Abstract
In Lyme borreliosis, the skin is the key site for bacterial inoculation by the infected tick and for cutaneous manifestations. We previously showed that different strains of Borrelia burgdorferi sensu stricto isolated from tick and from different clinical stages of the Lyme borreliosis (erythema migrans, and acrodermatitis chronica atrophicans) elicited a very similar transcriptional response in normal human dermal fibroblasts. In this study, using whole transcriptome microarray chips, we aimed to compare the transcriptional response of normal human dermal fibroblasts stimulated by 3 Borrelia burgdorferi sensu lato strains belonging to 3 main pathogenic species (B. afzelii, B. garinii and B. burgdorferi sensu stricto) in order to determine whether “species-related” inflammatory pathways could be identified. The three Borrelia strains tested exhibited similar transcriptional profiles, and no species-specific fingerprint of transcriptional changes in fibroblasts was observed. Conversely, a common core of chemokines/cytokines (CCL2, CXCL1, CXCL2, CXCL6, CXCL10, IL-6, IL-8) and interferon-related genes was stimulated by all the 3 strains. Dermal fibroblasts appear to play a key role in the cutaneous infection with Borrelia, inducing a homogeneous inflammatory response, whichever Borrelia species was involved.
Introduction
Lyme borreliosis is caused by spirochetes of the Borrelia burgdorferi sensu lato (sl) group that are transmitted by Ixodes spp. ticks: the bacteria are inoculated intradermally, multiply locally and eventually spread to various secondary organs throughout the body, especially the skin, the nervous system and the joints, inducing inflammatory lesions [1]. Lyme borreliosis is the most common tick-borne infectious disease in North America [2] and in countries with moderate climates in Eurasia [3]. Whereas B. burgdorferi sensu stricto (ss) is the only main Borrelia species responsible for Lyme borreliosis manifestations in North America, at least 8 species can be pathogenic for humans in Europe, with 5 clearly pathogenic species—B. burgdorferi ss, B. afzelii, B. garinii, B. bavariensis [4] and B. spielmanii [5]—, and 3 rarely, if at all, pathogenic species for humans—B. bissettii [6, 7], B. lusitaniae [8] and B. valaisiana [9]. The erythema migrans (EM), first clinical manifestation of Lyme borreliosis and considered the hallmark of the disease, can be caused by any pathogenic Borrelia species of the B. burgdorferi sl group. In contrast, acrodermatitis chronica atrophicans (ACA) is typically associated with B. afzelii, neurological manifestations most commonly involve B. garinii and arthritis is preferentially related to B. burgdorferi ss [10].
The skin is a key interface in Lyme borreliosis since it represents both the inoculation site of the spirochetes, a potential filter to select specific invasive clones injected at the tick-bite site [11, 12], and also the target of clinical manifestations at various stages of the disease [13]. However, factors determining the limitation of the disease to a benign localised EM or the appearance of systemic manifestations and the way the bacteria escape the local cutaneous immunity, spread, and reach distal target organs are unknown. Thus, understanding the interaction of the bacteria with skin cells is essential. The role of the skin’s immune cells in anti-Borrelia defense mechanisms was addressed in several previous works [14–16], but the skin’s resident cells (keratinocytes and fibroblasts) were also shown to be sensors of danger [13] and to play a key role in the anti-Borrelia response [17, 18]. In particular, we previously showed that Borrelia induces the transcription of numerous inflammatory genes in normal human dermal primary fibroblasts (NHDF) [19], including chemokines and cytokines and many transcription factors and components of the extracellular matrix. As the 3 B. burgdorferi ss strains tested in our model were isolated from different stages of the human infection and elicited very similar transcriptional modulation profiles, we concluded that Borrelia pathotype has little influence on the fibroblasts’ response. Since the 3 strains used in our previous study belonged to the same species, in this new study, we wanted to assess whether the species of Borrelia could influence the transcriptional modulations of fibroblasts. For this purpose, we tested 3 Borrelia strains, all isolated from erythema migrans lesions and belonging to 3 different pathogenic species (B. afzelii, B. garinii and B. burgdorferi ss) in our NHDF in vitro model, and compared the transcriptional profiles induced by the different species using whole transcriptome microarrays.
Materials and Methods
Spirochetes strains
Three different strains of Borrelia representing the 3 major European species were chosen among the collection of clinical isolates maintained in the Institute of Bacteriology of Strasbourg: B. afzelii strain IBS17, B. garinii strain IBS6 and B. burgdorferi ss strain IBS19. These 3 strains were isolated from patients presenting a solitary EM as a unique clinical manifestation (Table 1). Each strain was used at passage ≤ 7, cultured in BSK-H medium (Sigma, Saint Quentin Fallavier, France) at 33°C with 5% CO2, and washed twice before the assays as previously described [19]. Careful washing of Borrelia prior to every coincubation experiment was a prerequisite before every stimulation experiment since, in our preliminary experiments, we observed that the BSK medium by itself induces a nonspecific transcriptional response in human dermal fibroblasts that was significantly higher than that of negative control fibroblasts (data not shown).
Table 1. Origin of the Borrelia strains used in this study.
| n° | Species | Geographical origin, Country | Anatomical origin | Associated symptoms |
|---|---|---|---|---|
| IBS6 | B. garinii | Strasbourg, France | Skin biopsy of EMa | none |
| IBS17 | B. afzelii | La Roche Guyon, France | Skin biopsy of EM | none |
| IBS19 | B. burgdorferi ss | Metz, France | Skin biopsy of EM | none |
a Erythema migrans.
Fibroblast culture and stimulation
Primary human dermal fibroblasts (NHDF, Promocell, Heidelberg, Germany) were maintained in FGM2 medium (detailed characteristics of the fibroblast’s batches are presented in Table 2). To stimulate the cells, fibroblasts were used at passage 5 and seeded at 7.5 x 104 per well in a 24-well plate. At confluence and one day before Borrelia activation, FGM2 medium was replaced by FGM medium without fetal calf serum. If not otherwise stated, fibroblasts were stimulated with B. burgdorferi spirochetes at a multiplicity of infection (MOI) of 100:1 (100 Borrelia per fibroblast) for 24 hours.
Table 2. Characteristics of the fibroblast batches used in this study.
| NHDF | Batch n° | Anatomical origin | Agea | Sexe | Race |
|---|---|---|---|---|---|
| #1 | 3021904 | breast | 20 | female | caucasian |
| #2 | 3022702 | eyelid | 68 | female | caucasian |
| #3 | 10118012 | temple | 70 | male | caucasian |
| #4 | 10215061 | cheek | 56 | female | caucasian |
| #5 | 20612062 | cheek | 60 | male | caucasian |
| #6 | 70711061 | lip | 32 | female | caucasian |
a Age is given in years.
In order to provide evidence that our experimental methods and specifically the coincubation of Borrelia and fibroblasts in a BSK-free medium was compatible with the viability of Borrelia, viability experiments were conducted: Borrelia were washed twice, suspended in FGM medium and either incubated as is (at a concentration of 2.5 x 106 Borrelia per ml), or coincubated with fibroblasts at a MOI of 100:1. Twenty-four hours later, the culture medium (consisting of either Borrelia in FGM medium or Borrelia in FGM medium coincubated with human dermal fibroblasts), was recollected and instilled in BSK medium. We observed that both conditions provided viable Borrelia 96 hours later (data not shown).
IL-8 ELISA
IL-8 secretion levels were measured in culture supernatants of unstimulated and Borrelia-stimulated cells by ELISA. The protocol was based on sandwich techniques, as described by the manufacturer (R&D systems, Lille, France). Experiments of cell stimulation with spirochetes were carried out twice in independent experiments. Results are presented as means ± standard deviations (SDs) of triplicate values and are representative of the two independent experiments.
RNA extraction and semiquantitative real time RT-PCR
After removal of the supernatant, fibroblasts were directly resuspended in Trizol (Invitrogen, Cergy-Pontoise, France) and stored at -80°C until use. RNA extraction was performed with RN easy mini-kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s protocol, including treatment with DNase (Ambion, Courtaboeuf, France). Two μg of total RNA were reverse-transcribed with the Superscript II first-strand synthesis system (Invitrogen, Cergy-Pontoise, France). Semiquantitative reverse transcription PCR (QRT-PCR) was done on an ABI Prism 7000 (Applied Biosystems, Courtaboeuf, France) with specific primers (S1 Table). Expression levels of all transcripts studied were normalised to housekeeping gene level and the relative changes in gene expression were compared with those of untreated cells using the 2-ΔΔCt method. Two housekeeping genes were tested: β-actin and the RNA polymerase II genes [20].
Microarray analysis
Sample generation and DNA microarray hybridisation and analysis
The design of the microarray experiment included 4 different analytical conditions: unstimulated fibroblasts, and fibroblasts stimulated with B. burgdorferi ss IBS19, B. garinii IBS6 and B. afzelii IBS17 strains at MOI 100:1 for 24h. For each analytical condition, a total of 6 biological replicates represented by 6 different batches of human dermal primary fibroblasts (Table 2) were used. A total of 24 biological samples was therefore processed for this microarray experiment.
The HumanHT-12 V4.0 expression BeadChip (Illumina Inc., Cambridge, UK), comprising 47,232 transcripts was used to generate gene expression profiles of unstimulated and Borrelia-stimulated fibroblasts. RNA was extracted using RNeasy Mini kit (Qiagen, Courtaboeuf, France). Each sample contained 50–200 ng RNA, and the quality was assessed with the 2100 Agilent Bioanalyzer. The cRNA was synthesized, amplified and purified using the Illumina TotalPrep RNA Amplification Kit (Ambion, Foster City, CA, USA) following manufacturer recommendations. Briefly, 200 ng of RNA was reverse transcribed. After second strand synthesis, the cDNA was transcribed in vitro and cRNA labelled with biotin-16-UTP. Labelled probe hybridisation to the Beadchips was carried out using Illumina’s protocol. The Beadchips were scanned on the Illumina IScan using Illumina IScan image data acquisition software. Illumina GenomeStudio software was used for preliminary data analysis, data normalisation and quality controls. Raw microarray intensity data was background subtracted and normalised using the “normalise quantiles” function. We used the detection P-values as flags, flag = 0, if P> 0.05 and flag = 1, if P≤ 0.05. Each tested probe list was created after filtering probes flagged ‘1’ for at least half of the chips involved in the comparison. The group comparisons were made using Student’s t-test. We filtered the resulting P-values at 5% and at a 2-fold threshold for functions and pathway analyses. For each particular sample, a given transcript was considered regulated if the mean fold change exceeded 1.2 in comparison with the “unstimulated” condition. The microarray data have been deposited in the GEO repository (http://www.ncbi.nlm.nih.gov/geo/) with the record number GSE77058. Microarray experiments were performed according to the MIAME guidelines [21].
Results
Human dermal fibroblasts stimulated by B. burgdorferi ss IBS19, B. garinii IBS6 and B. afzelii IBS17 secrete IL-8
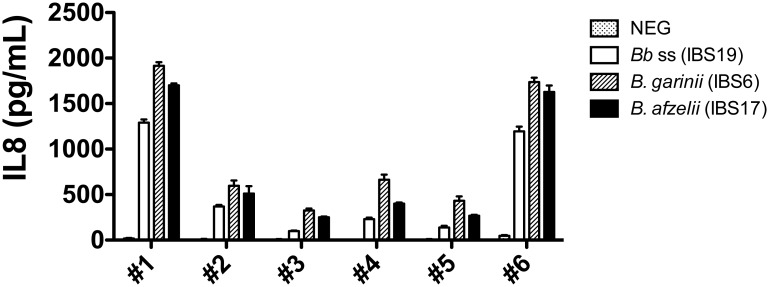
When coincubated with human primary dermal fibroblasts, different B. burgdorferi ss strains were shown to induce a pro-inflammatory response with IL-8 synthesis [18, 19]. To analyze the impact of the Borrelia species in our experimental model, we first measured the IL-8 synthesis by fibroblasts stimulated by B. burgdorferi ss IBS19, B. garinii IBS6 and B. afzelii IBS17. The 6 batches of fibroblasts tested in our study were able to produce the IL-8 chemokine. Even though absolute levels of IL-8 were highly variable between batches of cells tested, the levels of IL-8 synthesis obtained with the 3 Borrelia strains used for stimulation were comparable in each batch of fibroblasts (Fig 1).
Fig 1. Measurement of IL-8 secretion by 6 different batches of fibroblasts coincubated with strains of 3 different species of the B. burgdorferi sl group.
IL-8 secretion of fibroblasts stimulated by B. burgdorferi ss (Bb ss) IBS19, B. garinii IBS6, and B. afzelii IBS17 at MOI of 100:1 after 24h of stimulation. Each bar shows the mean ± SDs of triplicate values (technical replicates) obtained for each batch of fibroblasts—fibroblasts batch #1 to fibroblasts batch #6 (biological replicates). The results are representative of 2 independent experiments.
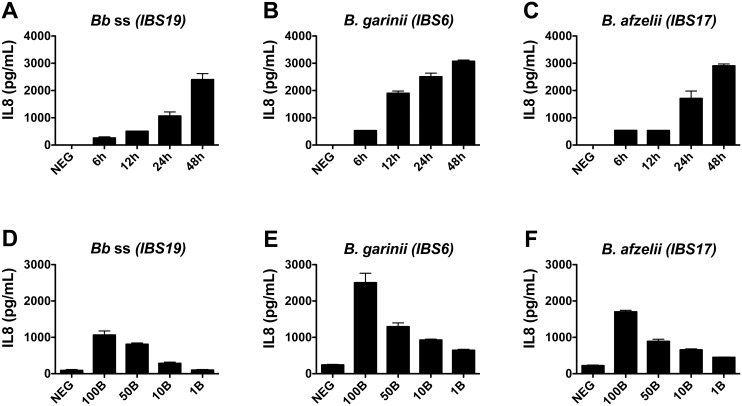
A complete kinetic and titration study was performed for fibroblasts batch #1, and showed that the chemokine was secreted in a dose- and time-dependent manner for each of the 3 tested Borrelia strains (Fig 2). Similar profiles for titration experiments were obtained with fibroblasts expressing lower levels of IL-8 (S1 Fig).
Fig 2. Kinetics and titration of IL-8 secretion by fibroblasts co-incubated with different species of the B. burgdorferi sl group.
(A-C) Kinetic studies of IL-8 secretion of fibroblasts stimulated by B. burgdorferi ss (Bb ss) IBS19, B. garinii IBS6, and B. afzelii IBS17 at MOI of 100:1. (D-F) Levels of IL-8 secretion by fibroblasts stimulated by increasing concentrations (MOI of 1:1 = 1B, MOI of 10:1 = 10B, 50:1 = 50B, and 100:1 = 100B) of the 3 Borrelia strains at 24 hours. NEG: unstimulated fibroblasts. (A-F) Each bar shows the mean ± SDs of duplicate values obtained for fibroblasts batch #1.
Global fibroblast transcriptional responses to B. burgdorferi ss IBS19, B. garinii IBS6 and B. afzelii IBS17
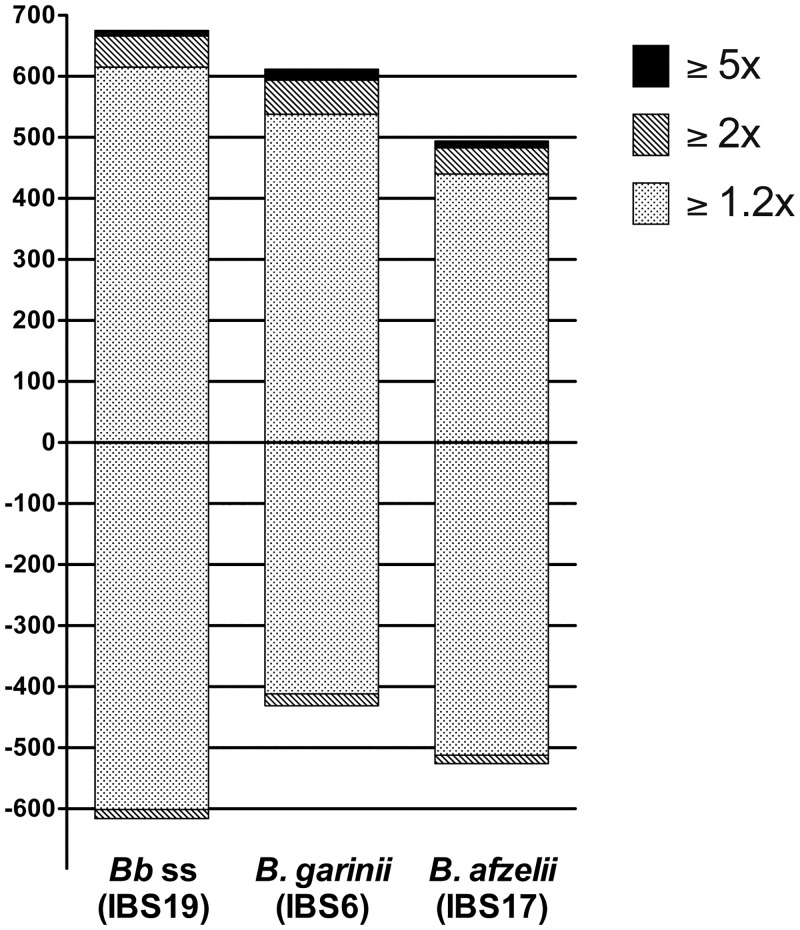
Transcriptional modifications were studied by the Human HT12 BeadChip microarray, allowing whole transcriptome analysis. Statistical analysis was then performed after normalization of the raw results obtained for the 6 tested biological replicates (fibroblasts batch #1 to fibroblasts batch #6) and the 4 analytical conditions (unstimulated, B. burgdorferi ss IBS19-stimulated, B. garinii IBS6-stimulated and B. afzelii IBS17-stimulated) by comparing Borrelia-stimulated vs unstimulated samples. Out of 47,231 transcripts present on the chip, 1,968 transcripts (4.2%) were significantly regulated (≥1.2 fold vs unstimulated fibroblasts) by at least one the 3 tested strains. Among them, 1,291 transcripts were differentially regulated by B. burgdorferi ss IBS19 (675 up- and 616 down-regulated), 1,043 by B. garinii IBS6 (612 up- and 431 down-regulated), and 1,020 by B. afzelii IBS17 (494 up- and 526 down-regulated). The majority of these transcripts were regulated with a fold-change ranging from 1.2 to 2 for all 3 tested strains (Fig 3).
Fig 3. Gene expression profiles obtained from human fibroblasts stimulated with the three species of the B. burgdorferi sl group.
Number of genes differentially expressed during fibroblast stimulation with Borrelia. The bars reflect the number of up-regulated genes (+) and down-regulated genes (-) for each strain. The light dotted areas correspond to gene expression changes of 1.2–2.0-fold, the gray hatched areas correspond to changes of 2.0–5.0-fold and black areas represent changes ≥5.0-fold.
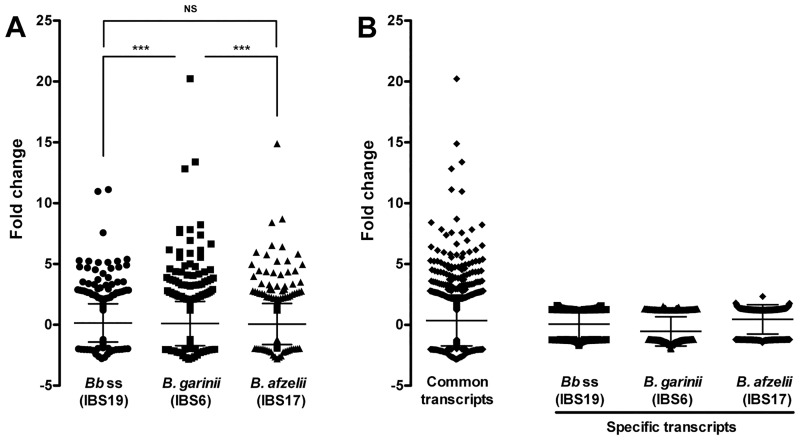
The global Borrelia-induced transcriptional modulation in fibroblasts was relatively similar in the 3 tested strains (similar number of up- and down-regulated genes) (Fig 3). Moreover, analyses of regulated genes showed that almost all the genes with high transcription modulation (≥2 fold vs unstimulated fibroblasts) were consistently stimulated by the 3 tested species (Fig 4B). The genes induced by only one of the tested species displayed mild transciptional modulations (between 1.2 and 2 fold vs unstimulated fibroblasts). However, it is noteworthy that the global level of modification induced by B. garinii IBS6 appeared significantly higher than that of B. afzelii IBS17 (P = 0.0007) and B. burgdorferi ss IBS19 (P = 0.0001), while the amplitude of activation induced by B. afzelii IBS17 and B. burgdorferi ss IBS19 did not statistically differ (Fig 4A).
Fig 4. Intensity of transcriptional modifications.
The distribution of regulated genes according to the level of regulation is expressed as fold-change in comparison to unstimulated cells. (A) Comparison of transcription levels between Borrelia strains. *** P <0.001, NS: not significant, tested by ANOVA using GraphPad Prism6 software (GraphPad, San Diego, CA). (B) Transcription levels of common (stimulated by the 3 strains) and specific (stimulated by only one of the strains) genes.
Highly up-regulated transcriptional responses are largely representative of pro-inflammatory pathways
We did not identify relevant specific strain-related transcriptional pathways. In contrast, we observed that the 3 strains belonging to 3 different species of the B. burgdorferi sl group elicited very similar transcriptional profiles in primary human dermal fibroblasts (see the global functional analyses and the signalisation pathway analyses results in S2 and S3 Figs). We then focused on co-regulated genes presenting the highest intensity of up-regulation. Among them, a common core of 42 genes were highly up-regulated in response to stimulation by all 3 Borrelia species. These mainly included pro-inflammatory genes involved in the innate immune response (Table 3). High levels of chemokines/cytokines were induced (CCL2, CXCL1, CXCL2, CXCL6, CXCL10, IL-6, IL-8), with fold-change values up to 20 for IL-8 in fibroblasts stimulated by B. garinii IBS6, along with a large number of genes representative of intracellular signalling pathways that sustain inflammatory responses, such as NF-κB- and interferon (IFN)-related genes. Whichever strain we tested, the IFN signalling and the activation of IRF by cytosolic pattern recognition receptors were found to be the two most overexpressed pathways in the signalisation pathway analyses (S2 Fig). Several other genes related to the metabolism, such as SOD2, were up-regulated by all 3 Borrelia species. Among all these genes, 7 of them (CXCL1, IL-6, IL-8, OAS2, STAT1, IFIH1 and SOD2) were already identified in our previous study as belonging to a common core of up-regulated genes stimulated in fibroblasts by different strains of the same species B. burgdorferi ss (strain N40 isolated from tick, strains Pbre and 1408 isolated from patients affected by EM or ACA respectively). When focusing on the most highly down-regulated genes, a common core of 9 genes was found to be down-regulated in response to all 3 Borrelia species. These genes are mainly involved in the cell division process (Table 4).
Table 3. Genes consistently stimulated by the 3 Borrelia strains with fold-change values ≥ 2.
| Annotation | B. afzelii (IBS17) | B. garinii (IBS6) | B. burgdorferi ss (IBS19) | Description/Function | |||
|---|---|---|---|---|---|---|---|
| Fold change | p-value | Fold change | p-value | Fold change | p-value | ||
| Inflammation and innate immune response | |||||||
| Chemokines/cytokines | |||||||
| CCL2 | 5.8 | 6.20E-04 | 8.2 | 2.10E-05 | 5.2 | 2.90E-04 | Chemoattractant for monocytes and basophils |
| CXCL1 | 8.7 | 3.80E-05 | 13.4 | 2.10E-05 | 7.6 | 1.90E-03 | GROα: chemoattractant for neutrophils |
| CXCL2 | 2.8 | 8.70E-04 | 3.6 | 3.30E-04 | 3.3 | 1.70E-03 | Hematoregulatory chemokine |
| CXCL6 | 6 | 5.10E-04 | 7.6 | 1.80E-04 | 4.7 | 3.20E-04 | Chemoattractant for neutrophils, strong antibacterial activity |
| CXCL10 | 6.5 | 3.90E-02 | 4.4 | 9.90E-03 | 2.5 | 5.30E-04 | Chemoattractant for monocytes and T-lymphocytes |
| IL6 | 3.3 | 2.30E-03 | 6.2 | 1.10E-05 | 2.6 | 3.90E-03 | Cytokine of the acute phase response |
| IL8 | 14.9 | 4.30E-04 | 20.2 | 1.90E-05 | 11 | 1.00E-03 | Chemoattractant for neutrophils |
| IFN-related pathway | |||||||
| DDX58 | 2.2 | 7.50E-03 | 2.5 | 1.60E-03 | 2.7 | 4.90E-02 | Innate immune receptor, induction of type I IFN and pro-inflammatory cytokines |
| GBP2 | 2.1 | 1.30E-03 | 2.4 | 3.80E-03 | 2.4 | 6.30E-03 | IFN-induced guanylate-binding protein 2: antiviral activity |
| HERC5 | 3.2 | 6.90E-03 | 3.8 | 6.00E-04 | 3.7 | 1.20E-02 | Positive regulator of innate antiviral response in cells induced by IFN |
| IFI6 | 2.3 | 9.10E-03 | 3.3 | 1.20E-03 | 2.6 | 1.60E-03 | IFN-α-inducible protein 6 |
| IFI27 | 2.7 | 1.10E-03 | 5.6 | 3.60E-05 | 3.4 | 3.60E-03 | IFN-α-inducible protein 27 |
| IFI44 | 2.6 | 2.50E-03 | 3.2 | 7.60E-05 | 2.7 | 7.40E-03 | IFN-induced protein 44 |
| IFI44L | 4.3 | 2.60E-03 | 7.4 | 1.90E-04 | 5.3 | 4.40E-03 | IFN-induced |
| IFIH1 | 2.9 | 2.40E-03 | 3.3 | 4.60E-04 | 3.3 | 7.60E-03 | Induction of type I IFN and pro-inflammatory cytokines |
| IFIT1 | 5.2 | 5.00E-03 | 5.6 | 7.00E-05 | 5.1 | 1.00E-03 | IFN-induced |
| IFIT2 | 6.4 | 1.80E-02 | 4.8 | 7.40E-05 | 4.8 | 3.00E-03 | IFN-induced, activation of IRF by cytosolic pattern recognition receptors |
| IFIT3 | 4.4 | 4.80E-03 | 5 | 8.60E-05 | 4.6 | 4.80E-03 | IFN-induced, inhibition of cell migration and proliferation |
| IFITM1 | 2.8 | 6.70E-04 | 4.6 | 9.10E-05 | 3.5 | 2.40E-04 | IFN-induced transmembrane protein 1 |
| IRF7 | 2.2 | 3.10E-03 | 2.8 | 9.60E-04 | 2.8 | 8.10E-04 | IFN regulatory factor 7: transcriptional regulator of type I IFN-dependent immune responses |
| ISG15 | 4.4 | 1.60E-03 | 6.9 | 2.10E-04 | 5.2 | 5.40E-04 | NK cell proliferation, chemoattractant for neutrophils, induce IFN-γ |
| MX1 | 8.4 | 5.10E-04 | 12.8 | 7.50E-05 | 11.1 | 4.60E-05 | IFN-induced GTP-binding protein |
| MX2 | 3.5 | 1.10E-02 | 4.9 | 2.50E-04 | 4.2 | 3.20E-03 | Interferon-induced GTP-binding protein |
| OAS1 | 2.9 | 6.80E-03 | 3.7 | 2.00E-04 | 3.3 | 3.70E-03 | Oligoadenylate synthetase-1: IFN-induced, innate immune response to viral infection |
| OAS2 | 4.1 | 3.00E-03 | 6 | 2.00E-04 | 5.1 | 4.90E-04 | Oligoadenylate synthetase-2: IFN-induced, innate immune response to viral infection |
| OAS3 | 2.8 | 1.10E-03 | 4.3 | 1.20E-04 | 3.5 | 6.30E-03 | Oligoadenylate synthetase-3: IFN-induced, dsRNA-activated antiviral enzyme |
| RSAD2 | 4 | 7.70E-03 | 4.5 | 3.40E-04 | 4.5 | 2.50E-02 | IFN-inducible iron-sulfur cluster-binding antiviral protein |
| STAT1 | 2.5 | 4.60E-04 | 3.6 | 5.50E-05 | 2.9 | 8.10E-03 | Signal transducer of activation-1, up-regulate genes in response to IFN type I, II or III |
| NF-kB pathway | |||||||
| NFKBIA | 2.2 | 3.20E-03 | 2.6 | 6.30E-04 | 2.2 | 3.90E-03 | Inhibits the activity of dimeric NF-kappa-B/REL complexes |
| NFKBIZ | 2.9 | 6.60E-03 | 3.2 | 2.50E-03 | 2.8 | 1.40E-02 | Induction of inflammatory genes activated through TLR/IL-1 receptor signalling |
| Complement pathway | |||||||
| CFB | 2.7 | 5.00E-03 | 4.2 | 6.30E-04 | 2.8 | 2.90E-03 | Factor B which is part of the alternate pathway of the complement system |
| Miscellaneous | |||||||
| C15orf48 | 2.2 | 3.90E-04 | 3.5 | 1.10E-04 | 2.5 | 2.60E-03 | Normal mucosa of esophagus-specific gene 1 protein |
| ECGF1 | 2 | 8.20E-03 | 2.5 | 2.00E-03 | 2 | 2.00E-03 | Thymidine phosphorylase: angiogenic activity |
| EPSTI1 | 4.4 | 7.30E-04 | 6.1 | 5.40E-05 | 5.4 | 8.70E-04 | Epithelial-stromal interaction protein 1 |
| HERC6 | 2.3 | 5.60E-03 | 3.3 | 3.50E-04 | 2.8 | 1.60E-02 | Probable E3 ubiquitin-protein ligase HERC6 |
| IER3 | 2.3 | 3.50E-03 | 2.5 | 3.20E-03 | 2.5 | 1.10E-02 | Role in the ERK signalling pathway |
| KYNU | 2.7 | 6.20E-03 | 3.9 | 3.00E-03 | 2 | 2.90E-03 | Putative uncharacterized protein KYNU: kynureninase activity |
| PRIC285 | 2 | 4.90E-03 | 2.7 | 3.10E-03 | 2.7 | 4.60E-03 | Helicase with zinc finger domain 2: acts as a transcriptional coactivator for nuclear receptors |
| SLC39A8 | 3.3 | 1.20E-03 | 4.4 | 5.70E-04 | 2.8 | 3.10E-03 | Zinc transporter ZIP8 |
| SOD2 | 5 | 8.80E-05 | 6.7 | 3.30E-05 | 4.7 | 1.60E-04 | Superoxide dismutase |
| TNFAIP6 | 5.3 | 3.40E-04 | 7.8 | 1.50E-05 | 4.9 | 1.20E-03 | Cell-cell and cell-matrix interactions |
| XAF1 | 2 | 2.70E-03 | 2.7 | 4.90E-04 | 2.8 | 6.30E-04 | Negative regulator of members of the IAP (Inhibitor of Apoptosis Protein) family |
Table 4. Genes consistently repressed by the 3 Borrelia strains with fold-change values ≤ 0.5.
| Annotation | B. afzelii (IBS 17) | B. garinii (IBS 6) | Bb ss (IBS 19) | Description/Function | |||
|---|---|---|---|---|---|---|---|
| Fold change | p-value | Fold change | p-value | Fold change | p-value | Cell division process | |
| CDC20 | 0,38 | 3,97E-06 | 0,35 | 1,53E-05 | 0,41 | 1,19E-04 | Activator of the anaphase promoting complex (APC/C) |
| PBK | 0,50 | 1,22E-05 | 0,50 | 6,95E-05 | 0,49 | 5,06E-04 | PDZ binding kinase: related to mitogen-activated protein kinase (MAPKK) family |
| TOP2A | 0,46 | 3,65E-06 | 0,44 | 8,88E-06 | 0,49 | 3,47E-04 | DNA topoisomerase 2-alpha: role in mitosis and meiosis for proper segregation of daughter chromosomes |
| KIF20A | 0,40 | 3,97E-05 | 0,37 | 3,21E-05 | 0,41 | 3,15E-05 | Mitotic kinesin required for chromosome passenger complex (CPC)-mediated cytokinesis |
| UBE2C | 0,37 | 1,10E-05 | 0,36 | 2,35E-05 | 0,37 | 9,67E-05 | Ubiquitin-conjugating enzyme E2 C: essential factor of the anaphase promoting complex/cyclosome (APC/C) |
| CEP55 | 0,49 | 1,39E-04 | 0,47 | 7,27E-05 | 0,48 | 1,72E-03 | Centrosomal protein of 55 kDa: role in mitotic exit and cytokinesis |
| LOC399942 | 0,44 | 3,17E-03 | 0,48 | 7,97E-03 | 0,49 | 2,20E-02 | Predicted: similar to Tubulin alpha-2 chain, major constituent of microtubules |
| ASPM | 0,46 | 8,55E-06 | 0,44 | 5,35E-05 | 0,48 | 6,88E-05 | Probable role in mitotic spindle regulation and coordination of mitotic processes |
| LOC100132394 | 0,36 | 8,45E-04 | 0,44 | 8,59E-03 | 0,36 | 4,50E-03 | Unknown function |
A focus on selected genes among those found to be differentially regulated by microarray analyses
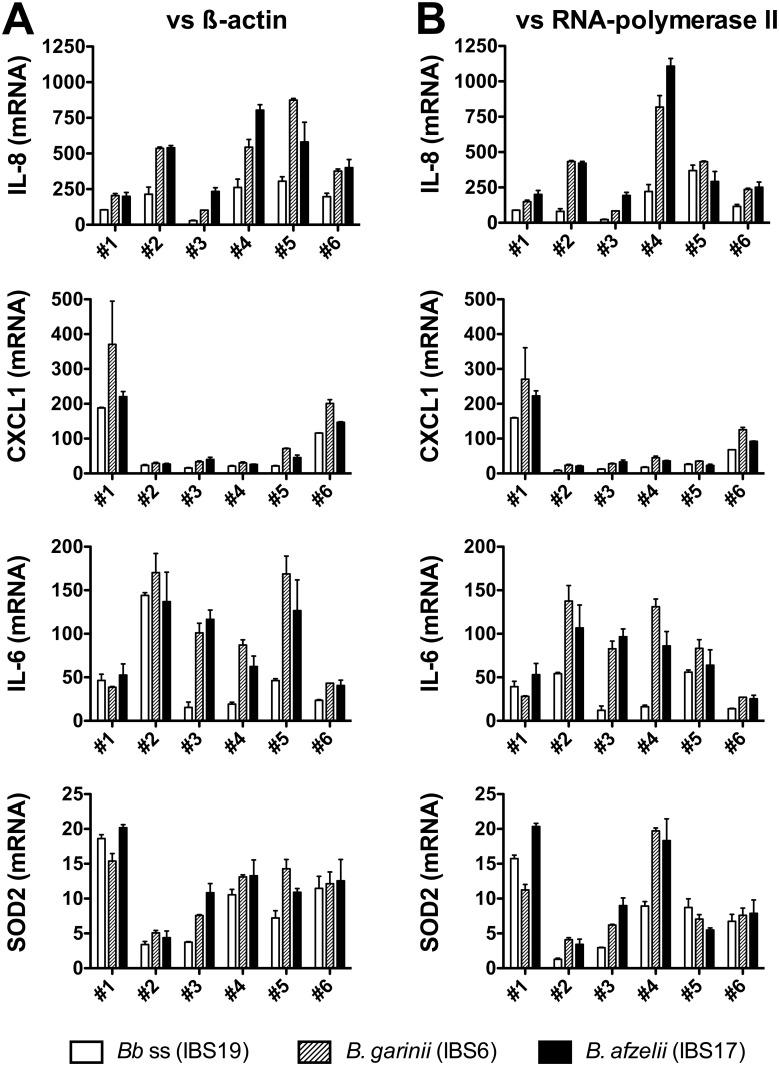
The mRNA transcription of selected genes found to be up-regulated by microarrays (IL-8, CXCL1, IL-6 and SOD2) was analyzed 24h after Borrelia stimulation at MOI 100:1. Using QRT-PCR assays, we confirmed the strong up-regulation of the genes encoding IL-8, CXCL1, IL-6, and SOD2 in all 6 batches of fibroblasts and the three Borrelia strains. The QRT-PCR results normalised to the β-actin (Fig 5A) are confirmed by normalising them to the expression of the RNA polymerase II (Fig 5B), another housekeeping gene known to be stable under various stimulatory conditions [20].
Fig 5. QRT-PCR analysis of mRNA levels induced in human dermal fibroblasts by different species of the B. burgdorferi sl group.
For each batch of fibroblasts,–fibroblasts batch #1 to fibroblasts batch #6 (biological replicates)–, the mRNA levels of IL-8, CXCL1, IL-6 and SOD2 stimulated during 24h at MOI 100:1 by B. burgdorferi ss IBS19 (white bars), B. garinii IBS6 (gray hatched bars) and B. afzelii IBS17 (black bars) were normalized to the β-actin (A) or RNA polymerase II (B) housekeeping gene levels and expressed as relative changes in gene transcription compared with untreated cells. Each bar shows the mean ± SDs of duplicate values (technical replicates).
The mRNA transcription of selected genes found to be down-regulated by microarrays (UBE2C, KIF20A, TOP2A, CEP55 and CDC20) was also analyzed under the same experimental conditions. Using QRT-PCR assays, we confirmed a moderate down-regulation of the genes encoding UBE2C, KIF20A, TOP2A, CEP55 and CDC20 in all 6 batches of fibroblasts induced by the three Borrelia strains (Fig 6).
Fig 6. QRT-PCR analysis of mRNA levels repressed in human dermal fibroblasts by different species of the B. burgdorferi sl group.
For each batch of fibroblasts,–fibroblasts batch #1 to fibroblasts batch #6 (biological replicates)–, the mRNA levels of UBE2C, KIF20A, TOP2A, CEP55 and CDC20 stimulated during 24h at MOI 100:1 by B. burgdorferi ss IBS19 (white bars), B. garinii IBS6 (gray hatched bars) and B. afzelii IBS17 (black bars) were normalized to the β-actin (A) or RNA polymerase II (B) housekeeping gene levels and expressed as relative changes in gene transcription compared with untreated cells. Each bar shows the mean ± SDs of duplicate values (technical replicates).
Discussion
Since the 3 strains used in our previous study belonged to the same species, in this new study, we wanted to assess whether the species of Borrelia could influence the transcriptional modulations of fibroblasts. For this purpose, we tested 3 Borrelia strains, all isolated from erythema migrans lesions and belonging to 3 different pathogenic species (B. afzelii, B. garinii and B. burgdorferi ss) in our NHDF in vitro model, and compared the transcriptional profiles induced by the different species using whole transcriptome microarrays. Exploring the interaction between Borrelia and fibroblasts, we extended a previous experimental work [19] to a selection of Borrelia strains belonging to the three main pathogenic species of Borrelia in the North hemisphere, and we increased the range of analyzed transcripts by using a whole genome microarray experiment.
The technical choices of the present study are important. First of all, Borrelia strains used were cautiously selected. We tested the most representative Borrelia species by selecting one strain of each of the main European Borrelia species: B. afzelii, B. garinii, and B. burgdorferi ss [3]. In order to avoid any bias that could be related to the specific environment from which the strains were isolated, we selected 3 clinical Borrelia strains that were all isolated from the same type of lesion (EM), the most frequent human clinical manifestation. Bias related to the disseminating potential of Borrelia strains was also limited by the selection of strains associated with similar associated clinical features (i.e. without any general symptom suggestive of dissemination associated with EM). All strains tested in our study were cultured in strictly identical conditions, and were all used at low passage (≤P7) in order to avoid the loss of plasmid-encoded virulence factors [22]. The MOI of 100:1 Borrelia used for fibroblast stimulations in microarray and QRT-PCR analyses was chosen to be representative of the physiologic spirochete load present in the dermis at the inoculation site a few days after transmission, when the inflammatory process is culminating. Indeed, although only a small number of Borrelia are present on tick salivary glands and are then inoculated in the dermis during the feeding process [23, 24], an intense replication of Borrelia occurs after inoculation in the host skin at the inoculation site, peaking at day 7 after inoculation [25, 26]. Moreover, setting the stimulation conditions at MOI of 100:1 for 24 hours enabled to yield a potent activation of human dermal fibroblasts while allowing the experimental conditions to emulate those of our first study assessing the early transcriptional response (at 24 hours) of human dermal fibroblasts stimulated by three strains of B. burgdorferi ss (MOI of 100:1) [19].
Secondly, the microarray experiments were precisely designed and the batches of fibroblasts tested were carefully chosen. A consensual number of 6 different batches of fibroblasts was used in order to reach a sufficient number of biological replicates [27]. Biases were limited by selecting NHDF batches issued from different donors of various sexes and ages, but all batches were issued from areas with similar transcriptional profiles. Indeed, fibroblasts derived from skin at different anatomical sites display distinct transcriptional patterns and this distinction was maintained in vitro when fibroblasts were isolated from the influence of other cell types, suggesting that fibroblasts at different locations in the body should be considered distinct differentiated cell types [28, 29]. Approximately 8% of all genes transcribed in fibroblasts were differentially expressed in a site-specific manner and this gene expression signature separates fibroblasts from anterior (rostral) and posterior (caudal) sites of the human body divided by the umbilicus [30]. Thus, in order to avoid the risk of missing transcriptional modifications induced by Borrelia, that could be concealed by the heterogeneity of fibroblasts, we chose, in our experiments, fibroblasts issued from a similar transcriptional area (head and chest).
In a previous study, analyzing the interaction between Borrelia and fibroblasts, we tested 3 strains of the same species (B. burgdorferi ss) that were isolated from various clinical environments (strain 1408 isolated from ACA; strain Pbre isolated from EM; strain N40 isolated from a tick). These 3 strains were shown to induce a very similar inflammatory profile in fibroblasts [19]. Therefore, no specific strain-related pathway has been identified that could be linked to the transcriptional responses elicited by clinical manifestations. We thus concluded that Borrelia pathotype has little influence on the fibroblasts response. Since the 3 strains used in our previous study belonged to the same species, this new study was designed to determine whether the fibroblasts transcriptional response could be differentially influenced by the Borrelia species. The data obtained in this study indicate that the transcriptional response of fibroblasts is largely comparable whichever the Borrelia species used for cell stimulation. Indeed, almost all the transcripts with the highest transcriptional modulation were consistently stimulated by the 3 tested species. Transcripts that were specifically modulated by only one strain showed only minor modifications. Thus, our results do not reveal the existence of a fingerprint of transcriptional changes in fibroblasts that could be associated with a particular Borrelia species. However, even if the overall Borrelia-induced transcriptional modulations in fibroblasts were very similar, an interesting finding of our study was that the level of modification induced by the B. garinii EM-inducing strain was significantly higher than that of B. afzelii and B. burgdorferi ss EM-inducing strains tested. Clinical observations previously reported B. garinii-induced EM to have shorter incubation and faster evolution than EM caused by B. afzelii [31, 32]. EM lesions caused by B. garinii were also significantly larger than EM lesions caused by B. burgdorferi ss [33] or B. afzelii [32]. Thus, our in-vitro observations are in accordance with the clinical features described by other authors.
Furthermore, the use of a whole genome microarray experiment combined with the analysis of a large number of replicates (3 strains tested with 6 different batches of fibroblasts) allowed an in-depth exploration of the early fibroblasts’ transcriptional response to Borrelia infection. The main observation was that the 3 Borrelia species induced the stimulation of a large number of pro-inflammatory genes involved in the innate immune response, with high levels of the chemokines/cytokines CCL2, CXCL1, CXCL2, CXCL6, CXCL10, IL-6, IL-8. This result corroborates previous studies reporting the induction of CXCL1, IL6 and IL8 in Borrelia-stimulated primary human fibroblasts in vitro [19, 34]. Borrelia-induced stimulation of dendritic cells and macrophage chemoattractants was also demonstrated in skin biopsies of murine models (MCP-1, also referred to as CCL2) [26], as well as in human biopsies of EM and ACA lesions (CCL2, CXCL1, CXCL9, CXCL10, and CCL20) [35, 36].
We also found that a large number of type I IFN-related genes was intensively stimulated in fibroblasts by Borrelia. Type I IFNs play a key role in linking the innate and adaptive immune responses. The stimulation of the type I IFN heteromeric receptor initiates an intracellular cascade leading to the phosphorylation of STAT proteins (especially STAT1) which in turn translocate to the nucleus and initiate transcription by binding specific sites in the promoters of IFN-stimulated genes [37]. The induction of type I IFN by Borrelia has been previously shown in human peripheral blood mononuclear cells [38]. A critical role of type I IFN has also been demonstrated in the pathogenesis of Borrelia in murine models of Lyme arthritis [39] involving multiple triggering ligands of the bacteria [40]. Fibroblasts were also previously shown to participate in the local propagation of a robust type I IFN response in joint tissues [41]. Since dermal fibroblasts represent the most abundant cells in the site of Borrelia inoculation by the tick biting pieces, the strong Borrelia-induced stimulation of type I IFN-related genes observed in our experimental model could indicate that fibroblasts actively participate in the initiation of the inflammatory response to Borrelia in the skin.
Interestingly, the cytosolic pattern recognition receptor pathway of type 1 IFN induction was also evidenced in fibroblasts stimulated by all 3 studied Borrelia strains. This system is triggered by the presence of double-stranded RNA or double-stranded DNA in the cell cytosol following infection by viruses or intracellular bacteria. The cytosolic pattern recognition receptor pathway eventually induces the activation of early phase type 1 IFNs and other proteins implicated in the innate immune response [42]. This finding is interesting since Borrelia is classically considered an extracellular pathogen and that only few experimental data currently support that it can be internalised by human dermal fibroblasts [43, 44]. However, considering the complex interplay between pattern recognition receptors and the various cross-regulation of the innate immune receptor signaling [45], this finding should be interpreted with caution since the same IFN-responsive genes and IFN-regulatory factors can be stimulated by pattern recognition receptors in different cellular locations. In the interaction between Borrelia and fibroblasts, the transcriptional response of fibroblasts do not seem to be related to the Borrelia species tested, since no species-related transcriptional fingerprint could be brought out. Conversely, the fibroblast response appears to be homogeneous whichever the Borrelia species or pathotype tested and this cell type seems to play a key role in the inflammatory response elicited by Borrelia. The question of the factors involved in the organotropism of Lyme borreliosis manifestations remains unanswered. Factors related to the host are probably more implicated in the pathogenesis of this complex disease and need to be explored in forthcoming studies.
Supporting Information
Levels of IL-8 secretion by fibroblasts stimulated by increasing concentrations (MOI of 1:1 = 1B, MOI of 10:1 = 10B, 50:1 = 50B, and 100:1 = 100B) of the 3 Borrelia strains at 24 hours for fibroblasts batch #3 (A-C) and fibroblasts batch #4 (D-F). NEG: unstimulated fibroblasts. NA: not available data. Each bar shows the mean ± SDs of duplicate values.
(TIF)
(A) Fibroblasts stimulated by B. burgdorferi ss IBS19. (B) Fibroblasts stimulated by B. garinii IBS6. (C) Fibroblasts stimulated by B. afzelii IBS17.
(PDF)
(A) Fibroblasts stimulated by B. burgdorferi ss IBS19. (B) Fibroblasts stimulated by B. garinii IBS6. (C) Fibroblasts stimulated by B. afzelii IBS17.
(PDF)
(PDF)
Acknowledgments
We thank Laurence Zilliox and Danièle Napolitano for their technical assistance in Borrelia cultures, and Abiba Doukani for her help in the submission of microarray data to GEO.
Abbreviations
- IFN
interferon
- EM
erythema migrans
- ACA
acrodermatitis chronica atrophicans
- NHDF
normal human dermal primary fibroblasts
- MOI
multiplicity of infection
- SDs
standard deviations
- QRT-PCR
semiquantitative reverse transcription PCR
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–73. Epub 2011/09/10. 10.1016/S0140-6736(11)60103-7 . [DOI] [PubMed] [Google Scholar]
- 2.Kuehn BM. CDC estimates 300,000 US cases of Lyme disease annually. JAMA. 2013;310(11):1110 10.1001/jama.2013.278331 . [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. Lyme borreliosis in Europe. Euro Surveill. 2011;16(27). Epub 2011/07/29. . [PubMed] [Google Scholar]
- 4.Markowicz M, Ladstatter S, Schotta AM, Reiter M, Pomberger G, Stanek G. Oligoarthritis caused by Borrelia bavariensis, Austria, 2014. Emerg Infect Dis. 2015;21(6):1052–4. 10.3201/eid2106.141516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maraspin V, Ruzic-Sabljic E, Strle F. Lyme borreliosis and Borrelia spielmanii. Emerg Infect Dis. 2006;12(7):1177 10.3201/eid1207.060077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudenko N, Golovchenko M, Mokracek A, Piskunova N, Ruzek D, Mallatova N, et al. Detection of Borrelia bissettii in cardiac valve tissue of a patient with endocarditis and aortic valve stenosis in the Czech Republic. J Clin Microbiol. 2008;46(10):3540–3. 10.1128/JCM.01032-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudenko N, Golovchenko M, Ruzek D, Piskunova N, Mallatova N, Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol Lett. 2009;292(2):274–81. 10.1111/j.1574-6968.2009.01498.x . [DOI] [PubMed] [Google Scholar]
- 8.Collares-Pereira M, Couceiro S, Franca I, Kurtenbach K, Schafer SM, Vitorino L, et al. First isolation of Borrelia lusitaniae from a human patient. J Clin Microbiol. 2004;42(3):1316–8. Epub 2004/03/09. 10.1128/JCM.42.3.1316-1318.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diza E, Papa A, Vezyri E, Tsounis S, Milonas I, Antoniadis A. Borrelia valaisiana in cerebrospinal fluid. Emerg Infect Dis. 2004;10(9):1692–3. Epub 2004/10/27. 10.3201/eid1009.030349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baranton G, De Martino SJ. Borrelia burgdorferi sensu lato diversity and its influence on pathogenicity in humans In: Lipsker D, Jaulhac B, editors. Curr Probl Dermatol. 37 Basel: Karger; 2009. p. 1–17. 10.1159/000213066 [DOI] [PubMed] [Google Scholar]
- 11.Brisson D, Baxamusa N, Schwartz I, Wormser GP. Biodiversity of Borrelia burgdorferi strains in tissues of Lyme disease patients. PloS ONE. 2011;6(8):e22926 Epub 2011/08/11. 10.1371/journal.pone.0022926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rego RO, Bestor A, Stefka J, Rosa PA. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PloS ONE. 2014;9(6):e101009 10.1371/journal.pone.0101009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9(10):679–91. 10.1038/nri2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filgueira L, Nestle FO, Rittig M, Joller HI, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157(7):2998–3005. Epub 1996/10/01. . [PubMed] [Google Scholar]
- 15.Suhonen J, Komi J, Soukka J, Lassila O, Viljanen MK. Interaction between Borrelia burgdorferi and immature human dendritic cells. Scand J Immunol. 2003;58(1):67–75. Epub 2003/06/28. . [DOI] [PubMed] [Google Scholar]
- 16.Talkington J, Nickell SP. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect Immun. 1999;67(3):1107–15. Epub 1999/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchal C, Schramm F, Kern A, Luft BJ, Yang X, Schuijt T, et al. Antialarmin effect of tick saliva during the transmission of Lyme disease. Infect Immun. 2011;79(2):774–85. Epub 2010/12/08. 10.1128/IAI.00482-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchal CM, Luft BJ, Yang X, Sibilia J, Jaulhac B, Boulanger NM. Defensin is suppressed by tick salivary gland extract during the in vitro interaction of resident skin cells with Borrelia burgdorferi. J Invest Dermatol. 2009;129(10):2515–7. 10.1038/jid.2009.73 [DOI] [PubMed] [Google Scholar]
- 19.Schramm F, Kern A, Barthel C, Nadaud S, Meyer N, Jaulhac B, et al. Microarray analyses of inflammation response of human dermal fibroblasts to different strains of Borrelia burgdorferi sensu stricto. PloS ONE. 2012;7(6):e40046 Epub 2012/07/07. 10.1371/journal.pone.0040046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313(4):856–62. Epub 2004/01/07. 10.1016/j.bbrc.2003.11.177 . [DOI] [PubMed] [Google Scholar]
- 21.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet. 2001;29(4):365–71. Epub 2001/12/01. 10.1038/ng1201-365 . [DOI] [PubMed] [Google Scholar]
- 22.Stewart PE, Byram R, Grimm D, Tilly K, Rosa PA. The plasmids of Borrelia burgdorferi: essential genetic elements of a pathogen. Plasmid. 2005;53(1):1–13. Epub 2005/01/06. 10.1016/j.plasmid.2004.10.006 . [DOI] [PubMed] [Google Scholar]
- 23.Dunham-Ems SM, Caimano MJ, Pal U, Wolgemuth CW, Eggers CH, Balic A, et al. Live imaging reveals a biphasic mode of dissemination of Borrelia burgdorferi within ticks. J Clin Invest. 2009;119(12):3652–65. Epub 2009/11/19. 10.1172/JCI39401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piesman J, Schneider BS, Zeidner NS. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J Clin Microbiol. 2001;39(11):4145–8. Epub 2001/10/30. 10.1128/JCM.39.11.4145-4148.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonara S, Ristow L, McCarthy J, Coburn J. Effect of Borrelia burgdorferi OspC at the site of inoculation in mouse skin. Infect Immun. 2010;78(11):4723–33. Epub 2010/08/11. 10.1128/IAI.00464-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kern A, Schnell G, Bernard Q, Boeuf A, Jaulhac B, Collin E, et al. Heterogeneity of Borrelia burgdorferi Sensu Stricto Population and Its Involvement in Borrelia Pathogenicity: Study on Murine Model with Specific Emphasis on the Skin Interface. PloS ONE. 2015;10(7):e0133195 10.1371/journal.pone.0133195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat Rev Genet. 2006;7(1):55–65. 10.1038/nrg1749 . [DOI] [PubMed] [Google Scholar]
- 28.Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99(20):12877–82. Epub 2002/09/26. 10.1073/pnas.162488599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinn JL, Wang JK, Liu H, Montgomery K, van de Rijn M, Chang HY. A systems biology approach to anatomic diversity of skin. J Invest Dermatol. 2008;128(4):776–82. Epub 2008/03/14. 10.1038/sj.jid.5700986 . [DOI] [PubMed] [Google Scholar]
- 30.Rinn JL, Bondre C, Gladstone HB, Brown PO, Chang HY. Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2006;2(7):e119 Epub 2006/08/10. 10.1371/journal.pgen.0020119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Logar M, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J, Jurca T, et al. Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection. 2004;32(1):15–9. 10.1007/s15010-004-3042-z . [DOI] [PubMed] [Google Scholar]
- 32.Carlsson SA, Granlund H, Jansson C, Nyman D, Wahlberg P. Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis. 2003;35(1):31–3. 10.1080/0036554021000026978 . [DOI] [PubMed] [Google Scholar]
- 33.Strle F, Ruzic-Sabljic E, Logar M, Maraspin V, Lotric-Furlan S, Cimperman J, et al. Comparison of erythema migrans caused by Borrelia burgdorferi and Borrelia garinii. Vector Borne Zoonotic Dis. 2011;11(9):1253–8. 10.1089/vbz.2010.0230 . [DOI] [PubMed] [Google Scholar]
- 34.Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158(7):3285–92. . [PubMed] [Google Scholar]
- 35.Müllegger RR, Means TK, Shin JJ, Lee M, Jones KL, Glickstein LJ, et al. Chemokine signatures in the skin disorders of Lyme borreliosis in Europe: predominance of CXCL9 and CXCL10 in erythema migrans and acrodermatitis and CXCL13 in lymphocytoma. Infect Immun. 2007;75(9):4621–8. Epub 2007/07/04. 10.1128/IAI.00263-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, et al. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46(1):85–92. 10.1086/524022 . [DOI] [PubMed] [Google Scholar]
- 37.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5(5):375–86. 10.1038/nri1604 . [DOI] [PubMed] [Google Scholar]
- 38.Krupna-Gaylord MA, Liveris D, Love AC, Wormser GP, Schwartz I, Petzke MM. Induction of type I and type III interferons by Borrelia burgdorferi correlates with pathogenesis and requires linear plasmid 36. PloS ONE. 2014;9(6):e100174 10.1371/journal.pone.0100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller JC, Ma Y, Bian J, Sheehan KC, Zachary JF, Weis JH, et al. A critical role for type I IFN in arthritis development following Borrelia burgdorferi infection of mice. J Immunol. 2008;181(12):8492–503. 10.4049/jimmunol.181.12.8492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller JC, Maylor-Hagen H, Ma Y, Weis JH, Weis JJ. The Lyme disease spirochete Borrelia burgdorferi utilizes multiple ligands, including RNA, for interferon regulatory factor 3-dependent induction of type I interferon-responsive genes. Infect Immun. 2010;78(7):3144–53. 10.1128/IAI.01070-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lochhead RB, Sonderegger FL, Ma Y, Brewster JE, Cornwall D, Maylor-Hagen H, et al. Endothelial cells and fibroblasts amplify the arthritogenic type I IFN response in murine Lyme disease and are major sources of chemokines in Borrelia burgdorferi-infected joint tissue. J Immunol. 2012;189(5):2488–501. 10.4049/jimmunol.1201095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abdullah Z, Knolle PA. Scaling of immune responses against intracellular bacterial infection. EMBO J. 2014;33(20):2283–94. 10.15252/embj.201489055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klempner MS, Noring R, Rogers RA. Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi. J Infect Dis. 1993;167(5):1074–81. Epub 1993/05/01. 10.1093/infdis/167.5.1074 . [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Weening EH, Faske JB, Hook M, Skare JT. Invasion of eukaryotic cells by Borrelia burgdorferi requires β1 integrins and Src kinase activity. Infect Immun. 2011;79(3):1338–48. Epub 2010/12/22. 10.1128/IAI.01188-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao X. Self-regulation and cross-regulation of pattern-recognition receptor signalling in health and disease. Nat Rev Immunol. 2016;16(1):35–50. 10.1038/nri.2015.8 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of IL-8 secretion by fibroblasts stimulated by increasing concentrations (MOI of 1:1 = 1B, MOI of 10:1 = 10B, 50:1 = 50B, and 100:1 = 100B) of the 3 Borrelia strains at 24 hours for fibroblasts batch #3 (A-C) and fibroblasts batch #4 (D-F). NEG: unstimulated fibroblasts. NA: not available data. Each bar shows the mean ± SDs of duplicate values.
(TIF)
(A) Fibroblasts stimulated by B. burgdorferi ss IBS19. (B) Fibroblasts stimulated by B. garinii IBS6. (C) Fibroblasts stimulated by B. afzelii IBS17.
(PDF)
(A) Fibroblasts stimulated by B. burgdorferi ss IBS19. (B) Fibroblasts stimulated by B. garinii IBS6. (C) Fibroblasts stimulated by B. afzelii IBS17.
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.