Abstract
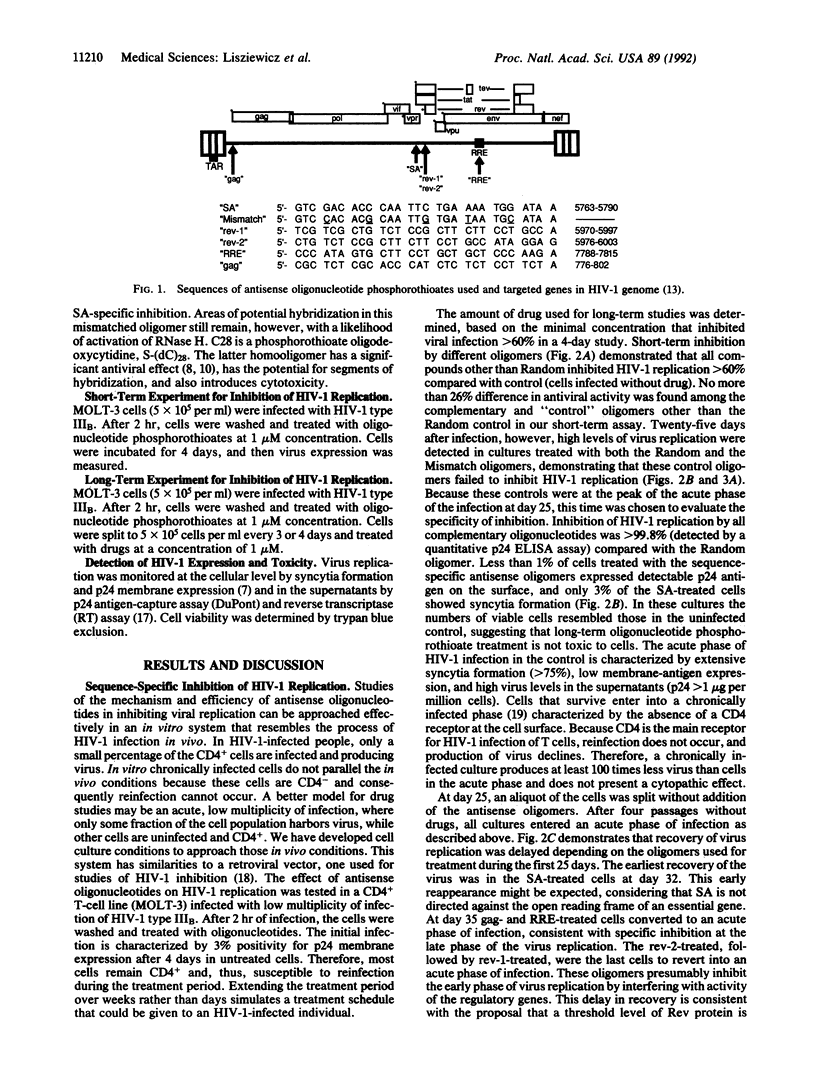
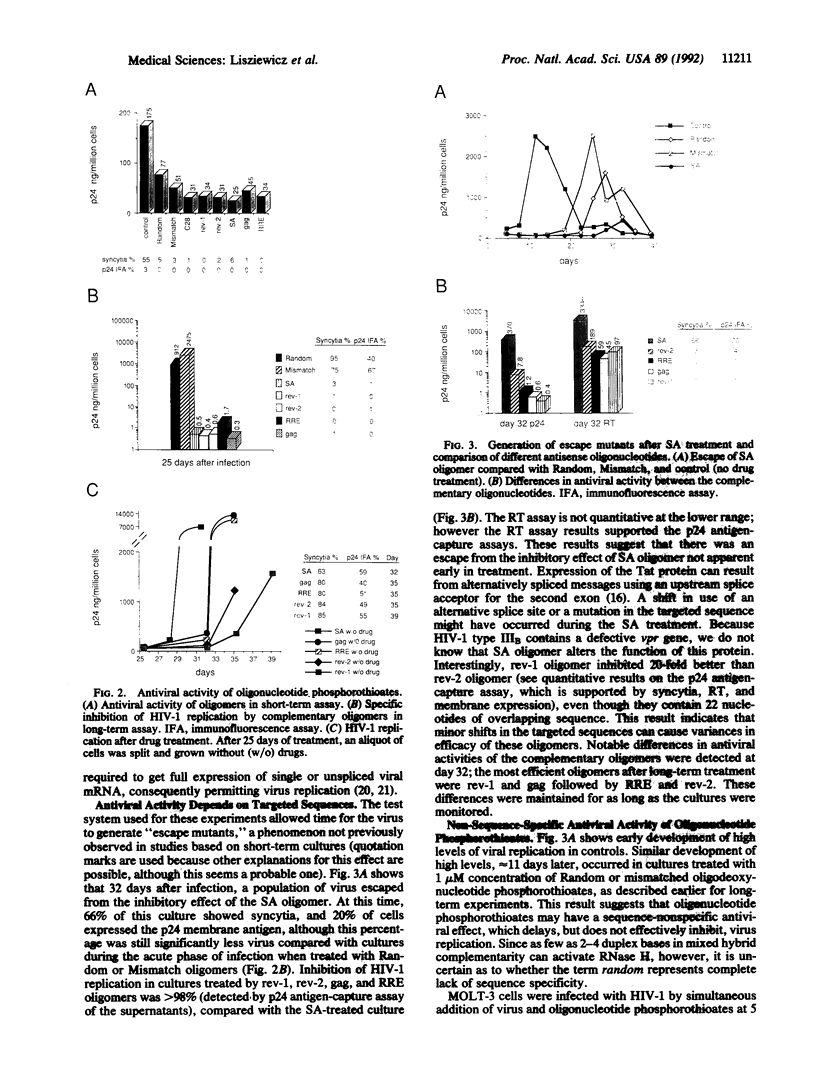
We have developed a culture system, simulating in vivo conditions of human immunodeficiency virus type 1 (HIV-1) infection, to evaluate the long-term efficacy of antisense oligonucleotide treatment. Five oligonucleotide phosphorothioates (28-mers), complementary to different regions of HIV-1 RNA, blocked replication of the virus in a sequence-specific manner at 1 microM concentration. Variations in antiviral activity were seen among the different oligonucleotides, revealing an effect of target selection. Mismatched or random oligonucleotide phosphorothioates delayed, but did not completely inhibit, HIV-1 replication. In the case of inhibition by a splice-acceptor-site antisense oligodeoxynucleotide, a break-through phenomenon occurred after 25 days of treatment, suggesting the development of an "escape mutant." This result did not occur when the inhibitory oligodeoxynucleotides were complementary to the primary-sequence areas of the rev-responsive element and rev-1 genes. Sequential treatment of HIV-1-infected cells with a combination of different antisense oligonucleotides, each administered once, also prevented the development of escape mutants. Our results suggest that chemotherapy based on specifically targeted antisense-oligonucleotide phosphorothioates may be an effective method for reducing the viral burden in HIV-1-infected individuals at clinically achievable oligonucleotide concentrations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S. Antisense oligonucleotides as antiviral agents. Trends Biotechnol. 1992 May;10(5):152–158. doi: 10.1016/0167-7799(92)90203-8. [DOI] [PubMed] [Google Scholar]
- Agrawal S., Goodchild J., Civeira M. P., Thornton A. H., Sarin P. S., Zamecnik P. C. Oligodeoxynucleoside phosphoramidates and phosphorothioates as inhibitors of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7079–7083. doi: 10.1073/pnas.85.19.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal S., Ikeuchi T., Sun D., Sarin P. S., Konopka A., Maizel J., Zamecnik P. C. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Chin D. J. Inhibition of human immunodeficiency virus type 1 Rev-Rev-response element complex formation by complementary oligonucleotides. J Virol. 1992 Jan;66(1):600–607. doi: 10.1128/jvi.66.1.600-607.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daefler S., Klotman M. E., Wong-Staal F. Trans-activating rev protein of the human immunodeficiency virus 1 interacts directly and specifically with its target RNA. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4571–4575. doi: 10.1073/pnas.87.12.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. Y., Jaroszewski J. W., Cohen J. S., Cheng Y. C. Mechanisms of inhibition of herpes simplex virus type 2 growth by 28-mer phosphorothioate oligodeoxycytidine. J Biol Chem. 1990 Nov 25;265(33):20172–20178. [PubMed] [Google Scholar]
- Goodchild J., Agrawal S., Civeira M. P., Sarin P. S., Sun D., Zamecnik P. C. Inhibition of human immunodeficiency virus replication by antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5507–5511. doi: 10.1073/pnas.85.15.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Cullen B. R. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell. 1991 Apr 19;65(2):241–248. doi: 10.1016/0092-8674(91)90158-u. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Tiley L. S., McCarn D. F., Rusche J. R., Hauber J., Cullen B. R. HIV-1 structural gene expression requires binding of the Rev trans-activator to its RNA target sequence. Cell. 1990 Feb 23;60(4):675–683. doi: 10.1016/0092-8674(90)90670-a. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Robert-Guroff M., Shimada T., Stein C. A., Mitsuya H., Wong-Staal F., Cohen J. S., Broder S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Stein C. A., Mitsuya H., Cohen J. S., Broder S. Synthesis of phosphorothioate analogues of oligodeoxyribonucleotides and their antiviral activity against human immunodeficiency virus (HIV). Gene. 1988 Dec 10;72(1-2):343–347. doi: 10.1016/0378-1119(88)90161-8. [DOI] [PubMed] [Google Scholar]
- Pomerantz R. J., Trono D., Feinberg M. B., Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990 Jun 29;61(7):1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Sarin P. S., Agrawal S., Civeira M. P., Goodchild J., Ikeuchi T., Zamecnik P. C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7448–7451. doi: 10.1073/pnas.85.20.7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarin P. S., Sun D., Thornton A., Müller W. E. Inhibition of replication of the etiologic agent of acquired immune deficiency syndrome (human T-lymphotropic retrovirus/lymphadenopathy-associated virus) by avarol and avarone. J Natl Cancer Inst. 1987 Apr;78(4):663–666. [PubMed] [Google Scholar]
- Sullenger B. A., Gallardo H. F., Ungers G. E., Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990 Nov 2;63(3):601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- Tiley L. S., Malim M. H., Tewary H. K., Stockley P. G., Cullen B. R. Identification of a high-affinity RNA-binding site for the human immunodeficiency virus type 1 Rev protein. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):758–762. doi: 10.1073/pnas.89.2.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers T., Baker B. F., Cook P. D., Zounes M., Buckheit R. W., Jr, Germany J., Ecker D. J. Inhibition of HIV-LTR gene expression by oligonucleotides targeted to the TAR element. Nucleic Acids Res. 1991 Jun 25;19(12):3359–3368. doi: 10.1093/nar/19.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. Oligodeoxynucleotide stability in subcellular extracts and culture media. J Biochem Biophys Methods. 1986 Sep;13(2):97–102. doi: 10.1016/0165-022x(86)90021-7. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. C., Goodchild J., Taguchi Y., Sarin P. S. Inhibition of replication and expression of human T-cell lymphotropic virus type III in cultured cells by exogenous synthetic oligonucleotides complementary to viral RNA. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4143–4146. doi: 10.1073/pnas.83.12.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]