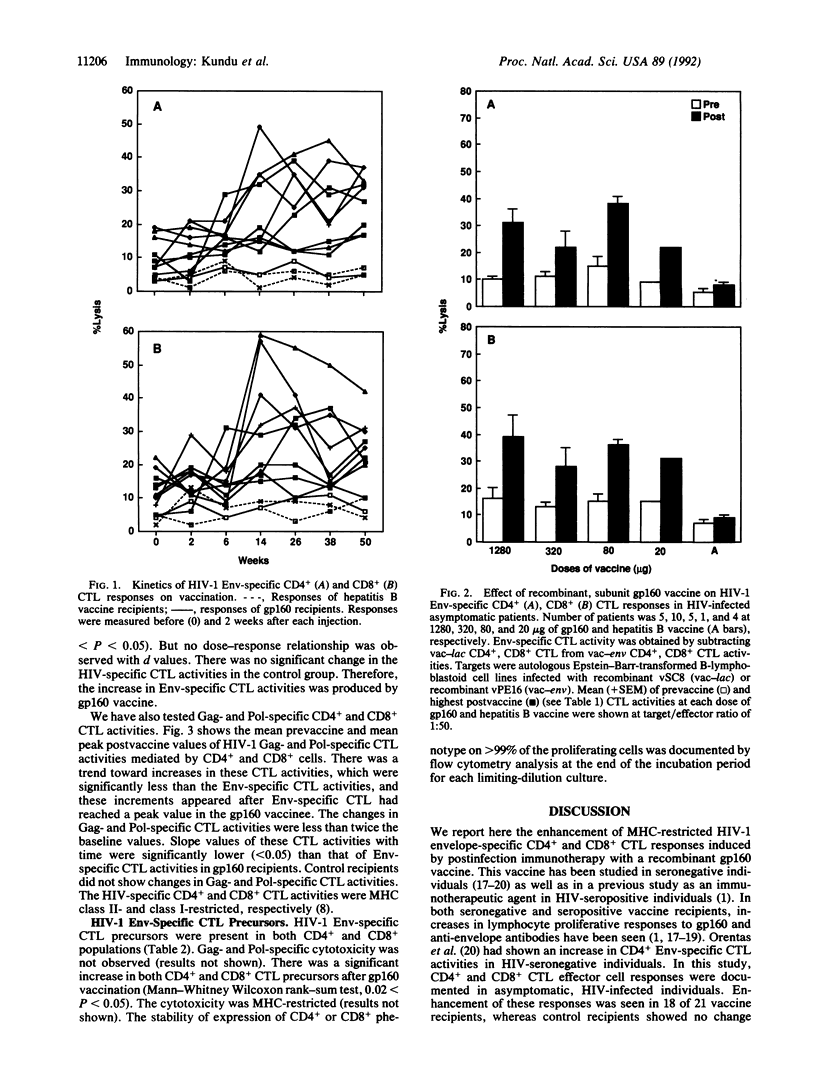
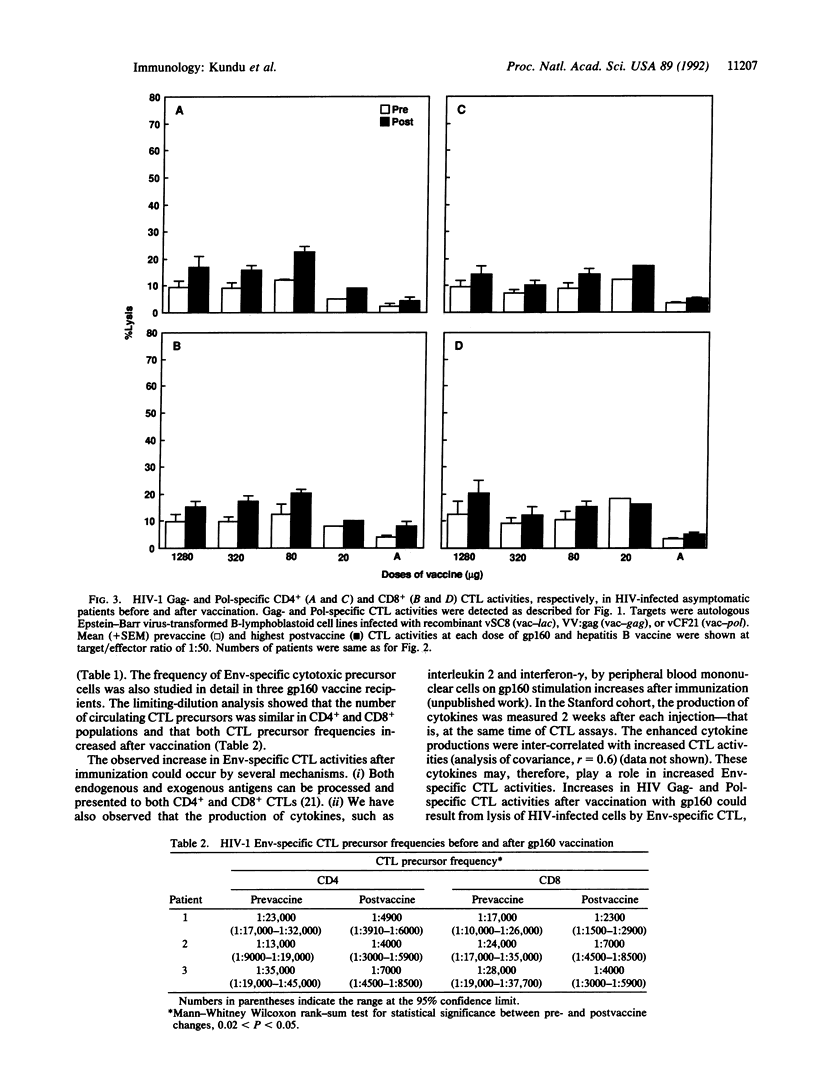
Abstract
Twenty-six human immunodeficiency virus (HIV)-infected asymptomatic patients with CD4+ lymphocytes > 400 per mm3 were randomly allocated to a range of doses of recombinant gp160 or a control (recombinant hepatitis B vaccine) on a double-blind basis. Each patient received an injection at 0, 4, 12, 24, 36, and 48 weeks. Treatment assignments were decoded when all patients reached 28 weeks of the study period. HIV-1-specific CD4+ and CD8+ cytotoxic T lymphocyte (CTL) activities were assessed in vitro before vaccination and 2 weeks after each injection. There were significant increases in major histocompatibility complex-restricted HIV-1 Env-specific CD4+ and CD8+ CTL activities in 18 of 21 gp160 vaccinees. No control-injected patients showed a significant change. Neither gp160 nor control recipients showed significant changes in HIV-1 Gag- and Pol-specific CTL activities. HIV-1 Env-specific CD4+ and CD8+ CTL precursor frequencies were also measured in three vaccinees before and at 24 weeks after vaccine was started. CTL precursor frequencies also increased in both CD4+ and CD8+ populations. This study shows that this gp160 vaccine is immunogenic in enhancing HIV-1 Env-specific cytotoxic T-cell-mediated immunity in HIV-seropositive individuals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Sharp M., Smith S., Koropchak C. M., Diaz P. S., Kinchington P., Ruyechan W., Hay J. Equivalent recognition of a varicella-zoster virus immediate early protein (IE62) and glycoprotein I by cytotoxic T lymphocytes of either CD4+ or CD8+ phenotype. J Immunol. 1991 Jan 1;146(1):257–264. [PubMed] [Google Scholar]
- Berzofsky J. A., Bensussan A., Cease K. B., Bourge J. F., Cheynier R., Lurhuma Z., Salaün J. J., Gallo R. C., Shearer G. M., Zagury D. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature. 1988 Aug 25;334(6184):706–708. doi: 10.1038/334706a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. HIV immunization. Fresh pathways to follow. Nature. 1990 Apr 26;344(6269):818–819. doi: 10.1038/344818a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Progress in vaccines against AIDS. Science. 1989 Dec 8;246(4935):1233–1234. doi: 10.1126/science.2555922. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Clerici M., Lucey D. R., Zajac R. A., Boswell R. N., Gebel H. M., Takahashi H., Berzofsky J. A., Shearer G. M. Detection of cytotoxic T lymphocytes specific for synthetic peptides of gp160 in HIV-seropositive individuals. J Immunol. 1991 Apr 1;146(7):2214–2219. [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Graham B. S., Greenberg S. B., Tacket C. O., Belshe R. B., Midthun K., Clements M. L., Gorse G. J., Horgan B. W., Atmar R. L. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med. 1991 Jan 15;114(2):119–127. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Downie A. W., Kempe C. H. Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967 Dec 18;202(12):1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- Golding H., Shearer G. M., Hillman K., Lucas P., Manischewitz J., Zajac R. A., Clerici M., Gress R. E., Boswell R. N., Golding B. Common epitope in human immunodeficiency virus (HIV) I-GP41 and HLA class II elicits immunosuppressive autoantibodies capable of contributing to immune dysfunction in HIV I-infected individuals. J Clin Invest. 1989 Apr;83(4):1430–1435. doi: 10.1172/JCI114034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmalin A., Clerici M., Houghten R., Pendleton C. D., Flexner C., Lucey D. R., Moss B., Germain R. N., Shearer G. M., Berzofsky J. A. An epitope in human immunodeficiency virus 1 reverse transcriptase recognized by both mouse and human cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2344–2348. doi: 10.1073/pnas.87.6.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly P., Guillon J. M., Mayaud C., Plata F., Theodorou I., Denis M., Debre P., Autran B. Cell-mediated suppression of HIV-specific cytotoxic T lymphocytes. J Immunol. 1989 Oct 1;143(7):2193–2201. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Keefer M. C., Bonnez W., Roberts N. J., Jr, Dolin R., Reichman R. C. Human immunodeficiency virus (HIV-1) gp160-specific lymphocyte proliferative responses of mononuclear leukocytes from HIV-1 recombinant gp160 vaccine recipients. J Infect Dis. 1991 Mar;163(3):448–453. doi: 10.1093/infdis/163.3.448. [DOI] [PubMed] [Google Scholar]
- Kundu S. K., Merigan T. C. Equivalent recognition of HIV proteins, Env, Gag and Pol, by CD4+ and CD8+ cytotoxic T-lymphocytes. AIDS. 1992 Jul;6(7):643–649. [PubMed] [Google Scholar]
- Kundu S. K., Merigan T. C. Inverse relationship of CD8+CD11+ suppressor T cells with human immunodeficiency virus (HIV)-specific cellular cytotoxicity and natural killer cell activity in HIV infection. Immunology. 1991 Dec;74(4):567–571. [PMC free article] [PubMed] [Google Scholar]
- Matthews T. J., Lyerly H. K., Weinhold K. J., Langlois A. J., Rusche J., Putney S. D., Gallo R. C., Bolognesi D. P. Prospects for development of a vaccine against HTLV-III-related disorders. AIDS Res Hum Retroviruses. 1987;3 (Suppl 1):197–206. doi: 10.1089/aid.1987.3.197. [DOI] [PubMed] [Google Scholar]
- Mittler R. S., Hoffmann M. K. Synergism between HIV gp120 and gp120-specific antibody in blocking human T cell activation. Science. 1989 Sep 22;245(4924):1380–1382. doi: 10.1126/science.2571187. [DOI] [PubMed] [Google Scholar]
- Moses L. E., Emerson J. D., Hosseini H. Analyzing data from ordered categories. N Engl J Med. 1984 Aug 16;311(7):442–448. doi: 10.1056/NEJM198408163110705. [DOI] [PubMed] [Google Scholar]
- Nixon D. F., McMichael A. J. Cytotoxic T-cell recognition of HIV proteins and peptides. AIDS. 1991 Sep;5(9):1049–1059. [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Plata F., Dadaglio G., Chenciner N., Hoffenbach A., Wain-Hobson S., Michel F., Langlade-Demoyen P. Cytotoxic T lymphocytes in HIV-induced disease: implications for therapy and vaccination. Immunodefic Rev. 1989;1(3):227–246. [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Germain R. N., Moss B., Berzofsky J. A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 class II-restricted help for itself. J Exp Med. 1990 Feb 1;171(2):571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981 Apr;126(4):1614–1619. [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Stanley S. D., Austin A. A., Matthews T. J., Bolognesi D. P. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J Immunol. 1989 May 1;142(9):3091–3097. [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]


