Fresh data on the timing and speed of the oceanic spawning migration of European eels suggest a new paradigm for spawning ecology.
Keywords: European eels, animal migration, spawning, escapement, electronic tags, vertical migration, predation
Abstract
The spawning migration of the European eel (Anguilla anguilla L.) to the Sargasso Sea is one of the greatest animal migrations. However, the duration and route of the migration remain uncertain. Using fishery data from 20 rivers across Europe, we show that most eels begin their oceanic migration between August and December. We used electronic tagging techniques to map the oceanic migration from eels released from four regions in Europe. Of 707 eels tagged, we received 206 data sets. Many migrations ended soon after release because of predation events, but we were able to reconstruct in detail the migration routes of >80 eels. The route extended from western mainland Europe to the Azores region, more than 5000 km toward the Sargasso Sea. All eels exhibited diel vertical migrations, moving from deeper water during the day into shallower water at night. The range of migration speeds was 3 to 47 km day−1. Using data from larval surveys in the Sargasso Sea, we show that spawning likely begins in December and peaks in February. Synthesizing these results, we show that the timing of autumn escapement and the rate of migration are inconsistent with the century-long held assumption that eels spawn as a single reproductive cohort in the springtime following their escapement. Instead, we suggest that European eels adopt a mixed migratory strategy, with some individuals able to achieve a rapid migration, whereas others arrive only in time for the following spawning season. Our results have consequences for eel management.
INTRODUCTION
There is global concern about the status of freshwater eel populations following a 40-year decline in recruitment and a 20-year decline in commercial catches in Europe, North America, and Asia (1–4). The European eel is listed as a critically endangered species (2, 5), and because it has been demonstrated to be a single Pan-European stock (6), it requires coordinated, continent-wide management measures (2, 4, 7) to understand and reverse the decline. However, despite decades of effort, the life cycles of temperate eels remain poorly understood because eels reproduce in remote areas of ocean (8, 9).
Adult European eels begin their spawning migration from European rivers and coasts during the autumn of each year [predominantly October to December (8)] and must migrate a distance of between 5000 and 10,000 km (depending on their departure locations) to the presumed spawning area in the Sargasso Sea. The duration of this journey is not known, but larval surveys have shown that the vast majority of the smallest larvae (≤5 mm) are present from February to late May (10); eels are therefore thought to achieve their spawning migration in a period of around 80 to 170 days (9). However, the migratory achievements of eels remain largely theoretical. Endurance swimming experiments have shown that eels can swim continuously at between 0.4 and 0.7 body lengths s−1 for periods of up to 173 days [a swimming speed of between 22 and 42 km day−1 for an eel of 70 cm (11)], covering a distance of more than 5500 km. Meanwhile, field studies have shown that adult eels travel at between 12 and 50 km day−1 [for European eel, (12–16); for American eel, (17)]. However, despite extending the period of tracking to more than 6 months, oceanic tracking experiments (15, 18) have not yet followed eels to the presumed spawning area of the Sargasso Sea.
Using data from a 5-year tagging study, we tracked the migrations of European eels released from five locations in Europe to determine the important features of the spawning migration. In total, 707 archival and satellite communicating tags were attached to escaping female silver eels. We analyzed the route that the eels took to the Atlantic Ocean and the speed of migration in coastal and oceanic waters. We also show that, as a consequence of diel vertical migrations between depths of 200 and 1000 m, silver European eels experienced a daily temperature range between 0° and 11°C during their oceanic migration, representing an expanded thermal niche. Finally, we place this new information in the context of the timing of the autumn migration from Europe and the occurrence of spawning in the Sargasso to test the hypothesis of a rapid migration to the Sargasso Sea. Our results provide evidence for a new paradigm of eel spawning migration, with consequences for eel stock management.
RESULTS
Recovery of data
The tagging program yielded 206 data sets (29%), of which 87 (12%) were from eels that escaped the coastal margins (fig. S1 shows the pop-up or recovery locations of these tags). These tags provided 4883 days of depth and temperature data from which we could reconstruct behavior and habitat use. All results that follow are derived from these data.
Migratory behavior
Thirty-three eels (more than one-third) migrated into oceanic waters (>200-m depth) in the Atlantic (Table 1), whereas 54 migrations ended in waters over the continental shelf. We were able to reconstruct the full migratory paths of the eels that migrated to the ocean and, additionally, five eels that migrated from Germany into the North Sea. The paths of the remaining 49 eels were treated as straight paths between the release and pop-off position of the tag. Overall, the migratory trajectories show that European eels follow routes that converge on the Azores region (Fig. 1). Eels released from the Swedish west coast and the Baltic outlet took a northerly route into the Norwegian Sea before turning west into the northeast Atlantic Ocean (Fig. 1 and fig. S2a). The trajectories of these eels were tightly clustered before reaching the Atlantic but spread out afterward. Eels released from the Celtic Sea (Ireland) and Bay of Biscay (France) followed a southwest bearing (Fig. 1 and fig. S2, b and c), with an extrapolated intercept near the Azores. One eel, which was released from Ireland and at liberty for 280 days, reached the Azores at the eastern margin of the Sargasso Sea. None of the eels released into the North Sea (Germany) reached oceanic waters, but four traveled south toward the English Channel, whereas two eels traveled north toward the Norwegian Sea (Fig. 1 and fig. S2d). From the west Mediterranean, eels traveled south and west toward the Straits of Gibraltar, with two eels reaching the Atlantic Ocean and one reaching the straits (Fig. 1 and fig. S2e).
Table 1. Migration metrics for eels that escaped the coast and entered the Atlantic Ocean.
Values are average ±1 SD of the mean. Figures in brackets show the maximum values observed. Two different measures of distance and migration speed were calculated, one representing the entire migration and one that took into account only the distance and speed during the oceanic portion of the migration (eel occupies water depth >200 m). A table providing details for all eel migrations is provided in table S3b.
|
Location (n) |
Duration (days) |
Full distance (km) |
Speed (km day−1) |
Oceanic distance (km) | Oceanic speed (km day−1) |
| Celtic Sea (8) | 106.8 ± 77.0 (273) | 2716.8 ± 1985.1 (6982.5) | 26.3 ± 7.2 (42.5) | 2277.3 ± 2066 (6709) | 24.4 ± 7.0 (33.8) |
| Mediterranean (3) | 157.1 ± 43.2 (183) | 1774.3 ± 434.7 (2245) | 11.5 ± 1.9 (12.9) | 1360.6 ± 498 (1905) | 14.5 ± 7.2 (21.8) |
| North Sea (5) | 27.8 ± 9.3 (34) | 533.6 ± 269.8 (794) | 21.4 ± 5.9 (29.1) | ||
| Baltic Sea (22) | 103.5 ± 42.8 (157) | 1768.2 ± 623.6 (2887) | 18.1 ± 5.5 (29.0) | 1574.6 ± 602.2 (2638) | 22.7 ± 8.3 (35.4) |
| Summary (38) | 98.5 ± 57.1 (273) | 1805.9 ± 1176.2 (6982.5) | 19.8 ± 6.9 (42.5) | 1725.5 ± 1136.4 (6709) | 22.4 ± 8.1 (35.4) |
Fig. 1. Reconstructed migrations of European eels.
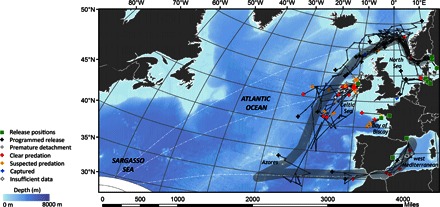
Reconstructed migrations or end positions of 87 eels that migrated into the Atlantic Ocean relative to the assumed spawning area in the Sargasso Sea (hatched area). Release positions are shown as green squares, whereas end positions are shown by crosses. Dashed lines show the most direct (great-circle) routes to the spawning area from the Celtic Sea, Baltic Sea, and Mediterranean Sea. The shaded gray lines shows the approximated routes of Atlantic and Mediterranean migrations used for extraction of temperature data shown in Fig. 4.
Most of the migration routes were consistent with the reverse direction of the northern part of the subtropical gyre in the North Atlantic Ocean, which branches east of Cape Hatteras into the North Atlantic and Azores Currents. The waters within these currents originate from the Gulf Stream, which passes along the western border of the Sargasso Sea. The routes did not conform to the shortest (great-circle) routes (Fig. 1). Overall, the average distance traveled by eels was 953 km (±1085 km; range, 77 to 6982 km; table S1). However, eels that reached the ocean traveled twice as far as the average distance over periods of up to 280 days (Table 1). From the easternmost release to the westernmost pop-up location, we tracked eels an end-to-end distance of ~5000 km, more than half the distance to the spawning area. In total, 14 eels migrated more than 2000 km, with one migrating more than 6900 km over a period of 10 months. The reconstructions of these long migrations show the existence of many meanders in the migration trajectories, leading to a greater estimated travel distance than simply release to pop-up position. The eel that migrated the longest distance length doubled back toward the Azores after traveling south (Fig. 1).
Migration speed varied between 3 and 47 km day−1 (19.4 ± 9.8 km day−1, n = 87; Fig. 2A and table S1) or between 0.03 and 0.56 body length s−1 (0.253 ± 0.123; Fig. 2B). There was no obvious clustering of migration speeds; the distribution conformed to a log-normal fit. Migration speed was independent of release location and date of release and did not vary depending on tag type (two-way analysis of variance, P > 0.05 in all cases; table S2). Eels that reached the ocean showed an overall migration speed between 7 and 35 km day−1, whereas those eels that did not reach the ocean showed overall migration speeds ranging from 3 to 47 km day−1 (Fig. 2A). Eels that reached the ocean exhibited faster migration speeds in the ocean than when they were migrating over the European shelf (22.4 km day−1 versus 19.5 km day−1; t1,32 = 2.6; P < 0.01, paired t test). Comparisons of average migration speed values were made with those available within the literature (Fig. 2C); there were no significant differences between the speeds that we observed and in situ studies using internal tags [t1,11 = 0.75; P > 0.05, t test (14, 19–26); table S3] or external pop-up satellite archival tags (PSATs) deployed on American eels (A. rostrata) [t1,16 = 1.6; P > 0.05, t test (17)], and the range of speed values was similar. The observed values of migration speed were lower than measured values of swimming speed measured in swim tunnel studies deployed on American eels (A. rostrata) [t1,8 = 5.21; P < 0.01, t test (11, 27–33); table S3], noting that these studies were undertaken at higher water temperatures than in situ (18°C versus ~10°C) and without the effect of adverse water currents.
Fig. 2. Migration speeds.
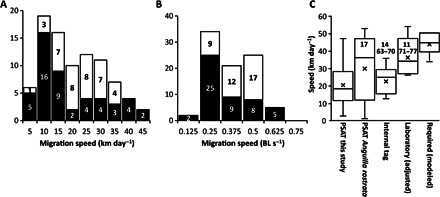
(A) Frequency distribution of observed migration speeds of tagged eels. Migration speed was calculated from the distance taken over either the fully reconstructed migration path (n = 38) or the distance between release and pop-up (n = 49) when full reconstruction was not possible. Labels show the midpoints of the bins. (B) As for (A), but expressed in body lengths per second. The migration speeds of eels that reached the ocean are shown in white, whereas the speeds of those eels that did not reach the ocean are shown in black. (C) Comparison of eel travel speeds reported here and in the literature (citations are given within or above the data). Box plots show the median value as a horizontal line and the mean as a cross. To enable comparison, swimming speeds observed in swim tunnel studies have been adjusted downward by 5 km day−1 to account for the average current speed that eels are likely to experience in the northeast Atlantic (45).
Of those eels that migrated for >1 week, approximately half (n = 41) suffered predation, occurring on average after 32 days (±39.2 days; Table 2 and table S4). Eels released from Ireland and the Mediterranean experienced the greatest risk of predation (27 of 44 and 5 of 8, respectively; fig. S2, b and e), whereas only two eels released from Sweden suffered the same fate (17 of 23 Swedish eels reached the scheduled tag pop-off date with only two definite predation events; figs. S2a and S3a). Tag data suggested that diverse predators were responsible (fig. S3, a to f). Ten predation events (~25%) occurred in oceanic waters, demonstrating a continued risk throughout migration.
Table 2. The fate of eels released from the European coast.
| Bay of Biscay |
Celtic Sea |
Mediterranean | North Sea | Baltic Sea | Total | |
| Pop-off | ||||||
| Premature | 1 | 7 | 0 | 2 | 2 | 12 |
| Scheduled | 0 | 2 | 3 | 1 | 17 | 23 |
| Predation | ||||||
| Strong evidence of predation | 2 | 13 | 5 | 1 | 2 | 23 |
| Suspected pelagic predation | 4 | 9 | 0 | 0 | 1 | 14 |
| Suspected benthic predation | 0 | 5 | 0 | 0 | 0 | 5 |
| Caught by fisherman | 0 | 0 | 0 | 1 | 0 | 0 |
| Unknown | 0 | 8 | 0 | 0 | 1 | 9 |
| Total | 7 | 44 | 8 | 5 | 23 | 87 |
Vertical migrations
Eels that reached oceanic depths exhibited a stereotypical vertical migration (Fig. 3), moving from shallow to deep water between night and day. Vertical migration resulted in a diel temperature change with experienced temperatures being cooler during the day than at night (Figs. 3 and 4B). Eels released from the Swedish west coast and the Baltic outlet experienced extreme ambient temperatures below 0°C when in the Norwegian Sea (fig. S4), whereas eels released from the Celtic Sea (Ireland) rarely experienced temperatures lower than 9°C. In the west Mediterranean, where the water column is homogeneous in depths <1000 m, temperature remained at 13.5°C (Fig. 4B). In general, light data were not recorded by the tags; their shallowest depths coincided with nighttime, and light levels at daytime depths were beyond the detection capability of the PSATs.
Fig. 3. Vertical migration behavior.
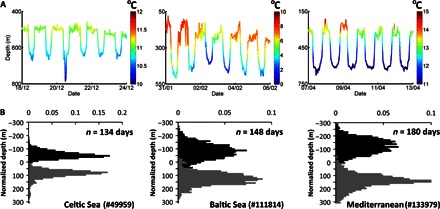
(A) Example vertical migrations of individuals measured over a 6-day period. Charts show an individual (#49559) released from Ireland that was migrating in the mid-Atlantic Ocean, an individual released from Sweden (#118814) that was migrating in the Norwegian Sea, and an individual from southern France (#133979) that was migrating in the eastern Atlantic Ocean. Depth and temperature data collected at between 15- and 30-min intervals are shown interpolated to a 1-min resolution. The color scales show temperature in degrees Celsius and vary between data sets in relation to eel location. (B) Vertical displacement histograms for the oceanic portion of migration for the same eels shown in (A). Because the average depth of eels increases considerably over the course of their migration, the depth measurements for each day were normalized to the average daily depth and separated into day/night (black/gray) periods. Negative values represent depths shallower than the mean.
Fig. 4. Depth and temperature experience of eels during the westward migration.
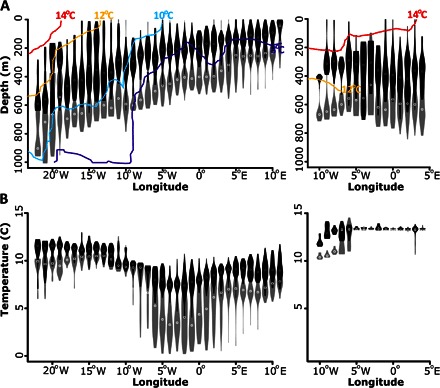
(A) Depth of eels during the oceanic migration. (B) Temperature experience. Data from eels migrating in the Atlantic Ocean are shown on the left, and the Mediterranean Sea on the right. The data are shown as a violin plot. Black violin symbols indicate the depths or temperatures occupied at nighttime, whereas gray symbols indicate the depths or temperatures occupied during the day. Maximal and minimal values for each degree longitude are indicated by the upper and lower limits of each violin symbol, whereas the width of the violin symbols shows the kernel density distribution of observations at that value. Circles within each violin represent the median value for each longitudinal bin. Colored contour lines in (A) indicate thermal structure of the water column within hydrographic sections (shown in Fig. 1) centered on the general oceanic migration paths of eels in the Atlantic Ocean and the Mediterranean Sea. Data are included for all longitude bins where there were more than 20 days of data.
As eels moved west in the Atlantic Ocean, regardless of where they originated, they occupied greater depths and used a larger vertical range (Fig. 4A), consistent with use of the mesopelagic zone. Occupied nighttime temperature in the Atlantic ranged between 8° and 11°C. The deepening of nighttime depths as eels moved west coincided with the deepening of thermal contours (Fig. 4A). Vertical migrations persisted until tag pop-off or predation; the eel at liberty for the longest period maintained the vertical migrations for the 276 days of liberty (fig. S5).
Timing of spawning
Average total length of leptocephali caught in the Sargasso Sea by day of the year is shown in Fig. 5A, which provides a growth rate from linear regression of 0.14 mm d−1 (total length = 0.140 × day − 0.94; R2 = 0.89). Because European eel larvae hatch 2 days after fertilization at a size of approximately 3 mm and grow rapidly for 6 days until the yolk sac is exhausted at a length of ~6 mm (34), the total length measurements of all leptocephali were projected backward in time to the date when a length of 6 mm was reached using the regression parameters; the day of fertilization was taken as this date minus 8 days. Figure 5B shows the frequency of spawning deduced in this way, with a clear peak in 22 February (day 53). Assuming no larval mortality, the spawning period appears to extend between early December and mid-May (90% of spawning activity occurs before 10 April). To account for larvae that die between spawning and capture, two values of instantaneous mortality, 3.8 year−1 (35) and 2.0 year−1 (36), were used to calculate the numbers of larvae required to account for the observed catches. This moves the time of peak spawning slightly earlier (Fig. 5B), between 14 and 18 February, respectively.
Fig. 5. Larval growth rates, timing of spawning, and timing of escapement of European eels.
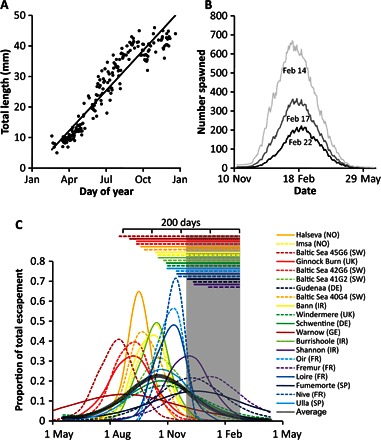
(A) Length measurements of Anguilla anguilla leptocephali belonging to the first year cohort. Each symbol represents the mean of length measurements of leptocephali sampled on each day of the calendar year. The weighted least-squares regression line between day of year and length is represented by a solid black line (length = 0.1401 × day − 0.94). (B) Frequency of spawning of European eels based on back calculation of growth rates. Timing is shown for three different larval instantaneous mortality rates (0, black; 2, dark gray; 3.6, light gray). (C) Timing of escapement of silver eels as a proportion of total measured escapement in 19 catchments between 70° and 43°N within Europe. Each colored line represents a catchment, with the thick black line denoting the average across all catchments. Lines are colored from red to purple according to the timing of peak escapement, whereas the legend is ordered by latitudinal position (north to south). The transparent gray shading shows the start of the spawning period to peak spawning. Colored lines at the top of the chart show the time between peak escapement and peak spawning. Sources of data are given in tables S5 and S6 (66, 93–107).
Timing of escapement
Silver eels are caught in most months in most locations, but the majority are caught between September and December (>80% of escapement was recorded between September and December in 9 of 20 data sets; table S5). Peak escapement occurred between 10 August and 20 December (days 222 to 354; average day, 287 ± 37.5; Fig. 5C) and was only weakly related to the distance required to travel to the presumed spawning area in the Sargasso Sea (Julian escapement date = 427 − 0.021 × distance; adjusted R2 = 0.29, F1,18 = 8.85, P < 0.05). The difference between the average dates of escapement and the timing of peak spawning was 209 days from the farthest locations, but potentially as short as 63 days from the closest (Fig. 5C and Table 3).
Table 3. The phenology and arrival success of eels leaving European catchments.
The proportion of eels arriving by peak spawning and 90% spawning was calculated using observed swimming speeds. The required average travel speed for 50% of eels to arrive by peak spawning was modeled by incrementing the observed speeds at intervals (1 km day−1) and recalculating. The percentage of eels arriving in time for the second spawning opportunity was calculated from observed migration speeds and represents the percentage arriving after the first spawning period has ended, and by 90% of the next. NA, not applicable.
|
Departure (day of year) |
Time to peak spawning (day) |
Peak spawning |
90% spawning |
Required speed (km day−1) |
Second spawning |
|
| All catchments | 288 | 130 | 5 | 12 | 45.9 | 65 |
| Imsa, Norway | 281 | 137 | 4 | 12 | 46.3 | 66 |
| Dee, Scotland | 246 | 172 | 12 | 23 | 43.1 | 61 |
| Lower Bann, Ireland | 276 | 142 | 10 | 21 | 33.8 | 65 |
| Windermere, U.K. | 293 | 125 | 8 | 16 | 36.3 | 65 |
| Schwentine River | 283 | 135 | 3 | 7 | 44.1 | 60 |
| Burrishoole, Ireland | 336 | 82 | 9 | 21 | NA | 66 |
| Shannon, Ireland | 307 | 111 | 2 | 9 | 36.3 | 74 |
| Oir, France | 355 | 63 | 3 | 12 | NA | 71 |
| Fremur, France | 309 | 109 | 1 | 5 | 44.1 | 71 |
| Loire, France | 317 | 101 | 3 | 12 | NA | 71 |
| Fume morte, Spain | 312 | 106 | 3 | 6 | 45.1 | 60 |
| Nive, France | 293 | 125 | 2 | 12 | NA | 71 |
| Gudena, Denmark | 268 | 150 | 3 | 7 | 45.1 | 68 |
| Ulla, Spain | 247 | 171 | 10 | 21 | NA | 67 |
| Halselva, Norway | 223 | 195 | 4 | 12 | 38.6 | 62 |
| ICES 41G2, Sweden | 296 | 122 | 2 | 7 | 41.3 | 64 |
| ICES 40G4, Sweden | 258 | 160 | 3 | 9 | NA | 62 |
| ICES 42G6, Sweden | 293 | 125 | 5 | 12 | 45.1 | 60 |
| ICES 45G6, Sweden | 333 | 85 | 7 | 15 | 41.3 | 58 |
| Warnow, German | 209 | 209 | 5 | 11 | 37.8 | 59 |
Journey duration and timing of arrival at the Sargasso Sea
Observations of migration speed from the tagging experiments were used to calculate the duration of migration and timing of arrival at the spawning area for eels from each catchment. First, a log-normal curve was fitted to the migration speed data. This distribution was then used in combination with the data on the proportion of eels escaping from each catchment in each month to calculate how many eels would arrive at the spawning area in time for the peak of the spawning period immediately following escapement (22 February or day 53), using the shortest distance to the spawning area (table S5). On average, only ~5% of eels would arrive at the spawning area by peak spawning (Table 3), and only ~12% by the time that 90% of larvae are spawned. The highest percentage of arrival was predicted for the Fremur, France (0.12), whereas the lowest was predicted for the river Bann, Ireland (0.01). In contrast, an average of 65% of eels would arrive at the spawning area between the end of the spawning period immediately after escapement and the peak of spawning of the next (Table 3). To assess the increase in migration speed required to enable 50% of eels to arrive by peak spawning in 22 February, the observed swimming speeds were incrementally translated along the x axis, before the log-normal distribution was refitted (truncated to a maximum migration speed of 52 km day−1; fig. S6). The percentage arriving in time for the spawning period immediately after escapement was then recalculated. This calculation is based on the assumptions that (i) eels migrate along the shortest route from escapement to the spawning area, (ii) there are no adverse or beneficial currents, and (iii) eels are aware of the distance to their goal and accordingly adjust swimming speed. The required increase in travel speed for 50% of eels to arrive by peak spawning varied between an additional 15 and 53 km day−1 above the observed travel speeds (Table 3 and Fig. 2C), with eels from six catchments failing to reach the 50% target even when all eels were traveling at 52 km day−1. These required speeds were well in excess of those exhibited by tagged eels described in this study and other field studies (14, 24–26), and of swimming speeds derived from unencumbered eels in swimming tunnel experiments (11, 27–33), as illustrated in Fig. 3C.
DISCUSSION
The data we present in this study are the most comprehensive empirical observations of the oceanic migratory behavior of anguillid eels yet made. Using data from eels released during autumn from the coasts of Sweden, France, Germany, and Ireland, we have mapped the main migration routes from Europe to the Azores region, approximately half the distance to the spawning area in the Sargasso Sea. The range of migration speeds shown by tagged eels (3 to 47 km day−1) was not consistent with the accepted hypothesis that silver eels leave rivers during autumn to spawn as a single reproductive cohort in the following spring (8–10, 17, 37–39). We therefore reanalyzed historic leptocephalus survey data and showed that, although eel spawning appears to begin as early as December and extend until April, the timings of silver eel escapement calculated from a meta-analysis of studies in European rivers are also inconsistent with the accepted hypothesis. Our results suggest that, although some eels may be capable of a rapid migration to the Sargasso Sea to spawn in early spring, it seems likely that many eels undertake a slower-paced migration that enables them to arrive in the Sargasso spawning area before spawning begins again the following December.
Migration route
In the early 20th century, J. Schmidt discovered the spawning area and mapped the spatial and temporal abundance of larvae (38). In doing so, he resolved a significant and enduring mystery of the eel life cycle. Since then, surveys of the Sargasso Sea have refined the probable location and hydrographic conditions for spawning (10, 40, 41), but the routes that silver eels take to get to the Sargasso Sea and how they achieve the migration have proved more difficult to assess because of the technical challenges of following eels in the ocean, and the range of different starting locations across Europe. The results of our tagging program demonstrated that eels released in four different locations in Europe were capable of long-distance migrations that would converge in the Azores region on a westerly route to the Sargasso Sea. The apparent route out of the North Sea via the English Channel is a hitherto unreported result, whereas the routes through the Norwegian Sea, out of the Mediterranean and west from Ireland, have been previously reported (15, 18, 42).
Historically, migration routes to the spawning area have been assumed to be “as the crow flies” from escapement to the Sargasso Sea [for example, (31, 39)]; however, as we have shown, migration routes were not simply error-free great-circle routes (that is, the shortest possible route to the Sargasso Sea from the departure points). Instead, many of the routes were in the reverse direction of the northern part of the subtropical gyre in the North Atlantic Ocean, which is consistent with the hypothesis that eels follow olfactory cues originating in the spawning area or that eels navigate using oceanic cues imprinted or learned during the leptocephalus phase. However, our reconstructions showed that the routes taken by eels contain meanders or deviations from a simple point-to-point migration along the shortest possible route. These meanderings are possibly related to entrapment within eddies or navigational responses to other hydrographic or bathymetric features [exemplified by leatherback turtle behavior (43) in contrast to the “perfect” migration assumed in modeling studies (44)].
Migration speed
Our study provides estimates of in situ migration speed over extended periods (up to ~280 days) and distances, and under natural conditions. In contrast, early studies of in situ migration used small implanted ultrasonic transmitters but could only follow the behavior of eels for a duration of a few days (24–26), whereas laboratory studies using swim tunnels have proved the endurance swimming capability of eels for up to 150 days (27–33), but without freedom of movement in three dimensions or the stressors of the natural environment. The distribution of migration speeds we observed was broad, ranging from 3 to 47 km day−1. Most of the observations fell between 10 and 30 km day−1 (0.25 to 0.5 body length s−1). In addition, our estimates of migration speed were derived from eels at the upper end of the size distribution; these are likely to be the fastest swimmers and therefore faster than the average migration speeds of the European eel population. These migration speeds and the rate of progress toward the Sargasso Sea were not sufficiently fast, and the variation in travel speed was too great, to support the hypothesis that all eels make a short and rapid migration to the Sargasso Sea.
Laboratory studies of eel swimming endurance (27–33) show that optimal swimming speeds are somewhat higher than our estimates (37 km day−1), but a distinction must be made between swimming speeds measured in warm (18°C) laboratory conditions and migration speeds measured in situ (10°C), when meandering, currents and the requirement for navigation and predator avoidance come into play. For example, eels swimming against the northeast Atlantic drift (for example, eels released from Ireland) face a current (5 km day−1; surface to 1000 m) against migration direction (45). Furthermore, eels will have a reduced optimal swimming speed at lower environmental temperatures. Our estimates of migration speed are consistent with most other field studies of European eel migration (14, 19–26) and also a recent study of American eel migration (17). Furthermore, it is not known whether eels in the ocean swim continuously at the same speed or modify their behavior in response to environmental cues or the day/night cycle. For example, silver European eels tagged in the Baltic Sea (14) were shown to rest completely during daylight hours and, if similar behavior occurred in the ocean, would considerably reduce average migration speeds.
Two methodological factors may lead to bias in our estimates. First, our estimated migration routes are simplified and likely shorter than the true length because it was not possible to map the migrations in great detail, with the consequence that we underestimate migration speed. Second, we cannot rule out that the eels in our study experienced some tagging effect (27). Previous studies on tagged eels have shown that tags increase drag and therefore increase the cost of transport (energy expended per unit distance) and reduce critical swimming speed (27, 30, 31, 46). However, the influence of external tags on optimal swimming speed and migratory period is thought to be negligible (30). Although the long-term effects of tag attachment on migrating eels are not known, we argue that, despite the added energetic cost of the increased drag, the migratory period of tagged eels is likely to be sufficiently similar to untagged eels to provide a good estimate of oceanic migration speeds. In support of this interpretation, our experiments used both internal and external tags, but eels tagged internally migrated no faster than eels with external tags. Thus, although much remains to be understood about eel migratory behavior in the ocean and assessments of tagging effect on oceanic migration speeds are difficult to make, our observations provide the most comprehensive description yet of eel oceanic migratory capability, both in terms of the overall migration speed and the broad distribution of migration speeds among eels (30). The evidence we present suggests that the duration of migration to the spawning area in the Sargasso Sea may be, for many eels, longer than the assumed 4 to 6 months, and that the a priori assumption that eels spawn in the spring following their departure from the continental habitat during autumn should be reconsidered.
Timing of spawning and escapement
Despite a number of studies, the onset and duration of the spawning season of the European eel are unclear. On the basis of the data from his early 20th century surveys, Schmidt (38) estimated that spawning commences in late winter or early spring and lasts to well on in summer. Van Ginneken and Maes (9) state a spawning period in March to June. Other estimates are March to May (35, 40). A recent review by Miller et al. (10) assessed that the most extensive spawning occurs in March to April, but other methods provide contrasting results (35). In common to all those estimates is that they rely on when the smallest leptocephali are found but underrepresent the simultaneous occurrence of larger leptocephali. Instead, our analysis was based on the occurrence and density of leptocephali and back calculation using regression-based estimates of growth rate. This suggests that, although spawning may extend until the end of May, the first larvae are likely to hatch in December, significantly earlier than currently thought. An early spawning time was originally proposed by Schmidt (38) and also by Tesch (47) on the basis of estimated growth rate calculated using the same methods as used here. Our estimates were based on the average length of larvae collected throughout the year and are therefore subject to sampling bias. This type of back calculation of spawning date is, of course, sensitive to estimates of growth rate. Higher estimates of larval eel growth rate based on otolith analysis, rather than our length-based method, support a later spawning date (48, 49). Resolving the differences in spawning date estimates from different methods is beyond the scope of this paper, but an in-depth analysis of leptocephalus survey data that incorporates information on eel growth rates from laboratory and field studies [for example, Castonguay (49)] will help to reduce uncertainty.
Nothing is known about the arrival of eels at the spawning area. Our analyses of larval growth rates suggest that half of the spawning will have occurred by mid-February, indicating that at least half the spawning stock needs to arrive before that. However, evidence from Anguilla japonica (50) suggests that eels may be batch spawners, and if European eels also have this spawning strategy, then it is possible that the distribution of arrival of spawners would need to be skewed further toward the beginning of the spawning period to explain the pattern of larval timing and abundance. Our recalculation of peak spawning dates suggests that, to have the best chance of spawning, the distribution of arrivals might therefore be biased toward an even earlier date.
On the basis of our observations of migration speed, the timing of spawning does not match well with the timing of escapement from many major European rivers. Our reanalysis of catch data provides evidence that, although eels departing from a greater distance from the Sargasso Sea tend to leave earlier, the timing of peak silver eel escapement lies within the autumn months (September to December) across Europe. Under the current migratory paradigm, in which eels reach the spawning area within a few months or fail to spawn (39), most of the European eels would therefore need to take no more than 3 or 4 months (and often considerably less) from escapement to arrive in time for the peak spawning period that we have identified. Our analysis suggests that, even if migration were perfect, eels would have to migrate at speeds well in excess of those observed in our study (and all others, including laboratory studies of swimming speed) to achieve an arrival in the period of peak spawning. Furthermore, our observations of migration speed provided no evidence of distance-compensated migration speed; eels released at the same time from different catchments swam at the same range of speeds, regardless of the distance to the Sargasso Sea, which reduces the potential for arrival in time for peak spawning. Vøllestad et al. (37) showed that much of the variation in escapement timing is explained by spatial and temporal differences in water temperature and water flow. The interannual variability in these parameters undermines the precise timing required for synchronous spawning in the Sargasso Sea the following spring and, combined with the evidence we present on the timing of spawning and escapement, suggests that escapement and spawning may not be as tightly coupled as previously assumed.
Vertical migrations and predation
European eels, like other species in the Anguilla genus, make stereotypical diel vertical migrations each day during their oceanic migrations (15, 51–53), and we have shown that this behavior persists up to 10 months after release. Diel migrations resulted in eels moving from cooler deep environments during the day to warmer shallower environments during the night. In other species of marine fish, occupation of deep water during the day is often related to predator avoidance (54), and this seems a strong possibility for eels, too. The gradual deepening of maximum depth as eels migrated west is consistent with a deepening light extinction point in the increasingly clear water further from the continental shelf. This is further supported by the gradual deepening and then shallowing of depth during each day (Fig. 3A and fig. S4), which is suggestive of an inverse daylight curve and is consistent with eels tracking an isolume. Similar deep migrations have been reported for A. japonica (51), Anguilla dieffenbacchi (53), Anguilla marmorata, and Anguilla megastoma (52). These vertical migrations often had a large effect on the temperatures that the eels experienced, particularly in the early portion of the migration when eels had not yet reached the oceanic waters of the Atlantic. In the case of eels migrating through the Norwegian Sea, these deep migrations resulted in occupation of extremely cold waters, well below the temperatures at which silver eels normally stop migrating downriver (37) or at which yellow eels normally experience torpor or hibernation (55). In contrast, there was essentially no change in temperature between the daytime and nighttime swimming depths in the Mediterranean. Occupation of deeper water during the day does not therefore indicate a requirement for a particular thermal environment [as also argued in the study by Jellyman and Tsukamoto (52)]. However, once in the oceanic Atlantic, eels released from different regions experienced similar and more stable temperatures (~10°C) during the day, but not at night, similar to the differences for A. japonica reported previously (51). This is consistent with eels tracking an isotherm during the day.
Despite their presumed antipredator behavior, a large proportion of tagged eels suffered predation. This is not unexpected and has been described elsewhere (56–58). In some cases, predation has been associated with behavioral disruption or lower critical swimming speeds brought on by the tagging procedure [for example, Béguer-Pon et al. (56)] or the transition to the marine environment (our additional suggestion). This may, at least in part, explain some of the predation mortalities suffered by tagged eels soon after release. However, in the cases of predation of eels away from the coast or when eels had been at liberty for some time, it is more likely that they migrated into “predator hot spots.” Future work mapping the density of the large predator guild (59) will provide a clearer idea of the risk that predation poses to the size of the European eel spawning stock.
Synthesis
The assumption that eels conduct a “short and rapid” migration between the continental habitat and the spawning area is long-held (8–10, 17, 37–39), despite the lack of empirical evidence to support it. Our data challenge this paradigm of European eel spawning ecology in two important ways. First, migration speeds of eels are not sufficiently fast to enable a large proportion of the eels to reach the Sargasso Sea in time for peak spawning. Second, the timings of autumn escapement and the timing of peak spawning are, respectively, too late and too early to enable any but the most rapid migrators to reach the spawning area in time to reproduce successfully. Our data and analyses provide a powerful argument for a mixed migratory strategy for European eels, with some individuals able to achieve a rapid migration to the Sargasso in time for the spawning season immediately after escapement, whereas others arrive only in time for the following spawning season one year later. Given that eels are semelparous, this strategy may give the best “bet-hedging” outcome.
The possibility that eels conduct migration lasting a year or more has previously been stated to be unlikely on the basis that eels do not feed during migration [(8, 39) and references therein] and therefore do not have sufficient energy stores to support migration over such a long period. However, long periods of starvation in advance of spawning occur in other long-distance migrators, such as Atlantic salmon (60, 61), and may even be necessary to enable development to full sexual maturity (50). A similar capacity cannot be ruled out for eels, which are much more efficient swimmers than salmon (9). In previous experiments in swim tunnels conducted at an average temperature of 18°C, eels lost only 20% of body mass after 150 days of continuous endurance swimming (28), which would rise to 60% if extrapolating to the extreme case of a 450-day migration. Our results show that eels occupy much lower temperatures (~10° to 12°C) for at least the first half of the migration; basal metabolic rate and the cost of travel would therefore be considerably lower. However, in contrast to laboratory studies, eels in the oceanic environment migrate with the additional energetic cost of vertical migrations. Although the added distance of the vertical migrations is relatively small compared to the horizontal distance being covered, buoyancy control is likely to incur additional energetic cost (62). The total cost of a long and slow migration may therefore appear large and unsustainable. Nevertheless, Tsukamoto et al. (50) provided evidence that spawning Japanese eels (A. japonica) are almost entirely wasted during spawning, suggesting that a significant proportion of energy is lost during the migrations and sexual maturation. Furthermore, if eels perform burst swimming (63) while migrating, or if eels change their rate of swimming between day and night [as for Westerberg et al. (14)], then the costs of covering the distance to the spawning ground would be considerably reduced. A long and slow migration may therefore be energetically possible and also confer other advantages, such as providing more time to sexually mature and find spawning aggregations. Conversely, migrating slowly would also bring disadvantages, such as the gradual metabolic depletion of energy stores over time (that could otherwise have been used for reproduction) and prolonged exposure to predation risk, although the abundance of predators in the Sargasso Sea is relatively low. At present though, there is insufficient data to draw firm conclusions, and additional research will be necessary to further resolve these uncertainties.
There are alternative explanations that can be suggested to account for the mismatch between migration speeds, migration onset, and spawning time. One of these is that silver eels departing from different locations across the continental range will not reach the Sargasso simultaneously and therefore spawn at either multiple different sites within the Atlantic or at different times within the Sargasso Sea. Another explanation, which is favored by some (39, 64), is that only eels that can reach the Sargasso Sea within a few months of escapement will be successful spawners. This would exclude almost all eels except the largest and those from the western edge of Europe. None of these explanations, or variants of them, provide a more parsimonious explanation than the mixed migration strategy that we suggest, which fits with the concept of a flexible spawning ecology that minimizes the risk of reproductive failure by ensuring arrival at the spawning area ahead of time and enabling the development of full sexual maturity before the spawning season begins in earnest.
Our conclusion, while opening a number of avenues for further research, has ramifications for population models used in stock assessment. For example, the predation risk and reproductive potential of eels migrating at different speeds may differ, with consequences for estimates of reproductive potential within the current European eel recovery plan (4). Although the decline in eel populations over the last 30 years (1–4) is unlikely to be related to changes in eel migratory strategy, changes in environmental factors that trigger the timing of escapement events such as climate-related rainfall patterns (65) that reduce the proportion of eels that are able to escape from catchments, barriers to migration (66), or factors that may influence the migratory endurance of eels [for example, fat content (67)] may well be contributory. Our results provide a new basis for exploring these factors, as well as a new paradigm for eel migration; further studies of eel migration, energetics, and swimming behavior will help to evaluate our conclusions.
MATERIALS AND METHODS
Study location
Female silver eels were caught during the autumn escapement period (September to December) in the lower reaches or estuaries of major European rivers between 2006 and 2012 (table S6). Eels in western France were caught by commercial stownet fisherman in the Loire River during the fishing season. Eels tagged in southern France were caught by fishermen in the Salses-Leucate and Gruissan lagoons during the authorized fishing season. Eels in Ireland were obtained from authorized commercial fisheries in the lakes Lough Mask and Lough Owel, and from the river Shannon. Eels in Sweden were captured in the river Enningdal drainage area by licensed commercial fishery and in a wolftrap near the outlet of Lake Fegen at the head of the river Ätran. Eels in Germany were caught by a commercial fyke net fisherman in the lower part of the river Eide and commercial stow net fisherman in the lower part of the river Havel (a tributary of the river Elbe) during the fishing season in the autumn of 2012. Eels in Spain were caught by professional eel fishermen operating in La Albufera. Additional tagging work was undertaken in the lagoons of Salses-Leucate and Gruissan, and Bages Sigean lagoon in France in 2011 and 2013.
Tags and data recovery
Two different tag types were used: PSATs and data storage tags (DSTs). The PSATs used were the Microwave Telemetry X-tag (www.microwavetelemetry.com/), which is 120 mm long with a 185-mm-long antenna (fig. S7a). The maximum diameter of the float is approximately 33 mm. Weight in air is 45 g, and net buoyancy in water was approximately 0.025° ± 0.006°N, corresponding to a negative weight of 2.6 g. The tags measure and store pressure, temperature, and light data every 2 min. A subset of these data are transmitted via Argos satellite when the tag pops up at either a predetermined date, or if any of the fail-safe features are triggered, for example, if a critical pressure is exceeded. The temperature measurement range is −4° to 40°C with a resolution of 0.23°C. The depth range is 0 to 1300 m with a resolution varying between 0.34 and 5.4 m depending on the gain value, which is automatically selected according to the depth measured at midnight each day. The time of release of PSATs was set to 3, 6, 10, 12, or 15 months after deployment. The constant pressure release feature that detaches the tag if the depth reading remains within 3 m for a period of 4 days was deactivated for the first 20 days after deployment to avoid premature release associated with limited movements in shallow water. After a tag pops up, surface position and a time series of depth and temperature were transmitted from the tag to low Earth-orbiting Argos satellites, where it can be downloaded.
We also used the Cefas Technology Ltd G5 long-life archival tag (www.cefastechnology.co.uk/) in combination with three different buoyant float designs. The first design was an implantable tag (hereafter termed as the i-DST; fig. S7a), which was fitted with a string of 11-mm-diameter floats (the same diameter as the tag). The total length with the floats was approximately 140 mm, weight in air was 9.8 g, and the net buoyancy was 0.009°N, corresponding to a negative weight in water of 0.9 g. The second design was an externally attached tag (hereafter termed as e-DST; fig. S7a), which combined a pop-off mechanism and the G5 tag in a cylindrical float with the diameter of 20 mm and length of 130 mm. The weight of this tag in air was 34.5 g, and the net buoyancy in water was 0.046°N. The third design of the e-DST was an externally attached tag (hereafter termed as e-DSTv2; fig. S7a), which was a two-float combination of a pop-off unit and the G5 tag with a diameter of 23 mm and total length of 93 mm. The weight of this tag in air was 31 g, and the net buoyancy in water was 0.0042°N. The floats of both i-DST and e-DST were made from syntactic foam and were painted in bright orange to facilitate detection once stranded at the coast. The depth sensor in the G5 is temperature-compensated in the range of 2° to 34°C. The pressure range used was 1000 m with ±10-m accuracy and 0.3-m resolution. Temperature accuracy is ±0.1°C, and resolution is 0.031°C. The memory available in the tags was nonvolatile flash memory of 2 megabytes. Data were sampled at an interval of 30 s for pressure and 5 min for temperature for the first 12 months. The sampling interval was increased to 5 min for both parameters after this time. Battery life allowed a total data storage time of more than 2 years. The time of release of e-DSTs was set to from 2 up to 5 months after deployment.
Tagging
On the basis of their larger size, all eels selected for tagging were female. A total of 707 eels were tagged. Their mean length was 90.4 cm (±9 cm; range, 63 to 116 cm), and their weight was 1.56 kg (±0.46 kg; range, 0.57 to 3.3 kg). Larger eels were used for PSAT tagging, and the smallest eels were used for i-DST implantation. Two indices of silvering were calculated for each eel: the Pankhurst index [the ratio of the mean eye area to the body length (68)] and the fin index [the percent of the length of the pelvic fin to the body length (69)]. In addition to being caught in gears that typically target silver eels during the autumn escapement, all eels in this study had Pankhurst and fin index values consistent with late-stage maturity and marine migratory status. Fat content of eels was consistent with values obtained for silver eels in other studies (70). Table S7 provides summary metrics of released eels.
Before tagging, eels were anesthetized using metomidate [d1-1-(1-phenylethyl)-5-(metoxycarbonyl) imidazole hydrochloride] at the concentration of 40 mg/liter (71). Eels tagged in the Mediterranean were anesthetized with Aqui-S (Aqua-S) at a concentration of 600 mg/liter. Fish were measured and weighed, and, where possible, their fat content was measured using a Distell Fatmeter [www.distell.com/ffm-general-description/; (72)]. Surgical implantation of i-DSTs was achieved by pushing the tags through a small (~1 cm) incision into the body cavity from the ventral surface. After tag insertion, the incision was closed with two independent single sutures and dusted with Cicatrin antibiotic. Previous long-term tests on tag insertion and effect had shown only limited effect in eels as small as 70 cm (73). For eels tagged before 2009, external tags were attached either using a bridle attached through the dorsal musculature using a stainless steel wire [as for (15)] or, for all eels tagged externally from 2009 onward, a three-point attachment with stainless steel wire inserted dorsally under the skin [fig. S7b; for details of both methods, see the study by Økland (74)]. The rationale behind this attachment technique is that the force exerted by the tag on the eel was concentrated on the anterior attachment point, allowing the two posterior attachment points to heal. If the anterior loop fails, then the tag is still attached by the remaining loops. In long-term tests, these attachment methods were successful in retaining the tags for at least 4 months (74).
Where possible, eels were released after 24 hours, although some were held in covered tanks for up to 3 weeks after tagging. Tagged eels were released at the coast or slightly offshore, near the capture locations [some eels captured in the Loire were transported offshore before release, and some eels captured in Sweden were translocated ~100 km to the coast before release; (18)]. Release positions are shown in Fig. 1 (and by ecoregion in fig. S2, a to e) and in full for all eels that escaped the coastal margins in table S8. Metrics of all eels are provided in table S9.
Determining the fate of migrating eels
Because all tag types rose to the surface once they became detached from their host eel, the data before this terminal point were assessed to determine host fate. Tags often detached prematurely, or the recorded pattern of depth, temperature, or light changed abruptly mid-record, indicating predation (fig. S3). In other less numerous cases, the patterns of depth and temperature changed gradually over a period of a few days before constant depth was recorded at the seabed or sea surface.
For each data set, the events before the final ascent of the tag were classified into the following categories: (i) programmed release (provides a known end point), (ii) premature release (provides a known end point), (iii) clear predation (swallowed as indicated by light or temperature data), (iv) suspected predation (pattern of depth shows abrupt change not consistent with eel behavior), (v) capture in fishing gear (known end point), and (vi) insufficient data, which included those tags that reported sensor failure or failed to transmit time series data.
Reconstructing oceanic migration route
Where possible, the trajectories of PSAT- and DST-tagged eels were reconstructed by using the timing of large vertical movements at dawn and dusk to estimate longitude, and temperature and depths to estimate latitude [as for (18)]. Longitude was calculated by using the diurnal swimming depth changes of the eel as proxy for sunset and sunrise, which provides an estimate of the time of local noon and thereby the longitude (75). All the eels displayed this diurnal cycle, and there was strong justification to believe that the depth changes are cued to dawn and dusk (14, 18, 76). Figure S8 gives an example of the diurnal depth change for one PSAT during the days before its pop-up, and a comparison of pop-up longitude to calculated longitude for 10 PSATs covering the full longitude range of pop-up positions, which provides a robust validation of this technique. The time series of longitude estimates were then used as a starting point for further reconstruction. In the first step, position estimates were refined by comparing eel maximum daily depths with the bathymetry of the general area. In a final refinement, we used specific hydrographic features that could be identified in most records and that were used as checkpoints. Examples are the point where the swimming depth exceeded 450 m simultaneous with a longitude estimate less than 6°E, which means that the eel must have reached the open Norwegian Sea at approximately 62°N, or the abrupt change in vertical temperature stratification passing the Strait of Gibraltar, where the thermally homogenous deep waters of the Mediterranean to the east meet the stratified waters of the Atlantic to the west, at a sill depth of around 300-m depth.
Three milestones were defined in the marine migration. The first was the release date. The second was the beginning of active oceanic migration, defined as the day when the maximum depth reached by the eel exceeded 200 m, which typified the onset of stereotypical large vertical diel migrations during the “oceanic” phase. The third milestone was the end position of the track taken as the position of first transmission of a PSAT or the pop-up position of the DST. The former had least-squares position error of less than 1.5 km, whereas the latter was found by comparing the temperature recorded by the DST with satellite measurements of sea surface temperature for the current date. This provided a relatively narrow latitude estimate, which was combined with the estimate of longitude at the end of the track. The position error depended mostly on the uncertainty of the longitude estimate and was estimated to be of the order of magnitude 100 km (18). In many cases, the migration of the eel could not be determined in this way, either because the data set was short (<10 days) or the eel had not reached the ocean and established daily vertical migrations for longitude estimation. In cases where the eel may have been taken by a predator, depth and temperature data were included until the final day on which the stereotypical diurnal migrations were recorded. The terminal positions of eels that were eaten by predators were uncertain because of the unknown postpredation movement of the predator. In cases where the eel was taken by a predator very soon after release, no attempt was made at reconstruction.
Describing habitat occupation and hydrography
For each data set recovered from an eel that reached oceanic depths, daily summary statistics of depth and temperature were calculated during the oceanic migration. Eels were classified as being in coastal waters when maximum depth remained below 200 m and oceanic when maximum depth exceeded 200 m. For the oceanic migration, deep and shallow phases (and the transitions between them) of the diel cycle were identified for each day using the points of inflection as eels made steep ascents or descents at dawn or dusk. Average occupied depths and temperatures during these phases were then calculated.
Data collected by the tagged eels were compared to long-term hydrographic data available from the World Ocean Atlas 2013 to help assess patterns in eel habitat occupation with general oceanographic characteristics. The horizontal resolution of the Atlas was one-fourth of a degree with 102 depth levels from surface to 5500 m. We used quarterly mean temperature and salinity for the time span of 1955–2012 to determine the average temperature stratification along a section following the general migration paths of the eels in the Atlantic Ocean (October to December; Fig. 1) and Mediterranean (January to March).
Determining the timing of spawning and escapement in European catchments
Historical data on eel larvae, which were compiled by J. D. McCleave and hosted by International Council for the Exploration of the Sea (ICES) (77), were analyzed to determine the changes of the length distribution of Anguilla anguilla leptocephalus larvae during the season. The database covers the result of eel larvae surveys from 1862 to 2007. The data set describes a total of 2375 hauls made using varying plankton and larval nets, which yielded a catch of more than 32,000 Anguilla anguilla or Anguilla rostrata leptocephali. The stations were spread across the North Atlantic but concentrated in the presumed spawning area in the Sargasso Sea. This database has been used in and is described in detail in a recent review by Miller et al. (10).
For our analysis, all Anguilla anguilla length measurements were first compiled into 10-day bins for the whole year. In most cases, the length distribution within each bin showed a peak in the small size range, which could be interpreted as the cohort spawned that season, and a secondary peak in a larger size range, which could be interpreted as the cohort spawned the previous year or years (fig. S9 shows the total number of measured leptocephali found in the database, and how these were split into a young of the year versus cohort spawned in previous years). The data from the secondary peak within each bin were removed from the data set; the number of measured leptocephali remaining in the data set that represented the young of the year was 14,631. Data were then rebinned by day, and the average total length per day was calculated to enable a regression analysis of the growth rate of leptocephali during their first few months of life. We used the slope of this regression together with the most recent estimate of size at hatching and growth rate during the yolk sac stage (34) to back-calculate the distribution of estimated spawning dates.
To determine timing of escapement, relevant literature in Web of Science was searched for evidence of the timing and variation in silver eel escapement across a north to south cline within Europe using the search terms “european silver eel catches” or “silver eel migration timing,” returning a total of 61 hits. Of these, nearly half (n = 29) did not provide suitable information or data and were considered no further, while a further 15 (78–92) were excluded after consideration because it was not possible to use the data within them to rebuild a full year’s catch time series. For the 17 remaining studies (table S6) (66, 93–107), silver eel escapement was typically measured monthly over a full annual cycle at the same location (table S5) for a total of 20 separate locations. Monthly values were normalized to proportions before performing a logistic regression on cumulative catch, using May as the first month in the annual escapement cycle.
Ethical statement
Eels were tagged using approved protocols by trained and individually licensed scientists working under national project authority in accordance with institutional and national guides for the care and use of laboratory animals. These guidelines are consistent with Institutional Review Board/Institutional Animal Care and Use Committee guidelines. Tagging in Ireland was performed under the authority of licenses B100/3922 and B100/3770 issued by the Department of Health and Children, Cruelty to Animals Act 1876, as amended by European Communities (Amendment of cruelty to Animals 1876) Regulation 2002 and 2005. Tagging in western France was conducted under the authority of the certificat capacitaire pour l’expérimentation animale (experimental animal certificate) no. A29-039-1 of the Muséum National d’Histoire Naturelle, Dinard. Tagging in southern France was conducted by trained and licensed scientists working under the authority of the certificat d’experimenter sur animaux vertébrés vivants (experimental animal certificate) no. 66.0801 of the Cefrem, University of Perpignan. Tagging in Sweden was conducted by trained and licensed scientists working under the authority of the Gothenburg ethics committee, which approved applications for tagging operations to study eel migrations on the 16 June 2008 (N178/08), 16 June 2010 (166-2010), and on the 14 September 2011 (250-2011). Tagging in Germany followed German legislation concerning care and use of laboratory animals, and ethical permission for the experiments was given by the Ministry of Energy, Agriculture, the Environment, and Rural Areas of the federal state Schleswig-Holstein [reference no. V311-7224.123.3(93-6/12)]. Tagging in Spain was performed under authority of the Consejeria de Medio Ambiente (Generalitat de Valencia).
Supplementary Material
Acknowledgments
We are very grateful to eel fishermen who helped catch eels for tagging or sampling and beachcombers who returned tags. We would like to thank colleagues who participated in the European eels in the Atlantic: Assessment of their decline (eeliad) project, but who we could not include in this study. The assistance of the editor and the comments and questions of two anonymous referees were extremely helpful during the review process and helped to improve the paper considerably. This paper is dedicated to F.-W. Tesch for laying the foundations for modern eel study and providing an original vision of how to track eels in the ocean. Funding: Most of the work described in this paper was funded under Grant Agreement GOCE-2008212133 (EELIAD) of the European Union FP7 research program on the environment (including climate change) and prepared under project no. 212133 (eeliad). National and regional governments also provided in-kind funding to support the work including the U.K. Department for Environment, Food, and Rural Affairs (to D.R., J.D.M., and A.W.); the French Ministry of Ecology, Sustainable Development, and Energy (to E.A.); the German Ministry of Agriculture and Environment of the federal state Saxony-Anhalt; the German Ministry of Agriculture, Environment, and Consumer Protection Mecklenburg-Vorpommern (to J.S. and U.B.); the Inland Fisheries Ireland (to P.G.); the Norwegian Institute for Nature Research (to F.Ø.); the Swedish Board of Fisheries (latterly Swedish University of Agricultural Sciences to H.W. and N.S.); the Villum Kann Rasmussen/Elisabeth and Knud Petersen’s Foundations (to K.A.); the Fundo Social Europeu/Ministério da Educação e Ciência; and the Portuguese Science and Technology Foundation (FCT) through PhD grant PD/BD/52603/2014 (to M.V.). In-kind support was provided by the Consejeria de Medio Ambiente de la Generalitat de Valencia, Spain. Author contributions: D.R. conceived and led the writing of the manuscript and analyzed data from archival tags to describe the environmental experience of eels. H.W. developed methods for and made the reconstructions of eel trajectories, contributed to the conception and writing, and analyzed the leptocephalus data. K.A. led the eel tagging program and contributed to the conception and writing of the manuscript. E.F. led the contribution to the eel tagging program in France and contributed to data interpretation and the writing of the manuscript. F.Ø. led the development of tagging methods and handling protocols and contributed to eel tagging programs in Ireland, France, Sweden, and Spain. P.G. led the eel tagging program in Ireland. E.A. led the contribution to the eel tagging program in the Mediterranean. J.M. contributed to the development of methods and eel tagging program in Ireland, France, and Spain. J.L.-C. led the contribution to the eel tagging program in Spain. N.S. led the eel tagging program in Sweden. J.S. led the eel tagging program in Germany and contributed to the analysis of data. A.A. contributed to the eel tagging program in France and the analysis of eel escapement data. M.V. contributed to data analysis and interpretation. A.W. contributed to the eel tagging program in Ireland and France. T.T. contributed to the analysis of eel escapement data. U.B. contributed to the German eel tagging program and data analysis. All coauthors contributed to and commented on the manuscript before submission. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1501694/DC1
fig. S1. Pop-off or recovery positions of DSTs and PSATs.
fig. S2a. Migration and end points of eels released from the Baltic Sea.
fig. S2b. Migration and end points of eels released from Ireland.
fig. S2c. Migration and end points of eels released from western France.
fig. S2d. Migration and end points of eels released from Germany.
fig. S2e. Migration and end points of eels released from the Mediterranean coast.
fig. S3a. Example of an eel (PSAT #89310, released from Ireland) being preyed upon by a (assumed) pelagic fish.
fig. S3b. Example of an eel (PSAT #49644, released from Ireland) being preyed upon by an endothermic fish.
fig. S3c. Example of an eel (PSAT #101445, released from Ireland) being preyed upon by a coastal pelagic predator.
fig. S3d. Example of an eel (PSAT #83156, released from Ireland) being preyed upon by a deep living fish.
fig. S3e. Example of an eel (PSAT #49559, released from Ireland) being preyed upon by a marine mammal.
fig. S3f. Example of an eel (PSAT #133984, released from southern France) being preyed upon by an unknown endotherm.
fig. S4. Example of daytime thermal experience of eels during migration along the Norwegian trench/deep in the Norwegian Sea.
fig. S5. Depth and temperature time series from PSAT #83140.
fig. S6. Relative frequency distribution of swimming speed.
fig. S7. Electronic tag types and attachment technique.
fig. S8. Principle and validation of behavioral geolocation.
fig. S9. Analysis of the total number of length-measured European eel leptocephali in the ICES database.
table S1. Details of reconstructed migrations of eels that reached the ocean.
table S2. Assessment of effect of tag and release country on speed, migration duration, and migration distance of eels.
table S3. The speed of European eels.
table S4a. The fate of eels that reached oceanic waters.
table S4b. The fate of all released eels for which data sets were recovered.
table S5. Summary of literature used to assess escapement date.
table S6. Silver eel escapement timing in the River Gudena, Denmark.
table S7. Release locations and numbers of eels released during the study.
table S8a. Summary metrics of tagged eels.
table S8b. Summary metrics of eels for which migratory data were recovered (±1 SD).
table S9. Metrics of all eels tagged (n = 707).
REFERENCES AND NOTES
- 1.Dekker W., Casselman J. M., The 2003 Québec Declaration of Concern about eel declines—11 years later: Are eels climbing back up the slippery slope?. Fisheries 39, 613–614 (2014). [Google Scholar]
- 2.Jacoby D. M. P., Casselman J. M., Crook V., DeLucia M.-B., Ahn H., Kaifu K., Kurwie T., Sasal P., Silfvergrip A. M. C., Smith K. G., Uchida K., Walker A., Gollock M., Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 4, 321–333 (2015). [Google Scholar]
- 3.Miller M. J., Feunteun E., Tsukamoto K., Did a “perfect storm” of oceanic changes and continental anthropogenic impacts cause northern hemisphere anguillid recruitment reductions?. ICES J. Mar. Sci. 73, 43–56 (2016). [Google Scholar]
- 4.Dekker W., Beaulaton L., Climbing back up what slippery slope? Dynamics of the European eel stock and its management in historical perspective. ICES J. Mar. Sci. 73, 5–13 (2016). [Google Scholar]
- 5.www.iucnredlist.org/details/60344/0 [accessed 7 August 2015].
- 6.Als T. D., Hansen M. M., Maes G. E., Castonguay M., Riemann L., Aarestrup K., Munk P., Sparholt H., Hanel R., Bernathez L., All roads lead to home: Panmixia of European eel in the Sargasso Sea. Mol. Ecol. 20, 1333–1346 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Council of the European Union , Council Regulation (EC) No 1100/2007 of 18 September 2007 establishing measures for the recovery of the stock of European eel. Official Journal of the European Union L248, 17–23 (2007). [Google Scholar]
- 8.F.-W. Tesch, The Eel J. E. Thorpe, Ed. (Blackwell Science, 2003). [Google Scholar]
- 9.van Ginneken V. J. T., Maes G. E., The European eel (Anguilla anguilla, Linnaeus), its lifecycle, evolution and reproduction: A literature review. Rev. Fish Biol. Fish. 15, 367–398 (2005). [Google Scholar]
- 10.Miller M. J., Bonhommeau S., Munk P., Castonguay M., Hanel R., McCleave J. D., A century of research on the larval distributions of the Atlantic eels: A re-examination of the data. Biol. Rev. Camb. Philos. Soc. 90, 1035–1064 (2015). [DOI] [PubMed] [Google Scholar]
- 11.van Ginneken V., Antonissen E., Müller U. K., Booms R., Eding E., Verreth J., van den Thillart G., Eel migration to the Sargasso: Remarkably high swimming efficiency and low energy costs. J. Exp. Biol. 208, 1329–1335 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Westerberg H., Counter-current orientation in the migration of the European eel. Rapp. P.-V. Reun. Cons. Int. Explor. Mer. 174, 134–143 (1979). [Google Scholar]
- 13.McCleave J. D., Arnold G. P., Movements of yellow-and silver-phase European eels (Anguilla anguilla L.) tracked in the western North Sea. ICES J. Mar. Sci. 56, 510–536 (1999). [Google Scholar]
- 14.Westerberg H., Lagenfelt I., Svedäng H., Silver eel migration behaviour in the Baltic. ICES J. Mar. Sci. 64, 1457–1462 (2007). [Google Scholar]
- 15.Aarestrup K., Økland F., Hansen M. M., Righton D., Gargan P., Castonguay M., Bernathez L., Howey P., Sparholt H., Pedersen M. I., McKinley R. S., Oceanic spawning migration of the European eel (Anguilla anguilla). Science 325, 1660 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Aarestrup K., Thorstad E. B., Koed A., Svendsen J. C., Jepsen N., Pedersen M. I., Økland F., Survival and progression rates of large European silver eel Anguilla anguilla in late freshwater and early marine phases. Aquat. Biol. 9, 263–270 (2010). [Google Scholar]
- 17.Béguer-Pon M., Castonguay M., Shan S., Benchetrit J., Dodson J. J., Direct observations of American eels migrating across the continental shelf to the Sargasso Sea. Nat. Commun. 6, 8705 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westerberg H., Sjöberg N., Lagenfelt I., Aarestrup K., Righton D., Behaviour of stocked and naturally recruited European eels during migration. Mar. Ecol. Prog. Ser. 496, 145–157 (2014). [Google Scholar]
- 19.Martinköwitz H., Ergebnisse von Blankaalmarkierungen an der ostrügischen Küste und Möglichkeit ihrer Nutzung für die Fangsteigerung durch neuartige Reusenkonstruktionen. Zeitschrift für Fischerei Neue Folge 10, 653–663 (1961). [Google Scholar]
- 20.Tesch F.-W., Telemetric observations on the spawning migration of the eel (Anguilla anguilla) west of the European continental shelf. Environ. Biol. Fish. 3, 203–209 (1978). [Google Scholar]
- 21.Lühmann M., Mann H., Wiederfänge markierter Elbaale vor der Küste Dänemarks. Arch. Fisc.Wiss. 9, 200–202 (1958). [Google Scholar]
- 22.Trybom F., Schneider G., Markierungsversuche mit Aalen und die Wanderungen gekennzeichneter Aale in der Ostsee. Rapp. P.-V. Reún. Cons. Perm. Int. Explor. Mer. 9, 51–59 (1908). [Google Scholar]
- 23.Määr A., Über die Aalwanderung im Baltischen Meer auf Grund der Wanderaalmarkierungsversuche im Finnischen und Livischen Meerbusen i. d. J. 1937–1939. Mitt. Anst. Binnenfisch. Drottningholm 27, 1–56 (1947). [Google Scholar]
- 24.Tesch F.-W., Insignificance of tidal currents for silver eel migration as studied by eel tracking and current measurements. Irish Fish. Invest. Ser. A 36, 105–109 (1992). [Google Scholar]
- 25.Tesch F.-W., Changes in swimming depth and direction of silver eels (Anguilla anguilla L.) from the continental shelf to the deep sea. Aquat. Living Res. 2, 9–20 (1989). [Google Scholar]
- 26.Tesch F.-W., Westerberg H., Karlsson L., Tracking studies on migrating silver eels in the Central Baltic. Meeresforschung 35, 193–196 (1991). [Google Scholar]
- 27.Methling C., Tudorache C., Skov P. V., Steffensen J. F., Pop up satellite tags impair swimming performance and energetics of the European eel (Anguilla anguilla). PLOS ONE 6, e20797 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van den Thillart G., van Ginneken V., Körner F., Heijmans R., van der Linden R., Gluvers A., Endurance swimming of European eel. J. Fish Biol. 65, 312–318 (2004). [Google Scholar]
- 29.Palstra A. P., Heppener D. F. M., van Ginneken V. J. T., Székely C., van den Thillart G. E. E. J. M., Swimming performance of silver eels is severely impaired by the swim-bladder parasite Anguillicola crassus. J. Exp. Mar. Biol. Ecol. 352, 244–256 (2007). [Google Scholar]
- 30.Tudorache C., Burgerhout E., Brittijn S., van den Thillart G., Comparison of swimming capacity and energetics of migratory European eel (Anguilla anguilla) and New Zealand short-finned eel (A. australis). Front. Physiol. 6, 256 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burgerhout E., Manabe R., Brittijn S. A., Aoyama J., Tsukamoto K., van den Thillart G. E. E. J. M., Dramatic effect of pop-up satellite tags on eel swimming. Naturwissenschaften 98, 631–634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palstra A., van Ginneken V., van den Thillart G., Cost of transport and optimal swimming speed in farmed and wild European silver eels (Anguilla anguilla). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 151, 37–44 (2008). [DOI] [PubMed] [Google Scholar]
- 33.van Ginneken V., Palstra A., Leonards P., Nieveen M., van den Berg H., Flik G., Spanings T., Niemantsverdriet P., van den Thillart G., Murk A., PCBs and the energy cost of migration in the European eel (Anguilla anguilla L.). Aquat. Toxicol. 92, 213–220 (2009). [DOI] [PubMed] [Google Scholar]
- 34.M. Davidsen, The effect of incubation temperature on embryonic development and muscle growth in yolk-sac larvae of the European eel (Anguilla anguilla L., 1758), Masters thesis, NTNU (2012). [Google Scholar]
- 35.Bonhommeau S., Castonguay M., Rivot E., Sabatié R., Le Pape O., The duration of migration of Atlantic Anguilla larvae. Fish Fish. 11, 289–306 (2010). [Google Scholar]
- 36.Pacariz S., Westerberg H., Björk G., Climate change and passive transport of European eel larvae. Ecol. Freshw. Fish 23, 86–94 (2014). [Google Scholar]
- 37.Vøllestad L. A., Jonsson B., Hvidsten N. A., Næsje T. F., Haraldstad Ø., Ruud-Hansen J., Environmental factors regulating the seaward migration of European silver eels (Anguilla anguilla). Can. J. Fish. Aquat. Sci. 43, 1909–1916 (1986). [Google Scholar]
- 38.Schmidt J., Breeding places and migrations of the eel. Nature 111, 51–54 (1923). [Google Scholar]
- 39.Capoccioni F., Costa C., Canali E., Aguzzi J., Antonucci F., Ragonese S., Bianchini M. L., The potential reproductive contribution of Mediterranean migrating eels to the Anguilla anguilla stock. Sci. Rep. 4, 7188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCleave J. D., Physical and behavioural controls on the oceanic distribution and migration of leptocephali. J. Fish Biol. 43, 243–273 (1993). [Google Scholar]
- 41.Munk P., Hansen M. M., Maes G. E., Nielsen T. G., Castonguay M., Riemann L., Sparholt H., Als T. D., Aarestrup K., Andersen N. G., Bachler M., Oceanic fronts in the Sargasso Sea control the early life and drift of Atlantic eels. Proc. Biol. Sci. 277, 3593–3599 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amilhat E., Aarestrup K., Faliex E., Simon G., Westerberg H., Righton D., First evidence of European eels exiting the Mediterranean Sea during spawning migration. Sci. Rep. 6, 21817 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hays G. C., Hobson V. J., Metcalfe J. D., Righton D., Sims D. W., Flexible foraging movements of leatherback turtles across the North Atlantic Ocean. Ecology 87, 2647–2656 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Béguer-Pon M., Shan S., Thompson K. R., Castonguay M., Sheng J., Dodson J. J., Exploring the role of the physical marine environment in silver eel migrations using a biophysical particle tracking model. ICES J. Mar. Sci. 73, 57–74 (2016). [Google Scholar]
- 45.Lavender K. L., Owens W. B., Davis R. E., The mid-depth circulation of the subpolar North Atlantic Ocean as measured by subsurface floats. Deep-Sea Res. I Oceanogr. Res. Pap. 52, 767–785 (2005). [Google Scholar]
- 46.Tudorache C., Burgerhout E., Brittijn S., van den Thillart G., The effect of drag and attachment site of external tags on swimming eels: Experimental quantification and evaluation tool. PLOS ONE 9, e112280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tesch F. W., Age and growth rates of North Atlantic eel larvae (Anguilla spp.), based on published length data. Helgol. Meeresunt. 52, 75–83 (1998). [Google Scholar]
- 48.McCleave J. D., Contrasts between spawning times of Anguilla species estimated from larval sampling at sea and from otolith analysis of recruiting glass eels. Mar. Biol. 155, 249–262 (2008). [Google Scholar]
- 49.Castonguay M., Growth of American and European eel leptocephali as revealed by otolith microstructure. Can. J. Zool. 65, 875–878 (1987). [Google Scholar]
- 50.Tsukamoto K., Chow S., Otake T., Kurogi H., Mochioka N., Miller M. J., Aoyama J., Kimura S., Watanabe S., Yoshinaga T., Shinoda A., Kuroki M., Oya M., Watanabe T., Hata K., Ijiri S., Kazeto Y., Nomura K., Tanaka H., Oceanic spawning ecology of freshwater eels in the western North Pacific. Nat. Commun. 2, 179 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manabe R., Aoyama J., Watanabe K., Kawai M., Miller M. J., Tsukamoto K., First observations of the oceanic migration of Japanese eel, from pop-up archival transmitting tags. Mar. Ecol. Prog. Ser. 437, 229–240 (2011). [Google Scholar]
- 52.Jellyman D., Tsukamoto K., Swimming depths of offshore migrating longfin eels Anguilla dieffenbachii. Mar. Ecol. Prog. Ser. 286, 261–267 (2005). [Google Scholar]
- 53.Schabetsberger R., Økland F., Aarestrup K., Kalfatak D., Sichrowsky U., Tambets M., Dall’Olmo G., Kaiser R., Miller P. I., Oceanic migration behaviour of tropical Pacific eels from Vanuatu. Mar. Ecol. Prog. Ser. 475, 177–190 (2013). [Google Scholar]
- 54.Brierley A. S., Diel vertical migration. Curr. Biol. 24, R1074–R1076 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Westerberg H., Sjöberg N., Overwintering dormancy behaviour of the European eel (Anguilla anguilla L.) in a large lake. Ecol. Freshw. Fish 24, 532–543 (2015). [Google Scholar]
- 56.Béguer-Pon M., Benchetrit J., Castonguay M., Aarestrup K., Campana S. E., Stokesbury M. J. W., Dodson J. J., Shark predation on migrating adult American eels (Anguilla rostrata) in the Gulf of St. Lawrence. PLOS ONE 7, e46830 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacroix G. L., Large pelagic predators could jeopardize the recovery of endangered Atlantic salmon. Can. J. Fish. Aquat. Sci. 71, 343–350 (2014). [Google Scholar]
- 58.Wahlberg M., Westerberg H., Aarestrup K., Feunteun E., Gargan P., Righton D., Evidence of marine mammal predation of the European eel (Anguilla anguilla L.) on its marine migration. Deep Sea Res. Part I Oceanogr. Res. Pap. 86, 32–38 (2014). [Google Scholar]
- 59.F. Velasco, M. Aanesen, H. Abreu, C. Armstrong, I. Bashmashnikov, M. Borges, J. M. Cabanas, D. Garza, T. J. Hegland, S. Lens, A. M. Martins, H. V. Mendes, A. Mendonca, J. Pereiro, M. Pérez, C. Porteiro, J. Raakjær, M. Rui Pinho, V. Samedy, A. Serrano, South Western Waters Atlas (University of Liverpool, 2009). [Google Scholar]
- 60.Fleming I. A., Reproductive strategies of Atlantic salmon: Ecology and evolution. Rev. Fish Biol. Fisher. 6, 379–416 (1996). [Google Scholar]
- 61.Klemetsen A., Amundsen P.-A., Dempson J. B., Jonsson B., Jonsson N., O’Connell M. F., Mortensen E., Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish 12, 1–59 (2003). [Google Scholar]
- 62.Pelster B., Swimbladder function and the spawning migration of the European eel Anguilla anguilla. Front. Physiol. 5, 486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weihs D., Energetic advantages of burst swimming of fish. J. Theor. Biol. 48, 215–229 (1974). [DOI] [PubMed] [Google Scholar]
- 64.Tucker D. W., A new solution to the Atlantic eel problem. Nature 183, 495–501 (1959). [DOI] [PubMed] [Google Scholar]
- 65.Kettle A. J., Vøllestad L. A., Wibig J., Where once the eel and the elephant were together: Decline of the European eel because of changing hydrology in southwest Europe and northwest Africa?. Fish Fish. 12, 380–411 (2011). [Google Scholar]
- 66.Pedersen M. I., Jepsen N., Aarestrup K., Koed A., Pedersen S., Økland F., Loss of European silver eel passing a hydropower station. J. Appl. Ichthyol. 28, 189–193 (2012). [Google Scholar]
- 67.Geeraerts C., Belpaire C., The effects of contaminants in European eel: A review. Ecotoxicology 19, 239–266 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Pankhurst N. W., Relation of visual changes to the onset of sexual maturation in the European eel Anguilla anguilla (L.). J. Fish Biol. 21, 127–140 (1982). [Google Scholar]
- 69.Durif C., Dufour S., Elie P., The silvering process of Anguilla anguilla: A new classification from the yellow resident to the silver migrating stage. J. Fish Biol. 66, 1025–1043 (2005). [Google Scholar]
- 70.Svedäng H., Wickström H., Low fat contents in female silver eels: Indications of insufficient energetic stores for migration and gonadal. J. Fish Biol. 50, 475–486 (1997). [Google Scholar]
- 71.Iversen M. H., Økland F., Thorstad E. B., Finstad B., The efficacy of Aqui-S vet. (iso-eugenol) and metomidate as anaesthetics in European eel (Anguilla anguilla L.), and their effects on animal welfare and primary and secondary stress responses. Aquac. Res. 44, 1307–1316 (2013). [Google Scholar]
- 72.Klefoth T., Skov C., Aarestrup K., Arlinghaus R., Reliability of non-lethal assessment methods of body composition and energetic status exemplified by applications to eel (Anguilla anguilla) and carp (Cyprinus carpio). Fish. Res. 146, 18–26 (2013). [Google Scholar]
- 73.Thorstad E. B., Økland F., Westerberg H., Aarestrup K., Metcalfe J. D., Evaluation of surgical implantation of electronic tags in European eel and effects of different suture materials. Mar. Freshw. Res. 64, 324–331 (2013). [Google Scholar]
- 74.Økland F., Thorstad E. B., Westerberg H., Aarestrup K., Metcalfe J. D., Development and testing of attachment methods for pop-up satellite archival transmitters in European eel. Anim. Biotelem. 1, 3 (2013). [Google Scholar]
- 75.Nielsen A., Bigelow K. A., Musyl M. K., Sibert J. R., Improving light-based geolocation by including sea surface temperature. Fish. Oceanogr. 15, 314–325 (2006). [Google Scholar]
- 76.Chow S., Okazaki M., Watanabe T., Segawa K., Yamamoto T., Kurogi H., Tanaka H. A. K.-I., Kawai M., Yamamoto S.-I., Mochioka N., Manabe R., Miyake Y., Light-sensitive vertical migration of the Japanese eel Anguilla japonica revealed by real-time tracking and its utilization for geolocation. PLOS ONE 10, e0121801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. www.ices.dk/marine-data/data-portals/Pages/Eggs-and-larvae.aspx [accessed 20 April 2015].
- 78.Aarestrup K., Thorstad E. B., Koed A., Jepsen N., Svendsen J. C., Pedersen M. I., Skov C., Økland F., Survival and behaviour of European silver eel in late freshwater and early marine phase during spring migration. Fisheries. Manag. Ecol. 15, 435–440 (2008). [Google Scholar]
- 79.Buysse D., Mouton A. M., Stevens M., Van den Neucker T., Coeck J., Mortality of European eel after downstream migration through two types of pumping stations. Fisheries. Manag. Ecol. 21, 13–21 (2014). [Google Scholar]
- 80.Calles O., Karlsson S., Vezza P., Comoglio C., Tielman J., Success of a low-sloping rack for improving downstream passage of silver eels at a hydroelectric plant. Freshwater Biol. 58, 2168–2179 (2013). [Google Scholar]
- 81.Charrier F., Mazel V., Caraguel J.-M., Abdallah Y., Le Gurun L. L., Legault A., Laffaille P., Escapement of silver-phase European eels, Anguilla anguilla, determined from fishing activities in a Mediterranean lagoon (Or, France). ICES J. Mar. Sci. 69, 30–33 (2012). [Google Scholar]
- 82.Haraldstad Ø., Vøllestad L. A., Jonsson B., Descent of European silver eels, Anguilla anguilla L., in a Norwegian watercourse. J. Fish Biol. 26, 37–41 (1985). [Google Scholar]
- 83.Breteler J. K., Vriese T., Borcherding J., Breukelaar A., Jörgensen L., Staas S., de Laak G., Ingendahl D., Assessment of population size and migration routes of silver eel in the River Rhine based on a 2-year combined mark-recapture and telemetry study. ICES J. Mar. Sci. 64, 1450–1456 (2007). [Google Scholar]
- 84.Breukelaar A. W., Ingendahl D., Vriese F. T., de Laak G., Staas S., Klein Breteler J. G. P., Route choices, migration speeds and daily migration activity of European silver eels Anguilla anguilla in the River Rhine, north-west Europe. J. Fish Biol. 74, 2139–2157 (2009). [DOI] [PubMed] [Google Scholar]
- 85.M. C. M. Bruijs, C. F. M. Durif, Silver eel migration and behaviour, In Spawning Migration of the European Eel: Reproduction Index, A Useful Tool for Conservation Management, G. van den Thillart, S. Dufour, J. C. Rankin, Eds. (Springer, 2009), pp. 65–95. [Google Scholar]
- 86.Cullen P., McCarthy T. K., The effects of artificial light on the distribution of catches of silver eel, Anguilla anguilla (L.), Across the Killaloe eel weir in the lower River Shannon. Biol. Environ. 100B, 165–169 (2000). [Google Scholar]
- 87.Cullen P., McCarthy T. K., Hydrometric and meteorological factors affecting the seaward migration of silver eels (Anguilla anguilla, L.) in the lower River Shannon. Environ. Biol. Fishes 67, 349–357 (2003). [Google Scholar]
- 88.Durif C. F. M., Elie P., Predicting downstream migration of silver eels in a large river catchment based on commercial fishery data. Fisheries Manag. Ecol. 15, 127–137 (2008). [Google Scholar]
- 89.Jansen H. M., Winter H. V., Bruijs M. C. M., Polman H. J. G., Just go with the flow? Route selection and mortality during downstream migration. ICES J. Mar. Sci. 64, 1437–1443 (2007). [Google Scholar]
- 90.Deelder C. L., Factors affecting the migration of the silver eel in Dutch inland waters. J. Cons. Int. Explor. Mer 20, 177–185 (1954). [Google Scholar]
- 91.Amilhat E., Farrugio H., Lecomte-Finiger R., Simon G., Sasal P., Silver eel population size and escapement in a Mediterranean lagoon: Bages-Sigean, France. Knowl. Manag. Aquatic. Ecosyst. 5, 390–391 (2008). [Google Scholar]
- 92.Lobón-Cerviá J. Carrascal M., Seasonal timing of silver eels (Anguilla anguilla L.) in a cantabrian stream (North Spain). Arch. Hydrobiol. 125, 121–126 (1992). [Google Scholar]
- 93.Acou A., Laffaille P., Legault A., Feunteun E., Migration pattern of silver eel (Anguilla anguilla, L.) in an obstructed river system. Ecol. Freshw. Fish 17, 432–442 (2008). [Google Scholar]
- 94.A. Acou, C. Boisneau, E. Feunteun, E. Prédiction des pics de dévalaison des anguilles argentées à partir des données environnementales : état des connaissances et développement d’un modèle opérationnel sur la Loire pour la gestion du turbinage (2009), available at http://www.onema.fr/sites/default/files/pdf/2009_066.pdf [accessed 14 September 2016].
- 95.Chadwick S., Knights B., Thorley J. L., Bark A., A long-term study of population characteristics and downstream migrations of the European eel Anguilla anguilla (L.) and the effects of a migration barrier in the Girnock Burn, north-east Scotland. J. Fish Biol. 70, 1535–1553 (2007). [Google Scholar]
- 96.Cobo F., Sánchez-Hernández J., Vieira R., Servia M., Seasonal downstream movements of the European eel in a Southwestern Europe river (River Ulla, NW Spain). Nova Acta Cient. Compostel. 21, 77–84 (2014). [Google Scholar]
- 97.Acou A., Lefebvre F., Contorunet P., Poizat G., Panfili J., Crivelli A. J., Silvering of Female Eels (Anguilla anguilla) in two sub-populations of the Rhone Delta. Bull. Fr.Pêche. Piscic. 368, 55–68 (2003). [Google Scholar]
- 98.Davidsen J. G., Finstad B., Økland F., Thorstad E. B., Mo T. A., Rikardsen A. H., Early marine migration of European silver eel Anguilla anguilla in northern Norway. J. Fish Biol. 78, 1390–1404 (2011). [DOI] [PubMed] [Google Scholar]
- 99.Frost W. E., The age and growth of eels (Anguilla anguilla) from the Windermere catchment area. J. Anim. Ecol. 14, 26–36 (1945). [Google Scholar]
- 100.Gosset C., Travade F., Durif C., Rives J., Elie P., Tests of two types of bypass for downstream migration of eels at a small hydroelectric power plant. River Res. Appl. 21, 1095–1105 (2005). [Google Scholar]
- 101.Hvidsten N., Yield of silver eel and factors effecting downstream migration in the stream Imsa, Norway. Rep. Ins. Freshw. Res. Drottningholm 62, 75–85 (1985). [Google Scholar]
- 102.Marohn L., Prigge E., Hanel R., Escapement success of silver eels from a German river system is low compared to management-based estimates. Freshwater Biol. 59, 64–72 (2014). [Google Scholar]
- 103.Moriarty C., Short note on the silver eel catch on the lower River Shannon. Int. Rev. Hydrobiol. 75, 817–818 (1990). [Google Scholar]
- 104.Parsons J., Vickers K. U., Warden Y., Relationship between elver recruitment and changes in the sex ratio of silver eels Anguilla anguilla L. migrating from Lough Neagh, Northern Ireland. J. Fish Biol. 10, 211–229 (1977). [Google Scholar]
- 105.Poole W. R., Reynolds J. D., Moriarty C., Observations on the silver eel migrations of the Burrishoole river system, Ireland, 1959 to 1988. Int. Rev. Hydrobiol. 75, 807–815 (1990). [Google Scholar]
- 106.Reckordt M., Ubl C., Wagner C., Frankowski J., Dorow M., Downstream migration dynamics of female and male silver eels (Anguilla anguilla L.) in the regulated German lowland Warnow River. Ecol. Freshw. Fish 23, 7–20 (2014). [Google Scholar]
- 107.Trancart T., Acou A., De Oliveira E., Feunteun E., Forecasting animal migration using SARIMAX: An efficient means of reducing silver eel mortality caused by turbines. Endanger. Species Res. 21, 181–190 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/10/e1501694/DC1
fig. S1. Pop-off or recovery positions of DSTs and PSATs.
fig. S2a. Migration and end points of eels released from the Baltic Sea.
fig. S2b. Migration and end points of eels released from Ireland.
fig. S2c. Migration and end points of eels released from western France.
fig. S2d. Migration and end points of eels released from Germany.
fig. S2e. Migration and end points of eels released from the Mediterranean coast.
fig. S3a. Example of an eel (PSAT #89310, released from Ireland) being preyed upon by a (assumed) pelagic fish.
fig. S3b. Example of an eel (PSAT #49644, released from Ireland) being preyed upon by an endothermic fish.
fig. S3c. Example of an eel (PSAT #101445, released from Ireland) being preyed upon by a coastal pelagic predator.
fig. S3d. Example of an eel (PSAT #83156, released from Ireland) being preyed upon by a deep living fish.
fig. S3e. Example of an eel (PSAT #49559, released from Ireland) being preyed upon by a marine mammal.
fig. S3f. Example of an eel (PSAT #133984, released from southern France) being preyed upon by an unknown endotherm.
fig. S4. Example of daytime thermal experience of eels during migration along the Norwegian trench/deep in the Norwegian Sea.
fig. S5. Depth and temperature time series from PSAT #83140.
fig. S6. Relative frequency distribution of swimming speed.
fig. S7. Electronic tag types and attachment technique.
fig. S8. Principle and validation of behavioral geolocation.
fig. S9. Analysis of the total number of length-measured European eel leptocephali in the ICES database.
table S1. Details of reconstructed migrations of eels that reached the ocean.
table S2. Assessment of effect of tag and release country on speed, migration duration, and migration distance of eels.
table S3. The speed of European eels.
table S4a. The fate of eels that reached oceanic waters.
table S4b. The fate of all released eels for which data sets were recovered.
table S5. Summary of literature used to assess escapement date.
table S6. Silver eel escapement timing in the River Gudena, Denmark.
table S7. Release locations and numbers of eels released during the study.
table S8a. Summary metrics of tagged eels.
table S8b. Summary metrics of eels for which migratory data were recovered (±1 SD).
table S9. Metrics of all eels tagged (n = 707).


