Abstract
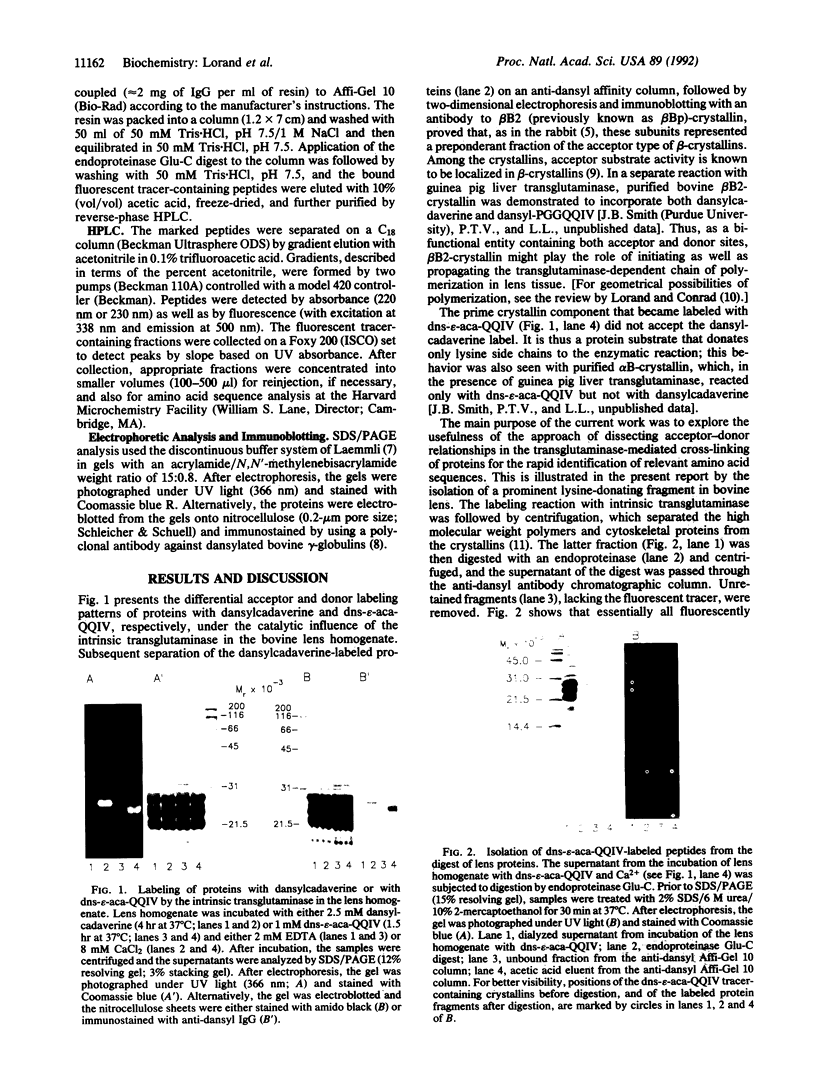
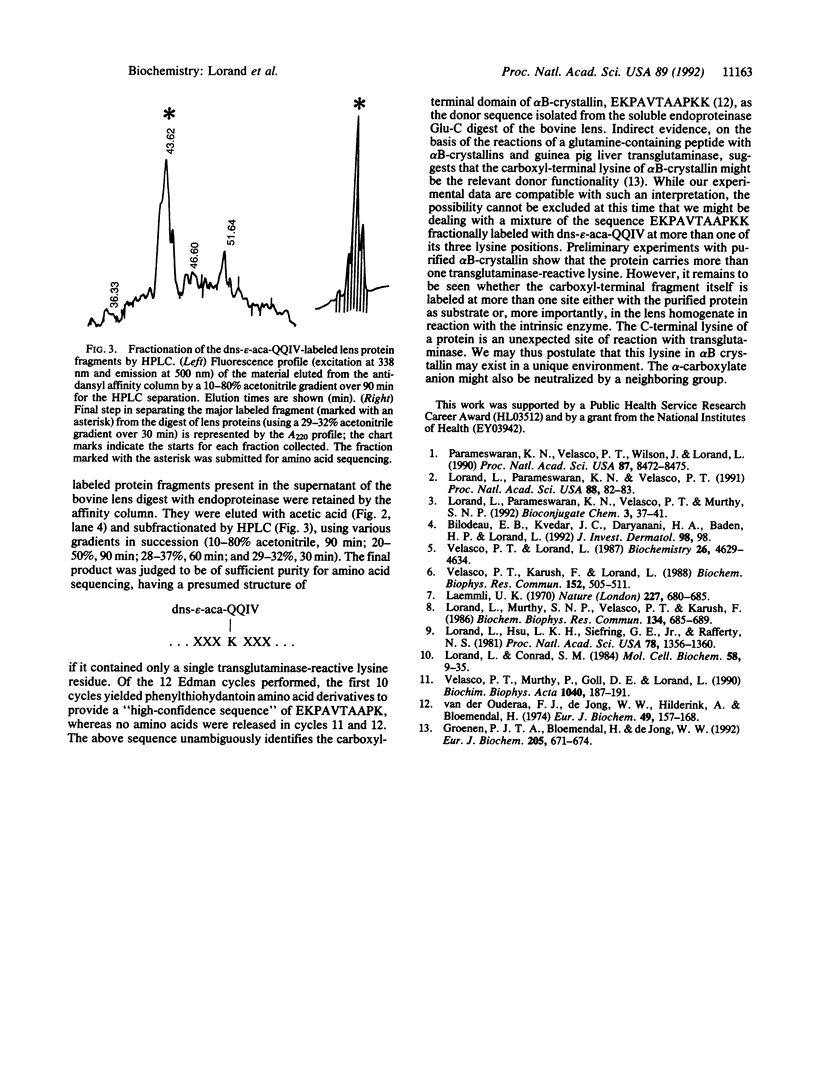
The transglutaminase (protein-glutamine: amine gamma-glutamyltransferase, EC 2.3.2.13)-catalyzed cross-linking of proteins in biological systems can often be inhibited by inclusion of small primary amines or glutamine-containing peptides, which act as site-specific blockers of the relevant acceptor (i.e., glutamine) and donor (i.e., lysine) functionalities of the natural substrates. Compounds such as dansylcadaverine and dansyl-epsilon-aminocaproyl-Gln-Gln-Ile-Val are particularly useful in sorting out acceptor-donor relationships among lens crystallins. Apart from its fluorescent properties, the dansyl hapten offered special advantages as a "handle" for the rapid isolation of transglutaminase targets even in the complex system of lens cortical homogenate. The dansylated peptide was incorporated into bovine lens proteins under the influence of the Ca(2+)-activated intrinsic transglutaminase and, after digestion by endoproteinase Glu-C, the tracer-containing fragments were isolated by affinity chromatography on an anti-dansyl antibody column. The major fluorescent peak was isolated by HPLC and sequenced by Edman degradation, which yielded phenylthiohydantoin amino acid derivatives for the first 10 cycles, EKPAVTAAPK, and none for the next 2. The sequence, corresponding to residues 165-174 of alpha B-crystallin, unambiguously identifies the known carboxyl-terminal domain, EK-PAVTAAPKK, as the prominent lysine-donating fragment in bovine lens.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Groenen P. J., Bloemendal H., de Jong W. W. The carboxy-terminal lysine of alpha B-crystallin is an amine-donor substrate for tissue transglutaminase. Eur J Biochem. 1992 Apr 15;205(2):671–674. doi: 10.1111/j.1432-1033.1992.tb16827.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Conrad S. M. Transglutaminases. Mol Cell Biochem. 1984;58(1-2):9–35. doi: 10.1007/BF00240602. [DOI] [PubMed] [Google Scholar]
- Lorand L., Hsu L. K., Siefring G. E., Jr, Rafferty N. S. Lens transglutaminase and cataract formation. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1356–1360. doi: 10.1073/pnas.78.3.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Murthy S. N., Velasco P. T., Karush F. Identification of transglutaminase substrates in inside-out vesicles from human erythrocytes: immunoblotting with anti-dansyl antibody. Biochem Biophys Res Commun. 1986 Jan 29;134(2):685–689. doi: 10.1016/s0006-291x(86)80474-0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Parameswaran K. N., Velasco P. T., Murthy S. N. Biotinylated peptides containing a factor XIIIa or a tissue transglutaminase-reactive glutaminyl residue that block protein cross-linking phenomena by becoming incorporated into amine donor sites. Bioconjug Chem. 1992 Jan-Feb;3(1):37–41. doi: 10.1021/bc00013a006. [DOI] [PubMed] [Google Scholar]
- Lorand L., Parameswaran K. N., Velasco P. T. Sorting-out of acceptor-donor relationships in the transglutaminase-catalyzed cross-linking of crystallins by the enzyme-directed labeling of potential sites. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):82–83. doi: 10.1073/pnas.88.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parameswaran K. N., Velasco P. T., Wilson J., Lorand L. Labeling of epsilon-lysine crosslinking sites in proteins with peptide substrates of factor XIIIa and transglutaminase. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8472–8475. doi: 10.1073/pnas.87.21.8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ouderaa F. J., De Jong W. W., Hilderink A., Bloemendal H. The amino-acids sequence of the alphaB2 chain of bovine alpha-crystallin. Eur J Biochem. 1974 Nov 1;49(1):157–168. doi: 10.1111/j.1432-1033.1974.tb03821.x. [DOI] [PubMed] [Google Scholar]
- Velasco P. T., Karush F., Lorand L. Transamidating activities of factor XIIIa and of transglutaminases, measured by an ELISA procedure. Biochem Biophys Res Commun. 1988 Apr 29;152(2):505–511. doi: 10.1016/s0006-291x(88)80066-4. [DOI] [PubMed] [Google Scholar]
- Velasco P. T., Lorand L. Acceptor-donor relationships in the transglutaminase-mediated cross-linking of lens beta-crystallin subunits. Biochemistry. 1987 Jul 28;26(15):4629–4634. doi: 10.1021/bi00389a006. [DOI] [PubMed] [Google Scholar]
- Velasco P. T., Murthy P., Goll D. E., Lorand L. Cross-linking and proteolysis in Ca2(+)-treated lens homogenates. Biochim Biophys Acta. 1990 Sep 3;1040(2):187–191. doi: 10.1016/0167-4838(90)90074-p. [DOI] [PubMed] [Google Scholar]