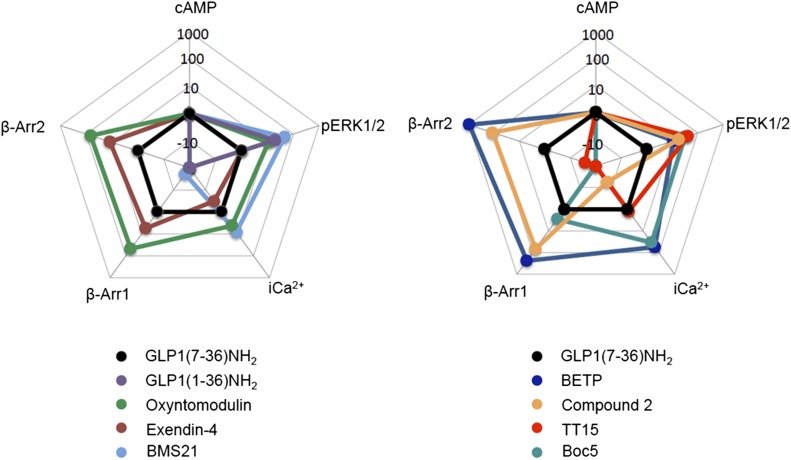
Abstract
The glucagon-like peptide (GLP)-1 receptor (GLP-1R) is a class B G protein–coupled receptor (GPCR) that mediates the action of GLP-1, a peptide hormone secreted from three major tissues in humans, enteroendocrine L cells in the distal intestine, α cells in the pancreas, and the central nervous system, which exerts important actions useful in the management of type 2 diabetes mellitus and obesity, including glucose homeostasis and regulation of gastric motility and food intake. Peptidic analogs of GLP-1 have been successfully developed with enhanced bioavailability and pharmacological activity. Physiologic and biochemical studies with truncated, chimeric, and mutated peptides and GLP-1R variants, together with ligand-bound crystal structures of the extracellular domain and the first three-dimensional structures of the 7-helical transmembrane domain of class B GPCRs, have provided the basis for a two-domain–binding mechanism of GLP-1 with its cognate receptor. Although efforts in discovering therapeutically viable nonpeptidic GLP-1R agonists have been hampered, small-molecule modulators offer complementary chemical tools to peptide analogs to investigate ligand-directed biased cellular signaling of GLP-1R. The integrated pharmacological and structural information of different GLP-1 analogs and homologous receptors give new insights into the molecular determinants of GLP-1R ligand selectivity and functional activity, thereby providing novel opportunities in the design and development of more efficacious agents to treat metabolic disorders.
I. Introduction
Glucagon-like peptide (GLP)-1 is a gastrointestinal peptide hormone secreted from three major tissues in humans, enteroendocrine L cells in the distal intestine, α cells in the pancreas, and the central nervous system, which has multiple therapeutic effects useful in the management of type 2 diabetes mellitus (T2DM). These include most prominently a glucose-dependent insulinotropic function and other actions on glucose homeostasis, as well as benefits to gastric emptying and appetite regulation valuable in reducing food intake and body weight. This hormone exerts its effects by binding to and activating a class B G protein–coupled receptor (GPCR), namely, GLP-1 receptor (GLP-1R). We review the current understanding of the structures of GLP-1 and GLP-1R, the molecular basis of their interaction, and the signaling events associated with it. We also discuss the peptide analogs and nonpeptidic ligands that have been developed to target GLP-1R, the molecular basis of their action, and the implications for ligand-biased activity and allosteric regulation of this hormone-receptor system. Some of these GLP-1R agonists are already in clinical use, with many more currently being developed, and likely to provide enhancements in their ease of administration, tolerability, and effectiveness.
II. Glucagon-Like Peptide-1
A. Discovery
GLP-1 is a member of the incretin family of gastrointestinal hormones (Creutzfeldt, 1979; Baggio and Drucker, 2007; Campbell and Drucker, 2013; Heppner and Perez-Tilve, 2015). In 1906, Moore and his colleagues tested the hypothesis that the pancreas might be stimulated by factors from the gut to help disposal of nutrients and started using porcine small intestine extract to treat diabetic patients (Moore, 1906). In 1928, Zunc and LaBarre were able to show a hypoglycemic effect following injection of secretin extracted from the small intestinal mucosa, and this effect was mediated through the pancreas (Zunz and LaBarre, 1928). Subsequently, the term incrétine (incretin) was introduced by LaBarre for a substance extracted from the upper gut mucosa, which produces hypoglycemia, but does not stimulate pancreatic exocrine secretion (LaBarre, 1932). It was later observed that orally administered glucose evoked a much stronger insulin release than that induced by i.v. injected glucose, supporting the concept of an entero-insular axis, that is, gut factor–stimulated insulin secretion (Elrick et al., 1964; McIntyre et al., 1964; Perley and Kipnis, 1967). The first discovered incretin hormone was gastric inhibitory polypeptide (GIP), which was isolated from crude extracts of the porcine small intestine for its activity to inhibit gastric acid secretion (Brown et al., 1975). This was followed by the observation that GIP could also stimulate insulin secretion in animals and humans, and thus, it was later renamed as glucose-dependent insulinotropic polypeptide, while retaining the same acronym (Dupre et al., 1973; Elahi et al., 1979; Sarson et al., 1984; Creutzfeldt and Ebert, 1985). GIP is released from the K cells of the small intestine. However, antibodies raised against GIP did not abolish the incretin effect, implying the existence of other prominent gut insulinotropic factors (Ebert and Creutzfeldt, 1982).
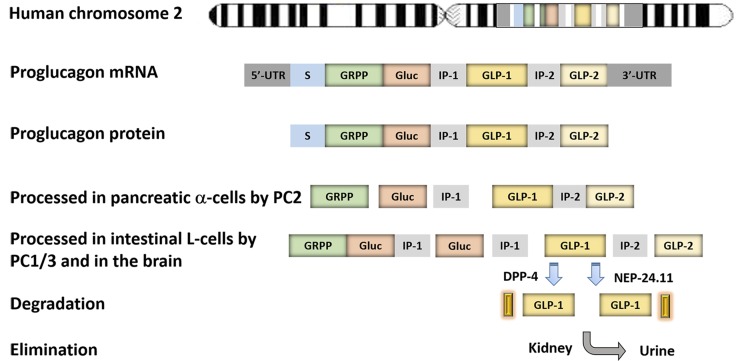
In 1981, GLP-1, the second incretin hormone, was identified in the translational products of mRNAs isolated from the pancreatic islets of anglerfish (Lund et al., 1981; Shields et al., 1981). Subsequently, both GLP-1 and GLP-2 were confirmed from cloned hamster and human preproglucagon cDNAs, but only GLP-1 was able to stimulate insulin secretion (Bell et al., 1983a, 1983b; Mojsov et al., 1987). The proglucagon gene is expressed in the α cells of the pancreas, the L cells of the intestine, and neurons in the caudal brainstem and hypothalamus (Mojsov et al., 1986; Drucker and Asa, 1988) (Fig. 1). Although its transcription produces the same single mRNA in these cell types, the 180-residue preproglucagon protein translated from it is cleaved differently in the pancreas than in the intestine (and brain) by differential posttranslational processing: the former releases glicentin-related pancreatic peptide, glucagon, intervening peptide 1 (IP1), and major proglucagon fragment (containing GLP-1, IP1, and GLP-2 as a single fusion peptide), whereas the latter releases glicentin, oxyntomodulin, GLP-1, IP1, and GLP-2 (Mojsov et al., 1986) (Fig. 1). Endogenous GLP-1 exists in two forms: one corresponds to proglucagon 78–107 with its C-terminal Arg amidated, that is, GLP-17–36 amide, and the other is longer and not amidated, GLP-17–37 (Holst et al., 1987; Orskov et al., 1989). Both have similar biologic activities, although the amide form may have slightly improved stability in the circulation (Wettergren et al., 1998). GLP-1, but not GLP-2, was later demonstrated to enhance glucose-stimulated insulin secretion in response to nutrient ingestion (Schmidt et al., 1985; Kreymann et al., 1987; Mojsov et al., 1987; Orskov et al., 1987).
Fig. 1.
Gene structure, expression, processing, degradation, and elimination of proglucagon. The proglucagon gene is located in human chromosome 2 and transcribed as one single mRNA in three major tissues, namely, the pancreas, the intestine, and the CNS. The mRNA is first translated into one single protein and then processed by prohormone convertase (PC) in different tissues. In the pancreatic α cells, proglucagon protein is processed by PC2 into glicentin-related polypeptide (GRPP), glucagon (Gluc), intervening peptide-1 (IP-1), and major proglucagon fragment, whereas in L cells of the small intestine and the brain, proglucagon is processed by PC1/3 into oxyntomodulin, intervening peptide-2 (IP-2), GLP-1, and GLP-2. GLP-1 is degraded by DPP-4 via cleavage of two amino acids from the N terminus, or by NEP-24.11 through cleavage of the C terminus in vivo. The cleaved products are eventually eliminated in the kidney. UTR, untranslated region.
The half-life of GLP-1 peptide in the circulation is very short (less than 2 minutes). Its rapid inactivation is mainly due to the cleavage of two amino acids from the N terminus by the ubiquitous proteolytic enzyme dipeptidyl peptidase-4 (DPP-4) (Deacon et al., 1995b) (Fig. 1). In addition, a membrane-bound zinc metallopeptidase, neutral endopeptidase 24.11 (NEP 24.11), has also been shown to cleave GLP-1 at its C terminus both in vitro and in vivo (Plamboeck et al., 2005). The metabolites of GLP-1 are then subject to renal clearance by several mechanisms (Ruiz-Grande et al., 1993).
B. Physiology
The main action of GLP-1 is to work as an incretin, that is, as a gut-derived hormone capable of potentiating insulin secretion in the presence of high plasma glucose levels. The incretin concept came from observations that insulin release was higher when the glucose was administered orally rather than i.v., even when the same plasma glucose concentration was achieved from both routes (McIntyre et al., 1964; Perley and Kipnis, 1967). Although many hormones were originally suspected to contribute to the incretin effect, the current view is that GLP-1 and GIP are responsible for most incretin activity normally observed (Holst and Orskov, 2001; Vilsboll and Holst, 2004; Creutzfeldt, 2005; Campbell and Drucker, 2013). Oral administration of glucose results in a two- to threefold greater insulin secretion than i.v. glucose. Both GLP-1 and GIP can enhance insulin secretion after a mixed meal, but GLP-1 is more potent than GIP (Nauck et al., 1993b; Elahi et al., 1994). In the human circulation, GIP concentration is eightfold higher than GLP-1; in type 2 diabetic patients, GLP-1 has more activity than GIP, but their effects on insulin secretion seem to be additive (Nauck et al., 1993a, 2004; Elahi et al., 1994).
1. Effect on Glucose Homeostasis.
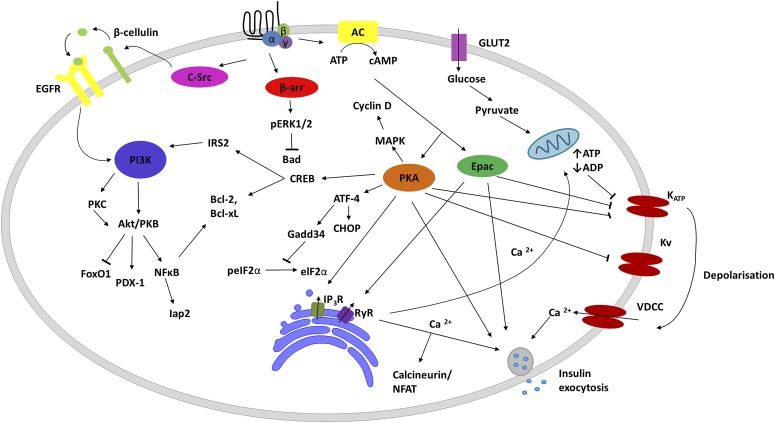
The insulinotropic activity of GLP-1 is strictly glucose-dependent mediated through its receptor at the membrane of pancreatic β cells (Kreymann et al., 1987; Mojsov et al., 1987; Holst, 2007). GLP-1 could not stimulate insulin secretion at low levels of glucose in humans (Kreymann et al., 1987). GLP-1R belongs to the class B GPCR subfamily whose members include receptors for peptidic hormones such as glucagon, secretin, GIP, etc. The binding of GLP-1 to its receptor activates heterotrimeric Gs protein, which subsequently elicits adenylate cyclase activity, resulting in cAMP formation (Gromada et al., 1998, 2004). The increased level of cAMP in turn leads to activation of protein kinase A (PKA) and the cAMP-regulated guanine nucleotide exchange factor II (Ozaki et al., 2000). In the presence of high levels of glucose, GLP-1 has an effect on ATP-sensitive (KATP) or voltage-gated potassium and calcium channels, resulting in membrane depolarization and Ca2+ release from both internal and extracellular stores. Increased Ca2+ together with cAMP will then promote exocytosis of vesicles containing insulin (Prentki and Matschinsky, 1987; Renstrom et al., 1997). This glucose-dependent insulinotropic action of GLP-1 involves the glucose transporter, metabolic ADP/ATP ratio, KATP inhibition, Ca2+ channel opening, and, ultimately, insulin secretion (Gromada et al., 2004; Dyachok et al., 2006).
Another major activity of GLP-1 to reduce blood glucose relates to the suppression of glucagon secretion from α cells of the endocrine pancreas (Gromada and Rorsman, 2004). Glucagon is a major hyperglycemic hormone, in addition to epinephrine, and its release is reciprocally correlated with insulin secretion in glucose oscillation to mobilize hepatic glucose in the fasting state, thereby helping to ensure the maintenance of normoglycemia. In T2DM, both fasting hyperglucagonemia and exaggerated glucagon responses, which most likely contribute to the hyperglycemia of patients, were observed (Shah et al., 2000; Toft-Nielsen et al., 2001). Interestingly, only GLP-1, but not GIP, inhibits glucagon secretion (Nauck et al., 1993b). However, the detailed mechanism(s) by which GLP-1 suppresses glucagon secretion remains unclear. Because the levels of GLP-1 mRNA detected in α cells varied between none and 20% of a cell population, it is generally thought that local elevated insulin and somatostatin in response to GLP-1 stimulation are capable of suppressing glucagon secretion in α cells (Orskov et al., 1988; Heller et al., 1997; de Heer et al., 2008; Godoy-Matos, 2014). Nonetheless, in type 1 diabetic patients with absent β cell activity who lack insulin and somatostatin, GLP-1 could still reduce glucose concentrations, suggesting a direct suppression of glucagon secretion (Creutzfeldt et al., 1996; Gromada and Rorsman, 2004).
2. Effect on Gastric Emptying.
GLP-1 also has an important inhibitory activity on gut motility and gastrointestinal secretion (Wettergren et al., 1993; Nauck et al., 1997). It not only inhibits meal-induced pancreatic secretion, but also the gastric emptying process in humans (Wettergren et al., 1993). Its suppression of gastrin-induced acid secretion was demonstrated by injection of GLP-1 and/or peptide YY. Both peptides are released from L cells in the ileal mucosa of healthy people (Wettergren et al., 1997) and can exhibit additive effects on gastrin-stimulated acid secretion, a function of unabsorbed nutrients in the ileum (Holst, 1997). It was subsequently shown that this inhibitory action of GLP-1 is mediated via a vagal pathway (Wettergren et al., 1994). This ileal-brake activity of GLP-1 was further demonstrated using the GLP-1R antagonist exendin9–39, and therefore, is believed to have physiologic relevance (Schirra et al., 2006; Maljaars et al., 2008).
3. Effect on Food Intake.
Another physiologic function of GLP-1 concerns inhibition of food intake that may have therapeutic value for body weight reduction. Whether this is related to its ileal brake effect is still debated. At least two neural mechanisms, central and peripheral, are involved in GLP-1 suppression of appetite and food intake. GLP-1 is expressed in the neurons of the brain stem, and GLP-1R is present in the hypothalamic areas that control energy homeostasis and food intake, including the arcuate nucleus, paraventricular nucleus, and dorsomedial nucleus (Jin et al., 1988; Kanse et al., 1988; Zheng et al., 2015). Intracerebroventricular injection of GLP-1 inhibits food intake in rats, and this activity is blocked by exendin9–39 (Tang-Christensen et al., 1996; Turton et al., 1996), or by the arcuate nucleus-damaging reagent, monosodium glutamate (Tang-Christensen et al., 1998). In contrast, GLP-1 released by L cells of the intestine after a meal inhibits gut mobility and gastric emptying, allowing nutrients in the ileum to reduce food intake (Read et al., 1994). Indeed, infusion of GLP-1 into normal human subjects significantly enhances satiety and decreases food intake (Flint et al., 1998). Consistent findings have shown that GLP-1R agonism promotes weight loss and improves glucose homeostasis in rodents, monkeys, and humans (Verdich et al., 2001; Barrera et al., 2011), and such weight-reducing properties have also been well-documented for two marketed GLP-1 mimetics, exenatide (exendin-4) and liraglutide (Moretto et al., 2008; Astrup et al., 2009; Norris et al., 2009; Lean et al., 2014). Although the exact mechanism mediating reduced food intake by peripherally administered GLP-1 has yet to be elucidated, it may involve signals generated by GLP-1 binding to its receptors on neurons in the gastrointestinal tract or hepatoportal bed (Burcelin et al., 2001; Holst, 2007).
4. Effect on Cardiovascular Activity.
Recently, there is increasing evidence suggesting that GLP-1 may play a crucial role in the cardiovascular system (Grieve et al., 2009; Ussher and Drucker, 2014). GLP-1R is widely expressed in the heart and blood vessels, such as vascular smooth muscle, cardiomyocytes, endocardium, and coronary endothelium/smooth muscle, in both rodents and humans (Campos et al., 1994; Wei and Mojsov, 1995; Bullock et al., 1996). In an early study, treatment of adult rat cardiac myocytes with GLP-1 increased cAMP levels, but did not lead to increased cardiomyocyte contractility, as would be anticipated in the heart (Vila Petroff et al., 2001). Interestingly, treatment of mouse cardiomyocytes with GLP-19–36 amide (a GLP-1 degradation product in vivo) resulted in Akt activation, extracellular regulated kinase (ERK) phosphorylation, and reduced apoptosis induced by hypoxia or hydrogen peroxide stress, implying an unconventional action of GLP-1 (Ban et al., 2010). In a rat ischemia-reperfusion model using isolated perfused heart and whole animal, GLP-1 significantly decreased infarction size, and this protection was abolished by exendin9–39, as well as inhibitors of adenylyl cyclase, phosphatidylinositol 3-kinase (PI3K), and p42/44 mitogen-activated protein kinase (MAPK), suggesting that these pathways were involved in GLP-1–mediated cardio-protection (Bose et al., 2005a). A direct action of GLP-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts was also observed (Zhao et al., 2006). GLP-1 treatment significantly increased glucose uptake and decreased left ventricular end-diastolic and developed pressures. Infusion of GLP-1 into live dogs increased myocardial glucose uptake, which could be blocked by p38 MAPK kinase or endothelial nitric oxide synthase inhibitors, pointing to a direct effect of GLP-1 in myocardium (Nikolaidis et al., 2004a; Bhashyam et al., 2010). Meanwhile, GLP-1R was detected in human coronary artery endothelial cells and umbilical vein endothelial cells, and GLP-1 or exendin-4 treatment led to nitric oxide production in both cell types, indicative of GLP-1 involvement in the vasculature (Nystrom et al., 2004; Erdogdu et al., 2010; Ishii et al., 2014). GLP-1 infusion into rats also increased heart rate and blood pressure, thereby reflecting its direct role in the heart (Barragan et al., 1996). In recent clinical trials with either GLP-1R agonists or DPP-4 inhibitors, general beneficial effects of GLP-1 on the cardiovascular system in both normal and diabetic subjects have been revealed, an interesting finding that may lead to potential new treatment of cardiovascular diseases, although these effects require a long-term validation (van Genugten et al., 2013; Avogaro et al., 2014; Ussher and Drucker, 2014; see V. Pharmaceutical Development and Therapeutics).
5. Effect on Immune Response.
GLP-1 can also regulate immune responses. Its receptor mRNA was discovered in multiple immune cell types from mice, including thymoyctes, splenocytes, bone marrow–derived cells, regulatory T cells, macrophages, and invariant natural killer T cells (Hadjiyanni et al., 2010; Hogan et al., 2011; Panjwani et al., 2013). Liraglutide treatment of patients with psoriasis, an inflammatory condition associated with metabolic diseases such as obesity, diabetes, and dyslipidemia, led to improvement of psoriasis area and severity index as well as decreased cytokine secretion from invariant natural killer T cells in a glycemic control-independent manner (Hogan et al., 2011). High-fat diet–fed mice treated with exendin-4 displayed decreased mRNA levels of the proinflammatory cytokines monocyte chemoattractant protein 1, tumor necrosis factor-α, and signal transducer and activator of transcription 3 (Koehler et al., 2009), and, in a type 1 diabetes animal model in which islets were transplanted into nonobese diabetic mice, GLP-1/gastrin treatment increased the number of transforming growth factor-β1–secreting lymphocytes and decreased IFN-γ–secreting lymphocytes with delayed onset of diabetes (Suarez-Pinzon et al., 2008).
6. Effect on Kidney Function.
Some experimental data point to the participation of GLP-1 in kidney function. Infusion of GLP-1 into healthy and obese human subjects enhanced sodium excretion, urinary secretion, and glomerular filtration rate, suggesting a renal protective effect of this peptide (Gutzwiller et al., 2004). In rats, GLP-1 was able to downregulate Na+/H+ exchanger isoform 3 (NHE3) in the renal proximal tubule, implying a potential therapeutic value for hypertension and disorders of sodium retention (Crajoinas et al., 2011). Rats administered with exendin-4 significantly improved renal function and reduced inflammation, proteinuria, and fibrosis in the kidney via a mechanism that was independent of glucose lowering (Kodera et al., 2011).
7. Effect on Nervous System.
In addition to the metabolic function in the brain, GLP-1 may also exert neuroprotective and neurotropic effects (Heppner and Perez-Tilve, 2015). The aggregation of amyloid β protein (Aβ) and the microtubule-associated protein Tau cause senile plaques and neurofibrillary tangles, resulting in loss of long-term potentiation, one of the major characteristics of Alzheimer’s disease (AD). Infusion of GLP-1 or exendin-4 into the lateral ventricles of mice decreased endogenous level of Aβ, and treatment of rat hippocampus neurons with GLP-1 and exendin-4 prevented Aβ-induced cell death (Perry et al., 2003). Intracerebroventricular dosing of GLP-1 enhanced synaptic plasticity in the hippocampus and completely reversed impairment in long-term potentiation caused by subsequent injection of Aβ (Gault and Holscher, 2008). GLP-1R was found in nigrostriatal neurons, and loss of these neurons is a feature of Parkinson’s disease (PD). GLP-1 or exendin-4 supported cell viability during hypoxic injury in primary neurons from rat cerebral cortical tissues; this activity was blocked by GLP-19–36 (a GLP-1R antagonist) and not observed in neurons from GLP-1−/− mice (Li et al., 2009). Exendin-4 also maintained cell viability and reduced apoptosis caused by H2O2-induced oxidative stress in NSC19 neuronal cells, a spinal cord cell line with similarities to cells in the central nervous system (CNS) (Li et al., 2012b).
Obviously, GLP-1 and GLP-1R are widely expressed in many tissues, and their physiologic actions have been incrementally elucidated, especially after the extensive clinical application of GLP-1 mimetics, including GLP-1R agonists and DPP-4 inhibitors, in the last decade. Although its roles in some tissues, such as adipocytes or skeletal muscles, are still illusive, GLP-1–related therapies have clearly provided multiple benefits to millions of patients suffering from metabolic disorders, and such effects are predominantly, but perhaps not always, based on signaling pathways mediated by its receptor.
C. Structure
Structurally, class B GPCRs consist of a large N-terminal extracellular domain (ECD) and a seven-transmembrane (7TM) helix domain, comprising the GPCR signature of seven membrane spanning α-helices [transmembrane (TM)1–7], connected by three extracellular (ECL) and intracellular (ICL) loops, and a C-terminal helix 8 (Schioth et al., 2003; Hollenstein et al., 2014). Pharmacological studies with truncated, chimeric, and mutated ligand and receptor variants, together with peptide ligand-bound ECD crystal structures and the first 7TM crystal structures of class B GPCRs, have provided the basis for a two-domain–binding mechanism of peptide hormone ligands to secretin-like class B GPCRs (Parthier et al., 2009; Donnelly, 2012; Hollenstein et al., 2014). According to this peptide ligand-binding mechanism: 1) the C terminus of the peptide ligand forms an initial complex with the ECD, and this allows 2) the N terminus of the peptide ligand to interact with the 7TM domain (7TMD) and activate the class B GPCR to couple to G proteins and other effectors to mediate intracellular signaling processes (see III. Glucagon-Like Peptide-1 Receptor). This section gives an overview of the structure–activity relationship (SAR) of GLP-1 peptides, thereby providing important information regarding the molecular determinants of ligand binding and functional activities at the GLP-1R. The development of GLP-1 analogs is described in detail in V. Pharmaceutical Development and Therapeutics, whereas SAR of GLP-1R will be discussed explicitly in III. Glucagon-Like Peptide-1 Receptor. Alignments of the sequences and structures of GLP-1 with GLP-2, glucagon, GIP, and exendin-4 are presented in Fig. 2. This also annotates the structural properties of GLP-1, effects of GLP-1 mutation studies, and GLP-1R and exendin-4 interaction sites in the corresponding GLP-1R ECD crystal structures (Runge et al., 2008; Underwood et al., 2010). Peptide ligand residues shown are annotated as three-letter amino acid codes with residue number as superscripts (e.g., His7, histidine at position 7), whereas receptor residue numbers are annotated as single-letter codes, Uniprot numbers, and Ballesteros-Weinstein/Wootten numbers/secondary structure motif as superscripts, according to IUPHAR guidelines (Pawson et al., 2014) and class B GPCR residue-numbering guidelines (Wootten et al., 2013c; Isberg et al., 2015), respectively. According to the Ballesteros–Weinstein class A GPCR (Ballesteros and Weinstein, 1995) and Wootten class B GPCR (Wootten et al., 2013c) residue-numbering schemes, the single most conserved residue in each TM helix is designated X.50 (Ballesteros-Weinstein number used for comparison within class A GPCRs as well as to compare across GPCR classes) or X.50b (Wootten number for comparison with class B GPCRs). X is the TM helix number, and all other residues in that helix are numbered relative to this conserved position (Hollenstein et al., 2014). GLP-1R residues that are most conserved in secretin-like class B GPCRs are S1551.50b, H1802.50b, E2473.50b, W2744.50b, N3205.50b, G3616.50b, and G3957.50b. GLP-1 and glucagon peptide ligands start with amino acid residue 7 (His7) due to post-translational processing (Fig. 2), whereas the homologous GLP-2, GIP, and exendin-4 start with His1 and Tyr1, respectively.
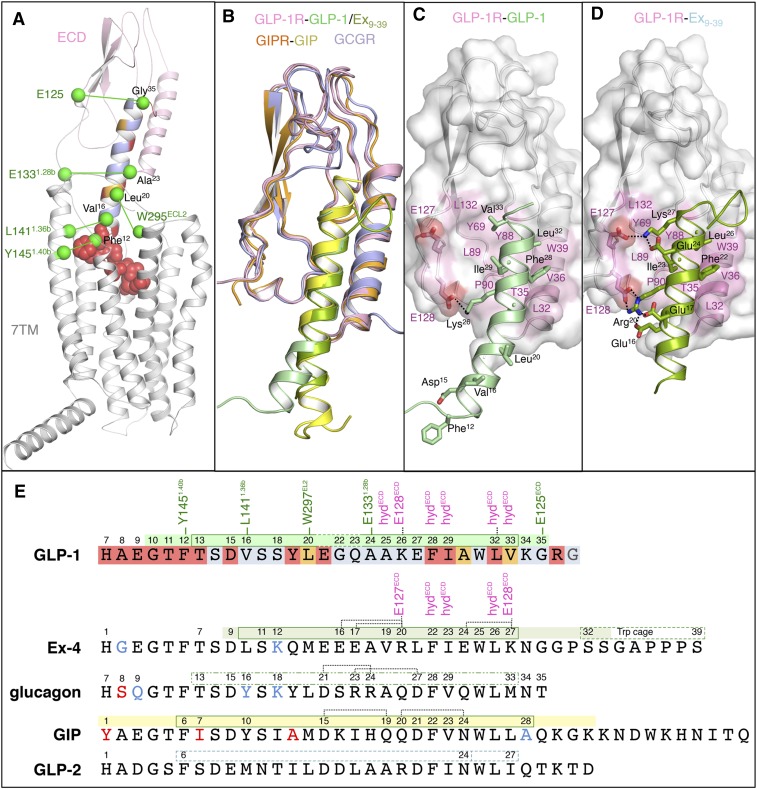
Fig. 2.
Structural characteristics of GLP-1 and its cognate receptor. (A) GLP-1–bound full-length GLP-1R homology model based on a previously published full-length glucagon-bound GCGR model combining structural and experimental information from the GCGR 7TMD crystal structure (PDB: 4L6R), the GCGR ECD structure (PDB: 4ERS), and the ECD structure of GLP-1–bound GLP-1R (PDB: 3IOL), complemented by site-directed mutagenesis, electron microscopy, hydrogen-deuterium exchange, and cross-linking studies (Siu et al., 2013; Yang et al., 2015b, 2016). The C-terminal helix of GLP-1 bound to the ECD region of GLP-1R is depicted as cartoon, whereas the atoms of the flexible N-terminal region of GLP-1 predicted to be bound to the 7TMD of GLP-1R are depicted as spheres. GLP-1 is color coded according to mutation effects (blue: <fourfold effect, orange: 4- to 10-fold effect, red: >10-fold effect IC50; see II. Glucagon-Like Peptide-1); mutation effects of GLP-1R are reported in Fig. 3 and Table 1. The Cα/Cβ atoms of GLP-1/GLP-1R residue pairs identified in photo cross-linking studies (Chen et al., 2009, 2010b; Miller et al., 2011) are depicted as green-colored spheres. (B) Structural alignment of the ECD structures of GLP-1 and exendin9–39–bound GLP-1R (PDB: 3IOL, 3C59), GIP-bound GIPR (PDB: 2QKH), and the mAb23-bound GCGR ECD structure (PDB: 4ERS). Comparison of the crystal structure binding modes of (C) GLP-1 and (D) exendin9–39. The surfaces of GLP-1R residues involved in important apolar interactions with GLP-1/apolar are colored pink, whereas residues involved in polar interactions described in II. Glucagon-Like Peptide-1 are also depicted as sticks (and their H-bond interaction networks are depicted as dashed lines). (E) Structure-based sequence alignment of GLP-1, exendin9–39, glucagon, GIP, and GLP-2. The regions of the peptide ligands solved in ECD–ligand complex crystal structures are marked above the amino acid sequences using the same color coding as in (B). Amino acids of GLP-1 are marked according to mutation study effects, as indicated in (A). The residues that are boxed are found in an α-helical conformation in the crystal structure complex (solid lines: GLP-1, exendin9–39, GIP) or in NMR studies in micelle DPC (dashed lines: glucagon, GLP-2), as described in II. Glucagon-Like Peptide-1.
1. α-Helical C-Terminal Region.
NMR, circular dichroism (CD) spectroscopy, and X-ray crystallography studies show that GLP-1 is unstructured in aqueous solution, but adopts a helical structure in a membrane-like environment and by binding to its receptor (Thornton and Gorenstein, 1994; Neidigh et al., 2001; Runge et al., 2007; Underwood et al., 2010). This conformational change upon receptor binding is also proposed for other class B GPCR peptide ligands, including glucagon (Braun et al., 1983; Siu et al., 2013), GIP (Alana et al., 2006; Parthier et al., 2007), and exendin-4 (Runge et al., 2007, 2008) (Fig. 2), implying a conserved receptor-ligand–binding mechanism (Parthier et al., 2009). Two-dimensional NMR experiments indicate that the C-terminal region of GLP-1 (Thr13-Lys34) is in an α-helical conformation in trifluorethanol and dodecylphosphocholine (DPC) micelles, with a less well-defined α-helical region around Gly22 (Thornton and Gorenstein, 1994; Neidigh et al., 2001; Underwood et al., 2010). NMR studies suggest that exendin-4 adopts a more well-defined single helix (Ser11-Lys27) than GLP-1 in DPC micelles (Neidigh et al., 2001). These NMR data are in line with ligand-bound GLP-1R ECD crystal structures showing that the GLP-1 is a kinked but continuous α-helix (Thr13–Val33), whereas the truncated exendin9–39 is a straighter helix (Leu10-Lys27) than GLP-1 when bound to GLP-1R (Runge et al., 2008; Underwood et al., 2010). In the ECD-bound crystal structure, the C-terminal α-helix of GLP-1 (Ala24-Val33) is stabilized by interaction with the ECD of GLP-1R, whereas the N-terminal helix (Thr13-Glu21) does not interact with the ECD. Although it cannot be excluded that the kink in GLP-1 observed in the crystal structure is a result of crystal packing between the N-terminal part of GLP-1 (Gly10-Glu21) and symmetry-related ECDs, the presence of two helical segments is consistent with the SAR studies with conformationally constrained GLP-1 analogs (Miranda et al., 2008; Murage et al., 2008). Cyclization of (mutated) residues Lys16-Glu20 and Met18-Ala22 by a lactam bridge (constraining these regions in an α-helical conformation) does not affect binding affinity and activity, whereas cyclization of residues Thr11-Asp15 even improved potency for GLP-1R compared with the corresponding linear analogs (Miranda et al., 2008; Murage et al., 2008).
Characterization of the stability and conformational changes of full-length, truncated, and GLP-1/exendin-4 chimeric peptides upon binding to the isolated ECD of GLP-1R by far-UV CD, differential scanning calorimetry, and fluorescence spectroscopy measurements demonstrated that exendin-4 has a higher α-helical propensity than GLP-1 in solution (Runge et al., 2007). Combination of these biophysical data with pharmacological studies showed that there is a positive correlation between the stability and α-helical propensity of the ligand in solution and its affinity for the ECD of GLP-1R (Runge et al., 2007). Comparison of the crystal structures of GLP-1–bound and exendin9–39-bound ECD provides possible explanations for the higher stability of the α-helix of exendin-4 as opposed to GLP-1 (Runge et al., 2008; Underwood et al., 2010). Although the α-helix of exendin-4 is stabilized by strong intramolecular ionic interactions between Glu16/Glu17 and Arg20 and between Glu24 and Lys27, corresponding (Gln23, Lys26) or differentially positioned (Glu27, Lys34) polar residues in GLP-1 do not form such intramolecular interactions when bound to the isolated ECD of GLP1-R [Protein Data Bank (PDB): 3IOL]. The GIP-bound GIP receptor (GIPR) ECD crystal structure indicates that the α-helix of GIP is stabilized by similar (Asp15-Gln19) and alternative (Gln20-Asn24) intramolecular H-bond interactions (Parthier et al., 2007), whereas glucagon may also be able to form helix-stabilizing intramolecular H-bond networks (e.g., Asp21-Arg24, Arg23-Asp27) (Siu et al., 2013). In addition to the higher α-helix propensity, the more pronounced amphiphilic nature of exendin-4 enables stronger polar and hydrophobic interactions with the ECD of GLP-1R via opposite sides of the α-helix than GLP-1 (vide infra). Moreover, exendin-4 is eight residues longer than GLP-1, and NMR studies have shown that this extended C-terminal region, comprising Ser32-Ser39, forms a stable tertiary structure that folds around Trp25 in trifluorethanol and glycol (Neidigh et al., 2001). This Trp-cage conformation is, however, not observed in NMR studies in DPC micelles (Neidigh et al., 2001) and has weak electron density in the exendin9–39-bound ECD GLP-1R crystal structure (Runge et al., 2008), suggesting that a stable Trp-cage conformation (which is absent in GLP-1) is only partially populated in the receptor-bound state of exendin-4.
2. Flexible N-Terminal Region.
NMR and X-ray crystallography studies indicate that the N-terminal regions preceding the conserved α-helix of GLP-1 (His7-Thr13), exendin-4 (His1-Thr7), GIP (Tyr1-Ile7), glucagon (His7-Thr13), and other class B GPCR peptide ligands are flexible in solution as well as in membrane-bound and ligand-bound states (Braun et al., 1983; Neidigh et al., 2001; Parthier et al., 2007; Runge et al., 2008; Underwood et al., 2010). The structure of the N-terminal region of GLP-1 (His7-Thr13) was not clearly elucidated in NMR studies in DPC micelles due to high conformational flexibility (Neidigh et al., 2001). In the ECD-bound GLP-1 crystal structure, this N-terminal region is unstructured, and no electron density was observed for His7-Glu9 (Underwood et al., 2010). Whereas the GLP-1R ECD crystal structures provide atomic details of the molecular interactions of the C-terminal regions of GLP-1 and exendin9–39 with the ECD of GLP-1R (Runge et al., 2008; Underwood et al., 2010), these structures do not give information on the receptor-bound conformation of the flexible ligand N terminus, nor on its interactions with GLP-1R. Ligand and receptor mutation studies suggest that an extended flexible conformation of these first residues allows the peptide ligands of class B GPCRs to reach deep into the pocket (Hollenstein et al., 2014). This receptor-bound peptide conformation is proposed to be stabilized by an amino acid motif (Thr11-Phe12-Thr13 in GLP) that is conserved in class B GPCR ligands (Neumann et al., 2008), which can induce an N-capping conformation similar to the one observed in the receptor-bound NMR structure of pituitary adenylate cyclase–activating polypeptide (PACAP) (Inooka et al., 2001). Substituting three residues in the flexible N terminus of GLP-1 by corresponding PACAP residues (Ala8Ser/Glu9Asp/Thr11Ile) does not affect affinity and potency for GLP-1R (Xiao et al., 2001), implying that GLP-1 adopts a similar conformation to PACAP upon binding to its receptor. Recently, NMR structures of an 11-mer GLP-1 analog were solved in alterative conformations containing a C-terminal α-helix (PDB: 2N08) and an N-terminal β-turn (PDB: 2N09), and stabilization of these conformations by cyclization cross-links (PDB: 2N0N and 2N0I) differentially influenced GLP-1R–binding affinity and agonist potency (Hoang et al., 2015). The accumulated ligand and receptor SAR (see II. Glucagon-Like Peptide-1 and III. Glucagon-Like Peptide-1 Receptor) suggests that a flexible conformation of the first seven residues allows GLP-1 to interact with residues in the 7TM-binding pocket of GLP-1R, and that the ligand N terminus may adopt a more constrained conformation to activate the receptor.
3. Interaction between the C-Terminal Helix and ECD.
Comparison of the crystal structures of GLP-1 and exendin9–39-bound GLP-1R ECD (Runge et al., 2008; Underwood et al., 2010), GIP-bound GIPR ECD (Parthier et al., 2007), and the antibody bound glucagon receptor (GCGR) ECD (Koth et al., 2012) provides information about similarities and differences in class B GPCR-bound peptide ligand conformations, and gives insights into the structural determinants of class B GPCR ligand recognition and selectivity (Fig. 2). The crystal structures of the ECDs of different class B GPCRs show that this domain has a conserved fold, including two central antiparallel β-sheets (β1-β4) and an N-terminal α-helix (α1) interconnected by several loops and stabilized by three conserved disulfide bonds (Donnelly, 2012; Pal et al., 2012; Hollenstein et al., 2014) (for more detailed description of the ECD of GLP-1R, see III. Glucagon-Like Peptide-1 Receptor). Class B GPCR ECD peptide ligand complexes exhibit overall a similar binding mode in which the C terminus of the peptide ligand adopts an α-helical conformation that binds between α1 and β1–β4 of the ECD (Parthier et al., 2009; Donnelly, 2012; Hollenstein et al., 2014). The conserved structural fold and binding orientation suggest that common mechanisms underlie ligand recognition of class B GPCRs and indicate that peptide ligand selectivity is in part determined by specific interactions of the C-terminal α-helix of the ligand with the ECD. The ligand-binding mode observed in the GLP-1R crystal structure is consistent with GLP-1 (vide infra) and GLP-1R (see III. Glucagon-Like Peptide-1 Receptor) mutation studies, as well as photo cross-linking experiments placing Gly35 located in the α-helix of GLP-1 in close proximity to E125ECD located in the linker region between the ECD and 7TMD of GLP-1R (Chen et al., 2009). The structural details of the ECD and 7TMD of GLP-1R are discussed in detail in III. Glucagon-Like Peptide-1 Receptor.
The α-helices of GLP-1, exendin-4, glucagon, and GIP are amphiphilic, containing a hydrophilic and hydrophobic region at opposite sides of the helix. Conserved apolar residues in the C-terminal part of GLP-1 (Phe28, Ile29, Leu32), exendin-4 (Phe22, Ile23, Leu26), GIP (Phe22, Val23, Leu26), and glucagon (Phe28, Val29, Leu32) share a similar hydrophobic interaction site with the ECD of the corresponding receptor (GLP-1R/GIPR/GCGR: L32ECD/A32/M29, T35ECD/L35/L32, V36ECD/Y36/F33, and W39ECD/39/36 in α1, Y69ECD/68/65 in β-turn 1 connecting β1-β2, Y88ECD/87/84, L89ECD/88/85, and P90ECD/89/86 in β-turn 2 connecting β3-β4) (Parthier et al., 2007; Runge et al., 2008; Underwood et al., 2010; Koth et al., 2012). Differences in hydrophobic/polar interactions with the N-terminal part of the ligand α-helix as well as ligand-specific ionic interactions with the ECD can partially explain the different relative affinities of GLP-1, exendin-4, GIP, and glucagon for the ECD of the corresponding receptor. The positively ionizable Lys26 of GLP-1 and homologous Arg20 of exendin9–39 both form ionic interactions with E128ECD in the C-terminal region of the ECD of GLP-1R. The Lys27 residue of exendin9–39 forms an additional ionic interaction with E128ECD in GLP-1R (Runge et al., 2008; Underwood et al., 2010), which in combination with the higher α-helical propensity of exendin-4 compared with GLP-1 (Runge et al., 2007) might explain the increased affinity of exendin-4/exendin9–39 for the isolated ECD of GLP-1R compared with GLP-1. There is no ionic interaction between peptide ligand and ECD side chains observed in the GIP-bound GIPR ECD crystal structure and the ECD GCGR-glucagon docking model based on the antibody-bound GCGR and GLP-1–bound GLP-1R crystal structures. It should be noted, however, that the apolar residues of GLP-1 (Val33), GIP (Leu27), and glucagon (Met33) that are aligned with Lys27 of exendin9–39 form an additional hydrophobic interaction site with the ECD of GLP-1 (Y69ECD/L123ECD), GIPR (Y68ECD /H115ECD), and GCGR (Y65ECD/A118ECD), respectively. Ala24 and Ala25 of GLP-1 and Val19 in exendin-4 form another hydrophobic interaction surface with α1 of the ECD of GLP-1R. The structurally aligned Gln19 of GIP forms an H-bond network with Asp15 and the N-terminal backbone of α1 of the GIPR ECD (Q30ECD and A32ECD), whereas corresponding residues in glucagon (Arg24, Ala25) may form a polar/apolar interaction site with the N-terminal region of the α1 helix of GCGR (Fig. 2).
4. Interaction between Flexible N terminus and 7TMD.
The peptide ligand-bound crystal structures of the ECDs of class B GPCRs (including GLP-1– and exendin-4–bound GLP-1R, GIP-bound GIPR, and antibody-bound GCGR) do not provide information about the molecular interactions between the receptor and the flexible N-terminal region of peptide ligands. Receptor/ligand mutagenesis and photo cross-linking studies nevertheless indicate that the 7TMD of class B GPCRs determines binding (selectivity) of the flexible N-terminal region of peptide ligands (Donnelly, 2012; Pal et al., 2012; Hollenstein et al., 2014). A recently reported full-length GCGR–glucagon model based on the crystal structures of the GCGR 7TMD (Siu et al., 2013), the antibody-bound GCGR ECD (Koth et al., 2012), the GLP-1–bound GLP-1R ECD (Underwood et al., 2010), and the N-capped conformation of PACAP (Inooka et al., 2001) offers a template for full-length GLP-1–bound GLP-1R models (Fig. 2A) that is consistent with the results of mutation studies of GLP-1 (vide infra), GLP-1R (see III. Glucagon-Like Peptide-1 Receptor), and other class B GPCRs (Hollenstein et al., 2014). This full-length GLP-1R model furthermore satisfies spatial constraints defined by GLP-1R cross-linking studies connecting the following: 1) Ala24 in the C-terminal part of the α-helix of GLP-1 to E133ECD in the region linking the ECD and 7TMD of GLP-1R (not solved in the GLP-1R crystal structure); 2) Leu20 in the N-terminal part of the α-helix of GLP-1 to W297ECL2 in ECL2 between TM4 and TM5 in GLP-1R; 3) Phe12 and Val16 in the flexible N-terminal region of GLP-1 that are positioned near Y1451.40b and L1411.36b in the TM1 of GLP-1R, respectively; and 4) a photolabile probe at position 6 (one position before His7) in GLP-1 to Y205ECL1 in ECL1 between TM2 and TM3 in GLP-1R (Chen et al., 2009, 2010b; Miller et al., 2011) (Fig. 2A).
D. Mutagenesis
The molecular determinants of GLP-1R ligand binding and functionality have been investigated extensively using truncated, chimeric, and site-specifically substituted GLP-1, exendin-4, and peptide ligands of closely related class B GPCRs (including glucagon and GIP). This section gives an overview as to how these data complement the structural information on GLP-1 (and the ECD–GLP-1 complex) described above. It should be noted that the current overview only focuses on changes to natural amino acids, as the incorporation of photoactive labels in GLP-1 for GLP-1/GLP-1R cross-linking studies (Chen et al., 2009, 2010b; Miller et al., 2011) is covered in this section, whereas the development of GLP-1 analogs by other modifications, for example, unnatural amino acid substitution and conjugation (Manandhar and Ahn, 2015), is discussed in V. Pharmaceutical Development and Therapeutics.
1. Truncated GLP-1 Analogs.
N-terminally truncated forms of GLP-1 (including des-[His7]-GLP-18–36 and des-[His7,Ala8]-GLP-19–36) are competitive antagonists of GLP-1R, whereas C-terminally truncated GLP-1 constructs (including des-[Gly37,Arg36]-GLP-17–37) remain agonists (Mojsov, 1992; Montrose-Rafizadeh et al., 1997). The binding affinities of N-terminally truncated GLP-1 constructs are not affected by mutations in the 7TMD of GLP-1R (Al-Sabah and Donnelly, 2003a,b; López de Maturana et al., 2004), indicating that interactions between the N-terminal region of GLP-1 and the 7TMD of GLP-1R are required for receptor activation. Most truncated GLP-1 constructs have a decreased affinity for GLP-1R (Mojsov, 1992; Montrose-Rafizadeh et al., 1997; Donnelly, 2012), suggesting that interactions with both the ECD and the 7TMD are important determinants of GLP-1 binding. Similar SARs have been observed for the endogenous ligands of related class B GPCRs, including glucagon/GCGR (Unson et al., 1989) and GIP/GIPR (Hinke et al., 2001), demonstrating that interactions of peptide ligands in the 7TMD of class B GPCRs are required for receptor activation. The N-terminally truncated des-[His7]-GLP-18–36 and des-[His7,Ala8]-GLP-19–36 variants of GLP-1 have a 100- and 1000-fold lower affinity than that of wild-type, whereas truncation of more than two N-terminal residues further diminishes GLP-1R binding (Mojsov, 1992; Montrose-Rafizadeh et al., 1997). Truncation of up to three C-terminal residues (Gly35, Arg36, and Gly37) only has a moderate effect on GLP-1R–binding affinity (up to fivefold decrease), but further deletion of C-terminal residues decreases binding affinity significantly. Nevertheless, several undecapeptide GLP-17–15 analogs in which the C-terminal 21 residues are replaced with a biphenylalanine dipeptide have been reported to possess almost the same potency as wild-type GLP-1 (Mapelli et al., 2009).
In contrast to GLP-1, which requires its N-terminal region for high-affinity binding to GLP-1R, the homologous agonist exendin-4 and the N-terminally truncated antagonist exendin9–39 have similar affinities for GLP-1R (Montrose-Rafizadeh et al., 1997). Although full-length GLP-1R binds exendin-4 and GLP-1 with similar high affinity, the isolated ECD maintains high affinity for exendin-4 and exendin9–39, but has decreased affinity for GLP-1 (Al-Sabah and Donnelly, 2003a; López de Maturana et al., 2003). In addition, GLP-1 binding is more sensitive to site-directed mutagenesis of the 7TMD of GLP-1R than exendin-4 (Al-Sabah and Donnelly, 2003a,b; López de Maturana et al., 2003; see III. Glucagon-Like Peptide-1 Receptor). Consistently, radioligand competition studies combining isolated C-terminal (ECD) and N-terminal (7TMD) GLP-1R constructs with native and N-terminally or C-terminally truncated GLP-1 and exendin-4 showed that: 1) GLP-1 binding is primarily determined by interactions with the ECD, but also requires interactions with the 7TMD of GLP-1R; and 2) exendin-4–binding affinity is mainly determined by interactions with the ECD of GLP-1R and does not heavily depend on interactions with the 7TMD (López de Maturana et al., 2003). Based on pharmacological studies with truncated GLP-1 and exendin-4, it was further postulated that the eight-residue C-terminal extension of exendin-4 (Trp-cage, see above) may play a role in its superior affinity for the ECD of GLP-1R (Al-Sabah and Donnelly, 2003a).
2. Chimeric GLP-1 Analogs.
Chimeric constructs of GLP-1 in combination with other class B GPCR peptide ligands (including exendin-4, glucagon, GIP, and PACAP) have been used to identify structural determinants of ligand selectivity for GLP-1R (and other class B GPCRs). Radioligand competition studies with truncated and chimeric GLP-1 and exendin-4 peptides suggested that divergent residues in the central part of GLP-1 (Val16-Arg36) and exendin-4 (Leu10-Gly30) determine GLP-1/exendin-4 selectivity for the ECD of GLP-1R, and indicated that the Trp-cage only plays a minor role in the increased affinity of exendin-4 for the ECD of GLP-1R compared with GLP-1 (Runge et al., 2003b). These pharmacological data are consistent with comparative CD and fluorescence spectroscopy, NMR, and X-ray crystallography analyses of (truncated and chimeric forms of) GLP-1 and exendin-4 (Runge et al., 2007), implying that the differential affinity for the ECD can be explained by the higher α-helical propensity of exendin-4 in solution and by stronger (ionic) interactions between GLP-1R and exendin-4, compared with GLP-1 (see above). GLP-1/glucagon chimera experiments showed that a peptide ligand consisting of the N-terminal part of glucagon combined with the C-terminal part of GLP-1 has high affinity for both GCGR and GLP-1R (Hjorth et al., 1994). Substitution of N-terminal GLP-1 residues with corresponding glucagon residues [e.g., (Ala8Ser/Glu9Gln)-GLP-1 and (Val16Tyr/Ser12Lys)-GLP-1] maintains high GLP-1R–binding and low GCGR-binding affinities, whereas substitution of N-terminal glucagon residues with corresponding GLP-1 residues decreases GCGR binding and maintains low GLP-1R–binding affinities. In contrast, substitution of C-terminal GLP-1 residues with corresponding glucagon residues decreases GLP-1R binding and maintains low GCGR-binding affinities, whereas replacement of, for example, the last three C-terminal residues of glucagon (Met33, Asn34, and Thr35) with the corresponding GLP-1 residues (Val33, Lys34, and Gly35) and an additional C-terminal GLP-1 residue (Arg36) increases GLP-1R binding with affinity for GCGR remaining moderate. Glucagon/GLP-1 chimeras were combined with GCGR/GLP-1R chimeras to identify the receptor domains that interact with N-terminal and C-terminal parts of glucagon and GLP-1 (Runge et al., 2003b). The chimeric GLP-1-(His7-Leu20)/glucagon-(Asp21-Thr35) is unable to bind and activate GCGR, but its binding affinity and potency are rescued by substituting the 7TMD of GCGR with the 7TMD of GLP-1R, suggesting that the N-terminal region of GLP-1 (His7-Leu20) interacts with the 7TMD of GLP-1R (Runge et al., 2003b). The GCGR(ECD)/GLP-1R(7TM) chimera has equal binding affinity and potency for GLP-1 and the chimeric glucagon-(His7-Leu20)/GLP-1-(Asp21-Gly37), although these are decreased compared with wild-type GLP-1R, indicating that the ECD of GLP-1R is the major determinant of GLP-1/glucagon selectivity by interacting with the C-terminal region of GLP-1. Chimeras combining different N-terminal and C-terminal regions of GLP-1 (His7-Leu20, Asp21-Arg36) and GIP (His1-Leu14, Asp14-Lys30), or substituting the middle parts of GIP with corresponding residues of GLP-1 (Ser18-Ala24, Glu21-Ala24), all had more than 100-fold lower binding affinity for GLP1-R compared with GLP-1-(His7-Arg36) (Hareter et al., 1997). Substituting three to five residues in the N-terminal part of GLP-1 by corresponding residues from GIP (His7Tyr/Thr13Ile/Val16Tyr), secretin (Ala8Ser/Glu9Asp/Asp15Glu/Val16Leu), vasoactive intestinal peptide (Ala8Ser/Glu9Asp/Gly10Ala/Thr11Val), or PACAP (Ala8Ser/Glu9Asp/Thr11Ile/Ser14Asp/Asp15Ser) diminished ligand potency and decreased binding affinity by more than 10-fold (Hareter et al., 1997). Substituting two to three residues in the N-terminal part of GLP-1 by corresponding residues from glucagon (Ala8Ser/Glu9Gln), peptide histidine isoleucine (Glu9Asp/Thr11Ile), or PACAP (Ala8Ser/Glu9Asp/Thr11Ile), in contrast, had only small effects on affinity for GLP-1R.
3. Substituted GLP-1 Analogs.
In addition to ligand (and receptor) truncation and chimera studies, several site-directed substitution experiments were performed to provide more detailed information regarding the molecular determinants of GLP-1/GLP-1R binding and selectivity.
a. GLP-1 hydrophobic region I.
Point substitution of Phe28, Ile29, and Leu32 residues into Ala all had a significant negative impact on GLP-1–binding affinity and potency, confirming the important role of this hydrophobic region I in the C terminus of GLP-1 in binding the ECD of GLP-1R (Adelhorst et al., 1994; Gallwitz et al., 1994). The effect of substitution on binding affinity (GLP-1 radioligand competition IC50) and potency (cAMP activity EC50) was, however, much larger for Phe28 (1300/1000-fold decrease in affinity/potency) and Ile29 (93/27-fold reduction) compared with Leu32 (17/twofold decrease), indicating that interactions of Phe28 and Ile29 with the conserved hydrophobic core of the ECD of GLP-1R are particularly important (Adelhorst et al., 1994). Substitution of Ala24 by Arg (the corresponding residue in glucagon) did not affect GLP-1 affinity, whereas Val33Ala (glucagon mimicking) and Ala30Gln mutations only had a moderate 5- to 6-fold effect on GLP-1 affinity (Adelhorst et al., 1994). These results imply that apolar interactions of Ala24 and Val33 with the ECD of GLP-1R [observed in the GLP-1–bound GLP-1R ECD crystal structure (Underwood et al., 2010)] are not essential for GLP-1R binding and suggest that GLP-1 residues at positions 24 (Ala/Arg) and 30 (Ala/Gln) are not key determinants of GLP-1R/GCGR selectivity.
b. GLP-1 hydrophobic region II.
Substitution of Val16, Tyr19, and Leu20 into Ala decreased GLP-1 affinity by six-fold (Val16, Leu20) to 19-fold (Tyr19), and had similar negative effects on potency (Adelhorst et al., 1994), showing that Tyr19 in particular is an important interaction site in hydrophobic region II located in the middle of the GLP-1 α-helix.
c. Polar residues in α-helix.
Alanine substitution of most polar residues in the α-helix of GLP-1 had either no significant (Ser14, Ser17, Ser18, Gly22, Gln23, Trp32) or only a moderate 6-fold effect (Lys26, Lys34) on GLP-1R–binding affinity and potency, with the exception of the three negatively charged residues Asp15, Glu21, and Glu27 (Adelhorst et al., 1994). Replacement of Asp15 by Ala resulted in a 41-fold decrease in affinity and loss of potency (Adelhorst et al., 1994). A recent systematic mutation study combining GLP-1 (Asp15Glu, Asp15Lys, Asp15Arg) and GLP-1R (residues L379ECL3R/E, R380ECL3D/G, F381ECL3R/E) demonstrated that an ionic interaction between Asp15 and R380ECL3 in the third extracellular loop of GLP-1R is indeed required for ligand recognition and receptor activation (Moon et al., 2015). The diminished GLP-1 binding of the GLP-1R R380ECL3D mutant was partially restored by Asp15Glu substitution and almost fully restored by the Asp15Lys and inverted Asp15Arg substitutions of GLP-1. The abolished potency of GLP-1 for the GLP-1R R380ECL3D was partially restored by the Asp15Lys and inverted Asp15Arg substitutions. Replacement of Glu21 by Ala and Gly significantly decreased GLP-1 affinity by 15-fold and 60-fold, respectively (Adelhorst et al., 1994; Watanabe et al., 1994), indicating that the side chain of Glu21 plays a key role in GLP-1 binding. Glu27Ala substitution had a moderate sixfold effect on binding affinity, but a greater, 240-fold, effect on potency, albeit with a large S.E.M. in the EC50 of the mutant (Adelhorst et al., 1994). Replacement of Glu27 by Lys did not have a significant effect on binding affinity or potency (Watanabe et al., 1994), suggesting that a charged/polar residue at position 27 may facilitate GLP-1R activation, possibly by stabilizing the α-helix of GLP-1 and/or stabilizing the active conformation of GLP-1R.
d. N-capping motif in GLP-1.
Alanine substitution of Thr11, Phe12, and Thr13, the three residues in GLP-1 that are proposed to stabilize the N-capped conformation (Inooka et al., 2001; Neumann et al., 2008) of the N terminus of GLP-1, had a significant impact on ligand affinity. The effect of the Thr11Ala substitution was, however, smaller (13-fold decrease) compared with Phe12Ala and Thr13Ala substitutions (133-fold decrease), whereas GLP-1 potency was only significantly affected by the latter two substitutions. These results suggest that Phe12 and Thr13 play a more important role in stabilizing the N-capped conformation via intramolecular interactions within GLP-1 and/or in stabilizing the activated conformation of GLP-1R via intermolecular interactions with the receptor.
e. N terminus of GLP-1.
Substitution studies indicate that the electrostatic, steric, and conformational properties of the four residues following the N-capping motif of GLP-1 (His7, Ala8, Glu9, Gly10) are important determinants for GLP-1R binding and/or activation. Substitution of His7 by Phe did not affect binding affinity or ligand potency, whereas substitution with positively ionizable (Arg, Lys), smaller (Ala), or larger (Trp, GIP-mimicking Tyr) residues diminished binding affinity (Adelhorst et al., 1994; Hareter et al., 1997; Gallwitz et al., 2000; Sarrauste de Menthiere et al., 2004). Altogether, these amino acid replacement data suggest that a small aromatic residue is required at position 7 that is sterically compatible with the GLP1-R binding site. The Ala8-Val and the exendin-4–mimicking Ala8-Gly mutants only showed a small (two- to threefold) decrease in binding affinity and potency, the Ala8-Ser analog displayed moderate to large impact (4- to 10-fold) on binding affinity, whereas substitution of Ala8 with Leu or Thr reduced GLP-1R binding (Adelhorst et al., 1994; Hareter et al., 1997; Deacon et al., 1998; Burcelin et al., 1999). These observations demonstrate that only small residues are tolerated at position 8, suggesting that steric constraints of the GLP-1R binding site and/or conformational flexibility of the N-terminal region around Ala8 play a crucial role in GLP-1 binding. Substitution of Glu9 with Asp, Met, or Leu did not significantly affect ligand affinity/potency; substitution with Ser, Tyr, Phe, or Pro moderately decreased affinity (5- to 10-fold); whereas replacement with Ala, Lys, and Val reduced ligand binding (Xiao et al., 2001; Green et al., 2003, 2004; Sarrauste de Menthiere et al., 2004). The substitution data of Glu9 indicate that residues with similar negative charge (Asp) or comparable size (Met, Leu) are preferred, and positively charged moieties (Lys) are not tolerated at position 9. Substitution of Gly10 by Ala, Asp, Glu, His, Lys, or Arg diminished both GLP-1 affinity and potency (Adelhorst et al., 1994; Moon et al., 2015), demonstrating the essential function of this residue in GLP-1R binding. Combined GLP-1 and GLP-1R mutation studies showed that diminished binding affinity and potency of GLP-1 for the GLP-1R R380ECL3D mutant could, for a small part, be recovered by the GLP-1 Gly10-Arg mutant (Moon et al., 2015), indicating that the Gly/Arg10 is in the vicinity of R/D380ECL3.
In summary, combining GLP-1 truncation, chimera, and point substitution studies presented in this section with structural information on GLP-1 conformation and interactions with GLP-1R and GLP-1R mutation data, a binding model for the full-length GLP-1–GLP-1R complex can be proposed (Fig. 2), in which: 1) hydrophobic region I in the C-terminal α-helix of GLP-1 consisting of Phe28, Ile29, and Leu32 binds the ECD (as observed in the GLP-1–bound GLP-1R ECD crystal structure); 2) a second hydrophobic region II in the middle of GLP-1 (Val16, Tyr19, Leu20) interacts with the region connecting the ECD and 7TMD (the so-called stalk region in the GCGR crystal structure); 3) the N terminus (His7-Thr13) of glucagon interacts with the 7TMD in a so-called N-capped conformation (stabilized by Thr11, Phe12, Thr13). In addition to these main determinants of GLP-1/GLP-1R binding, other residues in the C-terminal/α-helical region can play an important role in stabilizing and maintaining the amphiphilic character of the α-helix of GLP-1 or by forming additional hydrophobic (Ala24/Ala25 with the ECD) or ionic/polar (Lys26 with the ECD, D15 with ECL3) interaction sites with GLP-1R.
III. Glucagon-Like Peptide 1 Receptor
A. Discovery
Between 1979 and early 1980, Habener and colleagues found that 29–amino-acid pancreatic glucagon was the major bioactive peptide released from the pancreatic islets (Goodman et al., 1980a; Lund, 2005). The anglerfish islet was thus regarded as a rich source containing coding sequences for glucagon-related peptides. Hybrid arrest and hybrid selection of mRNAs encoding two preproglucagons led to the first identification of a cDNA encoding a 29-residue peptide highly homologous to mammalian glucagon (Goodman et al., 1980b; Lund et al., 1981), which was then used as a hybridization probe to screen the anglerfish islet cDNA library for other cDNAs to derive the entire coding sequence of anglerfish preproglucagon (Lund et al., 1982).
In 1982, the first mammalian (hamster) pancreatic preproglucagon cDNA was reported (Bell et al., 1983a), which resulted in the rapid isolation and sequencing of the human glucagon gene (Bell et al., 1983b), followed by bovine and rat preproglucagon cDNAs (Heinrich et al., 1984). These landmark discoveries led to our current concepts of GLP-1 as an incretin and a satiety hormone (Ebert and Creutzfeldt, 1987; Kreymann et al., 1987; Dupre et al., 1991; Mattson et al., 2003). It was found that truncated forms of GLP-1 are also biologically active in stimulating both insulin gene transcription and insulin secretion (Mojsov et al., 1986; Drucker et al., 1987; Fehmann and Habener, 1991; II. Glucagon-Like Peptide-1).
1. Receptor Cloning.
To better understand the action of GLP-1, Thorens isolated and characterized the first cDNA of the rat pancreatic β cell GLP-1 receptor in 1992 (Thorens, 1992), followed by cloning of the human receptor in 1993 (Dillon et al., 1993; Graziano et al., 1993; Thorens et al., 1993), revealing a sequence of 463 residues. This deduced primary sequence resembled that of the receptors for secretin, parathyroid hormone, and calcitonin, and hence enabled it to be classified within what was then a new branch of the GPCR superfamily (family B, class B, or secretin receptor-like) that now includes 15 members (Segre and Goldring, 1993; Hoare, 2005).
2. Receptor Expression.
Using RNA enzyme protection techniques, GLP-1R was found widely expressed in the human and mouse pancreas, lung, brain, stomach, heart, and kidney, but was not seen in tissues involved in glucose metabolism, such as the liver, skeletal muscle, and fat (Dunphy et al., 1998). DNA hybridization experiments showed that the human GLP-1R (hGLP-1R) gene is localized to chromosome 6p21, and the size is 40 kb, containing about 14 exons. Both human and rat receptors contain 463 amino acids, whereas that of the mouse has 489 amino acids, displaying a homology with hGLP-1R of 91% and 84%, respectively. Although the expression levels of GLP-1R vary among different tissues and cell types, Northern hybridization studies demonstrated that its expression in pancreatic islets, heart, and lungs is significantly higher (Dunphy et al., 1998). In addition, GLP-1R was also found in the lateral septum, thalamus, and hippocampus of the rat brain, and the cDNA fragment cloned from the brain, heart, and pancreas encodes the same amino acids of the receptor, suggesting its physiologic relevance in the cardiovascular and central nervous systems (Satoh et al., 2000).
It is known that the expression of GLP-1R in the islets of Langerhans is regulated by glucose and dexamethasone, but not by the PKA-dependent signaling pathway (Abrahamsen et al., 1995). Starvation and refeeding could influence its expression in the rat hypothalamus and other sites of the CNS (MacLusky et al., 2000). Although our knowledge about the transcriptional regulation mechanisms of GLP-1R is still limited, promoter analysis indicate that 1) transcription factors Sp1 and Sp3 may play important regulatory roles and 2) homologous desensitization and internalization are closely related to the intracellular sites of 441/442, 444/445, and 451/452 (Wildhage et al., 1999).
3. Receptor Biosynthesis.
About 5–10% of GPCRs contain an N-terminal cleavable signal peptide (Schulein et al., 2012). Most GPCRs mediate the integration of receptor into cell membrane by a signal anchor sequence that is located at the first TMD (Audigier et al., 1987; Wallin and von Heijne, 1995). Both types of signal sequences regulate the endoplasmic reticulum (ER) signal process at the beginning of the secretory pathway (Belin et al., 1996; Kochl et al., 2002).
The signal peptide is a sequence of approximately 20 amino acids in the N terminus of the receptor that includes a polar and charged N-terminal (n) region, a central hydrophobic (h) region, and a polar C-terminal (c) region (Schneider and Fechner, 2004; Clerico et al., 2008). The C-terminal side regularly contains helix-breaking proline and glycine residues and small uncharged residues at positions 1 and 3 of the cleavage site. After synthesis of the receptor's N-tail in the cytoplasm, the translation ceases by its binding to the signal recognition particle (SRP) (Halic and Beckmann, 2005; Shan and Walter, 2005). A translocon complex is attached to the membranes of ER that includes a GTP-dependent interaction between SRP and SRP receptor, translocon gating, protein synthesis, and integration of nascent chains with the bilayer (Kochl et al., 2002). The signal peptide is finally cleaved off by signal peptidases on the ER membrane. In contrast, signal anchor sequences are not cleaved and form a part of the mature protein (Audigier et al., 1987; Wallin and von Heijne, 1995; Higy et al., 2004; Schulein et al., 2012). In addition to recognition and binding of SRP and translocation to ER, the N-terminal signal sequence may play key roles in protein folding and trafficking. This is highly dependent upon whether the signal sequence is a cleaved signal peptide or an uncleaved signal anchor.
For GLP-1R, studies on the signal peptides of corticotropin-releasing factor-1 receptor (CRF1R) and corticotropin-releasing factor-2 receptor give a good model to compare. Both CRF receptors exhibit a high probability of N-terminal cleavable signal peptide by computational prediction. In reality, however, only the signal peptide of CRF1R is cleaved, whereas that of corticotropin-releasing factor-2 receptor is translocated and embedded to the plasma membrane by taking TM1 as a signal anchor sequence. It is thus integrated to be a part of the N-terminal ECD: not mediating ER targeting, but contributing to receptor activation (Alken et al., 2005). Interestingly, a mutant of rat CRF1R without the signal peptide sequence was able to translocate to the cell surface and display similar biologic function to the wild-type. The rat CRF2aR contains an uncleaved N-terminal signal peptide, and deletion of this resulted in a significant reduction in its membrane expression, suggesting that receptor transportation was affected (Rutz et al., 2006). The complexity of these signal sequences highlights the need for experimental verification of the role of the signal sequence in GLP-1R.
According to the signal peptide prediction program “SignalP 4.0” (Nielsen et al., 1997; Bendtsen et al., 2004), the sequence of the first 23 amino acids in GLP-1R fits all the criteria of an N-terminal signal peptide. The existence of a functional signal peptide was demonstrated experimentally by Huang et al. (2010), who showed that it was required for GLP-1R synthesis and was cleaved thereafter. Mutation of the signal peptide (A21-R) resulted in retention of the receptor within ER, whereas mutation of E34-R augmented its cell surface expression when the signal peptide was deleted. The amino acid sequence following the signal peptide in the GLP-1R, G27ECD-W39ECD, is relatively hydrophobic, and this region may be recognized by SRP (Huang et al., 2010). Ge et al. (2014) went deep into the function of this signal peptide by use of constructs containing epitope tags at the N and/or C terminus. A mutant GLP-1R without the signal peptide sequence was expressed in human embryonic kidney (HEK) 293 cells and displayed normal functionality with respect to ligand binding and cAMP activation, suggesting that the putative signal peptide may not be required for receptor synthesis. Immunoblotting analysis showed that the amount of GLP-1R synthesized in HEK293 cells was low without the signal peptide, indicating its role in facilitating receptor expression (Ge et al., 2014). Epitope tags at the N terminus of GLP-1R were detectable by immunofluorescence and immunoblotting, an observation that is inconsistent with another report that studied both signal peptide and the hydrophobic region after the signal peptide. It was found that the signal peptide was cleaved in the mature hGLP-1R, and cell surface expression was almost abolished by the mutation A21-R that prevented the cleavage, demonstrating that hydrophobic region after the signal peptide is necessary for efficient hGLP-1R trafficking to the cell surface. Because glycosylation is vital to cell surface expression, cleavage of the signal peptide will affect this process. In addition, mutating W39ECD, Y69ECD, and Y88ECD of hGLP-1R to alanine eliminated its cell surface expression without influencing N-linked glycosylation and cleavage of the signal peptide (Thompson and Kanamarlapudi, 2014).
Clearly, such discrepancies may not only reflect differences in the methods employed among these three studies, but also the cellular background and the complexity of the underlying mechanism(s).
B. Structure
The human GLP-1R is a 463-residue glycoprotein containing an N-terminal signal peptide and various glycosylation sites that are essential for the correct trafficking and processing of the receptor (Thorens, 1992; Dillon et al., 1993; Graziano et al., 1993; Thorens et al., 1993; Goke et al., 1994; Chen et al., 2010a; Huang et al., 2010). Like all class B GPCRs, GLP-1R possesses an N-terminal ECD of 100–150 residues connected to an integral TM domain (7TM) that is typical of all GPCRs, having seven α-helices (TM1–TM7) separated by ICL1–ICL3 and ECL1–ECL3 (Palczewski et al., 2000; Siu et al., 2013). However, despite this structural resemblance to other GPCRs, the sequence of the class B 7TMD is devoid of the consensus sequence motifs that typify the class A GPCRs (e.g., rhodopsin and adrenergic receptors). In contrast, the N-terminal ECD of class B GPCRs is a unique domain found only in this GPCR subfamily and is central to ligand recognition. Class B GPCRs are believed to all bind their peptide ligands via a common mechanism known as the two-domain model, in which the ECD first binds to the C-terminal region of the ligand, enabling a second interaction between the N-terminal region of the ligand and the 7TMD of the receptor (Bergwitz et al., 1996; Hoare, 2005).
1. N-Terminal Domain.
a. Structure determination of the N-terminal ECD.
The two-domain structure of GLP-1R enabled a strategy whereby the ECD could be expressed as an isolated domain suitable for structural and functional studies that were used to demonstrate its critical role in ligand binding (Wilmen et al., 1996; Xiao et al., 2000; Bazarsuren et al., 2002; Al-Sabah and Donnelly, 2003a; López de Maturana et al., 2003; Mann et al., 2007, 2010b; Runge et al., 2007, 2008; Underwood et al., 2010). The definitive study came from X-ray crystallography whereby the isolated ECD of hGLP-1R from an Escherichia coli inclusion body preparation was incubated with the peptide antagonist exendin9–39, before being further purified, crystallized, and analyzed using X-ray diffraction to yield a 2.2Å crystal structure (Runge et al., 2008). The protein fold closely resembles that of other class B GPCR ECD structures (Parthier et al., 2009) and contained two regions of antiparallel β-sheet, three disulfide bonds (46ECD–71ECD, 62ECD–104ECD, and 85ECD–126ECD), and an N-terminal α-helix. The core of the structure contains six conserved residues (D67ECD, W72ECD, P86ECD, R102ECD, G108ECD, and W110ECD), which are critical for the folding stability. Residues on the α-helix, turn 1, loop 2, and the C-terminal region form a ligand-binding groove for the antagonist’s well-defined α-helix (Leu10-Asn28) that interacts with the ECD using residues within the Glu15ECD-Ser32ECD region, the most critical residues being Val19ECD, Phe22ECD, Ile23ECD, and Leu26ECD, which are deeply buried in the ECD’s groove. The residues on the N-terminal side of Glu15 of exendin9–39 do not interact with the ECD, although residues 10–14 are nevertheless critical for high-affinity binding (Runge et al., 2007), presumably because they are required to stabilize the helical structure of the ligand. The nine-residue C-terminal extension of exendin9–39, which has no equivalent in GLP-1, plays no significant role in the peptide’s affinity at hGLP-1R (Runge et al., 2007; Mann et al., 2010b). A later crystal structure showed that GLP-1 also forms an α-helix when bound to the ECD, with the principal contacts being the equivalent hydrophobic interface formed by residues Ala24, Ala25, Phe28, Ile29, Leu32, and Val33 (Underwood et al., 2010). In contrast to that in exendin9–39, the ECD-bound α-helix of GLP-1 is kinked around Gly22, as observed in earlier NMR studies (Thornton and Gorenstein, 1994). The modest eightfold differential affinity between exendin-4 and GLP-1 at the isolated membrane-bound ECD of hGLP-1R (Mann et al., 2010b) could largely be explained through E127ECD, which interacts with exendin9–39 but not GLP-1, where mutagenesis to Ala resulted in a sevenfold reduction in affinity for exendin9–39 (Underwood et al., 2010; Patterson et al., 2013). Leu32ECD also appears to play a role in ligand selectivity, although it is less clear from the crystal structure how this occurs: substitution of Leu32ECD by Ala had no effect upon GLP-1 affinity or potency but did reduce exendin9–39 affinity by sevenfold and Gly2–GLP-1 by 10-fold (Underwood et al., 2010; Patterson et al., 2013).
b. Site-directed mutagenesis of the ECD.
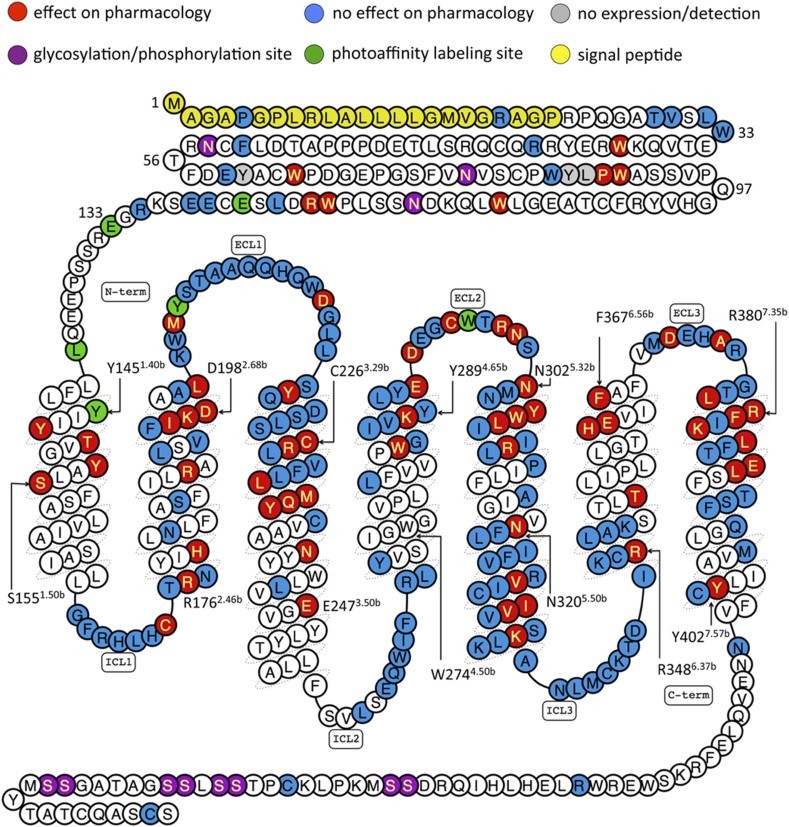
The N-terminal domain has been the subject of a number of mutagenesis studies summarized in Table 1 and Fig. 3 (Wilmen et al., 1997; Tibaduiza et al., 2001; Mann et al., 2010b; Underwood et al., 2010; Day et al., 2011; Koole et al., 2011; Patterson et al., 2013). Residues that have been mutated but do not greatly affect GLP-1 affinity/efficacy include P7ECDL, R20ECDK, L32ECDA, T35ECDA, V36ECDA, R44ECDH, E68ECDA, L123ECDA, E127ECDA, E127ECDQ, E128ECDA, E128ECDQ, and E128ECDM. To study a species-selective small-molecule antagonist, Tibaduiza et al. (2001) mutated W33ECD to Ser (the human to rat GLP-1R substitution), as well as to various other residue types, showing that there was no effect on GLP-1 albeit that the affinity and potency values were not given for the peptide ligands used. Wilmen et al. (1997) substituted six Trp residues in the ECD with Ala and found that, whereas W87A behaved like wild-type GLP-1R in binding and cAMP assays, the remaining substitutions at 39, 72, 91, 110, and 120 resulted in the abolition of detectable radioligand binding. Modest five- to eightfold reductions in GLP-1 potency have been observed for E68ECDK, P90ECDA, and R121ECDA (Underwood et al., 2010; Day et al., 2011). The ECD structures suggest that E68ECD is close to the C-terminal region of the peptide ligand, but, although it appears to have little significant interaction with exendin9–39 in the human receptor, the equivalent residue in rat GLP-1R, D68ECD, enhances exendin9–39 affinity by forming a hydrogen bond with Ser32 of the peptide (Mann et al., 2010b). Other mutations, Y69ECDA, Y88ECDA, and L89ECDA, have caused catastrophic effects on the ability of the receptor to be detected in binding or signaling assays (Underwood et al., 2010).
TABLE 1.
Summary of effects on GLP-1 pharmacology (affinity and ability to activate cAMP pathway) in published site-directed mutagenesis studies of GLP-1R
WT (wild-type) refers to mutations that resulted in either <fivefold or no statistically significant change from wild-type GLP-1R. ND (not determinable) refers to a property that was measured, but for which a value was not determinable. Blank cells mean that the assays used to estimate that particular pharmacological property were not carried out in the cited work. Residues with symbol † refer to data from rat GLP-1R (if different, the equivalent human residue number is displayed in the table to aid comparison). GLP-1 affinity or potency fold-change values with suffix M are from membrane preparations, whereas suffix C is from whole-cell assays. Cell surface expression values below 75% of WT are shown (>75% are shown as WT): a suffix E represents estimation from ELISAs; suffix mic was evaluated from immunofluorescent microscopy; suffix cyt was evaluated by flow cytometry with an anti-Flag antibody; suffix Ag refers to affinity or cell surface expression levels determined from agonist radioligand-binding assays, whereas suffix Ant was from antagonist radioligand-binding assays. ΔLog τc values relative to WT are shown where >0.5 and were calculated from data where the expression-corrected efficacy term τc had been calculated using the operational model of agonism, as defined in Wootten et al. (2013c). Residues with transmembrane helices are numbered according to Wootten et al. (2013c).
| Residue | Mutated to | -Fold Reduction Affinity | -Fold Reduction Potency‡ | Cell Surface Expression (% Wild-Type) | Comments and/or Other Observed Effects | Reference |
|---|---|---|---|---|---|---|
| P7ECD | L | WTC,Ant | WT | WTE | Koole et al., 2011 | |
| R20ECD | K | WTC,Ant | WT | WTE | Koole et al., 2011 | |
| L32ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| L32ECD | A | WT | Patterson et al., 2013 | |||
| W33ECD | S | WTC,Ant | Species change (human to rat)— the expected lack of effect on GLP-1 pharmacology was implied in text, but no data are shown | Tibaduiza et al., 2001 | ||
| T35ECD | A | WT | 7%Ag | Underwood et al., 2010 | ||
| Val-36ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| W39ECD† | NDC,Ag | Membrane expression confirmed via WB | Wilmen et al., 1997 | |||
| R44ECD | H | WTC,Ant | WT | WTE | Koole et al., 2011 | |
| N63ECD | L | WTC,Ag | WT | WTAg | Chen et al., 2010a | |
| E68ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| E68ECD | K | 8 | Day et al., 2011 | |||
| Y69ECD | A | NDM,Ag | ND | NDAg | Underwood et al., 2010 | |
| W72ECD† | NDC,Ag | Membrane expression confirmed via WB | Wilmen et al., 1997 | |||
| N82ECD | L | WTC,Ag | WT | WTAg | Chen et al., 2010a | |
| W87ECD | WTC,Ag | WT | 24–62% | Transient and stable cell lines analyzed | Wilmen et al., 1997 | |
| Y88ECD | A | NDM,Ag | ND | NDAg | Underwood et al., 2010 | |
| L89ECD | A | NDM,Ag | ND | NDAg | Underwood et al., 2010 | |
| Pro-90ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| W91ECD† | NDC,Ag | Membrane expression confirmed via WB | Wilmen et al., 1997 | |||
| W110ECD† | NDC,Ag | Membrane expression confirmed via WB | Wilmen et al., 1997 | |||
| N115ECD | L | WTC,Ag | WT | WTAg | Chen et al., 2010a | |
| W120ECD† | NDC,Ag | Membrane expression confirmed via WB | Wilmen et al., 1997 | |||
| R121ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| L123ECD | A | WTM,Ag | WT | 47%Ag | Underwood et al., 2010 | |
| E127ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| E127ECD | A | WT | Patterson et al., 2013 | |||
| E127ECD | E | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| E128ECD | A | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| E128ECD | A | WT | Patterson et al., 2013 | |||
| E128ECD | Q | WTM,Ag | WT | WTAg | Underwood et al., 2010 | |
| E128ECD | M | WT | Day et al., 2011 | |||
| R1311.26b | N | WTC,Ant | WT | WTE | Koole et al., 2011 | |
| L1411.36b | A | WTC,Ag | WT | 67%cyt | Yang et al., 2016 | |
| Y1451.40b | A | WTC,Ag | WT | 47%cyt | Yang et al., 2016 | |
| Y1481.43b | A | NDC,Ag | 26 | WTcyt | Yang et al., 2016 | |
| Y1481.43b | N | 15C,Ag | 8 | WTcyt | Yang et al., 2016 | |
| Y1481.43b | F | NDC,Ag | 14 | WTcyt | Yang et al., 2016 | |
| Y1481.43b | F | 10C,Ant | 14 | WTcyt | Yang et al., 2016 | |
| T1491.44b | M | 60C,Ant | 14–33 | WTE & Ant | Beinborn et al., 2005 | |
| T1491.44b | M* | 250C,Ant | 160 | <50%E | Emax = ND | Koole et al., 2011 |
| For additional residue substiutions, see Koole et al., 2015 | ||||||
| T1491.43b | M | NDC,Ag | 59 | WTcyt | Yang et al., 2016 | |
| T1491.43b | A | NDC,Ag | 82 | WTcyt | Yang et al., 2016 | |
| T1491.43b | S | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| Y1521.47b | A | 30M,Ant | ND | 7%Ant | Coopman et al., 2011 | |
| Y1521.47b | H | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| S1551.50b | A | WTC,Ant | 10 | 55%E, 48%Ant | ΔLog τc = 0.75 | Wootten et al., 2013c |
| G168ICL1 | S | WTC,Ant | WT | <50%E | Koole et al., 2011 | |
| F169ICL1† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| R170ICL1† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| H171ICL1† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| L172ICL1† | A | WTC,Ag | 35%Ag | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| H173ICL1† | A | WTC,Ag | 48%Ag | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| C174ICL1† | A | WTC,Ag | 45%Ag | 37% cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| C174ICL1 | A | 6 | Underwood et al., 2013 | |||
| C174ICL1 | S | 7 | Underwood et al., 2013 | |||
| T1752.45b† | A | WTC,Ag | 57%Ag | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| R1762.46b† | A | WTC,Ag | 13 | WTAg | 26% cAMP 10−7 M GLP-1 | Mathi et al., 1997 |
| N1772.47b† | A | WTC,Ag | 22%Ag | 43% cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| H1802.50b† | R | 21C,Ag | WTmic | Heller et al., 1996 | ||
| H1802.50b | A | NDC,Ant | 12 | 18%E, NDAnt | ΔLog τc = 0.86 | Wootten et al., 2013c |
| N1822.52b† | A | WTC,Ag | 24%Ag | “36% of WT” cAMP | Xiao et al., 2000 | |
| S1862.56b | A | WTC,Ant | WT | WTE,Ant | Wootten et al., 2013c | |
| F1872.57b | A | NDC,Ag | ND | 5%cyt | Yang et al., 2016 | |
| R1902.60b† | A | >20C,Ag | 21%Ag | “27% of WT” cAMP | Xiao et al., 2000 | |
| R1902.60b | A | 32M,Ant | 270 | 7%Ant | Coopman et al., 2011 | |
| R1902.60b | A | 20C,Ant | 34 | 53%E, 44Ant | ΔLog τc = 0.53 | Wootten et al., 2013c |
| R1902.60b | A | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| R1902.60b | K | NDC,Ag | 17 | WTcyt | Yang et al., 2016 | |
| R1902.60b | K | 32C,Ant | 17 | WTcyt | Yang et al., 2016 | |
| L1922.62b | S | WT | Underwood et al., 2011 | |||
| L1922.62b | S | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| V1942.64b | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| F1952.65b | L | WT | 59%Ag | Underwood et al., 2011 | ||
| I1962.66b† | S | WTC,Ag | ND | Moon et al., 2012 | ||
| K1972.67b | A | 28M,Ant | 630 | 57%Ant | Coopman et al., 2011 | |
| K1972.67b† | A | 5C,Ag | 30%Ag | “25% of WT” cAMP | Xiao et al., 2000 | |
| K1972.67b | A | NDC,Ag | ND | 51%cyt | Yang et al., 2016 | |
| K1972.67b | I | NDC,Ag | ND | 51%cyt | Yang et al., 2016 | |
| K1972.67b | I | 28C,Ant | ND | 51%cyt | Yang et al., 2016 | |
| K1972.67b | Q | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| K1972.67b | R | NDC,Ag | 23 | WTcyt | Yang et al., 2016 | |
| D1982.68b† | A | 10C,Ag | 16%Ag | “45% of WT” cAMP | Xiao et al., 2000 | |
| D1982.68b† | A | 63M,Ant | 44 | WTAnt | López de Maturana and Donnelly, 2002 | |
| D1982.68b | A | 43M,Ant | 977 | 66%Ant | Coopman et al., 2011 | |
| D1982.68b | A | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| D1982.68b† | N | WTM,Ag | López de Maturana and Donnelly, 2002 | |||
| D1982.68b | N | NDC,Ag | 89 | WTcyt | Yang et al., 2016 | |
| D1982.68b | E | NDC,Ag | 434 | WTcyt | Yang et al., 2016 | |
| D1982.68b | E | 58C,Ant | 434 | WTcyt | Yang et al., 2016 | |
| A200-L201† | V/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| L2012.68b | A | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| K202ECL1† | A | WTC,Ag | 45%Ag | “71% of WT” cAMP | Xiao et al., 2000 | |
| K202/W203† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| W203ECL1 | T | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| M204/Y205† | A/A | 37M,Ant | 51 | 28%Ant | López de Maturana et al., 2004 | |
| M204/Y205† | V/A | 23M,Ag | 32 | WTAnt | López de Maturana et al., 2004 | |
| M204/Y205† | A/V | 29M,Ag | 87 | WTAnt | López de Maturana et al., 2004 | |
| M204ECL1† | A | WTM,Ag | WT | 44%Ant | López de Maturana et al., 2004 | |
| M204ECL1 | A | NDC,Ag | 334 | WTcyt | Yang et al., 2016 | |
| M204ECL1 | R | NDC,Ag | 93 | WTcyt | Yang et al., 2016 | |
| Y205ECL1† | A | WTM,Ag | WT | WTAnt | López de Maturana et al., 2004 | |
| Y205ECL1 | A | NDC,Ag | 62 | WTcyt | Yang et al., 2016 | |
| S206/T207† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| A208/A209† | V/V | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| Q210Q211† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| Q211ECL1 | D | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| Q211ECL1 | R | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| H212ECL1† | A | WTC,Ag | WTAg | “WT cAMP” | Xiao et al., 2000 | |
| H212ECL1 | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| H212/Q213† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| D215ECL1† | A | WTC,Ag | 51%Ag | “57% of WT” cAMP | Xiao et al., 2000 | |
| W214ECL1 | V | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| W214/D215† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| G216/L217† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| L217ECL1 | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| L218/S219† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| Y220/Q221† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| Y220ECL1 | D | NDC,Ag | 105 | WTcyt | Yang et al., 2016 | |
| D2223.25b† | A | WTC,Ag | WTAg | “82% of WT” cAMP | Xiao et al., 2000 | |
| D222/S223† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| L224/G225† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| C2263.29b*† | A | 25M,Ant | 38 | 65%Ant | Mann et al., 2010a | |
| C2263.29b | A | >90 | Underwood et al., 2013 | |||
| R2273.30b† | A | >20C,Ag | 31%Ag | “90% WT” cAMP | Xiao et al., 2000 | |
| R227/L228† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| V229/F230† | A/A | WTM,Ag | WT | López de Maturana et al., 2004 | ||
| L232/M233† | V/T | 10C,Ag | 100 | Moon et al., 2012 | ||
| M2333.36b | A | NDC,Ag | 70 | WTcyt | Yang et al., 2016 | |
| M2333.36b | A | 16C,Ant | 70 | WTcyt | Yang et al., 2016 | |
| M2333.36b | T | NDC,Ag | 62 | WTcyt | Yang et al., 2016 | |
| M2333.36b | F | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| Q2343.37b | A | 13M,Ant | 45 | 27%Ant | Coopman et al., 2011 | |
| Q2343.37b | A | NDC,Ag | 151 | 35%cyt | Yang et al., 2016 | |
| Q2343.37b | N | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| Q2343.37b | E | NDC,Ag | 29 | 69%cyt | Yang et al., 2016 | |
| Q2343.37b | E | 17C,Ant | 29 | 69%cyt | Yang et al., 2016 | |
| Y2353.38b | A | 24M,Ant | 23 | 12%Ant | Coopman et al., 2011 | |
| C2363.39b | A | WT | Underwood et al., 2013 | |||
| N2403.43b† | A | >20C,Ag | 14%Ag | “8% WT” cAMP | Xiao et al., 2000 | |
| N2403.43b | A | WTC,Ant | 7 | WTE,Ant | ΔLog τc = 0.67 | Wootten et al., 2013c |
| N2403.43b | Q | WTC,Ant | WT | WTE,Ant | Wootten et al., 2016 | |
| N240/Q394 | A/A | WTC,Ant | WT | 71%E,Ant | ΔLog τc = 0.70 | Wootten et al., 2016 |
| N240/Q394 | Q/N | 5C,Ant | WT | 71%E,Ant | Wootten et al., 2016 | |
| Y2413.44b | A | WT | 42%Ag | Underwood et al., 2011 | ||
| Y2413.44b | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| E2473.50b | A | WTC,Ag | 64%Ag | “39% WT” cAMP | Xiao et al., 2000 | |
| E2473.50b | A | NDC,Ant | 14 | 18%E, NDAnt | ΔLog τc = 0.99 | Wootten et al., 2013c |
| F260ICL2 | L | WTC,Ant | WT | <50%E | Koole et al., 2011 | |
| E262ICL2† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| Q263ICL2† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| R264ICL2† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| I265ICL2† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| F2664.42b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| K2674.43b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| L2684.44b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| L2784.54b | M | WT | Underwood et al., 2011 | |||
| W2844.60b | A | 32M,Ant | 1349 | 34%Ant | Coopman et al., 2011 | |
| G2854.61b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| I2864.62b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| V2874.63b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| I286/V287 | A/A | WT | 85% SBM,Ag | Dods and Donnelly, 2015 | ||
| K2884.64b | A | 126C,Ant | ND | WTE | Koole et al., 2012a | |
| K2884.64b | A | 23M,Ant | 5732 | WTAnt | ΔLog τc = 1.39 | Dods and Donnelly, 2015 |
| K2884.64b† | A | 79M,Ant | 251 | Al-Sabah and Donnelly, 2003b | ||
| K2884.64b† | L | 63M,Ant | 79 | Al-Sabah and Donnelly, 2003b | ||
| K2884.64b | L | NDC,Ag | ND | 70%cyt | Yang et al., 2016 | |
| K2884.64b† | R | WTM,Ag | WT | Al-Sabah and Donnelly, 2003b | ||
| K288/Y289 | A, A | 2188 | 28% SBM,Ag | Dods and Donnelly, 2015 | ||
| Y2894.65b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| Y2894.65b | A | WTM,Ant | WT | 20%Ant | Dods and Donnelly, 2015 | |
| L2904.66b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| Y2914.67b | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| L290/Y291† | A/A | WTM,Ag | WT | Mann et al., 2010a | ||
| L290-Y291 | A/A | WT | 99% SBM,Ag | Dods and Donnelly, 2015 | ||
| E292ECL2 | A | 100C,Ant | 126 | WTE | ΔLog τc = 0.57 | Koole et al., 2012a |
| E292ECL2 | A | NDC,Ag | 33 | 39%cyt | Yang et al., 2016 | |
| D293ECL2 | A | 25C,Ant | 16 | 62%E | Koole et al., 2012a | |
| E292/D293† | A/A | 8M,Ag | 79 | Mann et al., 2010a | ||
| E292/D293 | A/A | 25 | 75% SBM,Ag | Dods and Donnelly, 2015 | ||
| E294ECL2 | A | WTC,Ant | WT | WTE | ΔLog τc = 0.65 | Koole et al., 2012a |
| G295ECL2 | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| E294-G295† | A/A | WTM,Ag | WT | Mann et al., 2010a | ||
| E294-G295 | A/A | WT | 97% SB M,Ag | Dods and Donnelly, 2015 | ||
| C296ECL2*† | A | 18M,Ant | WT | 23%Ant | Mann et al., 2010a | |
| C296ECL2 | A | 13C,Ant | 126 | 60E | Koole et al., 2012a | |
| C296ECL2 | S | NDC,Ag | 96 | WTcyt | Yang et al., 2016 | |
| W297ECL2 | A | 63C,Ant | 316 | WTE | ΔLog τc = 1.00 | Koole et al., 2012a |
| W297ECL2 | A | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| W297ECL2 | H | NDC,Ag | 50 | 40%cyt | Yang et al., 2016 | |
| T298ECL2 | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| W297/T298† | A/A | 100M,Ant | 50 | Mann et al., 2010a; | ||
| Donnelly, 2012 | ||||||
| W297/T298 | A/A | 22 | 57% SBM,Ag | Dods and Donnelly, 2015 | ||
| R299ECL2 | A | 32C,Ant | 85 | WTE | Koole et al., 2012a | |
| R299ECL2 | A | WTM,Ant | WT | 40%Ant | ΔLog τc = 0.60 | Dods and Donnelly, 2015 |
| R299ECL2 | S | NDC,Ag | 43 | WTcyt | Yang et al., 2016 | |
| N300ECL2 | A | 126C,Ant | 501 | WTE | ΔLog τc = 0.80 | Koole et al., 2012a |
| N300ECL2 | A | 36M,Ant | 104 | 18%Ant | Dods and Donnelly, 2015 | |
| N300ECL2 | A | NDC,Ag | ND | 16%cyt | Yang et al., 2016 | |
| R299/N300† | A/A | 25M,Ant | >3000 | Mann et al., 2010a; | ||
| Donnelly, 2012 | ||||||
| R299/N300 | A/A | 331 | 41% SBM,Ag | Dods and Donnelly, 2015 | ||
| S301ECL2 | A | WTC,Ant | WT | 63%E | Koole et al., 2012a | |
| N302ECL2 | A | 25C,Ant | 16 | WTE | ΔLog τc = 0.53 | Koole et al., 2012a |
| S301/N302† | A/A | WTM,Ag | WT | Mann et al., 2010a | ||
| S301/N302 | A/A | WT | 86% SBM,Ag | Dods and Donnelly, 2015 | ||
| N302/M303† | V/K | WTC,Ag | 10 | Moon et al., 2012 | ||
| M303ECL2 | A | WTC,Ant | WT | WTE | Koole et al., 2012a | |
| N304ECL2 | A | WTC,Ant | WT | 70%E | ΔLog τc = 0.74 | Koole et al., 2012a |
| N304ECL2 | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| M303/N304† | A/A | WTM,Ag | WT | Mann et al., 2010a | ||
| M303/N304 | A/A | WT | 91% SBM,Ag | Dods and Donnelly, 2015 | ||
| Y3055.35b | A | 79C,Ant | 40 | 52%E | Koole et al., 2012a | |
| Y3055.35b | A | WTM,Ant | WT | 22%Ant | Dods and Donnelly, 2015 | |
| W3065.36b | A | NDC,Ant | ND | ND | No receptor expression | Koole et al., 2012a |
| W3065.36 | A | 109M,Ant | 206 | 41%Ant | Dods and Donnelly, 2015 | |
| W3065.36 | A | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| †Y305/W306† | A/A | 316M,Ant | 50 | Mann et al., 2010a; | ||
| Donnelly, 2012 | ||||||
| Y305/W306 | A/A | 263 | 60% SBM,Ag | Dods and Donnelly, 2015 | ||
| L3075.37b | A | 13C,Ant | 25 | 47%E | Koole et al., 2012a | |
| L3075.37b | A | WT | 87% SBM,Ag | Dods and Donnelly, 2015 | ||
| I3085.38b | A | WT | 78% SBM,Ag | Dods and Donnelly, 2015 | ||
| L307/I308† | A/A | 251M,Ant | 6 | Mann et al., 2010a; | ||
| Donnelly 2012 | ||||||
| I3095.39b | A | WTM,Ant | WT | WTAnt | Dods and Donnelly, 2015 | |
| R3105.40b | A | 10M,Ant | 1259 | 17%Ant | Coopman et al., 2011 | |
| R3105.40b | A | WTM,Ant | 1290 | 19%Ant | ΔLog τc = 0.75 | Dods and Donnelly, 2015 |
| I309/R310† | A/A | 50M,Ant | >3000 | Mann et al., 2010a; | ||
| Donnelly, 2012 | ||||||
| I309/R310 | A/A | 13,490 | 13% SBM,Ag | Dods and Donnelly, 2015 | ||
| L311/P312 | A/A | WT | 91% SBM,Ag | Dods and Donnelly, 2015 | ||
| A3165.46b | T | WTC,Ant | WT | <25%E | Koole et al., 2011 | |
| N3205.50b | A | 18C,Ant | 10 | WTE,Ant | ΔLog τc = 0.50 | Wootten et al., 2013c |
| F3215.51b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| L3225.52b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| I3235.53b† | A | WTC,Ag | 35%Ant | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| F3245.54b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| I325/F326† | A/A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| V3275.57b† | A | WTC,Ag | 15 | WTAg | 42% cAMP 10−7 M GLP-1 | Mathi et al., 1997 |
| I3285.58b† | A | WTC,Ag | 9 | WTAnt | 39% cAMP 10−7 M GLP-1 | Mathi et al., 1997 |
| C3295.59b† | A | WTC,Ag | WTAnt | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| I3305.60b | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| V3315.61b† | A | WTC,Ag | 14 | WTAg | 45% cAMP 10−7 M GLP-1 | Mathi et al., 1997 |
| I3325.62b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| A3335.63b† | L | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Mathi et al., 1997 | |
| S3335.63b | C | WTC,Ant | WT | <50%E | For additional residue substiutions, see Koole et al., 2015 | Koole et al., 2011 |
| K3345.64b† | A | WTC,Ag | WTAg | 28% cAMP 10−7 M GLP-1 | Takhar et al., 1996; Mathi et al., 1997 | |
| L3355.65b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Takhar et al., 1996; Mathi et al., 1997 | |
| K3365.66b† | L | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Takhar et al., 1996; Mathi et al., 1997 | |
| K334/K351 | Deletions | WTC,Ag | Ag See paper for details | Takhar et al., 1996 | ||
| C3476.36b | A | WT | Underwood et al., 2013 | |||
| R3486.37b† | G | 12C,Ag | ND | WTmic | Heller et al., 1996 | |
| R3486.37b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Takhar et al., 1996 | |
| L3496.38b† | A | WTC,Ag | 76%Ag | WT cAMP 10−7 M GLP-1 | Takhar et al., 1996 | |
| A-3506.39b† | E | NDC,Ag | <10%Ag | 5% cAMP 10−7 M GLP-1 | Takhar et al., 1996 | |
| A-3506.39b† | K | WTC,Ag | 21%Ag | 2% cAMP 10−7 M GLP-1 | Takhar et al., 1996 | |
| K3516.40b† | A | WTC,Ag | WTAg | WT cAMP 10−7 M GLP-1 | Takhar et al., 1996 | |
| T3536.42b | A | NDC,Ant | 22 | 30%E, NDAnt | ΔLog τc = 0.84 | Wootten et al., 2013c |
| H3636.52b | A | 98M,Ant | ND | 24%Ant | Coopman et al., 2011 | |
| H3636.52b | A | 23C,Ant | 4 | 59%E, 53%Ant | ΔLog τc = 1.71 | Wootten et al., 2013c |
| E3646.53b | A | 58M,Ant | 15 | 42%Ant | Coopman et al., 2011 | |
| E3646.53b | A | 25C,Ant | 51%E,Ant | ΔLog τc = 0.66 | Wootten et al., 2016 | |
| E3646.53b | A | NDC,Ag | ND | 6%cyt | Yang et al., 2016 | |
| E3646.53b | Q | WTC,Ag | WT | 42%cyt | Yang et al., 2016 | |
| E3646.53b | Q | 0.2C,Ant | WT | 42%cyt | Yang et al., 2016 | |
| E364/E387 | N/Q | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| E3646.53b | Y | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| E3646.53b | D | WTC,Ag | WT | 53%cyt | Yang et al., 2016 | |
| F3676.56b | A | NDC,Ag | 72 | WTcyt | Yang et al., 2016 | |
| F3676.56b | A | 32C,Ant | 72 | WTcyt | Yang et al., 2016 | |
| F3676.56b | I | NDC,Ag | 20 | WTcyt | Yang et al., 2016 | |
| F3676.56b | H | 7C,Ag | 131 | WTcyt | Yang et al., 2016 | |
| M371ECL3 | A | WT M,Ant | WT | 54%Ant | Dods and Donnelly, 2015 | |
| D372ECL3 | A | WT M,Ant | 59 | 13%Ant | Dods and Donnelly, 2015 | |
| E373ECL3 | A | WTM,Ant | WT | 28%Ant | Dods and Donnelly, 2015 | |
| H374ECL3 | A | WTM,Ant | WT | 22%Ant | Dods and Donnelly, 2015 | |
| H374ECL3 | A | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| A-375ECL3 | A | 10M,Ant | WT | WTAnt | Dods and Donnelly, 2015 | |
| R376ECL3 | G | WTM,Ant | WT | WTAnt | Dods and Donnelly, 2015 | |
| R376ECL3 | Q | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| G377ECL3 | A | WTM,Ant | WT | 19%Ant | Dods and Donnelly, 2015 | |
| T3787.32b | A | WTM,Ant | WT | 12%Ant | Dods and Donnelly, 2015 | |
| L3797.33b | R | 12C,Ag | 141 | WTAg | Moon et al., 2015 | |
| L3797.33b | E | 11C,Ag | 165 | WTAg | Moon et al., 2015 | |
| L3797.33b | A | WTM,Ant | WT | 31%Ant | Dods and Donnelly, 2015 | |
| R3807.34b | D | 21C,Ag | 1853 | WTAg | Moon et al., 2015 | |
| R3807.34b | G | 4C,Ag | 40 | WTAg | Moon et al., 2015 | |
| R3807.34b | A | 128M,Ant | 263 | 68%Ant | Dods and Donnelly, 2015 | |
| R3807.34b | Q | NDC,Ag | ND | WTcyt | Yang et al., 2016 | |
| F3817.35b | R | WT C,Ag | WT | WTAg | Moon et al., 2015 | |
| F3817.35b | E | >200C,Ag | 234 | WTAg | Moon et al., 2015 | |
| F3817.35b | A | WTM,Ant | WT | 16%Ant | Dods and Donnelly, 2015 | |
| F3817.35b | S | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| I3827.36b | A | WTM,Ant | WT | 23%Ant | Dods and Donnelly, 2015 | |
| K3837.37b | A | WTM,Ant | 56 | WTAnt | ΔLog τc = 1.18 | Dods and Donnelly, 2015 |
| L3847.38b | A | WTM,Ant | WT | WTAnt | Dods and Donnelly, 2015 | |
| L3847.38b | A | NDC,Ag | 41 | WTcyt | Yang et al., 2016 | |
| L3847.38b | A | 48C,Ant | 41 | WTcyt | Yang et al., 2016 | |
| L3847.38b | V | NDC,Ag | 16 | WTcyt | Yang et al., 2016 | |
| F3857.40b | A | WTM,Ant | WT | WTAnt | Dods and Donnelly, 2015 | |
| T3867.41b | A | WTM,Ant | WT | 18%Ant | Dods and Donnelly, 2015 | |
| E3877.42b | A | WTM,Ant | WT | 43%Ant | ΔLog τc = 0.52 | Dods and Donnelly, 2015 |
| E3877.42b | A | WTM,Ant | WT | WTAnt | Coopman et al., 2011 | |
| E3877.42b | D | 13C,Ag | 10 | WTcyt | Yang et al., 2016 | |
| E3877.42b | D | 12C,Ant | 10 | WTcyt | Yang et al., 2016 | |
| E3877.42b | N | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| L3887.43b | A | NDC,Ag | 208 | WTcyt | Yang et al., 2016 | |
| L3887.43b | I | 5C,Ag | 81 | WTcyt | Yang et al., 2016 | |
| L3887.43b | F | WTC,Ag | WT | WTcyt | Yang et al., 2016 | |
| T3917.44b | A | WTM,Ant | WT | 64%M,Ant | Coopman et al., 2011 | |
| T3917.44b | A | WT | WTAg | Underwood et al., 2011 | ||
| S3927.47b | A | WTC,Ant | WT | WTE, Ant | Wootten et al., 2013c | |
| Q3947.49b | A | WTC,Ant | WT | WTE, Ant | Wootten et al., 2013c | |
| Q3947.49b | N | WTC,Ant | WTE,Ant | Wootten et al., 2016 | ||
| M3977.52b | L | WTC,Ag | WT | Dong et al., 2012 | ||
| Y4027.57b | A | NDC,Ant | 10 | 21%E, NDAnt | ΔLog τc = 1.59 | Wootten et al., 2013c |
| C4037.58b | A | 5.4 | Underwood et al., 2013 | |||
| N4067.61b | A | WTC,Ant | WT | WTE,Ant | Wootten et al., 2013c | |
| R421CTT | Q | WTC,Ant | WT | <50%E | Koole et al., 2011 | |
| C438 CTT | A | WT | Underwood et al., 2013 | |||
| C458 CTT | A | WT | Underwood et al., 2013 | |||
| C462 CTT | A | WT | Underwood et al., 2013 |
CTT, C-terminal tail; SB, specific binding of radiolabeled ligand; WB, Western blotting.
Note that potency changes can be due to changes in affinity, efficacy, and/or cell surface expression, and hence should be interpreted with caution, especially when either the affinity and/or expression levels are not known.
Also included in double mutations.
Fig. 3.
Summary of GLP-1R mutagenesis. A snake plot of GLP-1R from http://www.gpcrdb.org that has been colored (see Key) to highlight the location of signal peptide, glycosylation, and phosphorylation sites, as well as the mutated residues in Table 1. It should be noted that the color coding of 74 of the 195 mutated residues (W39, W72, W87, W91, W110, F169-C174, R176, N177, H180, N182, A200-Q213, W214-G225, R227-F230, L232, M233, E262-L268, F321-I332, K334-K336, and R348-K351) reflects the effects of rat GLP-1R mutations projected on the hGLP-1R amino acid sequence. The color coding of 27 of the 185 residues (K202, W203, S206-Q211, Q213, W214, G216-Q221, S223-G225, R227-F230, L332, M233, I325, and F326) reflects the effect of double mutations, not single-point mutations. Information on the fold change in ligand affinity and potency as well as expression levels of the GLP-1R mutants is reported in Table 1.
2. Seven-Transmembrane Domain.
a. Structure of the 7TMD.
To date, with the exception of a recently solved electron microscopy map of GCGR (Yang et al., 2015b), there are no experimentally determined three-dimensional structures available for any full-length class B GCPR, but, in addition to the isolated ECD structures mentioned above, there are now X-ray crystal structures for the 7TMD of human GCGR (Siu et al., 2013; Jazayeri et al., 2016) and CRF1R (Hollenstein et al., 2013). The partial CRF1R structure (PDB: 4K5Y) was solved in the presence of a small-molecule antagonist (CP-376395) and contained 12 thermo-stabilizing mutations and a T4 lysozyme fusion partner inserted into ICL2 (Hollenstein et al., 2013). The first GCGR 7TMD structure (PDB: 4L6R) was solved with an N-terminal fusion partner fused at residue 123 (Siu et al., 2013), and it is this structure that is of particular interest to understand GLP-1R because both the receptors and native ligands are closely related. Recently, another GCGR 7TMD crystal structure was solved that contained 11 thermo-stabilizing mutations and a T4 lysozyme fusion partner inserted into ICL2 (PDB: 5EE7) (Jazayeri et al., 2016). In this structure, a small-molecule antagonist (MK-0893) is bound to an extrahelical allosteric binding site that is distinct from the orthosteric peptide ligand binding site, and the receptor adopts a similar overall conformation as observed in the previously solved GCGR crystal structure. Analogs of MK-0893 bind GLP-1R with moderate micromolar affinity (Xiong et al., 2012), suggesting that the GCGR crystal structures may also offer a template for the design of small-molecule ligands that target the same extrahelical allosteric binding site in GLP-1R. Even though the general fold of the TM helices is similar to those observed in classes A and C GPCR crystal structures, both CRF1R and GCGR structures assume more open conformations toward the extracellular side than any other GPCR of known structure. In addition to this open 7TMD pocket, the GCGR structure revealed two other distinct structural features that are not observed in the CRF1R structure, namely, a long N-terminal extension of TM helix 1 (defined as stalk region) and a long helix 8 at the C-terminal end of the receptor that is differently oriented than helix 8 observed in the crystal structures of other GPCRs (Hollenstein et al., 2014). Although the distinct orientation of helix 8 in the GCGR structure may be a result of crystal lattice contacts, the stalk is proposed to play a role in the positioning of the ECD relative to the 7TMD (Siu et al., 2013). The electron microscopy map of the full-length GCGR stabilized by a monoclonal antibody (mAb23) suggests that mAb23 interacts with the ECD (preventing glucagon from binding to the receptor) and stabilizes GCGR in an open conformation in which the TM helix 1 stalk connects the ECD and 7TMD and the ECD is almost perpendicular to the membrane surface (Yang et al., 2015b). Hydrogen-deuterium exchange studies have indicated that des-His1-[Nle9-Ala11-Ala16]-glucagon-NH2 peptide antagonist binding protects the α-helical conformation of the stalk region of GCGR, consistent with molecular dynamics–simulation studies (Yang et al., 2015b). Full-length crystal structure of GLP-1R and other class B GPCRs are required to show whether the stalk region observed in the GCGR 7TMD crystal structure is indeed a conserved structural feature among secretin-like receptors. The GCGR structure lacks the presence of a peptide or small-molecule ligand, but there is clearly a deep and extensive binding pocket comprised of the TM helices at the extracellular side of the 7TM bundle. A glucagon-bound full-length GCGR model (Siu et al., 2013) and models of agonist-docked full-length GLP-1R (Dods and Donnelly, 2015; Wootten et al., 2016; Yang et al., 2016) have been proposed based on information from a variety of sources, including the crystal structures of the GCGR 7TMD (Siu et al., 2013), the antibody-bound GCGR ECD (Koth et al., 2012), the GLP-1–bound GLP-1R ECD (Underwood et al., 2010), and molecular dynamics simulations (Wootten et al., 2016; Yang et al., 2016). Although such models can be largely consistent with the results of mutation studies of GLP-1R (Table 1) (Dods and Donnelly, 2015; Wootten et al., 2016; Yang et al., 2016), and can provide insights into GLP-1R–specific mechanisms of ligand-binding selectivity (Yang et al., 2016) and biased signaling (Wootten et al., 2016), a complete interpretation of the mutagenesis data requires a high-resolution structure of the ligand-bound GLP-1R, and, hence, progress in this area is required and keenly anticipated.
b. Mutagenesis of the 7TMD.
Although there are numerous molecular pharmacological analyses of various ligands (both peptidic and nonpeptidic) acting at GLP-1R, this section of the review will focus only on those studies that highlight the action of GLP-1 itself. Likewise, whereas GLP-1R can signal through several pathways, this section will focus on the effects of site-directed mutagenesis on signaling through the Gs protein that raises intracellular cAMP levels. The ability of GLP-1R to activate other G proteins and pathways will be examined in more detail below. The mutagenesis data are summarized in Table 1 and Fig. 3, with the TM numbering following the convention described by Wootten et al. (2013c).
i. TM1 (S1291.24b-L1671.62b).
The first TM helix of GLP-1R (TM1) contains two single-nucleotide polymorphism (SNP) sites at positions 1311.26b (Arg/Asn) and 1491.44b (Thr/Met). Although the R1311.26bN mutation did not significantly affect GLP-1 action (Koole et al., 2011), Beinborn and colleagues showed that the T1491.44bM mutation (which has been associated with T2DM) led to a 60-fold reduction in GLP-1 affinity and a 30-fold reduction in potency (Tokuyama et al., 2004; Beinborn et al., 2005). The importance of this residue for GLP-1 action has been confirmed subsequently, demonstrating that the same mutation resulted in a 250-fold reduced affinity and a 160-fold reduced potency, whereas substitution by a variety of other residue types also led to compromised affinity and signaling (Koole et al., 2015), with the exception of the T1491.44bS mutant, which has similar ligand affinity and potency as wild-type GLP-1R (Yang et al., 2016). Mutation of Y1481.43b to Ala, Asn, or Phe diminished GLP-1 affinity and potency (Yang et al., 2016), while that of Y1521.47b to Ala reduced GLP-1 affinity by 30-fold—potency was compromised as well, although in this case the Bmax was low (Coopman et al., 2011), whereas Y1521.47bH does not affect either ligand affinity or potency (Yang et al., 2016). Mutation of the equivalent residue (Y1491.47b) in GCGR also caused low expression levels, whereas the mutation to His was expressed well and displayed sixfold reduced affinity. Mutation of S1551.50b to Ala, in contrast, reduced coupling [efficacy (Emax), i.e., the maximal response = 38%, ΔLog τc = 0.75] with a minor effect on affinity (Wootten et al., 2013c).
ii. ICL1 (G168-C174).
The first residue in the loop is the site of a known SNP (Gly to Ser), but the simulated mutation had no effect on GLP-1 action (Koole et al., 2011). Residues comprising the region between F169ICL1 and C174ICL1 in the first intracellular loop were systematically mutated to Ala, but no significant effect upon GLP-1 action was found (Mathi et al., 1997). However, C174ICL1 has also been mutated and exhibited a sixfold reduced potency when mutated to Ala and a sevenfold reduced potency when mutated to Ser (Underwood et al., 2013).
iii. TM2 (T1752.45b-L2012.71b).
A total of 17 sites within TM2 have been subjected to site-directed mutagenesis (Mathi et al., 1997; Xiao et al., 2000; López de Maturana and Donnelly, 2002; Underwood et al., 2011; Coopman et al., 2011; Moon et al., 2012; Wootten et al., 2013b; Yang et al., 2016). Of these, eight mutations displayed GLP-1 pharmacology similar to the wild-type receptor (T1752.45b, N1772.47b, N1822.52b, and A2002.70b in rat GLP-1R, and S1862.56b, L1922.62b, V1942.64b, and F1952.65b in hGLP-1R). In contrast, R1762.46b displayed compromised signaling with approximately 13-fold right-shifted potency and Emax reduced to 26% (Mathi et al., 1997). The mutation of the conserved H1802.50b to Arg resulted in a 21-fold reduced affinity (Heller et al., 1996), whereas substitution to Ala had led to no detectable binding, a 12-fold reduction in potency, and an Emax of 30% (ΔLog τc = 0.86) (Wootten et al., 2013c). R1902.60 has been mutated in multiple different studies: Xiao et al. (2000) found that its mutation to Ala in rat GLP-1R resulted in >20-fold reduced affinity and only 27% of the wild-type response to 100 nM GLP-1; Coopman et al. (2011) observed a more than 30-fold reduction in affinity and 270-fold reduction in potency; Wootten et al. (2013c) described a 20-fold reduction in GLP-1 affinity with a 34-fold reduction in potency and 56% Emax (ΔLog τc = 0.53); and Yang et al. (2016) reported a diminished GLP-1 affinity and potency for the R1902.60bA mutant, and a 32-fold reduction in GLP-1 affinity with a 17-fold reduction in potency for the R1902.60bK mutant. The latter GCGR-mimicking R1902.60bK mutant does not affect binding affinity of the glucagon mimetic GLP-1 Glu9Gln point mutant, suggesting that this residue plays a role in GLP-1 versus glucagon selectivity (Yang et al., 2016). Moon et al. (2012) mutated I1962.66b to Ser and observed normal affinity but no activity. K1972.67b has been mutated to Ala by both Xiao et al. (2000) and Coopman et al. (2011); the former (rat GLP-1R) showed a fivefold reduction in affinity but only 25% of the wild-type response to 100 nM GLP-1, whereas the latter (hGLP-1R) observed a 28-fold reduction in affinity and a 630-fold reduction in potency. Yang et al. (2016) showed that mutation of K1972.67b to Gln and Ile also diminished GLP-1 affinity and potency in hGLP-1R, whereas the K1972.67bR mutant reduced GLP-1 potency by 23-fold. The equivalent residues have been mutated in the closely related receptors GCGR (I1942.67b) and GIPR (R1902.67b), respectively, with significant impact on their pharmacology and, in the case of GCGR, an association with Asp3 of the ligand (Perret et al., 2002; Runge et al., 2003a; Yaqub et al., 2010; Siu et al., 2013). The neighboring residue D1982.68b has also been mutated by several groups: Xiao et al. (2000) observed a 10-fold reduction in affinity and only 20% of the wild-type response to 100 nM GLP-1 at rat GLP-1R (D1982.68bA); López de Maturana and Donnelly (2002) demonstrated a more than 60-fold reduction in affinity and a more than 40-fold reduction in potency for GLP-1 at rat GLP-1R (D1982.68bA); Coopman et al. (2011) described a more than 40-fold reduction in affinity but a more substantial 980-fold reduction in potency at hGLP-1R (D1982.68bA); Yang et al. (2016) showed that in addition to D1982.68bA, D1982.68bN and D1982.68bE mutants also diminished GLP-1 and exendin-4 affinity and GLP-1 potency.
iv. ECL1 (K202-L218).
The ECL1 of rat GLP-1R was scanned using double-Ala mutagenesis by López de Maturana et al. (2004), and only the double mutation of M204ECL1-Y205 ECL1 displayed some change from the wild-type (87-fold decrease in potency and 37-fold decrease in affinity). M204ECL1A and Y205ECL1A single mutants exhibited wild-type GLP-1 pharmacology in rat GLP-1R (López de Maturana et al., 2004), but diminished GLP-1 affinity and potency in hGLP-1R in another study (Yang et al., 2016). Although the W214ECL1-D215ECL1 double mutation to Ala showed wild-type properties, Xiao et al. (2000) have reported that the single D215ECL1-A mutation has a fourfold reduced affinity and only 57% of the wild-type cAMP production when treated with 100 nM GLP-1. Yang et al. (2016) showed that Q211ECL1D, Q211ECL1R, H212ECL1A, W214ECL1V, and L217ECL1A single amino acid mutants did not affect GLP-1R pharmacology, whereas W203ECL1A and Y220ECL1D diminished GLP-1 affinity and potency. In contrast, the equivalent region in GCGR plays a critical role in peptide ligand recognition with 15 residues between L198ECL1 and A220ECL1 affecting peptide response (Roberts et al., 2011; Siu et al., 2013).
v. TM3 (S2193.22b-A2563.59b).
Although the substitution of C2363.39b appeared to have minimal effects on GLP-1 pharmacology, the mutation of the conserved C2263.29b in two studies led to either no detectable activity or a 25-fold reduction in GLP-1 affinity with 38-fold lower potency; the latter was thought to be the result of the breaking of a disulfide bond and the creation of a hydrophobic side chain at position 296 (Mann et al., 2010a; Underwood et al., 2013). The equivalent cysteine in GCGR (C2243.39b) was also shown to be important for glucagon potency (Prevost et al., 2010; Roberts et al., 2011). The remaining residues spanning S2193.22b to F2303.33b were included in the double-alanine screening by López de Maturana et al. (2004), and all were found to display wild-type–like GLP-1 pharmacology, although Xiao et al. (2000) reported that the single R2273.30bA mutation exhibited a more than 20-fold reduced GLP-1 affinity; the equivalent mutation in GCGR (R2253.30bA) had no detectable peptide binding (Siu et al., 2013). The double substitution of L2323.35b/M2333.36b with V/T reduced affinity by 10-fold and potency by 100-fold (Moon et al., 2012), whereas the single substitution of M2333.36b with A and T abolished GLP-1 affinity and potency (Yang et al., 2016). The single-alanine substitutions at Q2343.37b and Y2353.38b, respectively, caused a 13- and 24-fold reduced affinity with 45- and 23-fold reduced potency (Coopman et al., 2011), whereas the M2333.36bF and Q2343.37bN mutants did not affect GLP-1 affinity or potency (Yang et al., 2016). The Q2343.37bE substitution diminished GLP-1 affinity, which was restored by the mutation of Glu9 of GLP-1 into the corresponding Gln residue in glucagon (Yang et al., 2016). Position 3.37b has also been implicated in playing a role in the ligand-binding affinity of GCGR and GIPR (Yaqub et al., 2010; Siu et al., 2013). Replacement of N2403.43b with Ala in rat GLP-1R reduced affinity by more than 20-fold and severely impeded cAMP production induced by 100 nM GLP-1 (Xiao et al., 2000), whereas the equivalent replacement in hGLP-1R had minimal effects on affinity but reduced Emax of GLP-1 to 78% (ΔLog τc = 0.67; Wootten et al., 2013c). Likewise, in GCGR the N2383.43bA mutation had no effect on affinity, whereas in GIPR the N2303.43bA mutant reduced potency by sevenfold. In contrast, substitution of the conserved E2473.50b to Ala in hGLP-1R reduced potency by 14-fold and Emax to 19% (ΔLog τc = 0.99; Wootten et al., 2013c), but the equivalent mutation in rat GLP-1R had no major effect (Xiao et al., 2000).
vi. ICL2 (F257-I265).
Mutations in the second intracellular loop to date have not shown any effects on GLP-1 pharmacology with the SNP swap Phe/Leu at 260 (Koole et al., 2011) and an Ala scan of E262ICL2-I265ICL2 (Mathi et al., 1997) displaying wild-type–like properties.
vii. TM4 (F2664.42b-Y2914.62b).
Two sites in TM4 have been highlighted as being key to GLP-1 activity. The first is W2844.60b, which, when mutated to Ala, led to a 32-fold reduced affinity and a 1350-fold reduced potency (Coopman et al., 2011). The second important site in this helix is K2884.64b, which, when mutated to Ala or Leu in rat GLP-1R, respectively, resulted in a 79- and 63-fold reduction in affinity with a 251- and 79-fold lower potency (Al-Sabah and Donnelly, 2003b). The K2884.64bL mutant also abolished GLP-1 affinity and potency in hGLP-1R (Yang et al., 2016). However, the K2884.64b to Ala mutation in hGLP-1R caused a 126-fold reduction in affinity with no detectable cAMP production (Koole et al., 2012a) and substitution of K2864.64b in GCGR abolished radiolabeled ligand binding (Siu et al., 2013). The following mutations did not affect GLP-1 pharmacology: F2664.42bA, R2674.43bA, L2684.44bA, L2784.54bM, G2854.61bA, I2864.62bA/V2874.63bA, I2864.62bA, V2874.63bA, Y2894.65bA, L2904.66bA/Y2914.67bA, L2904.66bA, and Y2914.67bA (Mathi et al., 1997; Mann et al., 2010a; Underwood et al., 2010; Koole et al., 2012a).
viii. ECL2 (E292-Y305).
The second extracellular loop is an important region for peptide ligand recognition (Mann et al., 2010a; Donnelly, 2012; Koole et al., 2012a; Moon et al., 2012; Dods and Donnelly, 2015). Aside from the key disulfide-forming C296ECL2, which has been shown to be crucial for affinity in rat GLP-1R and both affinity and coupling in hGLP-1R (Mann et al., 2010a; Koole et al., 2012a; Underwood et al., 2011), residues E292ECL2, D293ECL2, E294ECL2, W297ECL2, R299ECL2, N300ECL2, N302ECL2, and N304ECL2 all play a role in GLP-1 action (Y3055.35b is involved too—see TM5 below). Koole et al. (2012a) demonstrated that the individual substitutions E292ECL2 and D293ECL2 in hGLP-1R caused, respectively, ≥100-fold reduced affinity and potency (ΔLog τc = 0.57) and a 25-fold reduced affinity with 16-fold reduced potency, but no change in efficacy. Double mutations of both sites in rat GLP-1R and hGLP-1R support the importance of these residues in GLP-1 pharmacology (Mann et al., 2010a; Dods and Donnelly, 2015). Substitution by Ala of the equivalent E290ECL2 and N291ECL2 in GCGR led to, respectively, no detectable radioligand binding and a threefold reduction in affinity (Siu et al., 2013). The region encompassing W297ECL2-N300ECL2 was identified as essential in rat GLP-1R and hGLP-1R by double-Ala scanning, whereas the residues spanning S301ECL2-N304ECL2 did not alter GLP-1 pharmacology (Mann et al., 2010a; Donnelly, 2012; Dods and Donnelly, 2015). However, individual substitutions in hGLP-1R by Koole et al. (2012a) demonstrated that, along with E294ECL2, W297ECL2, and R299ECL2, amino acids N300ECL2, N302ECL2, and N304ECL2 were also important (Table 1). In GCGR, the equivalent tryptophan (W295ECL2) is indispensible for affinity (Siu et al., 2013). The double mutation of N302ECL2-M303ECL2 to Val-Lys caused a small affinity reduction with a 10-fold decrease in potency (Moon et al., 2012). Residues that appear to be unimportant for GLP-1–mediated production of cAMP are G295ECL2, T298ECL2, S301ECL2, and M303ECL2 (Mann et al., 2010a; Koole et al., 2012a; Dods and Donnelly, 2015), which correlates with available data for GCGR (Siu et al., 2013).
ix. TM5 (W3065.36b-K3365.66b).
Three double-Ala scan mutations have identified the extracellular end of TM5 of rat GLP-1R as being important for GLP-1 recognition, with substitutions of Y3055.35b/W3065.36b (this is the ECL2-TM5 interface), L3075.37b/I3085.38b, and I3095.39b/R3105.40b with Ala residues displaying significantly reduced affinity and potency (Mann et al., 2010a). The equivalent double substitutions in hGLP-1R supported a role for Y3055.35b/W3065.36b and I3095.39b/R3105.40b. However, whereas single mutations of Y3055.35b, L3075.37b, and I3085.38b to Ala did not significantly alter GLP-1 pharmacology in one study (Dods and Donnelly, 2015), the individual substitutions of Y3055.35b and L3075.37b with Ala resulted in 79-fold decreased affinity with 40-fold reduced potency and 13-fold reduced affinity with 25-fold lowered potency (ΔLog τc = 0.49), respectively, in a second (Koole et al., 2012a). Although W3065.36b was not expressed in levels sufficient for analysis in Chinese hamster ovary (CHO) cells (Koole et al., 2012a), in HEK293 cells its mutation to Ala resulted in >100-fold reduced affinity and >200-fold reduced potency (Dods and Donnelly, 2015). R3105.40bA resulted in a >1200-fold reduced potency, with little effect upon affinity, implying that it plays a key role in agonist-induced signaling (Coopman et al., 2011; Dods and Donnelly, 2015) (ΔLog τc = 0.75). The equivalent mutation in GIPR, R3005.40bA, caused a 42-fold reduced affinity and 86% Emax (Yaqub et al., 2010). This first part of TM5 is also involved in glucagon affinity in GCGR, with several mutations showing some effect on binding (Siu et al., 2013). N3205.50bA had a modest effect on GLP-1 action with an 18-fold reduced affinity and a 10-fold decreased potency (ΔLog τc = 0.50) (Wootten et al., 2013c). Takhar et al. (1996) and Mathi et al. (1997) Ala scanned the C-terminal end of TM5, demonstrating that V3275.57b, I3285.58b, V3315.61b, and K3345.64 had reduced GLP-1 affinity and activity. However, the mutations of A3165.46T, F3215.51A, L3225.52bA, I3235.53bA, F3245.54bA, V3255.55bA, R3265.56bA, C3295.59bA, I3305.60bA, V3325.62bA, S3335.63bA, S3335.63bC, L3355.65bA, and K3365.66bA did not greatly alter GLP-1 pharmacology (Mathi et al., 1997; Koole et al., 2011, 2012a).
x. ICL3 (A337-T343).
ICL3 does not appear to play a critical role in GLP-1R pharmacology because Takhar et al. (1996) screened the entire ICL3 region via deletion mutagenesis in three- or four-residue sections but found no effect on GLP-1 action, whereas Underwood et al. (2013) found that C341ICL3-A also had no effect (Takhar et al., 1996; Underwood et al., 2013).
xi. TM6 (D3446.33b-F3696.58b).
Takhar’s ICL3 Ala screening also extended into the first eight residues of TM6, but no significant effect on GLP-1 action was shown (Takhar et al., 1996). Whereas Underwood et al. (2013) confirmed that C3476.36bA also had no effect, Heller et al. (1996) showed that R3486.37bG resulted in a 12-fold reduced affinity and inability to produce cAMP. T3536.36bA led to a 22-fold reduction in potency (ΔLog τc = 0.84) (Wootten et al., 2013c). The Ala substitution at E3646.53b caused a 58-fold reduction in affinity with a 15-fold reduced potency (Coopman et al., 2011), whereas the individual substitutions of E3646.53b with Tyr, Asp, or Gln did not affect GLP-1 affinity or potency (Yang et al., 2016). Interestingly, the E3646.53bQ mutation resulted in a 50-fold and a 40-fold increase in GLP-1– and exendin-4–binding affinity, respectively, in exendin9–39 radioligand competition studies (Yang et al., 2016). Mutation of H3636.52b to Ala resulted in either a 100-fold reduced affinity with no detectable potency (Coopman et al., 2011) or a 23-fold reduced affinity with poor coupling (17% Emax, ΔLog τc = 1.71). Mutation of F3676.56b into Ala, Ile, and His decreased GLP-1 affinity and potency by 72-fold, 20-fold, and 131-fold, respectively (Yang et al., 2016).
xii. ECL3 (V370-G377).
Residues from M371ECL3 to G377ECL3 have been mutated to Ala (with A376 to Gly), showing only a modest effect upon GLP-1 pharmacology (Dods and Donnelly, 2015). D372ECL3A displayed wild-type affinity but a 60-fold reduced potency, although analysis with the operational model suggested no effect upon efficacy; hence, this is likely to be the result of reduced receptor expression (13% wild-type). A375ECL3G resulted in a 10-fold reduced affinity for GLP-1. ECL3 appears to play a role in ligand and/or ECD recognition in GCGR (Koth et al., 2012).
xiii. TM7 (Y3787.33b-Y4027.57b).
Residues from T3787.33b to E3877.42b have been mutated to Ala, suggesting that several residues play no major role in GLP-1 recognition—T3787.32b, L3797.33b, F3817.35b, I3827.36b, F3857.39b, and T3867.40b (Dods and Donnelly, 2015). However, Moon et al. (2015) showed that, although F3817.36bR had no effect, F3817.36bE reduced potency and affinity by >200-fold, and L3797.34bR and L3797.34bE resulted in a more than 10-fold reduced affinity and in the range of 150-fold decreased potency. R3807.35bA resulted in 128-fold reduced affinity, without reduced efficacy (Dods and Donnelly, 2015). The R3807.35bQ substitution resulted in a more than 15-fold reduction in GLP-1 affinity and abolished potency (Yang et al., 2016), whereas R3807.35bD lowered potency by 1850-fold, but affinity was only reduced by 21-fold, and R3807.35bG decreased potency by 40-fold and affinity by fourfold. K3837.38bA showed no significant change in agonist affinity, but displayed reduced potency and efficacy (56-fold reduced potency, ΔLog τc = 1.18) (Dods and Donnelly, 2015). Mutation of Y4027.57bA demonstrated a role for this residue in coupling because substitution to Ala resulted in a large decrease in efficacy (ΔLog τc = 1.59) (Wootten et al., 2013c), whereas the equivalent mutation in GIPR, Y3927.57bA, caused a fivefold reduction in potency (Yaqub et al., 2010). Substitutions at E3877.42b, T3917.46b, Q3947.49b, M3977.52b, and C4037.58b to Ala did not affect GLP-1 pharmacology (Coopman et al., 2011; Dong et al., 2012; Underwood et al., 2011; Wootten et al., 2013c). Substitution of L3847.39 (Ala, Val) and L3887.43 (Ala, Ile) into other aliphatic residues diminished GLP-1 affinity and potency in a similar way as corresponding mutants of homologous L3827.39b and L3867.43b residues in GCGR. The L3867.43bF did not affect GLP-1R affinity or potency, whereas the corresponding L3867.43bF mutant of GCGR abolished glucagon affinity and potency (Siu et al., 2013; Yang et al., 2016). The E3877.42bN mutant did not affect GLP-1 binding or potency, whereas the E3877.42bD mutant decreased GLP-1 affinity and potency by 13-fold and 10-fold, respectively, and the double E3646.53bN/E3877.42bQ mutant completely abolished GLP-1 binding (Yang et al., 2016). The reciprocal Ser8Ala substitution of glucagon restored binding of the GLP-1R–mimicking D3857.42bE mutant of GCGR (Runge et al., 2003a), indicating that this receptor–ligand residue pair plays an important role in ligand selectivity between GLP-1R and GCGR.
xiv. C terminus (C404-S463).
The mutations of N406-A, R421-Q, C438-A, C458-A, and C462-A had no major effect on GLP-1 action (Koole et al., 2011; Underwood et al., 2013; Wootten et al., 2013c). Widmann et al. (1996a) investigated the role of serine residues (four serine doublets in the C-terminal tail) in receptor desensitization. Single- and double-Ala mutations of the doublets S431/S432, S441/S442, S444/S445, and S451/S452 were analyzed to demonstrate their role in receptor phosphorylation and phorbol-12-myristate-13-acetate–induced desensitization (Widmann et al., 1996b).
C. Receptor Function
As a member of class B GPCRs, GLP-1R is highly conserved across species, thus underlining the physiologic importance (Huang et al., 2012). GLP-1R mediates the actions of GLP-1 via the incretin axis that is the functional connection between the intestine and the islets of Langerhans in the pancreas. Stimulation of the GLP-1R with GLP-1 primarily triggers the insulin release from islet β cells in a glucose-dependent manner and suppresses glucagon secretion from islet α cells, in addition to several other effects such as delay of gastric emptying and inhibition of appetite (Koole et al., 2013a; II. Glucagon-Like Peptide-1).
1. Signaling.
a. Recombinant cells.
The physiologic effects of GLP-1 are mediated by its interaction with GLP-1R and subsequent activation of its downstream signaling pathways. GLP-1R is pleiotropically coupled and signals through G-protein–dependent and independent mechanisms. It couples to Gαs-elevating cAMP when overexpressed in recombinant cell lines, including CHO, HEK cells, Chinese hamster lung (CHL) fibroblasts, and COS cells (Wheeler et al., 1993; Widmann et al., 1994; Montrose-Rafizadeh et al., 1999; Wootten et al., 2013b). Increased calcium mobilization has also been observed in CHO, HEK, and COS, but not CHL cells (Wheeler et al., 1993; Widmann et al., 1994; Montrose-Rafizadeh et al., 1999; Coopman et al., 2010; Koole et al., 2010). The mechanism behind this, however, is controversial. Azidoanilide-GTP cross-linking provided evidence for GLP-1R activation of Gαq/11 in CHO cells, whereas in HEK cells GTPγS binding and immunoprecipitation studies showed no activation of Gαq or Gαi (Montrose-Rafizadeh et al., 1999; Coopman et al., 2010). Furthermore, although Wheeler et al. observed a rapid increase in inositol trisphosphates, corresponding with increased calcium levels, and suggesting phospholipase C activation in GLP-1R/COS cells, these intermediates did not seem to be involved in other studies with COS and HEK cells (Wheeler et al., 1993; Widmann et al., 1994; Coopman et al., 2010).
Azidoanilide-GTP cross-linking developed by Montrose-Rafizadeh and colleagues also revealed GLP-1R activation of Gαi1,2 but not Gαi3 in CHO cells. In this cell line, GLP-1 also activates MAPKs, including ERK1/2 (Montrose-Rafizadeh et al., 1999; Koole et al., 2010), and p38, through a cholera toxin–dependent pathway (Montrose-Rafizadeh et al., 1999). GLP-1R can also elicit G-protein–independent signaling, via recruitment of the regulatory/scaffolding β-arrestin proteins (Sonoda et al., 2008; Quoyer et al., 2010). β-arrestin 2 recruitment has been observed in CHO, HEK293, and COS-7 cells, and β-arrestin 1 in CHO cells (Jorgensen et al., 2005; Schelshorn et al., 2012; Wootten et al., 2013b).
b. Pancreatic β cells.
Research has largely focused on characterizing the GLP-1R signaling in β cells, where it mediates increased insulin secretion, storage, and synthesis as well as increased β cell mass. Consistent with the findings in recombinant cells, elevated cAMP is vital in β cells for glucose-dependent insulin secretion mediated by GLP-1R, and, although calcium mobilization, phosphorylation of ERK, and β-arrestin are important, Gαq/11 does not seem to play a major role. A summary of known pathways involved in pancreatic β cell function is illustrated in Fig. 4.
Fig. 4.
Summary of the main characterized pathways of glucose and GLP-1 signaling in the pancreatic β cell. Glucose enters the cell through glucose transporter 2 and undergoes glycolysis to produce pyruvate that enters the mitochondria for oxidative metabolism and ATP production. This increase in cytosolic ATP closes the KATP channels, depolarizing the membrane and opening the voltage-dependent calcium channels, increasing calcium influx into the cell, causing insulin exocytosis. GLP-1 increases insulin exocytosis through a number of mechanisms. GLP-1R couples to Gαs, activates adenylate cyclase that converts ATP to cAMP, and mobilizes two downstream effectors, PKA and Epac. These have a range of effects, including closing KATP channels, enhancing fusion of insulin secretory granules with the membrane, whereas PKA also closes Kv channels, inhibiting membrane repolarization. PKA and Epac also increase intracellular calcium by facilitating CICR through the opening of inositol 1,4,5-trisphosphate receptor and ryanodine receptor calcium channels, respectively. This increase in calcium has also been proposed to upregulate mitochondrial ATP production and activate calcineurin, so nuclear factor of activated T cells promotes insulin gene transcription to increase insulin stores. Alongside increasing insulin synthesis and exocytosis, GLP-1 signals through a number of pathways to increase β cell mass. PKA reduces ER stress through ATF-Gadd34 signaling, increases β cell neogenesis by activating cyclin D, and elevates the expression of insulin receptor substrate 2 (IRS2), a β cell survival factor, as well as anti-apoptotic proteins Bcl-2 and Bcl-xL through CREB. PI3K is activated by IRS2 and transactivation of epidermal growth factor receptor, and this further promotes increased β cell mass through upregulation of PDX-1 and nuclear factor κB, which upregulates anti-apoptotic Bcl-2 /Bcl-xL and inhibitor of apoptosis protein-2.
i. Insulin Secretion.
β cells undergo glucose-stimulated insulin secretion. Glucose enters the β cell through glucose transporter 2 and is converted through glycolysis to pyruvate, which enters the mitochondria for oxidative phosphorylation, increasing the cytosolic ATP/ADP ratio (MacDonald et al., 2005). The increased ATP closes KATP channels, depolarizing the plasma membrane and increasing calcium influx through L-type voltage-dependent calcium channels, causing release of calcium from intracellular stores through calcium-induced calcium release (CICR) (MacDonald et al., 2005). Increased cytoplasmic calcium stimulates exocytosis of the insulin secretory granules.
GLP-1R signaling enhances this glucose-dependent insulin secretion through activation of Gαs, upregulation of cAMP, and subsequent activation of PKA and exchange protein activated by cAMP (Epac). PKA is a holoenzyme composed of both regulatory and catalytic subunits; the latter are released upon binding of cAMP to the regulatory subunits, leading to phosphorylation of downstream substrates, whereas Epac2 primarily functions as a guanine nucleotide exchange factor for small Ras-like G proteins, thereby regulating their activity. PKA phosphorylates the sulfonylurea receptor (SUR1) subunit of the KATP channels, closing them and further depolarizing the membrane (Light et al., 2002). Epac also inhibits KATP by increasing its sensitivity to ATP (Kang et al., 2008). The cAMP/PKA pathway, in concert with the PI3K/protein kinase C (PKC)ζ pathway (discussed in more detail below), also inhibits voltage-gated potassium channels, which in response to depolarization, opens and allows K+ efflux, repolarizing the cell (MacDonald et al., 2003). This delays repolarization, allowing increased calcium influx via voltage-dependent calcium channels (MacDonald et al., 2003). PKA and Epac1/2 are also involved in CICR from the ER, which is mediated by PKA via the inositol 1,4,5-trisphosphate receptor and by Epac1/2 through ryanodine receptors, increasing intracellular calcium (Kang et al., 2003; Tsuboi et al., 2003; Dyachok and Gylfe, 2004). These mechanisms, therefore, enhance the ability of β cells to exocytose insulin secretory granules. There is evidence that CICR also contributes to increased mitochondrial ATP, in conjunction with GLP-1–induced increases in ATP (Tsuboi et al., 2003). It was proposed that the intracellular calcium may enter the mitochondria to activate mitochondrial dehydrogenases to increase ATP production (Tsuboi et al., 2003). PKA and Epac also seem to have a role in the exocytosis of insulin secretory granules, with PKA phosphorylating snapin, a protein that is vital to the regulation of vesicle assembly, and Rab-3–interacting molecule (Rim) and Munc-131, which are involved in vesicle fusion (Kwan et al., 2007; Song et al., 2011). Epac2 also interacts with Rim2 and Piccolo, a calcium sensor, to form a complex that is important for insulin secretion (Fujimoto et al., 2002). Studies in islets of phospholipase C and Epac knockout mice also suggest that Epac2 acts via Rap1-regulated phospholipase Cε to cause calcium-induced insulin exocytosis (Shibasaki et al., 2007; Dzhura et al., 2011). GLP-1 enhances glucokinase activity through Epac2 in a Rim2/Rab3A-dependent manner, sensitizing β cells to glucose. Knockdown of β-arrestin 1 in cultured pancreatic cells resulted in a reduction of both GLP1-mediated cAMP production and insulin secretion (Sonoda et al., 2008). Evidence suggests that this occurs via an increase in β-arrestin 1–mediated ERK1/2 phosphorylation that enhances cAMP response element-binding protein (CREB) activation and cAMP levels, subsequently contributing to insulin secretion (Quoyer et al., 2010).
ii. Insulin Synthesis and Storage.
GLP-1 also acts to increase insulin stores in β cells by promoting insulin gene transcription, its mRNA stability, and biosynthesis. This occurs in both cAMP/PKA-dependent and independent manners (Baggio and Drucker, 2007). Studies with the rat insulin I promoter have implicated basic region leucine zipper proteins, similar in structure to CREB, which bind to the cAMP-responsive element site to upregulate insulin transcription in a cAMP/PKA-independent mechanism (Skoglund et al., 2000; Chepurny et al., 2002). The upregulation of the expression and activity of transcription factor pancreatic-duodenum homeobox-1 (PDX-1) and its increased activity also promote insulin transcription and biosynthesis via a PKA-mediated mechanism (Wang et al., 1999, 2001). Glucose and GLP-1 potentiate insulin gene transcription, by increasing calcium levels, activating calcineurin that dephosphorylates nuclear factor of activated T cells, resulting in its nuclear localization and the promotion of insulin gene transcription (Lawrence et al., 2002).
iii. β Cell Survival, Proliferation, and Neogenesis.
GLP-1 promotes β cell proliferation, neogenesis, and inhibition of apoptosis in rat models of T2DM (Buteau, 2011). GLP-1R activation facilitates the release of β-cellulin by membrane-bound metalloproteinases, inducing transactivation of epidermal growth factor receptor, which then signals through PI3K and activates Akt/protein kinase B (PKB), PKCζ, and p38 MAPK and ERK downstream (Buteau, 2011).
PDX-1 is also critical in increasing β cell mass due to its proliferative and anti-apoptotic effects (Li et al., 2005). As well as the increased activity mentioned above, GLP-1R enhances its expression. Akt inhibits forkhead transcription factor (Foxo1) action through its nuclear exclusion, relieving its inhibition on expression of forkhead box protein A2, which controls PDX-1 expression (Kitamura et al., 2002; Buteau et al., 2006). In addition, GLP-1–mediated activation of PKA upregulates CREB, increasing the expression of IRS2, a β cell survival factor, leading to activation of PI3K/PKB and the anti-apoptotic protein Bcl-2 (Wilson et al., 1996; Rhodes and White, 2002; Jhala et al., 2003). Bcl-2 and Bcl-xL are also upregulated through the activation of nuclear factor κB downstream of PKB (Buteau et al., 2004). Nuclear factor κB increases expression of inhibitor of apoptosis protein-2, thereby preventing apoptosis (Buteau et al., 2004). GLP-1 signaling has also been implicated in downregulation of proapoptotic caspase-3 and decrease in the cleavage of poly-ADP-ribose (Hui et al., 2003; D'Amico et al., 2005). ERK1/2 signaling via β-arrestin 1 also activates p90RSK, which phosphorylates proapoptotic Bad and inactivates it (Quoyer et al., 2010).
PKA activation of MAPK and cyclin D1 is important in the transition of the G1/Gs phase essential to cell cycle progression: they promote β cell neogenesis (Friedrichsen et al., 2006). GLP-1R agonists also improve β cell function and survival upon ER stress. This is believed to occur through PKA activation of C/EBP homologous protein and growth arrest and DNA damage-inducible protein (Gadd34), which prevents the dephosphorylation of translation initiator elF2α, allowing the ER to recover from stress (Yusta et al., 2006).
c. Extrapancreatic signaling.
Although GLP-1 signaling mediates many other physiologic effects, and GLP-1R is expressed in extrapancreatic tissues, the mechanistic basis for these effects is less well characterized. Therefore, there is limited knowledge regarding the underlying signaling mechanisms, although some important pathways and molecules have been identified in a limited subset of tissues.
i. Liver.
GLP-1 reduces hepatic gluconeogenesis and lipogenesis and increases glycogen formation, but there is some debate over whether these effects are mediated by GLP-1R in hepatocytes, or whether the effects may be indirectly mediated through CNS or insulin release. GLP-1 promotes glycogen synthesis and decreased gluconeogenesis in vitro through upregulation of glycogen synthase that occurs downstream of PI3K/PKB, PKC, and serine/threonine protein phosphatase 1, and also by reduced expression of gluconeogenetic enzyme phosphoenol pyruvate carboxykinase in rat hepatocytes (Redondo et al., 2003; Raab et al., 2009). In this system, GLP-1 also increases the phosphorylation of MAPK and p70s6k that perhaps are involved in other GLP-1 effects (Redondo et al., 2003). However, in both studies, the presence of GLP-1R in these hepatocytes was not confirmed.
Signaling mechanisms for fatty acid oxidation and insulin sensitization, important in reducing hepatic steatosis, however, have been characterized in rat hepatocytes confirmed to express GLP-1R (Svegliati-Baroni et al., 2011). Exendin-4 treatment resulted in increased peroxisome proliferator-activated receptor (PPAR) activity and PPARγ expression through the activation of PI3K and 5′ AMP-activated protein kinase (AMPK) pathways (Svegliati-Baroni et al., 2011). Increased PPAR activity induced the transcription of fatty acid β-oxidizing enzymes such as acyl-coenzyme A oxidase 1-palmitoyl and carnitine palmitoyltransferase 1 A, thereby reducing fatty acid levels in hepatocytes (Svegliati-Baroni et al., 2011). Increased PPARγ expression allowed increased insulin sensitization through the reduction of Ser307 c-Jun N-terminal kinase phosphorylation (Svegliati-Baroni et al., 2011). GLP-1R activation also seems to reduce insulin resistance by intersecting the insulin-signaling pathway. In HepG2 and Huh7 cells expressing GLP-1R, exendin-4 treatment led to the phosphorylation of key mediators of the insulin-signaling pathway; PDX-1, Akt1, and PKCζ and small interfering RNA of GLP-1R knocked down this phosphorylation for PDX-1 and PKCζ (Gupta et al., 2010).
ii. Kidney.
GLP-1R activation in the kidney mediates natriuretic and diuretic effects of GLP-1, including decreasing renal proximal tubule reabsorption, improving endothelial integrity, and reducing hypertension. The transporter NHE3, which largely mediates this reabsorption, is inhibited by GLP-1 through PKA and Epac. Exendin-4 causes decreased NHE3 function in the porcine kidney epithelial cell line, LLC-PK(1), with phosphorylation at serine 552, a PKA consensus site. The mechanism by which Epac inhibits the NHE3 transporter has not been determined (Carraro-Lacroix et al., 2009). It is known that angiotensin II signaling increases NHE3 activity, and oxidative stress is inhibited by GLP-1R–mediated cAMP elevation. In glomerular endothelial cells, exendin-4 promoted PKA-dependent phosphorylation of c-Raf(Ser259), preventing the activation of the angiotensin II–dependent pathway, p-c-Raf(Ser338)/ERK1/2/plasminogen activator inhibitor-1, a pathway upregulated in diabetes due to a hyperglycemic increase in PKC-β (Mima et al., 2012).
GLP-1R may also mediate renal protective effects through a reduction of PPARα as exendin-4 treatment decreased PPARα expression in both db/m and db/db mouse models (Park et al., 2007). This decreased expression of PPARα was paralleled by decreased transforming growth factor-β1, decreased type IV collagen, decreased caspase-3 expression, and reduced mesangial expansion (Park et al., 2007).
iii. Adipocytes.
GLP-1R signaling has been implicated in increased glucose uptake in human adipocytes, believed to be mediated by PI3K and MAPK (Sancho et al., 2007). GLP-1R also promotes increased adipocyte mass through preadipocyte proliferation and inhibition of apoptosis, mediated by ERK-, PKC-, and AKT-signaling pathways (Challa et al., 2012).
iv. Nervous System.
GLP-1R expressed in the nucleus tractus solitarius signals to suppress food intake and reduce body weight, and this is proposed to be mediated through PKA (Hayes et al., 2011). PKA decreases phosphorylation of AMPK and increases phosphorylation of p44/23 MAPKs/mitogen-activated protein kinase (MEK) (Hayes et al., 2011).
v. Cardiovascular System.
GLP-R is thought to confer cardioprotection through a number of mechanisms, including reducing damage caused by ischemia via inhibiting apoptosis and through oxidative stress, and improving energy utilization. GLP-1 is able to reduce infarct size in rat hearts, an effect that is attenuated by inhibition of cAMP, PI3K, and p42/44 MAPK and GLP-1R, and also requires activation of the mTOR/p70s6 kinase pathway (Bose et al., 2005b, 2007). Murine studies have implicated additional important signaling molecules. The GLP-1R–dependent cardioprotective effects of liraglutide in mouse cardiomyocytes were associated with an elevation of cAMP and increased caspase-3 activity (Noyan-Ashraf et al., 2009). In addition, liraglutide-mediated suppression of glycogen synthase kinase 3-β (GSK-3β) and caspase-3 activation in murine hearts was not evident in GLP-1R knockout mice (Noyan-Ashraf et al., 2009), confirming that these effects are receptor-dependent. Stimulation of GLP-1R also decreases H2O2-induced production of reactive oxygen species and upregulation of antioxidant enzymes in an Epac-dependent manner (Mangmool et al., 2015).
GLP-1 may signal via AMPK and Akt to improve glucose metabolism after an injury to promote recovery. These were important mediators upon exenatide treatment of TG9 mice that caused improved cardiac contractility, elevated myocardial GLUT4 expression, and increased uptake of 2-deoxyglucose (Vyas et al., 2011).
2. Ligand-Directed Signal Bias.
As described above, GLP-1R is pleiotropically coupled to a range of signaling effectors, each of which can impact on the physiologic response elicited by receptor activation. This allows the potential for individual ligands to evoke different patterns of response upon interaction with the receptor in what is termed ligand-directed biased signaling (Shonberg et al., 2014). Such biased signaling and regulation are thought to arise through the stabilization of distinct ensembles of receptor conformations that occur through the varying chemical contacts between ligands and the receptor. Although gross changes in signal bias can be recognized as a reversal of potency or efficacy (Kenakin and Miller, 2010; Koole et al., 2013b; Shonberg et al., 2014), less dramatic effects require a quantitative framework to identify significant differences in ligand response. The most robust method to quantify efficacy is the operational model of Black and Leff (Kenakin and Christopoulos, 2013), with the transduction ratio of τ/KA used to define strength of signaling of an individual ligand for a specific pathway, and this ratio can be determined from classic concentration-response experiments (Kenakin et al., 2012).
a. Peptide-mediated signal bias.
There are multiple endogenous peptides that can interact with and activate GLP-1R, including the fully processed GLP-17–36 amide and GLP-17–37 peptides, the extended 1–36 and 1–37 forms of these peptides, as well as oxyntomodulin. The search for more stable forms of GLP-1 has also provided a range of mimetic peptides, including exendin and modified forms of GLP-1 that have been developed for therapeutic use (V. Pharmaceutical Development and Therapeutics). Evidence for biased signaling requires measurement of multiple signaling endpoints. Early work characterizing ligands for GLP-1R was largely limited to measurement of cAMP, as the latter is the most well-coupled pathway and critical for the incretin effect on β cells. Therefore, it is only relatively recently that evidence for ligand-directed signaling has emerged.
Initial studies used heterologously expressed GLP-1R to more broadly examine three canonical pathways that have been linked to physiologic signaling in β cells, specifically cAMP accumulation, ERK phosphorylation, and intracellular calcium (iCa2+) mobilization. Even with this relatively limited assessment of signaling, quantitative evidence for peptide-mediated signal bias was observed, with oxyntomodulin exhibiting a relative bias toward pERK over cAMP and iCa2+ signaling compared with either GLP-1 or exendin (Koole et al., 2010), and this supported earlier observations of differences in cAMP signaling and β-arrestin recruitment between oxyntomodulin and GLP-17–36 amide (Jorgensen et al., 2007). Likewise, the extended form of GLP-11–36 amide had a relative bias toward pERK, but a loss of iCa2+ signaling. Thus, this work demonstrated GLP-1R was subject to the potential for peptide-mediated biased signaling, although no significant bias was observed between amidated and nonamidated forms of GLP-17–36 or between GLP-1 and exendin for these pathways (Koole et al., 2010). More extensive analysis of signaling and regulatory pathways that included recruitment of arrestins revealed additional tiers of biased signaling with both exendin and oxyntomdulin exhibiting stronger arrestin recruitment relative to GLP-17–36 amide, whereas GLP-11–36 did not recruit arrestins (Wootten et al., 2013b) (Fig. 5, left panel), at least in this recombinant system. The synthetic 11-mer peptide, BMS21, although much less potent than GLP-17–36 amide, had a relatively similar profile of activation of the canonical pathways (cAMP, pERK, and iCa2+), but did not elicit recruitment of arrestins (Wootten et al., 2013b). Intriguingly, the principal metabolite of GLP-1, GLP-19–36 amide, displays a relative preservation of pERK signaling (Li et al., 2012a; Wootten et al., 2012), despite a marked attenuation of cAMP production and ability to induce insulin secretion (Wootten et al., 2012), indicating that this peptide is strongly biased toward pERK and may be capable of signaling in a physiologically relevant manner. Additional evidence for peptide-mediated signaling bias is seen with a yeast assay of chimeric G protein activation (Weston et al., 2014), consisting of the yeast G protein GPA1 substituted by five amino acids from the human Gα sequence. In this system, exendin, oxyntomodulin, and glucagon exhibited relative bias toward the GPA1/Gαi chimera over the GPA1/Gαs chimera when compared with signaling by GLP-17–36, whereas liraglutide had a similar profile of signaling to GLP-1 across these two pathways (Weston et al., 2014). This indicates that biased signaling occurs even at the level of G protein recruitment and is consistent with stabilization of different ensembles of receptor conformations by different peptide ligands.
Fig. 5.
Web of bias illustrating distinctions in the pattern of signaling of different peptide agonists (left panel) or nonpeptidic modulators (right panel) at GLP-1R. The web of bias plots ΔΔτ/KA values on a logarithmic scale for each ligand and for every signaling pathway tested. Formation of these values included normalization to the reference ligand GLP-17–36 amide and the reference pathway, cAMP accumulation. The plots do not provide information on absolute potency, but on relative efficacy for signaling of individual pathways in comparison with that for cAMP. Data are from Koole et al., 2010; Willard et al., 2012a; and Wootten et al., 2013b.
b. Nonpeptide-mediated bias.
There has been considerable interest in the development of nonpeptidic ligands for GLP-1R as leads with improved bioavailability (Chen et al., 2007; Knudsen et al., 2007; Willard et al., 2012b). Although these ligands are principally tested in assays of cAMP formation and insulin secretion, a number of these compounds have now been evaluated across a more broad range of pathways, enabling assessment of the extent to which these compounds exhibit bias relative to native GLP-1 signaling. The most extensively studied are the Eli Lilly compound, 4-(3-benzyloxyphenyl)-2-ethylsulfinyl-6-(trifluoromethyl)pyrimidine (BETP), and the Novo Nordisk compound 2 (Coopman et al., 2010; Koole et al., 2010; Cheong et al., 2012; Willard et al., 2012a; Wootten et al., 2013b), but there are now some data comparing signaling for Boc5 and the TransTech Pharma compound TT15 (Wootten et al., 2013b). Although these compounds have low potency for cAMP production, relative to native GLP-17–36 amide, they also exhibit distinct patterns of signal bias (Koole et al., 2010; Wootten et al., 2013b). This can be illustrated in a web of bias (Fig. 5, right panel), which reveals that Boc5 and TT15 have similar patterns of signaling via GLP-1R for canonical pathways (cAMP, pERK, and iCa2+), but have diminished ability to recruit arrestin proteins. In contrast, compound 2 and BETP have a relatively enriched ability to recruit arrestins, but distinct effects on pERK and iCa2+ mobilization, where BETP trends towards reduced pERK but higher iCa2+, and compound 2 has the opposite profile (compared to the reference cAMP pathway and the signaling profile of GLP-1). Additional differences in the behavior of compound 2 and/or BETP have also been noted by others (Coopman et al., 2010; Cheong et al., 2012; Thompson and Kanamarlapudi, 2015), albeit that a lack of a quantitative framework for these analyses meant that potential implications for signaling bias were not fully explored. The fact that these nonpeptidic compounds have distinct profiles of signaling is not surprising, as their chemical diversity means that they interact with the receptor very differently from peptidic ligands. Indeed, BETP and compound 2 interact with the intracellular face of GLP-1R in a manner that involves covalent modification of C347 in ICL3/TM6 (Nolte et al., 2014).
3. Molecular Basis for Signal Bias.
There is increasing evidence demonstrating that the conformational change that drives activation transition involves reordering of hydrogen bond networks and intrahelical packing. Such a change promotes reorganization of the intracellular face of the receptor, thereby enabling interaction with intracellular effectors (Zhou et al., 2000; Curran and Engelman, 2003; Angel et al., 2009; Illergard et al., 2011). These networks are relatively well conserved within GPCR subfamilies and involve conserved polar residues (Venkatakrishnan et al., 2013). This is best studied for class A GPCRs, and suggests that there is evolutionary conservation of the mechanisms underpinning activation transition. Although there is limited direct homology in the amino acid sequence of the TM domains between the major receptor families, it is expected that such conserved polar residues within class B GPCRs play a similarly important structural role and contribute to the mechanism of ligand-directed biased signaling. This view is supported by experiments in which conserved intramembranous polar residues in GLP-1R were mutated to alanine. A cluster of residues, including R1902.60b, N2403.43b, H3636.52b, and Q3947.49b, when mutated, was shown to change the signaling profile of the receptor for cAMP, pERK, and iCa2+ in both a residue-specific and ligand-specific manner (Wootten et al., 2013c). Although differences were particularly marked between GLP-1 and oxyntomodulin, selective differences in the effect of mutation were also seen between GLP-1 and exendin, providing molecular evidence that the mechanism of receptor activation differs between these two peptides, despite relatively similar overall efficacy for the peptides across these pathways at the wild-type receptor (Wootten et al., 2013c). In addition to these residues that displayed ligand-specific effects, there was a series of serine residues (S1551.50b, S1862.56b, and S3927.47b) that played a role in the control of receptor-dependent signal bias; however, these had global effects on all peptides (Wootten et al., 2013c). These residues sit at the interface between either TM1 and TM7 (S1551.50b and S3927.47b) or TM2 and TM3 (S1862.56b) and are most likely involved in tight packing of these helices. With the solution of crystal structures of the TM domain of the related CRF1 and glucagon receptors (Hollenstein et al., 2013; Siu et al., 2013; Jazayeri et al., 2016), it is now possible to model the location of these residues with improved precision. Intriguingly, such modeling indicates that these residues reside at a fulcrum position of the receptor TM bundle, where the splayed helices of the open extracellular face of the receptor converge (Fig. 6), with the residues that contribute to ligand-dependent signaling forming a central interaction network and the smaller polar residues that are globally important for signaling external to this core.
Fig. 6.
Homology model of GLP-1R illustrating the relative position of key residues involved in the receptor-signaling bias. The modeling indicates that these residues reside at a fulcrum position of the receptor transmembrane bundle, where the splayed helices of the open extracellular face of the receptor converge, with the residues that contribute to ligand-dependent signaling forming a central interaction network (space fill, red) and the smaller polar residues that are globally important for signaling external to this core (space fill, blue). The receptor is displayed in three views at different horizontal rotation. Transmembrane helices are numbered with Roman numerals.
Although not broadly conserved across the class B subfamily, there is an additional polar threonine in TM1, at position 1491.44b, that is the site of a naturally occurring polymorphism leading to incorporation of a methionine in this position (Beinborn et al., 2005). Methionine at this position leads to a marked loss in coupling of the receptor to cAMP accumulation (Fortin et al., 2010; Koole et al., 2011) and iCa2+ mobilization, whereas ERK phosphorylation is relatively preserved (Koole et al., 2011). This is a global effect for peptide activators of the receptor (Koole et al., 2011). Exploration of the tolerance of this position to different amino acids revealed that serine substitution had relatively minimal detrimental effect, suggesting that maintenance of the polar nature of this residue is important. However, amino acids of similar size, although leading to attenuation of response in a pathway-specific manner, were the least affected, indicating that side chain packing was also important (Koole et al., 2015). Thus, this mutation changes the physiologic bias of the receptor in addition to markedly attenuating peptide-mediated signaling.
Although these experiments have been principally interpreted in the context of in cis signaling of receptor to effectors, GLP-1R, like most, if not all class B GPCRs, undergoes functionally important dimerization (Harikumar et al., 2006, 2010, 2012; Gao et al., 2009; Schelshorn et al., 2012). Intriguingly, disruption of the TM4 dimer interface of GLP-1R differentially impacted the signaling elicited by agonist peptides with a greater abrogation of iCa2+ mobilization relative to that of either cAMP accumulation or ERK phosphorylation (Harikumar et al., 2012). This indicates that differential ligand-mediated signaling could also involve the allosteric interaction of protomers within a receptor dimer.
In addition to an emerging appreciation of how ligands can selectively alter key hydrogen-bonding networks, there is some limited information on how extracellular loop residues contribute to peptide-mediated bias, in particular for ECL2 (Koole et al., 2012a,b). K288ECL2, C296ECL2, W297ECL2, and N300ECL2 had critical roles in governing signal bias of the receptor, but were also globally important for peptide signaling. Nonetheless, peptide-specific effects on relative efficacy and signal bias were most frequently observed for residues 301–305, although R299ECL2A mutation also exhibited different effects for individual peptides. M303ECL2 appeared to play a greater role for exendin and oxyntomodulin actions than those of GLP-1 peptides. Interestingly, ECL2 mutation was generally more detrimental to exendin-mediated iCa2+ mobilization than GLP-17–36 amide, providing additional support for subtle variances in receptor activation by these two peptides.
4. Allosteric Modulation.
As described above (II. Glucagon-Like Peptide-1 and III. Glucagon-Like Peptide 1 Receptor), peptide ligands engage GLP-1R via a diffuse pharmacophore that includes key interactions for affinity with the N-terminal extracellular domain, as well as poorly defined interactions with the extracellular loops and TM domain core that drive receptor activation. Nonpeptidic ligands have a distinct mode of binding, and in many cases can do so via topographically distinct, allosteric sites from those of the native peptides. Such ligands can bind simultaneously with peptide ligands and interact in a cooperative manner to alter the binding and/or efficacy of peptide signaling (and vice versa) (Leach et al., 2007; May et al., 2007; Keov et al., 2011; Wootten et al., 2013a; Gentry et al., 2015). Allosteric modulation of GPCRs is now a well-established paradigm that provides both advantages and challenges for drug discovery when compared with classic orthosteric ligands (Wootten et al., 2013a). The first characterized allosteric modulators of GLP-1R were identified by Novo Nordisk, and are exemplified by compound 2, which, as noted above, is an allosteric agonist of the receptor. In addition to its intrinsic efficacy, this series of compounds augmented the binding of radiolabeled GLP-1, indicating that it could act as a positive allosteric modulator (Knudsen et al., 2007). Nonetheless, there was only limited impact on the efficacy of GLP-1R for cAMP signaling. A hallmark of allosteric interactions is the phenomenon of probe dependence that describes the capacity for different effects, depending upon the orthosteric and allosteric ligand combination. This is also observed for ligands of GLP-1R. Although compound 2 has only limited effect on GLP-17–36 amide–induced cAMP production, it yields a ∼30-fold augmentation of oxyntomodulin signaling via this pathway (Koole et al., 2010; Willard et al., 2012b; Wootten et al., 2013b). Probe dependence is also seen for effects on the extended GLP-1 peptides and exendin, where very limited augmentation of cAMP signaling is observed. A similar pattern of effect is observed for BETP in that this compound also augments oxyntomodulin signaling, but has minimal effect on GLP-17–36 amide–, exendin-, or GLP-11–36 amide–mediated cAMP production (Willard et al., 2012b; Wootten et al., 2013b). A weak potentiation of compound 2–mediated cAMP production (or surrogate readouts such as cAMP response element–driven luciferase reporter) has also been reported for interaction of this ligand with truncated forms of exendin, including exendin5–39, exendin7–39, and exendin9–39, even in the absence of any measurable intrinsic activity (Coopman et al., 2010; Cheong et al., 2012).
Intriguingly, if the cooperative effect of BETP or compound 2 is studied over a broader array of signaling endpoints, then marked differences in effect are observed (Koole et al., 2012a; Li et al., 2012a; Wootten et al., 2012, 2013b). In contrast to the selective augmentation of cAMP response for oxyntomodulin, the allosteric ligands confer a similar, modest enhancement of arrestin recruitment for both GLP-1 and oxyntomodulin (Willard et al., 2012b; Wootten et al., 2013b), although a greater degree of negative cooperativity is seen on pERK responses of GLP-17–36 amide versus oxyntomodulin. Thus, the allosteric ligands also alter the signaling bias mediated by endogenous peptides. This is perhaps not surprising as both signaling bias and the cooperative effects of cobound ligands are driven through changes to the ensemble of conformations that the receptor samples (Wootten et al., 2013a). Indeed, the allosteric ligand-bound receptor can effectively be considered a distinct receptor for the interaction of the natural ligand(s).
Remarkably, both BETP and compound 2 very strikingly enhanced the cAMP response of the principal GLP-1 metabolite, GLP-19–36 amide, by up to ∼400-fold in the case of compound 2 (Li et al., 2012a; Wootten et al., 2012). Parallel augmentation of insulin secretion was observed in isolated rat islets and in vivo when pharmacological levels of the metabolite were coadministered with subthreshold levels of BETP (Wootten et al., 2012). The augmentation of signaling was principally limited to cAMP production, although weak potentiation of pERK and iCa2+ signaling was seen in HEK293 cells recombinantly expressing the receptor and INS-1E cells (Li et al., 2012a), but not in CHO cells recombinantly expressing the receptor (Wootten et al., 2012), providing further evidence that allosteric modulators can alter the signal bias of the receptor. Nonetheless, the lack of effect on the extended GLP-1 peptide indicates that the magnitude of the cooperative effect of BETP and compound 2 is not driven solely by the intrinsic efficacy of the activating peptide.
Ligands such as BETP and compound 2 are highly electrophilic and can form adducts with free cysteine residues (Eng et al., 2013). This is a feature of the action of these molecules to form, in particular, the covalent interaction with C3476.36b in ICL3/TM6 that is critical for both the intrinsic efficacy and the cooperative allosteric effect (Nolte et al., 2014). Nevertheless, the covalent modification of the cysteine is insufficient to explain the full extent of activity of these compounds as BETP and compound 2 have differences in their profile of intrinsic efficacy (see above) and also in their cooperative effect (Wootten et al., 2013b). Interestingly, there is a naturally occurring polymorphism that can appear at position 333 of the human receptor, being either a serine or a cysteine with the latter occurring only rarely (Koole et al., 2011, 2015). However, the C333ICL3 variant selectively abrogates compound 2–mediated cAMP production and cooperativity between compound 2 and oxyntomodulin for this pathway (Koole et al., 2011) while maintaining peptide-mediated signaling, suggesting that the environment surrounding C3476.36b is important for the action of compound 2. In contrast to the effect of the C333ICL3 polymorphism, the M1491.44b polymorphic variant has no effect on the intrinsic efficacy of compound 2, but, as noted above, causes a very marked attenuation of peptide-mediated cAMP signaling with over 100-fold loss of GLP-17–36 amide or exendin potency. At this mutant, compound 2 could restore the potency of both GLP-17–36 amide and exendin to that of the wild-type receptor (Koole et al., 2011). Thus, allosteric modulators have the capacity to alleviate genetic disease arising from the loss of function of GLP-1R.
Disruption of the TM4 dimer interface of GLP-1R also abolishes the intrinsic efficacy of BETP and compound 2, but the cooperative augmentation of cAMP signaling by oxyntomodulin is maintained, indicating that the allosteric effect occurs in cis at least for this class of ligands (Harikumar et al., 2012).
Although compounds such as BETP and compound 2 were among the first recognized and the most widely studied allosteric modulators of GLP-1R, a number of additional compounds have been reported to act as modulators of peptide response. This includes the flavonol, quercetin, which lacks intrinsic activity, but could selectively augment calcium signaling of efficacious peptides such as GLP-17–36 amide, GLP-17–37, and exendin, albeit that an inhibition of response was seen with high concentrations of quercetin (Koole et al., 2010; Wootten et al., 2011). This augmentation of iCa2+ was dependent upon the presence of a 3-hydroxyl group on the flavone backbone and was improved when a 3′4′-dihydroxyl modification was present (Wootten et al., 2011). An additional series of compounds that could selectively enhance iCa2+ signaling was recently reported following high-throughput screening (Morris et al., 2014). Unlike the flavonols, this series of compounds had significant intrinsic efficacy for mobilization of iCa2+. An exemplar from this series, termed (S)-9b, was examined in further detail, in which it was reported to also augment liraglutide-mediated GLP-1R internalization in recombinant cells and exendin-mediated insulin secretion in primary mouse islets (Morris et al., 2014). This latter effect occurred in both high and low glucose, which would be problematic from a therapeutic standpoint, but may also require further investigation to determine whether this is mediated by GLP-1R. Likewise, compound (S)-9b reduced haloperidol-induced catalepsy in rats (Morris et al., 2014), but, again, it needs to be confirmed whether this effect is mediated via GLP-1R. In other work, using virtual screening against a molecular model of GCGR, de Graaf et al. (2011) identified a number of compounds that had activity at both GCGR and GLP-1R, including weak antagonists and one compound that acted as an inhibitor of glucagon-mediated cAMP production at GCGR, but as a weak positive modulator of GLP-1R–mediated cAMP response.
At this point in time, the physiologic or therapeutic implications of biased signaling are largely unknown. Despite differences in the signaling/regulatory profile of compounds such as Boc5 or TT15, these compounds (or similar analogs) have demonstrated efficacy in modulation of ex vivo and in vivo insulin secretion (Chen et al., 2007; He et al., 2012). Allosteric compounds such as BETP or compound 2 likewise can augment insulin secretion, albeit that this is complicated by endogenous circulating peptides. Pharmacologically, both BETP and compound 2 can augment responses of oxyntomodulin and the GLP-1 metabolite (GLP-19–36 amide), and this is linked to increased in vivo insulin secretion, at least in the context of threshold doses of the allosteric and orthosteric peptides (Willard et al., 2012b; Wootten et al., 2012). In vitro assessment of the effect of the modulators on the signaling profile of oxyntomodulin or GLP-19–36 amide indicates that their most prominent effect is to augment cAMP production, suggesting that this alone may be sufficient to improve insulin secretion, at least in the context of the baseline signaling of the peptides/modulators. The significance for GLP-1R function outside of insulin secretion is even less clear, as there has been very limited assessment of these other functions and none controlled for the influence of biased signaling. A major current limitation is the range of tools available to probe the significance of signaling differences. Most nonpeptidic compounds have low potency, nonfavorable pharmacokinetic profiles, or unknown interaction with other targets that limits their utility. Exendin is biased relative to GLP-17–36 amide, but this difference is subtle in the context of canonical signaling, making interpretation of physiologic data difficult. Oxyntomodulin is the most biased of the characterized peptides, displays good affinity for GLP-1R, and has distinctions in its physiologic actions from GLP-1, but dissecting the importance of this bias is complicated by its significant interaction with GCGR (Pocai, 2013). Nonetheless, this may be a useful tool to study biased signaling in GCGR knockout animals. Additional approaches to infer the potential role of selective activation of signaling/regulatory pathways include the generation of knock-in mice that have receptors designed to selectively abrogate signaling via a specific pathway (e.g., G protein versus arrestin), although the development of potent biased peptide ligands could also provide novel scope for better understanding this phenomenon.
D. Receptor Regulation
1. Receptor Desensitization.
The activity of GLP-1R, like all GPCRs, is regulated by a coordinated balance between molecular mechanisms governing receptor signaling, desensitization, and resensitization. Receptor desensitization, or the reduced responsiveness of GPCR signaling to an agonist with time, is an important physiologic feedback mechanism that protects against both acute and chronic receptor overstimulation (Ferguson, 2001). This has important biologic and therapeutic implications.
GLP-1R undergoes rapid and reversible homologous and heterologous desensitization that has been observed in recombinant cell systems, in primary islets of Langerhans, and in insulinoma β cell lines (Fehmann and Habener, 1991; Gromada et al., 1996; Widmann et al., 1996a,b). Homologous desensitization refers to the loss of response to subsequent agonist stimulation following direct stimulation of the receptor and can occur through controlling the number of receptors present at the cell surface or by regulating the efficacy of the receptors at the cell surface. Heterologous desensitization describes receptor activation-independent regulation of receptors as well as mechanisms that occur after receptor activation that do not discriminate between activated, and nonactivated, receptors (Ferguson, 2001).
Acute incubation of islet cells with native GLP-1 induces rapid homologous GLP-1R desensitization in vitro (Fehmann and Habener, 1991; Gromada et al., 1996). In addition, exendin-4 also produces GLP-1R desensitization in islet cells following both acute and chronic exposure; however, this ligand is associated with a greater degree of desensitization compared with comparable incubations with GLP-1 (Baggio et al., 2004). Heterologous desensitization has been demonstrated in islets in response to multiple stimuli. For instance, GLP-1R heterologous desensitization was observed via acute exposure to phorbol myristate acetate (PMA), an activator of PKC (Widmann et al., 1996a,b; Baggio et al., 2004). In addition, prolonged hyperglycemia can induce desensitization, followed by downregulation with diminished responses to GLP-1R agonists that include weakened insulin secretion, reduced phosphorylation of CREB, impaired cAMP responses and PKA activity, and a reduction in GLP-1R mRNA (Rajan et al., 2015).
There is also the possibility that heterologous desensitization may occur due to activation of other receptors expressed in β cells that are involved in tightly controlling insulin secretion. To date, this has only been assessed with the ligand for GIPR. GLP-1R can heterodimerize with GIPR in vitro, with evidence that this heterodimerization can alter cellular signaling and trafficking profiles of GLP-1R (Schelshorn et al., 2012; Roed et al., 2015). However, despite their overlapping functions for signaling and insulin secretion, GIP does not produce meaningful heterologous desensitization of GLP-1R in islet cell studies (Rajan et al., 2015).
2. Underlying Mechanisms.
Desensitization is a consequence of a number of different mechanisms. They include uncoupling the receptor from heterotrimeric G proteins in response to receptor phosphorylation, the internalization of receptors to intramembranous compartments, and the downregulation of the total complement of receptors (Ferguson, 2001).
a. GLP-1R phosphorylation.
The most rapid mechanism by which GPCRs are desensitized is through the covalent modification of the receptor as a consequence of phosphorylation by intracellular kinases, either G protein–coupled receptor kinases (GRKs) or second messenger kinases (Ferguson, 2001). Both homologous and heterologous desensitization of GLP-1R are accompanied by phosphorylation of serine residues within the last 33 amino acids of the C-terminal tail (Widmann et al., 1996a,b).
b. GRKs.
For most GPCRs, homologous desensitization is thought to involve phosphorylation by GRKs that results in recruitment of β-arrestins. In vitro studies in fibroblast cells expressing GLP-1R revealed that homologous desensitization was associated with phosphorylation of serine residues 441/442, 444/445, and 451/452 in the C terminus (Widmann et al., 1997). Desensitization of GLP-1R–mediated cAMP responses after a first initial exposure of cells to GLP-1 was strictly dependent on the extent of phosphorylation with no, intermediate, or maximum phosphorylation observed in the presence of one, two, or three of the serine doublet phosphorylation sites, respectively.
Although various groups, using a number of techniques, have demonstrated that stimulation of GLP-1R leads to recruitment of both GRKs and β-arrestins (Jorgensen et al., 2005, 2011; Wootten et al., 2013a), to date, the direct involvement of these proteins in desensitization of GLP-1R response has not been demonstrated. In recombinant systems, GRK2 can interact with GLP-1R in response to stimulation by GLP-1 (Jorgensen et al., 2007, 2011). In addition, GRK2 has been linked to potentiation of β-arrestin 2 recruitment to the receptor. β-arrestin 1 is also recruited to GLP-1R upon activation by agonist ligands (Sonoda et al., 2008; Wootten et al., 2013a); however, whether recruitment of these proteins is required for internalization is currently not clear.
c. Second messenger protein kinases.
Heterologous desensitization of GLP-1R occurs at least in part via the second messenger kinases, cAMP-dependent PKA and PKC (Widmann et al., 1996b; Rajan et al., 2015). Direct activation of PKC by PMA markedly reduced the amplitude of GLP-1R–mediated calcium responses, whereas inhibitors of PKC slowed desensitization and increased the duration of calcium transients (Gromada et al., 1996). Using fusion proteins of wild-type and mutant GLP-1R C-terminal tails, in vitro phosphorylation experiments revealed that PKC-mediated phosphorylation occurred predominantly at residues 431/432, and, whereas positions 444/445 and 451/452 could be phosphorylated by PKC, they were poor substrates. In these studies, the serine doublet 441/442 was not a substrate for PKC (Widmann et al., 1996a). In contrast, studies performed in intact COS-7 cells showed all four of these doublets could be phosphorylated following PKC activation by PMA. This could be attributed to different isoforms of PKC in intact cells or the possibility that other kinases downstream of PKC activation may be important for PMA-induced GLP-1R phosphorylation. Phosphorylation of at least two of these serine doublets was required to engender PMA-induced heterologous desensitization, with removal of any pair of doublets leading to receptors that were completely resistant to PMA-induced GLP-1R desensitization (Widmann et al., 1996a).
PKA has been implicated as a mediator of desensitization and downregulation of GLP-1R from the cell surface in pancreatic islets in conditions of chronic hyperglycemia (Rajan et al., 2015). In MIN6 cells, reductions in cell surface receptor numbers in conditions of high glucose were mimicked by overexpression of a constitutively active PKA or continuous activation by forskolin. In addition, inhibition of PKA activity attenuated glucose-mediated downregulation of GLP-1R from the cell surface of these cells. Phosphorylation at serine 301, within the third intracellular loop of the receptor, is implicated in this PKA-mediated activity, with mutation of this residue abolishing glucose-dependent loss of the receptor from the cell surface. This was associated with a loss of an interaction between the receptor and the small ubiquitin-related modifier, SUMO (Rajan et al., 2015). Sumoylation of GLP-1R causes intracellular retention of the receptor and desensitization of receptor signaling as well as prevents resensitization of the receptor back to the cell surface (Rajan et al., 2012). This heterologous desensitization and subsequent downregulation of GLP-1R on the β cell surface by chronic hyperglycemic conditions have substantial implications for the efficacy of GLP-1–based therapies. Because SUMO expression is increased in mouse islets exposed to high glucose (Rajan et al., 2012), its interaction with GLP-1R may in part contribute to the reduced efficacy of incretin therapies in some T2DM patients with poorly controlled hyperglycemia (Fritsche et al., 2000; Stumvoll et al., 2002; Kjems et al., 2003).
3. In Vivo Evidence of Desensitization.
Given the clinical interest in the therapeutic benefits of achieving sustained chronic elevations of GLP-1R agonists in the plasma through repeated or continual administration, the scope of GLP-1R desensitization has direct therapeutic relevance. Up to now, only a few studies have addressed the relevance of GLP-1R desensitization in vivo. Chronic or intermittent intracerebroventricular GLP-1 administration inhibited food intake and reduced weight gain in rats (Davis et al., 1998). Similarly, chronic intermittent administration of GLP-1R agonists to diabetic rodents is associated with inhibition of food intake, improvement of glycemia, and reduction in hemoglobin A1c (HbA1c), demonstrating that repeated administration of GLP-1 is not associated with a diminished therapeutic response in vivo (Szayna et al., 2000; Rolin et al., 2002; Kim et al., 2003).
Considering the prolonged activity and stability of GLP-1 mimetics compared with native GLP-1 (Young et al., 1999), it seems likely that islet cells and extrapancreatic GLP-1R would be exposed for a greater period of time with these ligands than the endogenous peptides. In vitro studies showed that exendin-4 is more potent than GLP-1 in producing GLP-1R desensitization; however, chronic exposure to exendin-4 in normal or transgenic mice that express exendin-4 was not associated with significant downregulation of GLP-1R–dependent responses coupled to glucose homeostasis (Baggio et al., 2004). Furthermore, patients treated with twice-daily exendin-4 or once-weekly liraglutide continue to exhibit a decrease in HbA1c and marked reductions in postprandial glycemic excursion (Buse et al., 2004; de Wit et al., 2014). Therefore, although in vitro experiments clearly demonstrate that GLP-1R has the capacity for desensitization, there is little evidence that it undergoes clinically meaningful desensitization in vivo in terms of glucose regulation.
There is, however, some limited evidence for desensitization occurring in vivo (Nauck et al., 2011). Administering native GLP-1 continuously into healthy human subjects for 8.5 hours, followed by assessment of glucoregulatory responses to liquid test meals given 5 hours apart with ongoing continuous GLP-1 infusion, demonstrated a reduced ability of GLP-1 to suppress gastric emptying and glucagon levels by the second test meal. In addition, levels of pancreatic polypeptide, a marker of vagal activation, were not as inhibited during the second test meal compared with the first. However, C-peptide and insulin levels were preserved with only a small reduction in the second meal. These studies reveal that even short-term continuous GLP-1R stimulation may be associated with some degree of rapid tachyphylaxis, most evident in effects mediated through the vagus nerve and gastric emptying (Nauck et al., 2011). Despite this evidence for in vivo desensitization, the physiologic significance of this is still unclear.
4. Receptor Internalization.
GLP-1R rapidly internalizes as a complex associated with its bound ligand. This has been observed for GLP-1, exendin-4, liraglutide, and compound 2 (Widmann et al., 1995; Kuna et al., 2013; Roed et al., 2014). In BRIN-BD11 cells, antibody-labeled GLP-1R and fluorescently labeled GLP-1 were colocalized at the perinuclear space following internalization (Kuna et al., 2013). Furthermore, internalization of fluorescently labeled GLP-1, exendin-4, and liraglutide has been observed in both recombinant cell systems and primary mouse pancreatic islets (Roed et al., 2014). There is also some evidence of ligand-directed bias with GLP-1R internalization, although this has not been directly quantified. The potencies for internalization by GLP-1 and exendin-4 were 10-fold higher than liraglutide, although kinetics of internalization were similar for all three ligands (Roed et al., 2014).
Currently, the mechanism for GLP-1R internalization in vivo is unclear, as there is evidence that this mechanism may be cell-type dependent (Widmann et al., 1995; Vazquez et al., 2005; Syme et al., 2006; Thompson and Kanamarlapudi, 2015). As GLP-1R is expressed in multiple tissues throughout the body with distinct physiologic functions depending upon the location of the receptor, this implies that the mechanism and role of receptor internalization and desensitization may be tissue-dependent. In different cell backgrounds, two distinct mechanisms of internalization have been observed, with both mechanisms dependent on dynamin (Widmann et al., 1995; Syme et al., 2006; Kuna et al., 2013).
Studies performed in CHO and CHL cells recombinantly expressing GLP-1R revealed internalization via clathrin-coated pits, although these studies indicated that there might not be complete internalization via this method (Widmann et al., 1995; Vazquez et al., 2005). Studies in CHL fibroblasts demonstrated that the same three phosphorylation sites linked to homologous desensitization of GLP-1R–mediated cAMP responses (441/442, 444/445, and 451/452) are also important for internalization, supporting a correlation between phosphorylation at these sites and internalization (Widmann et al., 1997). However, the exact contribution of each phosphorylation site to desensitization of the cAMP response and internalization is different, indicating that the precise molecular basis for control of receptor desensitization and endocytosis is, at least in part, distinct. In addition to phosphorylation of these serine doublets, a region of the C-terminal tail close to the bottom of TMD7 is also of importance in mediating agonist-dependent GLP-1R internalization (Vazquez et al., 2005).
Traditionally for GPCRs, β-arrestin recruitment targets receptors for clathrin-mediated internalization (Ferguson, 2001). However, the literature indicates that this may not be the case for GLP-1R in some cell backgrounds, despite the ability of the receptor to interact with these proteins (Syme et al., 2006). Although there is little literature on the role that β-arrestin 2 plays in GLP-1R internalization and desensitization, there is some evidence that these processes can occur independently of β-arrestin recruitment, in a clathrin-independent mechanism. Studies performed in HEK293, COS-7, and MIN6 cells exhibited caveolin-1 regulation of internalization (Syme et al., 2006; Thompson and Kanamarlapudi, 2015). GLP-1R contains a classic caveolin-1–binding motif within the second intracellular loop such that it interacts and colocalizes with the protein intracellularly. Occurrence of this interaction was also supported by studies using inhibitors of caveolin-1. This mechanism of GLP-1R internalization has been proposed to occur through activation of Gαq proteins, followed by activation of PKC (Thompson and Kanamarlapudi, 2015). In support of β-arrestin–independent internalization, it was shown that knockdown of β-arrestin 1 in INS-1 cells had no effect on internalization or desensitization of GLP-1R (Sonoda et al., 2008).
Although internalization can contribute to desensitization of receptors, internalization and desensitization are two distinct phenomena. Internalization has the potential to contribute to termination of some GLP-1R–mediated signaling events; however, it does not terminate all components of this signaling (Kuna et al., 2013; Roed et al., 2015). A recent study revealed that inhibition of internalization using a dominant-negative form of dynamin (K44E) resulted in decreased cAMP formation, ERK1/2 phosphorylation, and calcium signaling, suggesting that at least some of the signaling mediated by these pathways occurs via internalized receptors. In addition, inhibition of GLP-1R internalization also attenuates insulin secretion, confirming that internalization is an integral part of the signaling process for this receptor (Kuna et al., 2013). Using fluorescently labeled ligands and receptors, studies in recombinant systems displayed colocalization of internalized GLP-1R with adenylate cyclase in endosomes (Kuna et al., 2013). Taken together, these findings all imply that GLP-1R continues to signal following internalization, and that internalization is not necessarily part of the mechanism for desensitization. This level of spatiotemporal control achieved by the compartmentalization of signaling through internalization may be vital to specific, fine-tuned responses from GLP-1R activation and may have substantial implications both physiologically and therapeutically for targeting this receptor in disease management.
5. Receptor Resensitization.
GLP-1R resensitization has not been extensively studied. Following desensitization, GLP-1R–mediated calcium signaling resensitizes after removal of extracellular ligand within 1 hour and does not require de novo protein synthesis (Gromada et al., 1996). After internalization, GLP-1R colocalizes with transferrin, a marker for recycling endosomes, suggesting that it is recycling GLP-1R that is returned back to the cell surface after internalization (Roed et al., 2014). Moreover, there is evidence in HEK293 cells that GLP-1R recycles back to the cell surface in response to GLP-1, exendin-4, and liraglutide, even after prolonged agonist treatment. However, recycling was two to three times slower for exendin-4 and liraglutide compared with GLP-1 (Roed et al., 2014). In the presence of GLP-1, receptors were found in transferrin-positive endosomes up to 45 minutes post ligand addition, whereas in the presence of exendin-4 and liraglutide colocalization of internalized GLP-1R and transferrin was observed for up to 60 minutes following ligand addition. Resensitization is important, as irreversible desensitization would leave a cell unable to respond to external stimuli, and therefore this mechanism protects against prolonged desensitization (Ferguson, 2001). Nonetheless, to date, the mechanism of GLP-1R resensitization remains largely unknown and requires further investigation.
There is also evidence of partial degradation of GLP-1R upon prolonged activation with exendin-4, liraglutide, and GLP-1, with colocalization observed between the receptor and lysosomes (Kuna et al., 2013; Roed et al., 2014). This mechanism leads to downregulation of receptor numbers.
IV. Pharmacological Tools
A. Traditional Bioassays
As GLP-1 exerts its vital physiologic and pharmacological functions through interacting with its receptor on the cell membrane, it is important to measure and determine the activities of the peptide and its analogs through the biologic actions of the protein. There are traditional bioassays (Table 2) to determine receptor-binding affinity and activation of the receptor, and several unique methods have been reported for assessing molecular recognition and biochemical consequences. In addition, molecular imaging of the receptor has demonstrated a potential for noninvasive monitoring of pancreatic β cells, which may be of great value in the diagnosis and prognosis of diabetes, tracking the disease progression, and evaluating therapeutic interventions, including islet regeneration and transplantation.
TABLE 2.
Summary of traditional biologic assays for GLP-1 analogs
| Molecular/Cellular Measurement | Description/Outcome | References |
|---|---|---|
| Receptor-binding assays: | ||
| Radioligand competition | Determines binding affinity (IC50 or Kd) by measuring radioactivity remaining on the receptor after competitive inhibition of radioligand with a GLP-1 analog. | Mathi et al., 1997; Tibaduiza et al., 2001; Xiao et al., 2001 |
| Time-resolved fluorescence resonance energy transfer (FRET) | Determines binding affinity (IC50) by measuring a decrease in FRET signal between Tb-labeled receptor and fluorescent ligand after competitive inhibition with a GLP-1 analog. | Maurel et al., 2008; Zwier et al., 2010; Roed et al., 2014 |
| Circular dichroism and fluorescence spectroscopies | Determines binding affinity by measuring conformational changes of a receptor protein upon ligand binding. | Runge et al., 2007 |
| Isothermal titration calorimetry | Determines thermodynamic parameters of binding, such as the dissociation constant, enthalpy change, entropy change, and reaction stoichiometry by measuring heat changes during receptor–ligand interaction. | Wiseman et al., 1989; Bazarsuren et al., 2002; Donnelly, 2012 |
| Total-internal reflection fluorescence imaging | Determines equilibrium constants and dissociation rates by monitoring fluorescence-labeled single molecule on lipid bilayer. | Fox et al., 2009; Myers et al., 2012 |
| Surface plasmon resonance | Determines dissociation constants by real-time measurement of receptor–ligand interaction. | Bazarsuren et al., 2002; Schroder-Tittmann et al., 2010; Locatelli-Hoops et al., 2013 |
| Photoaffinity labeling | Identifies residues of peptide and receptor at binding interface that are spatially proximal to each other. | Chowdhry and Westheimer, 1979; Ji et al., 1997; Vodovozova, 2007; Chen et al., 2009; Miller et al., 2011 |
| Receptor functional assays: | ||
| Homogeneous time-resolved fluorescence (HTRF) or alpha screen cAMP assays | HTRF technology is an immunoassay based on a FRET between a Tris-bipyridine europium cryptate used as a long-lived fluorescent donor and a chemically modified allophycocyanin used as acceptor. Alpha technology is a bead-based proximity assay. | Gesellchen et al., 2006; Einhorn and Krapfenbauer, 2015 |
| Radioimmunoassay | Determines receptor-stimulating potency (EC50) by quantitative analysis of cAMP production with immobilized anti-cAMP or anti-cGMP antibodies and radiolabeled cAMP/cGMP. | Farmer et al., 1975; Wheeler et al., 1995 |
| FRET-based cAMP assay | Determines receptor-stimulating potency (EC50) by measuring a decreased FRET signal between fluorescent proteins (CFP and YFP) and Epac protein. | Holz, 2004; Nikolaev et al., 2004; Landa et al., 2005; Harbeck et al., 2006; Sloop et al., 2010 |
| Luciferase reporter assay | Determines receptor-stimulating potency (EC50) by measuring luminescence that is increased by transcription of transfected luciferase reporter plasmid linked to cAMP response element. | Grynkiewicz et al., 1985; Cullinan et al., 1994; Bode et al., 1999; Miranda et al., 2008; Murage et al., 2008; Smale, 2010 |
| Intracellular calcium ion | Determines receptor activation by measuring intracellular Ca2+ level with calcium-sensitive dye, Fura-2. | Grynkiewicz et al., 1985; Cullinan et al., 1994; Bode et al., 1999 |
| Determination of incretin effects: | ||
| Glucose tolerance test (GTT; oral GTT, OGTT; intraperitoneal GTT, IPGTT; intravenous GTT, IVGTT) | Determines insulinotropic action of GLP-1 analogs by measuring glucose level after their administration. | Kreymann et al., 1987; Toft-Nielson et al., 1996 |
| Insulin secretion | Determines potency of GLP-1 analogs by measuring insulin secretagogue action. | Albano et al., 1972; Andersen et al., 1993; Goke et al., 1993b; Toft-Nielson et al., 1996; Kjems et al., 2003; Peyot et al., 2009 |
B. Other Bioassays
1. Receptor Trafficking.
Endocytosis of GPCRs and postendocytic trafficking between recycling and endosomal degradation are fundamental mechanisms that control the signaling capacity of receptors. Syme et al. (2006) studied the trafficking of green fluorescent protein–fused GLP-1R stably expressed on HEK293 cells in response to a GLP-1 agonist. It was found that GLP-1R associates with caveolin-1 in the lipid rafts of the cell membrane of HEK293 cells transfected with the green fluorescent protein–GLP-1R plasmid and MIN6 cells endogenously expressing the GLP-1 receptor. With confocal fluorescence microscopy and immunoprecipitation techniques, it was observed that stimulation by an agonist induces rapid and extensive internalization of the receptor in association with caveolin-1, and nearly all of the receptor was internalized after 15 minutes.
Similarly, Roed et al. (2014) combined microscopy and a time-resolved fluorescence resonance energy transfer–based technique for monitoring both receptor trafficking and signaling in living cells. The GLP-1 receptor rapidly and repeatedly internalizes and recycles upon ligand activation. GLP-1R recycling was found to be two to three times slower when induced by exendin-4 and liraglutide, compared with GLP-1 at equipotent concentrations. Independent of the ligand, activated receptors demonstrated cycling for a prolonged period of time as well as sustained cAMP signaling.
2. Immunoassays.
Determination of incretins, especially endogenous ones, by immunologic assays is considered difficult because differential processing of precursor molecules gives rise to a number of different peptides that may crossreact with antisera raised against GLP-1 (Deacon and Holst, 2009). Subsequent degradation of the peptides by DPP-4 and NEP 24.11 further complicates the assay process (Heijboer et al., 2011).
When the full processing pattern of proglucagon was not fully elucidated, the assays were directed to the middle region of GLP-1 and became independent to the processing pattern. This caused peptides with a deletion or extension at either the N or C terminus to be detected in addition to GLP-1 itself. Antisera were then made toward either the C terminus (Hendrick et al., 1993; Holst et al., 1994; Ørskov et al., 1994) or the N terminus (Gutniak et al., 1996). However, it was found that C-terminally directed antisera reacted with peptides truncated or extended at the N terminus and could not distinguish the full-length GLP-1 from the metabolite resulting from DPP-4 degradation. Similarly, N-terminally directed antisera could not differentiate the intact peptide from truncated or extended peptides at the C terminus.
Kirk and coworkers developed a sandwich enzyme-linked immunosorbent assay for determining exogenous GLP-17–36 amide in plasma samples collected from pharmacokinetic studies (Pridal et al., 1995). It is referred to as active GLP-1 assay. This experiment employs an N-terminally directed antibody (polyclonal rabbit antibody directed to the residues at position 7–14) and a C-terminally directed antibody (monoclonal mouse antibody directed to the residues at position 26–33). When compared with a radioimmunoassay employing a polyclonal rabbit antibody directed to an epitope in the midregion of the peptide, it was found to be superior due to reduced crossreactivity with GLP-1 fragments. It has a working range from 10 to 500 pmol/L.
3. Pancreatic β Cell Regeneration.
Besides insulin secretion from the pancreatic β cells in a glucose-dependent manner, GLP-1 can induce regeneration of the cells evidenced by immunohistochemical staining studies (Edvell and Lindstrom, 1999; Xu et al., 2006). PDX-1 is essential for pancreogenesis, pancreatic cell differentiation, and maturation. Perfetti and coworkers demonstrated that continuous infusion of GLP-1 to young and old rats upregulates PDX-1 expression in islets and total pancreas (Hui and Perfetti, 2002). They also found induced pancreatic cell proliferation and β cell neogenesis (Perfetti et al., 2000).
C. Molecular Imaging
Pancreatic β cells play an important role in glucose homeostasis, and their destruction is well documented with diabetes, in particular type 1. Whereas several clinical parameters, such as plasma glucose, glycated HbA1c, insulin, and C-peptide levels, indicate onset and progress of the disease, the majority of the β cells are destroyed by the time the symptoms appear (Matveyenko and Butler, 2008). Late diagnosis of the disease results from the remaining β cells compensating for the loss of insulin production due to cell death, and abnormalities in blood glucose concentrations are not typically observed until β cell mass (BCM) is diminished by more than 50% (Souza et al., 2006). Thus, noninvasive imaging of BCM would provide accurate and in-time status as well as useful prediction. β cell imaging has potential in monitoring the disease progression, evaluating efficacy of therapeutic interventions in preserving and restoring BCM, and following up β cell replacement therapies such as islet transplantation (Halban, 2004).
Imaging modalities, such as positron emission tomography (PET), magnetic resonance imaging, as well as other nuclear imaging techniques like single-photon emission-computed tomography (SPECT), and optical absorption or fluorescence spectroscopy and imaging, have shown promises in visualizing and quantitating molecular targets (Souza et al., 2006). However, assessing BCM in vivo has been challenging because the β cells that exist in pancreatic islets are small (50–300 μm in diameter), scarce (1–2% of pancreatic mass), and scattered throughout the organ (Ueberberg et al., 2009).
As nonselective imaging modalities like magnetic resonance imaging and computed tomography have failed to offer a reliable detection method, β cell–specific biomarkers have been investigated and evaluated (Shiue et al., 2004; Souza et al., 2006; Saudek et al., 2008; Malaisse et al., 2009; Moore, 2009; Ichise and Harris, 2010). Although a number of potential candidates for β cell imaging have been reported, such as vesicular monoamine transporter, GLP-1R, SUR1, glucose transporter 2, glycogen, zinc transporters, fluorodithizone, and monoclonal antibodies, many did not show promising results due to either low expression levels or insufficient β cell specificity (Murthy et al., 2008; Schneider, 2008; Moore, 2009). Vesicular monoamine transporter is expressed at dopamine nerve ends in the CNS and pancreatic β cells, and its ligands, 11C- and 18F-labeled dihydrotetrabenazine derivatives, have demonstrated a potential for β cell imaging (Souza et al., 2006; Harris et al., 2008; Kung et al., 2008). However, controversial findings of nonspecific binding of [11C]-dihydrotetrabenazine in human pancreas raised questions for its clinical application (Goland et al., 2009).
Compared with other β cell biomarkers, GLP-1R showed promise because of its highly specific expression in β cells and strong interaction with its ligands (Tornehave et al., 2008). However, its endogenous ligand, GLP-1, is difficult to be used as an imaging agent because of its extremely short half-life (II. Glucagon-Like Peptide-1) resulting from rapid metabolic degradation. This emphasizes that β cell imaging via GLP-1R as a biomarker requires GLP-1 analogs that have high metabolic stability as well as strong binding affinity.
Exendin-3 and exendin-4 are found to be resistant to DPP-4 degradation, and these stable GLP-1R agonists have been developed for β cell imaging (Gotthardt et al., 2006; Wild et al., 2006, 2010; Brom et al., 2010; Reiner et al., 2010; Wu et al., 2011; Connolly et al., 2012). Alternatively, bicyclic GLP-1 analogs developed by Ahn and coworkers have also demonstrated outstanding results in determining BCM (Ahn et al., 2011; Gao et al., 2011). A metal chelator, such as DOTA, NOTA, or DTPA, was conjugated to these peptides and subsequently labeled with a radiotracer (e.g., 64Cu, 68Ga, 99mTc, and 111In) for PET- and SPECT-imaging experiments.
A SPECT probe was developed based on exendin-4, [Lys40(Ahx-DTPA-111In)NH2]-exendin-4, to target GLP-1R for imaging insulinoma in Rip1Tag2 transgenic mice and showed high tumor uptake (Wild et al., 2006; Wicki et al., 2007). Although imaging agents derived from these stable GLP-1R agonists showed a high renal uptake, the potential of GLP-1R in visualizing β cells has been demonstrated by PET and SPECT imaging of insulinoma with radiolabeled exendin-3 and exendin-4 (Gotthardt et al., 2006; Brom et al., 2010; Wild et al., 2010). As an excellent PET tracer, 64Cu was used to label exendin-4, and the resulting 64Cu-labeled exendin-4 analog (64Cu-DO3A-VERSUS-Cys40-exendin-4) demonstrated a feasibility for in vivo imaging of intraportally transplanted islets in mice by showing high and specific uptake in INS-1 tumors despite substantial renal uptake (Wu et al., 2011). Remarkably, [Lys40(Ahx-DTPA-111In)NH2]-exendin-4 was successfully used to visualize autologous islets that have been transplanted into human muscle, proving a clinical potential of human β cell imaging via GLP-1R (Pattou et al., 2010).
In addition to GLP-1R agonists described above, antagonists, such as exendin9–39, were also developed as β cell imaging agents (Mukai et al., 2009). Radiolabeled at lysine residues with 125I-Bolton-Hunter reagent, exendin9–39 was examined for receptor specificity in vitro and in vivo. Accumulation of radioactive signals in β cells was observed, although the resolution of the imaging technique was low.
Ahn and colleagues have developed PET-imaging agents based on the GLP-1 sequence to quantitate BCM (Ahn et al., 2011; Gao et al., 2011). As the endogenous GLP-1 suffers from rapid metabolic degradation, they introduced two lactam bridges to GLP-1, one in the N-terminal region and the other near the C terminus. These two strategically placed lactam bridges were found to protect the cyclic peptides from proteolytic cleavages by NEP 24.11 (Murage et al., 2010). In addition, Ala at position 8 was replaced with D-Ala to prevent the DPP-4 degradation. These modifications not only enhanced metabolic stability of the GLP-1 analog, but also increased binding affinity to the receptor. Dynamic PET scans over 60 minutes showed a high pancreatic uptake of a bicyclic GLP-1 analog, [D-Ala8]-c[Glu18,Lys22]-c[Glu30,Lys34]-GLP-17–36, labeled with 64Cu or 68Ga, which disappeared in streptozotocin-induced type 1 diabetic mice or was competitively displaced by coinjection of unlabeled exendin-4 (Manandhar et al., 2013). Ex vivo scans and histology were also carried out to further confirm the PET-imaging findings (unpublished data).
V. Pharmaceutical Development and Therapeutics
As noted above, GLP-1 is a gastrointestinal peptide hormone that is secreted from L cells scattered in the distal small intestinal mucosa. It is released physiologically when ingested nutrients reach that level of the bowel, typically reflecting a meal large enough to benefit from the incretin effect of this hormone. Among its other physiologic effects is the slowing of gastric emptying, again reflecting the benefit of titration of the rate of delivery of nutrients to the absorptive surface so as not to overwhelm the absorptive capacity of the intestine. This peptide has a very short half-life in the circulation, with proteolysis, particularly by DPP-4, and renal clearance having prominent roles. This endoprotease cleaves the amino-terminal dipeptide from endogenous GLP-1 peptides; GLP-17–36 amide or GLP-17–37 is cleaved to yield GLP-19–36 amide and GLP-19–37, respectively, which are four orders of magnitude less active than the natural hormone (Montrose-Rafizadeh et al., 1997). Recognition of this led to the earliest strategy to increase endogenous GLP-1 levels using DPP-4 inhibitors, rather than acting at GLP-1R. This was ultimately followed by the development of molecules having intrinsic GLP-1R agonist activity. As described below, most of these are peptides and their derivatives, but small-molecule agonists have also surfaced. All of them have potential importance for diabetes, obesity, and associated metabolic and cardiovascular complications. Other candidate applications are also discussed.
The earliest recognition that GLP-1 exhibited effects useful in the management of diabetes came from rodent studies (Gutniak et al., 1992; Nauck et al., 1993c). This was followed by a seminal study in which GLP-1 itself was administered s.c. over a 6-week period in patients with T2DM (Zander et al., 2002). These patients displayed reduced levels of fasting glucose and HbA1c, as well as improved insulin sensitivity and β cell function. The proof-of-concept for the use of GLP-1 in diabetes demonstrated in this study was clear, yet major challenges existed that involved acceptable modes of administration and the need to improve duration of hormonal action.
There are now a wide variety of different peptidic GLP-1R agonists with variable durations of action and different advantages and disadvantages for clinical use. The first GLP-1R agonists to enter clinical use were peptide analogs and derivatives, as exemplified by exendin-4 (Eng et al., 2014), liraglutide, albiglutide, and dulaglutide (Table 3). Unfortunately, these peptides require parenteral administration, due to instability in the proteolytic milieu of the digestive tract, making it impossible to deliver adequate drug reproducibly via oral route. Technology has advanced so much, however, that easy-to-use pens for s.c. administration has become quite common. Semaglutide is a potent peptide that was recently tested with s.c. administration in a phase 2 clinical study of 411 patients (Nauck at al., 2016), showing improvement in HbA1c levels by 1.7%. This peptide is now being studied for oral dosing. This field has progressed so quickly that there are now even devices that allow longer-term activity being developed. Intarcia is an implantable device that releases exenatide over 6–12 months. This has been studied in two phase 3 trials (Mullard, 2014).
TABLE 3.
Peptidic GLP-1R agonists that have been launched or are in late-stage clinical trials
| Analog | Description | Company | Dosing Regimen | Status |
|---|---|---|---|---|
| Exenatide | Exendin-4 | Amylin/Eli Lilly; AstraZeneca | Twice daily | Launched |
| Exenatide once weekly | Exendin-4 | Amylin/Alkermes/Eli Lilly; AstraZeneca | Once weekly | Launched |
| Lixisenatide | Exendin-4 analog | Sanofi-Aventis | Once daily | Launched |
| Liraglutide | GLP-1 analog linked with a fatty acid | Novo Nordisk | Once daily | Launched |
| Albiglutide | GLP-1 analog fused to albumin | GlaxoSmithKline | Once weekly | Launched |
| Dulaglutide | GLP-1 analog fused to Fc | Eli Lilly | Once weekly | Launched |
| Semaglutide | GLP-1 analog linked with a fatty acid | Novo Nordisk | Once weekly | Phase 3 |
| Semaglutide (NN9924) | GLP-1 analog linked with a fatty di-acid | Novo Nordisk | Oral | Phase 2 |
| Taspoglutide | GLP-1 analog formulated for sustained release | Roche | Once weekly | Suspended |
We have finally entered an era in which small-molecule nonpeptidic GLP-1R agonists are now available (Fig. 7), although it still poses a tremendous challenge to make them adequately potent and bioavailable (Yang et al., 2015a). Most of these are thought to act within the helical bundle of GLP-1R. Crystallization of two members of the class B GPCR subfamily in 2013 (Hollenstein et al., 2013; Siu et al., 2013) provided a clue to why development of nonpeptidic modulators has been so difficult. Unlike the class A GPCRs that have tight helical bundles with a well-defined pocket for small-molecule ligands, the class B helical bundles appear to be much more open without defined small-molecule docking sites. Recently, one of the small-molecule agonists (BETP) had its site of action determined as covalently binding to a cysteine residue in intracellular loop 3 on the cytosolic side of the plasma membrane (Nolte et al., 2014), a molecular mechanism that was a major surprise, without precedent in other members of this subfamily. A molecule from TransTech Pharma, TTP054, is presently undergoing phase 2 clinical trials (Mullard, 2014).
Fig. 7.
Nonpeptidic GLP-1R modulators and peptide mimetics. Liraglutide (Novo Nordisk) was the first approved human GLP-1 analog to teat diabetes (European Union, 2009; United States, 2010) and obesity (United States, 2014; European Union, 2015). Liraglutide has 97% sequence homology to human GLP-1 and was designed to reversibly bind to albumin by attachment of palmatic acid via a L-γ-glutamic linker to lys26 in Arg34 GLP-17–37. The modification of Lys34 to Arg34 made it possible to produce Arg34 GLP-17–37 in yeast, followed by acylation of Lys26. The native GLP-1 peptide has a half-life of approximately 2 minutes due to rapid cleavage of GLP-17–37 to GLP-19–37 by DPP-4 (Deacon et al., 1995a,b; Vilsboll et al., 2003). Liraglutide comprises the natural GLP-1 N terminus, but has a half-life of about 11 hours after s.c. dosing to humans combined with a delayed absorption from sub cutis that gives a pharmacokinetic profile applicable for once-daily administration. The reason for extended circulation is due to reversible albumin binding that protects from DPP-4 degradation and glomerular filtration, whereas the delayed absorption is explained by the ability of liraglutide to form heptamers by self-assemble controlled by the fatty acid side-chain at position 26. Liraglutide is well tolerated and capable of substantially improving glycemic control with low risk of hypoglycemia and weight loss benefit (Knudsen et al., 2000; Knudsen, 2004; Madsen et al., 2007; Steensgaard et al., 2008; Dharmalingam et al., 2011; Wang et al., 2015).
A. Peptidic Analogs
1. GLP-1 Analogs.
Several GLP-1 analogs have successfully reached the market and become an important treatment of obesity and T2DM today (Table 3). Several reviews have been published during the past that describe the approved and emerging GLP-1R agonists and also summarize the most important clinical trials as well as toxicological considerations (Lund et al., 2011; Madsbad et al., 2011; Garber, 2012; Meier, 2012; Montanya, 2012; Lorenz et al., 2013; Trujillo and Nuffer, 2014; Trujillo et al., 2015; Madsbad, 2016; Zaccardi et al., 2016).
Taspoglutide (BIM-51077; Roche, Basel, Switzerland) is a potent GLP-1 analog with 93% homology to human GLP-17–36 amide as it has two amino acid substitutions in positions 8 and 35 with aminoisobutyric acid (Aib). Taspoglutide is protected against DPP-4 degradation by the Aib8/Aib35 substitution but is still cleared rapidly by the kidney. A sustained-release formulation that contains zinc-chloride and precipitates as a sub cutis deposition was developed. This once-weekly dosing formulation of taspoglutide has shown promising antidiabetic efficacy in clinical trials. Unfortunately, the analog was discontinued in phase 3 clinical trials due to severe gastrointestinal side effects (Retterstol, 2009; Dong et al., 2011; Garber, 2012).
The GLP-1 human serum albumin fusion protein, albiglutide, also known as albugon, discovered by Genentech (South San Francisco, CA) and developed by GlaxoSmithKline (Brentford, UK), was approved by the Food and Drug Administration (FDA) in 2014. To have a free N-terminal, which is important for GLP-1 activity, the peptide was fused via the C terminus to albumin. The construct is composed of a tandem repeat of Ala8 to Gly8 GLP-17–36 that is fused to the N terminus of human albumin. Gly8 GLP-1 was used to protect for DPP-4 degradation of the N terminus. The plasma half-life was extended to about 6–8 days, which made this fusion construct applicable for once-weekly dosing. One drawback relates to a significantly reduced potency that was probably due to a combination of the Gly8 modification and the covalent attachment to the larger protein human serum albumin. The tandem repeat of GLP-1 was used to obtain longer distance between albumin and the distal GLP-1 peptide, but the affinity for GLP-1R is 0.61 nM compared with 0.02 nM of the native peptide. The approved initial dose to treat T2DM is 30 mg given s.c. once per week, which may be increased to 50 mg on the same day of each week if the glycemic response is inadequate (Kim et al., 2003; Tomkin, 2009; St Onge and Miller, 2010).
Dulaglutide (LY2189265; Eli Lilly, Indianapolis, IN) is a GLP-1R agonist fused to a modified Fc fragment of immunoglobulin that acts as a carrier to secure long residence time by reduced in vivo clearance. The sequence of the fusion protein Gly8Glu22Gly36-GLP-17–37-(Gly4Ser)3Ala-Ala234,235Pro228-IgG4-Fc is modified in the N terminus of GLP-1 to protect against DPP-4 cleavage (Gly8), and the IgG4 Fc is modified to reduce interaction with high-affinity Fc receptors and avoid antibody-dependent cell-mediated cytotoxicity. The linker (Gly4Ser)3Ala was carefully selected to retain high GLP-1R affinity as direct fusion dramatically reduced in vitro activity by ∼95%. The half-life is above 1.5 days in rats and more than 2 days in monkeys. Dulaglutide was approved by the FDA in 2014 for once-weekly dosing of 0.75 mg s.c. (Glaesner et al., 2010).
There is still a need for optimization of once-weekly GLP-1 analogs because both albiglutide and dulaglutide are less efficacious than liraglutide with respect to weight loss (Dungan et al., 2014; Pratley et al., 2014).
The next generation of GLP-1 analogs using the reversible albumin affinity is represented by semaglutide (Novo Nordisk, Bagsværd, Denmark) currently in phase 3 clinical trials. This analog Aib8Lys26Arg34-GLP-17–37 has a more complex side chain composed of hexadecandinoyl attached to Lys26 via an L-γ-glutamic acid linker and a small hydrophilic spacer. The Aib8 was introduced to improve the DPP-4 stability beyond that of liraglutide, and the new side chain increased the albumin affinity, resulting in a half-life in humans long enough for once-weekly dosing. In a phase 2 study over 12 weeks, semaglutide dose-dependently reduced HbA1c and body weight, with higher doses being more effective than liraglutide (Nauck and Sesti, 2012; Trujillo and Nuffer, 2014; Lau et al., 2015).
2. Exenatide Experience.
In 1993, exendin-4 was isolated from the salivary glands of the gila monster (Heloderma suspectum) and found to be highly active on GLP-1R (Goke et al., 1993a). Exendin-4 is a 39–amino-acid peptide that shares approximately 50% amino acid sequence identity with human GLP-1. The discovery of the antidiabetic effects of exendin-4 increased the interest in this peptide, and several pharmaceutical companies thus focused on its clinical development as well as on discovery efforts in exendin-based GLP-1 analogs.
In 2005, the first GLP-1R agonist exenatide (AstraZeneca, Cambridge, UK) was approved by the FDA for treatment of T2DM. Exenatide, as well as other GLP-1R agonists, reduces blood glucose through multiple mechanisms, including enhancement of glucose-dependent insulin secretion, suppression of excess glucagon secretion, reduction of food intake, and slowing of gastric emptying. It is administered s.c. twice daily with doses between 5 and 10 μg (Cvetkovic and Plosker, 2007).
The very low therapeutic dose of exenatide made it an excellent candidate for an extended release formulation. Amylin Pharmaceuticals (San Diego, CA), Eli Lilly, and Alkermes (Dublin, Ireland) developed a formulation to prolong exenatide release, using biodegradable polymeric microspheres that entrap exenatide. The microspheres consist of exenatide incorporated into a matrix of poly (D,L-lactide-co-glycolide), which is a common biodegradable medical polymer with a history of safe use in humans. The final formulation was approved in 2011 as Bydureon for once-weekly dosing (Kim et al., 2007; Malone et al., 2009).
Lixisenatide (Sanofi, Paris, France) is a once-daily short-acting GLP-1R agonist used in the treatment of T2DM. The discovery of lixisenatide was inspired by the development of structure-inducing probe technology, which improves the half-life of structure-inducing probe conjugates compared with the parent peptide by enhancing stability and, as a consequence, creating resistance to proteolytic degradation. Lixisenatide, des-Pro36-exendin-41-39-Lys6 amide, comprises 44 amino acids and is based on the exendin-4 sequence with a proline deletion in position 36 and C-terminal extension with six lysines. Lixisenatide has an extended in vivo half-life of 91 minutes after i.v. administration (in rabbits) compared with 43 minutes for exendin-4. Lixisenatide was approved in 2013 and has a substantial sustained effect on gastric emptying and postprandial glucose excursions that may be related to the relatively shorter half-life. The combination with basal insulin to lower fasting plasma glucose is clinically valuable and could differentiate lixisenatide from other GLP-1R agonists, especially from those long-acting ones with little effect on gastric emptying and postprandial glucose (Werner et al., 2010; Christensen et al., 2014; Rosenstock et al., 2014).
3. GLP-1 Mimetics.
A novel class of 11–amino-acid GLP-1R agonists was discovered that consist of an analog of only the first 9 amino acids of GLP-1, in which the remaining 21 amino acids have been truncated and attached to an unnatural biphenyl diamino acid at the C terminus. The optimization of the sequence with several unnatural amino acids provided 11-mer GLP-1 mimetics with similar in vitro potency as the natural 30-mer peptide. The optimized 11-mers (e.g., BMS-686117) were reported to reduce plasma glucose and increase plasma insulin concentrations in diabetic ob/ob mice after a single relative high s.c. dose of the peptide (Mapelli et al., 2009; Haque et al., 2010). Based on NMR studies, the structures of these 11-mer peptides were determined and used to design lactam- and disulfide-based cyclic constrained peptides that retained high GLP-1R potency (Hoang et al., 2015).
BMS-686117 has a relatively shorter half-life of about 2 hours in dogs, and, accordingly, a sustained release formulation is required for clinical use. Formulation with Zn (II) forms a poorly water-soluble adduct such that the BMS-686117 content and reversible release of which can be tailored by varying the Zn:peptide ratio. An in vivo pharmacokinetics study in dogs of an optimized sustained release formulation concluded that a minimal initial burst and constant release are applicable to once-daily dosing (Qian et al., 2009).
B. Nonpeptidic Modulators
Even though more than two decades have passed since the cloning of the GLP-1R (Thorens, 1992), very few nonpeptidyl-based GLP-1R agonists have been published. One might speculate on the reason: the ligand–receptor interaction leading to activation requires multiple interfaces that are difficult to mimic with a small molecule. The majority of the nonpeptidyl-based GLP-1R agonists described in the literature are receptor modulators that bind to allosteric sites and do not compete with GLP-1 in the orthostatic site. Many such GLP-1R agonists were only mentioned in patents and are not included in this section, as their experimental data have not been peer-reviewed. There are also small numbers of nonpeptidic GLP-1R antagonists, and these will be discussed in the last part of this section. One review article is also referred concerning nonpeptidic GLP-1R modulators (Willard et al., 2012a).
To date, only one nonpeptidic GLP-1R agonist has entered into clinical trials. The compound has not been specifically disclosed, but a publication from the company described that it stimulated glucose-dependent insulin release in isolated islets from rodents and improved glycemic control in an oral glucose tolerance test (Gustavson et al., 2014).
Boc5, a substituted cyclobutane with four chiral centers, is among the few nonpeptidic GLP-1R agonists that have been comprehensively studied. It was discovered during a high-throughput screening against 48,160 small molecules using a luciferase reporter assay in HEK293 cells expressing the rat GLP-1R (Chen et al., 2007). Boc5 is the only nonpeptidic GLP-1R agonist that has been shown to compete for 125I-GLP-1 in binding assays and to be functionally antagonized by exendin9–39, thus acting like the natural peptide ligand.
The antidiabetic effect of Boc5 was demonstrated in various animal models. A 4-week dose-response study was performed in db/db mice with daily i.p. dosing of Boc5 at 0.1–3 mg. There was a dose-dependent decline in HbA1c during the treatment that continued to decrease until 2–3 weeks after the last dose. In contrast, Boc5 had no effect on HbA1c in nondiabetic C57BL/6J (wild-type) mice. Boc5 also inhibited food intake and decreased body weight of db/db mice by up to 16% during the study with reduced fat mass. In addition, it was demonstrated that Boc5 decreased the elevated levels of fasting insulin in db/db mice, and the insulin sensitivity was improved after 4 weeks of treatment. Thus, the effects of Boc5 seen both in vitro and in vivo suggest that this compound has similar pharmacological properties as peptidic GLP-1R agonists. Medicinal chemistry efforts were made to understand the SAR, and one analog, WB4-24, was found to be more potent in vitro compared with Boc5. WB4-24 was shown to have beneficial effects on glucose homeostasis and body weight in diet-induced obese mice (Su et al., 2008; He et al., 2010, 2012; Liu et al., 2012).
After an unsuccessful screening of 500,000 discrete small molecules with a GLP-1 competitive binding assay, a substituted quinoxaline was identified in subsequently performed functional screening of 250,000 compounds. This hit as well as some closely related analogs activated the formation of cAMP in BHK cells expressing the GLP-1R (Fig. 7, compounds 1 and 2). However, it did not compete with 125I-GLP-1 in a binding assay, but augmented the binding of the tracer. Furthermore, its functional effect could not be antagonized by exendin9–39. Functionality counter-screening analysis showed that the quinoxalines were inactive in BHK cells expressing GIPR, GLP-2 receptor, and GCGR. They were capable of releasing insulin from wild-type mouse islets, but not from islets isolated from GLP-1R knockout mice. These quinoxalines were thus concluded to be selective GLP-1R ago-allosteric modulators. Further SAR studies around these initial leads as well as some of the later identified nonpeptidic modulators have all been published (Knudsen et al., 2007; Teng et al., 2007; Coopman et al., 2010; Irwin et al., 2010; Koole et al., 2010; Cheong et al., 2012; Li et al., 2012a).
Pyrimidine-based ago-allosteric modulators (Fig. 7, compounds A and B) of GLP-1R were found by screening of a small library enriched with relevant pharmacophores for GPCRs. The screening was performed in HEK293 cells coexpressing hGLP-1R and a cAMP response element luciferase reporter. Like the quinoxaline-based modulators, the pyrimidines did not compete with 125I-GLP-1 in a binding assay and the functional effect was not antagonized with exendin9–39. They were shown to enhance GLP-1–induced cAMP signaling and insulin release in isolated rat islets. Compound B (BETP) increased plasma insulin levels in a glucose tolerance study using Sprague Dawley rats as well as hyperglycemic clamped Spraqgue Dawley rats. One drawback for further development of both quinoxaline- and pyrimidine-based leads is the chemical instability in the presence of nucleophiles (Sloop et al., 2010).
Recently, a tricyclic pyridoindole-based series of GLP-1R allosteric modulators was identified by high-throughput screening measuring intracellular calcium mobilization in the presence of low levels of GLP-1 or glucagon. These compounds were shown to potentiate glucose-dependent insulin release in primary mouse islets, but no in vivo studies were described (Morris et al., 2014).
The potential use of GLP-1R allosteric modulators to enhance the effect of endogenous GLP-1 was studied in vivo. An i.v. glucose tolerance study was carried out in which coadministration of BETP to rats dosed with subsaturating concentrations of oxyntomodulin markedly increased the insulinotropic effect of oxyntomodulin (Willard et al., 2012b).
C. Currently Approved Clinical Applications
A number of GLP-1R agonists have been approved for clinical use to improve glycemic control in adults with T2DM. Diet and exercise are the expected initial approach to treat these patients, with GLP-1R agonists prescribed as second-line drugs for patients who are refractory to the current standard of care. GLP-1R agonists have no current role in the management of patients with type 1 diabetes, and should be avoided in those patients having a history of pancreatitis or medullary thyroid cancer. The use of this category of drugs in T2DM reflects their direct effects on the β cells within the pancreatic islets to increase insulin biosynthesis and secretion, to stimulate cell proliferation, and to reduce apoptosis. A key benefit of GLP-1R agonists is the glucose-dependent effects on the β cell, with reduction in blood glucose only in patients exhibiting hyperglycemia. This eliminates the hypoglycemia risk of many other antidiabetic drugs. In fact, GLP-1R agonists have now largely replaced the use of thiazolidinediones (e.g., rosiglitazone). Adverse responses such as peripheral edema, weight gain, congestive heart failure, and osteoporosis associated with the latter are not observed in patients treated with GLP-1R agonists (Drucker et al., 2010). GLP-1R agonists also exhibited cardiovascular benefits, such as lowering blood pressure, improving lipid profiles, and possibly even enhancing cardiac contractility and endothelial function. In addition to its action on islet β cells, this hormone exerts effects on the following: 1) pancreatic α cells to reduce glucagon secretion and hepatic glucose output; 2) the cardiovascular system to elevate glucose utilization and protect the heart and vasculature against inflammation; 3) the brain to decrease food intake; and 4) the gut indirectly to slow gastric emptying.
1. Selection.
As the number of GLP-1R agonists in clinical use and trials expands, it will become clearer whether there are advantages of one drug over another in a specific clinical setting. We have already observed differences in the frequency of nausea, presumably reflecting the reduced gastric emptying, among these drugs. There is also variation in the pathway of inactivation and excretion of these drugs, making the selection of agent in patients with renal insufficiency important. Although biased agonism is now recognized for some of these agonists, there is still insufficient information on the broad pharmacodynamics properties of these drugs to differentiate the contribution of biased agonism from pharmacokinetic behavior to differential clinical responses, side effects, or toxicities, although this is likely to be an important consideration in future therapeutic development.
A very practical and useful categorization of GLP-1R agonists is based on their duration of action. The shortest-acting agents, like exenatide and lixisenatide with half-lives of 2–5 hours, seem to exert their beneficial effects predominantly by slowing gastric emptying and thereby reducing the rate of carbohydrate delivery, digestion, and absorption, leading to normal postprandial glucose levels. Of course, other nutrients also empty more slowly, and this has resulted in lower postprandial levels of free fatty acids and triglycerides, which has some metabolic benefits. For instance, decreased levels of chylomicrons are desired in patients with diabetes or metabolic syndrome. As the duration of GLP-1R agonist action is extended with peptides like albiglutide, dulaglutide, long-acting release exenatide, or liraglutide (half-lives of 12 hours to several days), the impact on blood glucose levels is enhanced as insulinotropic action and activity to reduce glucagon become more prominent than the effect on gastric emptying. Such prolonged activities have also been associated with increased side effects, such as diarrhea, tachycardia, immune responses, and injection site reactions. Of interest, nausea and vomiting appear to have greater incidence with the shorter-acting peptides. The longer-acting agents have typically provided more consistent effects on GLP-1R activation and blood glucose levels. Because the insulinotropic action of GLP-1 is dependent on the presence of hyperglycemia, long duration activity does not increase the risk of hypoglycemia. Therefore, long-acting release formulations have obvious advantages if the goal is to lower early morning glucose levels, because they retain efficacy throughout the night.
It is curious whether the induction of nausea, which is believed to be a result of slowed gastric emptying, is similar in the short-acting and longer-acting GLP-1R agonists, yet this seems to be attenuated quickly in the longer duration agonists and may take weeks to months to improve for the shorter duration agonists. Such a phenomenon most likely reflects differences in desensitization of GLP-1R, but the underlying mechanism has not been carefully studied. Although the weight loss effect seen with some of these GLP-1R agonists was thought to be related to slowed gastric emptying, the long-acting agents seem to result in greater weight reduction than the short-acting ones, suggesting certain other mechanism is dominant. This might relate to reduced appetite, potentially following GLP-1 action in the hypothalamus or other CNS regions.
2. Efficacy.
The first phase 2 trial to study a GLP-1R agonist in T2DM was started in 1999, comparing twice-daily administration of exenatide to saline (Kolterman et al., 2003). Not only was the exenatide effective in reducing glucose levels, but it did not induce hypoglycemia in patients who were on insulin therapy. Although exenatide was approved by the FDA for use in diabetes in 2005, concerns about hypoglycemia in insulin-requiring patients persisted, and this was finally studied in 2008 (Buse et al., 2011). The dramatic observations of enhanced reduction in HbA1c and no increase in hypoglycemic episodes, as well as weight loss, formally launched a new series of clinical studies to follow. A meta review published in Lancet in 2014 (Eng et al., 2014) chose 15 of 2905 studies that represented 4348 subjects to evaluate. HbA1c levels were decreased by an average of 0.44%, and weight was reduced by an average of 3.22 kg, without any increase in hypoglycemic episodes. Of note, the levels of HbA1c were not different using GLP-1R agonist with boluses of insulin, but the frequency of hypoglycemic episodes was significantly reduced with the former.
Short-acting GLP-1R agonists known to better control postprandial glucose levels have not been directly compared with that of long duration administered with basal insulin. Each has theoretical advantages and perhaps should be chosen based on the characteristics of the patient. Because long-acting agents can be given with short-acting insulin at meal time now, another important option exists, but needs to be evaluated.
The DURATION-3 trial (Diamant et al., 2014) compared long-acting release exenatide administered once per week to insulin glargine (a long-acting, man-made version of human insulin) in 456 patients with T2DM who had not achieved satisfactory glucose control with maximal tolerated doses of oral agents. Exenatide was able to better manage the blood glucose, with HbA1c levels being reduced by 1.01%, whereas glargine plus oral agents only reduced this by 0.81%. Again, the most notable observation was a much lower incidence of hypoglycemia in patients taking the GLP-1R agonist.
The AWARD-4 study did use dulaglutide injected only once per week and rapid-acting insulin with each meal to demonstrate improved levels of HbA1c than were observed in basal and bolus insulin administration (Jendle et al., 2014). Fixed-dose combinations of GLP-1R agonist and basal insulin have substantial appeal for convenience, although, at the current time, expense is a major concern. The DUAL-1 and DUAL-2 studies exhibited a 1.9% reduction in HbA1c in patients who followed a titration algorithm with a pen delivery device (Buse et al., 2011; Gough et al., 2014).
3. Benefits.
In addition to its action on blood glucose, mediated largely by impact on the pancreatic islet β and α cells, GLP-1R agonists have been shown to display favorable cardiovascular effects. This includes improvements in serum lipids, myocardial contractility, and endothelial function (Mudaliar and Henry, 2010). The effects on lipids are likely to reflect slowed gastric emptying and reduced delivery for absorption, as well as impact on hepatic and fat cell metabolism. Presumably, the direct cardiovascular benefit was achieved via abundant levels of GLP-1R expression in cardiomyocytes and blood vessels (Nolte et al., 2014). Experiments in rodents showed that GLP-1R agonists can reduce myocardial infarct size and improve left ventricular function, including better wall motility and ejection fractions (Nikolaidis et al., 2004b; Noyan-Ashraf et al., 2009). There also exists encouraging evidence in rodents of the neuroprotective role of GLP-1 for AD, PD, and even stroke (Harkavyi et al., 2008; McClean et al., 2011; Teramoto et al., 2011), although this has not been followed up by clinical studies.
A recent meta-analysis explored the impact of GLP-1R agonists on lipids in clinical trials (Sun et al., 2015). Thirty-five trials with 13 treatments lasting a minimum of 8 weeks were included, and GLP-1R agonists were associated with modest improvements in total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels, whereas there was no change in high-density lipoprotein cholesterol. It is not yet clear whether this translates into an improvement in cardiac and vascular events. It follows that a latest retrospective review of macrovascular outcomes in patients with T2DM who had been treated with insulin alone or with GLP-1R agonists (Paul et al., 2015) suggested that heart failure, myocardial infarction, and stroke were less common in patients who were taking the drug. Although such epidemiologic studies need to be interpreted carefully, this is inspiring and clearly worthy of prospective evaluation. A nested case-control study was also recently performed (Gejl et al., 2015). T2DM patients who developed one or more of these cardiovascular problems were compared with control patients with diabetes who did not develop such cardiovascular events. More than 10,000 patients were included, and 1,947 had one or more of these events. Liraglutide was associated with significant risk reduction, with the degree of reduction being dose- and duration-dependent. Another retrospective cohort study also showed a reduction in heart failure in patients with diabetes (Velez et al., 2015). More than 19,000 adult patients with diabetes were studied and matched with twice as many controls, and 1,426 patients with diabetes used GLP-1R agonists. This was also encouraging, but only points toward the need for prospective studies.
There is increasing excitement about the potential role for fixed-dose combinations of a long-acting insulin preparation and a GLP-1R agonist as an adjunct to diet and exercise to improve glycemic control in adults with T2DM. Two such preparations have recently been recommended for FDA approval by advisory committees, based on portfolios of clinical trials, including double-blinded designs. These include IDegLira from Novo Nordisk, combining Tresiba (insulin degludec) and Victoza (liraglutide) into a once-daily injectable product (http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm502074.pdf), and iGlarLixi from Sanofi, combining Lantus (insulin glargine) and Lyxumia (lixisenatide) into a similar product (http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm502559.pdf). Both of the component drugs in IDegLira and the insulin glargine in iGlarLixi are already FDA approved, whereas lixisenatide is currently undergoing independent regulatory review. The efficacy of both combination products was superior to that of the components alone, leading to the successful outcomes. The safety of them appears to be consistent with that of the individual components, and there are indications that some side effects like nausea and weight gain may actually be less in the combination preparations than for some individual components. It seems likely that at least one of these products or something alike will achieve a prominent role in therapy soon.
D. Potential Applications for Other Diseases
1. Neurologic Disorders.
The expression of GLP-1R in multiple sites of the nervous system and the involvement of GLP-1 in satiety and food intake regulation are well-established (II. Glucagon-Like Peptide-1). Because this incretin was also shown to improve learning in rats (Oka et al., 2000) and exert neurotrophic or neuroprotective effects when given intracerebroventricularly, its potential therapeutic value for AD, PD, and pain has been investigated (During et al., 2003; McClean et al., 2011; Gong et al., 2014b).
AD is characterized by progressive cognitive decline with a defined neuropathology of Aβ production and tau hyperphosphorylation (Claeysen et al., 2012). T2DM has been identified as a risk factor for AD, and an epidemiologic study indicated that 65% of T2DM patients showed evidence of featured AD plaques in their autopsied brains (Bassil et al., 2014), indicating that insulin desensitization in the periphery may be a factor in initiating or accelerating the development of neurodegenerative processes. It has led to the notion that drugs developed for the treatment of T2DM may be beneficial in modifying the pathophysiology of AD. In preclinical models of AD, GLP-1 decreases Aβ toxicity in vitro (During et al., 2003; Perry et al., 2003). A follow-up study demonstrated that GLP-1 and exendin-4 could protect cultured rat hippocampal neurons against glutamate-induced apoptosis (Perry et al., 2003). Furthermore, Val8GLP-1 blocked synaptic degradation and rescued synaptic plasticity in the hippocampus (Gengler et al., 2012). The neuroprotection effect of GLP-1 was further displayed in studies in which treatment with liraglutide, lixisenatide, or geniposide in the transgenic amyloid precursor peptide/presenilin 1 mice produced improvements in synaptic plasticity and reduced neuronal damage, tau hyperphosphorylation, microglial activation, plaque, and oligomer formation in the CNS (Perry et al., 2002; McClean et al., 2011; Lv et al., 2014; McClean and Holscher, 2014). These GLP-1 analogs also attenuated memory deficits, restored impaired signaling within the Akt/GSK-3β pathway, and reversed elevated levels of reactive oxygen species in amyloid precursor peptide/presenilin 1 mice. The recent reported effect of liraglutide on an early-stage sporadic AD model supports the notion that liraglutide-induced GLP-1R activity might be a viable target in AD therapy (Hansen et al., 2015).
PD is typified by a loss of dopaminergic neurons and cellular degeneration in the striatum (Le et al., 2009). Epidemiologic data suggest an association between T2DM and some neurodegenerative disorders, such as PD (Pressley et al., 2003). In patients with PD, it was found that the levels of insulin receptors were markedly reduced in the basal ganglia and the substantia nigra (Moroo et al., 1994). Furthermore, increased IRS2 phosphorylation, a marker of insulin resistance, was found in the basal ganglia of the 6-hydroxydopamine lesion rat PD model (Morris et al., 2008). An earlier report demonstrated that in high-fat-diet–fed rats, insulin resistance was accompanied by attenuated dopamine release and diminished dopamine clearance in the basal ganglia (Morris et al., 2011). Neuropathological studies in patients with PD also revealed that loss of insulin receptor immunoreactivity and mRNA coincides with loss of tyrosine hydroxylase mRNA (the rate-limiting enzyme in dopamine synthesis) (Takahashi et al., 1996).
GLP-1 and exendin-4 have been shown to exert a neurotrophic effect on 6-hydroxydopamine–treated dopamine neurons (Li et al., 2010). A growing body of evidence now exists to support the neurotrophic properties of GLP-1: activating GLP-1R–signaling pathways in neurons leads to proliferation and differentiation of cells from precursors into neurons, thereby drawing a striking similarity between cellular responses in pancreatic β cells and neurons (During et al., 2003). Indeed, such a protective action was observed in rodent models of PD: GLP-1 and its mimetics were effective to protect tyrosine hydroxylase–positive dopaminergic neurons and to preserve dopamine levels in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine–treated animals. Treatment with a GLP-1R agonist resulted in improved motor function, as demonstrated by rotarod and pole tests (Kim et al., 2009; Li et al., 2009). Recently, a single-blind clinical trial of exendin-4 in PD patients showed that exenatide was well tolerated, and the treated group exhibited clinically relevant improvements across motor and cognitive measures compared with the control group (Aviles-Olmos et al., 2013).
In a study to investigate glucoregulatory effects of exendins, a research group in Shanghai serendipitously discovered that GLP-1 produced a potent antinociception in the formalin test (Gong et al., 2011). They further examined the inhibitory role of the spinal GLP-1R signaling pathway in pain hypersensitivity states and its mechanism of action. Specific expression of GLP-1R on the spinal dorsal horn microglia was found, and the efficacy as well as potency of GLP-1, exenatide, and geniposide on antinociception in a variety of animal models of pain hypersensitivity versus acute nociceptive responses were evaluated (Gong et al., 2014b). The involvement of GLP-1 in antinociception was also verified by a nonpeptidic GLP-1R agonist (WB4-24) indicating that the effect is mediated by GLP-1R (Gong et al., 2014a; Fan et al., 2015).
2. Oncological Association.
As previously described above, GLP-1–based therapy is a long-term approach to the control of metabolic disorders exemplified by T2DM, whereby chronic adverse events become a major concern, including an increased risk of cancer (Esposito et al., 2012). Pancreas and thyroid are the main tissues of the concern.
Since the first case report of exenatide-induced pancreatitis in 2006 (Denker and Dimarco, 2006), increasing numbers of controversial observations were reported associated with the risk of pancreatitis when treating T2DM patients with GLP-1R agonists or DPP-4 inhibitors. Acute pancreatitis may potentially progress to chronic pancreatitis and ultimately develop pancreatic cancer (Yachida et al., 2010; Nauck and Friedrich, 2013). Safety alerts have been issued by the FDA for exenatide and sitagliptin in 2008 and 2009, respectively, for patients who have a history of pancreatitis or current symptoms suggestive of pancreatitis. In 2013, a workshop sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Cancer Institute on Pancreatitis-Diabetes-Pancreatic Cancer was held in Bethesda, MD (Andersen et al., 2013). Based on current data, neither the GLP-1R agonist liraglutide nor the DPP-4 inhibitor sitagliptin established a solid link with the increase of risk for pancreatic ductal adenocarcinoma in the therapy of patients with T2DM. Ongoing surveillance will be important in future outcome and epidemiologic studies that will be performed as the indications for use of these drugs expand; however, existing studies have been reassuring to date.
The effects of GLP-1R activation by liraglutide on human pancreatic cancer cells, MIA PaCa-2 and PANC-1, were studied recently (Zhao et al., 2014). Liraglutide dose- and time-dependently increased GLP-1R expression, arrested cell cycles in the S phase, and induced apoptosis through stimulation of cAMP production and inhibition of Akt and ERK1/2 pathways. Pancreatic tumor growth was also attenuated by liraglutide in a mouse xenograft model in vivo. In patients with pancreatic cancer, 43.3% were GLP-1R–negative (13 of 30) and, in GLP-1R–positive tissues, tumor size was inversely correlated with GLP-1R expression. This is the only report of the cytoreductive effect of GLP-1R signaling activated by liraglutide on pancreatic cancer cells (Zhao et al., 2014).
Thyroid cancer is a relatively rare disease, although its incidence has increased at an alarming rate in both men and women in the United States (Aschebrook-Kilfoy et al., 2011; Udelsman and Zhang, 2014). C cells constitute a minor fraction of the thyroid mass (0.1%) and are the only cells that produce calcitonin (Huang et al., 2006). GLP-1R agonists are capable of inducing increased calcitonin gene expression and C cell hyperplasia in the thyroid of wild-type, but not the GLP-1R knockout (Glp-1r−/−) mice (Yamada et al., 2008; Madsen et al., 2012). Long-term exposure to GLP-1R agonists resulted in C cell proliferation and the formation of C cell adenomas and (medullary thyroid) carcinomas in rodents (Elashoff et al., 2011; Bulchandani et al., 2012; Rosol, 2013). Data derived from the FDA Adverse Event Reporting System database also indicate a significantly elevated risk for thyroid cancer (histology not specified) with exenatide, but not sitagliptin treatment (Nauck and Friedrich, 2013). Several cases of thyroid cancer were reported during the liraglutide clinical development program (Neumiller et al., 2010; Elashoff et al., 2011). Thus, a Black Box warning regarding the risk of thyroid C cell cancer was labeled in all approved long-acting GLP-1 analogs (Parks and Rosebraugh, 2010; Andersen et al., 2013). However, a meta-analysis of serious adverse events reported with GLP-1R agonists indicates that neither liraglutide nor exenatide had an association with an increased risk of thyroid cancer (Alves et al., 2012). Such a discrepancy between humans and rodents may reside on the different expression level of GLP-1R in the thyroid because C cells are very sparse in primates when compared with rodents (Gallo, 2013; Pyke et al., 2014). Adequately powered long-term epidemiologic studies will be necessary to clarify the association between GLP-1–based therapies and the risk of thyroid cancer (Andersen et al., 2013).
Besides the pancreas and thyroid, GLP-1R is widely expressed in other tissues, such as the stomach, pituitary, heart, lung, kidney, and nervous system. Analysis of the FDA Adverse Event Reporting System database suggests that for several malignancies, excluding pancreatic and thyroid cancers, exenatide and sitagliptin apparently significantly reduced the odds ratios of some special cancers, such as lung cancer, prostate cancer, lymphoma/multiple myeloma for exenatide, and colon cancer and prostate cancer for sitagliptin (Vigneri et al., 2009; Koehler et al., 2011; Nauck and Friedrich, 2013). However, the data need to be interpreted with caution because the results are based on relatively small numbers of tumors and most likely are subject to reporting bias.
E. GLP-1R Antagonists
Under normal physiologic conditions, GLP-1 is secreted directly by the L cells of the gastrointestinal tract and, as described in this review, acts predominantly to enhance insulin secretion, decrease glucagon secretion, and inhibit gastric motility (II. Glucagon-Like Peptide-1). Based on these data, the main therapeutic strategy targeting GLP-1R has been development of agonists for the treatment of hyperglycemia.
It is established that the insulinotropic action of GLP-1 is highly glucose-dependent such that excessive GLP-1 secretion or sensitivity will not lead to hypoglycemia. However, clinical studies demonstrate that administration of GLP-1 in the presence of a nonglucose-dependent insulin secretagogue (e.g., a sulphonylurea that acts on the KATP channel) or even directly infusing supraphysiological levels of GLP-1 into normal subjects is associated with an increased risk of hypoglycemia (Toft-Nielsen et al., 1999; Buse et al., 2004). These data suggest that under rare conditions in which either the secretion of or sensitivity to GLP-1 is significantly enhanced, patients may display an increased incidence of insulin secretion and concomitant hypoglycemia. Furthermore, these conditions would be predicted to be responsive to treatment with a GLP-1R antagonist.
GLP-1R has a limited expression profile, and its agonists exhibit an excellent safety record, as indicated by the successful treatment of patients with T2DM. Furthermore, mouse GLP-1R knockouts are viable, develop normally, and demonstrate no overt phenotype (Scrocchi et al., 1996). Therefore, it is perhaps unsurprising that interest in the potential therapeutic opportunity for GLP-1R antagonists has emerged only recently, originating largely from a small number of reported clinical conditions that present severe hypoglycemia. These will be briefly discussed in the following paragraphs.
Congenital hyperinsulinism (CHI) represents the most frequent cause of severe, persistent hyperinsulinemic hypoglycemia in newborn babies and children, occurring in approximately 1/25,000 to 1/50,000 births (Lord et al., 2015). CHI is caused by genetic defects in key genes responsible for regulating insulin secretion and arises as a consequence of excess insulin secretion from the pancreatic β cells (Rahman et al., 2015). Insulin directly lowers blood sugars, causing hypoglycemia, but insulin hypersecretion also reduces the supply of alternative sources of energy substrates for oxidative metabolism in the CNS. As these normally act as a protective measure against hypoglycemia, adverse neurodevelopment outcomes affect approximately one-third of all patients (Avatapalle et al., 2013). The most severe forms of CHI accounting for approximately 45% of cases are due to recessive inactivating mutations in ABCC8 and KCJN11 that encode the two components of the β cell ATP-sensitive K+ channel (SUR1 and Kir6.2, respectively). The current treatment paradigm principally involves agonists of KATP channels (diazoxide) and somatostatin receptors. However, as the pathology of CHI mainly involves genetic defects in KATP channels, these patients (>50%) will therefore be refractory to diazoxide treatment that acts via this receptor. Both targets are expressed on β cells, but also many other cell types; and this widespread expression profile contributes to multiple off-target complications of these drugs. Pharmacologically unresponsive CHI requires surgical intervention that may be a limited pancreatectomy/lesionectomy, or in some cases children will require a near-total pancreatectomy (95–98%). Despite surgery, patients with the diffuse form of the disease often require further surgical episodes, and a significant majority of them will develop iatrogenic and early-onset diabetes.
The initial link between CHI and GLP-1 was first reported in rodent models of hyperinsulinism in which the KATP channel subunit ABCC8 was knocked out (De Leon et al., 2008). In this study, treatment with the GLP-1R antagonist exendin9–39 decreased cAMP levels, insulin secretion, and glucose disposal. Interestingly, these effects were generated in the fasting state (i.e., when glucose levels, and hence GLP-1 levels, should have been lowest) and in isolated islets to observe impact on both the basal and stimulated insulin secretion. Recently, these findings have been extended to testing exendin9–39 in adult human subjects with CHI owing to inactivating mutations in the KATP channel. Acute infusion of exendin9–39 significantly increased mean basal glucose levels and glucose area-under-the-curve, and markedly lowered the insulin:glucose ratios (Calabria et al., 2012). Currently, it is unclear in this setting whether the levels of GLP-1 are increased, or whether the responsiveness to exendin9–39 reflects the inverse agonist property of this peptide in controlling excessive insulin secretion by β cells. Recent published data from the Dunne laboratory in Manchester support the identification of a specific subpopulation with elevated GLP-1 levels that may at least partly explain the pathophysiological role of GLP-1 in this condition.
Roux-en-Y gastric bypass surgery is being used increasingly in the treatment of morbidly obese type 2 diabetic patients, and in the vast majority of patients this results in a very beneficial outcome with significant weight loss and resolution of diabetes. However, in a small minority of patients, gastric bypass surgery can lead to a profound postprandial hyperinsulinemic hypoglycemic state that emerges several years after surgery (Patti et al., 2005; Ashrafian et al., 2011). Typically, adult patients experience dizziness, weakness, headache, confusion, lethargy, slurred speech, coma, and seizures depending on the severity and duration of the hypoglycemic episode. In a landmark paper (Marsk et al., 2010), the incidence of severe hyperinsulinemic hypoglycemia was highlighted in approximately 0.2–0.5% of 5040 patients undergoing gastric bypass surgery in Sweden between 1986 and 2006. Discussion with clinical trust representatives of United Kingdom National Health Service confirmed this as an increasingly recognized unmet need, with the figure requiring pharmacological therapy after bypass surgery in the United Kingdom likely to be closer to 1%, in which by the end of 2012 a total of 18,577 procedures had been performed under the National Health Service. In the United States, the American Society for Metabolic and Bariatric Surgery reported the number of procedures increased from about 16,000 in the early 1990s to more than 103,000 in 2003, with the total number of surgeries performed by the end of 2005 exceeding 590,000. Based on such evidence, it is likely that this condition represents an area of considerable future commercial value that is predicted to increase in market size as the awareness and related diagnosis continue to expand.
A number of alternative hypotheses have been suggested to explain postprandial hyperinsulinemic hypoglycemia, as follows: 1) an exaggerated form of dumping syndrome; 2) the result of failure of the pancreas to revert after weight loss; and 3) an outcome from altered incretin secretion. Roux-en-Y gastric bypass surgery has been demonstrated to significantly increase the levels of GLP-1 secretion. Although the mechanism behind the postsurgery hyperinsulinemic hypoglycemia syndrome remains to be confirmed, an emerging number of affected patients present with higher insulin and GLP-1 responses (Service et al., 2005; Salehi et al., 2011). Furthermore, the effects of exogenously administered GLP-1 on gastrointestinal motility and secretion can be blocked by exendin9–39 (Schirra et al., 2006). Very recently, Salehi et al. (2014) have reported that this severe postprandial hypoglycemia in gastric bypass patients can be corrected by infusion of exendin9–39, consistent with a fundamental role for GLP-1 and its receptor in this mechanism.
Currently, no small-molecule GLP-1R antagonist is available that can be used to further understand the consequences of an overactive GLP-1 system. However, exendin9–39 is a selective, competitive GLP-1R antagonist that blocks GLP-1–mediated insulin secretion in vitro and in vivo and impairs glucose tolerance in response to endogenous and exogenous GLP-1 in humans and a variety of rodent models (Schirra et al., 1998; Edwards et al., 1999). Moreover, a number of groups have reported that exendin9–39 inhibits insulin secretion even in the absence of increased GLP-1 levels, suggesting that exendin9–39 may be an inverse agonist of GLP-1R (Serre et al., 1998; Salehi et al., 2011). A modified version of exendin9–39 has also been developed (Patterson et al., 2011) and was demonstrated to cause significant increases in glucose intolerance, food intake, and body weight in diet-induced obese mice, further supporting a role for endogenous GLP-1 as a key hormone in the obese state.
Clearly, the findings discussed above have a number of important implications that form the scientific basis for targeting GLP-1R with specific small-molecule antagonists. These not only highlight possible control of hyperinsulinemic hypoglycemia in different patient populations, but also suggest that an antagonist may reduce the enhanced rate of β cell expansion associated with CHI, thereby offering potential disease-modifying treatment opportunities.
VI. Conclusions
In this review, we have provided insights into the discovery, characterization, physiology, and pharmacology of GLP-1 and GLP-1R, a hormone-receptor system that seems to be ideally designed to facilitate the management of T2DM. This peptide hormone is secreted from enteroendocrine L cells in the distal intestine, exerting its effects through a class B GPCR on various target cells. Most prominent among these are the pancreatic islet β cell, where GLP-1 exerts a glucose-dependent insulinotropic action. It also has other roles in glucose homoeostasis, as well as in regulating gastric motility and appetite useful in the control of body weight. A series of analogs of the GLP-1 peptide have been developed to enhance activity and bioavailability, but efforts in obtaining therapeutically viable nonpeptidic GLP-1R modulators were less successful. The molecular basis of the docking and action of these ligands is reviewed, along with implications for their spectrum of biologic actions. Some of these GLP-1R agonists have already launched to the worldwide market with many more under development. Such an endeavor will definitely lead to better and more efficacious drugs to treat T2DM and obesity.
Acknowledgments
We thank Dr. Xiao Luo for information collection.
Abbreviations
- Aβ
amyloid β protein
- AD
Alzheimer’s disease
- Aib
aminoisobutyric acid
- AMPK
AMP-activated protein kinase
- BCM
β cell mass
- BETP
4-(3-benzyloxyphenyl)-2-ethylsulfinyl-6-(trifluoromethyl)pyrimidine
- CD
circular dichroism
- CHI
congenital hyperinsulinism
- CHL
Chinese hamster lung
- CHO
Chinese hamster ovary
- CICR
calcium-induced calcium release
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- CRF1R
corticotropin-releasing factor-1 receptor
- DPC
dodecylphosphocholine
- DPP-4
dipeptidyl peptidase-4
- ECD
extracellular domain
- ECL
extracellular loop
- Emax
maximal response
- Epac
exchange protein activated by cAMP
- ER
endoplasmic reticulum
- ERK
extracellular regulated kinase
- FDA
Food and Drug Administration
- GCGR
glucagon receptor
- GIP
gastric inhibitory polypeptide
- GIPR
GIP receptor
- GLP
glucagon-like peptide
- GLP-1R
GLP-1 receptor
- GPCR
G protein–coupled receptor
- GRK
G protein–coupled receptor kinase
- HbA1c
hemoglobin A1c
- HEK
human embryonic kidney
- hGLP-1R
human GLP-1R
- iCa2+
intracellular calcium
- ICL
intracellular loop
- IP1
intervening peptide 1
- IRS2
insulin receptor substrate 2
- KATP
ATP-sensitive potassium
- MAPK
mitogen-activated protein kinase
- NEP 24.11
neutral endopeptidase 24.11
- NHE3
Na+/H+ exchanger isoform 3
- PACAP
pituitary adenylate cyclase–activating polypeptide
- PD
Parkinson’s disease
- PDB
Protein Data Bank
- PDX-1
pancreatic and duodenal homeobox-1
- PET
positron emission tomography
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
- PKB
protein kinase B
- PKC
protein kinase C
- PMA
phorbol myristate acetate
- PPAR
peroxisome proliferator-activated receptor
- Rim
Rab-3–interacting molecule
- SAR
structure–activity relationship
- SNP
single-nucleotide polymorphism
- SPECT
single-photon emission-computed tomography
- SRP
signal recognition particle
- SUR1
sulfonylurea receptor
- T2DM
type 2 diabetes mellitus
- TM
transmembrane
- 7TM
seven transmembrane
- 7TMD
7TM domain
Authorship Contributions
Participated in research design: Wang.
Wrote or contributed to the writing of the manuscript: de Graaf, Donnelly, Wootten, Lau, Sexton, Miller, Ahn, Liao, Fletcher, Yang, Brown, Zhou, Deng, Wang.
Footnotes
This work was supported by National Health and Family Planning Commission of China [2012ZX09304-011, 2013ZX09401003-005, 2013ZX09507001, and 2013ZX09507-002 to M.-W.W.]; Chinese Academy of Sciences [to M.-W.W.]; Shanghai Science and Technology Development Fund [15DZ2291600 to M.-W.W.]; Thousand Talents Program in China [to M.-W.W.]; the CAS-Novo Nordisk Research Fund [to D.Y.]; National Natural Science Foundation of China [81373463 to D.Y.]; National Health and Medical Research Council of Australia (NHMRC) Principal Research Fellowship [to P.M.S.]; NHMRC Program [Grant 1055134 to P.M.S.]; NHMRC Career Development Fellowship [to D.W.]; NHMRC Project [Grant 1061044 to P.M.S. and Grant 1065410 to D.W.]; Welch Foundation [AT-1595 to J.-M.A.]; and Juvenile Diabetes Research Foundation [37-2011-20 to J.-M.A.].
References
- Abrahamsen N, Lundgren K, Nishimura E. (1995) Regulation of glucagon receptor mRNA in cultured primary rat hepatocytes by glucose and cAMP. J Biol Chem 270:15853–15857. [DOI] [PubMed] [Google Scholar]
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. (1994) Structure-activity studies of glucagon-like peptide-1. J Biol Chem 269:6275–6278. [PubMed] [Google Scholar]
- Ahn J-M, Murage EN, Lo S-T, Lin M, and Sun X (2011) Highly constrained GLP-1 analogues as non-invasive PET imaging agents for the assessment of pancreatic beta-cell mass, in Peptides: Building Bridges, Proceedings of the 22nd American Peptide Symposium (Lebl M ed); 2011 June 25-30; San Diego, CA. pp 220-221, American Peptide Society. [Google Scholar]
- Al-Sabah S, Donnelly D. (2003a) A model for receptor-peptide binding at the glucagon-like peptide-1 (GLP-1) receptor through the analysis of truncated ligands and receptors. Br J Pharmacol 140:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sabah S, Donnelly D. (2003b) The positive charge at Lys-288 of the glucagon-like peptide-1 (GLP-1) receptor is important for binding the N-terminus of peptide agonists. FEBS Lett 553:342–346. [DOI] [PubMed] [Google Scholar]
- Alaña I, Parker JC, Gault VA, Flatt PR, O’Harte FP, Malthouse JP, Hewage CM. (2006) NMR and alanine scan studies of glucose-dependent insulinotropic polypeptide in water. J Biol Chem 281:16370–16376. [DOI] [PubMed] [Google Scholar]
- Albano JD, Ekins RP, Maritz G, Turner RC. (1972) A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol 70:487–509. [DOI] [PubMed] [Google Scholar]
- Alken M, Rutz C, Köchl R, Donalies U, Oueslati M, Furkert J, Wietfeld D, Hermosilla R, Scholz A, Beyermann M, et al. (2005) The signal peptide of the rat corticotropin-releasing factor receptor 1 promotes receptor expression but is not essential for establishing a functional receptor. Biochem J 390:455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves C, Batel-Marques F, Macedo AF. (2012) A meta-analysis of serious adverse events reported with exenatide and liraglutide: acute pancreatitis and cancer. Diabetes Res Clin Pract 98:271–284. [DOI] [PubMed] [Google Scholar]
- Andersen DK, Andren-Sandberg Å, Duell EJ, Goggins M, Korc M, Petersen GM, Smith JP, Whitcomb DC. (2013) Pancreatitis-diabetes-pancreatic cancer: summary of an NIDDK-NCI workshop. Pancreas 42:1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen L, Dinesen B, Jørgensen PN, Poulsen F, Røder ME. (1993) Enzyme immunoassay for intact human insulin in serum or plasma. Clin Chem 39:578–582. [PubMed] [Google Scholar]
- Angel TE, Chance MR, Palczewski K. (2009) Conserved waters mediate structural and functional activation of family A (rhodopsin-like) G protein-coupled receptors. Proc Natl Acad Sci USA 106:8555–8560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. (2011) Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid 21:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafian H, Athanasiou T, Li JV, Bueter M, Ahmed K, Nagpal K, Holmes E, Darzi A, Bloom SR. (2011) Diabetes resolution and hyperinsulinaemia after metabolic Roux-en-Y gastric bypass. Obes Rev 12:e257–e272. [DOI] [PubMed] [Google Scholar]
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, Madsen J, Rasmussen MF, Lean ME, NN8022-1807 Study Group (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374:1606–1616. [DOI] [PubMed] [Google Scholar]
- Audigier Y, Friedlander M, Blobel G. (1987) Multiple topogenic sequences in bovine opsin. Proc Natl Acad Sci USA 84:5783–5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avatapalle HB, Banerjee I, Shah S, Pryce M, Nicholson J, Rigby L, Caine L, Didi M, Skae M, Ehtisham S, et al. (2013) Abnormal neurodevelopmental outcomes are common in children with transient congenital hyperinsulinism. Front Endocrinol 4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, et al. (2013) Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest 123:2730–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avogaro A, Vigili de Kreutzenberg S, Fadini GP. (2014) Cardiovascular actions of GLP-1 and incretin-based pharmacotherapy. Curr Diab Rep 14:483. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Kim JG, Drucker DJ. (2004) Chronic exposure to GLP-1R agonists promotes homologous GLP-1 receptor desensitization in vitro but does not attenuate GLP-1R-dependent glucose homeostasis in vivo. Diabetes 53 (Suppl 3):S205–S214. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. (1995) Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci 25:366–428. [Google Scholar]
- Ban K, Kim KH, Cho CK, Sauvé M, Diamandis EP, Backx PH, Drucker DJ, Husain M. (2010) Glucagon-like peptide (GLP)-1(9-36)amide-mediated cytoprotection is blocked by exendin(9-39) yet does not require the known GLP-1 receptor. Endocrinology 151:1520–1531. [DOI] [PubMed] [Google Scholar]
- Barragán JM, Rodríguez RE, Eng J, Blázquez E. (1996) Interactions of exendin-(9-39) with the effects of glucagon-like peptide-1-(7-36) amide and of exendin-4 on arterial blood pressure and heart rate in rats. Regul Pept 67:63–68. [DOI] [PubMed] [Google Scholar]
- Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. (2011) GLP-1 and energy balance: an integrated model of short-term and long-term control. Nat Rev Endocrinol 7:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil F, Fernagut PO, Bezard E, Meissner WG. (2014) Insulin, IGF-1 and GLP-1 signaling in neurodegenerative disorders: targets for disease modification? Prog Neurobiol 118:1–18. [DOI] [PubMed] [Google Scholar]
- Bazarsuren A, Grauschopf U, Wozny M, Reusch D, Hoffmann E, Schaefer W, Panzner S, Rudolph R. (2002) In vitro folding, functional characterization, and disulfide pattern of the extracellular domain of human GLP-1 receptor. Biophys Chem 96:305–318. [DOI] [PubMed] [Google Scholar]
- Beinborn M, Worrall CI, McBride EW, Kopin AS. (2005) A human glucagon-like peptide-1 receptor polymorphism results in reduced agonist responsiveness. Regul Pept 130:1–6. [DOI] [PubMed] [Google Scholar]
- Belin D, Bost S, Vassalli JD, Strub K. (1996) A two-step recognition of signal sequences determines the translocation efficiency of proteins. EMBO J 15:468–478. [PMC free article] [PubMed] [Google Scholar]
- Bell GI, Sanchez-Pescador R, Laybourn PJ, Najarian RC. (1983a) Exon duplication and divergence in the human preproglucagon gene. Nature 304:368–371. [DOI] [PubMed] [Google Scholar]
- Bell GI, Santerre RF, Mullenbach GT. (1983b) Hamster preproglucagon contains the sequence of glucagon and two related peptides. Nature 302:716–718. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795. [DOI] [PubMed] [Google Scholar]
- Bergwitz C, Gardella TJ, Flannery MR, Potts JT, Jr, Kronenberg HM, Goldring SR, Jüppner H. (1996) Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin: evidence for a common pattern of ligand-receptor interaction. J Biol Chem 271:26469–26472. [DOI] [PubMed] [Google Scholar]
- Bhashyam S, Fields AV, Patterson B, Testani JM, Chen L, Shen YT, Shannon RP. (2010) Glucagon-like peptide-1 increases myocardial glucose uptake via p38alpha MAP kinase-mediated, nitric oxide-dependent mechanisms in conscious dogs with dilated cardiomyopathy. Circ Heart Fail 3:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HP, Moormann B, Dabew R, Göke B. (1999) Glucagon-like peptide 1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology 140:3919–3927. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. (2005a) Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 54:146–151. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Yellon DM. (2005b) Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther 19:9–11. [DOI] [PubMed] [Google Scholar]
- Bose AK, Mocanu MM, Carr RD, Yellon DM. (2007) Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther 21:253–256. [DOI] [PubMed] [Google Scholar]
- Braun W, Wider G, Lee KH, Wüthrich K. (1983) Conformation of glucagon in a lipid-water interphase by 1H nuclear magnetic resonance. J Mol Biol 169:921–948. [DOI] [PubMed] [Google Scholar]
- Brom M, Oyen WJ, Joosten L, Gotthardt M, Boerman OC. (2010) 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. Eur J Nucl Med Mol Imaging 37:1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JC, Dryburgh JR, Ross SA, Dupré J. (1975) Identification and actions of gastric inhibitory polypeptide. Recent Prog Horm Res 31:487–532. [DOI] [PubMed] [Google Scholar]
- Bulchandani D, Nachnani JS, Herndon B, Molteni A, Pathan MH, Quinn T, Hamdan HA, Alba LM, Graves L. (2012) Effect of exendin (exenatide): GLP 1 receptor agonist on the thyroid and parathyroid gland in a rat model. Eur J Pharmacol 691:292–296. [DOI] [PubMed] [Google Scholar]
- Bullock BP, Heller RS, Habener JF. (1996) Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 137:2968–2978. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Da Costa A, Drucker D, Thorens B. (2001) Glucose competence of the hepatoportal vein sensor requires the presence of an activated glucagon-like peptide-1 receptor. Diabetes 50:1720–1728. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Dolci W, Thorens B. (1999) Long-lasting antidiabetic effect of a dipeptidyl peptidase IV-resistant analog of glucagon-like peptide-1. Metabolism 48:252–258. [DOI] [PubMed] [Google Scholar]
- Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. (2011) Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med 154:103–112. [DOI] [PubMed] [Google Scholar]
- Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, Exenatide-113 Clinical Study Group (2004) Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 27:2628–2635. [DOI] [PubMed] [Google Scholar]
- Buteau J. (2011) GLP-1 signaling and the regulation of pancreatic β-cells mass/function. Avances en Diabetología 27:3–8. [Google Scholar]
- Buteau J, El-Assaad W, Rhodes CJ, Rosenberg L, Joly E, Prentki M. (2004) Glucagon-like peptide-1 prevents beta cell glucolipotoxicity. Diabetologia 47:806–815. [DOI] [PubMed] [Google Scholar]
- Buteau J, Spatz ML, Accili D. (2006) Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes 55:1190–1196. [DOI] [PubMed] [Google Scholar]
- Calabria AC, Li C, Gallagher PR, Stanley CA, De León DD. (2012) GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes 61:2585–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JE, Drucker DJ. (2013) Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab 17:819–837. [DOI] [PubMed] [Google Scholar]
- Campos RV, Lee YC, Drucker DJ. (1994) Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134:2156–2164. [DOI] [PubMed] [Google Scholar]
- Carraro-Lacroix LR, Malnic G, Girardi AC. (2009) Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol 297:F1647–F1655. [DOI] [PubMed] [Google Scholar]
- Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C. (2012) Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem 287:6421–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, Zhang R, Zhang S, Lin L, Chen K, et al. (2007) A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA 104:943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Miller LJ, Dong M. (2010a) Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor. Am J Physiol Endocrinol Metab 299:E62–E68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pinon DI, Miller LJ, Dong M. (2009) Molecular basis of glucagon-like peptide 1 docking to its intact receptor studied with carboxyl-terminal photolabile probes. J Biol Chem 284:34135–34144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Pinon DI, Miller LJ, Dong M. (2010b) Spatial approximations between residues 6 and 12 in the amino-terminal region of glucagon-like peptide 1 and its receptor: a region critical for biological activity. J Biol Chem 285:24508–24518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Kim MK, Son MH, Kaang BK. (2012) Two small molecule agonists of glucagon-like peptide-1 receptor modulate the receptor activation response differently. Biochem Biophys Res Commun 417:558–563. [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Hussain MA, Holz GG. (2002) Exendin-4 as a stimulator of rat insulin I gene promoter activity via bZIP/CRE interactions sensitive to serine/threonine protein kinase inhibitor Ro 31-8220. Endocrinology 143:2303–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhry V, Westheimer FH. (1979) Photoaffinity labeling of biological systems. Annu Rev Biochem 48:293–325. [DOI] [PubMed] [Google Scholar]
- Christensen M, Miossec P, Larsen BD, Werner U, Knop FK. (2014) The design and discovery of lixisenatide for the treatment of type 2 diabetes mellitus. Expert Opin Drug Discov 9:1223–1251. [DOI] [PubMed] [Google Scholar]
- Claeysen S, Cochet M, Donneger R, Dumuis A, Bockaert J, Giannoni P. (2012) Alzheimer culprits: cellular crossroads and interplay. Cell Signal 24:1831–1840. [DOI] [PubMed] [Google Scholar]
- Clérico EM, Maki JL, Gierasch LM. (2008) Use of synthetic signal sequences to explore the protein export machinery. Biopolymers 90:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly BM, Vanko A, McQuade P, Guenther I, Meng X, Rubins D, Waterhouse R, Hargreaves R, Sur C, Hostetler E. (2012) Ex vivo imaging of pancreatic beta cells using a radiolabeled GLP-1 receptor agonist. Mol Imaging Biol 14:79–87. [DOI] [PubMed] [Google Scholar]
- Coopman K, Huang Y, Johnston N, Bradley SJ, Wilkinson GF, Willars GB. (2010) Comparative effects of the endogenous agonist glucagon-like peptide-1 (GLP-1)-(7-36) amide and the small-molecule ago-allosteric agent “compound 2” at the GLP-1 receptor. J Pharmacol Exp Ther 334:795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coopman K, Wallis R, Robb G, Brown AJ, Wilkinson GF, Timms D, Willars GB. (2011) Residues within the transmembrane domain of the glucagon-like peptide-1 receptor involved in ligand binding and receptor activation: modelling the ligand-bound receptor. Mol Endocrinol 25:1804–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, Girardi AC. (2011) Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol 301:F355–F363. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. (1979) The incretin concept today. Diabetologia 16:75–85. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W. (2005) The [pre-] history of the incretin concept. Regul Pept 128:87–91. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt W, Ebert R. (1985) New developments in the incretin concept. Diabetologia 28:565–573. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt WO, Kleine N, Willms B, Orskov C, Holst JJ, Nauck MA. (1996) Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care 19:580–586. [DOI] [PubMed] [Google Scholar]
- Cullinan CA, Brady EJ, Saperstein R, Leibowitz MD. (1994) Glucose-dependent alterations of intracellular free calcium by glucagon-like peptide-1(7-36amide) in individual ob/ob mouse beta-cells. Cell Calcium 15:391–400. [DOI] [PubMed] [Google Scholar]
- Curran AR, Engelman DM. (2003) Sequence motifs, polar interactions and conformational changes in helical membrane proteins. Curr Opin Struct Biol 13:412–417. [DOI] [PubMed] [Google Scholar]
- Cvetković RS, Plosker GL. (2007) Exenatide: a review of its use in patients with type 2 diabetes mellitus (as an adjunct to metformin and/or a sulfonylurea). Drugs 67:935–954. [DOI] [PubMed] [Google Scholar]
- D’Amico E, Hui H, Khoury N, Di Mario U, Perfetti R. (2005) Pancreatic beta-cells expressing GLP-1 are resistant to the toxic effects of immunosuppressive drugs. J Mol Endocrinol 34:377–390. [DOI] [PubMed] [Google Scholar]
- Davis HR, Jr, Mullins DE, Pines JM, Hoos LM, France CF, Compton DS, Graziano MP, Sybertz EJ, Strader CD, Van Heek M. (1998) Effect of chronic central administration of glucagon-like peptide-1 (7-36) amide on food consumption and body weight in normal and obese rats. Obes Res 6:147–156. [DOI] [PubMed] [Google Scholar]
- Day JW, Li P, Patterson JT, Chabenne J, Chabenne MD, Gelfanov VM, Dimarchi RD. (2011) Charge inversion at position 68 of the glucagon and glucagon-like peptide-1 receptors supports selectivity in hormone action. J Pept Sci 17:218–225. [DOI] [PubMed] [Google Scholar]
- de Graaf C, Rein C, Piwnica D, Giordanetto F, Rognan D. (2011) Structure-based discovery of allosteric modulators of two related class B G-protein-coupled receptors. ChemMedChem 6:2159–2169. [DOI] [PubMed] [Google Scholar]
- de Heer J, Rasmussen C, Coy DH, Holst JJ. (2008) Glucagon-like peptide-1, but not glucose-dependent insulinotropic peptide, inhibits glucagon secretion via somatostatin (receptor subtype 2) in the perfused rat pancreas. Diabetologia 51:2263–2270. [DOI] [PubMed] [Google Scholar]
- De León DD, Li C, Delson MI, Matschinsky FM, Stanley CA, Stoffers DA. (2008) Exendin-(9-39) corrects fasting hypoglycemia in SUR-1-/- mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion. J Biol Chem 283:25786–25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit HM, Vervoort GMM, Jansen HJ, de Grauw WJC, de Galan BE, Tack CJ. (2014) Liraglutide reverses pronounced insulin-associated weight gain, improves glycaemic control and decreases insulin dose in patients with type 2 diabetes: a 26 week, randomised clinical trial (ELEGANT). Diabetologia 57:1812–1819. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Holst JJ. (2009) Immunoassays for the incretin hormones GIP and GLP-1. Best Pract Res Clin Endocrinol Metab 23:425–432. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Johnsen AH, Holst JJ. (1995a) Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab 80:952–957. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Knudsen LB, Madsen K, Wiberg FC, Jacobsen O, Holst JJ. (1998) Dipeptidyl peptidase IV resistant analogues of glucagon-like peptide-1 which have extended metabolic stability and improved biological activity. Diabetologia 41:271–278. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. (1995b) Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 44:1126–1131. [DOI] [PubMed] [Google Scholar]
- Denker PS, Dimarco PE. (2006) Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care 29:471. [DOI] [PubMed] [Google Scholar]
- Dharmalingam M, Sriram U, Baruah MP. (2011) Liraglutide: a review of its therapeutic use as a once daily GLP-1 analog for the management of type 2 diabetes mellitus. Indian J Endocrinol Metab 15:9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamant M, Van Gaal L, Guerci B, Stranks S, Han J, Malloy J, Boardman MK, Trautmann ME. (2014) Exenatide once weekly versus insulin glargine for type 2 diabetes (DURATION-3): 3-year results of an open-label randomised trial. Lancet Diabetes Endocrinol 2:464–473. [DOI] [PubMed] [Google Scholar]
- Dillon JS, Tanizawa Y, Wheeler MB, Leng XH, Ligon BB, Rabin DU, Yoo-Warren H, Permutt MA, Boyd AE., 3rd (1993) Cloning and functional expression of the human glucagon-like peptide-1 (GLP-1) receptor. Endocrinology 133:1907–1910. [DOI] [PubMed] [Google Scholar]
- Dods RL, Donnelly D. (2015) The peptide agonist-binding site of the glucagon-like peptide-1 (GLP-1) receptor based on site-directed mutagenesis and knowledge-based modelling. Biosci Rep 36:e00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Pinon DI, Miller LJ. (2012) Site of action of a pentapeptide agonist at the glucagon-like peptide-1 receptor: insight into a small molecule agonist-binding pocket. Bioorg Med Chem Lett 22:638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong JZ, Shen Y, Zhang J, Tsomaia N, Mierke DF, Taylor JE. (2011) Discovery and characterization of taspoglutide, a novel analogue of human glucagon-like peptide-1, engineered for sustained therapeutic activity in type 2 diabetes. Diabetes Obes Metab 13:19–25. [DOI] [PubMed] [Google Scholar]
- Donnelly D. (2012) The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol 166:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker DJ, Asa S. (1988) Glucagon gene expression in vertebrate brain. J Biol Chem 263:13475–13478. [PubMed] [Google Scholar]
- Drucker DJ, Dritselis A, Kirkpatrick P. (2010) Liraglutide. Nat Rev Drug Discov 9:267–268. [DOI] [PubMed] [Google Scholar]
- Drucker DJ, Philippe J, Mojsov S, Chick WL, Habener JF. (1987) Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci USA 84:3434–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan KM, Povedano ST, Forst T, González JG, Atisso C, Sealls W, Fahrbach JL. (2014) Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet 384:1349–1357. [DOI] [PubMed] [Google Scholar]
- Dunphy JL, Taylor RG, Fuller PJ. (1998) Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol Cell Endocrinol 141:179–186. [DOI] [PubMed] [Google Scholar]
- Dupre J, Caussignac Y, McDonald TJ, Van Vliet S. (1991) Stimulation of glucagon secretion by gastric inhibitory polypeptide in patients with hepatic cirrhosis and hyperglucagonemia. J Clin Endocrinol Metab 72:125–129. [DOI] [PubMed] [Google Scholar]
- Dupre J, Ross SA, Watson D, Brown JC. (1973) Stimulation of insulin secretion by gastric inhibitory polypeptide in man. J Clin Endocrinol Metab 37:826–828. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, et al. (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9:1173–1179. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Gylfe E. (2004) Ca(2+)-induced Ca(2+) release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem 279:45455–45461. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Isakov Y, Sågetorp J, Tengholm A. (2006) Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439:349–352. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, Lu Y, Schwede F, Genieser HG, Smrcka AV, et al. (2011) Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets 3:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert R, Creutzfeldt W. (1982) Influence of gastric inhibitory polypeptide antiserum on glucose-induced insulin secretion in rats. Endocrinology 111:1601–1606. [DOI] [PubMed] [Google Scholar]
- Ebert R, Creutzfeldt W. (1987) Gastrointestinal peptides and insulin secretion. Diabetes Metab Rev 3:1–26. [DOI] [PubMed] [Google Scholar]
- Edvell A, Lindström P. (1999) Initiation of increased pancreatic islet growth in young normoglycemic mice (Umeå +/?). Endocrinology 140:778–783. [DOI] [PubMed] [Google Scholar]
- Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. (1999) Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes 48:86–93. [DOI] [PubMed] [Google Scholar]
- Einhorn L, Krapfenbauer K. (2015) HTRF: a technology tailored for biomarker determination-novel analytical detection system suitable for detection of specific autoimmune antibodies as biomarkers in nanogram level in different body fluids. EPMA J 6:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi D, Andersen DK, Brown JC, Debas HT, Hershcopf RJ, Raizes GS, Tobin JD, Andres R. (1979) Pancreatic alpha- and beta-cell responses to GIP infusion in normal man. Am J Physiol 237:E185–E191. [DOI] [PubMed] [Google Scholar]
- Elahi D, McAloon-Dyke M, Fukagawa NK, Meneilly GS, Sclater AL, Minaker KL, Habener JF, Andersen DK. (1994) The insulinotropic actions of glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (7-37) in normal and diabetic subjects. Regul Pept 51:63–74. [DOI] [PubMed] [Google Scholar]
- Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. (2011) Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 141:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrick H, Stimmler L, Hlad CJ, Jr, Arai Y. (1964) Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 24:1076–1082. [DOI] [PubMed] [Google Scholar]
- Eng C, Kramer CK, Zinman B, Retnakaran R. (2014) Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384:2228–2234. [DOI] [PubMed] [Google Scholar]
- Eng H, Sharma R, McDonald TS, Edmonds DJ, Fortin JP, Li X, Stevens BD, Griffith DA, Limberakis C, Nolte WM, et al. (2013) Demonstration of the innate electrophilicity of 4-(3-(benzyloxy)phenyl)-2-(ethylsulfinyl)-6-(trifluoromethyl)pyrimidine (BETP), a small-molecule positive allosteric modulator of the glucagon-like peptide-1 receptor. Drug Metab Dispos 41:1470–1479. [DOI] [PubMed] [Google Scholar]
- Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q. (2010) Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325:26–35. [DOI] [PubMed] [Google Scholar]
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. (2012) Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35:2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Gong N, Li TF, Ma AN, Wu XY, Wang MW, Wang YX. (2015) The non-peptide GLP-1 receptor agonist WB4-24 blocks inflammatory nociception by stimulating β-endorphin release from spinal microglia. Br J Pharmacol 172:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer RW, Harrington CA, Brown DH. (1975) A simple radioimmunoassay for 3′,5′, cyclic adenosine monophosphate. Anal Biochem 64:455–460. [DOI] [PubMed] [Google Scholar]
- Fehmann HC, Habener JF. (1991) Homologous desensitization of the insulinotropic glucagon-like peptide-I (7-37) receptor on insulinoma (HIT-T15) cells. Endocrinology 128:2880–2888. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. (2001) Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 53:1–24. [PubMed] [Google Scholar]
- Flint A, Raben A, Astrup A, Holst JJ. (1998) Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 101:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin JP, Schroeder JC, Zhu Y, Beinborn M, Kopin AS. (2010) Pharmacological characterization of human incretin receptor missense variants. J Pharmacol Exp Ther 332:274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CB, Wayment JR, Myers GA, Endicott SK, Harris JM. (2009) Single-molecule fluorescence imaging of peptide binding to supported lipid bilayers. Anal Chem 81:5130–5138. [DOI] [PubMed] [Google Scholar]
- Friedrichsen BN, Neubauer N, Lee YC, Gram VK, Blume N, Petersen JS, Nielsen JH, Møldrup A. (2006) Stimulation of pancreatic beta-cell replication by incretins involves transcriptional induction of cyclin D1 via multiple signalling pathways. J Endocrinol 188:481–492. [DOI] [PubMed] [Google Scholar]
- Fritsche A, Stefan N, Hardt E, Häring H, Stumvoll M. (2000) Characterisation of beta-cell dysfunction of impaired glucose tolerance: evidence for impairment of incretin-induced insulin secretion. Diabetologia 43:852–858. [DOI] [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. (2002) Piccolo, a Ca2+ sensor in pancreatic beta-cells: involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem 277:50497–50502. [DOI] [PubMed] [Google Scholar]
- Gallo M. (2013) Thyroid safety in patients treated with liraglutide. J Endocrinol Invest 36:140–145. [DOI] [PubMed] [Google Scholar]
- Gallwitz B, Ropeter T, Morys-Wortmann C, Mentlein R, Siegel EG, Schmidt WE. (2000) GLP-1-analogues resistant to degradation by dipeptidyl-peptidase IV in vitro. Regul Pept 86:103–111. [DOI] [PubMed] [Google Scholar]
- Gallwitz B, Witt M, Paetzold G, Morys-Wortmann C, Zimmermann B, Eckart K, Fölsch UR, Schmidt WE. (1994) Structure/activity characterization of glucagon-like peptide-1. Eur J Biochem 225:1151–1156. [DOI] [PubMed] [Google Scholar]
- Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A, Bordner A, Abagyan R, Miller LJ. (2009) Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol Pharmacol 76:264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Niu G, Yang M, Quan Q, Ma Y, Murage EN, Ahn J-M, Kiesewetter DO, Chen X. (2011) PET of insulinoma using ¹⁸F-FBEM-EM3106B, a new GLP-1 analogue. Mol Pharm 8:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber AJ. (2012) Novel GLP-1 receptor agonists for diabetes. Expert Opin Investig Drugs 21:45–57. [DOI] [PubMed] [Google Scholar]
- Gault VA, Hölscher C. (2008) GLP-1 agonists facilitate hippocampal LTP and reverse the impairment of LTP induced by beta-amyloid. Eur J Pharmacol 587:112–117. [DOI] [PubMed] [Google Scholar]
- Ge Y, Yang D, Dai A, Zhou C, Zhu Y, Wang MW. (2014) The putative signal peptide of glucagon-like peptide-1 receptor is not required for receptor synthesis but promotes receptor expression. Biosci Rep 34:e00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gejl M, Starup-Linde J, Scheel-Thomsen J, Gregersen S, Vestergaard P. (2015) Risk of cardiovascular disease: the effects of diabetes and anti-diabetic drugs: a nested case-control study. Int J Cardiol 178:292–296. [DOI] [PubMed] [Google Scholar]
- Gengler S, McClean PL, McCurtin R, Gault VA, Hölscher C. (2012) Val(8)GLP-1 rescues synaptic plasticity and reduces dense core plaques in APP/PS1 mice. Neurobiol Aging 33:265–276. [DOI] [PubMed] [Google Scholar]
- Gentry PR, Sexton PM, Christopoulos A. (2015) Novel allosteric modulators of G protein-coupled receptors. J Biol Chem 290:19478–19488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesellchen F, Prinz A, Zimmermann B, Herberg FW. (2006) Quantification of cAMP antagonist action in vitro and in living cells. Eur J Cell Biol 85:663–672. [DOI] [PubMed] [Google Scholar]
- Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, et al. (2010) Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev 26:287–296. [DOI] [PubMed] [Google Scholar]
- Godoy-Matos AF. (2014) The role of glucagon on type 2 diabetes at a glance. Diabetol Metab Syndr 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, Göke B. (1993a) Exendin-4 is a high potency agonist and truncated exendin-(9-39)-amide an antagonist at the glucagon-like peptide 1-(7-36)-amide receptor of insulin-secreting beta-cells. J Biol Chem 268:19650–19655. [PubMed] [Google Scholar]
- Göke R, Just R, Lankat-Buttgereit B, Göke B. (1994) Glycosylation of the GLP-1 receptor is a prerequisite for regular receptor function. Peptides 15:675–681. [DOI] [PubMed] [Google Scholar]
- Göke R, Wagner B, Fehmann HC, Göke B. (1993b) Glucose-dependency of the insulin stimulatory effect of glucagon-like peptide-1 (7-36) amide on the rat pancreas. Res Exp Med 193:97–103. [DOI] [PubMed] [Google Scholar]
- Goland R, Freeby M, Parsey R, Saisho Y, Kumar D, Simpson N, Hirsch J, Prince M, Maffei A, Mann JJ, et al. (2009) 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J Nucl Med 50:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Fan H, Ma AN, Xiao Q, Wang YX. (2014a) Geniposide and its iridoid analogs exhibit antinociception by acting at the spinal GLP-1 receptors. Neuropharmacology 84:31–45. [DOI] [PubMed] [Google Scholar]
- Gong N, Ma AN, Zhang LJ, Luo XS, Zhang YH, Xu M, Wang YX. (2011) Site-specific PEGylation of exenatide analogues markedly improved their glucoregulatory activity. Br J Pharmacol 163:399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Xiao Q, Zhu B, Zhang CY, Wang YC, Fan H, Ma AN, Wang YX. (2014b) Activation of spinal glucagon-like peptide-1 receptors specifically suppresses pain hypersensitivity. J Neurosci 34:5322–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RH, Jacobs JW, Chin WW, Lund PK, Dee PC, Habener JF. (1980a) Nucleotide sequence of a cloned structural gene coding for a precursor of pancreatic somatostatin. Proc Natl Acad Sci USA 77:5869–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RH, Lund PK, Jacobs JW, Habener JF. (1980b) Pre-prosomatostatins: products of cell-free translations of messenger RNAs from anglerfish islets. J Biol Chem 255:6549–6552. [PubMed] [Google Scholar]
- Gotthardt M, Lalyko G, van Eerd-Vismale J, Keil B, Schurrat T, Hower M, Laverman P, Behr TM, Boerman OC, Göke B, et al. (2006) A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regul Pept 137:162–167. [DOI] [PubMed] [Google Scholar]
- Gough SCL, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, Damgaard LH, Buse JB, NN9068-3697 (DUAL-I) Trial Investigators (2014) Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol 2:885–893. [DOI] [PubMed] [Google Scholar]
- Graziano MP, Hey PJ, Borkowski D, Chicchi GG, Strader CD. (1993) Cloning and functional expression of a human glucagon-like peptide-1 receptor. Biochem Biophys Res Commun 196:141–146. [DOI] [PubMed] [Google Scholar]
- Green BD, Gault VA, Irwin N, Mooney MH, Bailey CJ, Harriott P, Greer B, Flatt PR, O’Harte FP. (2003) Metabolic stability, receptor binding, cAMP generation, insulin secretion and antihyperglycaemic activity of novel N-terminal Glu9-substituted analogues of glucagon-like peptide-1. Biol Chem 384:1543–1551. [DOI] [PubMed] [Google Scholar]
- Green BD, Mooney MH, Gault VA, Irwin N, Bailey CJ, Harriott P, Greer B, Flatt PR, O’Harte FP. (2004) Lys9 for Glu9 substitution in glucagon-like peptide-1(7-36)amide confers dipeptidylpeptidase IV resistance with cellular and metabolic actions similar to those of established antagonists glucagon-like peptide-1(9-36)amide and exendin (9-39). Metabolism 53:252–259. [DOI] [PubMed] [Google Scholar]
- Grieve DJ, Cassidy RS, Green BD. (2009) Emerging cardiovascular actions of the incretin hormone glucagon-like peptide-1: potential therapeutic benefits beyond glycaemic control? Br J Pharmacol 157:1340–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Brock B, Schmitz O, Rorsman P. (2004) Glucagon-like peptide-1: regulation of insulin secretion and therapeutic potential. Basic Clin Pharmacol Toxicol 95:252–262. [DOI] [PubMed] [Google Scholar]
- Gromada J, Dissing S, Rorsman P. (1996) Desensitization of glucagon-like peptide 1 receptors in insulin-secreting beta TC3 cells: role of PKA-independent mechanisms. Br J Pharmacol 118:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Holst JJ, Rorsman P. (1998) Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch 435:583–594. [DOI] [PubMed] [Google Scholar]
- Gromada J, Rorsman P. (2004) New insights into the regulation of glucagon secretion by glucagon-like peptide-1. Horm Metab Res 36:822–829. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450. [PubMed] [Google Scholar]
- Gupta NA, Mells J, Dunham RM, Grakoui A, Handy J, Saxena NK, Anania FA. (2010) Glucagon-like peptide-1 receptor is present on human hepatocytes and has a direct role in decreasing hepatic steatosis in vitro by modulating elements of the insulin signaling pathway. Hepatology 51:1584–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson S, Burstein A, Valcarce C, Grimes I, Mjalli A. (2014) TTP273, an orally-available glucagon-like peptide-1 (GLP-1) agonist, notably reduces glycemia in subjects with type 2 diabetes mellitus (T2DM). Diabetes 63 (Suppl 1):A41. [Google Scholar]
- Gutniak MK, Juntti-Berggren L, Hellström PM, Guenifi A, Holst JJ, Efendic S. (1996) Glucagon-like peptide I enhances the insulinotropic effect of glibenclamide in NIDDM patients and in the perfused rat pancreas. Diabetes Care 19:857–863. [DOI] [PubMed] [Google Scholar]
- Gutniak M, Orskov C, Holst JJ, Ahrén B, Efendic S. (1992) Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med 326:1316–1322. [DOI] [PubMed] [Google Scholar]
- Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, et al. (2004) Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89:3055–3061. [DOI] [PubMed] [Google Scholar]
- Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. (2010) Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 53:730–740. [DOI] [PubMed] [Google Scholar]
- Halban PA. (2004) Cellular sources of new pancreatic beta cells and therapeutic implications for regenerative medicine. Nat Cell Biol 6:1021–1025. [DOI] [PubMed] [Google Scholar]
- Halic M, Beckmann R. (2005) The signal recognition particle and its interactions during protein targeting. Curr Opin Struct Biol 15:116–125. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, Pyke C, Knudsen LB, Farr SA, Vrang N. (2015) The GLP-1 receptor agonist liraglutide improves memory function and increases hippocampal CA1 neuronal numbers in a senescence-accelerated mouse model of Alzheimer’s disease. J Alzheimers Dis 46:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque TS, Lee VG, Riexinger D, Lei M, Malmstrom S, Xin L, Han S, Mapelli C, Cooper CB, Zhang G, et al. (2010) Identification of potent 11mer glucagon-like peptide-1 receptor agonist peptides with novel C-terminal amino acids: homohomophenylalanine analogs. Peptides 31:950–955. [DOI] [PubMed] [Google Scholar]
- Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. (2006) Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE 2006:pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hareter A, Hoffmann E, Bode HP, Göke B, Göke R. (1997) The positive charge of the imidazole side chain of histidine7 is crucial for GLP-1 action. Endocr J 44:701–705. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Ball AM, Sexton PM, Miller LJ. (2010) Importance of lipid-exposed residues in transmembrane segment four for family B calcitonin receptor homo-dimerization. Regul Pept 164:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. (2006) Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol 69:363–373. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Wootten D, Pinon DI, Koole C, Ball AM, Furness SG, Graham B, Dong M, Christopoulos A, Miller LJ, et al. (2012) Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signaling but does not affect small molecule allostery. Proc Natl Acad Sci USA 109:18607–18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. (2008) Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PE, Ferrara C, Barba P, Polito T, Freeby M, Maffei A. (2008) VMAT2 gene expression and function as it applies to imaging β-cell mass. J Mol Med 86:5–16. [DOI] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. (2011) Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Guan N, Gao WW, Liu Q, Wu XY, Ma DW, Zhong DF, Ge GB, Li C, Chen XY, et al. (2012) A continued saga of Boc5, the first non-peptidic glucagon-like peptide-1 receptor agonist with in vivo activities. Acta Pharmacol Sin 33:148–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Su H, Gao W, Johansson SM, Liu Q, Wu X, Liao J, Young AA, Bartfai T, Wang MW. (2010) Reversal of obesity and insulin resistance by a non-peptidic glucagon-like peptide-1 receptor agonist in diet-induced obese mice. PLoS One 5:e14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijboer AC, Frans A, Lomecky M, Blankenstein MA. (2011) Analysis of glucagon-like peptide 1; what to measure? Clin Chim Acta 412:1191–1194. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Gros P, Lund PK, Bentley RC, Habener JF. (1984) Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology 115:2176–2181. [DOI] [PubMed] [Google Scholar]
- Heller RS, Kieffer TJ, Habener JF. (1996) Point mutations in the first and third intracellular loops of the glucagon-like peptide-1 receptor alter intracellular signaling. Biochem Biophys Res Commun 223:624–632. [DOI] [PubMed] [Google Scholar]
- Heller RS, Kieffer TJ, Habener JF. (1997) Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes 46:785–791. [DOI] [PubMed] [Google Scholar]
- Hendrick GK, Gjinovci A, Baxter LA, Mojsov S, Wollheim CB, Habener JF, Weir GC. (1993) Glucagon-like peptide-I-(7-37) suppresses hyperglycemia in rats. Metabolism 42:1–6. [DOI] [PubMed] [Google Scholar]
- Heppner KM, Perez-Tilve D. (2015) GLP-1 based therapeutics: simultaneously combating T2DM and obesity. Front Neurosci 9:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higy M, Junne T, Spiess M. (2004) Topogenesis of membrane proteins at the endoplasmic reticulum. Biochemistry 43:12716–12722. [DOI] [PubMed] [Google Scholar]
- Hinke SA, Manhart S, Pamir N, Demuth H, W Gelling R, Pederson RA, McIntosh CHS. (2001) Identification of a bioactive domain in the amino-terminus of glucose-dependent insulinotropic polypeptide (GIP). Biochim Biophys Acta 1547:143–155. [DOI] [PubMed] [Google Scholar]
- Hjorth SA, Adelhorst K, Pedersen BB, Kirk O, Schwartz TW. (1994) Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. J Biol Chem 269:30121–30124. [PubMed] [Google Scholar]
- Hoang HN, Song K, Hill TA, Derksen DR, Edmonds DJ, Kok WM, Limberakis C, Liras S, Loria PM, Mascitti V, et al. (2015) Short hydrophobic peptides with cyclic constraints are potent glucagon-like peptide-1 receptor (GLP-1R) agonists. J Med Chem 58:4080–4085. [DOI] [PubMed] [Google Scholar]
- Hoare SR. (2005) Mechanisms of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discov Today 10:417–427. [DOI] [PubMed] [Google Scholar]
- Hogan AE, Tobin AM, Ahern T, Corrigan MA, Gaoatswe G, Jackson R, O’Reilly V, Lynch L, Doherty DG, Moynagh PN, et al. (2011) Glucagon-like peptide-1 (GLP-1) and the regulation of human invariant natural killer T cells: lessons from obesity, diabetes and psoriasis. Diabetologia 54:2745–2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K, de Graaf C, Bortolato A, Wang MW, Marshall FH, Stevens RC. (2014) Insights into the structure of class B GPCRs. Trends Pharmacol Sci 35:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K, Kean J, Bortolato A, Cheng RK, Doré AS, Jazayeri A, Cooke RM, Weir M, Marshall FH. (2013) Structure of class B GPCR corticotropin-releasing factor receptor 1. Nature 499:438–443. [DOI] [PubMed] [Google Scholar]
- Holst JJ. (1997) Enteroglucagon. Annu Rev Physiol 59:257–271. [DOI] [PubMed] [Google Scholar]
- Holst JJ. (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439. [DOI] [PubMed] [Google Scholar]
- Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. (1994) Proglucagon processing in porcine and human pancreas. J Biol Chem 269:18827–18833. [PubMed] [Google Scholar]
- Holst JJ, Orskov C. (2001) Incretin hormones: an update. Scand J Clin Lab Invest Suppl 234:75–85. [PubMed] [Google Scholar]
- Holst JJ, Orskov C, Nielsen OV, Schwartz TW. (1987) Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett 211:169–174. [DOI] [PubMed] [Google Scholar]
- Holz GG. (2004) Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Li J, Fu H, Yan Z, Bu G, He X, Wang Y. (2012) Characterization of glucagon-like peptide 1 receptor (GLP1R) gene in chickens: functional analysis, tissue distribution, and identification of its transcript variants. Domest Anim Endocrinol 43:1–15. [DOI] [PubMed] [Google Scholar]
- Huang CL, Sun L, Moonga BS, Zaidi M. (2006) Molecular physiology and pharmacology of calcitonin. Cell Mol Biol 52:33–43. [PubMed] [Google Scholar]
- Huang Y, Wilkinson GF, Willars GB. (2010) Role of the signal peptide in the synthesis and processing of the glucagon-like peptide-1 receptor. Br J Pharmacol 159:237–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui H, Nourparvar A, Zhao X, Perfetti R. (2003) Glucagon-like peptide-1 inhibits apoptosis of insulin-secreting cells via a cyclic 5′-adenosine monophosphate-dependent protein kinase A- and a phosphatidylinositol 3-kinase-dependent pathway. Endocrinology 144:1444–1455. [DOI] [PubMed] [Google Scholar]
- Hui H, Perfetti R. (2002) Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol 146:129–141. [DOI] [PubMed] [Google Scholar]
- Ichise M, Harris PE. (2010) Imaging of β-cell mass and function. J Nucl Med 51:1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illergård K, Kauko A, Elofsson A. (2011) Why are polar residues within the membrane core evolutionary conserved? Proteins 79:79–91. [DOI] [PubMed] [Google Scholar]
- Inooka H, Ohtaki T, Kitahara O, Ikegami T, Endo S, Kitada C, Ogi K, Onda H, Fujino M, Shirakawa M. (2001) Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat Struct Biol 8:161–165. [DOI] [PubMed] [Google Scholar]
- Irwin N, Flatt PR, Patterson S, Green BD. (2010) Insulin-releasing and metabolic effects of small molecule GLP-1 receptor agonist 6,7-dichloro-2-methylsulfonyl-3-N-tert-butylaminoquinoxaline. Eur J Pharmacol 628:268–273. [DOI] [PubMed] [Google Scholar]
- Isberg V, de Graaf C, Bortolato A, Cherezov V, Katritch V, Marshall FH, Mordalski S, Pin JP, Stevens RC, Vriend G, et al. (2015) Generic GPCR residue numbers: aligning topology maps while minding the gaps. Trends Pharmacol Sci 36:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Shibata R, Kondo K, Kambara T, Shimizu Y, Tanigawa T, Bando YK, Nishimura M, Ouchi N, Murohara T. (2014) Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric-oxide synthase-dependent mechanism. J Biol Chem 289:27235–27245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Doré AS, Lamb D, Krishnamurthy H, Southall SM, Baig AH, Bortolato A, Koglin M, Robertson NJ, Errey JC, et al. (2016) Extra-helical binding site of a glucagon receptor antagonist. Nature 533:274–277. [DOI] [PubMed] [Google Scholar]
- Jendle J, Rosenstock J, Blonde L, Woo V, Gross J, Jiang H, Milicevic Z. (2014) Better glycemic control and less weight gain with once-weekly dulaglutide vs. once-daily insulin glargine, both combined with premeal insulin lispro, in type 2 diabetes patients (AWARD-4). Diabetes 63 (Suppl 1):A246. [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. (2003) cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev 17:1575–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Hadac EM, Henne RM, Patel SA, Lybrand TP, Miller LJ. (1997) Direct identification of a distinct site of interaction between the carboxyl-terminal residue of cholecystokinin and the type A cholecystokinin receptor using photoaffinity labeling. J Biol Chem 272:24393–24401. [DOI] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. (1988) Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol 271:519–532. [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Kubale V, Vrecl M, Schwartz TW, Elling CE. (2007) Oxyntomodulin differentially affects glucagon-like peptide-1 receptor beta-arrestin recruitment and signaling through Galpha(s). J Pharmacol Exp Ther 322:148–154. [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Martini L, Schwartz TW, Elling CE. (2005) Characterization of glucagon-like peptide-1 receptor beta-arrestin 2 interaction: a high-affinity receptor phenotype. Mol Endocrinol 19:812–823. [DOI] [PubMed] [Google Scholar]
- Jorgensen R, Norklit Roed S, Heding A, Elling CE. (2011) Beta-arrestin2 as a competitor for GRK2 interaction with the GLP-1 receptor upon receptor activation. Pharmacology 88:174–181. [DOI] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. (2003) Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278:8279–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. (2008) Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic beta-cells and rat INS-1 cells. J Physiol 586:1307–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanse SM, Kreymann B, Ghatei MA, Bloom SR. (1988) Identification and characterization of glucagon-like peptide-1 7-36 amide-binding sites in the rat brain and lung. FEBS Lett 241:209–212. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. (2013) Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov 12:205–216. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Miller LJ. (2010) Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev 62:265–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. (2012) A simple method for quantifying functional selectivity and agonist bias. ACS Chem Neurosci 3:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keov P, Sexton PM, Christopoulos A. (2011) Allosteric modulation of G protein-coupled receptors: a pharmacological perspective. Neuropharmacology 60:24–35. [DOI] [PubMed] [Google Scholar]
- Kim JG, Baggio LL, Bridon DP, Castaigne JP, Robitaille MF, Jetté L, Benquet C, Drucker DJ. (2003) Development and characterization of a glucagon-like peptide 1-albumin conjugate: the ability to activate the glucagon-like peptide 1 receptor in vivo. Diabetes 52:751–759. [DOI] [PubMed] [Google Scholar]
- Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K. (2007) Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care 30:1487–1493. [DOI] [PubMed] [Google Scholar]
- Kim S, Moon M, Park S. (2009) Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol 202:431–439. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, 3rd, Wright CV, White MF, Arden KC, Accili D. (2002) The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 110:1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems LL, Holst JJ, Vølund A, Madsbad S. (2003) The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52:380–386. [DOI] [PubMed] [Google Scholar]
- Knudsen LB. (2004) Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem 47:4128–4134. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, Holst JJ, Jeppesen CB, Johnson MD, de Jong JC, et al. (2007) Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci USA 104:937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M, Agersø H. (2000) Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem 43:1664–1669. [DOI] [PubMed] [Google Scholar]
- Köchl R, Alken M, Rutz C, Krause G, Oksche A, Rosenthal W, Schülein R. (2002) The signal peptide of the G protein-coupled human endothelin B receptor is necessary for translocation of the N-terminal tail across the endoplasmic reticulum membrane. J Biol Chem 277:16131–16138. [DOI] [PubMed] [Google Scholar]
- Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, Kajitani N, Nishishita S, Sarai K, Hirota D, et al. (2011) Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia 54:965–978. [DOI] [PubMed] [Google Scholar]
- Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. (2009) Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 58:2148–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler JA, Kain T, Drucker DJ. (2011) Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology 152:3362–3372. [DOI] [PubMed] [Google Scholar]
- Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, et al. (2003) Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 88:3082–3089. [DOI] [PubMed] [Google Scholar]
- Koole C, Savage EE, Christopoulos A, Miller LJ, Sexton PM, Wootten D. (2013b) Minireview: signal bias, allosterism, and polymorphic variation at the GLP-1R: implications for drug discovery. Mol Endocrinol 27:1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Pabreja K, Savage EE, Wootten D, Furness SG, Miller LJ, Christopoulos A, Sexton PM. (2013a) Recent advances in understanding GLP-1R (glucagon-like peptide-1 receptor) function. Biochem Soc Trans 41:172–179. [DOI] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM. (2012a) Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) has a critical role in GLP-1 peptide binding and receptor activation. J Biol Chem 287:3642–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM. (2015) Differential impact of amino acid substitutions on critical residues of the human glucagon-like peptide-1 receptor involved in peptide activity and small-molecule allostery. J Pharmacol Exp Ther 353:52–63. [DOI] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Savage EE, Miller LJ, Christopoulos A, Sexton PM. (2012b) Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) differentially regulates orthosteric but not allosteric agonist binding and function. J Biol Chem 287:3659–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Valant C, Miller LJ, Christopoulos A, Sexton PM. (2011) Polymorphism and ligand dependent changes in human glucagon-like peptide-1 receptor (GLP-1R) function: allosteric rescue of loss of function mutation. Mol Pharmacol 80:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Valant C, Sridhar R, Woodman OL, Miller LJ, Summers RJ, Christopoulos A, Sexton PM. (2010) Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol Pharmacol 78:456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koth CM, Murray JM, Mukund S, Madjidi A, Minn A, Clarke HJ, Wong T, Chiang V, Luis E, Estevez A, et al. (2012) Molecular basis for negative regulation of the glucagon receptor. Proc Natl Acad Sci USA 109:14393–14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreymann B, Williams G, Ghatei MA, Bloom SR. (1987) Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet 2:1300–1304. [DOI] [PubMed] [Google Scholar]
- Kuna RS, Girada SB, Asalla S, Vallentyne J, Maddika S, Patterson JT, Smiley DL, DiMarchi RD, Mitra P. (2013) Glucagon-like peptide-1 receptor-mediated endosomal cAMP generation promotes glucose-stimulated insulin secretion in pancreatic β-cells. Am J Physiol Endocrinol Metab 305:E161–E170. [DOI] [PubMed] [Google Scholar]
- Kung MP, Hou C, Lieberman BP, Oya S, Ponde DE, Blankemeyer E, Skovronsky D, Kilbourn MR, Kung HF. (2008) In vivo imaging of beta-cell mass in rats using 18F-FP-(+)-DTBZ: a potential PET ligand for studying diabetes mellitus. J Nucl Med 49:1171–1176. [DOI] [PubMed] [Google Scholar]
- Kwan EP, Xie L, Sheu L, Ohtsuka T, Gaisano HY. (2007) Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes 56:2579–2588. [DOI] [PubMed] [Google Scholar]
- La Barre J. (1932) Sur les possibilite’s d’un traitement du diabète par l’incrétine. Bull Acad R Med Belg 12:14. [Google Scholar]
- Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. (2005) Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280:31294–31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, et al. (2015) Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 58:7370–7380. [DOI] [PubMed] [Google Scholar]
- Lawrence MC, Bhatt HS, Easom RA. (2002) NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes 51:691–698. [DOI] [PubMed] [Google Scholar]
- Le WD, Chen S, Jankovic J. (2009) Etiopathogenesis of Parkinson disease: a new beginning? Neuroscientist 15:28–35. [DOI] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A. (2007) Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28:382–389. [DOI] [PubMed] [Google Scholar]
- Lean ME, Carraro R, Finer N, Hartvig H, Lindegaard ML, Rössner S, Van Gaal L, Astrup A, NN8022-1807 Investigators (2014) Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes 38:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lu J, Willars GB. (2012a) Allosteric modulation of the activity of the glucagon-like peptide-1 (GLP-1) metabolite GLP-1 9-36 amide at the GLP-1 receptor. PLoS One 7:e47936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. (2005) beta-Cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1. Diabetes 54:482–491. [DOI] [PubMed] [Google Scholar]
- Li Y, Chigurupati S, Holloway HW, Mughal M, Tweedie D, Bruestle DA, Mattson MP, Wang Y, Harvey BK, Ray B, et al. (2012b) Exendin-4 ameliorates motor neuron degeneration in cellular and animal models of amyotrophic lateral sclerosis. PLoS One 7:e32008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. (2009) GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and parkinsonism. Proc Natl Acad Sci USA 106:1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH. (2010) Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem 113:1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. (2002) Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol 16:2135–2144. [DOI] [PubMed] [Google Scholar]
- Liu Q, Li N, Yuan Y, Lu H, Wu X, Zhou C, He M, Su H, Zhang M, Wang J, et al. (2012) Cyclobutane derivatives as novel nonpeptidic small molecule agonists of glucagon-like peptide-1 receptor. J Med Chem 55:250–267. [DOI] [PubMed] [Google Scholar]
- Locatelli-Hoops S, Yeliseev AA, Gawrisch K, Gorshkova I. (2013) Surface plasmon resonance applied to G protein-coupled receptors. Biomed Spectrosc Imaging 2:155–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Maturana R, Donnelly D. (2002) The glucagon-like peptide-1 receptor binding site for the N-terminus of GLP-1 requires polarity at Asp198 rather than negative charge. FEBS Lett 530:244–248. [DOI] [PubMed] [Google Scholar]
- López de Maturana R, Treece-Birch J, Abidi F, Findlay JB, Donnelly D. (2004) Met-204 and Tyr-205 are together important for binding GLP-1 receptor agonists but not their N-terminally truncated analogues. Protein Pept Lett 11:15–22. [DOI] [PubMed] [Google Scholar]
- López de Maturana R, Willshaw A, Kuntzsch A, Rudolph R, Donnelly D. (2003) The isolated N-terminal domain of the glucagon-like peptide-1 (GLP-1) receptor binds exendin peptides with much higher affinity than GLP-1. J Biol Chem 278:10195–10200. [DOI] [PubMed] [Google Scholar]
- Lord K, Radcliffe J, Gallagher PR, Adzick NS, Stanley CA, De León DD. (2015) High risk of diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J Clin Endocrinol Metab 100:4133–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M, Evers A, Wagner M. (2013) Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett 23:4011–4018. [DOI] [PubMed] [Google Scholar]
- Lund A, Knop FK, Vilsbøll T. (2011) Emerging GLP-1 receptor agonists. Expert Opin Emerg Drugs 16:607–618. [DOI] [PubMed] [Google Scholar]
- Lund PK. (2005) The discovery of glucagon-like peptide 1. Regul Pept 128:93–96. [DOI] [PubMed] [Google Scholar]
- Lund PK, Goodman RH, Dee PC, Habener JF. (1982) Pancreatic preproglucagon cDNA contains two glucagon-related coding sequences arranged in tandem. Proc Natl Acad Sci USA 79:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund PK, Goodman RH, Habener JF. (1981) Pancreatic pre-proglucagons are encoded by two separate mRNAs. J Biol Chem 256:6515–6518. [PubMed] [Google Scholar]
- Lv C, Liu X, Liu H, Chen T, Zhang W. (2014) Geniposide attenuates mitochondrial dysfunction and memory deficits in APP/PS1 transgenic mice. Curr Alzheimer Res 11:580–587. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, Joseph JW, Rorsman P. (2005) Glucose-sensing mechanisms in pancreatic beta-cells. Philos Trans R Soc Lond B Biol Sci 360:2211–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PE, Wang X, Xia F, El-kholy W, Targonsky ED, Tsushima RG, Wheeler MB. (2003) Antagonism of rat beta-cell voltage-dependent K+ currents by exendin 4 requires dual activation of the cAMP/protein kinase A and phosphatidylinositol 3-kinase signaling pathways. J Biol Chem 278:52446–52453. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Cook S, Scrocchi L, Shin J, Kim J, Vaccarino F, Asa SL, Drucker DJ. (2000) Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 141:752–762. [DOI] [PubMed] [Google Scholar]
- Madsbad S. (2016) Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 18:317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. (2011) An overview of once-weekly glucagon-like peptide-1 receptor agonists: available efficacy and safety data and perspectives for the future. Diabetes Obes Metab 13:394–407. [DOI] [PubMed] [Google Scholar]
- Madsen LW, Knauf JA, Gotfredsen C, Pilling A, Sjögren I, Andersen S, Andersen L, de Boer AS, Manova K, Barlas A, et al. (2012) GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology 153:1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen K, Knudsen LB, Agersoe H, Nielsen PF, Thøgersen H, Wilken M, Johansen NL. (2007) Structure-activity and protraction relationship of long-acting glucagon-like peptide-1 derivatives: importance of fatty acid length, polarity, and bulkiness. J Med Chem 50:6126–6132. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Louchami K, Sener A. (2009) Noninvasive imaging of pancreatic beta cells. Nat Rev Endocrinol 5:394–400. [DOI] [PubMed] [Google Scholar]
- Maljaars PW, Peters HP, Mela DJ, Masclee AA. (2008) Ileal brake: a sensible food target for appetite control: a review. Physiol Behav 95:271–281. [DOI] [PubMed] [Google Scholar]
- Malone J, Trautmann M, Wilhelm K, Taylor K, Kendall DM. (2009) Exenatide once weekly for the treatment of type 2 diabetes. Expert Opin Investig Drugs 18:359–367. [DOI] [PubMed] [Google Scholar]
- Manandhar B, Ahn JM. (2015) Glucagon-like peptide-1 (GLP-1) analogs: recent advances, new possibilities, and therapeutic implications. J Med Chem 58:1020–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manandhar B, Lo S-T, Murage E, Lin M, Sun X, and Ahn J-M (2013) Targeting GLP-1R for non-invasive assessment of pancreatic beta-cell mass, in Peptides Across The Pacific: Proceedings of the 23rd American Peptide Symposium (Lebl M ed); 2013 June 22-27; Big Island, Hawaii. pp 172-173, American Peptide Society. [Google Scholar]
- Mangmool S, Hemplueksa P, Parichatikanond W, Chattipakorn N. (2015) Epac is required for GLP-1R-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes. Mol Endocrinol 29:583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R, Nasr N, Hadden D, Sinfield J, Abidi F, Al-Sabah S, de Maturana RL, Treece-Birch J, Willshaw A, Donnelly D. (2007) Peptide binding at the GLP-1 receptor. Biochem Soc Trans 35:713–716. [DOI] [PubMed] [Google Scholar]
- Mann RJ, Al-Sabah S, de Maturana RL, Sinfield JK, Donnelly D. (2010a) Functional coupling of Cys-226 and Cys-296 in the glucagon-like peptide-1 (GLP-1) receptor indicates a disulfide bond that is close to the activation pocket. Peptides 31:2289–2293. [DOI] [PubMed] [Google Scholar]
- Mann RJ, Nasr NE, Sinfield JK, Paci E, Donnelly D. (2010b) The major determinant of exendin-4/glucagon-like peptide 1 differential affinity at the rat glucagon-like peptide 1 receptor N-terminal domain is a hydrogen bond from SER-32 of exendin-4. Br J Pharmacol 160:1973–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapelli C, Natarajan SI, Meyer JP, Bastos MM, Bernatowicz MS, Lee VG, Pluscec J, Riexinger DJ, Sieber-McMaster ES, Constantine KL, et al. (2009) Eleven amino acid glucagon-like peptide-1 receptor agonists with antidiabetic activity. J Med Chem 52:7788–7799. [DOI] [PubMed] [Google Scholar]
- Marsk R, Jonas E, Rasmussen F, Näslund E. (2010) Nationwide cohort study of post-gastric bypass hypoglycaemia including 5,040 patients undergoing surgery for obesity in 1986-2006 in Sweden. Diabetologia 53:2307–2311. [DOI] [PubMed] [Google Scholar]
- Mathi SK, Chan Y, Li X, Wheeler MB. (1997) Scanning of the glucagon-like peptide-1 receptor localizes G protein-activating determinants primarily to the N terminus of the third intracellular loop. Mol Endocrinol 11:424–432. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Perry T, Greig NH. (2003) Learning from the gut. Nat Med 9:1113–1115. [DOI] [PubMed] [Google Scholar]
- Matveyenko AV, Butler PC. (2008) Relationship between β-cell mass and diabetes onset. Diabetes Obes Metab 10 (Suppl 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, Bazin H, Tinel N, Durroux T, Prézeau L, et al. (2008) Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods 5:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May LT, Leach K, Sexton PM, Christopoulos A. (2007) Allosteric modulation of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 47:1–51. [DOI] [PubMed] [Google Scholar]
- McClean PL, Hölscher C. (2014) Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer’s disease. Neuropharmacology 86:241–258. [DOI] [PubMed] [Google Scholar]
- McClean PL, Parthsarathy V, Faivre E, Hölscher C. (2011) The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 31:6587–6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre N, Holdsworth CD, Turner DS. (1964) New interpretation of oral glucose tolerance. Lancet 2:20–21. [DOI] [PubMed] [Google Scholar]
- Meier JJ. (2012) GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 8:728–742. [DOI] [PubMed] [Google Scholar]
- Miller LJ, Chen Q, Lam PC, Pinon DI, Sexton PM, Abagyan R, Dong M. (2011) Refinement of glucagon-like peptide 1 docking to its intact receptor using mid-region photolabile probes and molecular modeling. J Biol Chem 286:15895–15907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima A, Hiraoka-Yamomoto J, Li Q, Kitada M, Li C, Geraldes P, Matsumoto M, Mizutani K, Park K, Cahill C, et al. (2012) Protective effects of GLP-1 on glomerular endothelium and its inhibition by PKCβ activation in diabetes. Diabetes 61:2967–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda LP, Winters KA, Gegg CV, Patel A, Aral J, Long J, Zhang J, Diamond S, Guido M, Stanislaus S, et al. (2008) Design and synthesis of conformationally constrained glucagon-like peptide-1 derivatives with increased plasma stability and prolonged in vivo activity. J Med Chem 51:2758–2765. [DOI] [PubMed] [Google Scholar]
- Mojsov S. (1992) Structural requirements for biological activity of glucagon-like peptide-I. Int J Pept Protein Res 40:333–343. [DOI] [PubMed] [Google Scholar]
- Mojsov S, Heinrich G, Wilson IB, Ravazzola M, Orci L, Habener JF. (1986) Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 261:11880–11889. [PubMed] [Google Scholar]
- Mojsov S, Weir GC, Habener JF. (1987) Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 79:616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanya E. (2012) A comparison of currently available GLP-1 receptor agonists for the treatment of type 2 diabetes. Expert Opin Pharmacother 13:1451–1467. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. (1999) Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology 140:1132–1140. [DOI] [PubMed] [Google Scholar]
- Montrose-Rafizadeh C, Yang H, Rodgers BD, Beday A, Pritchette LA, Eng J. (1997) High potency antagonists of the pancreatic glucagon-like peptide-1 receptor. J Biol Chem 272:21201–21206. [DOI] [PubMed] [Google Scholar]
- Moon MJ, Kim HY, Park S, Kim DK, Cho EB, Park CR, You DJ, Hwang JI, Kim K, Choe H, et al. (2012) Evolutionarily conserved residues at glucagon-like peptide-1 (GLP-1) receptor core confer ligand-induced receptor activation. J Biol Chem 287:3873–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon MJ, Lee YN, Park S, Reyes-Alcaraz A, Hwang JI, Millar RP, Choe H, Seong JY. (2015) Ligand binding pocket formed by evolutionarily conserved residues in the glucagon-like peptide-1 (GLP-1) receptor core domain. J Biol Chem 290:5696–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. (2009) Advances in beta-cell imaging. Eur J Radiol 70:254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B. (1906) On the treatment of diabetus mellitus by acid extract of duodenal mucous membrane. Biochem J 1:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG. (2008) Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 30:1448–1460. [DOI] [PubMed] [Google Scholar]
- Moroo I, Yamada T, Makino H, Tooyama I, McGeer PL, McGeer EG, Hirayama K. (1994) Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson’s disease. Acta Neuropathol 87:343–348. [DOI] [PubMed] [Google Scholar]
- Morris JK, Bomhoff GL, Gorres BK, Davis VA, Kim J, Lee PP, Brooks WM, Gerhardt GA, Geiger PC, Stanford JA. (2011) Insulin resistance impairs nigrostriatal dopamine function. Exp Neurol 231:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LC, Nance KD, Gentry PR, Days EL, Weaver CD, Niswender CM, Thompson AD, Jones CK, Locuson CW, Morrison RD, et al. (2014) Discovery of (S)-2-cyclopentyl-N-((1-isopropylpyrrolidin2-yl)-9-methyl-1-oxo-2,9-dihydro-1H-pyrrido[3,4-b]indole-4-carboxamide (VU0453379): a novel, CNS penetrant glucagon-like peptide 1 receptor (GLP-1R) positive allosteric modulator (PAM). J Med Chem 57:10192–10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Zhang H, Gupte AA, Bomhoff GL, Stanford JA, Geiger PC. (2008) Measures of striatal insulin resistance in a 6-hydroxydopamine model of Parkinson’s disease. Brain Res 1240:185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudaliar S, Henry RR. (2010) Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med 123:S19–S27. [DOI] [PubMed] [Google Scholar]
- Mukai E, Toyoda K, Kimura H, Kawashima H, Fujimoto H, Ueda M, Temma T, Hirao K, Nagakawa K, Saji H, et al. (2009) GLP-1 receptor antagonist as a potential probe for pancreatic beta-cell imaging. Biochem Biophys Res Commun 389:523–526. [DOI] [PubMed] [Google Scholar]
- Mullard A. (2014) Once-yearly device takes on daily and weekly diabetes drugs. Nat Biotechnol 32:1178. [DOI] [PubMed] [Google Scholar]
- Murage EN, Gao G, Bisello A, Ahn J-M. (2010) Development of potent glucagon-like peptide-1 agonists with high enzyme stability via introduction of multiple lactam bridges. J Med Chem 53:6412–6420. [DOI] [PubMed] [Google Scholar]
- Murage EN, Schroeder JC, Beinborn M, Ahn JM. (2008) Search for alpha-helical propensity in the receptor-bound conformation of glucagon-like peptide-1. Bioorg Med Chem 16:10106–10112. [DOI] [PubMed] [Google Scholar]
- Murthy R, Harris P, Simpson N, Van Heertum R, Leibel R, Mann JJ, Parsey R. (2008) Whole body [11C]-dihydrotetrabenazine imaging of baboons: biodistribution and human radiation dosimetry estimates. Eur J Nucl Med Mol Imaging 35:790–797. [DOI] [PubMed] [Google Scholar]
- Myers GA, Gacek DA, Peterson EM, Fox CB, Harris JM. (2012) Microscopic rates of peptide-phospholipid bilayer interactions from single-molecule residence times. J Am Chem Soc 134:19652–19660. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Baller B, Meier JJ. (2004) Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes 53 (Suppl 3):S190–S196. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Büsing M, Orskov C, Siegel EG, Talartschik J, Baartz A, Baartz T, Hopt UT, Becker HD, Creutzfeldt W. (1993a) Preserved incretin effect in type 1 diabetic patients with end-stage nephropathy treated by combined heterotopic pancreas and kidney transplantation. Acta Diabetol 30:39–45. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Friedrich N. (2013) Do GLP-1-based therapies increase cancer risk? Diabetes Care 36 (Suppl 2):S245–S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. (1993b) Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 91:301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Kemmeries G, Holst JJ, Meier JJ. (2011) Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60:1561–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. (1993c) Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia 36:741–744. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. (1997) Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol 273:E981–E988. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Petrie JR, Sesti G, Mannucci E, Courrèges JP, Lindegaard ML, Jensen CB, Atkin SL, Study 1821 Investigators (2016) A Phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care 39:231–241. [DOI] [PubMed] [Google Scholar]
- Nauck MPJ, Sesti G. (2012) The once-weekly human GLP1 analogue semaglutide provides significant reductions in HbA1c and body weight in patients with type 2 diabetes. Diabetologia 55 (Suppl 1):S7.22918257 [Google Scholar]
- Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH. (2001) Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry 40:13188–13200. [DOI] [PubMed] [Google Scholar]
- Neumann JM, Couvineau A, Murail S, Lacapère JJ, Jamin N, Laburthe M. (2008) Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem Sci 33:314–319. [DOI] [PubMed] [Google Scholar]
- Neumiller JJ, Sonnett TE, Wood LD, Setter SM, Campbell RK. (2010) Pharmacology, efficacy and safety of liraglutide in the management of type 2 diabetes. Diabetes Metab Syndr Obes 3:215–226. [PMC free article] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. (2004) Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem 279:37215–37218. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, Stolarski C, Shen YT, Shannon RP. (2004a) Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation 110:955–961. [DOI] [PubMed] [Google Scholar]
- Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. (2004b) Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 109:962–965. [DOI] [PubMed] [Google Scholar]
- Nolte WM, Fortin JP, Stevens BD, Aspnes GE, Griffith DA, Hoth LR, Ruggeri RB, Mathiowetz AM, Limberakis C, Hepworth D, et al. (2014) A potentiator of orthosteric ligand activity at GLP-1R acts via covalent modification. Nat Chem Biol 10:629–631. [DOI] [PubMed] [Google Scholar]
- Norris SL, Lee N, Thakurta S, Chan BK. (2009) Exenatide efficacy and safety: a systematic review. Diabet Med 26:837–846. [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ. (2009) GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 287:E1209–E1215. [DOI] [PubMed] [Google Scholar]
- Oka J, Suzuki E, Kondo Y. (2000) Endogenous GLP-1 is involved in beta-amyloid protein-induced memory impairment and hippocampal neuronal death in rats. Brain Res 878:194–198. [DOI] [PubMed] [Google Scholar]
- Orskov C, Bersani M, Johnsen AH, Højrup P, Holst JJ. (1989) Complete sequences of glucagon-like peptide-1 from human and pig small intestine. J Biol Chem 264:12826–12829. [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Nielsen OV. (1988) Effect of truncated glucagon-like peptide-1 [proglucagon-(78-107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology 123:2009–2013. [DOI] [PubMed] [Google Scholar]
- Orskov C, Holst JJ, Poulsen SS, Kirkegaard P. (1987) Pancreatic and intestinal processing of proglucagon in man. Diabetologia 30:874–881. [DOI] [PubMed] [Google Scholar]
- Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. (1994) Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes 43:535–539. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, et al. (2000) cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2:805–811. [DOI] [PubMed] [Google Scholar]
- Pal K, Melcher K, Xu HE. (2012) Structure and mechanism for recognition of peptide hormones by class B G-protein-coupled receptors. Acta Pharmacol Sin 33:300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745. [DOI] [PubMed] [Google Scholar]
- Panjwani N, Mulvihill EE, Longuet C, Yusta B, Campbell JE, Brown TJ, Streutker C, Holland D, Cao X, Baggio LL, et al. (2013) GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE(-/-) mice. Endocrinology 154:127–139. [DOI] [PubMed] [Google Scholar]
- Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, Han SW, Shin SJ, Bang BK, Breyer MD, et al. (2007) Long-term treatment of glucagon-like peptide-1 analog exendin-4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol 18:1227–1238. [DOI] [PubMed] [Google Scholar]
- Parks M, Rosebraugh C. (2010) Weighing risks and benefits of liraglutide: the FDA’s review of a new antidiabetic therapy. N Engl J Med 362:774–777. [DOI] [PubMed] [Google Scholar]
- Parthier C, Kleinschmidt M, Neumann P, Rudolph R, Manhart S, Schlenzig D, Fanghänel J, Rahfeld JU, Demuth HU, Stubbs MT. (2007) Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci USA 104:13942–13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. (2009) Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem Sci 34:303–310. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Li P, Day JW, Gelfanov VM, Dimarchi RD. (2013) A hydrophobic site on the GLP-1 receptor extracellular domain orients the peptide ligand for signal transduction. Mol Metab 2:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JT, Ottaway N, Gelfanov VM, Smiley DL, Perez-Tilve D, Pfluger PT, Tschöp MH, Dimarchi RD. (2011) A novel human-based receptor antagonist of sustained action reveals body weight control by endogenous GLP-1. ACS Chem Biol 6:135–145. [DOI] [PubMed] [Google Scholar]
- Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J, Hanto DW, Callery M, Arky R, Nose V, et al. (2005) Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48:2236–2240. [DOI] [PubMed] [Google Scholar]
- Pattou F, Kerr-Conte J, Wild D. (2010) GLP-1-receptor scanning for imaging of human beta cells transplanted in muscle. N Engl J Med 363:1289–1290. [DOI] [PubMed] [Google Scholar]
- Paul SK, Klein K, Maggs D, Best JH. (2015) The association of the treatment with glucagon-like peptide-1 receptor agonist exenatide or insulin with cardiovascular outcomes in patients with type 2 diabetes: a retrospective observational study. Cardiovasc Diabetol 14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson AJ, Sharman JL, Benson HE, Faccenda E, Alexander SP, Buneman OP, Davenport AP, McGrath JC, Peters JA, Southan C, et al. NC-IUPHAR (2014) The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands. Nucleic Acids Res 42:D1098–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti R, Zhou J, Doyle ME, Egan JM. (2000) Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141:4600–4605. [DOI] [PubMed] [Google Scholar]
- Perley MJ, Kipnis DM. (1967) Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest 46:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret J, Van Craenenbroeck M, Langer I, Vertongen P, Gregoire F, Robberecht P, Waelbroeck M. (2002) Mutational analysis of the glucagon receptor: similarities with the vasoactive intestinal peptide (VIP)/pituitary adenylate cyclase-activating peptide (PACAP)/secretin receptors for recognition of the ligand’s third residue. Biochem J 362:389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. (2002) Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther 302:881–888. [DOI] [PubMed] [Google Scholar]
- Perry T, Lahiri DK, Sambamurti K, Chen D, Mattson MP, Egan JM, Greig NH. (2003) Glucagon-like peptide-1 decreases endogenous amyloid-beta peptide (Abeta) levels and protects hippocampal neurons from death induced by Abeta and iron. J Neurosci Res 72:603–612. [DOI] [PubMed] [Google Scholar]
- Peyot ML, Gray JP, Lamontagne J, Smith PJ, Holz GG, Madiraju SR, Prentki M, Heart E. (2009) Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS One 4:e6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamboeck A, Holst JJ, Carr RD, Deacon CF. (2005) Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia 48:1882–1890. [DOI] [PubMed] [Google Scholar]
- Pocai A. (2013) Action and therapeutic potential of oxyntomodulin. Mol Metab 3:241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratley RE, Nauck MA, Barnett AH, Feinglos MN, Ovalle F, Harman-Boehm I, Ye J, Scott R, Johnson S, Stewart M, et al. HARMONY 7 Study Group (2014) Once-weekly albiglutide versus once-daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open-label, multicentre, non-inferiority phase 3 study. Lancet Diabetes Endocrinol 2:289–297. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM. (1987) Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev 67:1185–1248. [DOI] [PubMed] [Google Scholar]
- Pressley JC, Louis ED, Tang MX, Cote L, Cohen PD, Glied S, Mayeux R. (2003) The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology 60:87–93. [DOI] [PubMed] [Google Scholar]
- Prévost M, Vertongen P, Raussens V, Roberts DJ, Cnudde J, Perret J, Waelbroeck M. (2010) Mutational and cysteine scanning analysis of the glucagon receptor N-terminal domain. J Biol Chem 285:30951–30958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridal L, Ingwersen SH, Larsen FS, Holst JJ, Adelhorst K, Kirk O. (1995) Comparison of sandwich enzyme-linked immunoadsorbent assay and radioimmunoassay for determination of exogenous glucagon-like peptide-1(7-36)amide in plasma. J Pharm Biomed Anal 13:841–850. [DOI] [PubMed] [Google Scholar]
- Pyke C, Heller RS, Kirk RK, Ørskov C, Reedtz-Runge S, Kaastrup P, Hvelplund A, Bardram L, Calatayud D, Knudsen LB. (2014) GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155:1280–1290. [DOI] [PubMed] [Google Scholar]
- Qian F, Ni N, Burton LS, Wang YF, Desikan S, Hussain M, Smith RL. (2009) Sustained release subcutaneous delivery of BMS-686117, a GLP-1 receptor peptide agonist, via a zinc adduct. Int J Pharm 374:46–52. [DOI] [PubMed] [Google Scholar]
- Quoyer J, Longuet C, Broca C, Linck N, Costes S, Varin E, Bockaert J, Bertrand G, Dalle S. (2010) GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem 285:1989–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab EL, Vuguin PM, Stoffers DA, Simmons RA. (2009) Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol 297:R1785–R1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman SA, Nessa A, Hussain K. (2015) Molecular mechanisms of congenital hyperinsulinism. J Mol Endocrinol 54:R119–R129. [DOI] [PubMed] [Google Scholar]
- Rajan S, Dickson LM, Mathew E, Orr CM, Ellenbroek JH, Philipson LH, Wicksteed B. (2015) Chronic hyperglycemia downregulates GLP-1 receptor signaling in pancreatic β-cells via protein kinase A. Mol Metab 4:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Torres J, Thompson MS, Philipson LH. (2012) SUMO downregulates GLP-1-stimulated cAMP generation and insulin secretion. Am J Physiol Endocrinol Metab 302:E714–E723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read N, French S, Cunningham K. (1994) The role of the gut in regulating food intake in man. Nutr Rev 52:1–10. [DOI] [PubMed] [Google Scholar]
- Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Peñacarrillo ML. (2003) Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol 204:43–50. [DOI] [PubMed] [Google Scholar]
- Reiner T, Kohler RH, Liew CW, Hill JA, Gaglia J, Kulkarni RN, Weissleder R. (2010) Near-infrared fluorescent probe for imaging of pancreatic β cells. Bioconjug Chem 21:1362–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renström E, Eliasson L, Rorsman P. (1997) Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol 502:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retterstøl K. (2009) Taspoglutide: a long acting human glucagon-like polypeptide-1 analogue. Expert Opin Investig Drugs 18:1405–1411. [DOI] [PubMed] [Google Scholar]
- Rhodes CJ, White MF. (2002) Molecular insights into insulin action and secretion. Eur J Clin Invest 32 (Suppl 3):3–13. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Vertongen P, Waelbroeck M. (2011) Analysis of the glucagon receptor first extracellular loop by the substituted cysteine accessibility method. Peptides 32:1593–1599. [DOI] [PubMed] [Google Scholar]
- Roed SN, Nøhr AC, Wismann P, Iversen H, Bräuner-Osborne H, Knudsen SM, Waldhoer M. (2015) Functional consequences of glucagon-like peptide-1 receptor cross-talk and trafficking. J Biol Chem 290:1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roed SN, Wismann P, Underwood CR, Kulahin N, Iversen H, Cappelen KA, Schäffer L, Lehtonen J, Hecksher-Soerensen J, Secher A, et al. (2014) Real-time trafficking and signaling of the glucagon-like peptide-1 receptor. Mol Cell Endocrinol 382:938–949. [DOI] [PubMed] [Google Scholar]
- Rolin B, Larsen MO, Gotfredsen CF, Deacon CF, Carr RD, Wilken M, Knudsen LB. (2002) The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab 283:E745–E752. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Hanefeld M, Shamanna P, Min KW, Boka G, Miossec P, Zhou T, Muehlen-Bartmer I, Ratner RE. (2014) Beneficial effects of once-daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal-S). J Diabetes Complications 28:386–392. [DOI] [PubMed] [Google Scholar]
- Rosol TJ. (2013) On-target effects of GLP-1 receptor agonists on thyroid C-cells in rats and mice. Toxicol Pathol 41:303–309. [DOI] [PubMed] [Google Scholar]
- Ruiz-Grande C, Alarcón C, Alcántara A, Castilla C, López Novoa JM, Villanueva-Peñacarrillo ML, Valverde I. (1993) Renal catabolism of truncated glucagon-like peptide 1. Horm Metab Res 25:612–616. [DOI] [PubMed] [Google Scholar]
- Runge S, Gram C, Brauner-Osborne H, Madsen K, Knudsen LB, Wulff BS. (2003a) Three distinct epitopes on the extracellular face of the glucagon receptor determine specificity for the glucagon amino terminus. J Biol Chem 278:28005–28010. [DOI] [PubMed] [Google Scholar]
- Runge S, Schimmer S, Oschmann J, Schiødt CB, Knudsen SM, Jeppesen CB, Madsen K, Lau J, Thøgersen H, Rudolph R. (2007) Differential structural properties of GLP-1 and exendin-4 determine their relative affinity for the GLP-1 receptor N-terminal extracellular domain. Biochemistry 46:5830–5840. [DOI] [PubMed] [Google Scholar]
- Runge S, Thøgersen H, Madsen K, Lau J, Rudolph R. (2008) Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem 283:11340–11347. [DOI] [PubMed] [Google Scholar]
- Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. (2003b) Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol 138:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz C, Renner A, Alken M, Schulz K, Beyermann M, Wiesner B, Rosenthal W, Schülein R. (2006) The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J Biol Chem 281:24910–24921. [DOI] [PubMed] [Google Scholar]
- Salehi M, Gastaldelli A, D'Alessio DA. (2014) Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146:669-680.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi M, Prigeon RL, D’Alessio DA. (2011) Gastric bypass surgery enhances glucagon-like peptide 1-stimulated postprandial insulin secretion in humans. Diabetes 60:2308–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho V, Nuche B, Arnés L, Cancelas J, González N, Díaz-Miguel M, Martín-Duce A, Valverde I, Villanueva-Peñacarrillo ML. (2007) The action of GLP-1 and exendins upon glucose transport in normal human adipocytes, and on kinase activity as compared to morbidly obese patients. Int J Mol Med 19:961–966. [PubMed] [Google Scholar]
- Sarrauste de Menthière C, Chavanieu A, Grassy G, Dalle S, Salazar G, Kervran A, Pfeiffer B, Renard P, Delagrange P, Manechez D, et al. (2004) Structural requirements of the N-terminal region of GLP-1-[7-37]-NH2 for receptor interaction and cAMP production. Eur J Med Chem 39:473–480. [DOI] [PubMed] [Google Scholar]
- Sarson DL, Wood SM, Kansal PC, Bloom SR. (1984) Glucose-dependent insulinotropic polypeptide augmentation of insulin: physiology or pharmacology? Diabetes 33:389–393. [DOI] [PubMed] [Google Scholar]
- Satoh F, Beak SA, Small CJ, Falzon M, Ghatei MA, Bloom SR, Smith DM. (2000) Characterization of human and rat glucagon-like peptide-1 receptors in the neurointermediate lobe: lack of coupling to either stimulation or inhibition of adenylyl cyclase. Endocrinology 141:1301–1309. [DOI] [PubMed] [Google Scholar]
- Saudek F, Brogren CH, Manohar S. (2008) Imaging the beta-cell mass: why and how. Rev Diabet Stud 5:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelshorn D, Joly F, Mutel S, Hampe C, Breton B, Mutel V, Lütjens R. (2012) Lateral allosterism in the glucagon receptor family: glucagon-like peptide 1 induces G-protein-coupled receptor heteromer formation. Mol Pharmacol 81:309–318. [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Lagerström MC, Watanobe H, Jonsson L, Vergoni AV, Ringholm A, Skarphedinsson JO, Skuladottir GV, Klovins J, Fredriksson R. (2003) Functional role, structure, and evolution of the melanocortin-4 receptor. Ann N Y Acad Sci 994:74–83. [DOI] [PubMed] [Google Scholar]
- Schirra J, Nicolaus M, Roggel R, Katschinski M, Storr M, Woerle HJ, Göke B. (2006) Endogenous glucagon-like peptide 1 controls endocrine pancreatic secretion and antro-pyloro-duodenal motility in humans. Gut 55:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. (1998) Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 101:1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WE, Siegel EG, Creutzfeldt W. (1985) Glucagon-like peptide-1 but not glucagon-like peptide-2 stimulates insulin release from isolated rat pancreatic islets. Diabetologia 28:704–707. [DOI] [PubMed] [Google Scholar]
- Schneider G, Fechner U. (2004) Advances in the prediction of protein targeting signals. Proteomics 4:1571–1580. [DOI] [PubMed] [Google Scholar]
- Schneider S. (2008) Efforts to develop methods for in vivo evaluation of the native β-cell mass. Diabetes Obes Metab 10 (Suppl 4):109–118. [DOI] [PubMed] [Google Scholar]
- Schröder-Tittmann K, Bosse-Doenecke E, Reedtz-Runge S, Ihling C, Sinz A, Tittmann K, Rudolph R. (2010) Recombinant expression, in vitro refolding, and biophysical characterization of the human glucagon-like peptide-1 receptor. Biochemistry 49:7956–7965. [DOI] [PubMed] [Google Scholar]
- Schülein R, Westendorf C, Krause G, Rosenthal W. (2012) Functional significance of cleavable signal peptides of G protein-coupled receptors. Eur J Cell Biol 91:294–299. [DOI] [PubMed] [Google Scholar]
- Scrocchi LA, Brown TJ, MaClusky N, Brubaker PL, Auerbach AB, Joyner AL, Drucker DJ. (1996) Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258. [DOI] [PubMed] [Google Scholar]
- Segre GV, Goldring SR. (1993) Receptors for secretin, calcitonin, parathyroid hormone (PTH)/PTH-related peptide, vasoactive intestinal peptide, glucagonlike peptide 1, growth hormone-releasing hormone, and glucagon belong to a newly discovered G-protein-linked receptor family. Trends Endocrinol Metab 4:309–314. [DOI] [PubMed] [Google Scholar]
- Serre V, Dolci W, Schaerer E, Scrocchi L, Drucker D, Efrat S, Thorens B. (1998) Exendin-(9-39) is an inverse agonist of the murine glucagon-like peptide-1 receptor: implications for basal intracellular cyclic adenosine 3′,5′-monophosphate levels and beta-cell glucose competence. Endocrinology 139:4448–4454. [DOI] [PubMed] [Google Scholar]
- Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. (2005) Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 353:249–254. [DOI] [PubMed] [Google Scholar]
- Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. (2000) Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab 85:4053–4059. [DOI] [PubMed] [Google Scholar]
- Shan SO, Walter P. (2005) Co-translational protein targeting by the signal recognition particle. FEBS Lett 579:921–926. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, et al. (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104:19333–19338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D, Warren TG, Roth SE, Brenner MJ. (1981) Cell-free synthesis and processing of multiple precursors to glucagon. Nature 289:511–514. [DOI] [PubMed] [Google Scholar]
- Shiue CY, Schmitz A, Schirrmacher R, Shiue GG, Alavi A. (2004) Potential approaches for beta cell imaging with PET and SPECT. Curr Med Chem 4:271–280. [Google Scholar]
- Shonberg J, Lopez L, Scammells PJ, Christopoulos A, Capuano B, Lane JR. (2014) Biased agonism at G protein-coupled receptors: the promise and the challenges--a medicinal chemistry perspective. Med Res Rev 34:1286–1330. [DOI] [PubMed] [Google Scholar]
- Siu FY, He M, de Graaf C, Han GW, Yang D, Zhang Z, Zhou C, Xu Q, Wacker D, Joseph JS, et al. (2013) Structure of the human glucagon class B G-protein-coupled receptor. Nature 499:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund G, Hussain MA, Holz GG. (2000) Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes 49:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop KW, Willard FS, Brenner MB, Ficorilli J, Valasek K, Showalter AD, Farb TB, Cao JX, Cox AL, Michael MD, et al. (2010) Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes 59:3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale ST. (2010) Luciferase assay. Cold Spring Harb Protoc 2010:pdb.prot5421. [DOI] [PubMed] [Google Scholar]
- Song WJ, Seshadri M, Ashraf U, Mdluli T, Mondal P, Keil M, Azevedo M, Kirschner LS, Stratakis CA, Hussain MA. (2011) Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab 13:308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, Imamura T, Yoshizaki T, Babendure JL, Lu JC, Olefsky JM. (2008) Beta-arrestin-1 mediates glucagon-like peptide-1 signaling to insulin secretion in cultured pancreatic beta cells. Proc Natl Acad Sci USA 105:6614–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza F, Freeby M, Hultman K, Simpson N, Herron A, Witkowsky P, Liu E, Maffei A, Harris PE. (2006) Current progress in non-invasive imaging of beta cell mass of the endocrine pancreas. Curr Med Chem 13:2761–2773. [DOI] [PubMed] [Google Scholar]
- St Onge EL, Miller SA. (2010) Albiglutide: a new GLP-1 analog for the treatment of type 2 diabetes. Expert Opin Biol Ther 10:801–806. [DOI] [PubMed] [Google Scholar]
- Steensgaard DB, Thomsen JK, Olsen HB, Knudsen LB. (2008) The molecular basis for the delayed absorption of the once-daily human GLP-1 analoge, liraglutide. Diabetes 57 (Suppl 1):A164. [Google Scholar]
- Stumvoll M, Fritsche A, Häring HU. (2002) Clinical characterization of insulin secretion as the basis for genetic analyses. Diabetes 51 (Suppl 1):S122–S129. [DOI] [PubMed] [Google Scholar]
- Su H, He M, Li H, Liu Q, Wang J, Wang Y, Gao W, Zhou L, Liao J, Young AA, et al. (2008) Boc5, a non-peptidic glucagon-like peptide-1 receptor agonist, invokes sustained glycemic control and weight loss in diabetic mice. PLoS One 3:e2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Pinzon WL, Power RF, Yan Y, Wasserfall C, Atkinson M, Rabinovitch A. (2008) Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 57:3281–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Wu S, Wang J, Guo S, Chai S, Yang Z, Li L, Zhang Y, Ji L, Zhan S. (2015) Effect of glucagon-like peptide-1 receptor agonists on lipid profiles among type 2 diabetes: a systematic review and network meta-analysis. Clin Ther 37:225-241.e8. [DOI] [PubMed] [Google Scholar]
- Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, et al. (2011) Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int 31:1285–1297. [DOI] [PubMed] [Google Scholar]
- Syme CA, Zhang L, Bisello A. (2006) Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Mol Endocrinol 20:3400–3411. [DOI] [PubMed] [Google Scholar]
- Szayna M, Doyle ME, Betkey JA, Holloway HW, Spencer RG, Greig NH, Egan JM. (2000) Exendin-4 decelerates food intake, weight gain, and fat deposition in Zucker rats. Endocrinology 141:1936–1941. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Yamada T, Tooyama I, Moroo I, Kimura H, Yamamoto T, Okada H. (1996) Insulin receptor mRNA in the substantia nigra in Parkinson’s disease. Neurosci Lett 204:201–204. [DOI] [PubMed] [Google Scholar]
- Takhar S, Gyomorey S, Su RC, Mathi SK, Li X, Wheeler MB. (1996) The third cytoplasmic domain of the GLP-1[7-36 amide] receptor is required for coupling to the adenylyl cyclase system. Endocrinology 137:2175–2178. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Göke R, Fink-Jensen A, Jessop DS, Møller M, Sheikh SP. (1996) Central administration of GLP-1-(7-36) amide inhibits food and water intake in rats. Am J Physiol 271:R848–R856. [DOI] [PubMed] [Google Scholar]
- Tang-Christensen M, Vrang N, Larsen PJ. (1998) Glucagon-like peptide 1(7-36) amide’s central inhibition of feeding and peripheral inhibition of drinking are abolished by neonatal monosodium glutamate treatment. Diabetes 47:530–537. [DOI] [PubMed] [Google Scholar]
- Teng M, Johnson MD, Thomas C, Kiel D, Lakis JN, Kercher T, Aytes S, Kostrowicki J, Bhumralkar D, Truesdale L, et al. (2007) Small molecule ago-allosteric modulators of the human glucagon-like peptide-1 (hGLP-1) receptor. Bioorg Med Chem Lett 17:5472–5478. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Miyamoto N, Yatomi K, Tanaka Y, Oishi H, Arai H, Hattori N, Urabe T. (2011) Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 31:1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Kanamarlapudi V. (2014) The regions within the N-terminus critical for human glucagon like peptide-1 receptor (hGLP-1R) cell surface expression. Sci Rep 4:7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A, Kanamarlapudi V. (2015) Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem Pharmacol 93:72–84. [DOI] [PubMed] [Google Scholar]
- Thorens B. (1992) Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA 89:8641–8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Porret A, Bühler L, Deng SP, Morel P, Widmann C. (1993) Cloning and functional expression of the human islet GLP-1 receptor: demonstration that exendin-4 is an agonist and exendin-(9-39) an antagonist of the receptor. Diabetes 42:1678–1682. [DOI] [PubMed] [Google Scholar]
- Thornton K, Gorenstein DG. (1994) Structure of glucagon-like peptide (7-36) amide in a dodecylphosphocholine micelle as determined by 2D NMR. Biochemistry 33:3532–3539. [DOI] [PubMed] [Google Scholar]
- Tibaduiza EC, Chen C, Beinborn M. (2001) A small molecule ligand of the glucagon-like peptide 1 receptor targets its amino-terminal hormone binding domain. J Biol Chem 276:37787–37793. [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, Michelsen BK, Holst JJ. (2001) Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86:3717–3723. [DOI] [PubMed] [Google Scholar]
- Toft-Nielson M, Madsbad S, Holst JJ. (1996) The effect of glucagon-like peptide I (GLP-I) on glucose elimination in healthy subjects depends on the pancreatic glucoregulatory hormones. Diabetes 45:552–556. [DOI] [PubMed] [Google Scholar]
- Toft-Nielsen MB, Madsbad S, Holst JJ. (1999) Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care 22:1137–1143. [DOI] [PubMed] [Google Scholar]
- Tokuyama Y, Matsui K, Egashira T, Nozaki O, Ishizuka T, Kanatsuka A. (2004) Five missense mutations in glucagon-like peptide 1 receptor gene in Japanese population. Diabetes Res Clin Pract 66:63–69. [DOI] [PubMed] [Google Scholar]
- Tomkin GH. (2009) Albiglutide, an albumin-based fusion of glucagon-like peptide 1 for the potential treatment of type 2 diabetes. Curr Opin Mol Ther 11:579–588. [PubMed] [Google Scholar]
- Tornehave D, Kristensen P, Rømer J, Knudsen LB, Heller RS. (2008) Expression of the GLP-1 receptor in mouse, rat, and human pancreas. J Histochem Cytochem 56:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. (2003) Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J 369:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo JM, Nuffer W. (2014) GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy 34:1174–1186. [DOI] [PubMed] [Google Scholar]
- Trujillo JM, Nuffer W, Ellis SL. (2015) GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 6:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, et al. (1996) A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379:69–72. [DOI] [PubMed] [Google Scholar]
- Udelsman R, Zhang Y. (2014) The epidemic of thyroid cancer in the United States: the role of endocrinologists and ultrasounds. Thyroid 24:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueberberg S, Meier JJ, Waengler C, Schechinger W, Dietrich JW, Tannapfel A, Schmitz I, Schirrmacher R, Köller M, Klein HH, et al. (2009) Generation of novel single-chain antibodies by phage-display technology to direct imaging agents highly selective to pancreatic β- or α-cells in vivo. Diabetes 58:2324–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R, Reedtz-Runge S. (2010) Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem 285:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood CR, Knudsen LB, Garibay PW, Peters GH, Reedtz-Runge S. (2013) Development of a cysteine-deprived and C-terminally truncated GLP-1 receptor. Peptides 49:100–108. [DOI] [PubMed] [Google Scholar]
- Underwood CR, Knudsen SM, Wulff BS, Brauner-Osborne H, Lau J, Knudsen LB, Peters GH, Reedtz-Runge S. (2011) Transmembrane alpha-helix 2 and 7 are important for small molecule-mediated activation of the GLP-1 receptor. Pharmacology 88:340–348. [DOI] [PubMed] [Google Scholar]
- Unson CG, Gurzenda EM, Iwasa K, Merrifield RB. (1989) Glucagon antagonists: contribution to binding and activity of the amino-terminal sequence 1-5, position 12, and the putative alpha-helical segment 19-27. J Biol Chem 264:789–794. [PubMed] [Google Scholar]
- Ussher JR, Drucker DJ. (2014) Cardiovascular actions of incretin-based therapies. Circ Res 114:1788–1803. [DOI] [PubMed] [Google Scholar]
- van Genugten RE, Möller-Goede DL, van Raalte DH, Diamant M. (2013) Extra-pancreatic effects of incretin-based therapies: potential benefit for cardiovascular-risk management in type 2 diabetes. Diabetes Obes Metab 15:593–606. [DOI] [PubMed] [Google Scholar]
- Vázquez P, Roncero I, Blázquez E, Alvarez E. (2005) The cytoplasmic domain close to the transmembrane region of the glucagon-like peptide-1 receptor contains sequence elements that regulate agonist-dependent internalisation. J Endocrinol 186:221–231. [DOI] [PubMed] [Google Scholar]
- Velez M, Peterson EL, Wells K, Swadia T, Sabbah HN, Williams LK, Lanfear DE. (2015) Association of antidiabetic medications targeting the glucagon-like peptide 1 pathway and heart failure events in patients with diabetes. J Card Fail 21:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. (2013) Molecular signatures of G-protein-coupled receptors. Nature 494:185–194. [DOI] [PubMed] [Google Scholar]
- Verdich C, Flint A, Gutzwiller JP, Näslund E, Beglinger C, Hellström PM, Long SJ, Morgan LM, Holst JJ, Astrup A. (2001) A meta-analysis of the effect of glucagon-like peptide-1 (7-36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab 86:4382–4389. [DOI] [PubMed] [Google Scholar]
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. (2009) Diabetes and cancer. Endocr Relat Cancer 16:1103–1123. [DOI] [PubMed] [Google Scholar]
- Vila Petroff MG, Egan JM, Wang X, Sollott SJ. (2001) Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res 89:445–452. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Agersø H, Krarup T, Holst JJ. (2003) Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88:220–224. [DOI] [PubMed] [Google Scholar]
- Vilsbøll T, Holst JJ. (2004) Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia 47:357–366. [DOI] [PubMed] [Google Scholar]
- Vodovozova EL. (2007) Photoaffinity labeling and its application in structural biology. Biochemistry 72:1–20. [DOI] [PubMed] [Google Scholar]
- Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs A, Jay PY, Hruz PW. (2011) Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PLoS One 6:e17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin E, von Heijne G. (1995) Properties of N-terminal tails in G-protein coupled receptors: a statistical study. Protein Eng 8:693–698. [DOI] [PubMed] [Google Scholar]
- Wang X, Cahill CM, Piñeyro MA, Zhou J, Doyle ME, Egan JM. (1999) Glucagon-like peptide-1 regulates the beta cell transcription factor, PDX-1, in insulinoma cells. Endocrinology 140:4904–4907. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lomakin A, Kanai S, Alex R, Benedek GB. (2015) Transformation of oligomers of lipidated peptide induced by change in pH. Mol Pharm 12:411–419. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou J, Doyle ME, Egan JM. (2001) Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology 142:1820–1827. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kawai K, Ohashi S, Yokota C, Suzuki S, Yamashita K. (1994) Structure-activity relationships of glucagon-like peptide-1(7-36)amide: insulinotropic activities in perfused rat pancreases, and receptor binding and cyclic AMP production in RINm5F cells. J Endocrinol 140:45–52. [DOI] [PubMed] [Google Scholar]
- Wei Y, Mojsov S. (1995) Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett 358:219–224. [DOI] [PubMed] [Google Scholar]
- Werner U, Haschke G, Herling AW, Kramer W. (2010) Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept 164:58–64. [DOI] [PubMed] [Google Scholar]
- Weston C, Poyner D, Patel V, Dowell S, Ladds G. (2014) Investigating G protein signalling bias at the glucagon-like peptide-1 receptor in yeast. Br J Pharmacol 171:3651–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettergren A, Maina P, Boesby S, Holst JJ. (1997) Glucagon-like peptide-1 7-36 amide and peptide YY have additive inhibitory effect on gastric acid secretion in man. Scand J Gastroenterol 32:552–555. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Petersen H, Orskov C, Christiansen J, Sheikh SP, Holst JJ. (1994) Glucagon-like peptide-1 7-36 amide and peptide YY from the L-cell of the ileal mucosa are potent inhibitors of vagally induced gastric acid secretion in man. Scand J Gastroenterol 29:501–505. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Pridal L, Wøjdemann M, Holst JJ. (1998) Amidated and non-amidated glucagon-like peptide-1 (GLP-1): non-pancreatic effects (cephalic phase acid secretion) and stability in plasma in humans. Regul Pept 77:83–87. [DOI] [PubMed] [Google Scholar]
- Wettergren A, Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. (1993) Truncated GLP-1 (proglucagon 78-107-amide) inhibits gastric and pancreatic functions in man. Dig Dis Sci 38:665–673. [DOI] [PubMed] [Google Scholar]
- Wheeler MB, Gelling RW, McIntosh CH, Georgiou J, Brown JC, Pederson RA. (1995) Functional expression of the rat pancreatic islet glucose-dependent insulinotropic polypeptide receptor: ligand binding and intracellular signaling properties. Endocrinology 136:4629–4639. [DOI] [PubMed] [Google Scholar]
- Wheeler MB, Lu M, Dillon JS, Leng XH, Chen C, Boyd AE., 3rd (1993) Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology 133:57–62. [DOI] [PubMed] [Google Scholar]
- Wicki A, Wild D, Storch D, Seemayer C, Gotthardt M, Behe M, Kneifel S, Mihatsch MJ, Reubi J-C, Mäcke HR, et al. (2007) [Lys40(Ahx-DTPA-111In)NH2]-Exendin-4 is a highly efficient radiotherapeutic for glucagon-like peptide-1 receptor-targeted therapy for insulinoma. Clin Cancer Res 13:3696–3705. [DOI] [PubMed] [Google Scholar]
- Widmann C, Bürki E, Dolci W, Thorens B. (1994) Signal transduction by the cloned glucagon-like peptide-1 receptor: comparison with signaling by the endogenous receptors of beta cell lines. Mol Pharmacol 45:1029–1035. [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B. (1995) Agonist-induced internalization and recycling of the glucagon-like peptide-1 receptor in transfected fibroblasts and in insulinomas. Biochem J 310:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B. (1996a) Heterologous desensitization of the glucagon-like peptide-1 receptor by phorbol esters requires phosphorylation of the cytoplasmic tail at four different sites. J Biol Chem 271:19957–19963. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B. (1996b) Desensitization and phosphorylation of the glucagon-like peptide-1 (GLP-1) receptor by GLP-1 and 4-phorbol 12-myristate 13-acetate. Mol Endocrinol 10:62–75. [DOI] [PubMed] [Google Scholar]
- Widmann C, Dolci W, Thorens B. (1997) Internalization and homologous desensitization of the GLP-1 receptor depend on phosphorylation of the receptor carboxyl tail at the same three sites. Mol Endocrinol 11:1094–1102. [DOI] [PubMed] [Google Scholar]
- Wild D, Béhé M, Wicki A, Storch D, Waser B, Gotthardt M, Keil B, Christofori G, Reubi JC, Mäcke HR. (2006) [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J Nucl Med 47:2025–2033. [PubMed] [Google Scholar]
- Wild D, Wicki A, Mansi R, Béhé M, Keil B, Bernhardt P, Christofori G, Ell PJ, Mäcke HR. (2010) Exendin-4-based radiopharmaceuticals for glucagonlike peptide-1 receptor PET/CT and SPECT/CT. J Nucl Med 51:1059–1067. [DOI] [PubMed] [Google Scholar]
- Wildhage I, Trusheim H, Göke B, Lankat-Buttgereit B. (1999) Gene expression of the human glucagon-like peptide-1 receptor is regulated by Sp1 and Sp3. Endocrinology 140:624–631. [DOI] [PubMed] [Google Scholar]
- Willard FS, Bueno AB, Sloop KW. (2012a) Small molecule drug discovery at the glucagon-like peptide-1 receptor. Exp Diabetes Res 2012:709893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Wootten D, Showalter AD, Savage EE, Ficorilli J, Farb TB, Bokvist K, Alsina-Fernandez J, Furness SG, Christopoulos A, et al. (2012b) Small molecule allosteric modulation of the glucagon-like peptide-1 receptor enhances the insulinotropic effect of oxyntomodulin. Mol Pharmacol 82:1066–1073. [DOI] [PubMed] [Google Scholar]
- Wilmen A, Göke B, Göke R. (1996) The isolated N-terminal extracellular domain of the glucagon-like peptide-1 (GLP)-1 receptor has intrinsic binding activity. FEBS Lett 398:43–47. [DOI] [PubMed] [Google Scholar]
- Wilmen A, Van Eyll B, Göke B, Göke R. (1997) Five out of six tryptophan residues in the N-terminal extracellular domain of the rat GLP-1 receptor are essential for its ability to bind GLP-1. Peptides 18:301–305. [DOI] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. (1996) Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol 16:5546–5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman T, Williston S, Brandts JF, Lin LN. (1989) Rapid measurement of binding constants and heats of binding using a new titration calorimeter. Anal Biochem 179:131–137. [DOI] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Sexton PM. (2013a) Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov 12:630–644. [DOI] [PubMed] [Google Scholar]
- Wootten D, Savage EE, Valant C, May LT, Sloop KW, Ficorilli J, Showalter AD, Willard FS, Christopoulos A, Sexton PM. (2012) Allosteric modulation of endogenous metabolites as an avenue for drug discovery. Mol Pharmacol 82:281–290. [DOI] [PubMed] [Google Scholar]
- Wootten D, Savage EE, Willard FS, Bueno AB, Sloop KW, Christopoulos A, Sexton PM. (2013b) Differential activation and modulation of the glucagon-like peptide-1 receptor by small molecule ligands. Mol Pharmacol 83:822–834. [DOI] [PubMed] [Google Scholar]
- Wootten D, Simms J, Koole C, Woodman OL, Summers RJ, Christopoulos A, Sexton PM. (2011) Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids. J Pharmacol Exp Ther 336:540–550. [DOI] [PubMed] [Google Scholar]
- Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM. (2013c) Polar transmembrane interactions drive formation of ligand-specific and signal pathway-biased family B G protein-coupled receptor conformations. Proc Natl Acad Sci USA 110:5211–5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Reynolds CA, Koole C, Smith KJ, Mobarec JC, Simms J, Quon T, Coudrat T, Furness SG, Miller LJ, et al. (2016) A hydrogen-bonded polar network in the core of the glucagon-like peptide-1 receptor is a fulcrum for biased agonism: lessons from class B crystal structures. Mol Pharmacol 89:335–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Todorov I, Li L, Bading JR, Li Z, Nair I, Ishiyama K, Colcher D, Conti PE, Fraser SE, et al. (2011) In vivo imaging of transplanted islets with 64Cu-DO3A-VS-Cys40-Exendin-4 by targeting GLP-1 receptor. Bioconjug Chem 22:1587–1594. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Giguere J, Parisien M, Jeng W, St-Pierre SA, Brubaker PL, Wheeler MB. (2001) Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry 40:2860–2869. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Jeng W, Wheeler MB. (2000) Characterization of glucagon-like peptide-1 receptor-binding determinants. J Mol Endocrinol 25:321–335. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Guo J, Candelore MR, Liang R, Miller C, Dallas-Yang Q, Jiang G, McCann PE, Qureshi SA, Tong X, et al. (2012) Discovery of a novel glucagon receptor antagonist N-[(4-(1S)-1-[3-(3, 5-dichlorophenyl)-5-(6-methoxynaphthalen-2-yl)-1H-pyrazol-1-yl]ethylphenyl)carbonyl]-β-alanine (MK-0893) for the treatment of type II diabetes. J Med Chem 55:6137–6148. [DOI] [PubMed] [Google Scholar]
- Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. (2006) GLP-1/exendin-4 facilitates β-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract 73:107–110. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N. (2008) The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology 149:574–579. [DOI] [PubMed] [Google Scholar]
- Yang D, de Graaf C, Yang L, Song G, Dai A, Cai X, Feng Y, Reedtz-Runge S, Hanson MA, Yang H, et al. (2016) Structural determinants of binding the seven-transmembrane domain of the glucagon-like peptide-1 receptor. J Biol Chem 291:12991-13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Yang D, de Graaf C, Moeller A, West GM, Dharmarajan V, Wang C, Siu FY, Song G, Reedtz-Runge S, et al. (2015b) Conformational states of the full-length glucagon receptor. Nat Commun 6:7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DH, Zhou CH, Liu Q, Wang MW. (2015a) Landmark studies on the glucagon subfamily of GPCRs: from small molecule modulators to a crystal structure. Acta Pharmacol Sin 36:1033–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqub T, Tikhonova IG, Lättig J, Magnan R, Laval M, Escrieut C, Boulègue C, Hewage C, Fourmy D. (2010) Identification of determinants of glucose-dependent insulinotropic polypeptide receptor that interact with N-terminal biologically active region of the natural ligand. Mol Pharmacol 77:547–558. [DOI] [PubMed] [Google Scholar]
- Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, Denaro M. (1999) Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta). Diabetes 48:1026–1034. [DOI] [PubMed] [Google Scholar]
- Yusta B, Baggio LL, Estall JL, Koehler JA, Holland DP, Li H, Pipeleers D, Ling Z, Drucker DJ. (2006) GLP-1 receptor activation improves beta cell function and survival following induction of endoplasmic reticulum stress. Cell Metab 4:391–406. [DOI] [PubMed] [Google Scholar]
- Zaccardi F, Htike ZZ, Webb DR, Khunti K, Davies MJ. (2016) Benefits and harms of once-weekly glucagon-like peptide-1 receptor agonist treatments: a systematic review and network meta-analysis. Ann Intern Med 164:102–113. [DOI] [PubMed] [Google Scholar]
- Zander M, Madsbad S, Madsen JL, Holst JJ. (2002) Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359:824–830. [DOI] [PubMed] [Google Scholar]
- Zheng H, Cai L, Rinaman L. (2015) Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Struct Funct 220:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. (2006) Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317:1106–1113. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wei R, Wang L, Tian Q, Tao M, Ke J, Liu Y, Hou W, Zhang L, Yang J, et al. (2014) Activation of glucagon-like peptide-1 receptor inhibits growth and promotes apoptosis of human pancreatic cancer cells in a cAMP-dependent manner. Am J Physiol Endocrinol Metab 306:E1431–E1441. [DOI] [PubMed] [Google Scholar]
- Zhou FX, Cocco MJ, Russ WP, Brunger AT, Engelman DM. (2000) Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat Struct Biol 7:154–160. [DOI] [PubMed] [Google Scholar]
- Zunz E, LaBarre J. (1928) Hyperinsulinémie consécutive a l’injection de solution de secrétine non hypotensive. C R Soc Biol 98:1435–1438. [Google Scholar]
- Zwier JM, Roux T, Cottet M, Durroux T, Douzon S, Bdioui S, Gregor N, Bourrier E, Oueslati N, Nicolas L, et al. (2010) A fluorescent ligand-binding alternative using Tag-lite® technology. J Biomol Screen 15:1248–1259. [DOI] [PubMed] [Google Scholar]