ, Lusciks J, Zayas JP, et al. NK cells and CD8+ T cells cooperate to improve therapeutic responses in melanoma treated with interleukin-2 (IL-2) and CTLA-4 blockade. Journal for immunotherapy of cancer. 2015;3:18. doi: 10.1186/s40425-015-0063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertucci F, Finetti P, Mamessier E, Pantaleo MA, Astolfi A, Ostrowski J, et al. PDL1 expression is an independent prognostic factor in localized GIST. Oncoimmunology. 2015;4(5):e1002729. doi: 10.1080/2162402X.2014.1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, et al. TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of clinical investigation. 2015;125(11):4053–62. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, et al. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. The Journal of clinical investigation. 2015;125(5):2046–58. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, et al. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. European journal of immunology. 2013;43(8):2138–50. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerboni C, Fionda C, Soriani A, Zingoni A, Doria M, Cippitelli M, et al. The DNA Damage Response: A Common Pathway in the Regulation of NKG2D and DNAM-1 Ligand Expression in Normal, Infected, and Cancer Cells. Frontiers in immunology. 2014;4:508. doi: 10.3389/fimmu.2013.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Zhang H, Li M, Hu D, Li C, Ge B, et al. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell death and differentiation. 2013;20(3):456–64. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inozume T, Yaguchi T, Furuta J, Harada K, Kawakami Y, Shimada S. Melanoma Cells Control Anti-Melanoma CTL Responses via Interaction between TIGIT and CD155 in the Effector Phase. The Journal of investigative dermatology. 2015 doi: 10.1038/JID.2015.404. [DOI] [PubMed] [Google Scholar]
- 20.Wang F, Hou H, Wu S, Tang Q, Liu W, Huang M, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. European journal of immunology. 2015;45(10):2886–97. doi: 10.1002/eji.201545480. [DOI] [PubMed] [Google Scholar]
- 21.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56. doi: 10.1016/j.immuni.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56CD57NKG2C NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 2015 doi: 10.1038/leu.2015.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. The Journal of clinical investigation. 2015;125(9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of immunology. 2009;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. Journal of immunology. 2010;185(4):2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20(15):4096–106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- 27.Warren HS, Campbell AJ, Waldron JC, Lanier LL. Biphasic response of NK cells expressing both activating and inhibitory killer Ig-like receptors. Int Immunol. 2001;13(8):1043–52. doi: 10.1093/intimm/13.8.1043. [DOI] [PubMed] [Google Scholar]
- 28.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105(8):3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 29.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. The New England journal of medicine. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. The Lancet Oncology. 2015;16(5):522–30. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 34.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature immunology. 2013;14(10):1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50(3):799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–26. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 axis regulates human T cell function. Journal of immunology. 2012;188(8):3869–75. doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, et al. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nature reviews Immunology. 2015;15(4):243–54. doi: 10.1038/nri3799. [DOI] [PubMed] [Google Scholar]
- 40.Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. Journal of immunology. 2009;182(9):5693–701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solecki D, Bernhardt G, Lipp M, Wimmer E. Identification of a nuclear respiratory factor-1 binding site within the core promoter of the human polio virus receptor/CD155 gene. The Journal of biological chemistry. 2000;275(17):12453–62. doi: 10.1074/jbc.275.17.12453. [DOI] [PubMed] [Google Scholar]
- 42.Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, et al. Induction of myelodysplasia by myeloid-derived suppressor cells. The Journal of clinical investigation. 2013;123(11):4595–611. doi: 10.1172/JCI67580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis ZB, Cooley SA, Cichocki F, Felices M, Wangen R, Luo X, et al. Adaptive Natural Killer Cell and Killer Cell Immunoglobulin-Like Receptor-Expressing T Cell Responses are Induced by Cytomegalovirus and Are Associated with Protection against Cytomegalovirus Reactivation after Allogeneic Donor Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2015;21(9):1653–62. doi: 10.1016/j.bbmt.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. Journal of immunology. 2012;189(10):5082–8. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, et al. Vaccine prevention of maternal cytomegalovirus infection. The New England journal of medicine. 2009;360(12):1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garu A, Moku G, Gulla SK, Chaudhuri A. Genetic Immunization with In Vivo Dendritic Cell Targeting Liposomal DNA Vaccine Carrier Induces Long-lasting Anti-tumor Immune Response. Molecular therapy: the journal of the American Society of Gene Therapy. 2015 doi: 10.1038/mt.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakamura R, Rosa CL, Longmate J, Drake J, Slape C, Zhou Q, et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. The Lancet Haematology. doi: 10.1016/S2352-3026(15)00246-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.