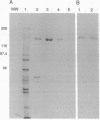
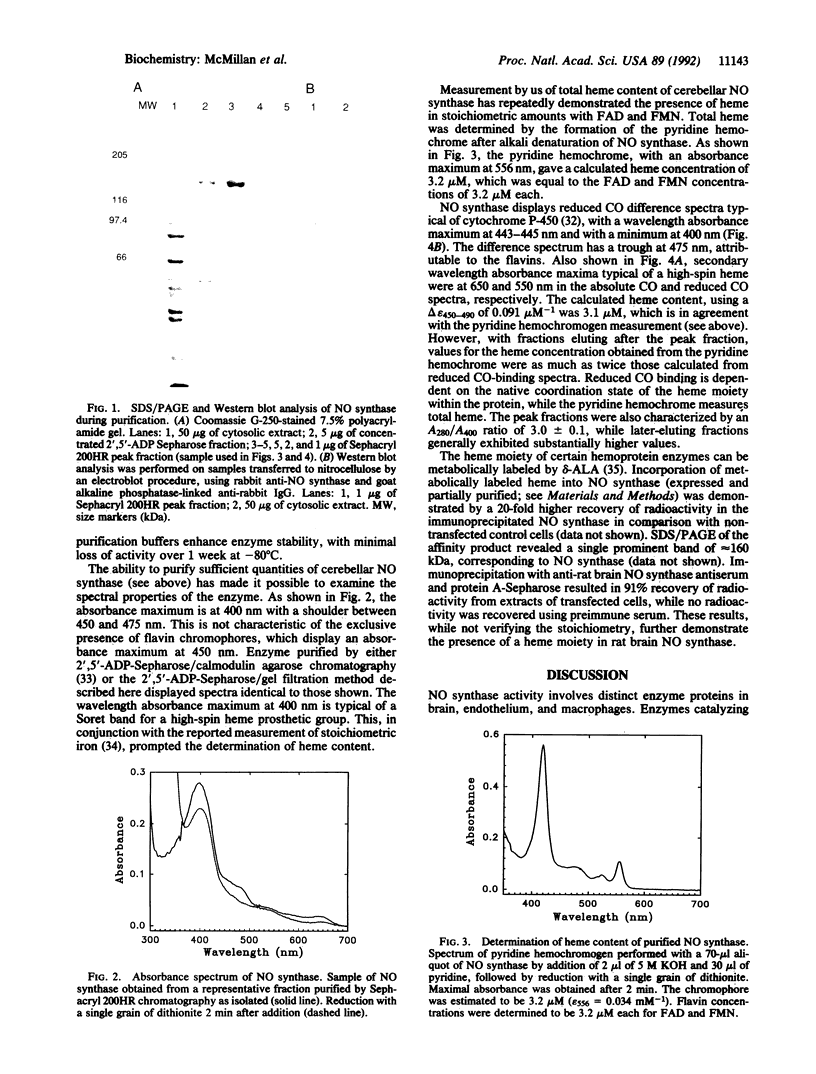
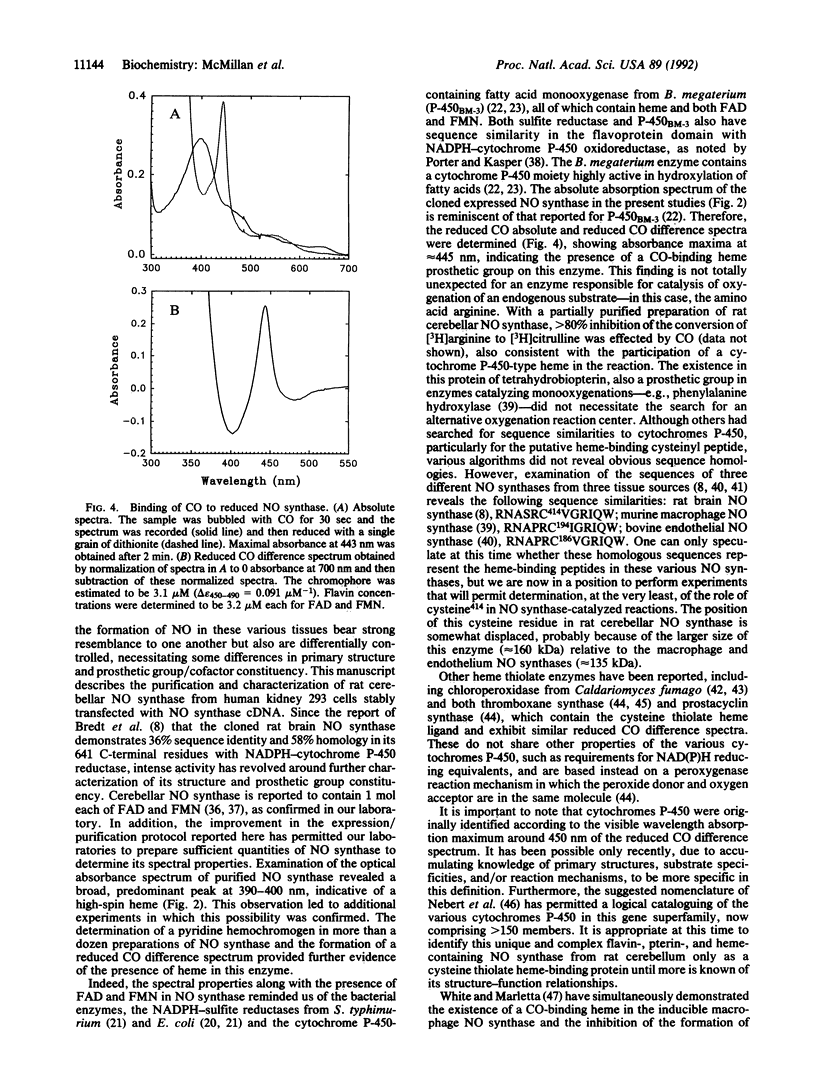
Abstract
The endogenous formation of nitric oxide (NO) has become an area of intense interest as evidence for its biological functions has been obtained in three distinct tissues: circulating macrophages, in which it exerts cytotoxic effects; blood vessels, in which it has been identified as endothelium-derived relaxing factor; and neuronal cells, in which it functions as a neurotransmitter. The formation of NO in brain extracts has been shown to be catalyzed by an enzyme, termed NO synthase, which generates the NO responsible for stimulation of cGMP formation, the highest levels of which occur in the cerebellum. NO synthase catalyzes the formation of citrulline from arginine with the coincident production of NO and has been shown to be a flavoprotein, containing 1 mol each of FAD and FMN, tetrahydrobiopterin, and iron. It is also reported to contain an alpha-helical, calmodulin-binding consensus sequence consistent with its stimulation by calmodulin in the presence of Ca2+. The formation of NO requires incorporation of one of the atoms of molecular oxygen into one of the guanidinium nitrogen atoms of arginine with the coincident formation of citrulline. This communication reports that rat cerebellar NO synthase, cloned and stably expressed in human kidney 293 cells, contains heme in amounts stoichiometric with the flavins FAD and FMN as evidenced by the appearance of a pyridine hemochrome and a reduced CO difference spectrum with an absorbance maximum at approximately 445 nm. The finding of a CO-binding heme moiety explains the presence of iron in the enzyme and suggests a role for prosthetic heme as an oxygenase reaction center. This report also presents evidence for incorporation of delta-[14C]aminolevulinate specifically into immunoprecipitable NO synthase in stably transfected human kidney 293 cells but not in nontransfected cells. Simultaneously, K. A. White and M. A. Marletta [(1992) Biochemistry 31, 6627-6631] have demonstrated a CO-binding heme prosthetic group in purified murine macrophage NO synthase and have suggested the identity of these reaction centers in both the constitutive (cerebellar) and inducible (macrophage) forms of NO synthase.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billiar T. R., Curran R. D., Stuehr D. J., West M. A., Bentz B. G., Simmons R. L. An L-arginine-dependent mechanism mediates Kupffer cell inhibition of hepatocyte protein synthesis in vitro. J Exp Med. 1989 Apr 1;169(4):1467–1472. doi: 10.1084/jem.169.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate-linked enhancement of cGMP levels in the cerebellum. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9030–9033. doi: 10.1073/pnas.86.22.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenais B., Yapo A., Lepoivre M., Tenu J. P. High-performance liquid chromatographic analysis of the unusual pathway of oxidation of L-arginine to citrulline and nitric oxide in mammalian cells. J Chromatogr. 1991 Feb 22;539(2):433–441. doi: 10.1016/s0021-9673(01)83952-2. [DOI] [PubMed] [Google Scholar]
- Curran R. D., Billiar T. R., Stuehr D. J., Hofmann K., Simmons R. L. Hepatocytes produce nitrogen oxides from L-arginine in response to inflammatory products of Kupffer cells. J Exp Med. 1989 Nov 1;170(5):1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Gardner L. C., Cox T. M. Biosynthesis of heme in immature erythroid cells. The regulatory step for heme formation in the human erythron. J Biol Chem. 1988 May 15;263(14):6676–6682. [PubMed] [Google Scholar]
- Hauschildt S., Lückhoff A., Mülsch A., Kohler J., Bessler W., Busse R. Induction and activity of NO synthase in bone-marrow-derived macrophages are independent of Ca2+. Biochem J. 1990 Sep 1;270(2):351–356. doi: 10.1042/bj2700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hollenberg P. F., Hager L. P. The P-450 nature of the carbon monoxide complex of ferrous chloroperoxidase. J Biol Chem. 1973 Apr 10;248(7):2630–2633. [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas S., Marsden P. A., Li G. K., Tempst P., Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6348–6352. doi: 10.1073/pnas.89.14.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., Marletta M. A. Analytical and preparative high-performance liquid chromatography separation of flavin and flavin analog coenzymes. Anal Biochem. 1980 Nov 15;109(1):87–93. doi: 10.1016/0003-2697(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Mayer B., John M., Heinzel B., Werner E. R., Wachter H., Schultz G., Böhme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett. 1991 Aug 19;288(1-2):187–191. doi: 10.1016/0014-5793(91)81031-3. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., Moncada S. Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem J. 1989 Jul 1;261(1):293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Characterization of a catalytically self-sufficient 119,000-dalton cytochrome P-450 monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1986 Jun 5;261(16):7160–7169. [PubMed] [Google Scholar]
- Narhi L. O., Fulco A. J. Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J Biol Chem. 1987 May 15;262(14):6683–6690. [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Ostrowski J., Barber M. J., Rueger D. C., Miller B. E., Siegel L. M., Kredich N. M. Characterization of the flavoprotein moieties of NADPH-sulfite reductase from Salmonella typhimurium and Escherichia coli. Physicochemical and catalytic properties, amino acid sequence deduced from DNA sequence of cysJ, and comparison with NADPH-cytochrome P-450 reductase. J Biol Chem. 1989 Sep 25;264(27):15796–15808. [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Moncada S. A novel citrulline-forming enzyme implicated in the formation of nitric oxide by vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):348–352. doi: 10.1016/s0006-291x(89)80219-0. [DOI] [PubMed] [Google Scholar]
- Porter T. D. An unusual yet strongly conserved flavoprotein reductase in bacteria and mammals. Trends Biochem Sci. 1991 Apr;16(4):154–158. doi: 10.1016/0968-0004(91)90059-5. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Smith R. M., Nakane M., Murad F. Ca2+/calmodulin-dependent NO synthase type I: a biopteroflavoprotein with Ca2+/calmodulin-independent diaphorase and reductase activities. Biochemistry. 1992 Mar 31;31(12):3243–3249. doi: 10.1021/bi00127a028. [DOI] [PubMed] [Google Scholar]
- Sono M., Hager L. P., Dawson J. H. Electron paramagnetic resonance investigations of exogenous ligand complexes of low-spin ferric chloroperoxidase: further support for endogenous thiolate ligation to the heme iron. Biochim Biophys Acta. 1991 Jul 12;1078(3):351–359. doi: 10.1016/0167-4838(91)90156-t. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich V., Castle L., Weber P. Spectral evidence for the cytochrome P450 nature of prostacyclin synthetase. Biochem Pharmacol. 1981 Jul 15;30(14):2033–2036. doi: 10.1016/0006-2952(81)90218-5. [DOI] [PubMed] [Google Scholar]
- White K. A., Marletta M. A. Nitric oxide synthase is a cytochrome P-450 type hemoprotein. Biochemistry. 1992 Jul 28;31(29):6627–6631. doi: 10.1021/bi00144a001. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]